Structure and Binding Properties to Blood Co-Factors of the Least Sulfated Galactan Found in the Cell Wall of the Red Alga Botryocladia occidentalis
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Preliminary Physicochemical Analyses of BoSG Fr1
2.2. Structural Characterization of BoSG Fr1 by NMR Spectroscopy
2.3. SPR-Based Binding Properties of BoSG Fr1 and Derivatives
3. Materials and Methods
3.1. Reagents
3.2. Extraction of BoSGs
3.3. Fractionation of BoSGs
3.4. Desalting of BoSG Fr1
3.5. Desulfation of BoSG Fr1
3.6. Oversulfation of BoSG Fr1
3.7. Alkaline Treatment
3.8. PAGE and HPSEC/MALS
3.9. NMR Spectroscopy
3.10. SPR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.J.; Morais, A.M.B.; Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Huang, X.; Cheong, K. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.A.; Pomin, V.H. Marine carbohydrate-based compounds with medicinal properties. Mar. Drugs 2018, 16, 233. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.A.; Pomin, V.H. The sea as a rich source of structurally unique glycosaminoglycans and mimetics. Microorganisms 2017, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Mourão, P.A.S. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Structural and functional insights into sulfated galactans: A systematic review. Glycoconj. J. 2010, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ciancia, M.; Matulewicz, M.C.; Tuvikene, R. Structural diversity in galactans from red seaweeds and its influence on rheological properties. Front. Plant Sci. 2020, 11, 559986. [Google Scholar] [CrossRef]
- Pomin, V.H. Phylogeny, structure, function, biosynthesis and evolution of sulfated galactose-containing glycans. Int. J. Biol. Macromol. 2016, 84, 372–379. [Google Scholar] [CrossRef]
- Farias, E.H.C.; Pomin, V.H.; Valente, A.; Nader, H.B.; Rocha, H.A.O.; Mourão, P.A.S. A preponderantly 4-sulfated, 3-linked galactan from the green alga Codium isthmocladum. Glycobiology 2008, 18, 250–259. [Google Scholar] [CrossRef]
- Gu, D.; Huang, L.; Chen, X.; Wu, Q.; Ding, K. Structural characterization of a galactan from Ophiopogon japonicus and anti-pancreatic cancer activity of its acetylated derivative. Int. J. Biol. Macromol. 2018, 113, 907–915. [Google Scholar] [CrossRef]
- Maurya, A.K.; Sharma, P.; Samanta, P.; Shami, A.A.; Misra, S.K.; Zhang, F.; Thara, R.; Kumar, D.; Shi, D.; Linhardt, R.J.; et al. Structure, anti-SARS-CoV-2, and anticoagulant effects of two sulfated galactans from the red alga Botryocladia occidentalis. Int. J. Biol. Macromol. 2023, 238, 124168. [Google Scholar] [CrossRef]
- Pomin, V.H. Fucanomics and galactanomics: Marine distribution, medicinal impact, conceptions, and challenges. Mar. Drugs 2012, 10, 793–811. [Google Scholar] [CrossRef]
- Pomin, V.H. Fucanomics and galactanomics: Current status in drug discovery, mechanisms of action and role of the well-defined structures. Biochim. Biophys. Acta 2012, 1820, 1971–1979. [Google Scholar] [CrossRef]
- Pomin, V.H. How to analyze the anticoagulant and antithrombotic mechanisms of action in fucanome and galactanome? Glycoconj. J. 2014, 31, 89–99. [Google Scholar] [CrossRef]
- Mourão, P.A.S. Perspective on the use of sulfated polysaccharides from marine organisms as a source of new antithrombotic drugs. Mar. Drugs 2015, 13, 2770–2784. [Google Scholar] [CrossRef] [PubMed]
- Farias, W.R.L.; Valente, A.P.; Pereira, M.S.; Mourão, P.A.S. Structure and Anticoagulant Activity of Sulfated Galactans. Isolation of a unique sulfated galactan from the red algae Botryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. J. Biol. Chem. 2000, 275, 29299–29307. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Zoepfl, M.; Samanta, P.; Zhang, F.; Xia, K.; Thara, R.; Linhardt, R.J.; Doerksen, R.J.; McVoy, M.A.; Pomin, V.H. Fractionation of sulfated galactan from the red alga Botryocladia occidentalis separates its anticoagulant and anti-SARS-CoV-2 properties. J. Biol. Chem. 2022, 298, 101856. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, T.B.P.; Costa, B.B.; Moreira, T.A.; Cabral, L.M.; Silva, L.C.R.P.; Mourão, P.A.S.; Vilanova, E.; Cinelli, L.P. Insights on chemical-biological correlations learned from investigations on the sulfated galactan from the marine alga Bothryocladia occidentalis. Int. J. Biol. Macromol. 2020, 158, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.G.; Benevides, N.M.B.; Melo, M.R.S.; Valente, A.P.; Melo, F.R.; Mourão, P.A.S. Structure and anticoagulant activity of a sulfated galactan from the red alga, Gelidium crinale. Is there a specific structural requirement for the anticoagulant action? Carbohydr. Res. 2005, 340, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Quindere, A.L.G.; Santos, G.R.C.; Oliveira, S.N.M.C.G.; Glauser, B.F.; Fontes, B.P.; Queiroz, I.N.L.; Benevides, N.M.B.; Pomin, V.H.; Mourão, P.A.S. Is the antithrombotic effect of sulfated galactans independent of serpin? J. Thromb. Haemost. 2013, 12, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Farias, W.R.L.; Nazareth, R.A.; Mourão, P.A.S. Dual effects of sulfated D-galactans from the red algae Botryocladia occidentalis preventing thrombosis and inducing platelet aggregation. Thromb. Haemost. 2001, 86, 1540–1546. [Google Scholar] [CrossRef]
- Pomin, V.H.; Mahdi, F.; Jin, W.; Zhang, F.; Linhardt, R.J.; Paris, J.J. Red algal sulfated galactan binds and protects neural cells from HIV-1 gp120 and Tat. Pharmaceuticals 2021, 14, 714. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.J.C.; Oliveira, S.M.C.G.; Melo, F.R.; Pereira, M.G.; Benevides, N.M.B.; Mourão, P.A.S. Slight differences in sulfation of algal galactans account for differences in their anticoagulant and venous antithrombotic activities. Thromb. Haemost. 2008, 99, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Melo, F.R.; Mourão, P.A.S. An algal sulfated galactan has an unusual dual effect on venous thrombosis due to activation of factor XII and inhibition of the coagulation proteases. Thromb. Haemost. 2008, 99, 531–538. [Google Scholar] [CrossRef]
- Pomin, V.H.; Mourão, P.A.S. Specific sulfation and glycosylation-a structural combination for the anticoagulation of marine carbohydrates. Front. Cell. Infect. Microbiol. 2014, 4, 33. [Google Scholar] [CrossRef]
- Glauser, B.F.; Mourão, P.A.S.; Pomin, V.H. Marine sulfated glycans with serpin-unrelated anticoagulant properties. Adv. Clin. Chem. 2013, 62, 269–303. [Google Scholar]
- Pomin, V.H. Review: An overview about the structure-function relationship of marine sulfated homopolysaccharides with regular chemical structures. Biopolymers 2009, 91, 601–609. [Google Scholar] [CrossRef]
- Pomin, V.H. Chapter 12—Structure-function relationship of anticoagulant and antithrombotic well-defined sulfated polysaccharides from marine invertebrates. Adv. Food Nutr. Res. 2012, 65, 195–209. [Google Scholar]
- Pomin, V.H. Marine non-glycosaminoglycan sulfated glycans as potential pharmaceuticals. Pharmaceuticals 2015, 8, 848–864. [Google Scholar] [CrossRef]
- Gong, G.; Zhao, J.; Wang, C.; Wei, M.; Dang, T.; Deng, Y.; Sun, J.; Song, S.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of the degradation products from Porphyra haitanensis polysaccharides. Process Biochem. 2018, 74, 185–193. [Google Scholar] [CrossRef]
- Sanchez, R.A.R.; Matulewicz, M.C.; Ciancia, M. NMR spectroscopy for structural elucidation of sulfated polysaccharides from red seaweeds. Int. J. Biol. Macromol. 2022, 199, 386–400. [Google Scholar] [CrossRef]
- Albano, R.M.; Pavão, M.S.G.; Mourão, P.A.S.; Mulloy, B. Structural studies of a sulfated L-galactan from Styela plicata (Tunicate): Analysis of the smith-degraded polysaccharide. Carbohydr. Res. 1990, 208, 163–l74. [Google Scholar] [CrossRef]
- Castro, M.O.; Pomin, V.H.; Santos, L.L.; Vilela-Silva, A.E.S.; Hirohashi, N.; Pol-Fachin, L.; Verli, H.; Mourão, P.A.S. A unique 2-sulfated β-galactan from the egg jelly of the sea urchin Glyptocidaris crenularis: Confirmation flexibility versus induction of the sperm acrosome reaction. J. Biol. Chem. 2009, 284, 18790–18800. [Google Scholar] [CrossRef] [PubMed]
- Mourão, P.A.S.; Perlin, A.S. Structural features of sulfated glycans from the tunic of Styela plicata (Chordata-Tunicata). A unique occurrence of L-galactose in sulfated polysaccharides. Glycobiology 2005, 15, 11–20. [Google Scholar] [CrossRef]
- Bilan, M.I.; Ustyuzhanina, N.E.; Shashkov, A.S.; Thanh, T.T.T.; Bui, M.L.; Tran, T.T.V.; Bui, V.N.; Usov, A.I. Sulfated polysaccharides of the Vietnamese brown alga Sargassum aquifolium (Fucales, Sargassaceae). Carbohydr. Res. 2017, 449, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, K.; Uchiyama, H.; Wajima, N. Chemical sulfation of preparations of chondroitin 4- and 6-sulfates, and dermatan sulfate. Preparation of chondroitin sulfate E-like materials from chondroitin 4-sulfate. Carbohydr. Res. 1986, 158, 183–190. [Google Scholar] [CrossRef]
- Fonseca, R.J.C.; Oliveira, S.M.C.G.; Pomin, V.H.; Mecawi, A.S.; Araujo, I.G.; Mourão, P.A.S. Effects of oversulfated and fucosylated chondroitin sulfates on coagulation. Thromb. Haemost. 2010, 103, 994–1004. [Google Scholar] [CrossRef]
- Freile-Plegrin, Y.; Robledo, D. Influence of alkali treatment on agar from Gracilaria cornea from Yucatan, Mexico. J. Appl. Phycol. 1997, 9, 533–539. [Google Scholar]
- Matsuhiro, B.; Conte, A.F.; Damonte, E.B.; Kolender, A.A.; Matulewicz, M.C.; Mejías, E.G.; Pujol, C.A.; Zúñiga, E.A. Structural analysis and antiviral activity of a sulfated galactan from the red seaweed Schizymenia binderi (Gigartinales, Rhodophyta). Carbohydr. Res. 2005, 340, 2392–2402. [Google Scholar] [CrossRef]
- Dwivedi, R.; Samanta, P.; Sharma, P.; Zhang, F.; Mishra, S.K.; Kucheryavy, P.; Kim, S.B.; Aderibigbe, A.O.; Linhardt, R.J.; Tandon, R.; et al. Structural and kinetic analyses of holothurian sulfated glycans suggest potential treatment for SARS-CoV-2 infection. J. Biol. Chem. 2021, 297, 101207. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Sharma, P.; Eilts, F.; Zhang, F.; Linhardt, R.J.; Tandon, R.; Pomin, V.H. Anti-SARS-CoV-2 and anticoagulant properties of Pentacta pygmaea fucosylated chondroitin sulfate depend on high molecular weight structures. Glycobiology 2023, 33, 75–85. [Google Scholar] [CrossRef] [PubMed]

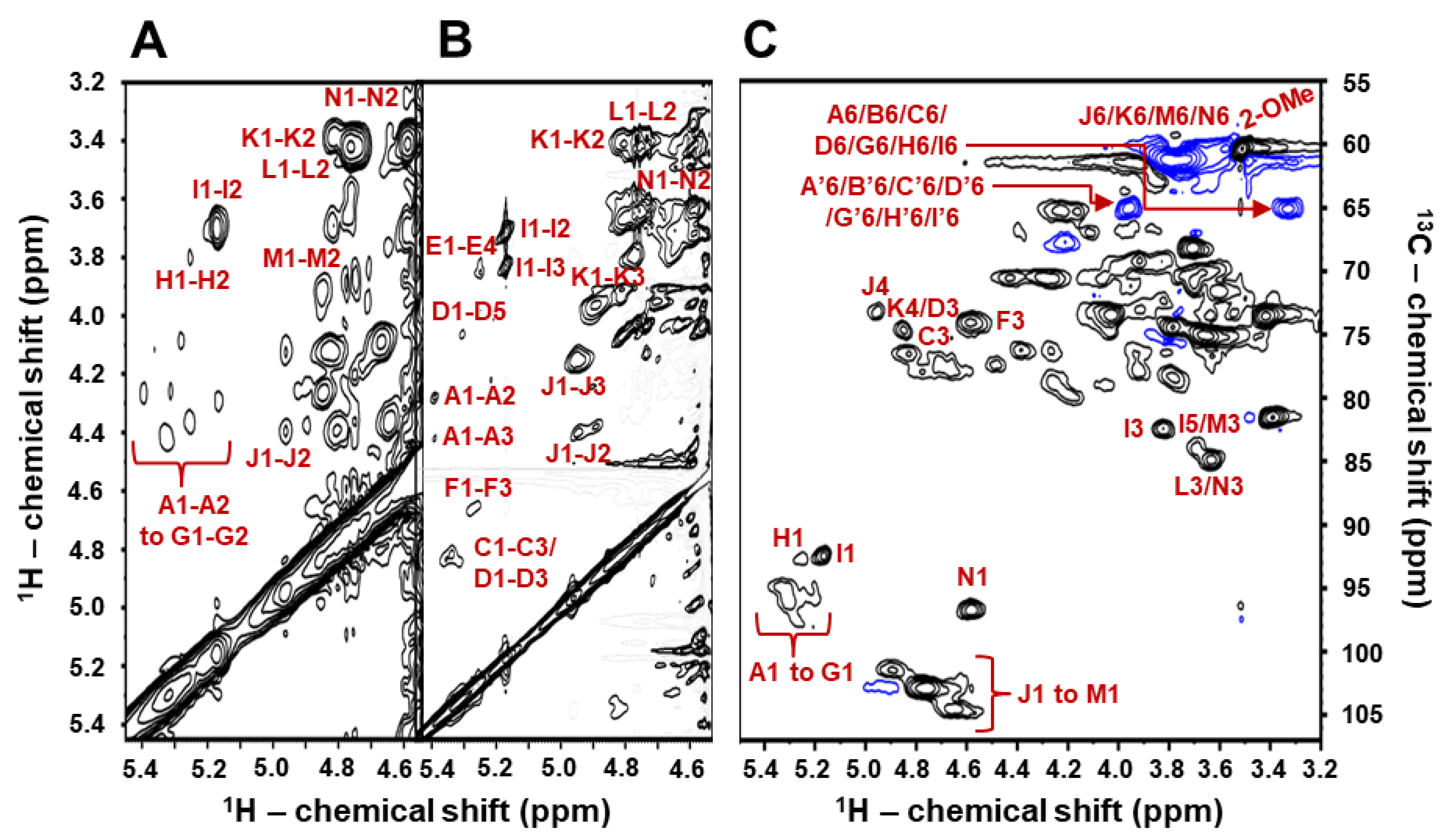
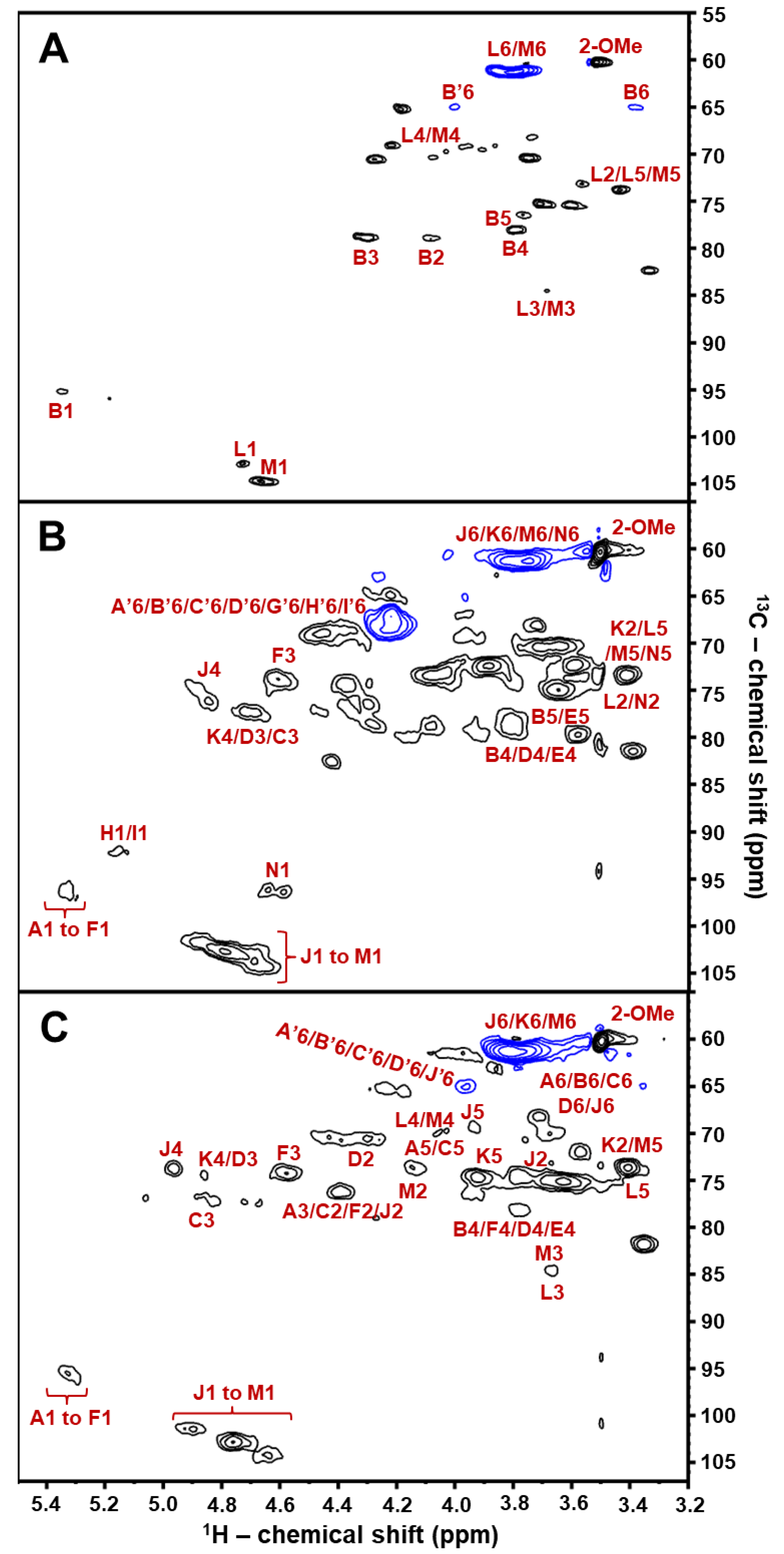


| Unit: [Structure] and (Letter Notation) | Source | 1H1/13C1 | 1H2/13C2 | 1H3/13C3 | 1H4/13C4 | 1H5/13C5 | 1H6/13C6 | 2-O-Me |
|---|---|---|---|---|---|---|---|---|
| [→4)-α-AnGal-2-(SO3−)-(1→] (A1) | Fr1 | 5.39/94.8 | 4.27/78.7 | 4.41/76.1 | 4.16/79.7 | 4.03/74.0 | 3.32, 3.94/64.9 | - |
| [→4)-α-AnGal-2(SO3−)-(1→] | [12] | 5.35/94.7 | 4.40/76.7 | 4.81/78.7 | - | - | - | - |
| [→4)-α-AnGal-2-O-Me-(1→] b (B1) | Fr1 | 5.35/96.0 | 4.09/79.2 | 4.24/78.9 | 3.83/78.5 | 3.74/76.1 | 3.32, 3.94/64.9 | 3.51/60.1 |
| [→4)-α-AnGal-2-O-Me-(1→] | [31] | 5.31/98.7 | 3.5578.8 | 3.85/78.4 | 3.65/77.6 | 3.4575.3 | 3.33/69.8 | 3.51/60.1 |
| [→4)-α-AnGal-2(SO3−)-(1→] (C1) | Fr1 | 5.32/95.4 | 4.41/76.1 | 4.81/77.3 | 4.16/79.7 | 4.05/74.0 | 3.32, 3.94/64.9 | - |
| [→4)-α-AnGal-2(SO3−)-(1→] | [12] | 5.35/94.7 | 4.40/76.7 | 4.81/78.7 | - | - | - | - |
| [→4)-α-AnGal-(1→] (D1) | Fr1 | 5.31/95.6 | 4.26/70.6 | 4.87/74.4 | 3.83/78.5 | 4.29/78.9 | 3.32, 3.94/64.9 | - |
| [→4)-α-AnGal-(1→] | [32] | 5.07/94.7 | 4.08/70.4 | 4.53/79.7 | 4.60/78.5 | 4.67/77.1 | 3.32, 3.94/64.9 | - |
| [→4)-α-AnGal-2-O-Me-(1→] (E1) | Fr1 | 5.27/97.0 | 4.09/78.7 | 4.22/78.9 | 3.83/78.5 | 3.74/76.1 | 3.32, 3.94/64.9 | 3.51/60.1 |
| [→4)-α-AnGal-2-O-Me-(1→] | [33] | 5.31/98.7 | 3.5578.8 | 3.85/78.4 | 3.65/77.6 | 3.4575.3 | 3.33/69.8 | 3.51/60.1 |
| [→4)-α-AnGal-2(SO3−)-(1→] (F1) | Fr1 | 5.25/95.4 | 4.36/76.1 | 4.64/74.3 | 3.98/78.7 | - | - | - |
| [→4)-α-AnGal-2(SO3−)-(1→] | [33] | 5.27/94.7 | 4.66/75.4 | 4.78/78.1 | 4.72/78.3 | 4.70/78.1 | 4.06. 4.21/70.0 | - |
| [→4)-α-AnGal-2(SO3−)-(1→] (G1) | Fr1 | 5.16/95.6 | 4.29/78.5 | 4.41/76.1 | 4.13/79.0 | 3.96/77.5 | 3.32, 3.94/64.9 | - |
| [→4)-α-AnGal-2(SO3−)-(1→] | [33] | 5.27/94.7 | 4.66/75.4 | 4.78/78.1 | 4.72/78.3 | 4.70/78.1 | 4.06. 4.21/70.0 | - |
| [→4)-α-AnGal-(1→] (H1) | Fr1 | 5.25/92.5 | 3.79/70.0 | 4.33/76.2 | 4.02/79.2 | 4.21/79.0 | 3.32, 3.94/64.9 | - |
| [→4)-α-AnGal-(1→] | [32] | 5.07/94.7 | 4.08/70.4 | 4.53/79.7 | 4.60/78.5 | 4.67/77.1 | 3.32, 3.94/64.9 | - |
| [→4)-α-AnGal-(1→] (I1) | Fr1 | 5.17/92.3 | 3.69/71.1 | 3.82/82.3 | 4.05/79.2 | 3.70/83.6 | 3.32, 3.94/64.9 | - |
| [→4)-α-AnGal-(1→] | [32] | 5.07/94.7 | 4.08/70.4 | 4.53/79.7 | 4.60/78.5 | 4.67/77.1 | 3.32, 3.94/64.9 | - |
| [→3)-β-D-Gal-2,4(SO3−)-(1→] (J1) | Fr1 | 4.94/101.4 | 4.39/76.1 | 4.15/79.7 | 4.97/73.5 | 3.95/69.6 | 3.70/61.1 | - |
| [→3)-β-D-Gal-2,4(SO3−)-(1→] | [12] | 4.94/102.4 | 4.41/76.7 | 4.18/81.1 | 4.99/74.2 | - | 3.77/62.4 | - |
| [→3)-β-D-Gal-4(SO3−)-(1→] (K1) | Fr1 | 4.80/102.8 | 3.37/73.2 | 4.02/79.2 | 4.88/74.4 | 3.82/74.4 | 3.70/61.1 | - |
| [→3)-β-D-Gal-4(SO3−)-(1→] | [32] | 4.70/102.5 | 3.65/69.4 | 4.06/78.2 | 4.91/73.7 | 3.87/74.8 | 3.86/61.2 | - |
| [→3)-β-D-Gal-(1→] (L1) | Fr1 | 4.75/102.0 | 3.43/73.7 | 3.62/84.9 | 4.01/70.0 | 3.38/73.5 | - | - |
| [→3)-β-D-Gal-(1→] | [34] | 4.79/103.5 | 3.90/69.9 | 3.93/81.9 | 4.29/68.3 | 3.82/74.8 | 3.87/60.5 | |
| [→3)-β-D-Gal-(1→] (M1) | Fr1 | 4.65/103.7 | 4.08/73.2 | 3.71/83.9 | 4.02/70.1 | 3.38/73.2 | 3.62/61.1 | - |
| [→3)-β-D-Gal-(1→] | [20] | 4.57/104.5 | 3.63/72.1 | 3.73/84.1 | 4.12/71.4 | 3.76/73.7 | 3.77/63.4 | - |
| [→3)-β-D-Gal-(1→] (N1) | Fr1 | 4.58/97.0 | 3.42/73.7 | 3.65/85.0 | 4.01/70.1 | 3.37/73.5 | 3.61/61.3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurya, A.K.; Ahmed, H.A.; DeWitt, A.; Shami, A.A.; Misra, S.K.; Pomin, V.H. Structure and Binding Properties to Blood Co-Factors of the Least Sulfated Galactan Found in the Cell Wall of the Red Alga Botryocladia occidentalis. Mar. Drugs 2024, 22, 81. https://doi.org/10.3390/md22020081
Maurya AK, Ahmed HA, DeWitt A, Shami AA, Misra SK, Pomin VH. Structure and Binding Properties to Blood Co-Factors of the Least Sulfated Galactan Found in the Cell Wall of the Red Alga Botryocladia occidentalis. Marine Drugs. 2024; 22(2):81. https://doi.org/10.3390/md22020081
Chicago/Turabian StyleMaurya, Antim K., Hoda Al. Ahmed, Anderson DeWitt, Anter A. Shami, Sandeep K. Misra, and Vitor H. Pomin. 2024. "Structure and Binding Properties to Blood Co-Factors of the Least Sulfated Galactan Found in the Cell Wall of the Red Alga Botryocladia occidentalis" Marine Drugs 22, no. 2: 81. https://doi.org/10.3390/md22020081
APA StyleMaurya, A. K., Ahmed, H. A., DeWitt, A., Shami, A. A., Misra, S. K., & Pomin, V. H. (2024). Structure and Binding Properties to Blood Co-Factors of the Least Sulfated Galactan Found in the Cell Wall of the Red Alga Botryocladia occidentalis. Marine Drugs, 22(2), 81. https://doi.org/10.3390/md22020081








