Shark IgNAR: The Next Broad Application Antibody in Clinical Diagnoses and Tumor Therapies?
Abstract
:1. Preface
2. Structural Characteristics and Advantages of IgNAR
2.1. Limitations of mAbs
2.2. Advantages of Shark-Derived VNAR
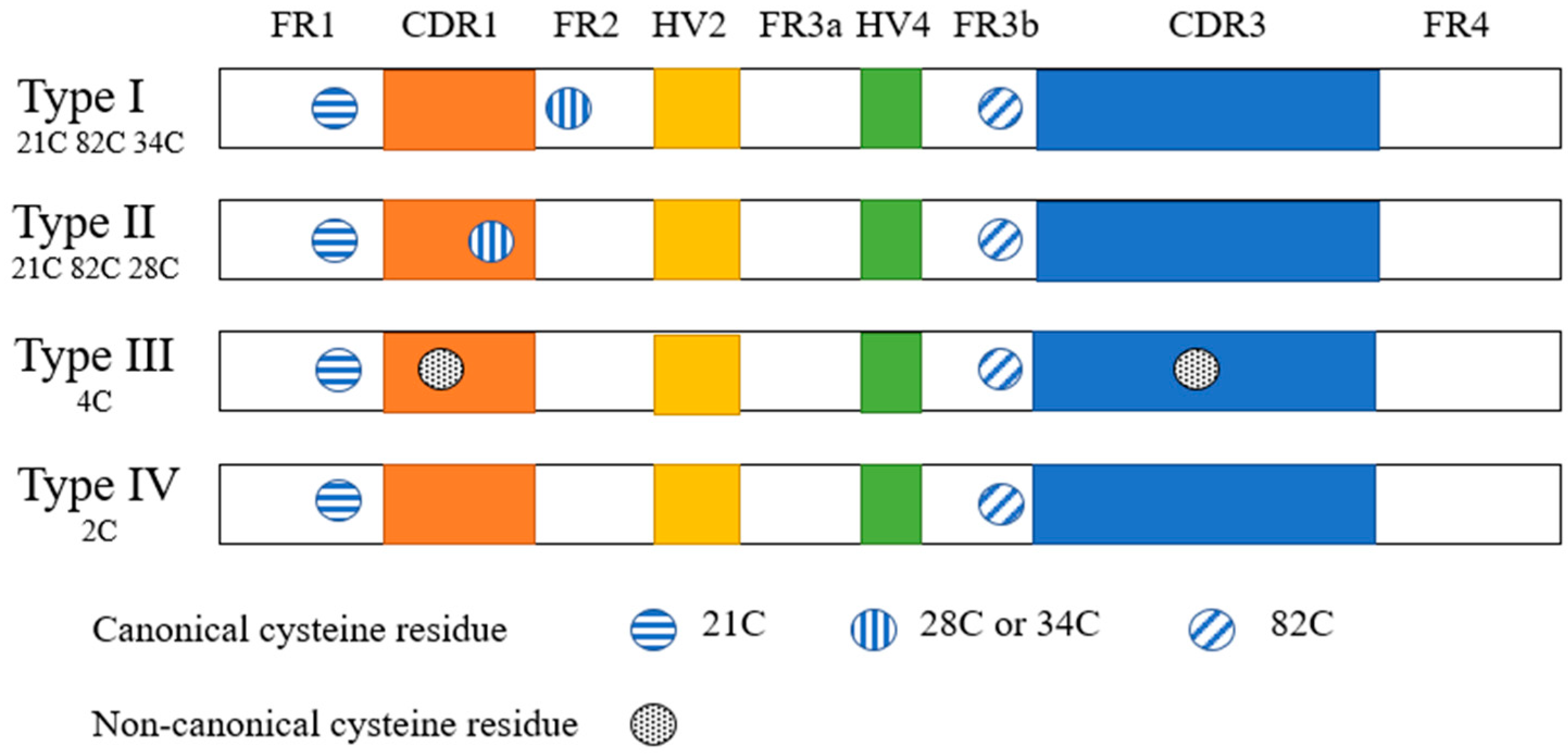
2.2.1. Structural Characteristics of Shark Monodomain Antibodies
2.2.2. Biochemical Characteristics of IgNAR
2.2.3. IgNAR Can Be Humanized
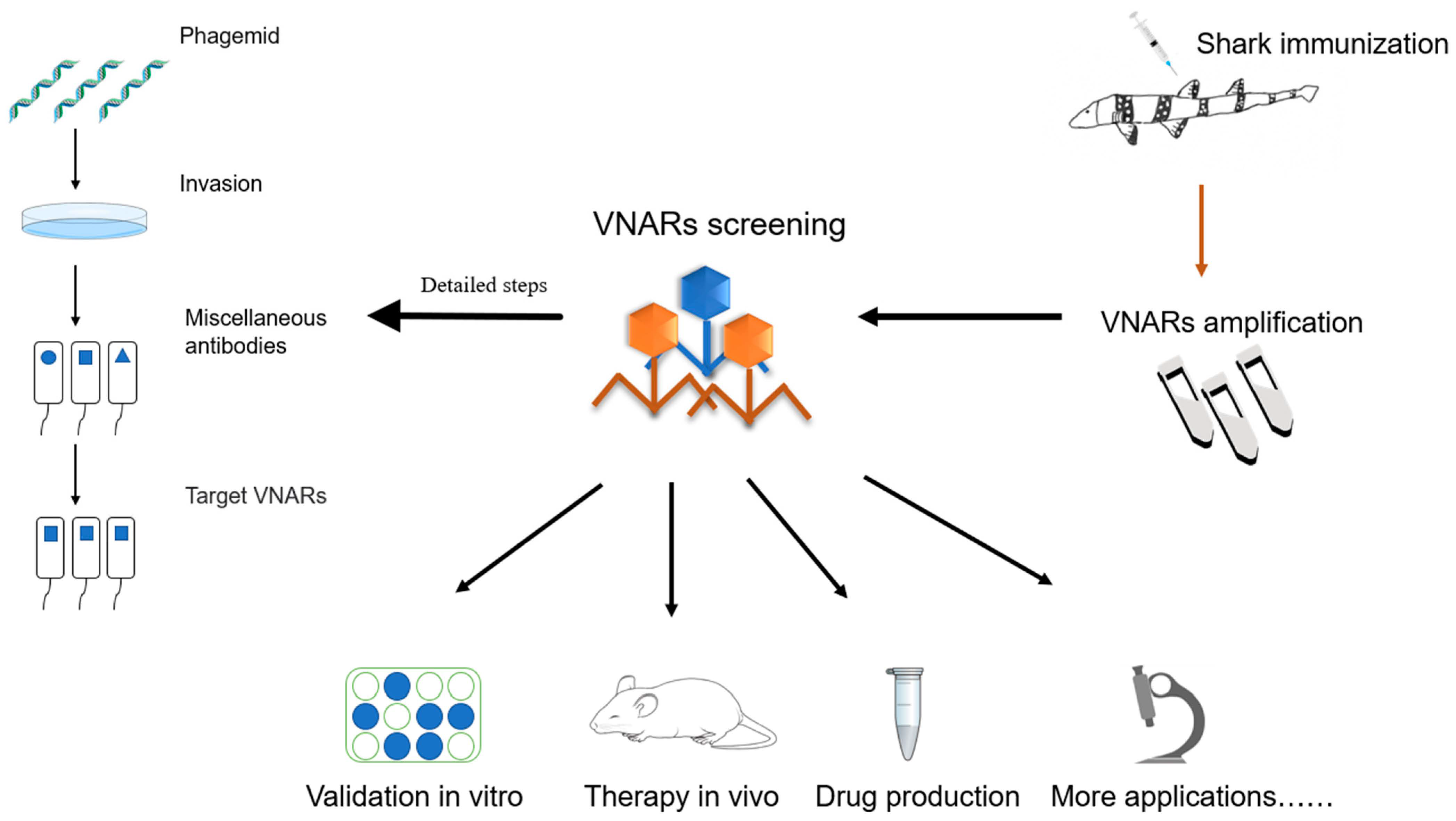
2.3. Preparation and Mass Production of Specific VNARs
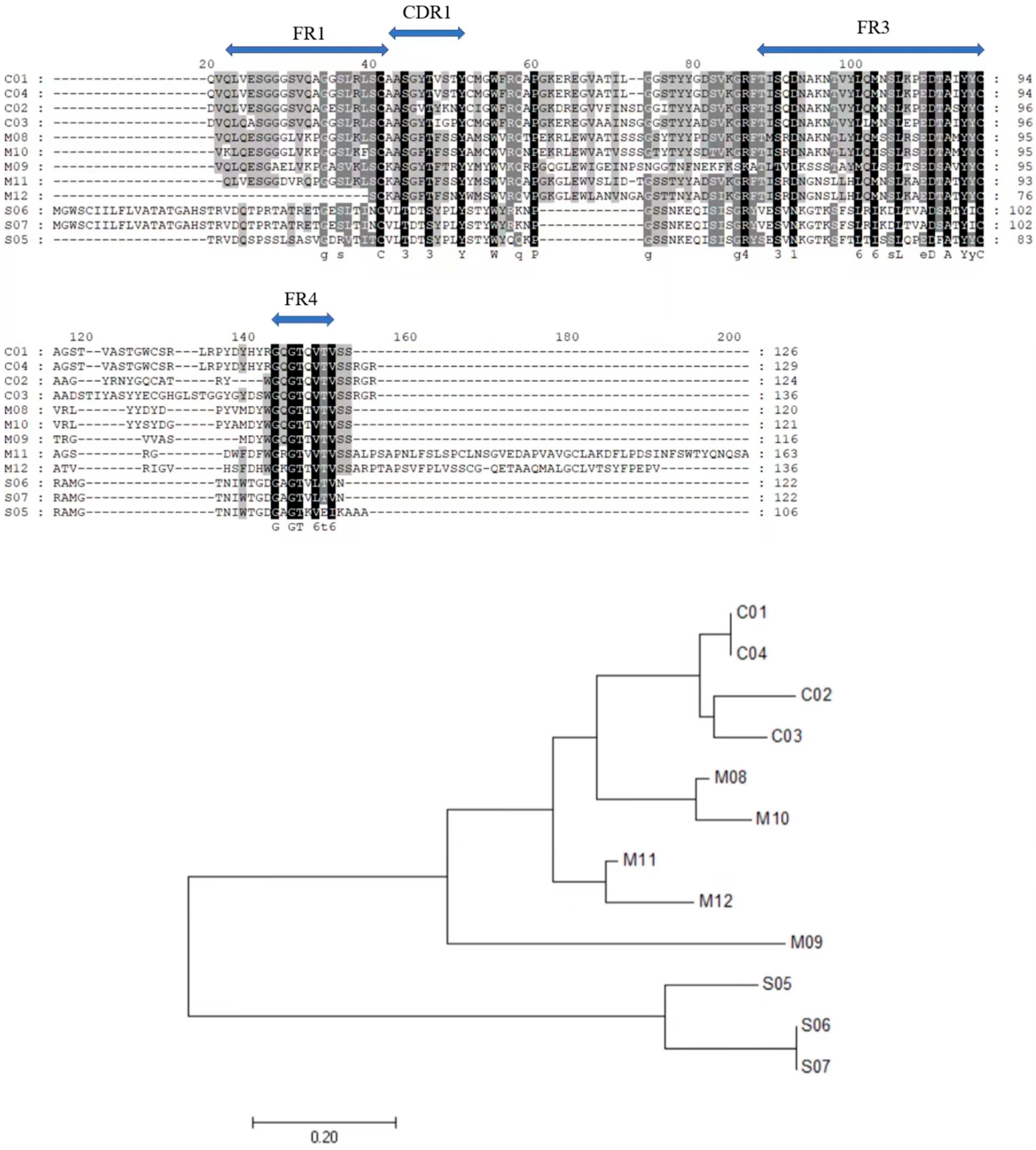
2.3.1. Use of Chiloscyllium plagiosum as a Model Organism for VNAR Preparation
2.3.2. Phage Library Screening of Specific VNARs
2.3.3. Recombinant VNAR Production
3. Potential Applications of VNAR in Preclinical Test
3.1. Advantages in Drug Development and Clinical Treatment
3.2. Exploration and Development of VNAR-Based New Drugs
3.3. Application of VNAR as an Enzyme Inhibitor
3.4. VNARs Can Penetrate Tissues and Deliver Drugs across the Blood–Brain Barrier
4. Applications in Anti-Tumor Preclinical Studies
4.1. VNARs Have Stronger Affinity to Cancer-Specific Targeting Antigens
4.2. Strong and Rapid Tissue Permeability
4.3. The Next-Generation Antibody for Anti-Tumor Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kaplon, H.; Crescioli, S.; Chenoweth, A.; Visweswaraiah, J.; Reichert, J.M. Antibodies to watch in 2023. MAbs 2023, 15, 2153410. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, M.; Ferguson, L.; Steven, J.; Porter, A.; Barelle, C. Shark variable new antigen receptor biologics—A novel technology platform for therapeutic drug development. Expert Opin. Biol. Ther. 2014, 14, 1527–1539. [Google Scholar] [CrossRef]
- Adams, G.P.; Weiner, L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.K.; Gasser, R.A.; Magill, A.J.; Miller, R.S. Update on rapid diagnostic testing for malaria. Clin. Microbiol. Rev. 2008, 21, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.R.; Liu, W.; Michelich, C.R.; Dewhirst, M.W.; Yuan, F.; Chilkoti, A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl. Cancer Inst. 2006, 98, 335–344. [Google Scholar] [CrossRef]
- Sokolov, P.; Nifontova, G.; Samokhvalov, P.; Karaulov, A.; Sukhanova, A.; Nabiev, I. Nontoxic Fluorescent Nanoprobes for Multiplexed Detection and 3D Imaging of Tumor Markers in Breast Cancer. Pharmaceutics 2023, 15, 946. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Hajduczki, A.; Martinez, E.J.; Bai, H.; Matz, H.; Hill, T.M.; Lewitus, E.; Chang, W.C.; Dawit, L.; Peterson, C.E.; et al. Shark nanobodies with potent SARS-CoV-2 neutralizing activity and broad sarbecovirus reactivity. Nat. Commun. 2023, 14, 580. [Google Scholar] [CrossRef]
- Kok, B.H.; Lim, H.T.; Lim, C.P.; Lai, N.S.; Leow, C.Y.; Leow, C.H. Dengue virus infection—A review of pathogenesis, vaccines, diagnosis and therapy. Virus Res. 2023, 324, 17. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Harb, W.; Peer, C.J.; Hua, Q.; Xu, S.Y.; Lu, H.L.; Lu, N.; He, Y.; Xu, T.; Dong, R.P.; et al. First-in-Human Phase I Study of Envafolimab, a Novel Subcutaneous Single-Domain Anti-PD-L1 Antibody, in Patients with Advanced Solid Tumors. Oncologist 2021, 26, E1514–E1525. [Google Scholar] [CrossRef]
- Andre, A.S.; Dias, J.N.R.; Aguiar, S.; Nogueira, S.; Bule, P.; Carvalho, J.I.; Antonio, J.P.M.; Cavaco, M.; Neves, V.; Oliveira, S.; et al. Rabbit derived VL single-domains as promising scaffolds to generate antibody-drug conjugates. Sci. Rep. 2023, 13, 4837. [Google Scholar] [CrossRef]
- Ward, E.S.; Gussow, D.; Griffiths, A.D.; Jones, P.T.; Winter, G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature 1989, 341, 544–546. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Avila, D.; Hughes, M.; Hughes, A.; McKinney, E.C.; Flajnik, M.F. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature 1995, 374, 168–173. [Google Scholar] [CrossRef]
- English, H.; Hong, J.; Ho, M. Ancient species offers contemporary therapeutics: An update on shark VNAR single domain antibody sequences, phage libraries and potential clinical applications. Antib. Ther. 2020, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Bian, H.; Wu, X.; Fu, T.; Fu, Y.; Hong, J.; Fleming, B.D.; Flajnik, M.F.; Ho, M. Construction and next-generation sequencing analysis of a large phage-displayed VNAR single-domain antibody library from six naive nurse sharks. Antib. Ther. 2019, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstaele, F.; Holz, J.B.; Revets, H. The development of nanobodies for therapeutic applications. Curr. Opin. Investig. Drugs 2009, 10, 1212–1224. [Google Scholar]
- Zinn, S.; Vazquez-Lombardi, R.; Zimmermann, C.; Sapra, P.; Jermutus, L.; Christ, D. Advances in antibody-based therapy in oncology. Nat. Cancer 2023, 4, 165–180. [Google Scholar] [CrossRef]
- Dooley, H.; Stanfield, R.L.; Brady, R.A.; Flajnik, M.F. First molecular and biochemical analysis of in vivo affinity maturation in an ectothermic vertebrate. Proc. Natl. Acad. Sci. USA 2006, 103, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Stanfield, R.L.; Dooley, H.; Verdino, P.; Flajnik, M.F.; Wilson, I.A. Maturation of shark single-domain (IgNAR) antibodies: Evidence for induced-fit binding. J. Mol. Biol. 2007, 367, 358–372. [Google Scholar] [CrossRef]
- Li, D.; English, H.; Hong, J.; Liang, T.Y.Z.; Merlino, G.; Day, C.P.; Ho, M. A novel PD-L1-targeted shark V-NAR single-domain-based CAR-T cell strategy for treating breast cancer and liver cancer. Mol. Ther. Oncolytics 2022, 24, 849–863. [Google Scholar] [CrossRef]
- Pothin, E.; Lesuisse, D.; Lafaye, P. Brain Delivery of Single-Domain Antibodies: A Focus on VHH and VNAR. Pharmaceutics 2020, 12, 937. [Google Scholar]
- Henderson, K.A.; Streltsov, V.A.; Coley, A.M.; Dolezal, O.; Hudson, P.J.; Batchelor, A.H.; Gupta, A.; Bai, T.; Murphy, V.J.; Anders, R.F.; et al. Structure of an IgNAR-AMA1 complex: Targeting a conserved hydrophobic cleft broadens malarial strain recognition. Structure 2007, 15, 1452–1466. [Google Scholar] [CrossRef] [PubMed]
- Barelle, C.; Gill, D.S.; Charlton, K. Shark Novel Antigen Receptors-The Next Generation of Biologic Therapeutics? In Pharmaceutical Biotechnology; Guzman, C.A., Feuerstein, G.Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 49–62. [Google Scholar]
- Zielonka, S.; Empting, M.; Grzeschik, J.; Konning, D.; Barelle, C.J.; Kolmar, H. Structural insights and biomedical potential of IgNAR scaffolds from sharks. MAbs 2015, 7, 15–25. [Google Scholar] [CrossRef]
- Jorgensen, P.; Chanthap, L.; Rebueno, A.; Tsuyuoka, R.; Bell, D. Malaria rapid diagnostic tests in tropical climates: The need for a cool chain. Am. J. Trop. Med. Hyg. 2006, 74, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Lin, J.J.; Ma, H.; Zhong, N.; Xie, X.X.; Yang, Y.R.; Zheng, P.Y.; Zhang, L.J.; Jin, T.C.; Cao, M.J. Screening and Characterization of Shark-Derived VNARs against SARS-CoV-2 Spike RBD Protein. Int. J. Mol. Sci. 2022, 23, 10904. [Google Scholar] [CrossRef] [PubMed]
- Ubah, O.C.; Lake, E.W.; Gunaratne, G.S.; Gallant, J.P.; Fernie, M.; Robertson, A.J.; Marchant, J.S.; Bold, T.D.; Langlois, R.A.; Matchett, W.E.; et al. Mechanisms of SARS-CoV-2 neutralization by shark variable new antigen receptors elucidated through X-ray crystallography. Nat. Commun. 2021, 12, 7325. [Google Scholar] [CrossRef] [PubMed]
- An EUA for Bamlanivimab-A Monoclonal Antibody for COVID-19. Med. Lett. Drugs Ther. 2021, 325, 880–881.
- Juma, S.N.; Gong, X.X.; Hu, S.J.; Lv, Z.B.; Shao, J.Z.; Liu, L.L.; Chen, G.Q. Shark New Antigen Receptor (IgNAR): Structure, Characteristics and Potential Biomedical Applications. Cells 2021, 10, 1140. [Google Scholar] [CrossRef]
- Kovalenko, O.V.; Olland, A.; Piche-Nicholas, N.; Godbole, A.; King, D.; Svenson, K.; Calabro, V.; Muller, M.R.; Barelle, C.J.; Somers, W.; et al. Atypical Antigen Recognition Mode of a Shark Immunoglobulin New Antigen Receptor (IgNAR) Variable Domain Characterized by Humanization and Structural Analysis. J. Biol. Chem. 2013, 288, 17408–17419. [Google Scholar] [CrossRef]
- Dooley, H.; Flajnik, M.F.; Porter, A.J. Selection and characterization of naturally occurring single-domain (IgNAR) antibody fragments from immunized sharks by phage display. Mol. Immunol. 2003, 40, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.R.; Saunders, K.; Grace, C.; Jin, M.; Piche-Nicholas, N.; Steven, J.; O’Dwyer, R.; Wu, L.Y.; Khetemenee, L.; Vugmeyster, Y.; et al. Improving the pharmacokinetic properties of biologics by fusion to an anti-HSA shark VNAR domain. MAbs 2012, 4, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Streltsov, V.A.; Carmichael, J.A.; Nuttall, S.D. Structure of a shark IgNAR antibody variable domain and modeling of an early-developmental isotype. Protein Sci. 2005, 14, 2901–2909. [Google Scholar] [CrossRef]
- Jia, L.; Wang, Y.; Shen, Y.J.; Zhong, B.; Luo, Z.; Yang, J.J.; Chen, G.D.; Jiang, X.F.; Chen, J.Q.; Lyu, Z. IgNAR characterization and gene loci identification in whitespotted bamboo shark (Chiloscyllium plagiosum) genome. Fish Shellfish Immunol. 2023, 133, 9. [Google Scholar] [CrossRef]
- Wei, L.K.; Wang, M.N.; Xiang, H.T.; Jiang, Y.; Gong, J.H.; Su, D.; Al Azad, M.A.R.; Dong, H.M.; Feng, L.M.; Wu, J.J.; et al. Bamboo Shark as a Small Animal Model for Single Domain Antibody Production. Front. Bioeng. Biotechnol. 2021, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Dolezal, O.; Parisi, K.; Angerosa, J.; Dogovski, C.; Barraclough, M.; Sanalla, A.; Casey, J.; González, I.; Perugini, M.; et al. Shark Variable New Antigen Receptor (VNAR) Single Domain Antibody Fragments: Stability and Diagnostic Applications. Antibodies 2013, 2, 66–81. [Google Scholar] [CrossRef]
- Ho, M. Inaugural Editorial: Searching for Magic Bullets. Antib. Ther. 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural Single-Domain Antibodies. In Annual Review of Biochemistry; Kornberg, R.D., Ed.; Annual Reviews: Palo Alto, CA, USA, 2013; Volume 82, pp. 775–797. [Google Scholar]
- Ho, M. Perspectives on the development of neutralizing antibodies against SARS-CoV-2. Antib. Ther. 2020, 3, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Dooley, H.; Flajnik, M.F. Antibody repertoire development in cartilaginous fish. Dev. Comp. Immunol. 2006, 30, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, S.D. Overview and discovery of IgNARs and generation of VNARs. Single Domain Antibodies Methods Protoc. 2012, 911, 27–36. [Google Scholar]
- Steven, J.; Muller, M.R.; Carvalho, M.F.; Ubah, O.C.; Kovaleva, M.; Donohoe, G.; Baddeley, T.; Cornock, D.; Saunders, K.; Porter, A.J.; et al. In Vitro Maturation of a humanized shark Vnar Domain to improve its Biophysical Properties to Facilitate clinical Development. Front. Immunol. 2017, 8, 15. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Sun, Y.; Hong, J.; Ho, M. Humanization of the Shark VNAR Single Domain Antibody Using CDR Grafting. Curr. Protoc. 2023, 3, e630. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Quintero, M.L.; Fischer, A.L.M.; Kokot, J.; Waibl, F.; Seidler, C.A.; Liedl, K.R. The influence of antibody humanization on shark variable domain (VNAR) binding site ensembles. Front. Immunol. 2022, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Rossotti, M.A.; Belanger, K.; Henry, K.A.; Tanha, J. Immunogenicity and humanization of single-domain antibodies. FEBS J. 2022, 289, 4304–4327. [Google Scholar] [CrossRef] [PubMed]
- De Greve, H.; Virdi, V.; Bakshi, S.; Depicker, A. Simplified monomeric VHH-Fc antibodies provide new opportunities for passive immunization. Curr. Opin. Biotechnol. 2020, 61, 96–101. [Google Scholar] [CrossRef]
- Liu, H.P.; Schittny, V.; Nash, M.A. Removal of a Conserved Disulfide Bond Does Not Compromise Mechanical Stability of a VHH Antibody Complex. Nano Lett. 2019, 19, 5524–5529. [Google Scholar] [CrossRef]
- Flajnik, M.F. A cold-blooded view of adaptive immunity. Nat. Rev. Immunol. 2018, 18, 438–453. [Google Scholar] [CrossRef]
- Leow, C.H.; Fischer, K.; Leow, C.Y.; Braet, K.; Cheng, Q.; McCarthy, J. Isolation and characterization of malaria PfHRP2 specific V-NAR antibody fragments from immunized shark phage display library. Malar. J. 2018, 17, 15. [Google Scholar] [CrossRef]
- Burciaga-Flores, M.; Marquez-Aguirre, A.L.; Duenas, S.; Gasperin-Bulbarela, J.; Licea-Navarro, A.F.; Camacho-Villegas, T.A. First pan-specific vNAR against human TGF-beta as a potential therapeutic application: In silico modeling assessment. Sci. Rep. 2023, 13, 3596. [Google Scholar] [CrossRef]
- Crouch, K.; Smith, L.E.; Williams, R.; Cao, W.; Lee, M.; Jensen, A.; Dooley, H. Humoral immune response of the small-spotted catshark, Scyliorhinus canicular. Fish Shellfish Immunol. 2013, 34, 1158–1169. [Google Scholar] [CrossRef]
- Chen, W.K.; Liu, M.K. Reproductive biology of whitespotted bamboo shark Chiloscyllium plagiosum in northern waters off Taiwan. Fisheries Science. 2006, 72, 1215–1224. [Google Scholar] [CrossRef]
- Zhao, L.F.; Chen, M.L.; Wang, X.N.; Kang, S.K.; Xue, W.W.; Li, Z.P. Identification of Anti-TNF alpha VNAR Single Domain Antibodies from Whitespotted Bambooshark (Chiloscyllium plagiosum). Mar. Drugs 2022, 20, 307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Gao, H.Y.; Li, H.B.; Guo, J.; Ouyang, B.J.; Wang, M.N.; Xu, Q.W.; Wang, J.H.; Lv, M.Q.; Guo, X.Y.; et al. The White-Spotted Bamboo Shark Genome Reveals Chromosome Rearrangements and Fast-Evolving Immune Genes of Cartilaginous Fish. iScience 2020, 23, 53. [Google Scholar] [CrossRef]
- Dong, H.M.; Zhang, Y.L.; Wang, J.H.; Xiang, H.T.; Lv, T.H.; Wei, L.K.; Yang, S.S.; Liu, X.P.; Ren, B.Z.; Zhang, X.Q.; et al. Cas9-Based Local Enrichment and Genomics Sequence Revision of Megabase-Sized Shark IgNAR Loci. J. Immunol. 2022, 208, 181–189. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.P.N.; Tan, E.; Mizuno, N.; Hosoya, S.; Reza, M.S.; Watabe, S.; Kinoshita, S.; Asakawa, S. Transcriptomic analysis of immunoglobulin novel antigen receptor (IgNAR) heavy chain constant domains of brownbanded bamboo shark (Chiloscyllium punctatum). Fish Shellfish Immunol. 2019, 84, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.D.; Ding, W.; Zhu, L.; Zhou, Y.H.; Dong, Y.K.; Li, L.; Liu, J.J.; Wang, Y.Z.; Li, Z.H.; Zhu, L.N.; et al. Screening and characterization of inhibitory vNAR targeting nanodisc-assembled influenza M2 proteins. iScience 2023, 26, 21. [Google Scholar] [CrossRef] [PubMed]
- Ryckaert, S.; Pardon, E.; Steyaert, J.; Callewaert, N. Isolation of antigen-binding camelid heavy chain antibody fragments (nanobodies) from an immune library displayed on the surface of Pichia pastoris. J. Biotechnol. 2010, 145, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Fleetwood, F.; Devoogdt, N.; Pellis, M.; Wernery, U.; Muyldermans, S.; Stahl, S.; Lofblom, J. Surface display of a single-domain antibody library on Gram-positive bacteria. Cell. Mol. Life Sci. 2013, 70, 1081–1093. [Google Scholar] [CrossRef]
- Konning, D.; Zielonka, S.; Grzeschik, J.; Empting, M.; Valldorfl, B.; Krah, S.; Schroter, C.; Sellmann, C.; Hock, B.; Kolmarl, H. Camelid and shark single domain antibodies: Structural features and therapeutic potential. Curr. Opin. Struct. Biol. 2017, 45, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Yau, K.Y.F.; Groves, M.A.T.; Li, S.; Sheedy, C.; Lee, H.; Tanha, J.; MacKenzie, C.R.; Jermutus, L.; Hall, J.C. Selection of hapten-specific single-domain antibodies from a non-immunized llama ribosome display library. J. Immunol. Methods 2003, 281, 161–175. [Google Scholar] [CrossRef]
- Monegal, A.; Ami, D.; Martinelli, C.; Huang, H.; Aliprandi, M.; Capasso, P.; Francavilla, C.; Ossolengo, G.; de Marco, A. Immunological applications of single-domain llama recombinant antibodies isolated from a naive library. Protein Eng. Des. Sel. 2009, 22, 273–280. [Google Scholar] [CrossRef]
- Goldman, E.R.; Anderson, G.P.; Liu, J.L.; Delehanty, J.B.; Sherwood, L.J.; Osborn, L.E.; Cummins, L.B.; Hayhurst, A. Facile generation of heat-stable antiviral and antitoxin single domain antibodies from a semisynthetic llama library. Anal. Chem. 2006, 78, 8245–8255. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.R.; Li, G.H.; Hu, Y.H.; Ou, W.J.; Wan, Y.K. Construction of a synthetic phage-displayed Nanobody library with CDR3 regions randomized by trinucleotide cassettes for diagnostic applications. J. Transl. Med. 2014, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Palacios, A.M.; Grzeschik, J.; Deweid, L.; Krah, S.; Zielonka, S.; Rosner, T.; Peipp, M.; Valerius, T.; Kolmar, H. Specific Targeting of Lymphoma Cells Using Semisynthetic Anti-Idiotype Shark Antibodies. Front. Immunol. 2020, 11, 15. [Google Scholar]
- McCafferty, J.; Griffiths, A.D.; Winter, G.; Chiswell, D.J. Phage antibodies: Filamentous phage displaying antibody variable domains. Nature 1990, 348, 552–554. [Google Scholar] [CrossRef]
- Muller, M.R.; O’Dwyer, R.; Kovaleva, M.; Rudkin, F.; Dooley, H.; Barelle, C.J. Generation and isolation of target-specific single-domain antibodies from shark immune repertoires. Methods Mol. Biol. 2012, 907, 177–194. [Google Scholar] [PubMed]
- Shao, C.Y.; Secombes, C.J.; Porter, A.J. Rapid isolation of IgNAR variable single-domain antibody fragments from a shark synthetic library. Mol. Immunol. 2007, 44, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.; Nuttall, S.; Revill, P.; Colledge, D.; Cabuang, L.; Soppe, S.; Dolezal, O.; Griffiths, K.; Bartholomeusz, A.; Locarnini, S. Targeting the hepatitis B virus precore antigen with a novel IgNAR single variable domain intrabody. Virology 2011, 411, 132–141. [Google Scholar] [CrossRef]
- Ohtani, M.; Hikima, J.; Jung, T.S.; Kondo, H.; Hirono, I.; Aoki, T. Construction of an Artificially Randomized IgNAR Phage Display Library: Screening of Variable Regions that Bind to Hen Egg White Lysozyme. Mar. Biotechnol. 2013, 15, 56–62. [Google Scholar] [CrossRef]
- Ohtani, M.; Hikima, J.; Jung, T.S.; Kondo, H.; Hirono, I.; Takeyama, H.; Aoki, T. Variable domain antibodies specific for viral hemorrhagic septicemia virus (VHSV) selected from a randomized IgNAR phage display library. Fish Shellfish. Immunol. 2013, 34, 724–728. [Google Scholar] [CrossRef]
- Duan, Z.; Buffington, J.; Hong, J.; Ho, M. Production and Purification of Shark and Camel Single-Domain Antibodies from Bacterial and Mammalian Cell Expression Systems. Curr. Protoc. 2022, 2, e459. [Google Scholar] [CrossRef]
- Leow, C.H.; Fischer, K.; Leow, C.Y.; Cheng, Q.; Chuah, C.; McCarthy, J. Single Domain Antibodies as New Biomarker Detectors. Diagnostics 2017, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Konning, D.; Kolmar, H. Beyond antibody engineering: Directed evolution of alternative binding scaffolds and enzymes using yeast surface display. Microb. Cell. Fact. 2018, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Ubah, O.C.; Porter, A.J.; Barelle, C.J. In Vitro ELISA and Cell-Based Assays Confirm the Low Immunogenicity of VNAR Therapeutic Constructs in a Mouse Model of Human RA: An Encouraging Milestone to Further Clinical Drug Development. J. Immunol. Res. 2020, 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Cataland, S.R.; Peyvandi, F.; Coppo, P.; Knobl, P.; Hovinga, J.A.K.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Callewaert, F.; et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2019, 380, 335–346. [Google Scholar] [CrossRef]
- Cao, Q.H.; Huang, C.L.; Yi, H.; Gill, A.J.; Chou, A.; Foley, M.; Hosking, C.G.; Lim, K.K.; Triffon, C.F.; Shi, Y.; et al. A single-domain i-body, AD-114, attenuates renal fibrosis through blockade of CXCR4. JCI Insight 2022, 7, 19. [Google Scholar] [CrossRef]
- Clarke, E.; Stocki, P.; Sinclair, E.H.; Gauhar, A.; Fletcher, E.J.R.; Krawczun-Rygmaczewska, A.; Duty, S.; Walsh, F.S.; Doherty, P.; Rutkowski, J.L. A Single Domain Shark Antibody Targeting the Transferrin Receptor 1 Delivers a TrkB Agonist Antibody to the Brain and Provides Full Neuroprotection in a Mouse Model of Parkinson’s Disease. Pharmaceutics 2022, 14, 1335. [Google Scholar] [CrossRef]
- Jain, S.; Doshi, A.S.; Iyer, A.K.; Amiji, M.M. Multifunctional nanoparticles for targeting cancer and inflammatory diseases. J. Drug Target 2013, 21, 888–903. [Google Scholar] [CrossRef]
- Peyvandi, F.; Scully, M.; Hovinga, J.A.K. Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2016, 374, 511–522. [Google Scholar] [CrossRef]
- Cunningham, S.P.A.; Piedra, F.; Martinon-Torres, H.; Szymanski, B.; Brackeva, E.; Dombrecht, L.; Detalle, C.; Fleurinck, R.S. Grp, Nebulised ALX-0171 for respiratory syncytial virus lower respiratory tract infection in hospitalised children: A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Resp. Med. 2021, 9, 21–32. [Google Scholar] [CrossRef]
- Kim, G.W.; Lee, N.R.; Pi, R.H.; Lim, Y.S.; Lee, Y.M.; Lee, J.M.; Jeong, H.S.; Chung, S.H. IL-6 inhibitors for treatment of rheumatoid arthritis: Past, present, and future. Arch. Pharm. Res. 2015, 38, 575–584. [Google Scholar] [CrossRef]
- Stanfield, R.L.; Dooley, H.; Flajnik, M.F.; Wilson, I.A. Crystal structure of a shark single-domain antibody V region in complex with lysozyme. Science 2004, 305, 1770–1773. [Google Scholar] [CrossRef]
- Husain, B.; Ellerman, D. Expanding the Boundaries of Biotherapeutics with Bispecific Antibodies. Biodrugs 2018, 32, 441–464. [Google Scholar] [CrossRef]
- Feldwisch, J.; Tolmachev, V. Engineering of affibody molecules for therapy and diagnostics. Methods Mol. Biol. 2012, 899, 103–126. [Google Scholar] [PubMed]
- Torchia, J.; Weiskopf, K.; Levy, R. Targeting lymphoma with precision using semisynthetic anti-idiotype peptibodies. Proc. Natl. Acad. Sci. USA 2016, 113, 5376–5381. [Google Scholar] [CrossRef]
- Mir, M.A.; Mehraj, U.; Sheikh, B.A.; Hamdani, S.S. Nanobodies: The “Magic Bullets” in therapeutics, drug delivery and diagnostics. Hum. Antibodies 2020, 28, 29–51. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Zhu, J.; Nie, Y.; Hu, R.; Wang, T.; Li, P.; Zhang, Q.; Yang, Y. Nanobody Technology for Mycotoxin Detection in the Field of Food Safety: Current Status and Prospects. Toxins 2018, 10, 180. [Google Scholar] [CrossRef]
- Chakravarty, R.; Goel, S.; Cai, W.B. Nanobody: The “Magic Bullet” for Molecular Imaging? Theranostics 2014, 4, 386–398. [Google Scholar] [CrossRef]
- Kong, Q.; Yao, Y.; Chen, R.; Lu, S. Progress in nanobody and its application in diagnosis. Chin. J. Biotechnol. 2014, 30, 1351–1361. [Google Scholar]
- Siontorou, C.G. Nanobodies as novel agents for disease diagnosis and therapy. Int. J. Nanomed. 2013, 8, 4215–4227. [Google Scholar] [CrossRef]
- Gauhar, A.; Privezentzev, C.V.; Demydchuk, M.; Gerlza, T.; Rieger, J.; Kungl, A.J.; Walsh, F.S.; Rutkowski, J.L.; Stocki, P. Single domain shark VNAR antibodies neutralize SARS-CoV-2 infection in vitro. FASEB J. 2021, 35, 13. [Google Scholar] [CrossRef]
- Valdovino-Navarro, B.J.; Duenas, S.; Flores-Acosta, G.I.; Gasperin-Bulbarela, J.; Bernaldez-Sarabia, J.; Cabanillas-Bernal, O.; Cervantes-Luevano, K.E.; Licea-Navarro, A.F. Neutralizing Ability of a Single Domain VNAR Antibody: In Vitro Neutralization of SARS-CoV-2 Variants of Concern. Int. J. Mol. Sci. 2022, 23, 12267. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Chen, Z.L.; Sun, J.; Xu, T.T.; Wang, Q.; Yi, H.S.; Niu, X.F.; Zhu, J.B.; Fan, M.Z.; Hou, R.T.; et al. A Class of Shark-Derived Single-Domain Antibodies can Broadly Neutralize SARS-Related Coronaviruses and the Structural Basis of Neutralization and Omicron Escape. Small Methods 2022, 6, 12. [Google Scholar] [CrossRef]
- Lauwereys, M.; Ghahroudi, M.A.; Desmyter, A.; Kinne, J.; Holzer, W.; De Genst, E.; Wyns, L.; Muyldermans, S. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J. 1998, 17, 3512–3520. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.G.; Oleksy, A.; Cavazza, T.; Richards, M.W.; Vernos, I.; Matthews, D.; Bayliss, R. Allosteric inhibition of Aurora-A kinase by a synthetic vNAR domain. Open Biol. 2016, 6, 10. [Google Scholar] [CrossRef]
- Stocki, P.; Szary, J.; Rasmussen, C.L.M.; Demydchuk, M.; Northall, L.; Logan, D.B.; Gauhar, A.; Thei, L.; Moos, T.; Walsh, F.S.; et al. Blood-brain barrier transport using a high affinity, brain-selective VNAR antibody targeting transferrin receptor 1. FASEB J. 2021, 35, 19. [Google Scholar] [CrossRef]
- Kovaleva, M.; Johnson, K.; Steven, J.; Barelle, C.J.; Porter, A. Therapeutic Potential of Shark Anti-ICOSL VNAR Domains is Exemplified in a Murine Model of Autoimmune Non-Infectious Uveitis. Front. Immunol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Camacho-Villegas, T.A.; Mata-Gonzalez, M.T.; Garcia-Ubbelohd, W.; Nunez-Garcia, L.; Elosua, C.; Paniagua-Solis, J.F.; Licea-Navarro, A.F. Intraocular Penetration of a vNAR: In Vivo and In Vitro VEGF(165) Neutralization. Mar. Drugs 2018, 16, 13. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Chen, L.P. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2019, 175, 313–326. [Google Scholar] [CrossRef]
- Xenaki, K.T.; Dorrestijn, B.; Muns, J.A.; Adamzek, K.; Doulkeridou, S.; Houthoff, H.; Oliveira, S.; Henegouwen, P. Homogeneous tumor targeting with a single dose of HER2-targeted albumin-binding domain-fused nanobody-drug conjugates results in long-lasting tumor remission in mice. Theranostics 2021, 11, 5525–5538. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Munn, L.L.; Jain, R.K. Reengineering the Physical Microenvironment of Tumors to Improve Drug Delivery and Efficacy: From Mathematical Modeling to Bench to Bedside. Trends Cancer 2018, 4, 292–319. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Vasalou, C.; Helmlinger, G.; Gomes, B. A Mechanistic Tumor Penetration Model to Guide Antibody Drug Conjugate Design. PLoS ONE 2015, 10, 20. [Google Scholar] [CrossRef]
- Thurber, G.M.; Schmidt, M.M.; Wittrup, K.D. Antibody tumor penetration: Transport opposed by systemic and antigen-mediated clearance. Adv. Drug Deliv. Rev. 2008, 60, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Xenaki, K.T.; Oliveira, S.; Henegouwen, P. Antibody or Antibody Fragments: Implications for Molecular Imaging and Targeted Therapy of Solid Tumors. Front. Immunol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Adams, G.P.; Schier, R.; Marshall, K.; Wolf, E.J.; McCall, A.M.; Marks, J.D.; Weiner, L.M. Increased affinity leads to improved selective tumor delivery of single-chain Fv antibodies. Cancer Res. 1998, 58, 485–490. [Google Scholar]
- Cilliers, C.; Guo, H.; Liao, J.S.; Christodolu, N.; Thurber, G.M. Multiscale Modeling of Antibody-Drug Conjugates: Connecting Tissue and Cellular Distribution to Whole Animal Pharmacokinetics and Potential Implications for Efficacy. AAPS J. 2016, 18, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Leach, A.; Smyth, P.; Ferguson, L.; Steven, J.; Greene, M.K.; Branco, C.M.; McCann, A.P.; Porter, A.; Barelle, C.J.; Scott, C.J. Anti-DLL4 VNAR targeted nanoparticles for targeting of both tumour and tumour associated vasculature. Nanoscale 2020, 12, 14751–14763. [Google Scholar] [CrossRef]
- Ubah, O.C.; Steven, J.; Kovaleva, M.; Ferguson, L.; Barelle, C.; Porter, A.J.R.; Barelle, C.J. Novel, Anti-hTNF-alpha Variable New Antigen Receptor Formats with Enhanced Neutralizing Potency and Multifunctionality, Generated for Therapeutic Development. Front. Immunol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Ubah, O.C.; Steven, J.; Porter, A.J.; Barelle, C.J. An Anti-hTNF-alpha Variable New Antigen Receptor Format Demonstrates Superior in vivo Preclinical Efficacy to Humira (R) in a Transgenic Mouse Autoimmune Polyarthritis Disease Model. Front. Immunol. 2019, 10, 12. [Google Scholar] [CrossRef]
- Broos, K.; Lecocq, Q.; Xavier, C.; Bridoux, J.; Nguyen, T.T.; Corthals, J.; Schoonooghe, S.; Lion, E.; Raes, G.; Keyaerts, M.; et al. Evaluating a Single Domain Antibody Targeting Human PD-L1 as a Nuclear Imaging and Therapeutic Agent. Cancers 2019, 11, 872. [Google Scholar] [CrossRef]
- Awad, R.M.; Lecocq, Q.; Zeven, K.; Ertveldt, T.; De Beck, L.; Ceuppens, H.; Broos, K.; De Vlaeminck, Y.; Goyvaerts, C.; Verdonck, M.; et al. Formatting and gene-based delivery of a human PD-L1 single domain antibody for immune checkpoint blockade. Mol. Ther. Methods Clin. Dev. 2021, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nishikawa, M.; Kamisaki, K.; Hachiya, S.; Nakamura, M.; Kuwazuru, T.; Tanimura, S.; Soyano, K.; Takeda, K. Marine-derived microbes and molecules for drug discovery. Inflamm. Regen. 2022, 42, 12. [Google Scholar] [CrossRef] [PubMed]
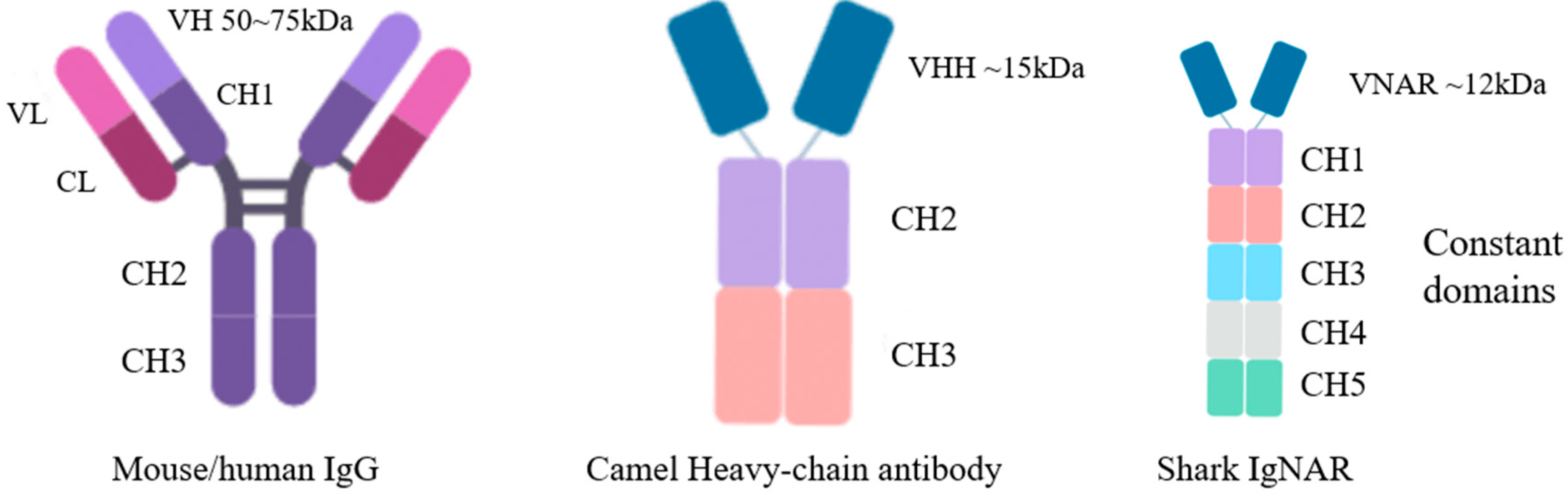

| V Region of mAbs | VHH | VNAR | ||
|---|---|---|---|---|
| Structural complexity | Complex post-translational modifications (glycoproteins) | +++ | — | — |
| Expression in prokaryotic cells | — | +++ | +++ | |
| Molecular weight | molecular weight (~kDa) | 50~75 | ~15 | ~12 |
| Tissue penetration (BBB, ocular model, placental barrier) | — | ++ | +++ | |
| Structural stability | Physiological and biochemical environment (high temperature, pH, urea) | — | +++ | +++ |
| Shortcomings | Difficulty of humanization | — | + | +++ |
| Drug | Application | Source | Clinical Trial Phase | Ref. |
|---|---|---|---|---|
| Caplacizumab | aTTP | VHH | 2019 FDA-approved | [76] |
| AD214 | Idiopathic pulmonary fibrosis | VNAR | Phase I | [77] |
| TXB4 | Primary central nervous system Lymphoma | VNAR | Preclinical | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Sun, L.; Hu, C.; Zheng, F.; Lyu, Z.; Shao, J. Shark IgNAR: The Next Broad Application Antibody in Clinical Diagnoses and Tumor Therapies? Mar. Drugs 2023, 21, 496. https://doi.org/10.3390/md21090496
Jiang X, Sun L, Hu C, Zheng F, Lyu Z, Shao J. Shark IgNAR: The Next Broad Application Antibody in Clinical Diagnoses and Tumor Therapies? Marine Drugs. 2023; 21(9):496. https://doi.org/10.3390/md21090496
Chicago/Turabian StyleJiang, Xiaofeng, Ling Sun, Chengwu Hu, Feijian Zheng, Zhengbing Lyu, and Jianzhong Shao. 2023. "Shark IgNAR: The Next Broad Application Antibody in Clinical Diagnoses and Tumor Therapies?" Marine Drugs 21, no. 9: 496. https://doi.org/10.3390/md21090496
APA StyleJiang, X., Sun, L., Hu, C., Zheng, F., Lyu, Z., & Shao, J. (2023). Shark IgNAR: The Next Broad Application Antibody in Clinical Diagnoses and Tumor Therapies? Marine Drugs, 21(9), 496. https://doi.org/10.3390/md21090496







