New Three-Finger Protein from Starfish Asteria rubens Shares Structure and Pharmacology with Human Brain Neuromodulator Lynx2
Abstract
:1. Introduction
2. Results
2.1. Identification of TFPs in Starfish Genomes
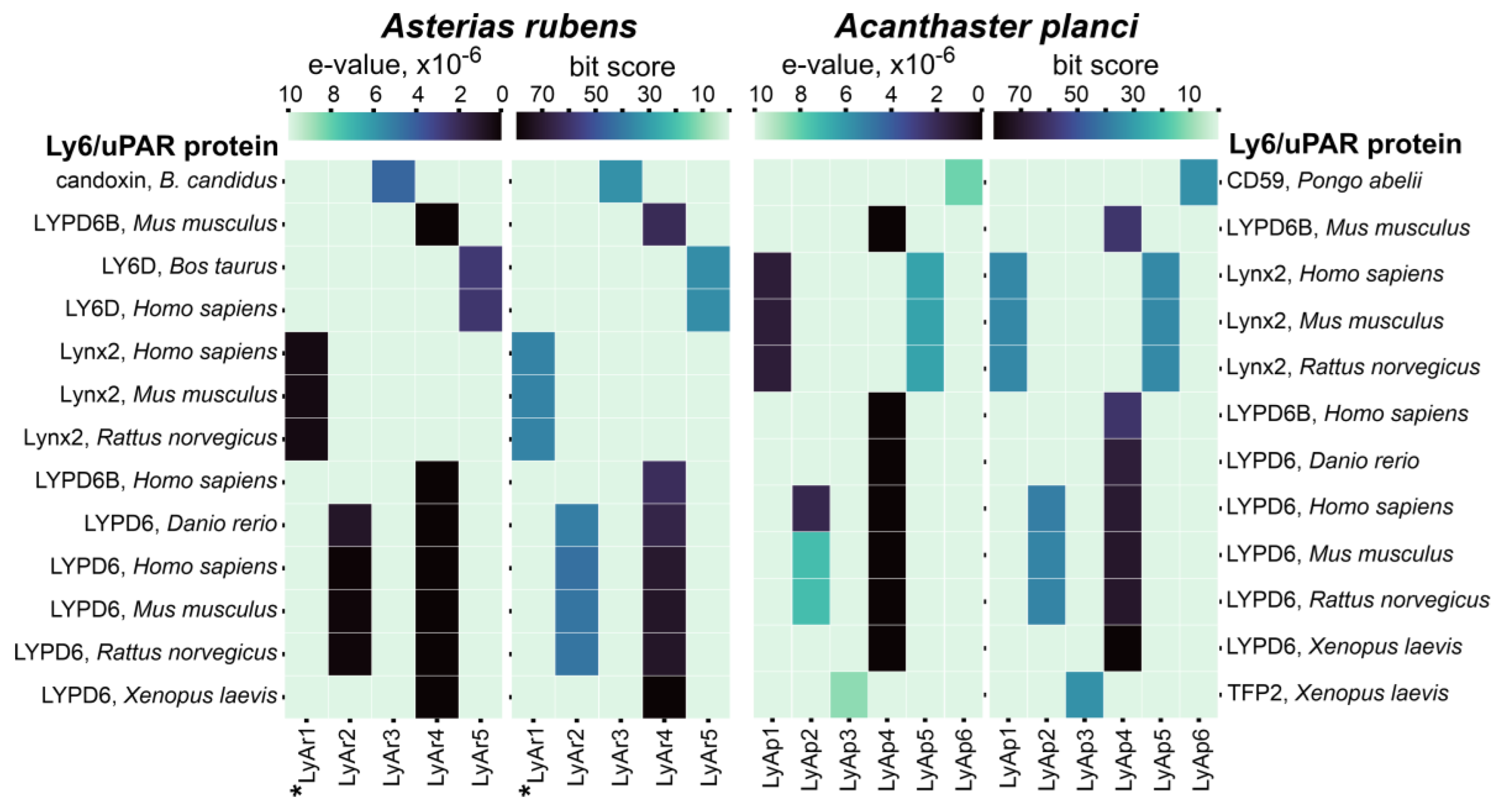
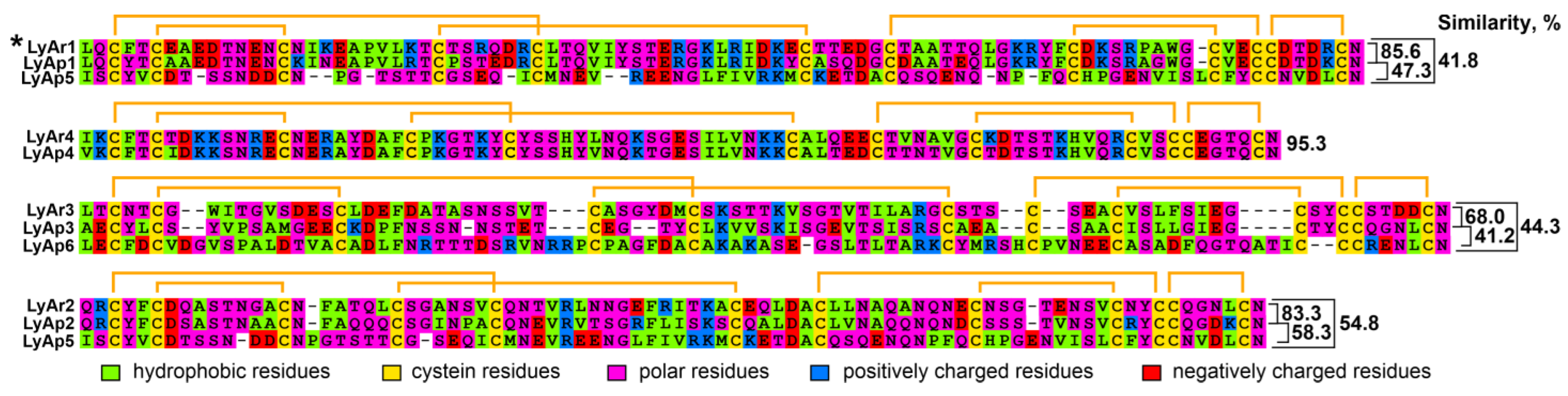
2.2. Relationships of TFPs from Starfishes and Other Animals
2.3. Protein LyAr1 (Lystar5) Is Expressed in Asteria rubens
2.4. Recombinant Production and Purification of Lystar5
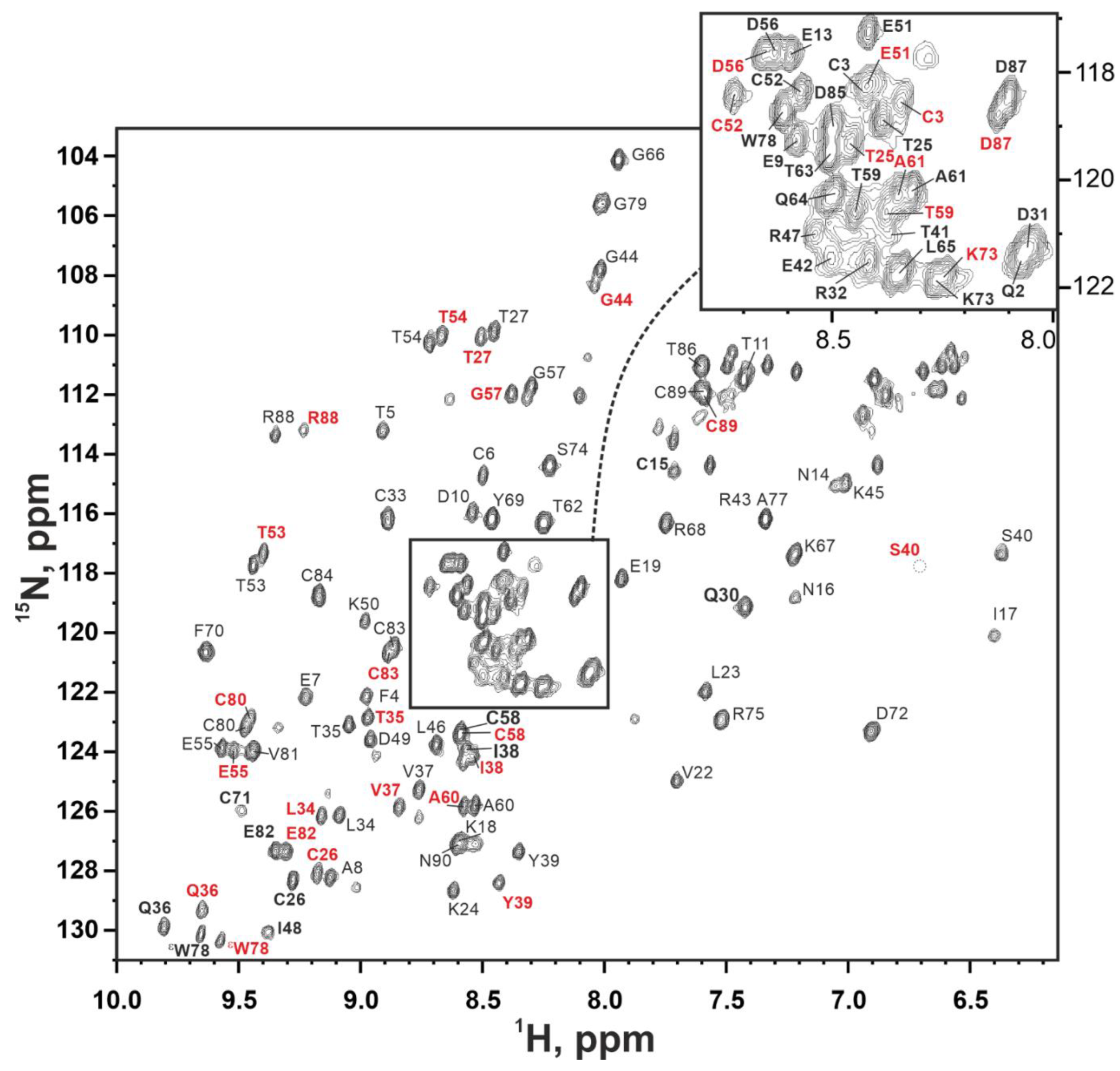
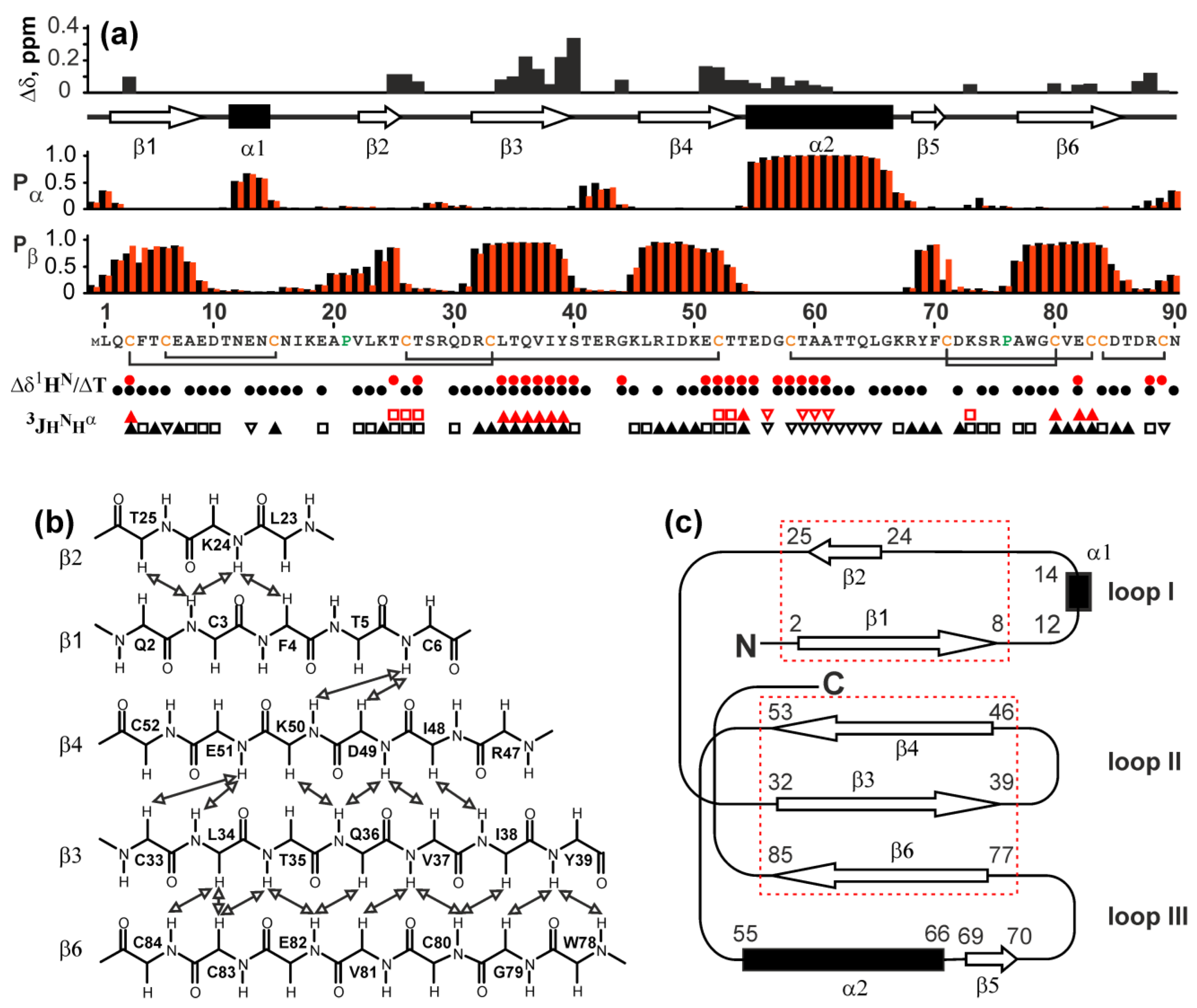
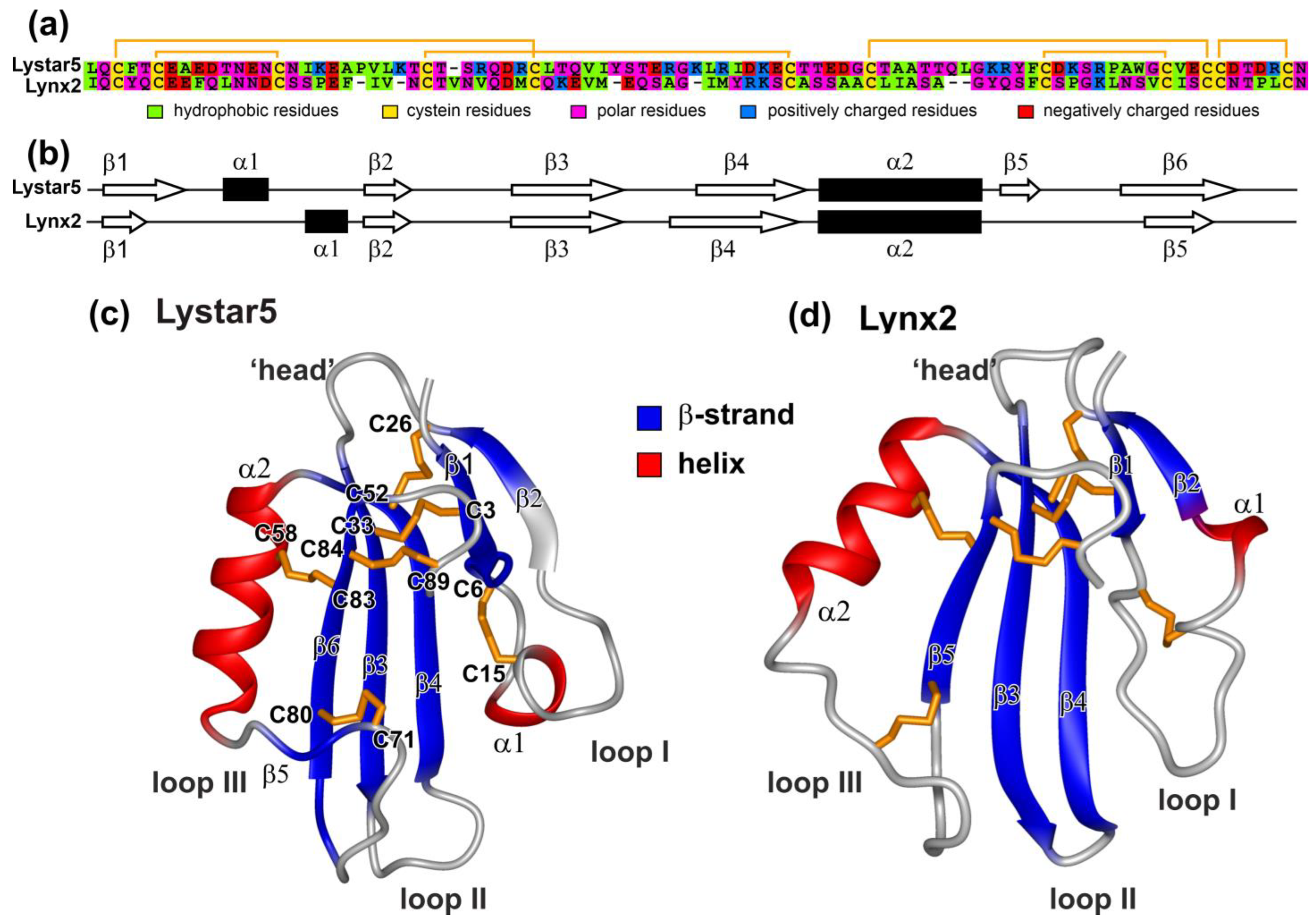
2.5. NMR Study Confirms the Three-Finger Fold of Lystar5
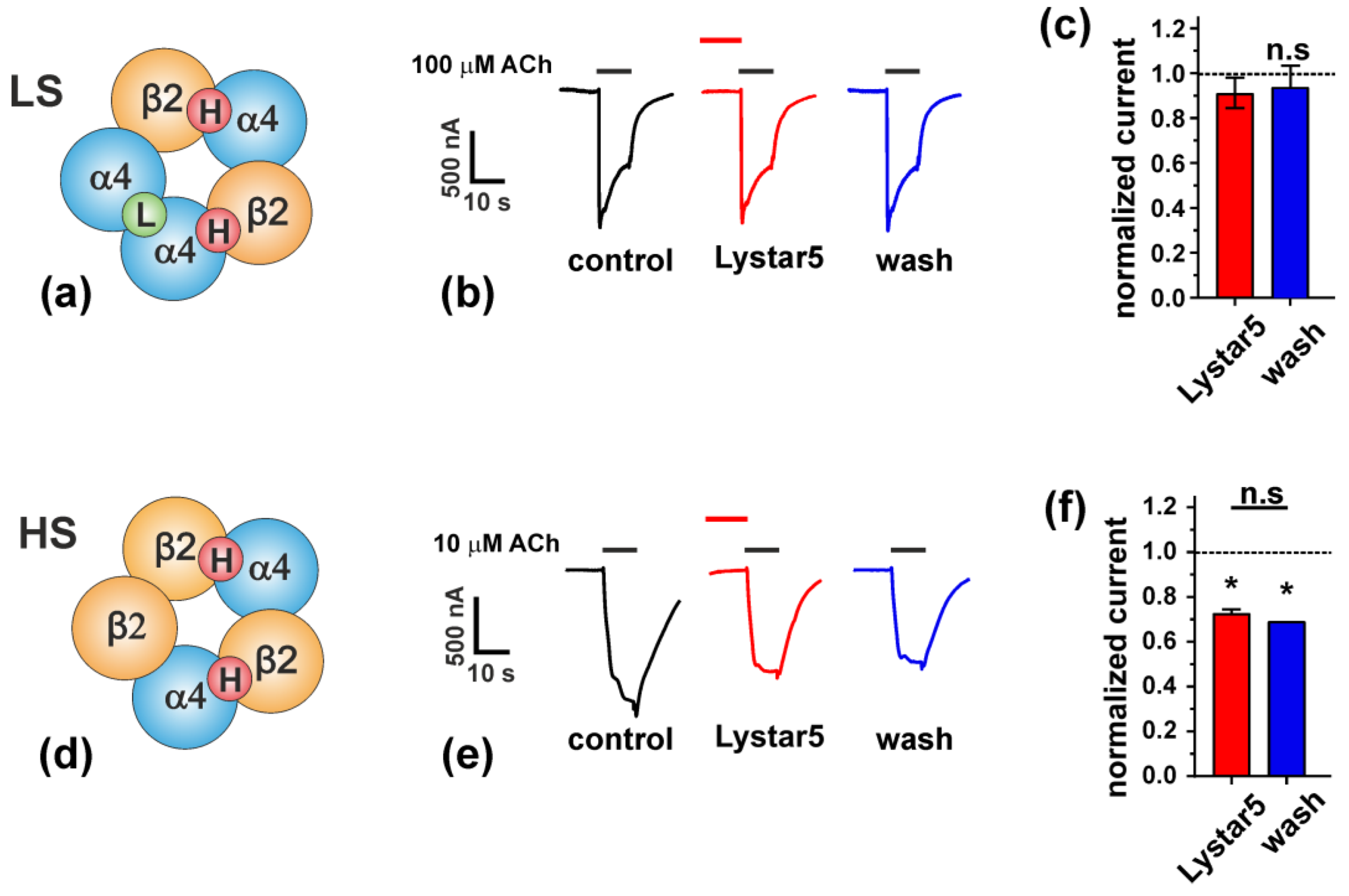
2.6. Study of Lystar5 Pharmacology at nAChRs
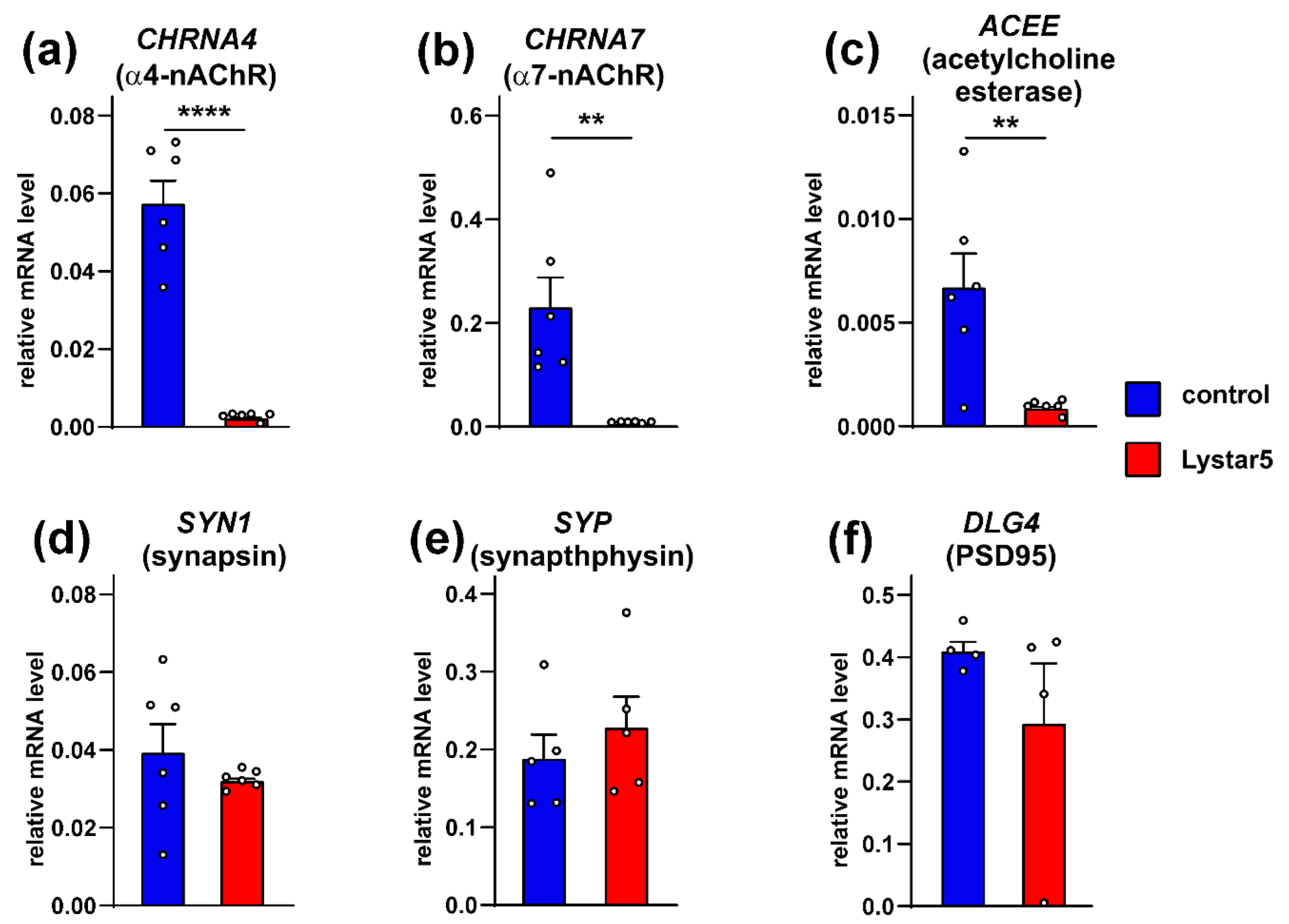
2.7. Lystar5 Down-Regulates Expression of nAChRs and Acetylcholine Esterase
3. Discussion
4. Materials and Methods
4.1. Prediction of TFPs from Starfishes
4.2. Animals and Tissue Isolation
4.3. Confirmation of Lystar5 Expression in A. rubens
4.4. Design of the Lystar5 Gene for the Recombinant Production
4.5. Bacterial Production of Lystar5
4.6. Assignment of 13C-15N-NMR Spectra of Lystar5 and Secondary Structure Determination
4.7. Electrophysiological Recordings in X. laevis Oocytes
4.8. Primary Neuron Culture
4.9. Real-Time PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vasilyeva, N.A.; Loktyushov, E.V.; Bychkov, M.L.; Shenkarev, Z.O.; Lyukmanova, E.N. Three-Finger Proteins from the Ly6/UPAR Family: Functional Diversity within One Structural Motif. Biochemistry (Mosc.) 2017, 82, 1702–1715. [Google Scholar] [CrossRef] [PubMed]
- Galat, A.; Gross, G.; Drevet, P.; Sato, A.; Ménez, A. Conserved Structural Determinants in Three-Fingered Protein Domains. FEBS. J. 2008, 275, 3207–3225. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.M.; Harrison, R.A.; Lachmann, P.J.; Neuhaus, D. Structure of a Soluble, Glycosylated Form of the Human Complement Regulatory Protein CD59. Structure 1994, 2, 185–199. [Google Scholar] [CrossRef] [Green Version]
- Arredondo, J.; Chernyavsky, A.I.; Grando, S.A. SLURP-1 and -2 in Normal, Immortalized and Malignant Oral Keratinocytes. Life Sci. 2007, 80, 2243–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loughner, C.L.; Bruford, E.A.; McAndrews, M.S.; Delp, E.E.; Swamynathan, S.; Swamynathan, S.K. Organization, Evolution and Functions of the Human and Mouse Ly6/UPAR Family Genes. Hum. Genomics 2016, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyukmanova, E.N.; Shenkarev, Z.O.; Shulepko, M.A.; Paramonov, A.S.; Chugunov, A.O.; Bychkov, M.L.; Dolgikh, D.A. Structure-Function Study of Human SLURP-1 and SLURP-2 Suggests Multiple Molecular Targets. FEBS. J. 2015, 282, 168. [Google Scholar]
- Lyukmanova, E.N.; Shulepko, M.A.; Shenkarev, Z.O.; Bychkov, M.L.; Paramonov, A.S.; Chugunov, A.O.; Kulbatskii, D.S.; Arvaniti, M.; Dolejsi, E.; Schaer, T.; et al. Secreted Isoform of Human Lynx1 (SLURP-2): Spatial Structure and Pharmacology of Interactions with Different Types of Acetylcholine Receptors. Sci. Rep. 2016, 6, 30698. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, V.; George, A.A.; Nishi, R.; Whiteaker, P. The Prototoxin LYPD6B Modulates Heteromeric A3β4-Containing Nicotinic Acetylcholine Receptors, but Not A7 Homomers. FASEB. J. 2016, 30, 1109–1119. [Google Scholar] [CrossRef] [Green Version]
- Tekinay, A.B.; Nong, Y.; Miwa, J.M.; Lieberam, I.; Ibanez-Tallon, I.; Greengard, P.; Heintz, N. A Role for LYNX2 in Anxiety-Related Behavior. Proc. Natl. Acad. Sci. USA 2009, 106, 4477–4482. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, M.S.; Cinar, B.; Jensen, M.M.; Lyukmanova, E.N.; Shulepko, M.A.; Tsetlin, V.; Klein, A.B.; Mikkelsen, J.D. Expression of the Ly-6 Family Proteins Lynx1 and Ly6H in the Rat Brain Is Compartmentalized, Cell-Type Specific, and Developmentally Regulated. Brain Struct. Funct. 2014, 219, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ren, J.; Lu, W.; Harlos, K.; Jones, E.Y. Structure of the Wnt Signaling Enhancer LYPD6 and Its Interactions with the Wnt Coreceptor LRP6. FEBS. Lett. 2018, 592, 3152–3162. [Google Scholar] [CrossRef] [PubMed]
- Lyukmanova, E.N.; Shenkarev, Z.O.; Shulepko, M.A.; Mineev, K.S.; D’Hoedt, D.; Kasheverov, I.E.; Filkin, S.Y.; Krivolapova, A.P.; Janickova, H.; Dolezal, V.; et al. NMR Structure and Action on Nicotinic Acetylcholine Receptors of Water-Soluble Domain of Human LYNX1. J. Biol. Chem. 2011, 286, 10618–10627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvaniti, M.; Jensen, M.M.; Soni, N.; Wang, H.; Klein, A.B.; Thiriet, N.; Pinborg, L.H.; Muldoon, P.P.; Wienecke, J.; Imad Damaj, M.; et al. Functional Interaction between Lypd6 and Nicotinic Acetylcholine Receptors. J. Neurochem. 2016, 138, 806–820. [Google Scholar] [CrossRef] [PubMed]
- Ibañez-Tallon, I.; Miwa, J.M.; Wang, H.L.; Adams, N.C.; Crabtree, G.W.; Sine, S.M.; Heintz, N. Novel Modulation of Neuronal Nicotinic Acetylcholine Receptors by Association with the Endogenous Prototoxin Lynx1. Neuron 2002, 33, 893–903. [Google Scholar] [CrossRef] [Green Version]
- Kulbatskii, D.; Shenkarev, Z.; Bychkov, M.; Loktyushov, E.; Shulepko, M.; Koshelev, S.; Povarov, I.; Popov, A.; Peigneur, S.; Chugunov, A.; et al. Human Three-Finger Protein Lypd6 Is a Negative Modulator of the Cholinergic System in the Brain. Front. Cell Dev. Biol. 2021, 9, 662227. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.A.; Henderson, B.J.; Yu, C.; Parker, R.L.; Richards, C.I.; Lester, H.A.; Miwa, J.M. Lynx1 Shifts A4β2 Nicotinic Receptor Subunit Stoichiometry by Affecting Assembly in the Endoplasmic Reticulum. J. Biol. Chem. 2014, 289, 31423–31432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shulepko, M.A.; Bychkov, M.L.; Lyukmanova, E.N.; Kirpichnikov, M.P. Recombinant Analogue of the Human Protein SLURP-1 Inhibits the Growth of U251 MG and A172 Glioma Cells. Dokl. Biochem. Biophys. 2020, 493, 211–214. [Google Scholar] [CrossRef]
- Blasi, F.; Carmeliet, P. UPAR: A Versatile Signalling Orchestrator. Nat. Rev. Mol. Cell. Biol. 2002, 3, 932–943. [Google Scholar] [CrossRef]
- Mar, K.B.; Rinkenberger, N.R.; Boys, I.N.; Eitson, J.L.; McDougal, M.B.; Richardson, R.B.; Schoggins, J.W. LY6E Mediates an Evolutionarily Conserved Enhancement of Virus Infection by Targeting a Late Entry Step. Nat. Commun. 2018, 9, 3603. [Google Scholar] [CrossRef] [PubMed]
- Pfaender, S.; Mar, K.B.; Michailidis, E.; Kratzel, A.; Boys, I.N.; V’kovski, P.; Fan, W.; Kelly, J.N.; Hirt, D.; Ebert, N.; et al. LY6E Impairs Coronavirus Fusion and Confers Immune Control of Viral Disease. Nat. Microbiol. 2020, 5, 1330–1339. [Google Scholar] [CrossRef]
- Yu, J.; Murthy, V.; Liu, S.-L. Relating GPI-Anchored Ly6 Proteins UPAR and CD59 to Viral Infection. Viruses 2019, 11, 1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özhan, G.; Sezgin, E.; Wehner, D.; Pfister, A.S.; Kühl, S.J.; Kagermeier-Schenk, B.; Kühl, M.; Schwille, P.; Weidinger, G. Lypd6 Enhances Wnt/β-Catenin Signaling by Promoting Lrp6 Phosphorylation in Raft Plasma Membrane Domains. Dev. Cell 2013, 26, 331–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNally, J.D.; Wu, S.-B.; Sturgeon, C.M.; Storey, K.B. Identification and Characterization of a Novel Freezing Inducible Gene, Li16, in the Wood Frog Rana Sylvatica. FASEB. J. 2002, 16, 902–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Cao, X.; Wang, S.; Ji, G.; Zhang, S.; Li, H. Identification of Ly2 Members as Antimicrobial Peptides from Zebrafish Danio Rerio. Biosci. Rep. 2017, 37, BSR20160265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garza-Garcia, A.; Harris, R.; Esposito, D.; Gates, P.B.; Driscoll, P.C. Solution Structure and Phylogenetics of Prod1, a Member of the Three-Finger Protein Superfamily Implicated in Salamander Limb Regeneration. PLoS ONE 2009, 4, e7123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilburn, D.B.; Kunkel, C.L.; Feldhoff, R.C.; Feldhoff, P.W.; Searle, B.C. Recurrent Co-Option and Recombination of Cytokine and Three Finger Proteins in Multiple Reproductive Tissues Throughout Salamander Evolution. Front. Cell Dev. Biol. 2022, 10, 828947. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yao, X.; Gu, S.; Zhang, Y.; Liu, Z.; Zhang, Y. Selectivity of Lynx Proteins on Insect Nicotinic Acetylcholine Receptors in the Brown Planthopper, Nilaparvata Lugens. Insect. Mol. Biol. 2010, 19, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Joiner, W.J.; Wu, M.N.; Yue, Z.; Smith, C.J.; Sehgal, A. Identification of SLEEPLESS, a Sleep-Promoting Factor. Science 2008, 321, 372–376. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Robinson, J.E.; Joiner, W.J. SLEEPLESS Is a Bifunctional Regulator of Excitability and Cholinergic Synaptic Transmission. Curr. Biol. 2014, 24, 621–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabelnikov, S.V.; Bobkov, D.E.; Sharlaimova, N.S.; Petukhova, O.A. Injury Affects Coelomic Fluid Proteome of the Common Starfish, Asterias rubens. J. Exp. Biol. 2019, 222, jeb198556. [Google Scholar] [CrossRef] [Green Version]
- Hennebert, E.; Leroy, B.; Wattiez, R.; Ladurner, P. An Integrated Transcriptomic and Proteomic Analysis of Sea Star Epidermal Secretions Identifies Proteins Involved in Defense and Adhesion. J. Proteomics 2015, 128, 83–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reich, A.; Dunn, C.; Akasaka, K.; Wessel, G. Phylogenomic Analyses of Echinodermata Support the Sister Groups of Asterozoa and Echinozoa. PLoS ONE 2015, 10, e0119627. [Google Scholar] [CrossRef] [Green Version]
- Semmens, D.C.; Mirabeau, O.; Moghul, I.; Pancholi, M.R.; Wurm, Y.; Elphick, M.R. Transcriptomic Identification of Starfish Neuropeptide Precursors Yields New Insights into Neuropeptide Evolution. Open Biol. 2016, 6, 150224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, M.R.; Kocot, K.M.; Baughman, K.W.; Fernandez-Valverde, S.L.; Gauthier, M.E.A.; Hatleberg, W.L.; Krishnan, A.; McDougall, C.; Motti, C.A.; Shoguchi, E.; et al. The Crown-of-Thorns Starfish Genome as a Guide for Biocontrol of This Coral Reef Pest. Nature 2017, 544, 231–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, E.K.; Garm, A.L.; Ullrich-Lüter, E.; Cuomo, C.; Arnone, M.I. The Crowns Have Eyes: Multiple Opsins Found in the Eyes of the Crown-of-Thorns Starfish Acanthaster Planci. BMC. Evol. Biol. 2018, 18, 168. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.J.; Stewart, P.; Rivera-Posada, J. De Novo Assembly of the Transcriptome of Acanthaster Planci Testes. Mol. Ecol. Resour. 2015, 15, 953–966. [Google Scholar] [CrossRef]
- Paramonov, A.S.; Kocharovskaya, M.V.; Tsarev, A.V.; Kulbatskii, D.S.; Loktyushov, E.V.; Shulepko, M.A.; Kirpichnikov, M.P.; Lyukmanova, E.N.; Shenkarev, Z.O. Structural Diversity and Dynamics of Human Three-Finger Proteins Acting on Nicotinic Acetylcholine Receptors. Int. J. Mol. Sci. 2020, 21, 7280. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Puddifoot, C.A.; Taylor, P.; Joiner, W.J. Mechanisms of Inhibition and Potentiation of A4β2 Nicotinic Acetylcholine Receptors by Members of the Ly6 Protein Family. J. Biol. Chem. 2015, 290, 24509–24518. [Google Scholar] [CrossRef] [Green Version]
- Pratchett, M.; Caballes, C.; Wilmes, J.; Matthews, S.; Mellin, C.; Sweatman, H.; Nadler, L.; Brodie, J.; Thompson, C.; Hoey, J.; et al. Thirty Years of Research on Crown-of-Thorns Starfish (1986–2016): Scientific Advances and Emerging Opportunities. Diversity 2017, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Vogler, C.; Benzie, J.; Lessios, H.; Barber, P.; Wörheide, G. A Threat to Coral Reefs Multiplied? Four Species of Crown-of-Thorns Starfish. Biol. Lett. 2008, 4, 696–699. [Google Scholar] [CrossRef] [Green Version]
- Paramonov, A.S.; Shulepko, M.A.; Kocharovskaya, M.V.; Alenkin, A.E.; Evdokimova, A.O.; Akentiev, P.I.; Shenkarev, Z.O.; Kirpichnikov, M.P.; Lyukmanova, E.N. Bacterial Production and Structural Study of Human Neuromodulator Lynx2. Russ. J. Bioorg. Chem. 2020, 46, 1261–1269. [Google Scholar] [CrossRef]
- Shulepko, M.A.; Lyukmanova, E.N.; Shenkarev, Z.O.; Dubovskii, P.V.; Astapova, M.V.; Feofanov, A.V.; Arseniev, A.S.; Utkin, Y.N.; Kirpichnikov, M.P.; Dolgikh, D.A. Towards Universal Approach for Bacterial Production of Three-Finger Ly6/UPAR Proteins: Case Study of Cytotoxin I from Cobra N. Oxiana. Protein Expr. Purif. 2017, 130, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Bax, A. Protein Backbone and Sidechain Torsion Angles Predicted from NMR Chemical Shifts Using Artificial Neural Networks. J. Biomol. NMR. 2013, 56, 227–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyukmanova, E.N.; Shulepko, M.A.; Tikhonov, R.V.; Shenkarev, Z.O.; Paramonov, A.S.; Wulfson, A.N.; Kasheverov, I.E.; Ustich, T.L.; Utkin, Y.N.; Arseniev, A.S.; et al. Bacterial Production and Refolding from Inclusion Bodies of a “Weak” Toxin, a Disulfide Rich Protein. Biochemistry 2009, 74, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Shenkarev, Z.O.; Shulepko, M.A.; Bychkov, M.L.; Kulbatskii, D.S.; Shlepova, O.V.; Vasilyeva, N.A.; Andreev-Andrievskiy, A.A.; Popova, A.S.; Lagereva, E.A.; Loktyushov, E.V.; et al. Water-Soluble Variant of Human Lynx1 Positively Modulates Synaptic Plasticity and Ameliorates Cognitive Impairment Associated with A7-NAChR Dysfunction. J. Neurochem 2020, 155, 45–61. [Google Scholar] [CrossRef]
- Bychkov, M.L.; Shulepko, M.A.; Shlepova, O.V.; Kulbatskii, D.S.; Chulina, I.A.; Paramonov, A.S.; Baidakova, L.K.; Azev, V.N.; Koshelev, S.G.; Kirpichnikov, M.P.; et al. SLURP-1 Controls Growth and Migration of Lung Adenocarcinoma Cells, Forming a Complex With A7-NAChR and PDGFR/EGFR Heterodimer. Front. Cell. Dev. Biol. 2021, 9, 739391. [Google Scholar] [CrossRef]
- Bychkov, M.L.; Vasilyeva, N.A.; Shulepko, M.A.; Balaban, P.M.; Kirpichnikov, M.; Lyukmanova, E.N. Lynx1 Prevents Long-Term Potentiation Blockade and Reduction of Neuromodulator Expression Caused by Aβ1-42 and JNK Activation. Acta Naturae 2018, 10. [Google Scholar] [CrossRef]
- Franco, C.F.; Santos, R.; Coelho, A.V. Exploring the proteome of an echinoderm nervous system: 2-DE of the sea star radial nerve cord and the synaptosomal membranes subproteome. Proteomics 2011, 11, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.F.; Santos, R.; Coelho, A.V. Proteolytic events are relevant cellular responses during nervous system regeneration of the starfish Marthasterias glacialis. J. Proteom. 2014, 99, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.F.; Santos, R.; Coelho, A.V. Proteome characterization of sea star coelomocytes—The innate immune effector cells of echinoderms. PROTEOMICS 2011, 11, 3587–3592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lang, Q.; Li, J.; Xie, F.; Wan, B.; Yu, L. Identification and characterization of human LYPD6, a new member of the Ly-6 superfamily. Mol. Biol. Rep. 2009, 37, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.M., Jr.; Roh, S.-H.; Gharpure, A.; Morales-Perez, C.L.; Teng, J.; Hibbs, R.E. Structural principles of distinct assemblies of the human α4β2 nicotinic receptor. Nature 2018, 557, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Taly, A.; Corringer, P.-J.; Guedin, D.; Lestage, P.; Changeux, J.-P. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 2009, 8, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Devlin, C.; Schlosser, W.; Belz, D.T.; Kodiak, K.; Nash, R.F.; Zitomer, N. Pharmacological identification of acetylcholine receptor subtypes in echinoderm smooth muscle (Sclerodactyla briareus). Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 2000, 125, 53–64. [Google Scholar] [CrossRef]
- Arredondo, J.; Nguyen, V.T.; Chernyavsky, A.I.; Bercovich, D.; Orr-Urtreger, A.; Kummer, W.; Lips, K.S.; Vetter, D.E.; Grando, S.A. Central role of α7 nicotinic receptor in differentiation of the stratified squamous epithelium. J. Cell Biol. 2002, 159, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Shulepko, M.A.; Kulbatskii, D.S.; Bychkov, M.L.; Lyukmanova, E.N. Human Nicotinic Acetylcholine Receptors: Part II. Non-Neuronal Cholinergic System. Russ. J. Bioorganic Chem. 2019, 45, 66–75. [Google Scholar] [CrossRef]
- Tracey, K.J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Investig. 2007, 117, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosur, V.; Loring, R.H. α4β2 Nicotinic Receptors Partially Mediate Anti-Inflammatory Effects through Janus Kinase 2-Signal Transducer and Activator of Transcription 3 but Not Calcium or cAMP Signaling. Mol. Pharmacol. 2010, 79, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, U.N. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med. Sci. Monit. 2007, 13, RA214-21. [Google Scholar] [PubMed]
- Ben Khadra, Y.; Ferrario, C.; Di Benedetto, C.; Said, K.; Bonasoro, F.; Carnevali, M.D.C.; Sugni, M. Wound repair during arm regeneration in the red starfish Echinaster sepositus. Wound Repair Regen. 2015, 23, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorshkov, A.N.; Blinova, M.I.; Pinaev, G.P. Ultrastructure of coelomic epithelium and coelomocytes of the starfish Asterias rubens L. in norm and after wounding. Cell Tissue Biol. 2009, 3, 477–490. [Google Scholar] [CrossRef]
- Olsen, T.B.; Christensen, F.E.G.; Lundgreen, K.; Dunn, P.H.; Levitis, D.A. Coelomic Transport and Clearance of Durable Foreign Bodies by Starfish (Asterias rubens). Biol. Bull. 2015, 228, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharlaimova, N.; Shabelnikov, S.; Petukhova, O. Small coelomic epithelial cells of the starfish Asterias rubens L. that are able to proliferate in vivo and in vitro. Cell Tissue Res. 2014, 356, 83–95. [Google Scholar] [CrossRef]
- Sharlaimova, N.; Shabelnikov, S.; Bobkov, D.; Martynova, M.; Bystrova, O.; Petukhova, O. Coelomocyte replenishment in adult Asterias rubens: The possible ways. Cell Tissue Res. 2020, 383, 1043–1060. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 Predicts All Five Types of Signal Peptides Using Protein Language Models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Pierleoni, A.; Martelli, P.L.; Casadio, R. PredGPI: A GPI-Anchor Predictor. BMC Bioinform. 2008, 9, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanungo, K. In vitro studies on the effects of cell-free coelomic fluid, calcium, and/or magnesium on clumping of coelomocytes of the sea star Asterias forbesi (echinodermata: Asteroidea). Biol. Bull. 1982, 163, 438–452. [Google Scholar] [CrossRef]
- Cavanagh, J.; Fairbrother, W.J.; Palmer, A.G., III; Skelton, N.J. Protein NMR Spectroscopy: Principles and Practice. Elsevier: San Diego, CA, USA, 1995; ISBN 978-0-08-051529-8. [Google Scholar]
- Bax, A.; Vuister, G.W.; Grzesiek, S.; Delaglio, F.; Wang, A.C.; Tschudin, R.; Zhu, G. Measurement of homo- and heteronuclear J couplings from quantitative J correlation. Methods Enzymol. 1994, 239, 79–105. [Google Scholar] [CrossRef] [PubMed]
- Suntsova, M.; Gogvadze, E.V.; Salozhin, S.; Gaifullin, N.; Eroshkin, F.; Dmitriev, S.E.; Martynova, N.; Kulikov, K.; Malakhova, G.; Tukhbatova, G.; et al. Human-specific endogenous retroviral insert serves as an enhancer for the schizophrenia-linked gene PRODH. Proc. Natl. Acad. Sci. USA 2013, 110, 19472–19477. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paramonov, A.S.; Shulepko, M.A.; Makhonin, A.M.; Bychkov, M.L.; Kulbatskii, D.S.; Chernikov, A.M.; Myshkin, M.Y.; Shabelnikov, S.V.; Shenkarev, Z.O.; Kirpichnikov, M.P.; et al. New Three-Finger Protein from Starfish Asteria rubens Shares Structure and Pharmacology with Human Brain Neuromodulator Lynx2. Mar. Drugs 2022, 20, 503. https://doi.org/10.3390/md20080503
Paramonov AS, Shulepko MA, Makhonin AM, Bychkov ML, Kulbatskii DS, Chernikov AM, Myshkin MY, Shabelnikov SV, Shenkarev ZO, Kirpichnikov MP, et al. New Three-Finger Protein from Starfish Asteria rubens Shares Structure and Pharmacology with Human Brain Neuromodulator Lynx2. Marine Drugs. 2022; 20(8):503. https://doi.org/10.3390/md20080503
Chicago/Turabian StyleParamonov, Alexander S., Mikhail A. Shulepko, Alexey M. Makhonin, Maxim L. Bychkov, Dmitrii S. Kulbatskii, Andrey M. Chernikov, Mikhail Yu. Myshkin, Sergey V. Shabelnikov, Zakhar O. Shenkarev, Mikhail P. Kirpichnikov, and et al. 2022. "New Three-Finger Protein from Starfish Asteria rubens Shares Structure and Pharmacology with Human Brain Neuromodulator Lynx2" Marine Drugs 20, no. 8: 503. https://doi.org/10.3390/md20080503
APA StyleParamonov, A. S., Shulepko, M. A., Makhonin, A. M., Bychkov, M. L., Kulbatskii, D. S., Chernikov, A. M., Myshkin, M. Y., Shabelnikov, S. V., Shenkarev, Z. O., Kirpichnikov, M. P., & Lyukmanova, E. N. (2022). New Three-Finger Protein from Starfish Asteria rubens Shares Structure and Pharmacology with Human Brain Neuromodulator Lynx2. Marine Drugs, 20(8), 503. https://doi.org/10.3390/md20080503








