Abstract
Algae have emerged as fascinating subjects of study due to their vast potential as sources of valuable metabolites with diverse biotechnological applications, including their use as fertilizers, feed, food, and even pharmaceutical precursors. Among the numerous compounds found in algae, lectins have garnered special attention for their unique structures and carbohydrate specificities, distinguishing them from lectins derived from other sources. Here, a comprehensive overview of the latest scientific and technological advancements in the realm of algal lectins with a particular focus on their antiviral properties is provided. These lectins have displayed remarkable effectiveness against a wide range of viruses, thereby holding great promise for various antiviral applications. It is worth noting that several alga species have already been successfully commercialized for their antiviral potential. However, the discovery of a diverse array of lectins with potent antiviral capabilities suggests that the field holds immense untapped potential for further expansion. In conclusion, algae stand as a valuable and versatile resource, and their lectins offer an exciting avenue for developing novel antiviral agents, which may lead to the development of cutting-edge antiviral therapies.
1. Introduction
Viruses are the most abundant biological entities on Earth (though the term “biological” may still offer doubts to some biologists). Their diversity, accompanied by their rapid rates of mutation and adaptability, makes them an important threat to humanity [1]. Although many viruses are considered harmful to humans, only a few can cause serious health problems [2]. To the best of our knowledge, there are more than 1000 known species of viruses that are capable of infecting humans [3]. Antiviral drugs are used for the treatment of viral infections [4]; however, only drugs for ten viral species known to infect humans are clinically approved [5]. This underscores the significance of discovering new sources for antiviral compounds. A diverse range of molecules with anticancer, antiviral, and antibiotic activities has been identified and isolated from algal species. In fact, algae represent one of the richest known sources of natural antivirals, as well as a significant source of other bioactive compounds [6].
3. Lectins
Lectins, a class of molecules prevalent in various algae, are renowned for their antiviral properties and offering promising avenues for the development of novel pharmaceuticals [10]. In the current study, we delve into the primary groups extracted from algae that hold potential as antiviral agents (Table 1).
These are natural, bioactive proteins of non-immune origin, characterized by their remarkable capacity to bind polysaccharides, glycans, glycoproteins, and glycolipids with a high degree of specificity [18,19]. These compounds hold significant importance owing to their biological properties, which include interactions with specific blood groups such as lymphocyte agglutination, sperm, erythrocytes, and platelets. Additionally, they can induce mitosis in lymphocytes and exhibit the capability to induce cytotoxic effects on T-lymphocytes [20]. They possess two or more carbohydrate-binding sites that have the ability to agglutinate erythrocytes without altering the properties of carbohydrates [21,22]. Some applications include the assessment of lymphoproliferative and cytotoxic functions in mononuclear cells, the detection of chromosomal abnormalities, the utilization of fluorescent markers to investigate structural alterations in glyco-conjugates located on cell surfaces, and other applications in antiviral research [22]. Algal lectins are presently employed in biomedical research for a range of purposes, including their roles in antiviral, antinociceptive, anti-inflammatory, and antitumor activities, among other applications [19,21].
The origins of lectin research trace back to 1888, when the agglutination of red blood cells by ricin was first demonstrated. This compound found notoriety during the World Wars as a weapon of choice [23]. The term lectin was introduced by Boyd in 1954 and derives from the Latin word “legere”, which means “to choose” [24]. The first pure hemagglutinin was obtained from a type of bean in 1919 and is the most studied lectin to date [20,24]. The initial documentation of lectins being employed as antiviral agents dates back to the 1980s, a crucial period during the emergence of HIV. During this time, research showcased their capacity to inhibit reverse transcriptase in HIV-1 patients, positioning lectins as significant contenders in the realm of antiviral treatments [24].
Regarding innate immunity, lectins can function as Pattern Recognition Receptors (PRRs), enabling them to identify Pathogen-Associated Molecular Patterns (PAMPs) and Damage-Associated Molecular Patterns (DAMPs). The principal classes of lectins involved in innate immunity include C-type lectin receptors (CLRs), siglecs, and galectins [25]. For instance, galectins (carbohydrate (glycan)-binding proteins that are expressed by a wide range of cells and bind to galactose-containing glycans) can modulate not only innate but also adaptive immune cells through binding to glycans on the surface of immune cells or intracellularly (by carbohydrate-dependent or -independent interactions). This family of lectins expressed by immune cells can also be involved in host responses to infection. They have the capacity to directly bind to microorganisms and can modulate various antimicrobial functions, including processes like autophagy [26].
These different classes of lectins exhibit diverse functions in antimicrobial defense and immune homeostasis, making the use of lectins a promising strategy for modeling immune responses in contexts such as infections, autoimmunity, cancer, and vaccination [25].
3.1. Algal Lectins
Algae represent an extensive reservoir of lectins characterized by their distinctive properties [27]. Certain alga species have been documented to harbor lectins that exhibit carbohydrate specificity towards complex glycoproteins or high-mannose N-glycans [27]. These interactions give them the ability to bind terminal mannoses present in high-mannose oligosaccharides and crosslink these glycans on the surface of the viral envelope glycoprotein gp120 [28]. The presence of lectins has been demonstrated in about 838 alga species, according to Web of Science. However, it is anticipated that this number will continue to grow, given the existence of thousands of marine alga species worldwide yet to be studied.
Lectins are classified according to their structure into the following: merolectins, possessing one carbohydrate recognition domain (CRD), small proteins that due to their monovalent nature are unable to precipitate glycoconjugates or agglutinate cells; hololectins, containing two or more CRDs with homologous structure; chimeric lectins, fusion proteins that have a CRD in conjunction with a domain with catalytic activity; and super lectins, possessing at least two different CRDs [23].
Production of Algal Lectins
Some algae can be cultivated in photobioreactors, such as bubble column, airlift, stirred tank, and tubular recycle photobioreactors [29]. The most important prerequisites for mass-producing lectins are the identification of sources with elevated lectin content and the implementation of straightforward purification methods [30]. The presence of cell walls and intracellular polysaccharides causes high viscosity and ionic interactions, making the extraction process difficult, as does some features of the morphology of the marine algae themselves; for example, marine algae with a tougher thallus may require more processing [31]. Lectins are typically isolated from marine algae by grinding the algal tissue with liquid nitrogen and extracting with appropriate buffers and alcohol, as described by Maliki and co-workers [32]. This method can produce higher yields, but it is not ideal for large-scale production, since a large amount of biomass is required to extract a small quantity of compound and a significant amount of waste is generated during the extraction process [32,33]. The products of biosynthesis also depend on several other factors such as compound sensitivity to shear forces, the tissue growth rate, cell aggregation, and cell flotation [32]. As an alternative to invasive and destructive techniques, the cold steeping infusion (CSI) method is available. This allows the recovery of lectins from algal cultures without causing harm to the producing tissues. In this case, algae release extracellular bioactive compounds—lectins included—that are continuously retrieved from buffer solutions, allowing the continuous growth of the algal biomass to subsequent culture cycles [34].
3.2. Algal Lectins with Antiviral Potential
3.2.1. Griffithsin
The lectin griffithsin (GRFT) is a red-alga-derived lectin known as a potent antiviral agent capable of preventing and treating infections caused by several enveloped viruses. GRFT is thermostable and resists a large pH range, exhibits little toxicity or immunogenicity, and is available for large-scale manufacturing [35].
Griffithsin was isolated for the first time from an aqueous extract of Griffithsia sp. (Rhodophyta). It is a protein widely known for its ability to inhibit HVI1 in vitro. This compound is considered one of the most powerful entry inhibitors to date [10]. Its antiviral activity is correlated to its structural characteristics since it presents a unique structure (Figure 1) [28] that is extremely selective to a sugar present in the envelope of many pathogenic viruses, such as hepatitis C virus (HCV), herpes simplex virus 2 (HSV2), Japanese encephalitis virus (JEV), and porcine epidemic diarrhea virus (PEDV) [36]. Griffithsin is not only an extremely effective HIV entry inhibitor, but also improves antibody responses and avoids cell fusion and cell-to-cell transmission of HIV [37]. According to an in silico study by Naik and colleagues [38], lectins have a high binding affinity for the glycans of the SARS-CoV-2 spike glycoprotein (found on the surface of some enveloped viruses). These authors found that an interaction between the model lectin Lablab purpureus and the amino acid residues Asn487, Tyr489, Gln493, Lys417, and Tyr505 of the receptor binding domain (RBD) of SARS-CoV-2 was formed, and an analogous interaction for SARS-CoV-2 spike protein was observed with griffithsin, demonstrating the potential of these molecules for neutralizing coronavirus infection [38].
Although lectins are known for their broad-spectrum activity, high specificity, and local delivery (topical application), there are also concerns regarding their toxicity, as they can induce mitogenic activity after long exposures. Among the broad range of assessed lectins, griffithsin (GRFT) distinguishes itself in several aspects. Notably, it deviates from the norm with its lack of mitogenic activity. Furthermore, it has demonstrated promising outcomes in Phase 1 clinical trials, confirming its safety when used in topical formulations. Additionally, it holds the potential for cost-effective production in substantial quantities [39,40].
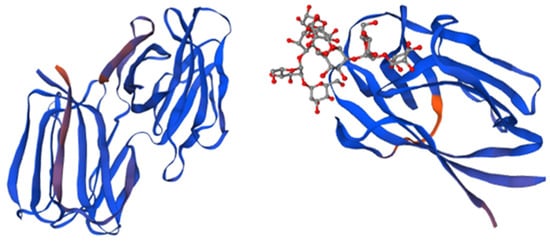
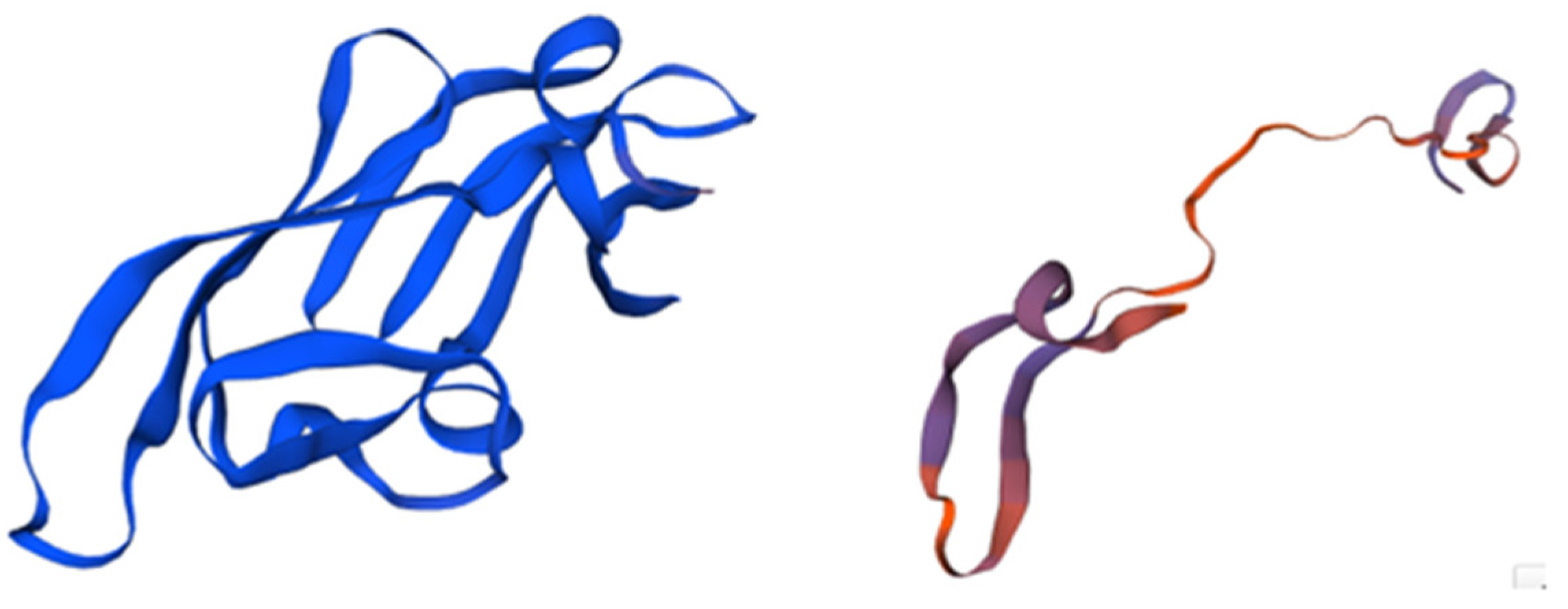
Figure 1.
The structure of Griffithsin and the domains of this protein. Amino acids located within beta strands are highlighted in magenta to indicate their secondary structure, while each monomer of the domain-swapped dimer is depicted in blue. The crystal structure of a GRFT dimer with six mannoses is depicted, with each GRFT monomer shown in blue, and the N-terminal extension resulting from the cloning procedure colored in orange. Adapted from Pubmed and Micewicz et al. [41] (PDB entry code 2GTY).
Figure 1.
The structure of Griffithsin and the domains of this protein. Amino acids located within beta strands are highlighted in magenta to indicate their secondary structure, while each monomer of the domain-swapped dimer is depicted in blue. The crystal structure of a GRFT dimer with six mannoses is depicted, with each GRFT monomer shown in blue, and the N-terminal extension resulting from the cloning procedure colored in orange. Adapted from Pubmed and Micewicz et al. [41] (PDB entry code 2GTY).
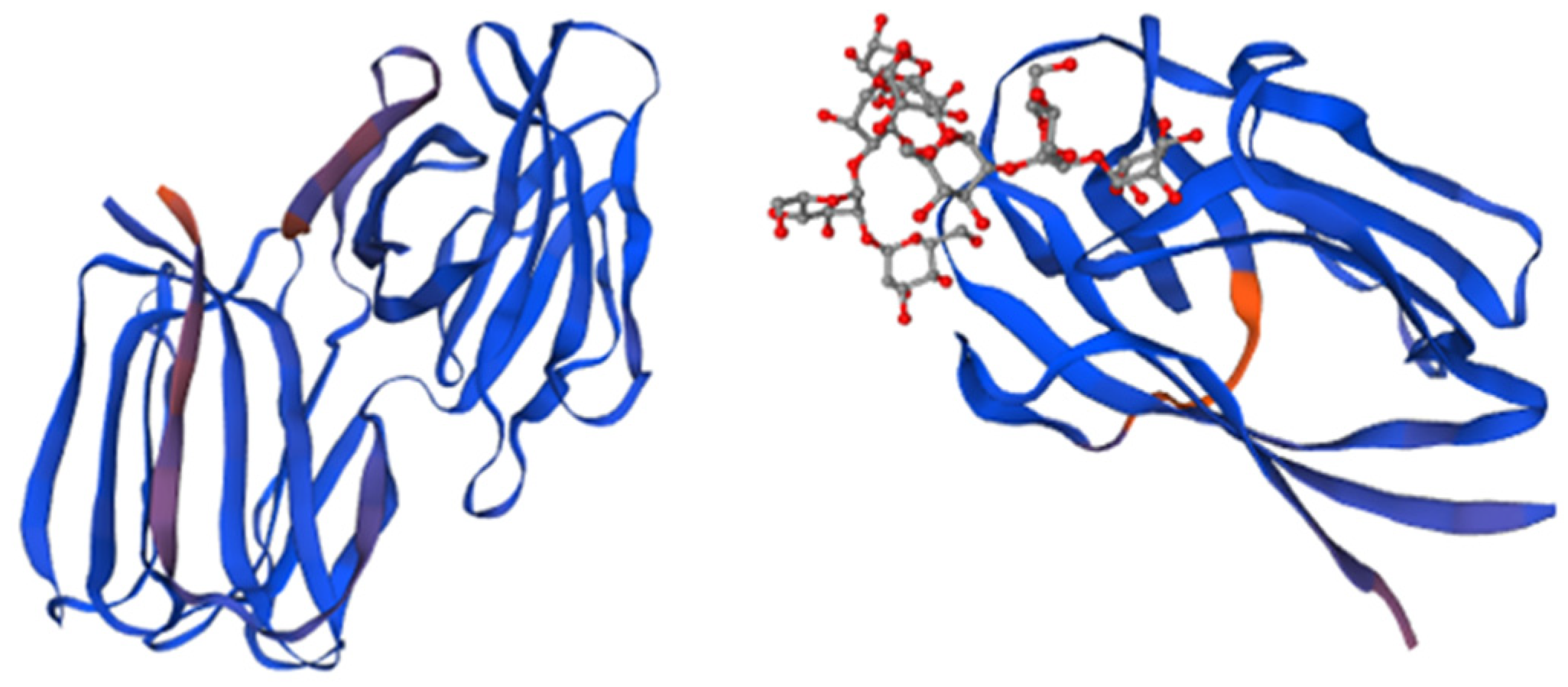
Griffithsin’s antiviral activity stems from its ability to bind terminal mannoses present in high-mannose oligosaccharides and crosslink these glycans on the surface of the viral envelope glycoproteins [28]. GRFT is capable of inhibiting gp120 (a glycoprotein that is part of the outer layer of the virus) from binding to the 2G12 mAb, which targets N-linked glycans at positions 332, 339, and 392 on gp120.
The mechanisms of action are based on the exposure of the CD4 binding site of gp120 through the glycan at position 386 and blockage of the coreceptor binding step [37]; inhibition of mannose binding to gp120 and improvement of the humoral immune response to gp120 [42]; inhibition of gp120 binding to DC-SIGN and expulsion of gp120 from the gp120/DC-SIGN complex [43]; alteration of the gp120 structure through the exposure of the CD4 binding site [43]; intra-virion crosslinking of gp120 [35]; and inter-virion aggregation or clustering of gp120 [29].
Using the GRFT molecule as a base, Micewicz et al. [41] successfully constructed a model for a peptide referred to as grifonin1. This peptide consists of three covalently linked beta sheets, which exhibit a distinctive triple symmetry. Even though it shows a less potent antiviral activity than its base protein, it is capable of inhibiting viral cycles at low concentrations. To enrich the capacity of GRFT to inhibit HIV infection, Kagiampakis et al. [44] created a covalently linked fusion protein based on GRFT and a notorious virus entry inhibitor known as GRFT-C37. GRFT-C37 is capable of blocking virus fusion by binding to the terminal helices of the gp41, with a capacity between 5 and 8 times greater than that of GRFT [43,45].
3.2.2. Cyanovirin-N
Cyanovirin-N (CV-N) is a 101-amino-acid protein extremely resistant to physicochemical degradation, and it is capable of supporting treatment with denaturants, detergents, organic solvents, and heat up to 100 °C with no apparent loss of antiviral properties [46].
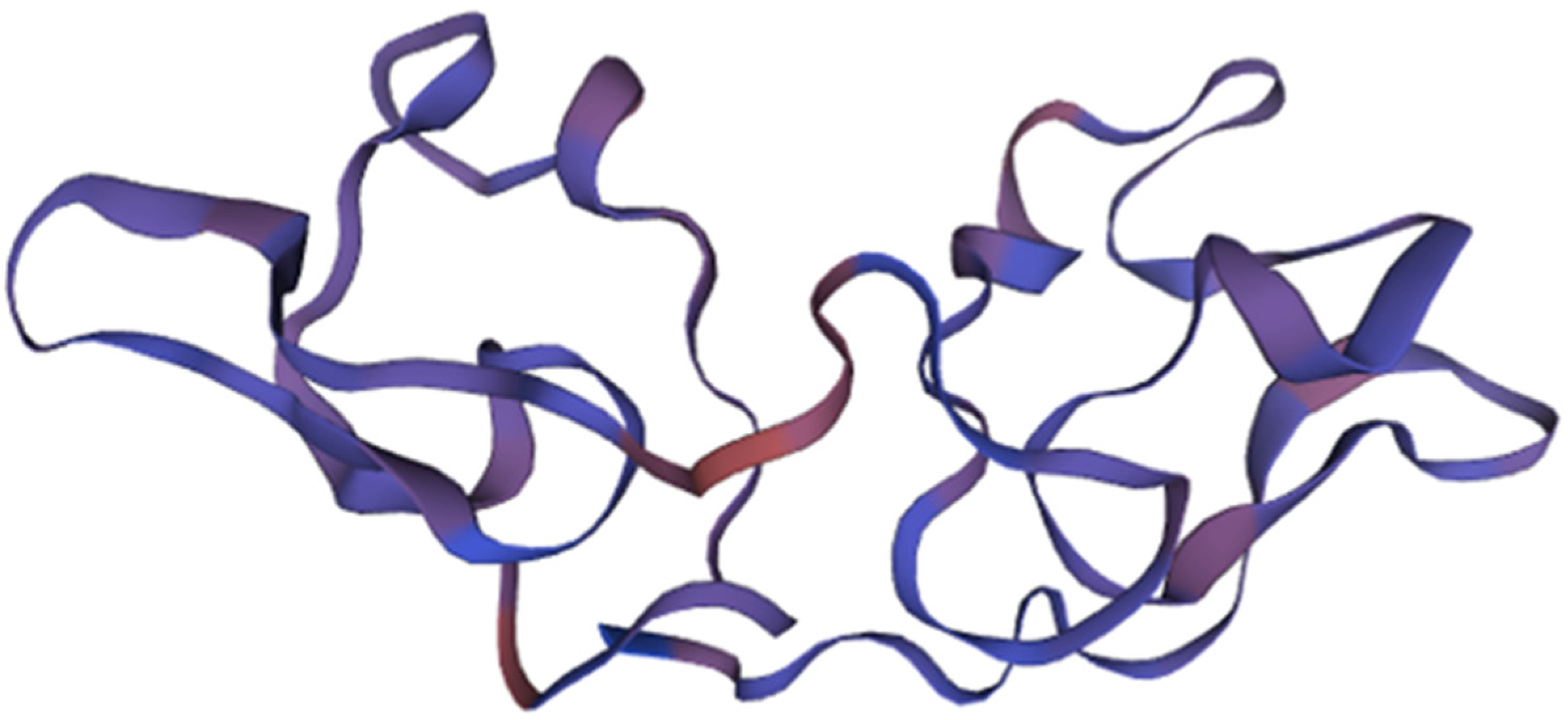
This compound was initially isolated in 1997 from the cyanobacterium Nostoc ellipsosporum (Cyanobacteria) as part of efforts to identify agents from natural sources capable of inhibiting human immunodeficiency virus (HIV) infection [47,48]. The antiviral activity of CV-N is closely linked to its structural characteristics. Specifically, it possesses a duplicated internal sequence that includes four cysteine (Cys) group residues, leading to the formation of two disulfide bridges. This structural arrangement is depicted in Figure 2 [49]. These bonds are fundamental for the stabilization of the protein’s structure, determining its antiviral activity.
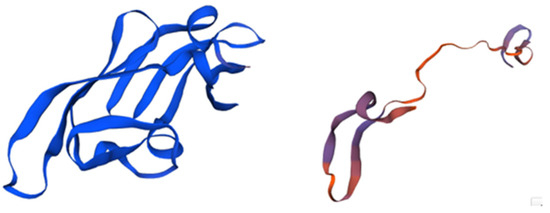
Figure 2.
The structure of Cyanovirin and its domains. The protein, consisting of 101 amino acids, is depicted in blue, with its β-strands and helical turns also highlighted in blue. The stereo view shows superpositions of the ensemble of the final 40 simulated annealing structures of cyanovirin-N. The backbone is shown in magenta, the disulfide bridges are depicted in orange, and all other side chains are represented in blue. Adapted from Pubmed and Botos et al. [50] (PDB entry code 1M5J).
Cyanovirin has demonstrated antiviral activity against several viruses, including HIV-1 and HIV-2. It achieves this by inhibiting cell fusion and interrupting the viral transmission cycle through its binding to gp120, a glycoprotein that constitutes a part of the outer layer of the virus and is found in the viral envelope [51].
CV-N interacts with high-mannose oligosaccharide structures on gp120 and gp41 and is thus capable of inactivating various virus strains. CV-N possesses two carbohydrate-binding sites, namely, 186 and 189, showing the highest affinity for oligomannose oligosaccharides such as Man8 and Man9. Several studies revealed that the binding of a nonamannoside to CV-N was multivalent in nature, and nonamannoside was found to cross-link CV-N molecules through this multivalent binding [52]. CV-N binds to gp120 in a manner that does not occlude the CD4 binding site or other domains on gp120 and does not interfere with soluble CD4-induced conformational changes in gp120. CV-N could prevent essential interactions between the envelope glycoprotein and target cell receptors acting at the level of the virus and not the target cell, to abort the initial infection process [53]. CV-N shows enhanced cytotoxicity effects against HIV-infected gp120-expressing H9 cells and exerts its activity by binding to high-mannose oligosaccharides, located predominantly in the C2–C4 region of gp120, preventing the virus from entering by blocking the fusion with the cell membrane and cell-to-cell transmission [54].
Using the cyanovirin molecule as a base, Lei et al. [55] modeled a peptide by modifying the α-amine group of the N-terminus of LCV-N (linker cyanovirin) with 10 kDa polyethylene glycol propionaldehyde (mPEG-ALD). The new compound, known as a monoPEGylated derivative of CV-N, exhibits potent inhibitory activity against acyclovir-resistant strains of the herpes simplex virus (HSV), with IC50 values in the nanomolar (nM) range.
3.2.3. Scytovirin
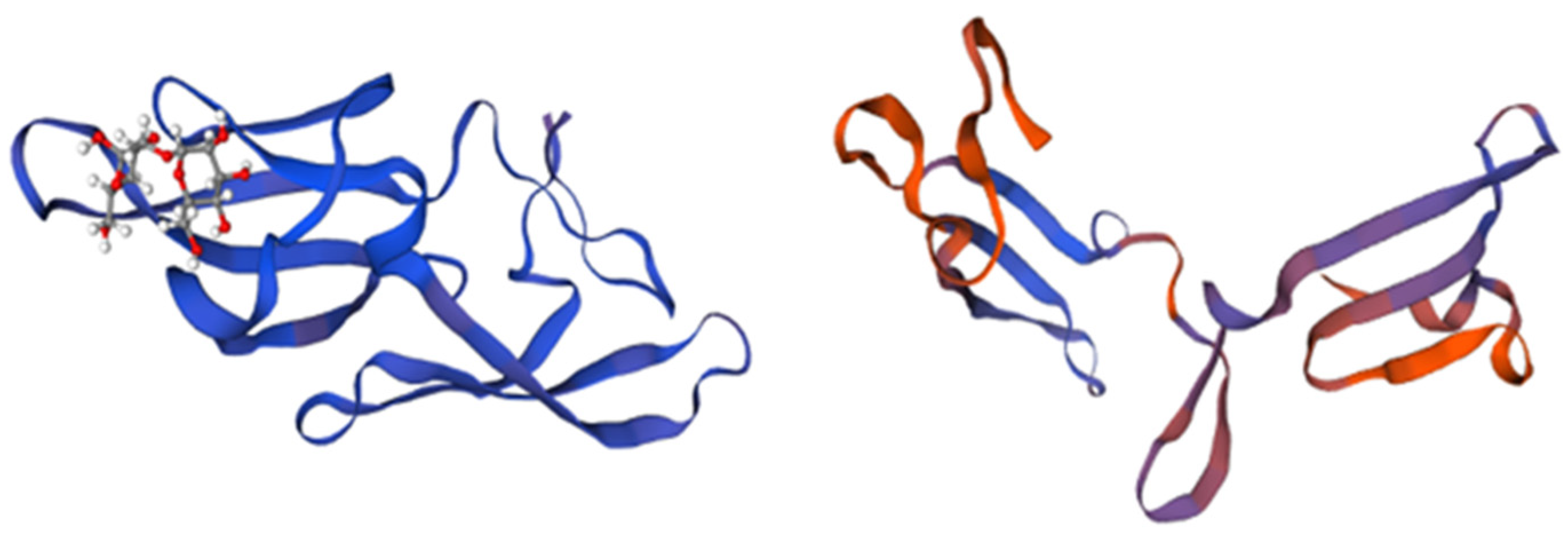
Scytovirin (SVN) is a cyanobacterium-derived carbohydrate-binding protein formed by a single chain of 95 amino acids. It has demonstrated very low toxicity in both human hepatocyte carcinoma cell lines and mouse models [56]. This compound was isolated for the first time in 2007 from Scytonema varium (Cyanobacteria), strain HG-24-1, by McFeeters et al. [57]. Its antiviral activity is correlated to the two sequence repeats, forming two identical structural domains, SD1: 3-43 and SD2: 51-89, divided by a pro-rich linker [58]. Each domain contains three aromatic amino acids involved in carbohydrate binding and two intra-domain disulphide bonds. The fifth inter-domain disulphide bond links Cys-7 and Cys-55. The two carbohydrate-binding domains are specific for Man α (1-2)Man α (1-6)Man α (1-6)Man tetramannose, as shown in Figure 3, conferring the ability to block viral and fungal glycans with high mannose content [10,59].
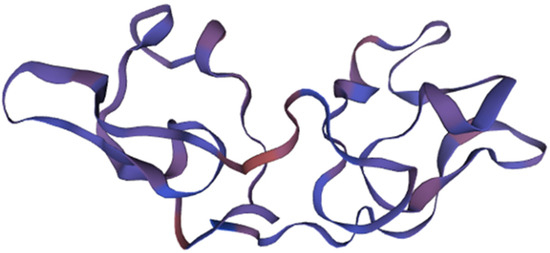
Figure 3.
The structure and domains of scytovirin. Structural domain 1 is represented in blue, structural domain 2 is represented in purple, and the disulfide bonds are highlighted in magenta. Adapted from Pubmed and Moulaei et al. [59] (PDB entry code 2JMVJ).
SVN has two carbohydrate-binding sites with substantially different affinities, capable of binding to the envelope glycoproteins gp160, gp120, and gp41, but it does not bind to the T-cell extracellular CD4 receptor or to other common cell surface proteins [57].
3.2.4. Microvirin
Microvirin (MNV) is an α (1,2)-mannose-specific lectin that possesses anti-HIV activity comparable to that of cyanovirin. However, it has been shown to be 50 times less cytotoxic [60]. This 108-amino-acid-long lectin is a monomer in solution with a single glycan-binding site that also recognizes terminal α (1,2)-mannose sugars, as presented in Figure 4 [61].
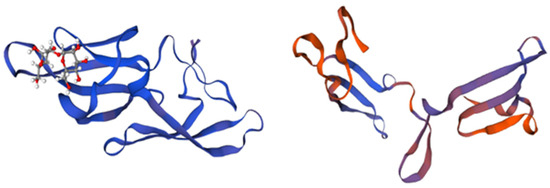
Figure 4.
Structure and domains of microvirin. The structure is divided into two structural domains, depicted in blue and magenta, while the bound glycan is colored in orange. The insertion of four amino acids in domain A, as compared to domain B, is indicated in blue and magenta. Adapted from Pubmed and Shahzad-ul-Hussan et al. [61] (PDB entry code 2y1s).
Microvirin was first extracted from Microcystis aeruginosa (Cyanobacteria), a species known for forming algal blooms [60], and it displayed a robust affinity with certain viruses [62]. It also inhibits syncytium formation (the accumulation of infected cells with neighboring cells, leading to the formation of multinucleate enlarged cells) between persistently HIV-1-infected T cells and uninfected CD4(+) T cells and inhibits transmission to CD4(+) T cells [61]. It irreversibly inactivates a wide range of immunodeficiency viruses, such as HIV-1 and HIV-2 [51].
Using the MVN molecule as a base, Shahid et al. [62] modeled a variant with two domains exhibiting 100% sequence identity. This alteration served to decrease the chemical heterogeneity of the molecule. The new compound, known as LUMS1, has potent inhibitory activity against HIV-1 and HCV, showing EC50 values of 37.2 and 45.3 nM, respectively [62]. There is also an inhibitory role of MVN during the attachment of gp120 to cellular receptors and subsequent fusion steps. This compound has preference for the co-receptor CCR5, the main co-receptor used by HIV for transmission [63,64].
3.2.5. Others
The Brown-Alga-Derived OAAH (Oscillatoria agardhii Agglutinin Homolog) Lectin Family
The Planktothrix agardhii (formerly Oscillatoria agardhii) (Cyanobacteria) agglutinin homolog (OAAH) is a family of proteins belonging to lectins with a sequence repeat of 66 amino acids [65]. The first member of this family was isolated for the first time in 2011 from Planktothrix agardhii, by Koharudin et al. [64]. These lectins can withstand a wide pH range from 4 to 11 and high temperatures, remaining active even at temperatures of at least 80 °C [66]. They are recognized for their capacity to inhibit HIV [27].
The antiviral activity of these lectins is closely tied to their structural characteristics. Specifically, they feature 10 β-strands that fold into a single, compact, β-barrel-like domain, resulting in a distinctive topology characterized by two symmetric carbohydrate-binding sites with a preference for man α(1–6)man-linked sugars [65]. The OAAH family proteins also interact with high-mannose oligosaccharide structures on gp120; thus, they are capable of inactivating several virus strains [27].
The Yellow-Alga-Derived Legume-Lectin-like Family
This lectin family possesses properties that are effective against HIV, and it also shows potential utility in anticancer applications [27]. The scaffold found in legume lectins (Fabaceae) also occurs in various alga species, including Ostreococcus tauri (Chlorophyta), Gracilaria fisheri (formerly Hydropuntia fisheri) (Rhodophyta), Microchloropsis gaditana (formerly Nannochloropsis gaditana) (Eustigmatophyceae), and Porphyra umbilicalis (Rhodophyta) [27]. They have two undistinguishable mannose-binding sites and differ from the single-chain legume lectins, which results from the non-covalent association of four protomers to give homotetrameric mannose-binding lectins [67]. Their antiviral activity is correlated to their affinity for mannose and high-mannose glycans forming complexes with glycoproteins or high-mannose N-glycan [27]. These interactions give them the ability to bind the terminal mannoses present in high-mannose oligosaccharides and crosslink these glycans on the surface of the viral envelope glycoproteins [28].
Green-Alga-Derived Galanthus nivalis Agglutinin (GNA)
Monocot-lectin or GNA-like are a family of lectins containing three bundles of four β-strands arranged into a flattened β-prism structure around a central pseudoaxis. The GNA-like seaweed lectin from the brown alga Boodlea coacta showed inhibitory activity against HIV-1 and influenza virus H1N1 [27]. Their antiviral activity is correlated to their carbohydrate-binding propensity, conferring the ability to block viral and fungal glycans with high mannose content. Their high affinity for HIV contributes to the potency of their antiviral activity, presenting an EC50 of 8.2 nM, improving the antibody responses and avoiding cell fusion and HIV cell-to-cell transmission [68].
The Multifunctional Protein in Peroxisomal β-Oxidation (MFP2)-like Families
The lectin from the green alga Bryopsis plumosa has been characterized as a Man-specific lectin structurally related to the Multifunctional Protein in Peroxisomal β-Oxidation (MFP2), consisting of three β-harpins that adopt a triangular disposition to form a sort of β-trefoil structure, presenting antiviral activity against HIV [27]. Their antiviral activity is correlated to the presence of a network of hydrogen bonds of mannose and amino acids Lys123, Asp125, Ser154, Asp163, and Val164, forming a monosaccharide-binding pocket [27]. This interaction improves the antibody responses and avoids cell fusion and cell-to-cell transmission of HIV [27].
Mannose-Binding Lectin from Grateloupia chiangii (G. chiangii Lectin, GCL)
In 2020, Hwang et al. [69] purified a novel mannose-binding lectin from the macroalga Grateloupia chiangii (Rhodophyta) (GCL) using antiviral screens and affinity chromatography. Its activity could be assumed due to its specificity for high-mannan N-glycans, which are related to antiviral abilities. This interaction can confer the ability to block viral and fungal glycans with high mannose content. The preliminary tests demonstrated antiviral activity of GCL against the influenza virus and HSV but not against HIV [69]. However, further investigation is necessary to fully understand the behavior of this lectin.

Table 1.
Summary of lectins extracted from algae with antiviral potential, outlining their specificity and the corresponding targets they interact with.
Table 1.
Summary of lectins extracted from algae with antiviral potential, outlining their specificity and the corresponding targets they interact with.
| Alga | Lectin | Specificity | Virus | Reference |
|---|---|---|---|---|
| Griffithsia sp. (Rhodophyta) | Griffithsin | Mannose | HIV | [37,70] |
| HSV | [36] | |||
| HCV | [29] | |||
| SARS-CoV1 and MERS | [71,72] | |||
| EBOV | [73] | |||
| JEV | [37] | |||
| HPV | [36] | |||
| Nostoc ellipsosporum (Cyanobacteria) | CV-N | High-mannose glycans | HIV | [74] |
| HCV | [75] | |||
| Influenza virus | [76] | |||
| Rhinoviruses | [59] | |||
| SARS-CoV2 | [77] | |||
| EBOV | [52] | |||
| Measles virus | [55] | |||
| HHV6 | ||||
| SIV | [52] | |||
| Trichomonas vaginalis | [78] | |||
| Cytonema varium (Cyanobacteria) | SVN | High-mannose glycans | HIV | [79] |
| HCV | ||||
| SARS-CoV1 | [77] | |||
| EBOV | [79] | |||
| Microcystis viridis and Microcystis aeruginosa (Cyanobacteria) | MVN | High-mannose glycans | HIV-1 | [62] |
| HCV | ||||
| Oscillatoria agardhii (Cyanobacteria) | OAAH | High-mannose glycans | HIV-1 | [80] |
| Ostreococcus tauri (Chlorophyta), Gracilaria fisheri (Rhodophyta), Microchloropsis gaditana (Eustigmatophycae), and Porphyra umbilicalis (Rhodophyta) | Yellow-alga-derived legume-lectin-like family | Mannose and high-mannose glycans | HIV | [28] |
| Boodlea coacta (Chlorophyta) | Green-alga-derived Galanthus nivalis agglutinin (GNA) | High mannose (HM)-type N-glycans | HIV | [28] |
| H1N1 | ||||
| Bryopsis plumosa (Chlorophyta) | MFP2-like families | Mannose and high-mannose glycans | HIV-1 | |
| Grateloupia chiangii (Rhodophyta) | Mannose-binding lectin from Grateloupia chiangii (G. chiangii lectin, GCL) | High-mannan N-glycans | HSV | [69] |
4. Materials and Methods
This literature review includes the available information regarding lectins from algae with antiviral capacities up to the 13th of July of 2023, using the SCOPUS, WEB OF SCIENCE, and NCBI databases. The search was performed using the keyword combination “Antiviral* AND (Lectins* OR Algae* AND (Macroalga* OR seaweed)” to compile the works including alga extracts with antiviral potential/activity against viruses. In addition, for the review of works reporting the lectin production of algae, the keyword combination “Algae* OR macroalgae* OR microalgae* OR seaweed AND (Lectin OR production* OR * OR content*) AND (virus*) AND (Macroalga* OR seaweed)” was used. For protein visualization and design, sequences were downloaded from NCBI Structure and were designed and visualized using Biozentrum’s SWISS-MODEL online database.
5. Conclusions
The study of antiviral activities within algae has encompassed a broad range of viral diseases. However, the major focus of research investment has been directed towards HIV, a virus that has afflicted millions of people worldwide, with an estimated 37 million people carrying the virus. As one of the most extensively studied viruses globally, HIV has prompted significant efforts to explore potential antiviral agents from diverse sources, including algae. Notably, almost all classes of algae have shown remarkable antiviral potential, making them distinctive resources for the detection and development of new antiviral drugs targeting a wide range of viruses. Lectin proteins have emerged as pivotal contributors in this undertaking, showcasing remarkable antiviral capabilities. Two prominent examples are cyanovirin and griffithsin, which have emerged as main antiviral references and hold immense promise as potential treatments against viruses like HIV. Among the different groups of seaweeds, the Rhodophyta and Phaeophyta groups have been more extensively scrutinized in comparison to Chlorophyta. The substantial number of patents registered in this domain underscores the keen interest of major economic powers in harnessing the potential of alga-based antiviral solutions. As research continues to progress, the field of alga-based antiviral agents is constantly expanding, with a growing number of new research initiatives and patents. Encouraging clinical trials are already in progress, offering the potential for the development of alga-based treatments for challenging viruses, such as HIV and coronaviruses. While none of these compounds have made it to the market thus far, the evident interest in lectins underscores their pivotal role as potential drug candidates. The ongoing exploration of algae and their antiviral potential holds significant promise for unveiling novel therapeutic options to combat viral infections. This provides hope for the enhancement of healthcare outcomes in the future.
Author Contributions
Conceptualization, C.A., C.F. and M.F.L.L.; methodology, C.A. and C.F.; software, C.A.; investigation, C.A. and C.F.; writing—original draft preparation, C.A.; writing—review and editing, C.F. and M.F.L.L.; supervision, C.F. and M.F.L.L.; project administration, M.F.L.L.; funding acquisition, M.F.L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Fundação para a Ciência e a Tecnologia (FCT) to MARE (UID/MAR/04292/2020), the Associate Laboratory ARNET (LA/P/0069/2020), through national funds. The authors also acknowledge the support of project DisCovEr+—Desenvolvimento e otimização do diagnóStico COVid19 como resposta de EmeRgência à pandemia (SAICTCOVID/72543/2020), supported by FEDER—Fundo Europeu de Desenvolvimento Regional da União Europeia in the framework of Portugal 2020 through COMPETE2020. C. Félix was supported by an FCT researcher contract (2021.03113.CEECIND).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pan, D.; Nolan, J.; Williams, K.H.; Robbins, M.J.; Weber, K.A. Abundance and Distribution of Microbial Cells and Viruses in an Alluvial Aquifer. Front. Microbiol. 2017, 8, 1199. [Google Scholar] [CrossRef] [PubMed]
- Zürcher, S.J.; Kerksieck, P.; Adamus, C.; Burr, C.M.; Lehmann, A.I.; Huber, F.K.; Richter, D. Prevalence of Mental Health Problems During Virus Epidemics in the General Public, Health Care Workers and Survivors: A Rapid Review of the Evidence. Front. Public Health 2020, 8, 560389. [Google Scholar] [CrossRef] [PubMed]
- Lasso, G.; Mayer, S.V.; Winkelmann, E.R.; Chu, T.; Elliot, O.; Patino-Galindo, J.A.; Park, K.; Rabadan, R.; Honig, B.; Shapira, S.D. A Structure-Informed Atlas of Human-Virus Interactions. Cell 2019, 178, 1526–1541.e16. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Khan, F.S.; Rehman, M.I.M.U.; Akram, M.; Riaz, M.; Rasool, G.; Khan, A.H.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef]
- Adamson, C.S.; Chibale, K.; Goss, R.J.M.; Jaspars, M.; Newman, D.J.; Dorrington, R.A. Antiviral drug discovery: Preparing for the next pandemic. Chem. Soc. Rev. 2021, 50, 3647–3655. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Obaidi, I.; Nagar, S.; Scalabrino, G.; Sheridan, H. The antiviral potential of algal-derived macromolecules. Curr. Res. Biotechnol. 2021, 3, 120–134. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; De Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef]
- Rahman, A.; Agrawal, S.; Nawaz, T.; Pan, S.; Selvaratnam, T. A Review of Algae-Based Produced Water Treatment for Biomass and Biofuel Production. Water 2020, 12, 2351. [Google Scholar] [CrossRef]
- Pagarete, A.; Ramos, A.S.; Puntervoll, P.; Allen, M.J.; Verdelho, V. Antiviral Potential of Algal Metabolites—A Comprehensive Review. Mar. Drugs 2021, 19, 94. [Google Scholar] [CrossRef]
- Chathuranga, K.; Weerawardhana, A.; Dodantenna, N.; Ranathunga, L.; Cho, W.-K.; Ma, J.Y.; Lee, J.-S. Inhibitory Effect of Sargassum fusiforme and Its Components on Replication of Respiratory Syncytial Virus In Vitro and In Vivo. Viruses 2021, 13, 548. [Google Scholar] [CrossRef]
- Ciancia, M.; Matulewicz, M.C.; Tuvikene, R. Structural Diversity in Galactans from Red Seaweeds and Its Influence on Rheological Properties. Front. Plant Sci. 2020, 11, 559986. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.-X.; Guan, H.-S. The Antiviral Activities and Mechanisms of Marine Polysaccharides: An Overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Lee, J.-B.; Hayashi, K.; Hirata, M.; Kuroda, E.; Suzuki, E.; Kubo, Y.; Hayashi, T. Antiviral Sulfated Polysaccharide from Navicula directa, a Diatom Collected from Deep-Sea Water in Toyama Bay. Biol. Pharm. Bull. 2006, 29, 2135–2139. [Google Scholar] [CrossRef]
- Sansone, C.; Brunet, C.; Noonan, D.M.; Albini, A. Marine Algal Antioxidants as Potential Vectors for Controlling Viral Diseases. Antioxidants 2020, 9, 392. [Google Scholar] [CrossRef]
- Kanekiyo, K.; Hayashi, K.; Takenaka, H.; Lee, J.-B.; Hayashi, T. Anti-herpes Simplex Virus Target of an Acidic Polysaccharide, Nostoflan, from the Edible Blue-Green Alga Nostoc flagelliforme. Biol. Pharm. Bull. 2007, 30, 1573–1575. [Google Scholar] [CrossRef] [PubMed]
- Cardellina, J.H.; Munro, M.H.G.; Fuller, R.W.; Manfredi, K.P.; McKee, T.C.; Tischler, M.; Bokesch, H.R.; Gustafson, K.R.; Beutler, J.A.; Boyd, M.R. A Chemical Screening Strategy for the Dereplication and Prioritization of HIV-Inhibitory Aqueous Natural Products Extracts. J. Nat. Prod. 1993, 56, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.A. Lectin Histochemistry: Historical Perspectives, State of the Art, and the Future. Methods Mol. Biol. 2017, 1560, 93–107. [Google Scholar] [CrossRef]
- Singh, R.S.; Thakur, S.R.; Bansal, P. Algal lectins as promising biomolecules for biomedical research. Crit. Rev. Microbiol. 2015, 41, 77–88. [Google Scholar] [CrossRef]
- Gorakshakar, A.; Ghosh, K. Use of lectins in immunohematology. Asian J. Transfus. Sci. 2016, 10, 12–21. [Google Scholar] [CrossRef]
- Ahmed, N.; Jahan, R.; Nissapatorn, V.; Wilairatana, P.; Rahmatullah, M. Plant lectins as prospective antiviral biomolecules in the search for COVID-19 eradication strategies. BioMedicine 2022, 146, 112507. [Google Scholar] [CrossRef]
- Lam, S.K.; Ng, T.B. Lectins: Production and practical applications. Appl. Microbiol. Biotechnol. 2011, 89, 45–55. [Google Scholar] [CrossRef]
- Mishra, A.; Behura, A.; Mawatwal, S.; Kumar, A.; Naik, L.; Mohanty, S.S.; Manna, D.; Dokania, P.; Mishra, A.; Patra, S.K.; et al. Structure-function and application of plant lectins in disease biology and immunity. Food Chem. Toxicol. 2019, 134, 110827. [Google Scholar] [CrossRef]
- Dan, X.; Liu, W.; Ng, T.B. Development and Applications of Lectins as Biological Tools in Biomedical Research. Med. Res. Rev. 2016, 36, 221–247. [Google Scholar] [CrossRef]
- Lepenies, B.; Lang, R. Editorial: Lectins and Their Ligands in Shaping Immune Responses. Front. Immunol. 2019, 10, 2379. [Google Scholar] [CrossRef]
- Liu, F.-T.; Stowell, S.R. The role of galectins in immunity and infection. Nat. Rev. Immunol. 2023, 23, 479–494. [Google Scholar] [CrossRef]
- Barre, A.; Simplicien, M.; Benoist, H.; Van Damme, E.J.; Rougé, P. Mannose-Specific Lectins from Marine Algae: Diverse Structural Scaffolds Associated to Common Virucidal and Anti-Cancer Properties. Mar. Drugs 2019, 17, 440. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, N.E.; O’Keefe, B.R.; Mori, T.; Zhu, C.; Giomarelli, B.; Vojdani, F.; Palmer, K.E.; McMahon, J.B.; Wlodawer, A. Domain-Swapped Structure of the Potent Antiviral Protein Griffithsin and Its Mode of Carbohydrate Binding. Structure 2006, 14, 1127–1135. [Google Scholar] [CrossRef]
- Lusvarghi, S.; Lohith, K.; Morin-Leisk, J.; Ghirlando, R.; Hinshaw, J.E.; Bewley, C.A. Binding Site Geometry and Subdomain Valency Control Effects of Neutralizing Lectins on HIV-1 Viral Particles. ACS Infect. Dis. 2016, 2, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Pagliolico, S.L.; Verso, V.R.L.; Bosco, F.; Mollea, C.; La Forgia, C. A Novel Photo-bioreactor Application for Microalgae Production as a Shading System in Buildings. Energy Procedia 2017, 111, 151–160. [Google Scholar] [CrossRef]
- Mu, J.; Hirayama, M.; Sato, Y.; Morimoto, K.; Hori, K. A Novel High-Mannose Specific Lectin from the Green Alga Halimeda renschii Exhibits a Potent Anti-Influenza Virus Activity through High-Affinity Binding to the Viral Hemagglutinin. Mar. Drugs 2017, 15, 255. [Google Scholar] [CrossRef]
- Maliki, I.M.; Misson, M.; Teoh, P.L.; Rodrigues, K.F.; Yong, W.T.L. Production of Lectins from Marine Algae: Current Status, Challenges, and Opportunities for Non-Destructive Extraction. Mar. Drugs 2022, 20, 102. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Extraction of protein from the macroalga Palmaria palmata. LWT—Food. Sci. Technol. 2013, 51, 375–382. [Google Scholar] [CrossRef]
- Polzin, J.; Rorrer, G.L. Selective production of the acyclic monoterpene β-myrcene by microplantlet suspension cultures of the macrophytic marine red alga Ochtodes secundiramea under nutrient perfusion cultivation with bromide-free medium. Algal Res. 2018, 36, 159–166. [Google Scholar] [CrossRef]
- Moulaei, T.; Alexandre, K.B.; Shenoy, S.R.; Meyerson, J.R.; Krumpe, L.R.; Constantine, B.; Wilson, J.; Buckheit, R.W., Jr.; McMahon, J.B.; Subramaniam, S.; et al. Griffithsin tandemers: Flexible and potent lectin inhibitors of the human immunodeficiency virus. Retrovirology 2015, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Griffithsin, a Highly Potent Broad-Spectrum Antiviral Lectin from Red Algae: From Discovery to Clinical Application. Mar. Drugs 2019, 17, 567. [Google Scholar] [CrossRef]
- Zeitlin, L.; Pauly, M.; Whaley, K.J. Second-generation HIV microbicides: Continued development of griffithsin. Proc. Natl. Acad. Sci. USA 2009, 106, 6029–6030. [Google Scholar] [CrossRef]
- Naik, S.; Kumar, S. Lectins from plants and algae act as anti-viral against HIV, influenza and coronaviruses. Mol. Biol. Rep. 2022, 49, 12239–12246. [Google Scholar] [CrossRef]
- Romero, J.A.F.; Paglini, M.G.; Priano, C.; Koroch, A.; Rodríguez, Y.; Sailer, J.; Teleshova, N. Algal and Cyanobacterial Lectins and Their Antimicrobial Properties. Mar. Drugs 2021, 19, 687. [Google Scholar] [CrossRef]
- Singh, R.S.; Walia, A.K. Lectins from red algae and their biomedical potential. J. Appl. Phycol. 2018, 30, 1833–1858. [Google Scholar] [CrossRef]
- Micewicz, E.D.; Cole, A.L.; Jung, C.-L.; Luong, H.; Phillips, M.L.; Pratikhya, P.; Sharma, S.; Waring, A.J.; Cole, A.M.; Ruchala, P. Grifonin-1: A Small HIV-1 Entry Inhibitor Derived from the Algal Lectin, Griffithsin. PLoS ONE 2010, 5, e14360. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Michael, E.; Eggink, D.; van Montfort, T.; Lasnik, A.B.; Palmer, K.E.; Sanders, R.W.; Moore, J.P.; Klasse, P.J.; Pritchard, L.K.; et al. Occluding the Mannose Moieties on Human Immunodeficiency Virus Type 1 gp120 with Griffithsin Improves the Antibody Responses to Both Proteins in Mice. AIDS Res. Hum. Retroviruses 2012, 28, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Hoorelbeke, B.; Xue, J.; LiWang, P.J.; Balzarini, J. Role of the Carbohydrate-Binding Sites of Griffithsin in the Prevention of DC-SIGN-Mediated Capture and Transmission of HIV-1. PLoS ONE 2013, 8, e64132. [Google Scholar] [CrossRef]
- Kagiampakis, I.; Gharibi, A.; Mankowski, M.K.; Snyder, B.A.; Ptak, R.G.; Alatas, K.; LiWang, P.J. Potent Strategy To Inhibit HIV-1 by Binding both gp120 and gp41. Antimicrob. Agents Chemother. 2011, 55, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Hoorelbeke, B.; Kagiampakis, I.; Demeler, B.; Balzarini, J.; LiWang, P.J. The Griffithsin Dimer Is Required for High-Potency Inhibition of HIV-1: Evidence for Manipulation of the Structure of gp120 as Part of the Griffithsin Dimer Mechanism. Antimicrob. Agents Chemother. 2013, 57, 3976–3989. [Google Scholar] [CrossRef]
- Mori, T.; Boyd, M.R. Cyanovirin-N, a Potent Human Immunodeficiency Virus-Inactivating Protein, Blocks both CD4-Dependent and CD4-Independent Binding of Soluble gp120 (sgp120) to Target Cells, Inhibits sCD4-Induced Binding of sgp120 to Cell-Associated CXCR4, and Dissociates Bound sgp120 from Target Cells. Antimicrob. Agents Chemother. 2001, 45, 664–672. [Google Scholar] [CrossRef][Green Version]
- Boyd, M.R.; Gustafson, K.R.; McMahon, J.B.; Shoemaker, R.H.; O’Keefe, B.R.; Mori, T.; Gulakowski, R.J.; Wu, L.; Rivera, M.I.; Laurencot, C.M.; et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob. Agents Chemother. 1997, 41, 1521–1530. [Google Scholar] [CrossRef]
- Matei, E.; Basu, R.; Furey, W.; Shi, J.; Calnan, C.; Aiken, C.; Gronenborn, A.M. Structure and Glycan Binding of a New Cyanovirin-N Homolog. J. Biol. Chem. 2016, 291, 18967–18976. [Google Scholar] [CrossRef]
- Bewley, C.A.; Gustafson, K.R.; Boyd, M.R.; Covell, D.G.; Bax, A.; Clore, G.M.; Gronenborn, A.M. Solution structure of cyanovirin-N, a potent HIV-inactivating protein. Nat. Struct. Biol. 1998, 5, 571–578. [Google Scholar] [CrossRef]
- Botos, I.; O’keefe, B.R.; Shenoy, S.R.; Cartner, L.K.; Ratner, D.M.; Seeberger, P.H.; Boyd, M.R.; Wlodawer, A. Structures of the Complexes of a Potent Anti-HIV Protein Cyanovirin-N and High Mannose Oligosaccharides. J. Biol. Chem. 2002, 277, 34336–34342. [Google Scholar] [CrossRef]
- Huskens, D.; Férir, G.; Vermeire, K.; Kehr, J.-C.; Balzarini, J.; Dittmann, E.; Schols, D. Microvirin, a Novel α(1,2)-Mannose-specific Lectin Isolated from Microcystis aeruginosa, Has Anti-HIV-1 Activity Comparable with That of Cyanovirin-N but a Much Higher Safety Profile. J. Biol. Chem. 2010, 285, 24845–24854. [Google Scholar] [CrossRef]
- Dam, T.; Brewer, C. Fundamentals of lectin-carbohydrate interactions. In Comprehensive Glycoscience; Kamerling, J.P., Boons, G.-J., Lee, Y.C., Suzuki, A., Taniguchi, N., Voragen, A.G., Eds.; Elsevier: Oxford, UK, 2007; pp. 397–452. [Google Scholar] [CrossRef]
- Esser, M.T.; Mori, T.; Mondor, I.; Sattentau, Q.J.; Dey, B.; Berger, E.A.; Boyd, M.R.; Lifson, J.D. Cyanovirin-N Binds to gp120 To Interfere with CD4-Dependent Human Immunodeficiency Virus Type 1 Virion Binding, Fusion, and Infectivity but Does Not Affect the CD4 Binding Site on gp120 or Soluble CD4-Induced Conformational Changes in gp120. J. Virol. 1999, 73, 4360–4371. [Google Scholar] [CrossRef]
- Lotfi, H.; Sheervalilou, R.; Zarghami, N. An update of the recombinant protein expression systems of Cyanovirin-N and challenges of preclinical development. BioImpacts 2018, 8, 139–151. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, W.; Liang, H.; Wang, Z.; Chen, J.; Hong, H.; Xie, L.; Nie, H.; Xiong, S. Preparation of a monoPEGylated derivative of cyanovirin-N and its virucidal effect on acyclovir-resistant strains of herpes simplex virus type 1. Arch. Virol. 2019, 164, 1259–1269. [Google Scholar] [CrossRef]
- Jones, T.H.; McClelland, E.E.; McFeeters, H.; McFeeters, R.L. Novel Antifungal Activity for the Lectin Scytovirin: Inhibition of Cryptococcus neoformans and Cryptococcus gattii. Front. Microbiol. 2017, 8, 755. [Google Scholar] [CrossRef] [PubMed]
- McFeeters, R.L.; Xiong, C.; O’keefe, B.R.; Bokesch, H.R.; McMahon, J.B.; Ratner, D.M.; Castelli, R.; Seeberger, P.H.; Byrd, R.A. The Novel Fold of Scytovirin Reveals a New Twist for Antiviral Entry Inhibitors. J. Mol. Biol. 2007, 369, 451–461. [Google Scholar] [CrossRef]
- O’Keefe, B.R.; Smee, D.F.; Turpin, J.A.; Saucedo, C.J.; Gustafson, K.R.; Mori, T.; Blakeslee, D.; Buckheit, R.; Boyd, M.R. Potent Anti-Influenza Activity of Cyanovirin-N and Interactions with Viral Hemagglutinin. Antimicrob. Agents Chemother. 2003, 47, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Moulaei, T.; Botos, I.; Ziółkowska, N.E.; Bokesch, H.R.; Krumpe, L.R.; McKee, T.C.; O’Keefe, B.R.; Dauter, Z.; Wlodawer, A. Atomic-resolution crystal structure of the antiviral lectin scytovirin. Protein Sci. 2007, 16, 2756–2760. [Google Scholar] [CrossRef] [PubMed]
- Gondim, A.C.S.; da Silva, S.R.; Mathys, L.; Noppen, S.; Liekens, S.; Sampaio, A.H.; Nagano, C.S.; Rocha, C.R.C.; Nascimento, K.S.; Cavada, B.S.; et al. Potent antiviral activity of carbohydrate-specific algal and leguminous lectins from the Brazilian biodiversity. MedChemComm 2019, 10, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Shahzad-Ul-Hussan, S.; Gustchina, E.; Ghirlando, R.; Clore, G.M.; Bewley, C.A. Solution Structure of the Monovalent Lectin Microvirin in Complex with Manα(1–2)Man Provides a Basis for Anti-HIV Activity with Low Toxicity. J. Biol. Chem. 2011, 286, 20788–20796. [Google Scholar] [CrossRef]
- Shahid, M.; Qadir, A.; Yang, J.; Ahmad, I.; Zahid, H.; Mirza, S.; Windisch, M.P.; Shahzad-Ul-Hussan, S. An Engineered Microvirin Variant with Identical Structural Domains Potently Inhibits Human Immunodeficiency Virus and Hepatitis C Virus Cellular Entry. Viruses 2020, 12, 199. [Google Scholar] [CrossRef]
- Hladik, F.; McElrath, M.J. Setting the stage: Host invasion by HIV. Nat. Rev. Immunol. 2008, 8, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, K.; Princen, K.; Hatse, S.; De Clercq, E.; Dey, K.; Bell, T.W.; Schols, D. CADA, a novel CD4-targeted HIV inhibitor, is synergistic with various anti-HIV drugs in vitro. AIDS 2004, 18, 2115–2125. [Google Scholar] [CrossRef]
- Koharudin, L.M.I.; Kollipara, S.; Aiken, C.; Gronenborn, A.M. Structural Insights into the Anti-HIV Activity of the Oscillatoria agardhii Agglutinin Homolog Lectin Family. J. Biol. Chem. 2012, 287, 33796–33811. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Murakami, M.; Miyazawa, K.; Hori, K. Purification and characterization of a novel lectin from a freshwater cyanobacterium, Oscillatoria agardhii. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 125, 169–177. [Google Scholar] [CrossRef]
- Buts, L.; Garcia-Pino, A.; Wyns, L.; Loris, R. Structural basis of carbohydrate recognition by a Man(α1-2)Man-specific lectin from Bowringia milbraedii. Glycobiology 2006, 16, 635–640. [Google Scholar] [CrossRef]
- Sato, Y.; Hirayama, M.; Morimoto, K.; Yamamoto, N.; Okuyama, S.; Hori, K. High Mannose-binding Lectin with Preference for the Cluster of α1–2-Mannose from the Green Alga Boodlea coacta Is a Potent Entry Inhibitor of HIV-1 and Influenza Viruses. J. Biol. Chem. 2011, 286, 19446–19458. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Han, J.-W.; Jeon, H.; Cho, K.; Kim, J.-H.; Lee, D.-S.; Han, J.W. Characterization of a Novel Mannose-Binding Lectin with Antiviral Activities from Red Alga, Grateloupia chiangii. Biomolecules 2020, 10, 333. [Google Scholar] [CrossRef]
- Tyo, K.M.; Lasnik, A.B.; Zhang, L.; Jenson, A.B.; Fuqua, J.L.; Palmer, K.E.; Steinbach-Rankins, J.M. Rapid-Release Griffithsin Fibers for Dual Prevention of HSV-2 and HIV-1 Infections. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Alsaidi, S.; Cornejal, N.; Mahoney, O.; Melo, C.; Verma, N.; Bonnaire, T.; Chang, T.; O’keefe, B.R.; Sailer, J.; Zydowsky, T.M.; et al. Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model. Mar. Drugs 2021, 19, 418. [Google Scholar] [CrossRef]
- Millet, J.K.; Séron, K.; Labitt, R.N.; Danneels, A.; Palmer, K.E.; Whittaker, G.R.; Dubuisson, J.; Belouzard, S. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antivir. Res. 2016, 133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Ebola virus (EBOV) infection: Therapeutic strategies. Biochem. Pharmacol. 2015, 93, 1–10. [Google Scholar] [CrossRef]
- Dey, B.; Lerner, D.L.; Lusso, P.; Boyd, M.R.; Elder, J.H.; Berger, E.A. Multiple Antiviral Activities of Cyanovirin-N: Blocking of Human Immunodeficiency Virus Type 1 gp120 Interaction with CD4 and Coreceptor and Inhibition of Diverse Enveloped Viruses. J. Virol. 2000, 74, 4562–4569. [Google Scholar] [CrossRef] [PubMed]
- Helle, F.; Wychowski, C.; Vu-Dac, N.; Gustafson, K.R.; Voisset, C.; Dubuisson, J. Cyanovirin-N Inhibits Hepatitis C Virus Entry by Binding to Envelope Protein Glycans. J. Biol. Chem. 2006, 281, 25177–25183. [Google Scholar] [CrossRef] [PubMed]
- Smee, D.F.; Wandersee, M.K.; Checketts, M.B.; O’Keefe, B.R.; Saucedo, C.; Boyd, M.R.; Mishin, V.P.; Gubareva, L.V. Influenza a (H1N1) Virus Resistance to Cyanovirin-N Arises Naturally during Adaptation to Mice and by Passage in Cell Culture in the Presence of the Inhibitor. Antivir. Chem. Chemother. 2007, 18, 317–327. [Google Scholar] [CrossRef]
- Naidoo, D.; Kar, P.; Roy, A.; Mutanda, T.; Bwapwa, J.; Sen, A.; Anandraj, A. Structural Insight into the Binding of Cyanovirin-N with the Spike Glycoprotein, Mpro and PLpro of SARS-CoV-2: Protein–Protein Interactions, Dynamics Simulations and Free Energy Calculations. Molecules 2021, 26, 5114. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ratner, D.M.; Ryan, C.M.; Johnson, P.J.; O’keefe, B.R.; Secor, W.E.; Anderson, D.J.; Robbins, P.W.; Samuelson, J. Anti-Retroviral Lectins Have Modest Effects on Adherence of Trichomonas vaginalis to Epithelial Cells In Vitro and on Recovery of Tritrichomonas foetus in a Mouse Vaginal Model. PLoS ONE 2015, 10, e0135340. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A.R.; Giomarelli, B.G.; Lear-Rooney, C.M.; Saucedo, C.J.; Yellayi, S.; Krumpe, L.R.H.; Rose, M.; Paragas, J.; Bray, M.; Olinger, G.G., Jr.; et al. The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against Zaire Ebola virus. Antivir. Res. 2014, 112, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Koharudin, L.M.; Gronenborn, A.M. Structural Basis of the Anti-HIV Activity of the Cyanobacterial Oscillatoria Agardhii Agglutinin. Structure 2011, 19, 1170–1181. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).