The Antiviral Potential of Algal Lectins
Abstract
1. Introduction
2. Algal Antiviral Compounds
3. Lectins
3.1. Algal Lectins
Production of Algal Lectins
3.2. Algal Lectins with Antiviral Potential
3.2.1. Griffithsin
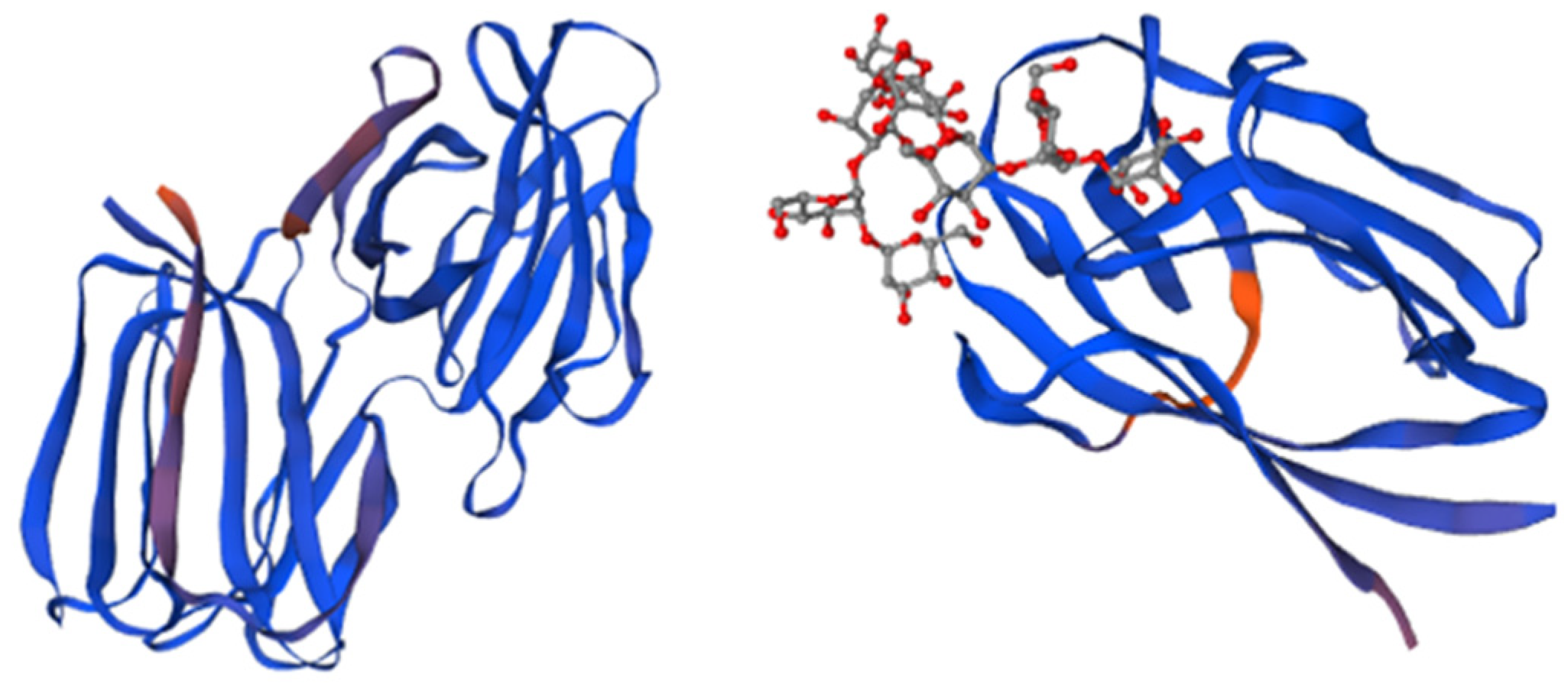
3.2.2. Cyanovirin-N
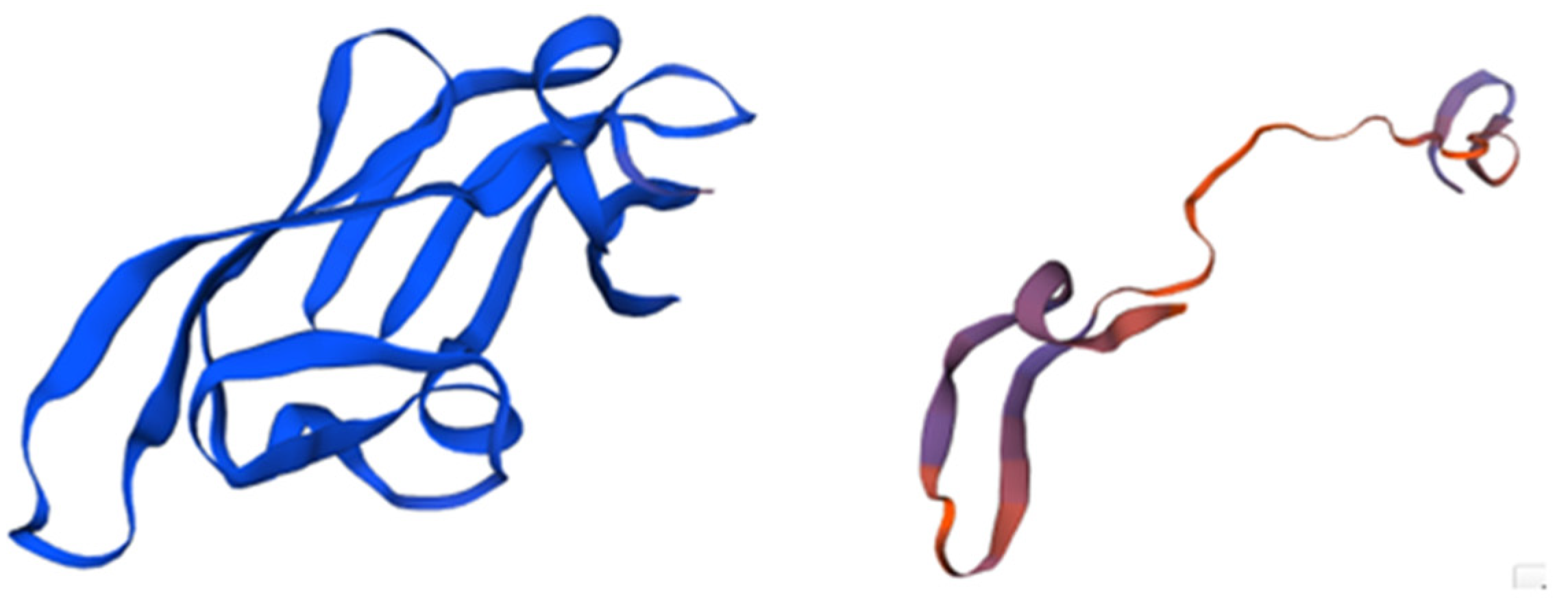
3.2.3. Scytovirin
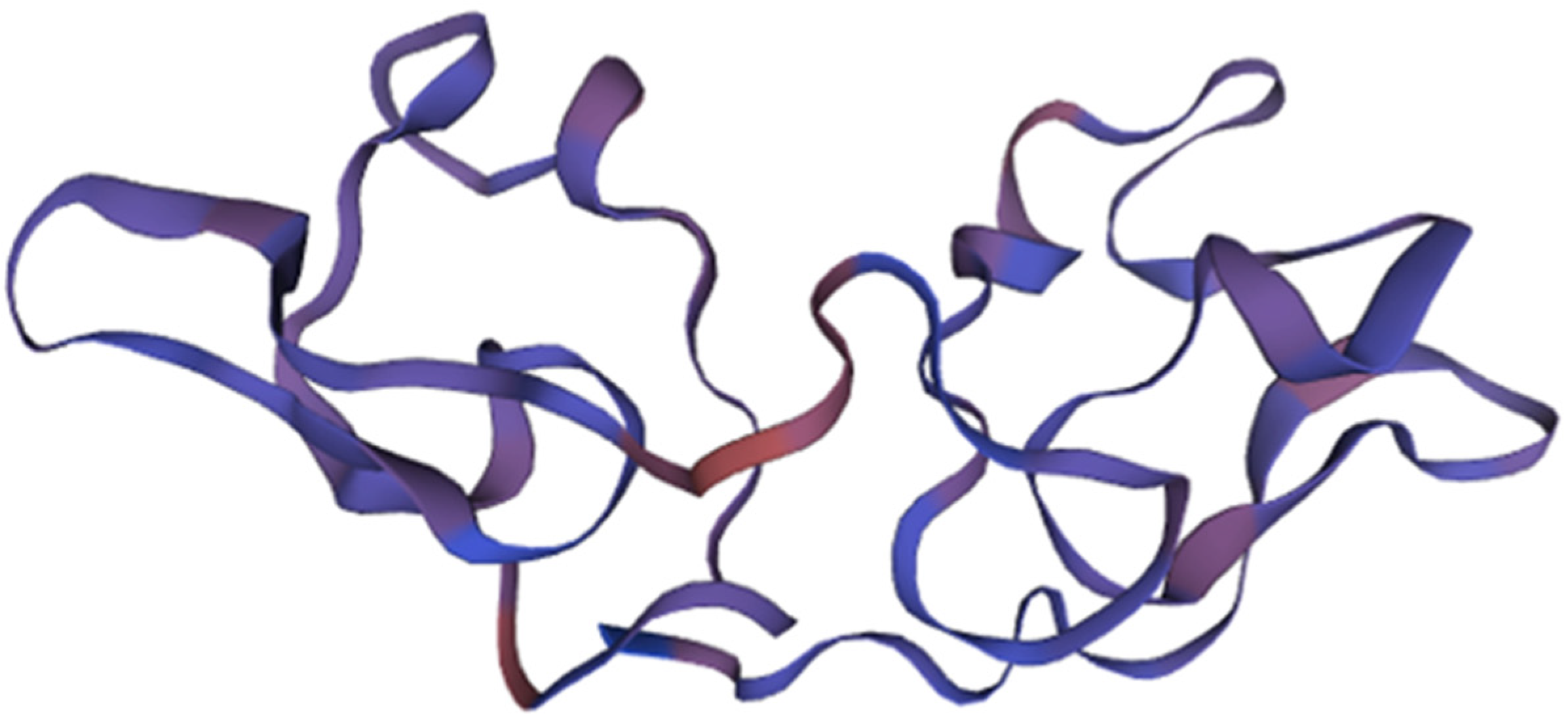
3.2.4. Microvirin
3.2.5. Others
The Brown-Alga-Derived OAAH (Oscillatoria agardhii Agglutinin Homolog) Lectin Family
The Yellow-Alga-Derived Legume-Lectin-like Family
Green-Alga-Derived Galanthus nivalis Agglutinin (GNA)
The Multifunctional Protein in Peroxisomal β-Oxidation (MFP2)-like Families
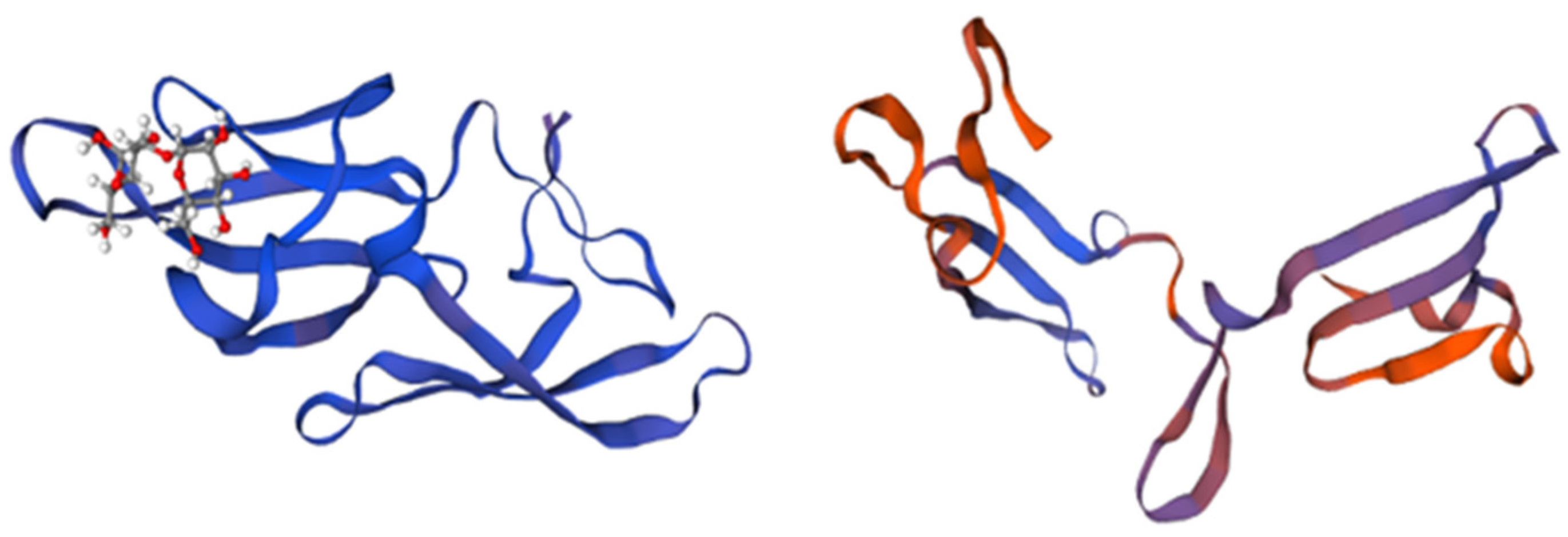
Mannose-Binding Lectin from Grateloupia chiangii (G. chiangii Lectin, GCL)
| Alga | Lectin | Specificity | Virus | Reference |
|---|---|---|---|---|
| Griffithsia sp. (Rhodophyta) | Griffithsin | Mannose | HIV | [37,70] |
| HSV | [36] | |||
| HCV | [29] | |||
| SARS-CoV1 and MERS | [71,72] | |||
| EBOV | [73] | |||
| JEV | [37] | |||
| HPV | [36] | |||
| Nostoc ellipsosporum (Cyanobacteria) | CV-N | High-mannose glycans | HIV | [74] |
| HCV | [75] | |||
| Influenza virus | [76] | |||
| Rhinoviruses | [59] | |||
| SARS-CoV2 | [77] | |||
| EBOV | [52] | |||
| Measles virus | [55] | |||
| HHV6 | ||||
| SIV | [52] | |||
| Trichomonas vaginalis | [78] | |||
| Cytonema varium (Cyanobacteria) | SVN | High-mannose glycans | HIV | [79] |
| HCV | ||||
| SARS-CoV1 | [77] | |||
| EBOV | [79] | |||
| Microcystis viridis and Microcystis aeruginosa (Cyanobacteria) | MVN | High-mannose glycans | HIV-1 | [62] |
| HCV | ||||
| Oscillatoria agardhii (Cyanobacteria) | OAAH | High-mannose glycans | HIV-1 | [80] |
| Ostreococcus tauri (Chlorophyta), Gracilaria fisheri (Rhodophyta), Microchloropsis gaditana (Eustigmatophycae), and Porphyra umbilicalis (Rhodophyta) | Yellow-alga-derived legume-lectin-like family | Mannose and high-mannose glycans | HIV | [28] |
| Boodlea coacta (Chlorophyta) | Green-alga-derived Galanthus nivalis agglutinin (GNA) | High mannose (HM)-type N-glycans | HIV | [28] |
| H1N1 | ||||
| Bryopsis plumosa (Chlorophyta) | MFP2-like families | Mannose and high-mannose glycans | HIV-1 | |
| Grateloupia chiangii (Rhodophyta) | Mannose-binding lectin from Grateloupia chiangii (G. chiangii lectin, GCL) | High-mannan N-glycans | HSV | [69] |
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pan, D.; Nolan, J.; Williams, K.H.; Robbins, M.J.; Weber, K.A. Abundance and Distribution of Microbial Cells and Viruses in an Alluvial Aquifer. Front. Microbiol. 2017, 8, 1199. [Google Scholar] [CrossRef] [PubMed]
- Zürcher, S.J.; Kerksieck, P.; Adamus, C.; Burr, C.M.; Lehmann, A.I.; Huber, F.K.; Richter, D. Prevalence of Mental Health Problems During Virus Epidemics in the General Public, Health Care Workers and Survivors: A Rapid Review of the Evidence. Front. Public Health 2020, 8, 560389. [Google Scholar] [CrossRef] [PubMed]
- Lasso, G.; Mayer, S.V.; Winkelmann, E.R.; Chu, T.; Elliot, O.; Patino-Galindo, J.A.; Park, K.; Rabadan, R.; Honig, B.; Shapira, S.D. A Structure-Informed Atlas of Human-Virus Interactions. Cell 2019, 178, 1526–1541.e16. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Khan, F.S.; Rehman, M.I.M.U.; Akram, M.; Riaz, M.; Rasool, G.; Khan, A.H.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef]
- Adamson, C.S.; Chibale, K.; Goss, R.J.M.; Jaspars, M.; Newman, D.J.; Dorrington, R.A. Antiviral drug discovery: Preparing for the next pandemic. Chem. Soc. Rev. 2021, 50, 3647–3655. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Obaidi, I.; Nagar, S.; Scalabrino, G.; Sheridan, H. The antiviral potential of algal-derived macromolecules. Curr. Res. Biotechnol. 2021, 3, 120–134. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; De Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef]
- Rahman, A.; Agrawal, S.; Nawaz, T.; Pan, S.; Selvaratnam, T. A Review of Algae-Based Produced Water Treatment for Biomass and Biofuel Production. Water 2020, 12, 2351. [Google Scholar] [CrossRef]
- Pagarete, A.; Ramos, A.S.; Puntervoll, P.; Allen, M.J.; Verdelho, V. Antiviral Potential of Algal Metabolites—A Comprehensive Review. Mar. Drugs 2021, 19, 94. [Google Scholar] [CrossRef]
- Chathuranga, K.; Weerawardhana, A.; Dodantenna, N.; Ranathunga, L.; Cho, W.-K.; Ma, J.Y.; Lee, J.-S. Inhibitory Effect of Sargassum fusiforme and Its Components on Replication of Respiratory Syncytial Virus In Vitro and In Vivo. Viruses 2021, 13, 548. [Google Scholar] [CrossRef]
- Ciancia, M.; Matulewicz, M.C.; Tuvikene, R. Structural Diversity in Galactans from Red Seaweeds and Its Influence on Rheological Properties. Front. Plant Sci. 2020, 11, 559986. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.-X.; Guan, H.-S. The Antiviral Activities and Mechanisms of Marine Polysaccharides: An Overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Lee, J.-B.; Hayashi, K.; Hirata, M.; Kuroda, E.; Suzuki, E.; Kubo, Y.; Hayashi, T. Antiviral Sulfated Polysaccharide from Navicula directa, a Diatom Collected from Deep-Sea Water in Toyama Bay. Biol. Pharm. Bull. 2006, 29, 2135–2139. [Google Scholar] [CrossRef]
- Sansone, C.; Brunet, C.; Noonan, D.M.; Albini, A. Marine Algal Antioxidants as Potential Vectors for Controlling Viral Diseases. Antioxidants 2020, 9, 392. [Google Scholar] [CrossRef]
- Kanekiyo, K.; Hayashi, K.; Takenaka, H.; Lee, J.-B.; Hayashi, T. Anti-herpes Simplex Virus Target of an Acidic Polysaccharide, Nostoflan, from the Edible Blue-Green Alga Nostoc flagelliforme. Biol. Pharm. Bull. 2007, 30, 1573–1575. [Google Scholar] [CrossRef] [PubMed]
- Cardellina, J.H.; Munro, M.H.G.; Fuller, R.W.; Manfredi, K.P.; McKee, T.C.; Tischler, M.; Bokesch, H.R.; Gustafson, K.R.; Beutler, J.A.; Boyd, M.R. A Chemical Screening Strategy for the Dereplication and Prioritization of HIV-Inhibitory Aqueous Natural Products Extracts. J. Nat. Prod. 1993, 56, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.A. Lectin Histochemistry: Historical Perspectives, State of the Art, and the Future. Methods Mol. Biol. 2017, 1560, 93–107. [Google Scholar] [CrossRef]
- Singh, R.S.; Thakur, S.R.; Bansal, P. Algal lectins as promising biomolecules for biomedical research. Crit. Rev. Microbiol. 2015, 41, 77–88. [Google Scholar] [CrossRef]
- Gorakshakar, A.; Ghosh, K. Use of lectins in immunohematology. Asian J. Transfus. Sci. 2016, 10, 12–21. [Google Scholar] [CrossRef]
- Ahmed, N.; Jahan, R.; Nissapatorn, V.; Wilairatana, P.; Rahmatullah, M. Plant lectins as prospective antiviral biomolecules in the search for COVID-19 eradication strategies. BioMedicine 2022, 146, 112507. [Google Scholar] [CrossRef]
- Lam, S.K.; Ng, T.B. Lectins: Production and practical applications. Appl. Microbiol. Biotechnol. 2011, 89, 45–55. [Google Scholar] [CrossRef]
- Mishra, A.; Behura, A.; Mawatwal, S.; Kumar, A.; Naik, L.; Mohanty, S.S.; Manna, D.; Dokania, P.; Mishra, A.; Patra, S.K.; et al. Structure-function and application of plant lectins in disease biology and immunity. Food Chem. Toxicol. 2019, 134, 110827. [Google Scholar] [CrossRef]
- Dan, X.; Liu, W.; Ng, T.B. Development and Applications of Lectins as Biological Tools in Biomedical Research. Med. Res. Rev. 2016, 36, 221–247. [Google Scholar] [CrossRef]
- Lepenies, B.; Lang, R. Editorial: Lectins and Their Ligands in Shaping Immune Responses. Front. Immunol. 2019, 10, 2379. [Google Scholar] [CrossRef]
- Liu, F.-T.; Stowell, S.R. The role of galectins in immunity and infection. Nat. Rev. Immunol. 2023, 23, 479–494. [Google Scholar] [CrossRef]
- Barre, A.; Simplicien, M.; Benoist, H.; Van Damme, E.J.; Rougé, P. Mannose-Specific Lectins from Marine Algae: Diverse Structural Scaffolds Associated to Common Virucidal and Anti-Cancer Properties. Mar. Drugs 2019, 17, 440. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, N.E.; O’Keefe, B.R.; Mori, T.; Zhu, C.; Giomarelli, B.; Vojdani, F.; Palmer, K.E.; McMahon, J.B.; Wlodawer, A. Domain-Swapped Structure of the Potent Antiviral Protein Griffithsin and Its Mode of Carbohydrate Binding. Structure 2006, 14, 1127–1135. [Google Scholar] [CrossRef]
- Lusvarghi, S.; Lohith, K.; Morin-Leisk, J.; Ghirlando, R.; Hinshaw, J.E.; Bewley, C.A. Binding Site Geometry and Subdomain Valency Control Effects of Neutralizing Lectins on HIV-1 Viral Particles. ACS Infect. Dis. 2016, 2, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Pagliolico, S.L.; Verso, V.R.L.; Bosco, F.; Mollea, C.; La Forgia, C. A Novel Photo-bioreactor Application for Microalgae Production as a Shading System in Buildings. Energy Procedia 2017, 111, 151–160. [Google Scholar] [CrossRef]
- Mu, J.; Hirayama, M.; Sato, Y.; Morimoto, K.; Hori, K. A Novel High-Mannose Specific Lectin from the Green Alga Halimeda renschii Exhibits a Potent Anti-Influenza Virus Activity through High-Affinity Binding to the Viral Hemagglutinin. Mar. Drugs 2017, 15, 255. [Google Scholar] [CrossRef]
- Maliki, I.M.; Misson, M.; Teoh, P.L.; Rodrigues, K.F.; Yong, W.T.L. Production of Lectins from Marine Algae: Current Status, Challenges, and Opportunities for Non-Destructive Extraction. Mar. Drugs 2022, 20, 102. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Extraction of protein from the macroalga Palmaria palmata. LWT—Food. Sci. Technol. 2013, 51, 375–382. [Google Scholar] [CrossRef]
- Polzin, J.; Rorrer, G.L. Selective production of the acyclic monoterpene β-myrcene by microplantlet suspension cultures of the macrophytic marine red alga Ochtodes secundiramea under nutrient perfusion cultivation with bromide-free medium. Algal Res. 2018, 36, 159–166. [Google Scholar] [CrossRef]
- Moulaei, T.; Alexandre, K.B.; Shenoy, S.R.; Meyerson, J.R.; Krumpe, L.R.; Constantine, B.; Wilson, J.; Buckheit, R.W., Jr.; McMahon, J.B.; Subramaniam, S.; et al. Griffithsin tandemers: Flexible and potent lectin inhibitors of the human immunodeficiency virus. Retrovirology 2015, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Griffithsin, a Highly Potent Broad-Spectrum Antiviral Lectin from Red Algae: From Discovery to Clinical Application. Mar. Drugs 2019, 17, 567. [Google Scholar] [CrossRef]
- Zeitlin, L.; Pauly, M.; Whaley, K.J. Second-generation HIV microbicides: Continued development of griffithsin. Proc. Natl. Acad. Sci. USA 2009, 106, 6029–6030. [Google Scholar] [CrossRef]
- Naik, S.; Kumar, S. Lectins from plants and algae act as anti-viral against HIV, influenza and coronaviruses. Mol. Biol. Rep. 2022, 49, 12239–12246. [Google Scholar] [CrossRef]
- Romero, J.A.F.; Paglini, M.G.; Priano, C.; Koroch, A.; Rodríguez, Y.; Sailer, J.; Teleshova, N. Algal and Cyanobacterial Lectins and Their Antimicrobial Properties. Mar. Drugs 2021, 19, 687. [Google Scholar] [CrossRef]
- Singh, R.S.; Walia, A.K. Lectins from red algae and their biomedical potential. J. Appl. Phycol. 2018, 30, 1833–1858. [Google Scholar] [CrossRef]
- Micewicz, E.D.; Cole, A.L.; Jung, C.-L.; Luong, H.; Phillips, M.L.; Pratikhya, P.; Sharma, S.; Waring, A.J.; Cole, A.M.; Ruchala, P. Grifonin-1: A Small HIV-1 Entry Inhibitor Derived from the Algal Lectin, Griffithsin. PLoS ONE 2010, 5, e14360. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Michael, E.; Eggink, D.; van Montfort, T.; Lasnik, A.B.; Palmer, K.E.; Sanders, R.W.; Moore, J.P.; Klasse, P.J.; Pritchard, L.K.; et al. Occluding the Mannose Moieties on Human Immunodeficiency Virus Type 1 gp120 with Griffithsin Improves the Antibody Responses to Both Proteins in Mice. AIDS Res. Hum. Retroviruses 2012, 28, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Hoorelbeke, B.; Xue, J.; LiWang, P.J.; Balzarini, J. Role of the Carbohydrate-Binding Sites of Griffithsin in the Prevention of DC-SIGN-Mediated Capture and Transmission of HIV-1. PLoS ONE 2013, 8, e64132. [Google Scholar] [CrossRef]
- Kagiampakis, I.; Gharibi, A.; Mankowski, M.K.; Snyder, B.A.; Ptak, R.G.; Alatas, K.; LiWang, P.J. Potent Strategy To Inhibit HIV-1 by Binding both gp120 and gp41. Antimicrob. Agents Chemother. 2011, 55, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Hoorelbeke, B.; Kagiampakis, I.; Demeler, B.; Balzarini, J.; LiWang, P.J. The Griffithsin Dimer Is Required for High-Potency Inhibition of HIV-1: Evidence for Manipulation of the Structure of gp120 as Part of the Griffithsin Dimer Mechanism. Antimicrob. Agents Chemother. 2013, 57, 3976–3989. [Google Scholar] [CrossRef]
- Mori, T.; Boyd, M.R. Cyanovirin-N, a Potent Human Immunodeficiency Virus-Inactivating Protein, Blocks both CD4-Dependent and CD4-Independent Binding of Soluble gp120 (sgp120) to Target Cells, Inhibits sCD4-Induced Binding of sgp120 to Cell-Associated CXCR4, and Dissociates Bound sgp120 from Target Cells. Antimicrob. Agents Chemother. 2001, 45, 664–672. [Google Scholar] [CrossRef][Green Version]
- Boyd, M.R.; Gustafson, K.R.; McMahon, J.B.; Shoemaker, R.H.; O’Keefe, B.R.; Mori, T.; Gulakowski, R.J.; Wu, L.; Rivera, M.I.; Laurencot, C.M.; et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob. Agents Chemother. 1997, 41, 1521–1530. [Google Scholar] [CrossRef]
- Matei, E.; Basu, R.; Furey, W.; Shi, J.; Calnan, C.; Aiken, C.; Gronenborn, A.M. Structure and Glycan Binding of a New Cyanovirin-N Homolog. J. Biol. Chem. 2016, 291, 18967–18976. [Google Scholar] [CrossRef]
- Bewley, C.A.; Gustafson, K.R.; Boyd, M.R.; Covell, D.G.; Bax, A.; Clore, G.M.; Gronenborn, A.M. Solution structure of cyanovirin-N, a potent HIV-inactivating protein. Nat. Struct. Biol. 1998, 5, 571–578. [Google Scholar] [CrossRef]
- Botos, I.; O’keefe, B.R.; Shenoy, S.R.; Cartner, L.K.; Ratner, D.M.; Seeberger, P.H.; Boyd, M.R.; Wlodawer, A. Structures of the Complexes of a Potent Anti-HIV Protein Cyanovirin-N and High Mannose Oligosaccharides. J. Biol. Chem. 2002, 277, 34336–34342. [Google Scholar] [CrossRef]
- Huskens, D.; Férir, G.; Vermeire, K.; Kehr, J.-C.; Balzarini, J.; Dittmann, E.; Schols, D. Microvirin, a Novel α(1,2)-Mannose-specific Lectin Isolated from Microcystis aeruginosa, Has Anti-HIV-1 Activity Comparable with That of Cyanovirin-N but a Much Higher Safety Profile. J. Biol. Chem. 2010, 285, 24845–24854. [Google Scholar] [CrossRef]
- Dam, T.; Brewer, C. Fundamentals of lectin-carbohydrate interactions. In Comprehensive Glycoscience; Kamerling, J.P., Boons, G.-J., Lee, Y.C., Suzuki, A., Taniguchi, N., Voragen, A.G., Eds.; Elsevier: Oxford, UK, 2007; pp. 397–452. [Google Scholar] [CrossRef]
- Esser, M.T.; Mori, T.; Mondor, I.; Sattentau, Q.J.; Dey, B.; Berger, E.A.; Boyd, M.R.; Lifson, J.D. Cyanovirin-N Binds to gp120 To Interfere with CD4-Dependent Human Immunodeficiency Virus Type 1 Virion Binding, Fusion, and Infectivity but Does Not Affect the CD4 Binding Site on gp120 or Soluble CD4-Induced Conformational Changes in gp120. J. Virol. 1999, 73, 4360–4371. [Google Scholar] [CrossRef]
- Lotfi, H.; Sheervalilou, R.; Zarghami, N. An update of the recombinant protein expression systems of Cyanovirin-N and challenges of preclinical development. BioImpacts 2018, 8, 139–151. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, W.; Liang, H.; Wang, Z.; Chen, J.; Hong, H.; Xie, L.; Nie, H.; Xiong, S. Preparation of a monoPEGylated derivative of cyanovirin-N and its virucidal effect on acyclovir-resistant strains of herpes simplex virus type 1. Arch. Virol. 2019, 164, 1259–1269. [Google Scholar] [CrossRef]
- Jones, T.H.; McClelland, E.E.; McFeeters, H.; McFeeters, R.L. Novel Antifungal Activity for the Lectin Scytovirin: Inhibition of Cryptococcus neoformans and Cryptococcus gattii. Front. Microbiol. 2017, 8, 755. [Google Scholar] [CrossRef] [PubMed]
- McFeeters, R.L.; Xiong, C.; O’keefe, B.R.; Bokesch, H.R.; McMahon, J.B.; Ratner, D.M.; Castelli, R.; Seeberger, P.H.; Byrd, R.A. The Novel Fold of Scytovirin Reveals a New Twist for Antiviral Entry Inhibitors. J. Mol. Biol. 2007, 369, 451–461. [Google Scholar] [CrossRef]
- O’Keefe, B.R.; Smee, D.F.; Turpin, J.A.; Saucedo, C.J.; Gustafson, K.R.; Mori, T.; Blakeslee, D.; Buckheit, R.; Boyd, M.R. Potent Anti-Influenza Activity of Cyanovirin-N and Interactions with Viral Hemagglutinin. Antimicrob. Agents Chemother. 2003, 47, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Moulaei, T.; Botos, I.; Ziółkowska, N.E.; Bokesch, H.R.; Krumpe, L.R.; McKee, T.C.; O’Keefe, B.R.; Dauter, Z.; Wlodawer, A. Atomic-resolution crystal structure of the antiviral lectin scytovirin. Protein Sci. 2007, 16, 2756–2760. [Google Scholar] [CrossRef] [PubMed]
- Gondim, A.C.S.; da Silva, S.R.; Mathys, L.; Noppen, S.; Liekens, S.; Sampaio, A.H.; Nagano, C.S.; Rocha, C.R.C.; Nascimento, K.S.; Cavada, B.S.; et al. Potent antiviral activity of carbohydrate-specific algal and leguminous lectins from the Brazilian biodiversity. MedChemComm 2019, 10, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Shahzad-Ul-Hussan, S.; Gustchina, E.; Ghirlando, R.; Clore, G.M.; Bewley, C.A. Solution Structure of the Monovalent Lectin Microvirin in Complex with Manα(1–2)Man Provides a Basis for Anti-HIV Activity with Low Toxicity. J. Biol. Chem. 2011, 286, 20788–20796. [Google Scholar] [CrossRef]
- Shahid, M.; Qadir, A.; Yang, J.; Ahmad, I.; Zahid, H.; Mirza, S.; Windisch, M.P.; Shahzad-Ul-Hussan, S. An Engineered Microvirin Variant with Identical Structural Domains Potently Inhibits Human Immunodeficiency Virus and Hepatitis C Virus Cellular Entry. Viruses 2020, 12, 199. [Google Scholar] [CrossRef]
- Hladik, F.; McElrath, M.J. Setting the stage: Host invasion by HIV. Nat. Rev. Immunol. 2008, 8, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, K.; Princen, K.; Hatse, S.; De Clercq, E.; Dey, K.; Bell, T.W.; Schols, D. CADA, a novel CD4-targeted HIV inhibitor, is synergistic with various anti-HIV drugs in vitro. AIDS 2004, 18, 2115–2125. [Google Scholar] [CrossRef]
- Koharudin, L.M.I.; Kollipara, S.; Aiken, C.; Gronenborn, A.M. Structural Insights into the Anti-HIV Activity of the Oscillatoria agardhii Agglutinin Homolog Lectin Family. J. Biol. Chem. 2012, 287, 33796–33811. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Murakami, M.; Miyazawa, K.; Hori, K. Purification and characterization of a novel lectin from a freshwater cyanobacterium, Oscillatoria agardhii. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 125, 169–177. [Google Scholar] [CrossRef]
- Buts, L.; Garcia-Pino, A.; Wyns, L.; Loris, R. Structural basis of carbohydrate recognition by a Man(α1-2)Man-specific lectin from Bowringia milbraedii. Glycobiology 2006, 16, 635–640. [Google Scholar] [CrossRef]
- Sato, Y.; Hirayama, M.; Morimoto, K.; Yamamoto, N.; Okuyama, S.; Hori, K. High Mannose-binding Lectin with Preference for the Cluster of α1–2-Mannose from the Green Alga Boodlea coacta Is a Potent Entry Inhibitor of HIV-1 and Influenza Viruses. J. Biol. Chem. 2011, 286, 19446–19458. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Han, J.-W.; Jeon, H.; Cho, K.; Kim, J.-H.; Lee, D.-S.; Han, J.W. Characterization of a Novel Mannose-Binding Lectin with Antiviral Activities from Red Alga, Grateloupia chiangii. Biomolecules 2020, 10, 333. [Google Scholar] [CrossRef]
- Tyo, K.M.; Lasnik, A.B.; Zhang, L.; Jenson, A.B.; Fuqua, J.L.; Palmer, K.E.; Steinbach-Rankins, J.M. Rapid-Release Griffithsin Fibers for Dual Prevention of HSV-2 and HIV-1 Infections. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Alsaidi, S.; Cornejal, N.; Mahoney, O.; Melo, C.; Verma, N.; Bonnaire, T.; Chang, T.; O’keefe, B.R.; Sailer, J.; Zydowsky, T.M.; et al. Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model. Mar. Drugs 2021, 19, 418. [Google Scholar] [CrossRef]
- Millet, J.K.; Séron, K.; Labitt, R.N.; Danneels, A.; Palmer, K.E.; Whittaker, G.R.; Dubuisson, J.; Belouzard, S. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antivir. Res. 2016, 133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Ebola virus (EBOV) infection: Therapeutic strategies. Biochem. Pharmacol. 2015, 93, 1–10. [Google Scholar] [CrossRef]
- Dey, B.; Lerner, D.L.; Lusso, P.; Boyd, M.R.; Elder, J.H.; Berger, E.A. Multiple Antiviral Activities of Cyanovirin-N: Blocking of Human Immunodeficiency Virus Type 1 gp120 Interaction with CD4 and Coreceptor and Inhibition of Diverse Enveloped Viruses. J. Virol. 2000, 74, 4562–4569. [Google Scholar] [CrossRef] [PubMed]
- Helle, F.; Wychowski, C.; Vu-Dac, N.; Gustafson, K.R.; Voisset, C.; Dubuisson, J. Cyanovirin-N Inhibits Hepatitis C Virus Entry by Binding to Envelope Protein Glycans. J. Biol. Chem. 2006, 281, 25177–25183. [Google Scholar] [CrossRef] [PubMed]
- Smee, D.F.; Wandersee, M.K.; Checketts, M.B.; O’Keefe, B.R.; Saucedo, C.; Boyd, M.R.; Mishin, V.P.; Gubareva, L.V. Influenza a (H1N1) Virus Resistance to Cyanovirin-N Arises Naturally during Adaptation to Mice and by Passage in Cell Culture in the Presence of the Inhibitor. Antivir. Chem. Chemother. 2007, 18, 317–327. [Google Scholar] [CrossRef]
- Naidoo, D.; Kar, P.; Roy, A.; Mutanda, T.; Bwapwa, J.; Sen, A.; Anandraj, A. Structural Insight into the Binding of Cyanovirin-N with the Spike Glycoprotein, Mpro and PLpro of SARS-CoV-2: Protein–Protein Interactions, Dynamics Simulations and Free Energy Calculations. Molecules 2021, 26, 5114. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ratner, D.M.; Ryan, C.M.; Johnson, P.J.; O’keefe, B.R.; Secor, W.E.; Anderson, D.J.; Robbins, P.W.; Samuelson, J. Anti-Retroviral Lectins Have Modest Effects on Adherence of Trichomonas vaginalis to Epithelial Cells In Vitro and on Recovery of Tritrichomonas foetus in a Mouse Vaginal Model. PLoS ONE 2015, 10, e0135340. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A.R.; Giomarelli, B.G.; Lear-Rooney, C.M.; Saucedo, C.J.; Yellayi, S.; Krumpe, L.R.H.; Rose, M.; Paragas, J.; Bray, M.; Olinger, G.G., Jr.; et al. The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against Zaire Ebola virus. Antivir. Res. 2014, 112, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Koharudin, L.M.; Gronenborn, A.M. Structural Basis of the Anti-HIV Activity of the Cyanobacterial Oscillatoria Agardhii Agglutinin. Structure 2011, 19, 1170–1181. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez, C.; Félix, C.; Lemos, M.F.L. The Antiviral Potential of Algal Lectins. Mar. Drugs 2023, 21, 515. https://doi.org/10.3390/md21100515
Alvarez C, Félix C, Lemos MFL. The Antiviral Potential of Algal Lectins. Marine Drugs. 2023; 21(10):515. https://doi.org/10.3390/md21100515
Chicago/Turabian StyleAlvarez, Christian, Carina Félix, and Marco F. L. Lemos. 2023. "The Antiviral Potential of Algal Lectins" Marine Drugs 21, no. 10: 515. https://doi.org/10.3390/md21100515
APA StyleAlvarez, C., Félix, C., & Lemos, M. F. L. (2023). The Antiviral Potential of Algal Lectins. Marine Drugs, 21(10), 515. https://doi.org/10.3390/md21100515









