New Chlorinated Metabolites and Antiproliferative Polyketone from the Mangrove Sediments-Derived Fungus Mollisia sp. SCSIO41409
Abstract
1. Introduction
2. Results
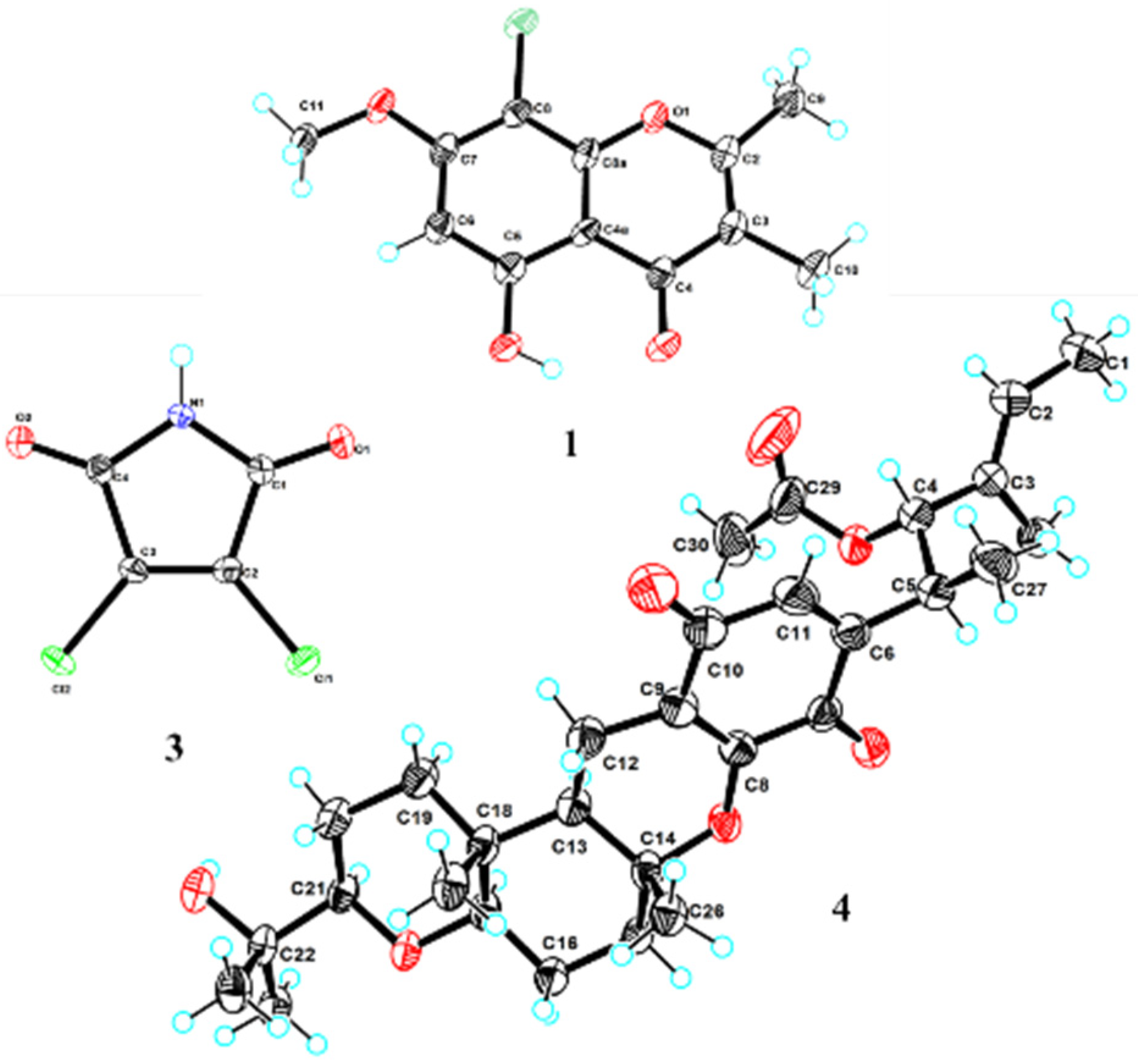
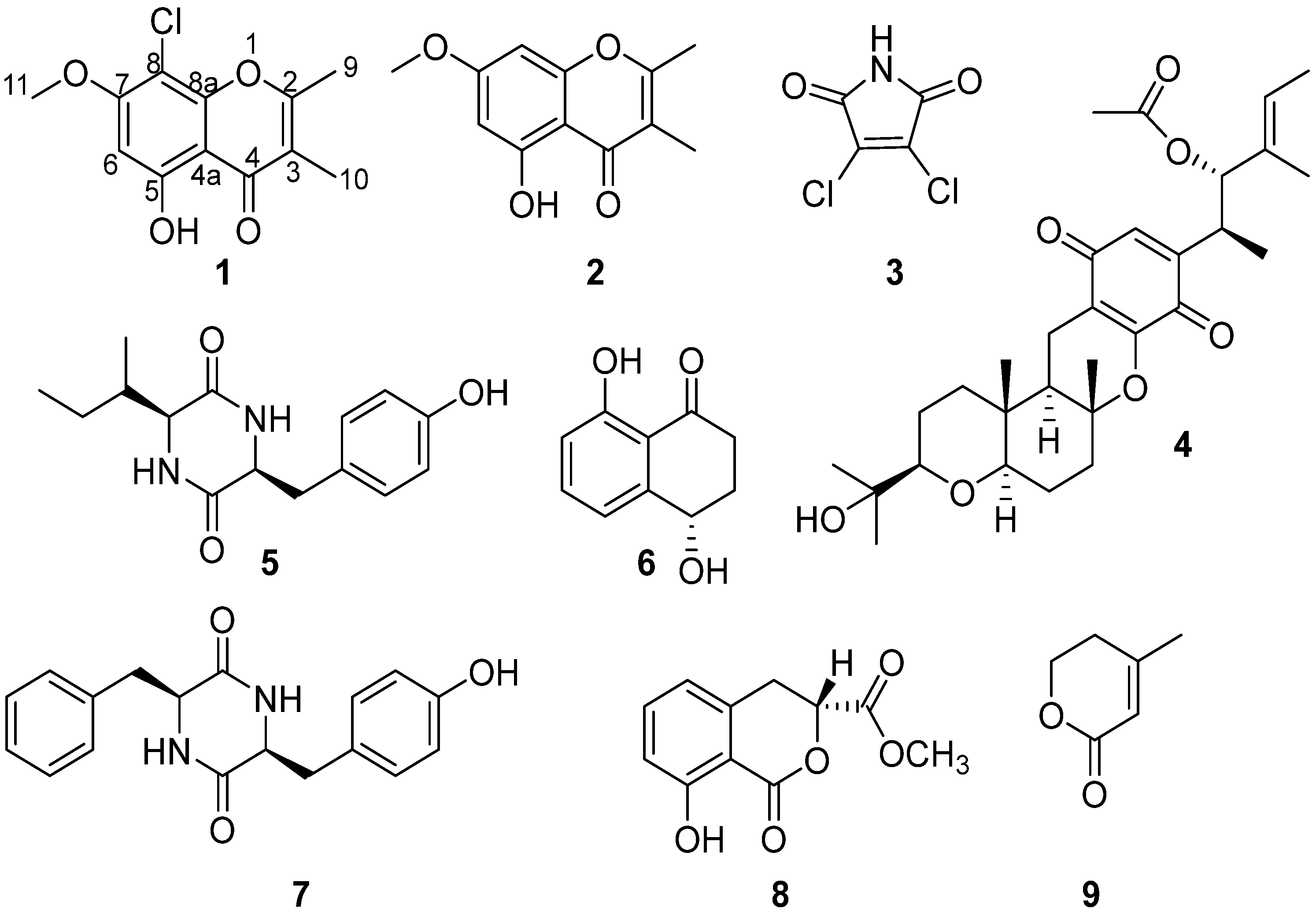
2.1. Structural Determination

| Pos. | δC Type | δH (J in Hz) | HMBC |
|---|---|---|---|
| 2 | 163.9, C | ||
| 3 | 114.6, C | ||
| 4 | 181.1, C | ||
| 4a | 103.7, C | ||
| 5 | 160.0, C | ||
| 6 | 95.9, CH | 6.62 (s) | 4a, 5, 7, 8 |
| 7 | 159.9, C | ||
| 8 | 97.4, C | ||
| 8a | 151.6, C | ||
| 9 | 18.3, CH3 | 2.42 (s) | 2, 3 |
| 10 | 8.8, CH3 | 1.91 (s) | 2, 3, 4 |
| 11 | 57.0, CH3 | 3.94 (s) | 7 |
| 5-OH | 13.09 (s) | 4a, 5, 6 |
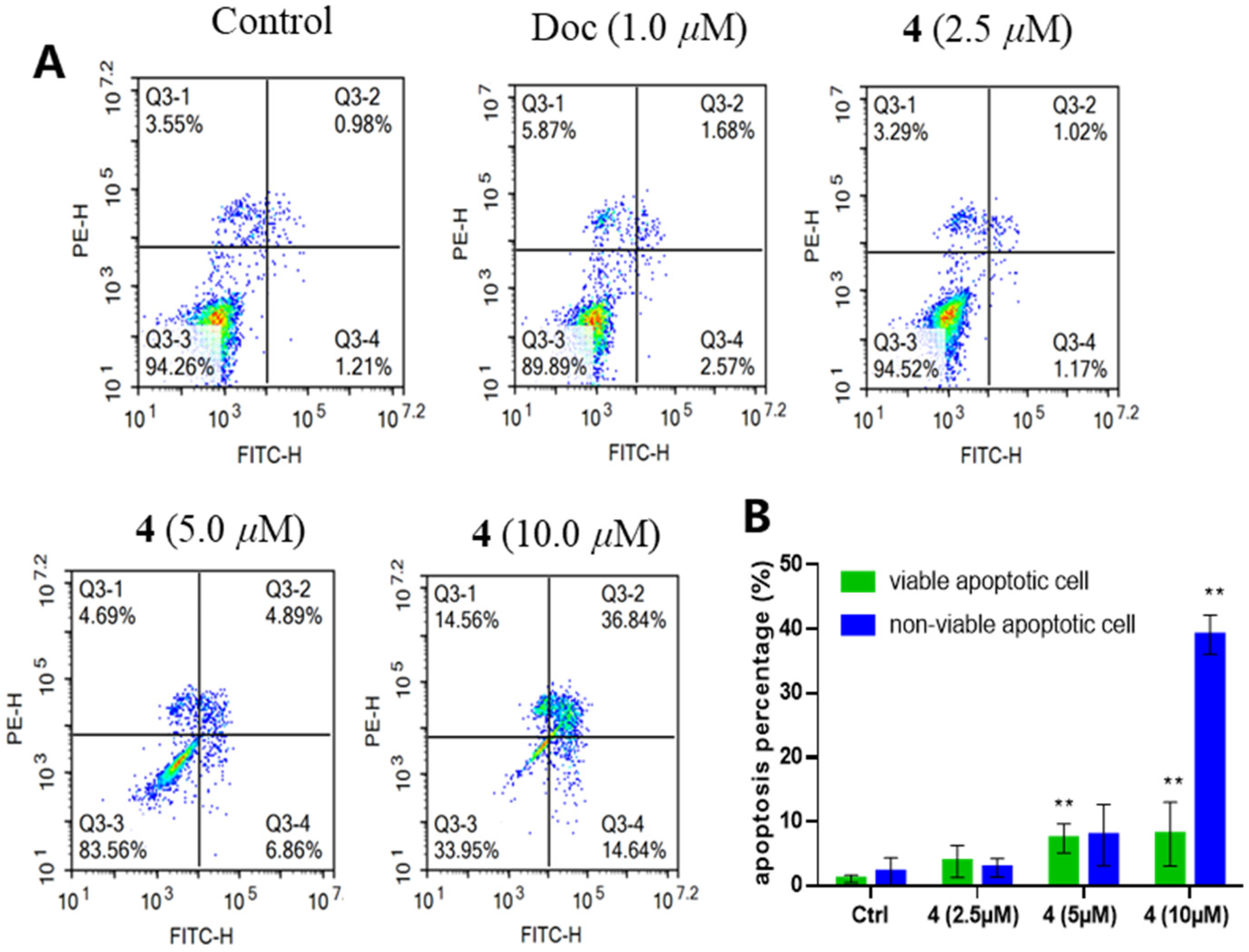
2.2. Antimicrobial and Antiproliferative Activities
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungal Material
4.3. Fermentation and Extraction
4.4. Isolation and Purification
4.5. Spectroscopic Data of Compounds
4.6. X-ray Crystallographic Analysis
4.7. Antibacterial Activity Assay
4.8. Cytotoxicity Bioassay
4.9. Plate Clone Formation Assay
4.10. Apoptosis and Cell Cycle Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Li, K.; Chen, S.; Pang, X.; Cai, J.; Zhang, X.; Liu, Y.; Zhu, Y.; Zhou, X. Natural products from mangrove sediments-derived microbes: Structural diversity, bioactivities, biosynthesis, and total synthesis. Eur. J. Med. Chem. 2022, 230, 114117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhu, T.; Cai, S.; Gu, Q.; Li, D. Drimane Sesquiterpenoids from the Mangrove-Derived Fungus Aspergillus ustus. Chem. Pharm. Bull. 2011, 59, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.W.; Chang, H.S.; Cheng, M.J.; Chan, H.Y.; Hsieh, S.Y.; Liu, T.W.; Chen, S.W.; Yuan, G.F.; Chen, I.S. New Metabolites from the Endophytic Fungus Mollisia sp. Chem. Nat. Compd. 2016, 52, 585–590. [Google Scholar] [CrossRef]
- Li, K.; Su, Z.; Gao, Y.; Lin, X.; Pang, X.; Yang, B.; Tao, H.; Luo, X.; Liu, Y.; Zhou, X. Cytotoxic Minor Piericidin Derivatives from the Actinomycete Strain Streptomyces psammoticus SCSIO NS126. Mar. Drugs 2021, 19, 428. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Li, H.-Y.; Hu, C.; Sheng, H.-F.; Zhang, Y.; Lin, B.-R.; Zhou, G.-X. Ergosterols from the Culture Broth of Marine Streptomyces anandii H41-59. Mar. Drugs 2016, 14, 84. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.-M.; Meng, L.-H.; Jiang, W.-L.; Xu, G.-M.; Huang, C.-G.; Wang, B.-G. Bisthiodiketopiperazines and Acorane Sesquiterpenes Produced by the Marine-Derived Fungus Penicillium adametzioides AS-53 on Different Culture Media. J. Nat. Prod. 2015, 78, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, A.N.; Smetanina, O.F.; Ivanets, E.V.; Kalinovsky, A.I.; Dyshlovoy, S.A. Pretrichodermamides D–F from a marine algicolous fungus Penicillium sp. KMM 4672. Mar. Drugs 2016, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Chen, A.; Zhu, M.; Qi, X.; Tang, W.; Liu, M.; Li, D.; Gu, Q.; Li, J. Penicisulfuranol A, a novel C-terminal inhibitor disrupting molecular chaperone function of Hsp90 independent of ATP binding domain. Biochem. Pharmacol. 2019, 163, 404–415. [Google Scholar] [CrossRef]
- Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2021, 39, 560–595. [Google Scholar] [CrossRef]
- Luo, X.; Lin, X.; Tao, H.; Wang, J.; Li, J.; Yang, B.; Zhou, X.; Liu, Y. Isochromophilones A–F, cytotoxic chloroazaphilones from the marine mangrove endophytic fungus Diaporthe sp. SCSIO 41011. J. Nat. Prod. 2018, 81, 934–941. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.; Ye, Y.; Xu, Z.; Zhao, Q.; Luo, X.; Liu, Y.; Guo, P. Anti-inflammatory compounds from the mangrove endophytic fungus Amorosia sp. SCSIO 41026. Front. Microbiol. 2022, 13, 976399. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, H.; Herath, K.B.; Ondeyka, J.G.; Polishook, J.D.; Bills, G.F.; Dombrowski, A.W.; Springer, M.S.; Siciliano, S.; Malkowitz, L.; Sanchez, M.; et al. Isolation and Structure of Antagonists of Chemokine Receptor (CCR5). J. Nat. Prod. 2004, 67, 1036–1038. [Google Scholar] [CrossRef]
- Nakanishi, S.; Ando, K.; Kawamoto, I.; Yasuzawa, T.; Sano, H.; Kase, H. KS-504 compounds, novel inhibitors of Ca2+ and calmodulin-dependent cyclic nucleotide phosphodiesterase from mollisia ventosa. J. Antibiot. 1989, 42, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Sterner, O.; Anke, T. Mollisianitrile, a New Antibiotic from Mollisia sp. A59-96. Z. Für Nat. C 2007, 62, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Thines, E.; Arendholz, W.-R.; Anke, H.; Sterner, O. Benesudon, a New Antibiotic Fungal Metabolite from Cultures of Mollisia benesuada (Tul.) Phill. J. Antibiot. 1997, 50, 13–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Der Kerk, G.J.M.; Overeem, J.C. Mollisin, a dichloronaphthoquinone derivative produced by the fungus: Mollisia caesia. Recl. Trav. Chim. Pays-Bas. 1957, 76, 425–436. [Google Scholar] [CrossRef]
- Cai, J.; Chen, C.; Tan, Y.; Chen, W.; Luo, X.; Luo, L.; Yang, B.; Liu, Y.; Zhou, X. Bioactive Polyketide and Diketopiperazine Derivatives from the Mangrove-Sediment-Derived Fungus Aspergillus sp. SCSIO41407. Molecules 2021, 26, 4851. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, X.; Yang, Z.; Tan, Y.; Peng, B.; Liu, Y.; Zhou, X. Thiodiketopiperazines and Alkane Derivatives Produced by the Mangrove Sediment–Derived Fungus Penicillium ludwigii SCSIO 41408. Front. Microbiol. 2022, 13, 857041. [Google Scholar] [CrossRef]
- Chen, C.; Chen, W.; Tao, H.; Yang, B.; Zhou, X.; Luo, X.; Liu, Y. Diversified Polyketides and Nitrogenous Compounds from the Mangrove Endophytic Fungus Penicillium steckii SCSIO 41025. Chin. J. Chem. 2021, 39, 2132–2140. [Google Scholar] [CrossRef]
- Song, X.; Liu, C.; Chen, P.; Zhang, H.; Sun, R. Natural Product-Based Pesticide Discovery: Design, Synthesis and Bioactivity Studies of N-Amino-Maleimide Derivatives. Molecules. 2018, 23, 1521. [Google Scholar] [CrossRef]
- Koyama, N.; Nagahiro, T.; Yamaguchi, Y.; Masuma, R.; Tomoda, H.; Omura, S. Stemphones, Novel Potentiators of Imipenem Activity against Methicillin-resistant Staphylococcus aureus, Produced by Aspergillus sp. FKI-2136. J. Antibiot. 2005, 58, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, T.; Takenaka, Y.; Nagakura, N.; Hamada, N. 2,3-Dialkylchromones from Mycobiont Cultures of the Lichen Graphis scripta. Heterocycles 2000, 53, 1589. [Google Scholar] [CrossRef]
- Harizani, M.; Katsini, E.; Georgantea, P.; Roussis, V.; Ioannou, E. New Chlorinated 2,5-Diketopiperazines from Marine-Derived Bacteria Isolated from Sediments of the Eastern Mediterranean Sea. Molecules 2020, 25, 1509. [Google Scholar] [CrossRef]
- Kokubun, T.; Veitch, N.C.; Bridge, P.D.; Simmonds, M.S. Dihydroisocoumarins and a tetralone from Cytospora eucalypticola. Phytochemistry 2003, 62, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Shen, Y.; Zhu, Y.; Xu, Q.; Liu, X.; Ni, K.; Cao, X.; Zhang, W.; Jiao, B. Diketopiperazine constituents of marine Bacillus subtilis. Chem. Nat. Compd. 2009, 45, 290–292. [Google Scholar] [CrossRef]
- Fang, Z.F.; Yu, S.S.; Zhou, W.Q.; Chen, X.G.; Ma, S.G.; Li, Y.; Qu, J. A new isocoumarin from metabolites of the endophytic fungus Alternaria tenuissima (Nees & T. Nees: Fr.) Wiltshire. Chin. Chem. Lett. 2012, 23, 317–320. [Google Scholar] [CrossRef]
- Mao, Z.; Zhang, W.; Wu, C.; Feng, H.; Peng, Y.; Shahid, H.; Cui, Z.; Ding, P.; Shan, T. Diversity and antibacterial activity of fungal endophytes from Eucalyptus exserta. BMC Microbiol. 2021, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Liu, D.; Shao, Z.; Huang, J.; Fan, A.; Lin, W. Chlorinated metabolites with antibacterial activities from a deep-sea-derived Spiromastix fungus. RSC Adv. 2021, 11, 29661–29667. [Google Scholar] [CrossRef]
- Yamazaki, H.; Koyama, N.; Omura, S.; Tomoda, H. Structure-activity Relationships of Stemphones, Potentiators of Imipenem Activity against Methicillin-resistant Staphylococcus aureus. J. Antibiot. 2008, 61, 426–441. [Google Scholar] [CrossRef]
- Nobe, K.; Miyatake, M.; Nobe, H.; Sakai, Y.; Takashima, J.; Momose, K. Novel diacylglycerol kinase inhibitor selectively suppressed an U46619-induced enhancement of mouse portal vein contraction under high glucose conditions. J. Cereb. Blood Flow Metab. 2004, 143, 166–178. [Google Scholar] [CrossRef]
- Koyama, N.; Kobayashi, K.; Yamazaki, H.; Hiroshi, T. Inhibition of Lipid Droplet Accumulation in Mouse Macrophages by Stemphone Derivatives. J. Antibiot. 2008, 61, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Fang, W.; Shi, L.; Wang, K.; Zhang, Y.; Zhang, Z.; Wu, Z.; Yang, Z.; Gu, Y. Novonestmycins A and B, two new 32-membered bioactive macrolides from Streptomyces phytohabitans HBERC-20821. J. Antibiot. 2014, 68, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, J.; Yan, H.; Shi, M.; Zheng, Q.; Wang, Y.; Zhu, Y.; Miao, L.; Gao, X. Kaempferol inhibits benign prostatic hyperplasia by resisting the action of androgen. Eur. J. Pharmacol. 2021, 907, 174251. [Google Scholar] [CrossRef] [PubMed]


| Activities | Strains or Cells | 3 | 4 | Positive |
|---|---|---|---|---|
| Antifungal (MIC, μg/mL) | B. cinerea | 25 | >100 | 12.50 a |
| V. dahlia | 25 | >100 | 12.50 a | |
| F. graminearum | 50 | >100 | 12.50 a | |
| F. oxysporum | 50 | >100 | >100 a | |
| R. solani | 50 | >100 | 100 a | |
| S. nodorum | 25 | 50 | 12.50 a | |
| Antibacterial (MIC, μg/mL) | E. rhusiopathiae | >100 | 1.56 | 6.25b |
| S. aureus | 100 | >100 | 6.25 b | |
| S. suis | 100 | 6.25 | 1.56 b | |
| E. coli | >100 | >100 | 50.00 c | |
| Cytotoxic (IC50, μM) | 22Rv1 | 8.35 | 5.81 | 0.03 d |
| PC-3 | 9.60 | 2.77 | 0.12 d | |
| HepG2 | / | 7.11 | 178.60 d | |
| A549 | / | 11.68 | 29.95 d | |
| Hela | / | 11.47 | / | |
| WPMY-1 | / | 5.53 | 0.51 d | |
| MC3T3-E1 | / | 3.63 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Wang, X.; Gan, X.; Zhou, Q.; Luo, X.; Yang, B.; Liu, Y.; Ratnasekera, D.; Zhou, X. New Chlorinated Metabolites and Antiproliferative Polyketone from the Mangrove Sediments-Derived Fungus Mollisia sp. SCSIO41409. Mar. Drugs 2023, 21, 32. https://doi.org/10.3390/md21010032
Cai J, Wang X, Gan X, Zhou Q, Luo X, Yang B, Liu Y, Ratnasekera D, Zhou X. New Chlorinated Metabolites and Antiproliferative Polyketone from the Mangrove Sediments-Derived Fungus Mollisia sp. SCSIO41409. Marine Drugs. 2023; 21(1):32. https://doi.org/10.3390/md21010032
Chicago/Turabian StyleCai, Jian, Xueni Wang, Xia Gan, Qian Zhou, Xiaowei Luo, Bin Yang, Yonghong Liu, Disna Ratnasekera, and Xuefeng Zhou. 2023. "New Chlorinated Metabolites and Antiproliferative Polyketone from the Mangrove Sediments-Derived Fungus Mollisia sp. SCSIO41409" Marine Drugs 21, no. 1: 32. https://doi.org/10.3390/md21010032
APA StyleCai, J., Wang, X., Gan, X., Zhou, Q., Luo, X., Yang, B., Liu, Y., Ratnasekera, D., & Zhou, X. (2023). New Chlorinated Metabolites and Antiproliferative Polyketone from the Mangrove Sediments-Derived Fungus Mollisia sp. SCSIO41409. Marine Drugs, 21(1), 32. https://doi.org/10.3390/md21010032












