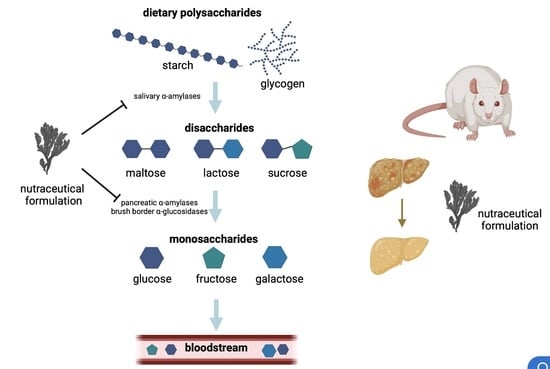
A Nutraceutical Formulation Containing Brown Algae Reduces Hepatic Lipid Accumulation by Modulating Lipid Metabolism and Inflammation in Experimental Models of NAFLD and NASH
Abstract
:1. Introduction
2. Results
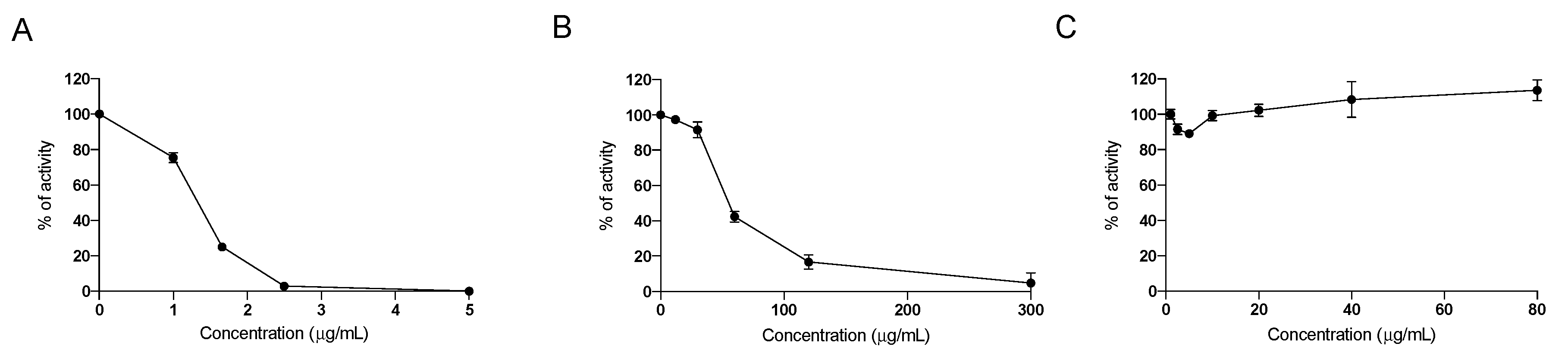
2.1. In Vitro Effect of the Nutraceutical Formulation on Digestive Enzymes
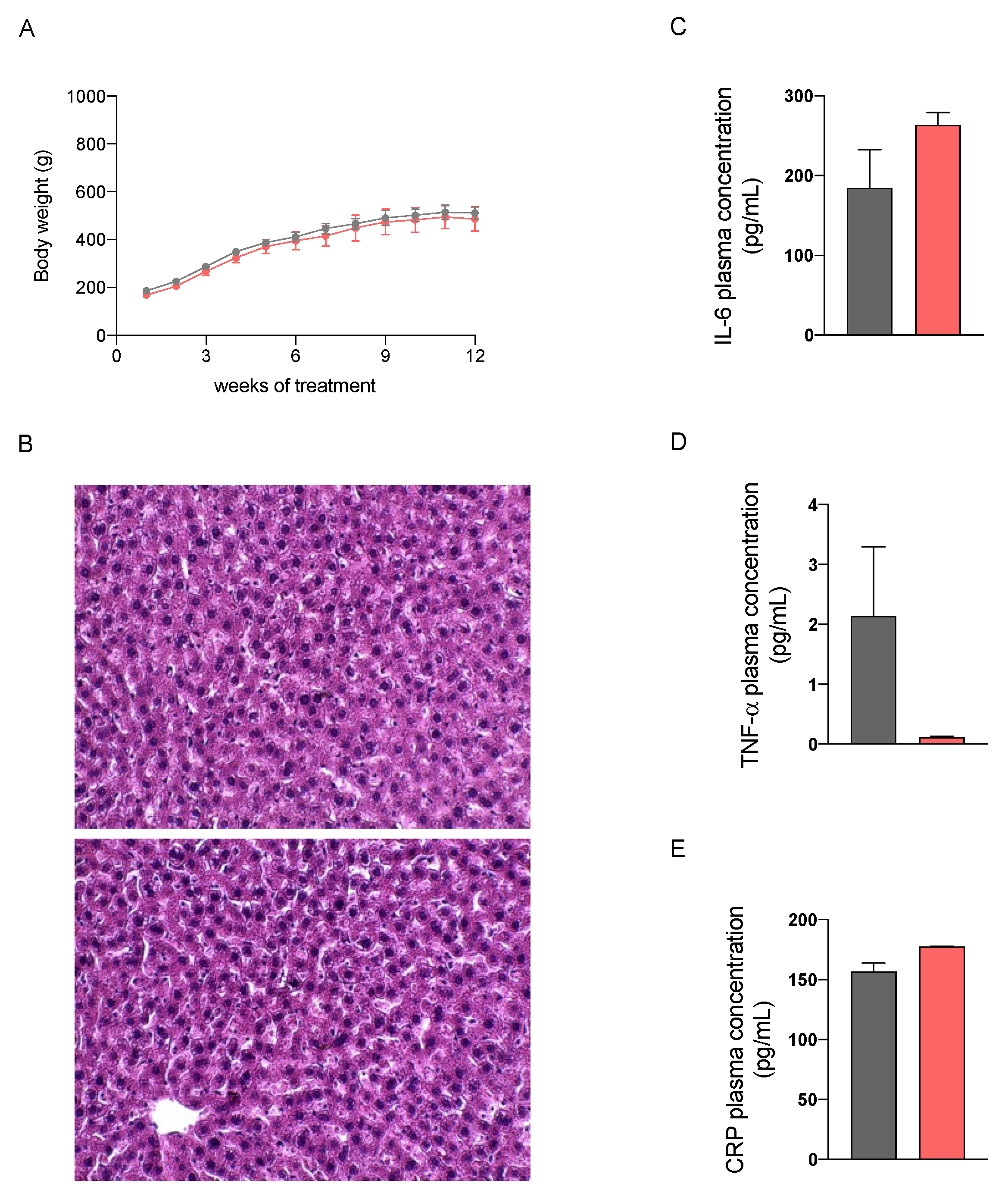
2.2. In Vivo Safety Assessment of the Nutraceutical Formulation on the Liver
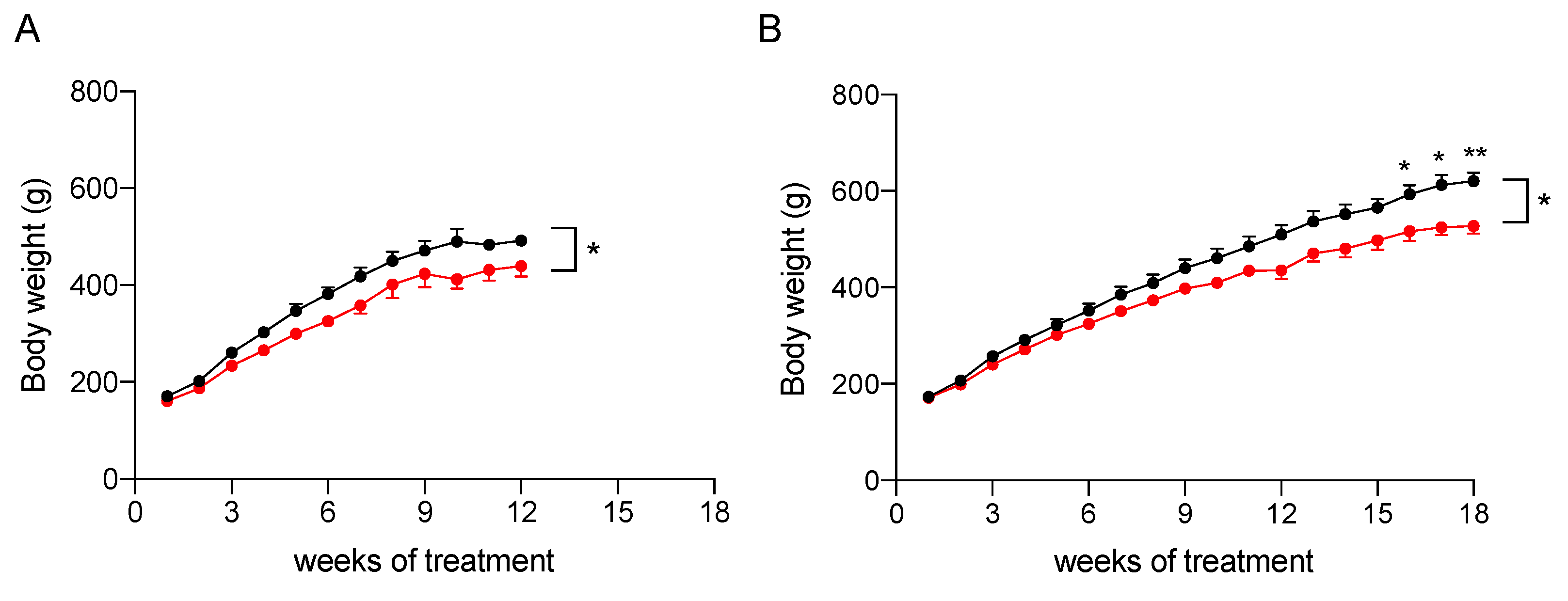
2.3. In Vivo Assessment of the Efficacy of the Nutraceutical Formulation against Liver Steatosis
2.3.1. Effect on Body Weight
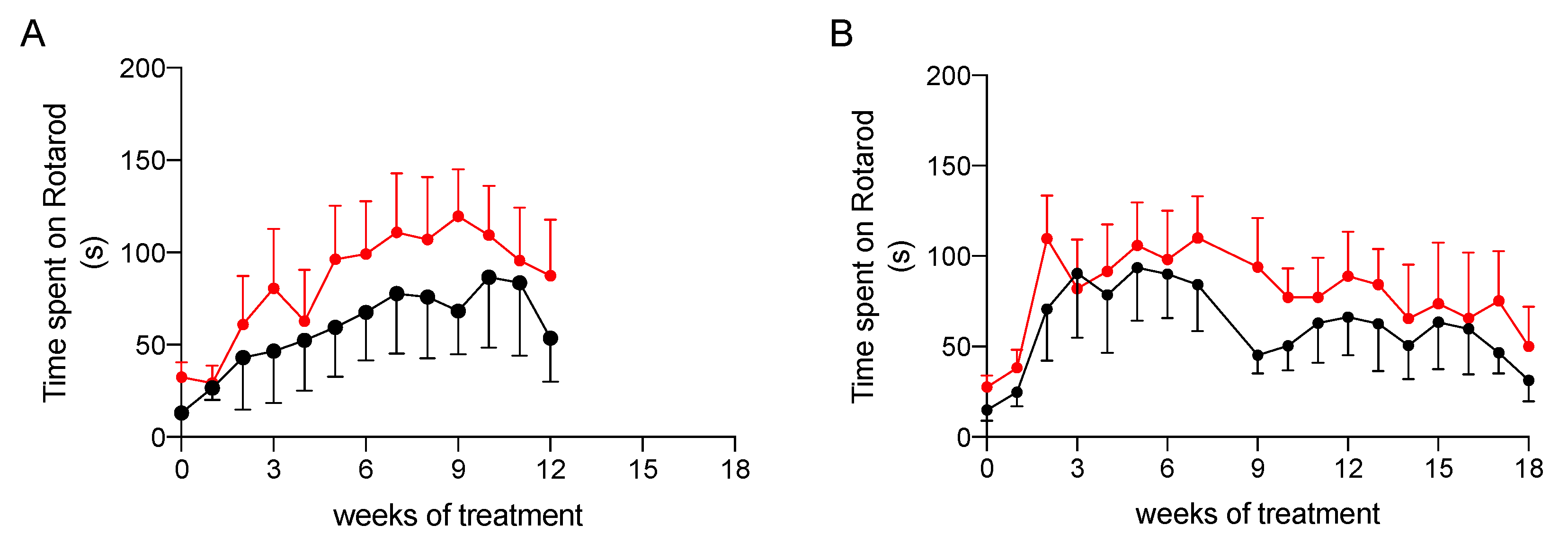
2.3.2. Effect on Physical Performance
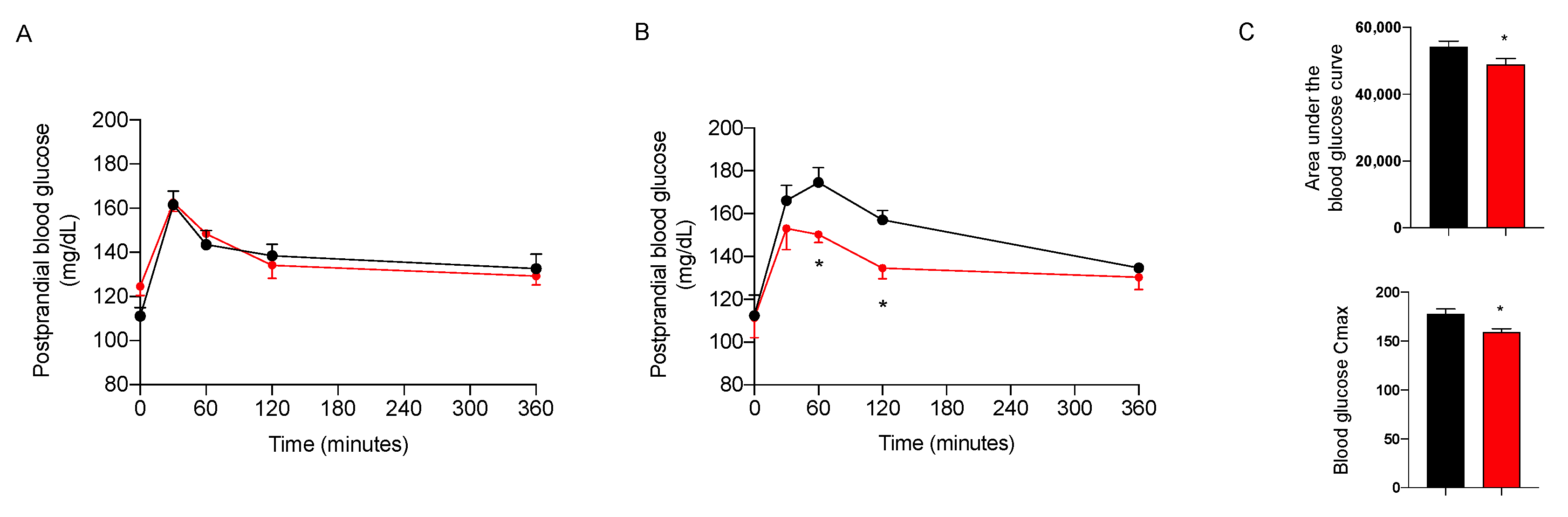
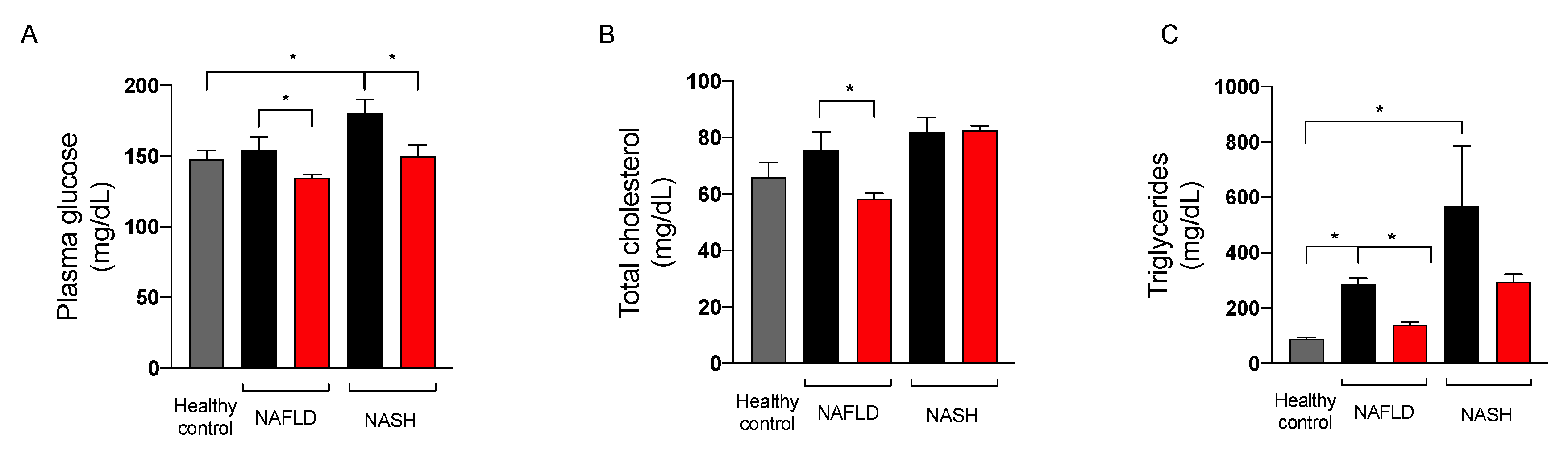
2.3.3. Effect on Glycemic Control
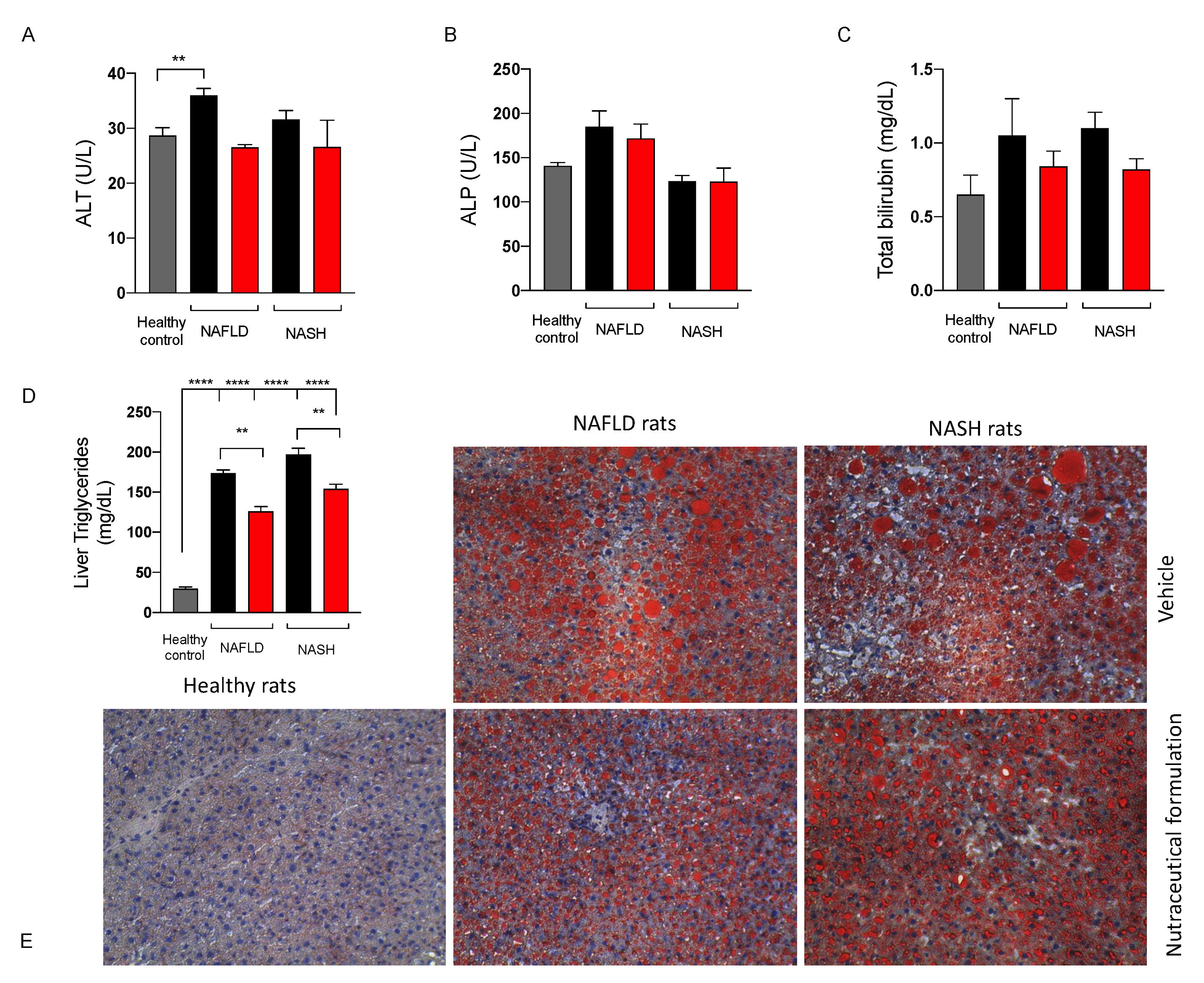
2.3.4. Effect on Liver Function
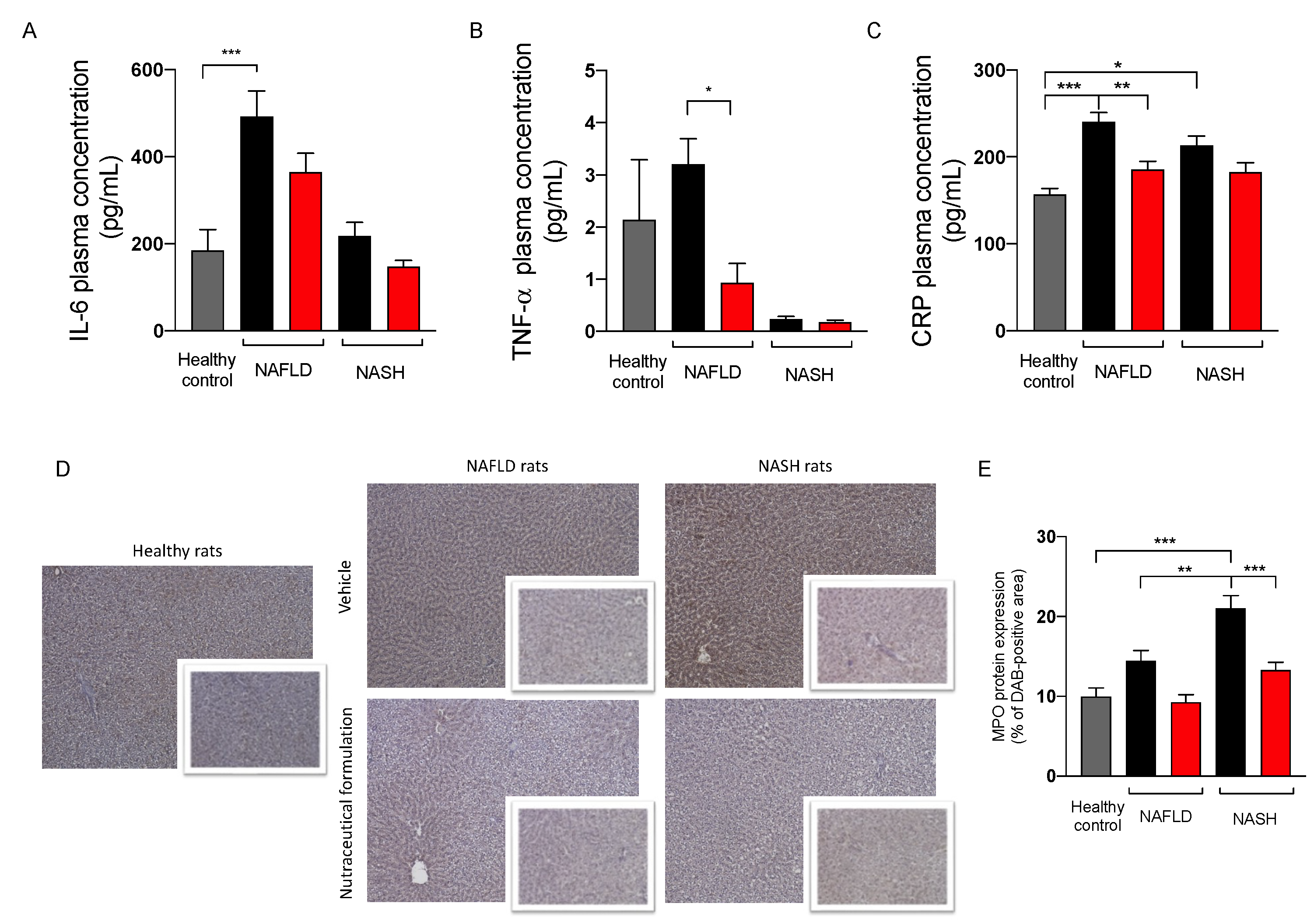
2.3.5. Effect on Inflammation
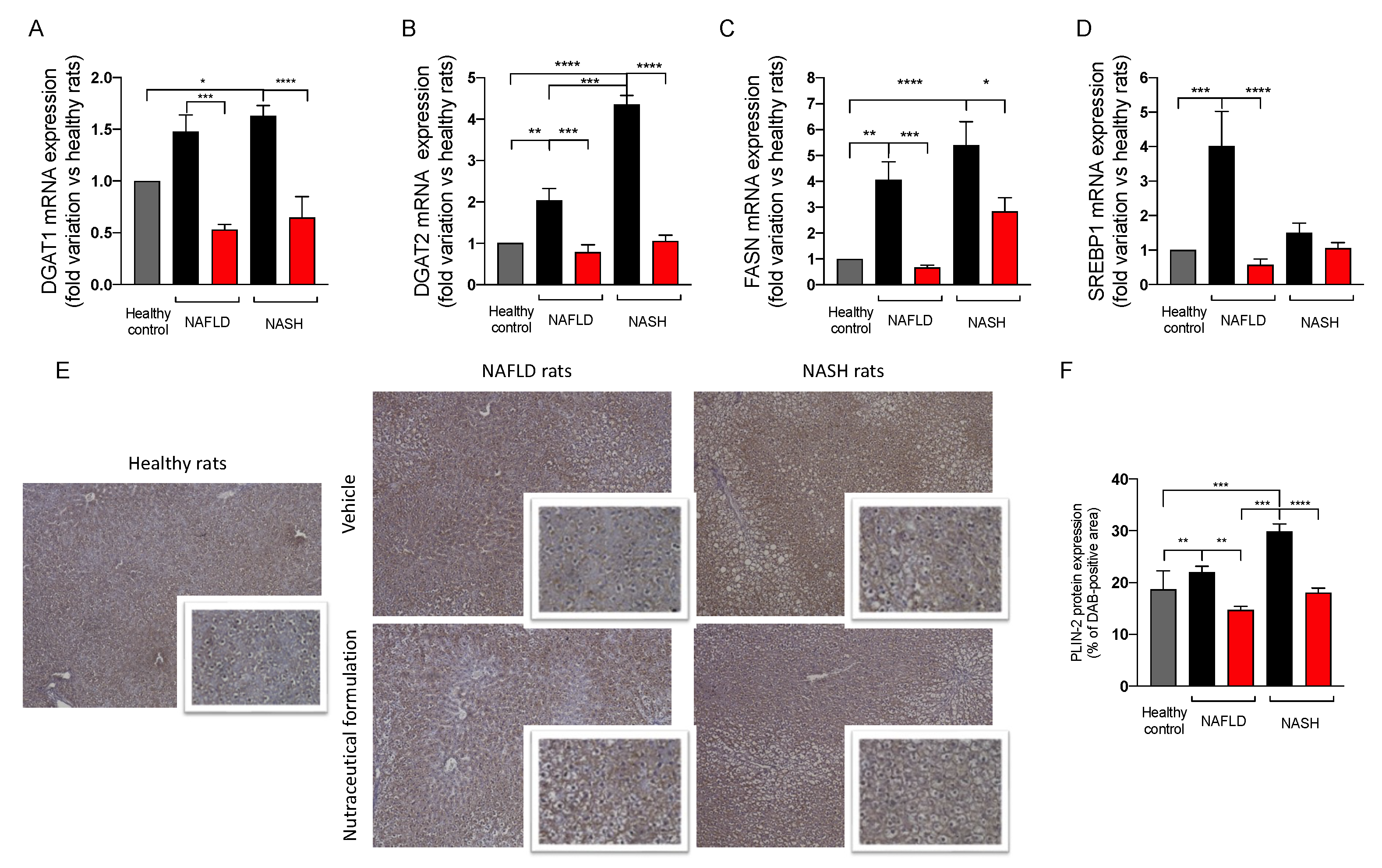
2.3.6. Effect on Hepatic Lipid Metabolism
3. Discussion
4. Materials and Methods
4.1. In Vitro Assessement of Inhibition of Digestive Enzymes
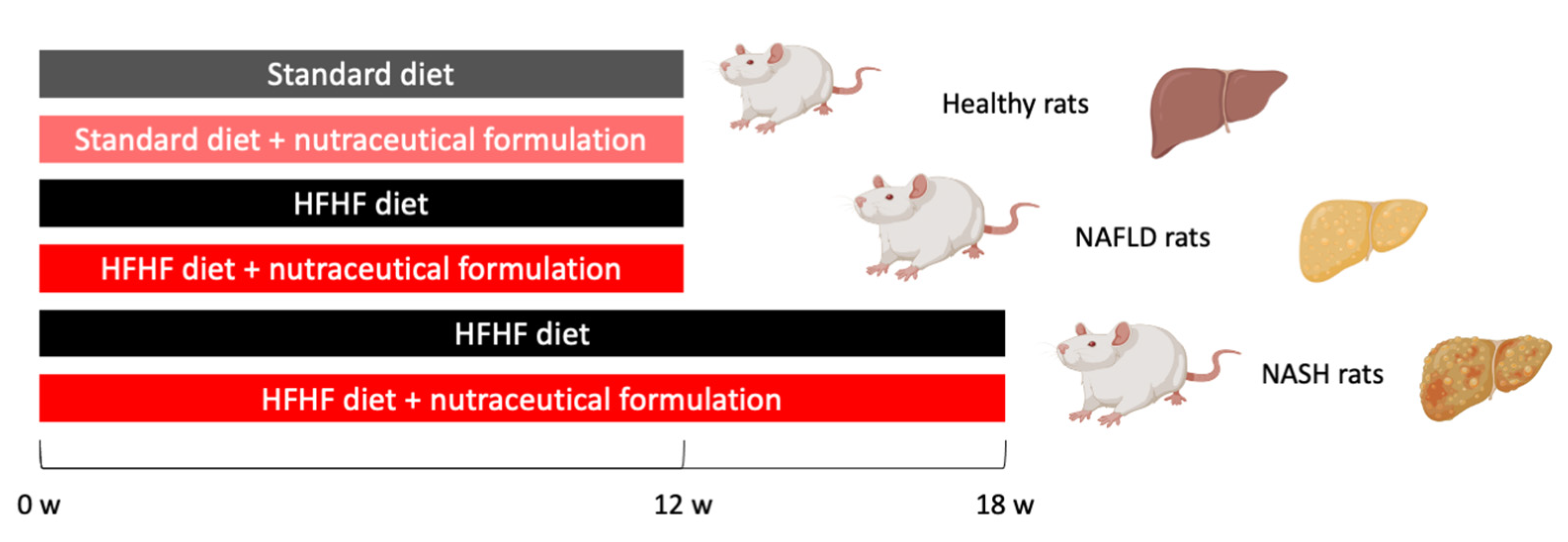
4.2. Animal Model of NAFLD/NASH
4.3. Postprandial Blood Glucose Levels, Biochemical and Histological Analysis
4.4. Gene Expression Analysis of Hepatic Tissues
4.5. Protein Quantification of IL-6, TNF-α, and CRP in Plasma Samples
4.6. Immunohistochemical Analisys of PLIN-2 and MPO in Hepatic Tissues
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asrih, M.; Jornayvaz, F.R. Metabolic Syndrome and Nonalcoholic Fatty Liver Disease: Is Insulin Resistance the Link? Mol. Cell. Endocrinol. 2015, 418, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Burra, P.; Becchetti, C.; Germani, G. NAFLD and Liver Transplantation: Disease Burden, Current Management and Future Challenges. JHEP Rep. 2020, 2, 100192. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.; Gallego-Durán, R.; Gallego, P.; Grande, L. Genetic and Epigenetic Regulation in Nonalcoholic Fatty Liver Disease (NAFLD). Int. J. Mol. Sci. 2018, 19, 911. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V. Non-Alcoholic Fatty Liver Disease: What the Clinician Needs to Know. World J. Gastroenterol. 2014, 20, 12956–12980. [Google Scholar] [CrossRef]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V. Lipid Droplets and Liver Disease: From Basic Biology to Clinical Implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Cobbina, E.; Akhlaghi, F. Non-Alcoholic Fatty Liver Disease (NAFLD)—Pathogenesis, Classification, and Effect on Drug Metabolizing Enzymes and Transporters. Drug Metab. Rev. 2017, 49, 197–211. [Google Scholar] [CrossRef]
- Gabbia, D.; De Martin, S. Brown Seaweeds for the Management of Metabolic Syndrome and Associated Diseases. Molecules 2020, 25, 4182. [Google Scholar] [CrossRef]
- Keleszade, E.; Patterson, M.; Trangmar, S.; Guinan, K.J.; Costabile, A. Clinical Efficacy of Brown Seaweeds Ascophyllum nodosum and Fucus vesiculosus in the Prevention or Delay Progression of the Metabolic Syndrome: A Review of Clinical Trials. Molecules 2021, 26, 714. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Hu, F.B. Role of Chromium in Human Health and in Diabetes. Diabetes Care 2004, 27, 2741–2751. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Zhang, X.; Hu, L.; Fang, Z.; Huang, Z.; Shi, P. Trivalent Chromium Alleviates Oleic Acid Induced Steatosis in SMMC-7721 Cells by Decreasing Fatty Acid Uptake and Triglyceride Synthesis. Biometals 2016, 29, 881–892. [Google Scholar] [CrossRef]
- Kooshki, F.; Moradi, F.; Karimi, A.; Niazkar, H.R.; Khoshbaten, M.; Maleki, V.; Pourghassem Gargari, B. Chromium Picolinate Balances the Metabolic and Clinical Markers in Nonalcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1298–1306. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Chen, C.-J.; Liu, C.-H.; Mao, F.C. Chromium Attenuates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in KK/HlJ Mice. Biochem. Biophys. Res. Commun. 2010, 397, 459–464. [Google Scholar] [CrossRef]
- Matt, G.E.; Quintana, P.J.E.; Hoh, E.; Dodder, N.G.; Mahabee-Gittens, E.M.; Padilla, S.; Markman, L.; Watanabe, K. Tobacco Smoke Is a Likely Source of Lead and Cadmium in Settled House Dust. J. Trace Elem. Med. Biol. 2021, 63, 126656. [Google Scholar] [CrossRef]
- Carreres, L.; Jílková, Z.M.; Vial, G.; Marche, P.N.; Decaens, T.; Lerat, H. Modeling Diet-Induced NAFLD and NASH in Rats: A Comprehensive Review. Biomedicines 2021, 9, 378. [Google Scholar] [CrossRef]
- Gabbia, D.; Dall’Acqua, S.; Di Gangi, I.M.; Bogialli, S.; Caputi, V.; Albertoni, L.; Marsilio, I.; Paccagnella, N.; Carrara, M.; Giron, M.C.; et al. The Phytocomplex from Fucus vesiculosus and Ascophyllum nodosum Controls Postprandial Plasma Glucose Levels: An In Vitro and In Vivo Study in a Mouse Model of NASH. Mar. Drugs 2017, 15, 41. [Google Scholar] [CrossRef]
- Van Herck, M.; Vonghia, L.; Francque, S. Animal Models of Nonalcoholic Fatty Liver Disease—A Starter’s Guide. Nutrients 2017, 9, 1072. [Google Scholar] [CrossRef]
- De Martin, S.; Gabbia, D.; Carrara, M.; Ferri, N. The Brown Algae Fucus vesiculosus and Ascophyllum nodosum Reduce Metabolic Syndrome Risk Factors: A Clinical Study. Nat. Prod. Commun. 2018, 13, 1691–1694. [Google Scholar] [CrossRef]
- Nicolucci, A.; Rossi, M.C.; Petrelli, M. Effectiveness of Ascophyllum nodosum and Fucus vesiculosus on Metabolic Syndrome Components: A Real-World, Observational Study. J. Diabetes Res. 2021, 2021, 3389316. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Cicero, A.F.G.; D’Angelo, A.; Maffioli, P. Ascophyllum nodosum and Fucus vesiculosus on Glycemic Status and on Endothelial Damage Markers in Dysglicemic Patients. Phytother. Res. 2019, 33, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Colognesi, M.; Gabbia, D.; De Martin, S. Depression and Cognitive Impairment-Extrahepatic Manifestations of NAFLD and NASH. Biomedicines 2020, 8, 229. [Google Scholar] [CrossRef]
- Khan, A.; Alsahli, M.; Rahmani, A. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T. Role of Lipid Droplet Proteins in Liver Steatosis. J. Physiol. Biochem. 2011, 67, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, F.; Faghihimani, E.; Adibi, P. Nonalcoholic Fatty Liver Disease: Diagnostic Biomarkers. World J. Gastrointest. Pathophysiol. 2017, 8, 11–26. [Google Scholar] [CrossRef]
- Gabbia, D.; Saponaro, M.; Sarcognato, S.; Guido, M.; Ferri, N.; Carrara, M.; De Martin, S. Fucus vesiculosus and Ascophyllum nodosum Ameliorate Liver Function by Reducing Diet-Induced Steatosis in Rats. Mar. Drugs 2020, 18, 62. [Google Scholar] [CrossRef]
- Kim, K.Y.; Nguyen, T.H.; Kurihara, H.; Kim, S.M. α-Glucosidase Inhibitory Activity of Bromophenol Purified from the Red Alga Polyopes Lancifolia. J. Food Sci. 2010, 75, H145–H150. [Google Scholar] [CrossRef]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Magalhães, P.O.; Silveira, D. α-Amylase Inhibitors: A Review of Raw Material and Isolated Compounds from Plant Source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef]
- Dechakhamphu, A.; Wongchum, N. Screening for Anti-Pancreatic Lipase Properties of 28 Traditional Thai Medicinal Herbs. Asian Pac. J. Trop. Biomed. 2015, 5, 1042–1045. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- André, R.; Pacheco, R.; Bourbon, M.; Serralheiro, M.L. Brown Algae Potential as a Functional Food against Hypercholesterolemia: Review. Foods 2021, 10, 234. [Google Scholar] [CrossRef]
- Negara, B.F.S.P.; Sohn, J.H.; Kim, J.-S.; Choi, J.-S. Effects of Phlorotannins on Organisms: Focus on the Safety, Toxicity, and Availability of Phlorotannins. Foods 2021, 10, 452. [Google Scholar] [CrossRef]
- Gabbia, D.; Roverso, M.; Guido, M.; Sacchi, D.; Scaffidi, M.; Carrara, M.; Orso, G.; Russo, F.P.; Floreani, A.; Bogialli, S.; et al. Western Diet-Induced Metabolic Alterations Affect Circulating Markers of Liver Function before the Development of Steatosis. Nutrients 2019, 11, 1602. [Google Scholar] [CrossRef] [PubMed]
- Bhatt-Wessel, B.; Jordan, T.W.; Miller, J.H.; Peng, L. Role of DGAT Enzymes in Triacylglycerol Metabolism. Arch. Biochem. Biophys. 2018, 655, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.; Vander Steen, T.; Espinoza, I.; Venkatapoorna, C.M.K.; Hu, Z.; Silva, F.M.; Regan, K.; Cuyàs, E.; Meng, X.W.; Verdura, S.; et al. Fatty Acid Synthase (FASN) Regulates the Mitochondrial Priming of Cancer Cells. Cell Death Dis. 2021, 12, 977. [Google Scholar] [CrossRef]
- Eberlé, D.; Hegarty, B.; Bossard, P.; Ferré, P.; Foufelle, F. SREBP Transcription Factors: Master Regulators of Lipid Homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef]
- Carr, R.M.; Ahima, R.S. Pathophysiology of Lipid Droplet Proteins in Liver Diseases. Exp. Cell Res. 2016, 340, 187–192. [Google Scholar] [CrossRef]
- Pawella, L.M.; Hashani, M.; Eiteneuer, E.; Renner, M.; Bartenschlager, R.; Schirmacher, P.; Straub, B.K. Perilipin Discerns Chronic from Acute Hepatocellular Steatosis. J. Hepatol. 2014, 60, 633–642. [Google Scholar] [CrossRef]
- Gabbia, D.; Cannella, L.; De Martin, S. The Role of Oxidative Stress in NAFLD–NASH–HCC Transition—Focus on NADPH Oxidases. Biomedicines 2021, 9, 687. [Google Scholar] [CrossRef]
- Ramírez-Moreno, E.; Arias-Rico, J.; Jiménez-Sánchez, R.C.; Estrada-Luna, D.; Jiménez-Osorio, A.S.; Zafra-Rojas, Q.Y.; Ariza-Ortega, J.A.; Flores-Chávez, O.R.; Morales-Castillejos, L.; Sandoval-Gallegos, E.M. Role of Bioactive Compounds in Obesity: Metabolic Mechanism Focused on Inflammation. Foods 2022, 11, 1232. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Liu, Y.; Li, H.; Wang, Q.; Huang, Z.; Liu, W.; Shi, P. Trivalent Chromium Supplementation Ameliorates Oleic Acid-Induced Hepatic Steatosis in Mice. Biol. Trace Elem. Res. 2019, 187, 192–201. [Google Scholar] [CrossRef]
- Sahin, K.; Tuzcu, M.; Orhan, C.; Sahin, N.; Kucuk, O.; Ozercan, I.H.; Juturu, V.; Komorowski, J.R. Anti-Diabetic Activity of Chromium Picolinate and Biotin in Rats with Type 2 Diabetes Induced by High-Fat Diet and Streptozotocin. Br. J. Nutr. 2013, 110, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Martino, F.; Puddu, P.E.; Pannarale, G.; Colantoni, C.; Martino, E.; Niglio, T.; Zanoni, C.; Barillà, F. Low Dose Chromium-Polynicotinate or Policosanol Is Effective in Hypercholesterolemic Children Only in Combination with Glucomannan. Atherosclerosis 2013, 228, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.-R.; Shin, T.-S.; Lee, M.-S.; Park, J.-Y.; Park, K.-E.; Yoon, N.-Y.; Kim, J.-S.; Choi, J.-S.; Jang, B.-C.; Byun, D.-S.; et al. Isolation and Identification of Phlorotannins from Ecklonia Stolonifera with Antioxidant and Anti-Inflammatory Properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.J.P.; Ahn, G.; Lee, W.-W.; Kang, M.-C.; Kim, E.-A.; Jeon, Y.-J. Anti-Inflammatory Activity of Phlorotannin-Rich Fermented Ecklonia Cava Processing by-Product Extract in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. J. Appl. Phycol. 2013, 25, 1207–1213. [Google Scholar] [CrossRef]
- Dong, X.; Bai, Y.; Xu, Z.; Shi, Y.; Sun, Y.; Janaswamy, S.; Yu, C.; Qi, H. Phlorotannins from Undaria Pinnatifida Sporophyll: Extraction, Antioxidant, and Anti-Inflammatory Activities. Mar. Drugs 2019, 17, 434. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, K.; Fu, J. HDAC6 Mediates Macrophage INOS Expression and Excessive Nitric Oxide Production in the Blood During Endotoxemia. Front. Immunol. 2020, 11, 1893. [Google Scholar] [CrossRef]
- Ahmadinejad, F.; Geir Møller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.-S. Molecular Mechanisms behind Free Radical Scavengers Function against Oxidative Stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef]
- Han, J.; Jiang, Y.; Li, Z.; Kravchenko, V.V.; Ulevitch, R.J. Activation of the Transcription Factor MEF2C by the MAP Kinase P38 in Inflammation. Nature 1997, 386, 296–299. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kuznetsova, T.A.; Kryzhanovsky, S.P.; Ermakova, S.P.; Galkina, I.V.; Shchelkanov, M.Y. Molecular Targets of Brown Algae Phlorotannins for the Therapy of Inflammatory Processes of Various Origins. Mar. Drugs 2022, 20, 243. [Google Scholar] [CrossRef]
- Bahar, B.; O’Doherty, J.V.; Smyth, T.J.; Sweeney, T. A Comparison of the Effects of an Ascophyllum Nodosum Ethanol Extract and Its Molecular Weight Fractions on the Inflammatory Immune Gene Expression In-Vitro and Ex-Vivo. Innov. Food Sci. Emerg. Technol. 2016, 37, 276–285. [Google Scholar] [CrossRef]
- Schmidt-Arras, D.; Rose-John, S. IL-6 Pathway in the Liver: From Physiopathology to Therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [Green Version]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic Syndrome: A Comprehensive Perspective Based on Interactions between Obesity, Diabetes, and Inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Y.; Liu, J. Tumor Necrosis Factor-α Signaling in Nonalcoholic Steatohepatitis and Targeted Therapies. J. Genet. Genom. 2022, 49, 269–278. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; Dalla Pozza, A.; Albertoni, L.; Lazzari, R.; Zigiotto, G.; Carrara, M.; Baldo, V.; Baldovin, T.; Floreani, A.; De Martin, S. Pregnane X Receptor and Constitutive Androstane Receptor Modulate Differently CYP3A-Mediated Metabolism in Early- and Late-Stage Cholestasis. World J. Gastroenterol. 2017, 23, 7519–7530. [Google Scholar] [CrossRef]
| Parameter | Healthy Rats (Vehicle) | Healthy Rats (Nutraceutical Formulation) |
|---|---|---|
| Blood glucose (mg/dL) | 147.5 ± 13.1 | 140.3 ± 8.1 |
| Urea (mmol/L) | 5.4 ± 0.5 | 5.1 ± 0.2 |
| Creatinine (μmol/L) | 14.5 ± 1.9 | 12.7 ± 1.5 |
| Sodium (mmol/L) | 143 ± 2.2 | 142 ± 1.0 |
| Potassium (mmol/L) | 4.3 ± 0.5 | 4.2 ± 0.4 |
| Chloride (mmol/L) | 100.0 ± 2.4 | 100.7 ± 1.2 |
| Total bilirubin (μmol/L) | 0.7 ± 0.3 | 0.8 ± 0.1 |
| Albumin (g/L) | 12.0 ± 0.0 | 12.3 ± 0.6 |
| Calcium (mmol/L) | 2.6 ± 0.1 | 2.6 ± 0.1 |
| Inorganic phosphate (mmol/L) | 1.9 ± 0.2 | 1.8 ± 0.1 |
| AST (U/L) | 103.3 ± 17.1 | 94.3 ± 7.6 |
| ALT (U/L) | 29.8 ± 3.0 | 17.0 ± 9.5 |
| ALP (U/L) | 156.5 ± 31.4 | 119.0 ± 22.3 |
| Total cholesterol (mg/dL) | 66.0 ± 10.2 | 70.0 ± 13.5 |
| TG (mg/dL) | 89.0 ± 12.8 | 114.7 ± 23.6 |
| Parameter | NAFLD Rats | NASH Rats |
|---|---|---|
| Time | p < 0.0001 | p < 0.0001 |
| Treatment | p < 0.05 | p < 0.05 |
| Time × Treatment | ns | p < 0.0001 |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| DGAT-1 | TCCTGAATTGGTGCGTGGTG | GAAACAGAGACACCACCTGGA |
| DGAT-2 | GCAGCGAGAACAAGAATAAAGGA | CCACCTTGGATCTGTTGAGC |
| GPAT-4 | TGTGGGACGGTGGATTGAAG | GCTCCGGTCCTCATGGTTAC |
| FASN | GCATTTCCACAACCCCAACC | AACGAGTTGATGCCCACGAT |
| SREBP-1 | CATGGACGAGCTACCCTTCG | GGGCATCAAATAGGCCAGGG |
| SREBP-2 | CGAACTGGGCGATGGATGAGA | TCTCCCACTTGATTGCTGACA |
| β-actin | GCCACCAGTTCGCCATGGA | TTCTGACCCATACCCACCAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabbia, D.; Roverso, M.; Zanotto, I.; Colognesi, M.; Sayaf, K.; Sarcognato, S.; Arcidiacono, D.; Zaramella, A.; Realdon, S.; Ferri, N.; et al. A Nutraceutical Formulation Containing Brown Algae Reduces Hepatic Lipid Accumulation by Modulating Lipid Metabolism and Inflammation in Experimental Models of NAFLD and NASH. Mar. Drugs 2022, 20, 572. https://doi.org/10.3390/md20090572
Gabbia D, Roverso M, Zanotto I, Colognesi M, Sayaf K, Sarcognato S, Arcidiacono D, Zaramella A, Realdon S, Ferri N, et al. A Nutraceutical Formulation Containing Brown Algae Reduces Hepatic Lipid Accumulation by Modulating Lipid Metabolism and Inflammation in Experimental Models of NAFLD and NASH. Marine Drugs. 2022; 20(9):572. https://doi.org/10.3390/md20090572
Chicago/Turabian StyleGabbia, Daniela, Marco Roverso, Ilaria Zanotto, Martina Colognesi, Katia Sayaf, Samantha Sarcognato, Diletta Arcidiacono, Alice Zaramella, Stefano Realdon, Nicola Ferri, and et al. 2022. "A Nutraceutical Formulation Containing Brown Algae Reduces Hepatic Lipid Accumulation by Modulating Lipid Metabolism and Inflammation in Experimental Models of NAFLD and NASH" Marine Drugs 20, no. 9: 572. https://doi.org/10.3390/md20090572
APA StyleGabbia, D., Roverso, M., Zanotto, I., Colognesi, M., Sayaf, K., Sarcognato, S., Arcidiacono, D., Zaramella, A., Realdon, S., Ferri, N., Guido, M., Russo, F. P., Bogialli, S., Carrara, M., & De Martin, S. (2022). A Nutraceutical Formulation Containing Brown Algae Reduces Hepatic Lipid Accumulation by Modulating Lipid Metabolism and Inflammation in Experimental Models of NAFLD and NASH. Marine Drugs, 20(9), 572. https://doi.org/10.3390/md20090572