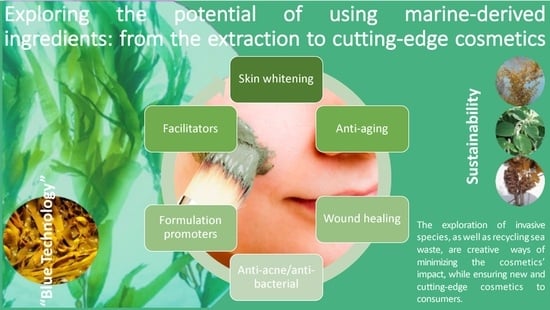
Exploring the Potential of Using Marine-Derived Ingredients: From the Extraction to Cutting-Edge Cosmetics
Abstract
:1. Introduction
2. Methods
3. Main Marine-Derived Cosmetic Ingredients
3.1. Skin-Whitening Agents
3.2. Anti-Aging Activity
3.2.1. Photoaging and Photo-Protective Activity
3.2.2. Inhibition of MMPs (Matrix Metalloproteinases)
3.2.3. Antioxidant Activity
Carotenoids
MAAs and Other Pigments
Polysaccharides and Oligosaccharides
3.2.4. Anti-Inflammatory and Wound-Healing Ingredients
Polysaccharides and Oligosaccharides
Phlorotannins
Coral-Derived Pseudopterosins
Sea Cucumber-Derived Fatty Acids
3.2.5. Collagen
3.3. Anti-Acne Activity
3.4. Formulation Promoters and Facilitators
3.4.1. Sea Water
3.4.2. Polysaccharides as Gel-Forming Agents and Viscosity Controllers
3.4.3. Carotenoids, Chlorophylls and Phycobilins as Colorant and Dyes
3.4.4. Marine Biosurfactants
3.4.5. Preservatives
4. Extraction of Marine-Derived Cosmetic Ingredients: Challenges and Implications
4.1. Challenges Regarding the Extraction and Utilization of Cosmetic Ingredients
4.1.1. Biological and Technical Challenges
4.1.2. Sustainable Supply
4.1.3. Reproducibility of Extracts and Challenges in the Scale-Up Process
4.1.4. Potential Contamination of Raw Material and Extracts
Biological Contamination
Chemical Contamination
4.2. Public Policy and Regulatory Framework
CEN/TR 17611 Algae and Algae Products—Specifications for Cosmetic Sector Applications
5. Sustainability and Marine Biotechnology in the Cosmetic Industry
5.1. Ensuring Sustainability in Marine-Derived Cosmetics: A Life-Cycle Approach
Fundamentals of Sustainability and Its Application in the Cosmetic Industry
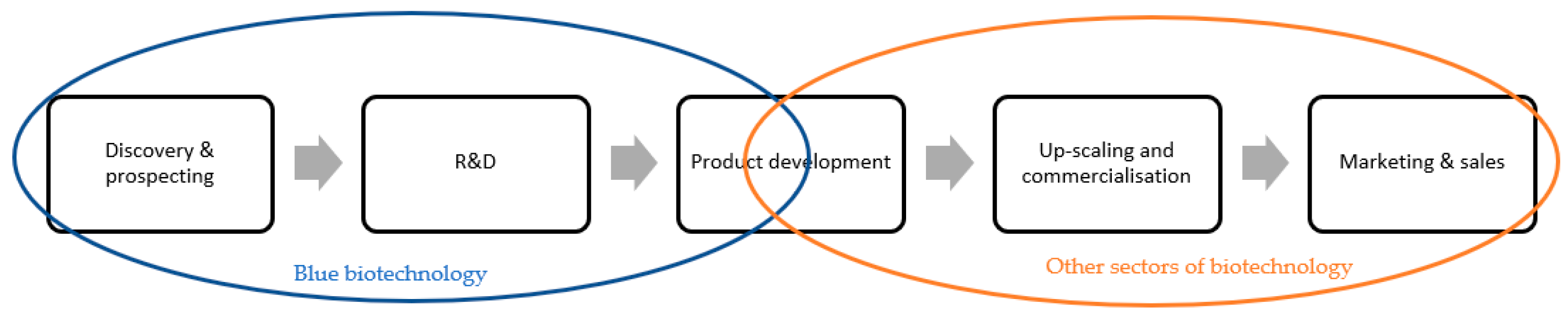
5.2. “Blue Biotechnology” and Sustainability
5.2.1. Biotechnology, Marine Biotechnology and “Blue Biotechnology”
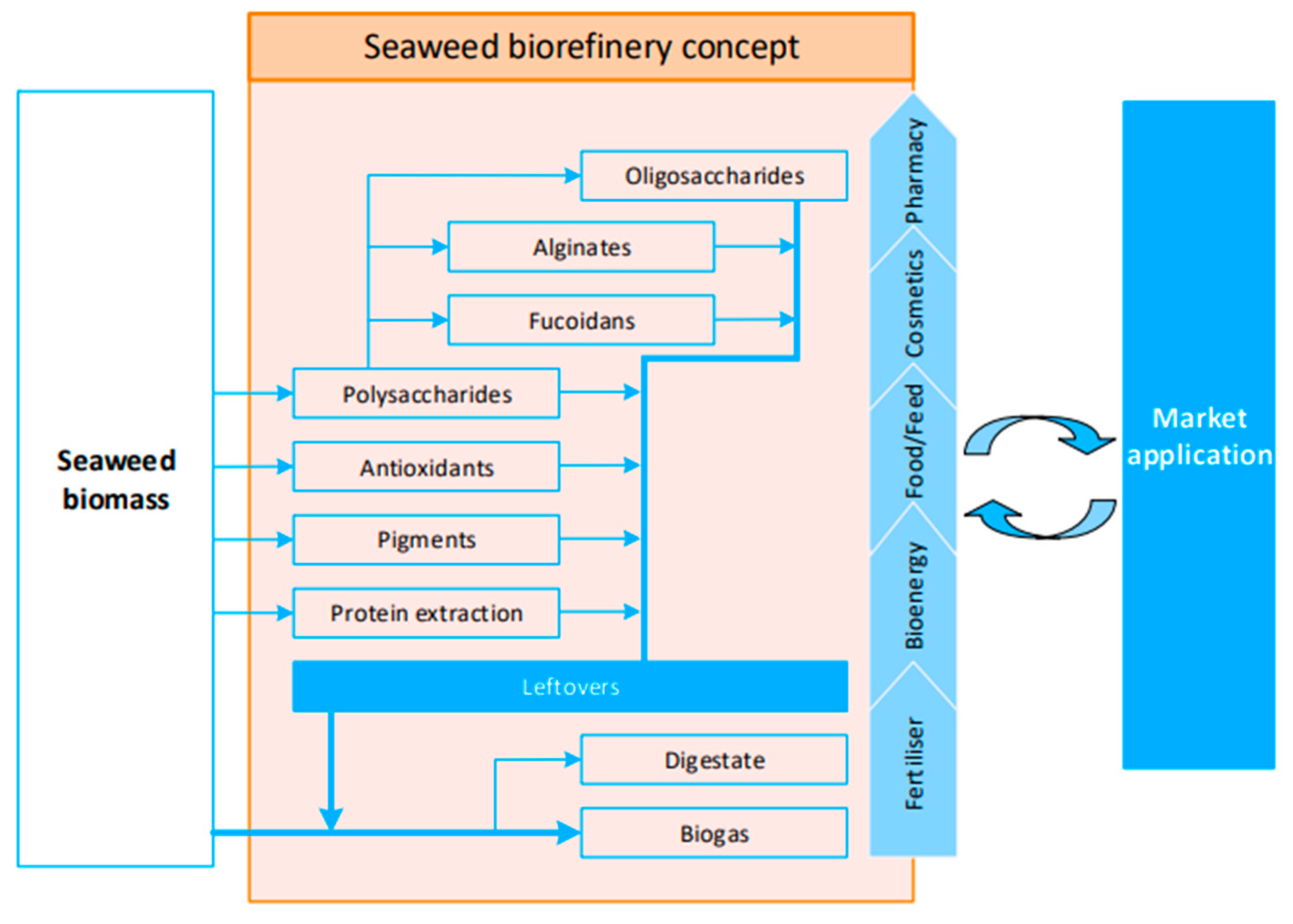
5.2.2. Biorefineries in the Sustainability Assessment of “Blue Biotechnology” Processes
5.2.3. “Blue Biotechnology”-Based Applications in the Cosmetic Industry
Marine Viruses as a Source of Ceramides
Marine-Derived Biosurfactants
Immobilized Lipases from Antarctic Fungi and Yeasts
5.3. Invasive Species as a Source of Compounds of Interest
5.3.1. Sargassum spp.
5.3.2. Ulva lactuca and Ulvans
5.3.3. Undaria pinnatifida
5.4. Marine Waste Products as a Source of Compounds of Interest
5.4.1. Chitin and Chitosan
5.4.2. Collagen
5.4.3. Natural Calcium Phosphates
5.4.4. Beach-Cast Seaweeds
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillerme, J.B.; Couteau, C.; Coiffard, L. Applications for marine resources in cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Thiyagarasaiyar, K.; Goh, B.H.; Jeon, Y.J.; Yow, Y.Y. Algae metabolites in cosmeceutical: An overview of current applications and challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.; Amaral, M.N.; Reis, C.P.; Custódio, L. Marine Natural Products as Innovative Cosmetic Ingredients. Mar. Drugs 2023, 21, 170. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. Metabolites from macroalgae and its applications in the cosmetic industry: A circular economy approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, E.; Kijjoa, A.; Pinto, M. Marine-derived compounds with potential use as cosmeceuticals and nutricosmetics. Molecules 2020, 25, 2536. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, E.B.; Oztan, A.; Fisher, D.E. Effects of Ultraviolet Exposure Behaviors on Skin Pigmentation and Melanoma. J. Pigment. Disord. 2014, 1, 2. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef]
- Azam, M.S.; Choi, J.; Lee, M.S.; Kim, H.R. Hypopigmenting effects of brown algae-derived phytochemicals: A review on molecular mechanisms. Mar. Drugs 2017, 15, 297. [Google Scholar] [CrossRef]
- Li, X.; Jeong, J.H.; Lee, K.T.; Rho, J.R.; Choi, H.D.; Kang, J.S.; Son, B.W. γ-Pyrone derivatives, kojic acid methyl ethers from a marine-derived fungus Altenaria sp. Arch. Pharmacal Res. 2003, 26, 532–534. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Yadav, V.K.; Yadav, K.K.; Tirth, V.; Algahtani, A.; Islam, S.; Gupta, N.; Jeon, B.H. Seaweed-based molecules and their potential biological activities: An eco-sustainable cosmetics. Molecules 2021, 26, 5313. [Google Scholar] [CrossRef] [PubMed]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive properties of marine phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Jung, D.W.; Hillman, P.F.; Nam, S.J.; Lee, C.S. SNA077, an Extract of Marine Streptomyces sp., Inhibits Melanogenesis by Downregulating Melanogenic Proteins via Inactivation of cAMP/PKA/CREB Signaling. Int. J. Mol. Sci. 2022, 23, 14922. [Google Scholar] [CrossRef] [PubMed]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Puizina-Ivi, N. Skin aging. Acta Dermatovenerol. APA 2008, 17, 47–54. [Google Scholar]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- McCallion, R.; Po, A.L.W. Dry and photo–aged skin: And manifestations management. J. Clin. Pharm. Ther. 1993, 18, 15–32. [Google Scholar] [CrossRef]
- Yalcinkaya, E.; Celik, M.; Bugan, B. Extracellular matrix turnover: A balance between MMPs and their inhibitors. Arq. Bras. De Cardiol. 2014, 102, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Coiffard, L. Phycocosmetics and other marine cosmetics, specific cosmetics formulated using marine resources. Mar. Drugs 2020, 18, 322. [Google Scholar] [CrossRef] [PubMed]
- Pallela, R.; Na-Young, Y.; Kim, S.K. Anti-photoaging and photoprotective compounds derived from marine organisms. Mar. Drugs 2010, 8, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Pons, L.; Avila, C.; Romano, G.; Verde, C.; Giordano, D. UV-protective compounds in marine organisms from the southern ocean. Mar. Drugs 2018, 16, 336. [Google Scholar] [CrossRef] [PubMed]
- Losantos, R.; Churio, M.S.; Sampedro, D. Computational Exploration of the Photoprotective Potential of Gadusol. ChemistryOpen 2015, 4, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kim, S.K. Matrix metalloproteinase inhibitors (MMPIs) from marine natural products: The current situation and future prospects. Mar. Drugs 2009, 7, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Joe, M.J.; Kim, S.N.; Choi, H.Y.; Shin, W.S.; Park, G.M.; Kang, D.W.; Kim, Y.K. The Inhibitory Effects of Eckol and Dieckol from Ecklonia stolonifera on the Expression of Matrix Metalloproteinase-1 in Human Dermal Fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.Y.; Sun, Z.W.; Shin, H.S.; Lee, D.G.; Yi, T.H. The Protective Effects of Fucosterol Against Skin Damage in UVB-Irradiated Human Dermal Fibroblasts. Mar. Biotechnol. 2014, 16, 361–370. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, E.Y.; Yoon, J.; Hong, A.; Nam, S.J.; Ko, J. Sarmentosamide, an anti-aging compound from a marine-derived Streptomyces sp. APmarine042. Mar. Drugs 2020, 18, 463. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, Y.W.; Liu, Q.; Liu, L.P.; Luo, F.L.; Zhou, H.C.; Isoda, H.; Ohkohchi, N.; Li, Y.M. Reactive Oxygen Species in Skin Repair, Regeneration, Aging, and Inflammation. In Reactive Oxygen Species (ROS) in Living Cells; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Vitale, G.A.; Coppola, D.; Esposito, F.P.; Buonocore, C.; Ausuri, J.; Tortorella, E.; de Pascale, D. Antioxidant molecules from marine fungi: Methodologies and perspectives. Antioxidants 2020, 9, 1183. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Rathod, N.B.; Čagalj, M.; Hamed, I.; Mekinić, I.G. Astaxanthin from Crustaceans and Their Byproducts: A Bioactive Metabolite Candidate for Therapeutic Application. Mar. Drugs 2022, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Lyons, N.M.; O’Brien, N.M. Modulatory effects of an algal extract containing astaxanthin on UVA-irradiated cells in culture. J. Dermatol. Sci. 2002, 30, 73–84. [Google Scholar] [CrossRef]
- Cahyana, A.H.; Shuto, Y.; Kinoshita, Y. Pyropheophytin a as an Antioxidative Substance from the Marine Alga, Arame (Eisenia bicyclis). Biosci. Biotechnol. Biochem. 1992, 56, 1533–1535. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.E.; Kim, K.H.; Kang, N.J. Beneficial effects of marine algae-derived carbohydrates for skin health. Mar. Drugs 2018, 16, 459. [Google Scholar] [CrossRef] [PubMed]
- Anestopoulos, I.; Kiousi, D.E.; Klavaris, A.; Maijo, M.; Serpico, A.; Suarez, A.; Sanchez, G.; Salek, K.; Chasapi, S.A.; Zompra, A.A.; et al. Marine-derived surface active agents: Health-promoting properties and blue biotechnology-based applications. Biomolecules 2020, 10, 885. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, S.H.; Park, S.J.; Lee, Y.J.; Park, J.H.; Song, P.H.; Cho, C.M.; Ku, S.K.; Song, C.H. Promoting wound healing using low molecular weight fucoidan in a full-thickness dermal excision rat model. Mar. Drugs 2017, 15, 112. [Google Scholar] [CrossRef]
- Jung, W.K.; Heo, S.J.; Jeon, Y.J.; Lee, C.M.; Park, Y.M.; Byun, H.G.; Choi, Y.H.; Park, S.G.; Choi, I.W. Inhibitory Effects and Molecular Mechanism of Dieckol Isolated from Marine Brown Alga on COX-2 and iNOS in Microglial Cells. J. Agric. Food Chem. 2009, 57, 4439–4446. [Google Scholar] [CrossRef]
- Zohdi, R.M.; Zakaria, Z.A.B.; Yusof, N.; Mustapha, N.M.; Abdullah, M.N.H. Sea cucumber (Stichopus hermanii) based hydrogel to treat burn wounds in rats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 30–37. [Google Scholar] [CrossRef]
- Ogai, K.; Nakagami, G.; Oe, M.; Nakatani, T.; Okuwa, M.; Sanada, H.; Sugama, J. A prospective observational study using sea cucumber and honey as topical therapy for diabetic foot ulcers in Indonesia. J. Wellness Health Care 2017, 41, 41–56. [Google Scholar]
- Geahchan, S.; Baharlouei, P.; Rahman, M.A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Mikhal’chik, E.V.; Suprun, M.V.; Papacharalambous, M.; Truhanov, A.I.; Korkina, L.G. Skin antiageing and systemic Redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: A single-blind case-control clinical study. Oxidative Med. Cell. Longev. 2016, 2016, 4389410. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS scavenging activity, photoprotective, and wound-healing properties of collagen-derived peptides from the marine sponge Chondrosia reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef] [PubMed]
- Januário, A.P.; Félix, R.; Félix, C.; Reboleira, J.; Valentão, P.; Lemos, M.F.L. Red seaweed-derived compounds as a potential new approach for acne vulgaris care. Pharmaceutics 2021, 13, 1930. [Google Scholar] [CrossRef] [PubMed]
- Tanghetti, E.A. The Role of Inflammation in the Pathology of Acne. J. Clin. Aesthetic Dermatol. 2013, 6, 27–35. [Google Scholar]
- Kamei, Y.; Sueyoshi, M.; Hayashi, K.I.; Terada, R.; Nozaki, H. The novel anti-Propionibacterium acnes compound, Sargafuran, found in the marine brown alga Sargassum macrocarpum. J. Antibiot. 2009, 62, 259–263. [Google Scholar] [CrossRef]
- Chen, L.W.; Chung, H.L.; Wang, C.C.; Su, J.H.; Chen, Y.J.; Lee, C.J. Anti-acne effects of cembrene diterpenoids from the cultured soft coral Sinularia flexibilis. Mar. Drugs 2020, 18, 487. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, G.; Zhang, Y.H.; Chen, F.Q.; Weng, H.F.; Xiao, A.F. Agarose stearate-carbomer940 as stabilizer and rheology modifier for surfactant-free cosmetic formulations. Mar. Drugs 2021, 19, 344. [Google Scholar] [CrossRef]
- Siahaan, E.A.; Pangestuti, R.; Pratama, I.S.; Putra, Y.; Kim, S.K. Beneficial effects of astaxanthin in cosmeceuticals with focus on emerging market trends. In Global Perspectives on Astaxanthin; Elsevier: Amsterdam, The Netherlands, 2021; pp. 557–568. [Google Scholar] [CrossRef]
- Martins, B.T.; da Silva, M.C.; Pinto, M.; Cidade, H.; Kijjoa, A. Marine natural flavonoids: Chemistry and biological activities. Nat. Prod. Res. 2019, 33, 3260–3272. [Google Scholar] [CrossRef]
- Lyu, X.; Lyu, Y.; Yu, H.; Chen, W.; Ye, L.; Yang, R. Biotechnological advances for improving natural pigment production: A state-of-the-art review. Bioresour. Bioprocess. 2022, 9, 1–38. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Kubicki, S.; Bollinger, A.; Katzke, N.; Jaeger, K.E.; Loeschcke, A.; Thies, S. Marine biosurfactants: Biosynthesis, structural diversity and biotechnological applications. Mar. Drugs 2019, 17, 408. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Kim, S.K. Introduction of Marine Algae Extracts. In Marine Algae Extracts: Processes, Products, and Applications; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Matos, G.S.; Pereira, S.G.; Genisheva, Z.A.; Gomes, A.M.; Teixeira, J.A.; Rocha, C.M.R. Advances in Extraction Methods to Recover Added-Value Compounds from Seaweeds: Sustainability and Functionality. Foods 2021, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Łęska, B.; Messyasz, B.; Schroeder, G. Application of Algae Biomass and Algae Extracts in Cosmetic Formulations. In Algae Biomass: Characteristics and Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 89–101. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Gupta, A.K.; Seth, K.; Maheshwari, K.; Baroliya, P.K.; Meena, M.; Kumar, A.; Vinayak, V.; Harish. Biosynthesis and extraction of high-value carotenoid from algae. Front. Biosci. 2021, 26, 171–190. [Google Scholar] [CrossRef]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef]
- United Nations. Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from Their Utilization to the Convention on Biological Diversity. 2011. Available online: https://www.cbd.int/abs/doc/protocol/nagoya-protocol-en.pdf (accessed on 17 March 2023).
- Pangestuti, R.; Siahaan, E.A.; Kim, S.K. Photoprotective Substances Derived from Marine Algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef]
- Power, D.M.; Anjos, L.; Manchado, M.; Letsiou, S.; Cardoso CR, J.; Félix, R.; Pinto, P.; Infante, C.; Carballo, C.; Mantecon, L.; et al. Handbook for Microalgae-Based Novel High Added-Value Products for Cosmetic and Aquaculture; Under the Algae 4a-b Project; European Union’s Horizon: Brussels, Belgium, 2020. [Google Scholar]
- Da Silva, T.L.; Reis, A. Scale-up Problems for the Large Scale Production of Algae. In Algal Biorefinery: An Integrated Approach; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 125–149. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, J.; Fa, Y. Overcoming the Biological Contamination in Microalgae and Cyanobacteria Mass Cultivations for Photosynthetic Biofuel Production. Molecules 2020, 25, 5220. [Google Scholar] [CrossRef] [PubMed]
- Chang, V.S.; Teo, S.S. Evaluation of heavy metal, antioxidant and anti-tyrosinase activities of red seaweed (Eucheuma cottonii). Int. Food Res. J. 2016, 23, 2370. [Google Scholar]
- Caliceti, M.; Argese, E.; Sfriso, A.; Pavoni, B. Heavy metal contamination in the seaweeds of the Venice lagoon. Chemosphere 2002, 47, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) N° 1223/2009 on Cosmetic Products. Available online: https://eur-lex.europa.eu/legal-content/EN (accessed on 18 July 2023).
- Grillo, G.; Tabasso, S.; Solarino, R.; Cravotto, G.; Toson, C.; Ghedini, E.; Menegazzo, F.; Signoretto, M. From seaweeds to cosmeceutics: A multidisciplinar approach. Sustainability 2021, 13, 13443. [Google Scholar] [CrossRef]
- COSING–Cosmetic Ingredient Database. Available online: https://ec.europa.eu/growth/tools-databases/cosing (accessed on 18 July 2023).
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European Union legislation on macroalgae products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- ISO 16128-1:2016; Guidelines on Technical Definitions and Criteria for Natural and Organic Cosmetic Ingredients and Products: Part 1 Definitions for Ingredients. International Organization for Standardization (ISO): Geneva, Switzerland. Available online: www.iso.org/standard/62503.html (accessed on 11 May 2023).
- ISO 16128-2:2017; Guidelines on Technical Definitions and Criteria for Natural and Organic Cosmetic Ingredients and Products: Part 2 Criteria for Ingredients and Products. International Organization for Standardization (ISO): Geneva, Switzerland. Available online: www.iso.org/standard/65197.html (accessed on 11 May 2023).
- CEN/TR 454; Algae and Algae Products. European Commitee for Standardization (CEN): Brussels, Belgium. Technical Report 454. 2021. Available online: https://standards.iteh.ai/catalog/tc/cen/716626c7-934a-467a-b1bb-6ccad1e57d61/cen-tc-454 (accessed on 13 May 2023).
- CEN/TR 17611; Algae and algae products–Specifications for cosmetic sector applications. European Commitee for Standardization (CEN): Brussels, Belgium. Technical Report 17611. 2021. Available online: https://standards.iteh.ai/catalog/standards/cen/92afec03-63c7-4699-b1e5-27fb516ad3fb/cen-tr-17611-2021 (accessed on 13 May 2023).
- UN Environment Programme–Plastic Pollution. Available online: https://www.unep.org/plastic-pollution (accessed on 20 June 2023).
- Cosmetics Europe. Good Sustainability Practice (GSP) for the Cosmetics Industry. Available online: https://www.cosmeticseurope.eu/files/4214/6521/4452/GSP_Brochure (accessed on 20 June 2023).
- Tolnay, A.; Koris, A.; Magda, R. Sustainable Development of Cosmetic Products in the Frame of the Laboratory Market. Visegrad J. Bioecon. Sustain. Dev. 2019, 7, 62–66. [Google Scholar] [CrossRef]
- Pérez-López, P.; Feijoo, G.; Moreira, M.T. Sustainability Assessment of Blue Biotechnology Processes: Addressing Environmental, Social and Economic Dimensions. In Designing Sustainable Technologies, Products and Policies; Springer: Berlin/Heidelberg, Germany, 2018; pp. 475–486. [Google Scholar]
- World Commission on Environment and Development. Brundtland Report of the World Commission on Environment and Development: Our Common Future; United Nations: Geneva, Switzerland, 1987. [Google Scholar]
- De Carvalho, A.P.; Barbieri, J.C. Innovation and Sustainability in the Supply Chain of a Cosmetics Company: A Case Study. J. Technol. Manag. Innov. 2012, 7, 144–156. [Google Scholar] [CrossRef]
- Pagels, F.; Arias, A.; Guerreiro, A.; Guedes, A.C.; Moreira, M.T. Seaweed Cosmetics under the Spotlight of Sustainability. Phycology 2022, 2, 374–383. [Google Scholar] [CrossRef]
- Murray, P.M.; Moane, S.; Collins, C.; Beletskaya, T.; Thomas, O.P.; Duarte, A.W.F.; Nobre, F.S.; Owoyemi, I.O.; Pagnocca, F.C.; Sette, L.D.; et al. Sustainable production of biologically active molecules of marine based origin. New Biotechnol. 2013, 30, 839–850. [Google Scholar] [CrossRef]
- Bom, S.; Ribeiro, H.M.; Marto, J. Sustainability calculator: A tool to assess sustainability in cosmetic products. Sustainability 2020, 12, 1437. [Google Scholar] [CrossRef]
- Collins, J.; Broggiato, A.; Vanagt, T. Blue Biotechnology. In Building Industries at Sea—‘Blue Growth’ and the New Maritime Economy; River Publishers: Aalborg, Denmark, 2022; pp. 39–71. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Procedia 2017, 128, 504–511. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.N.; McHugh, E.; Walsh, D.; Murray, P.; Moane, S.; Feijoo, G.; Moreira, M.T. Life cycle assessment of the production of the red anti-oxidant carotenoid astaxanthin by microalgae: From lab to pilot scale. J. Clean. Prod. 2014, 64, 332–344. [Google Scholar] [CrossRef]
- Sánchez-Paz, A.; Muhlia-Almazan, A.; Saborowski, R.; García-Carreño, F.; Sablok, G.; Mendoza-Cano, F. Marine Viruses: The Beneficial Side of a Threat. Appl. Biochem. Biotechnol. 2014, 174, 2368–2379. [Google Scholar] [CrossRef]
- Ansorge-Schumacher, M.B.; Thum, O. Immobilised lipases in the cosmetics industry. Chem. Soc. Rev. 2013, 42, 6475–6490. [Google Scholar] [CrossRef] [PubMed]
- Susano, P.; Silva, J.; Alves, C.; Martins, A.; Pinteus, S.; Gaspar, H.; Goettert, M.I.; Pedrosa, R. Mitigating the negative impacts of marine invasive species—Sargassum muticum—A key seaweed for skincare products development. Algal Res. 2022, 62, 102634. [Google Scholar] [CrossRef]
- Andreakis, N.; Schaffelke, B. Invasive Marine Seaweeds: Pest or Prize? Springer: Berlin/Heidelberg, Germany, 2012; pp. 235–262. [Google Scholar] [CrossRef]
- Lopresto, C.G.; Paletta, R.; Filippelli, P.; Galluccio, L.; de la Rosa, C.; Amaro, E.; Jáuregui-Haza, U.; de Frias, J.A. Sargassum Invasion in the Caribbean: An Opportunity for Coastal Communities to Produce Bioenergy Based on Biorefinery—An Overview. Waste Biomass Valorization 2022, 13, 2769–2793. [Google Scholar] [CrossRef]
- Dominguez, H.; Loret, E.P. Ulva lactuca, A Source of Troubles and Potential Riches. Mar. Drugs 2019, 17, 357. [Google Scholar] [CrossRef]
- Selvasudha, N.; Goswami, R.; Subi, M.T.M.; Rajesh, S.; Kishore, K.; Vasanthi, H.R. Seaweeds derived ulvan and alginate polysaccharides encapsulated microbeads–Alternate for plastic microbeads in exfoliating cosmetic products. Carbohydr. Polym. Technol. Appl. 2023, 6, 100342. [Google Scholar] [CrossRef]
- Gan, A.; Baroutian, S. Subcritical water extraction for recovery of phenolics and fucoidan from New Zealand Wakame (Undaria pinnatifida) seaweed. J. Supercrit. Fluids 2022, 190, 105732. [Google Scholar] [CrossRef]
- Siahaan, E.A.; Agusman; Pangestuti, R.; Shin, K.H.; Kim, S.K. Potential Cosmetic Active Ingredients Derived from Marine By-Products. Mar. Drugs 2022, 20, 734. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; De Lima, M.A.B.; de Oliveira Franco, L.; De Campos-Takaki, G.M. Seafood waste as attractive source of chitin and chitosan production and their applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef] [PubMed]
- Oslan, S.N.H.; Li, C.X.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Extraction and Characterization of Bioactive Fish By-Product Collagen as Promising for Potential Wound Healing Agent in Pharmaceutical Applications: Current Trend and Future Perspective. Int. J. Food Sci. 2022, 2022, 9437878. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; He, J.; Chen, J.; Li, Y.; Zhang, X.; Zheng, Y.; Jia, L. Valorization of Fish Processing By-Products: Microstructural, Rheological, Functional, and Properties of Silver Carp Skin Type I Collagen. Foods 2022, 11, 2985. [Google Scholar] [CrossRef]
- Righi, S.; Prato, E.; Magnani, G.; Lama, V.; Biandolino, F.; Parlapiano, I.; Carella, F.; Iafisco, M.; Adamiano, A. Calcium phosphates from fish bones in sunscreen: An LCA and toxicity study of an emerging material for circular economy. Sci. Total. Environ. 2023, 862, 160751. [Google Scholar] [CrossRef] [PubMed]
- Harb, T.B.; Chow, F. An overview of beach-cast seaweeds: Potential and opportunities for the valorization of underused waste biomass. Algal Res. 2022, 62, 102643. [Google Scholar] [CrossRef]
- Zárate, R.; Portillo, E.; Teixidó, S.; de Carvalho, M.A.A.P.; Nunes, N.; Ferraz, S.; Seca, A.M.L.; Rosa, G.P.; Barreto, M.C. Pharmacological and Cosmeceutical Potential of Seaweed Beach-Casts of Macaronesia. Appl. Sci. 2020, 10, 5831. [Google Scholar] [CrossRef]
| Polysaccharides | Cosmetic Properties to CosIng Database | Species Where These Polysaccharides Are Found (e.g.,) |
|---|---|---|
| Alginates and their salts (calcium, sodium, magnesium, ammonium and potassium) | Binding Emulsion stabilizing Film-forming Humectant Viscosity-controlling | Brown macroalgae (e.g., Ascophyllum nodosum, Laminaria hyperborea, Laminaria digitata) [4] |
| Carrageenans (including hydrolyzed carrageenan) | Binding Emulsion-stabilizing Film-forming Skin-conditioning Viscosity-controlling | Kappaphycus and Eucheuma genera [4] |
| Agar and agarose | Binding Fragrance Viscosity-controlling Skin conditioning | Red macroalgae (Gelidium and Gracilaria species) [4] |
| Fucoidan | Skin-conditioning Skin protector | Brown macroalgae (e.g., Sargassum stenophyllum, Fucus vesiculosus) [4] |
| Xylans | Film-forming Skin-conditioning | Green macroalgae (Bryopsidales order) [4] |
| Mannans | Film-forming Skin-conditioning | Green macroalgae (Bryopsidales order) [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.P.; Custódio, L.; Reis, C.P. Exploring the Potential of Using Marine-Derived Ingredients: From the Extraction to Cutting-Edge Cosmetics. Mar. Drugs 2023, 21, 620. https://doi.org/10.3390/md21120620
Costa JP, Custódio L, Reis CP. Exploring the Potential of Using Marine-Derived Ingredients: From the Extraction to Cutting-Edge Cosmetics. Marine Drugs. 2023; 21(12):620. https://doi.org/10.3390/md21120620
Chicago/Turabian StyleCosta, João Pedro, Luísa Custódio, and Catarina Pinto Reis. 2023. "Exploring the Potential of Using Marine-Derived Ingredients: From the Extraction to Cutting-Edge Cosmetics" Marine Drugs 21, no. 12: 620. https://doi.org/10.3390/md21120620
APA StyleCosta, J. P., Custódio, L., & Reis, C. P. (2023). Exploring the Potential of Using Marine-Derived Ingredients: From the Extraction to Cutting-Edge Cosmetics. Marine Drugs, 21(12), 620. https://doi.org/10.3390/md21120620