Antioxidant Activity of Fucoidan Modified with Gallic Acid Using the Redox Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of Fucoidans
2.2. Analysis of In Vitro Antioxidant Activity
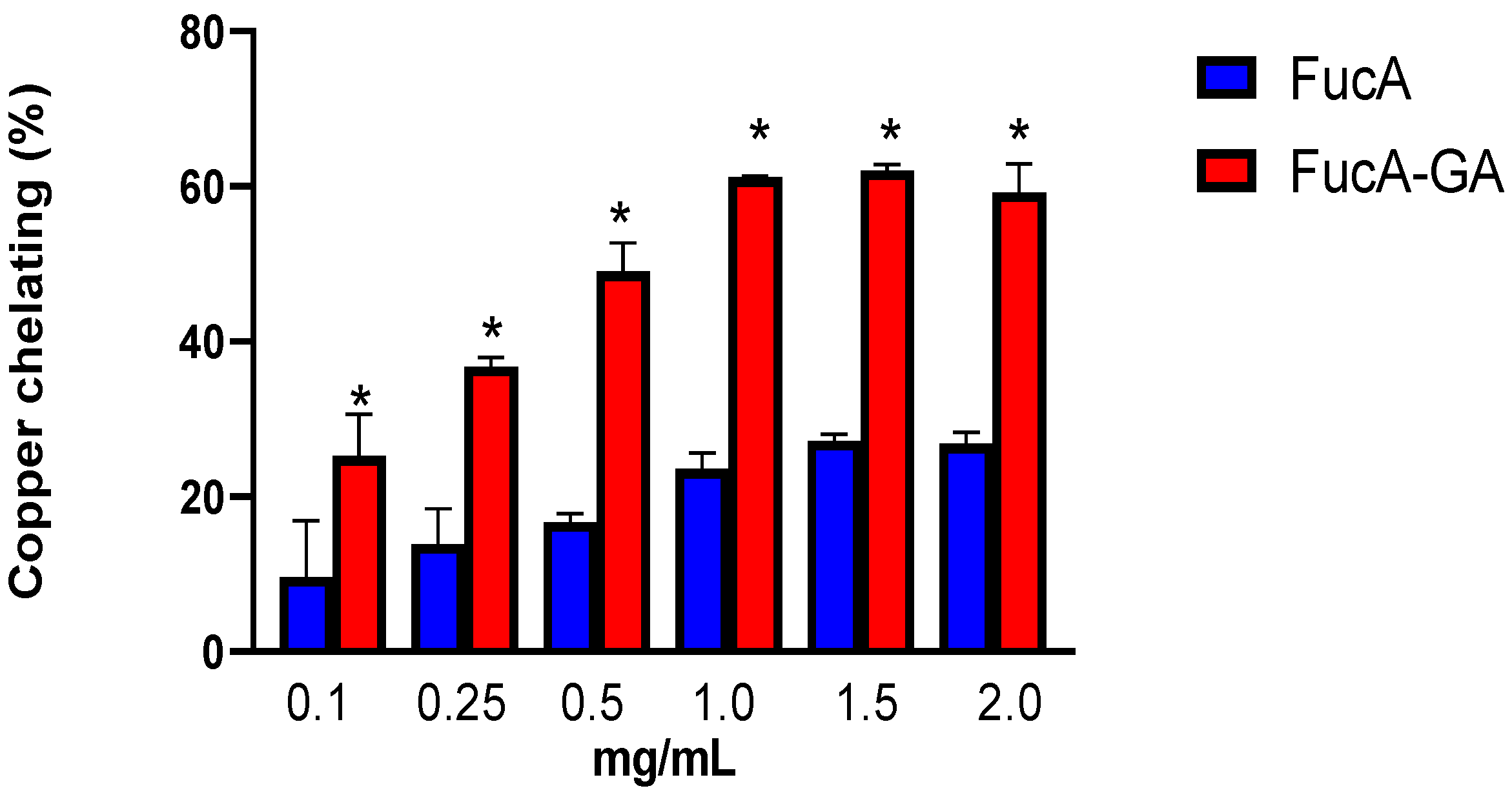
2.2.1. Copper and Ferrous Ions Chelating Ability
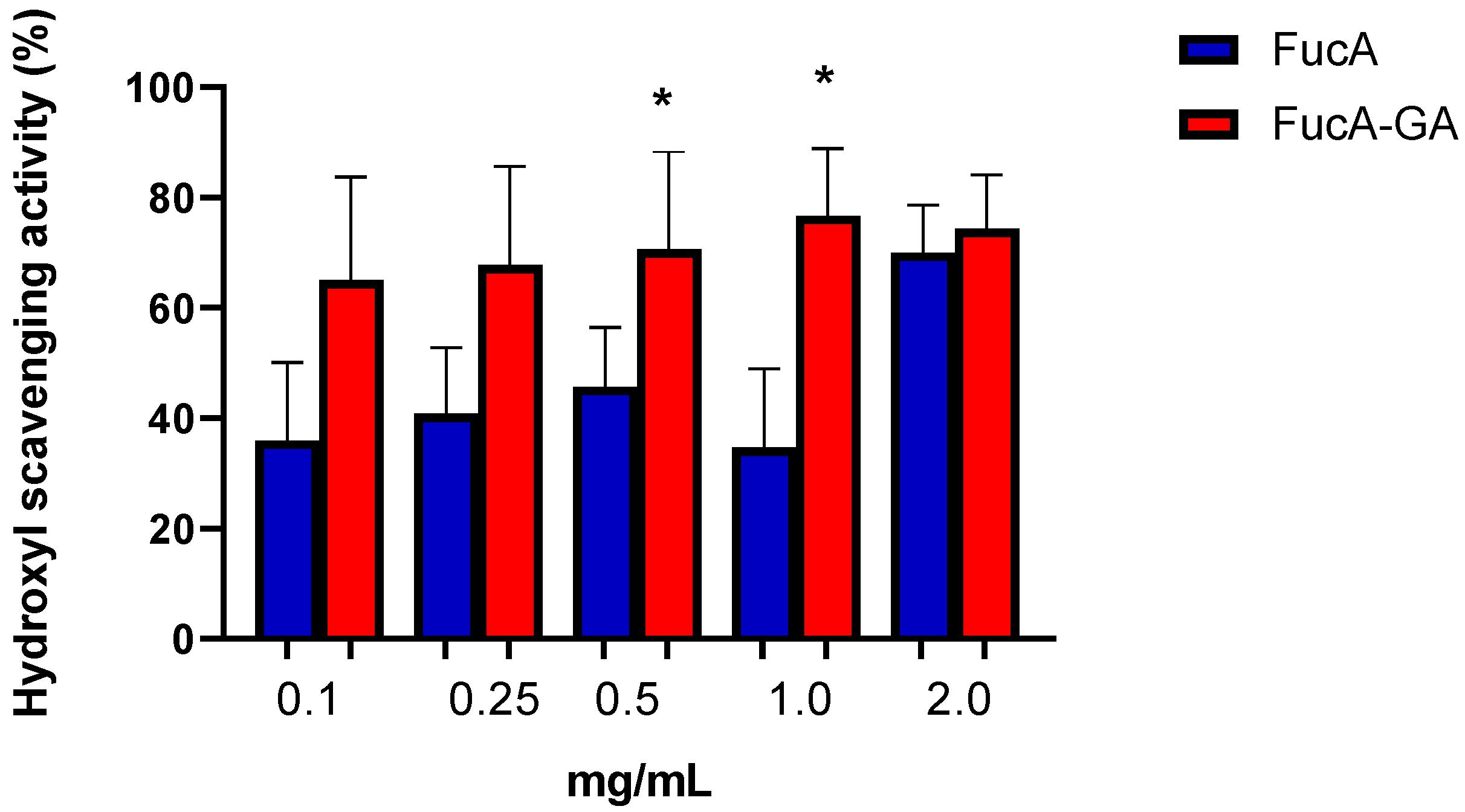
2.2.2. Evaluation of Hydroxyl (OH) Radical Scavenging
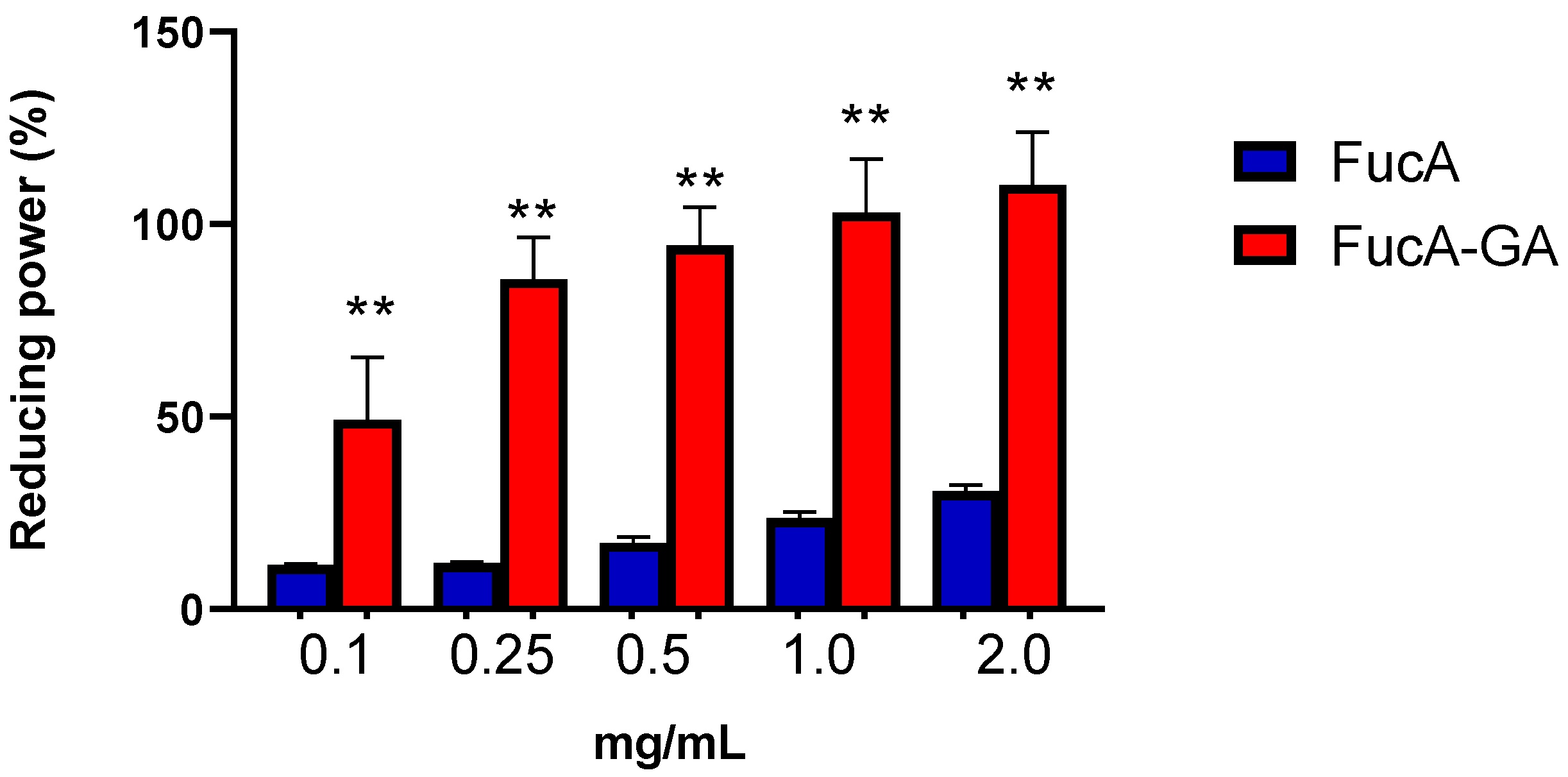
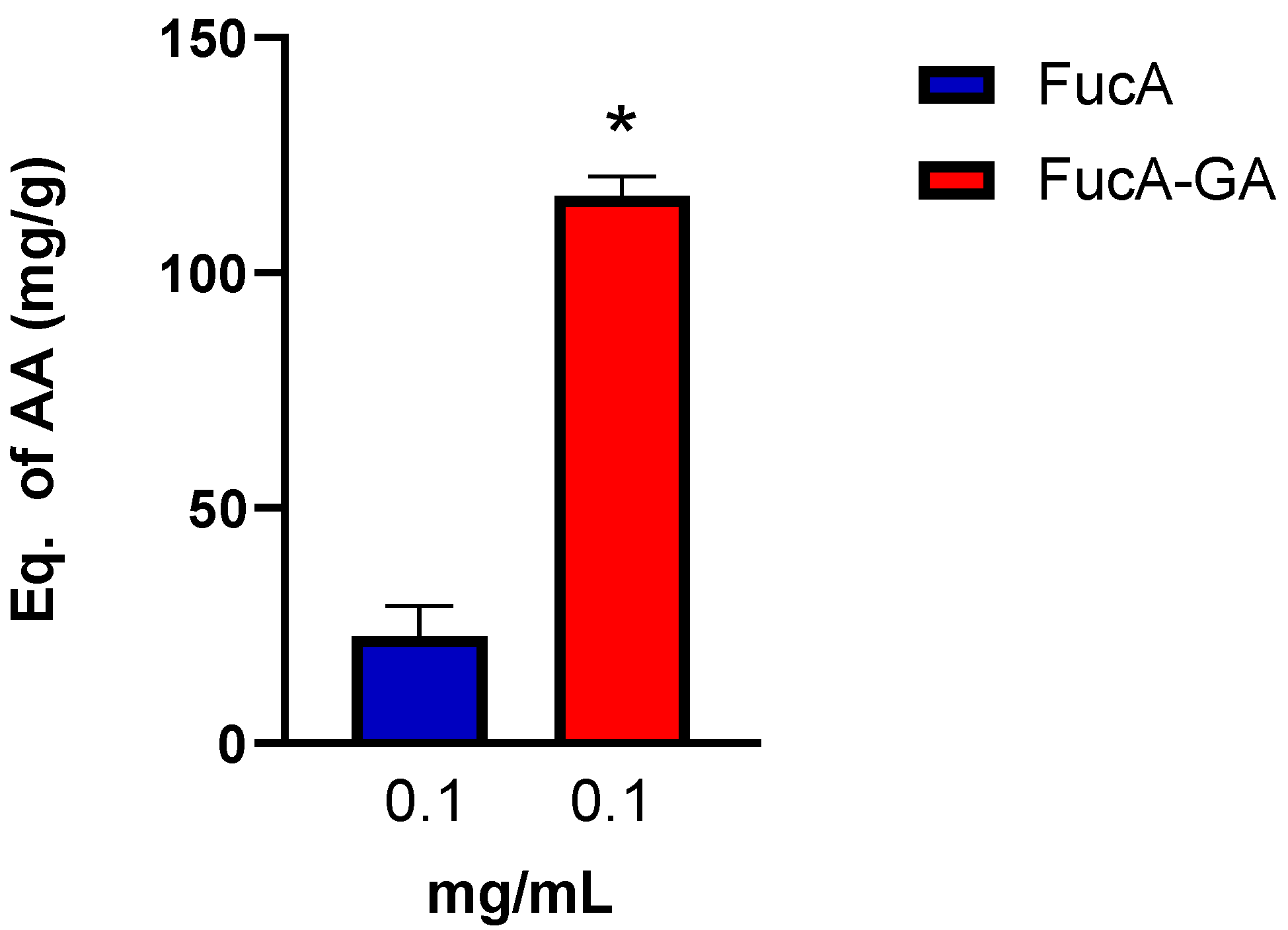
2.2.3. Assessment of Reducing Power and Determination of Total Antioxidant Capacity (TAC)
2.3. Effect of FucA and Fuc-GA on Pre-Osteoblastic Cells MC3T3
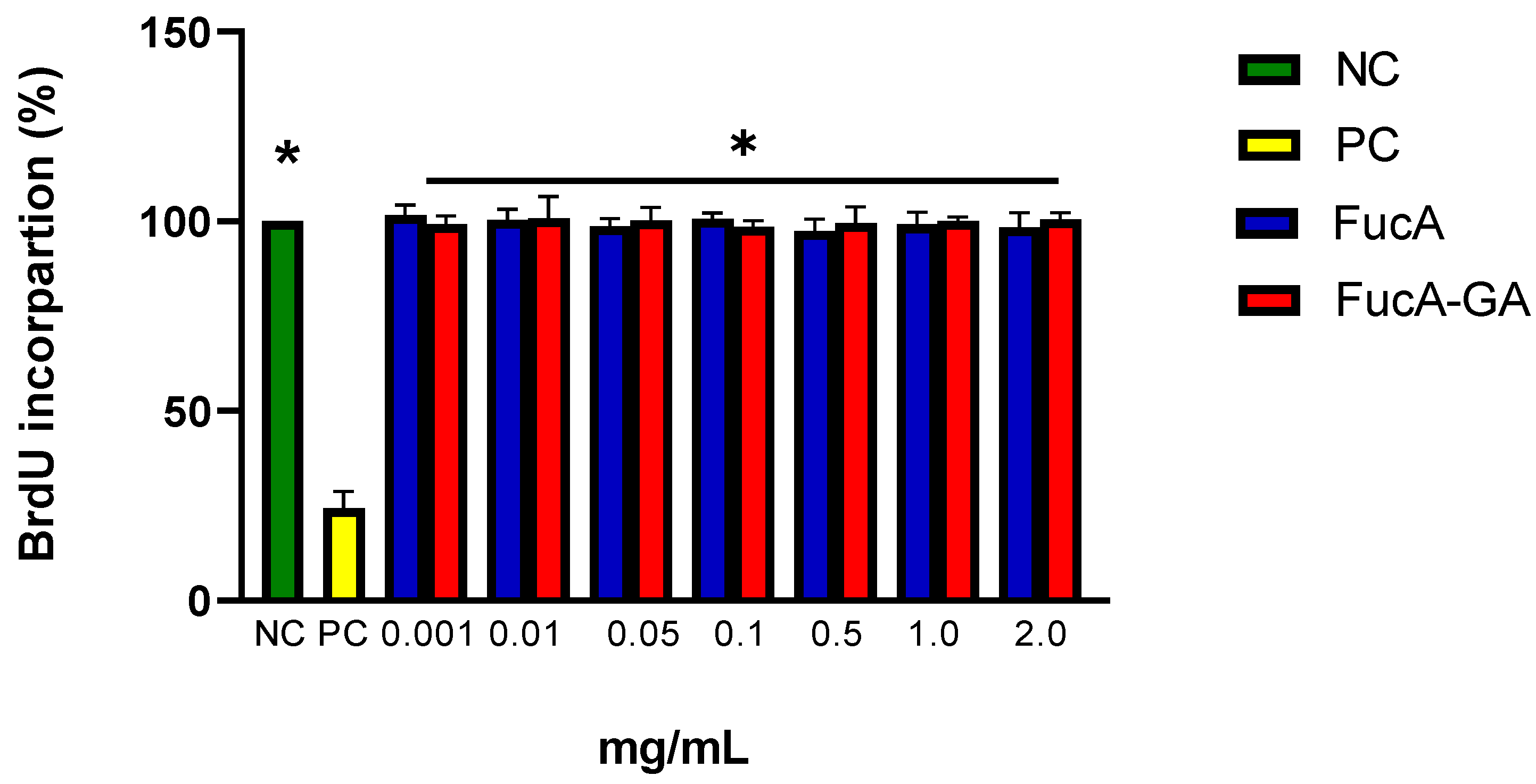
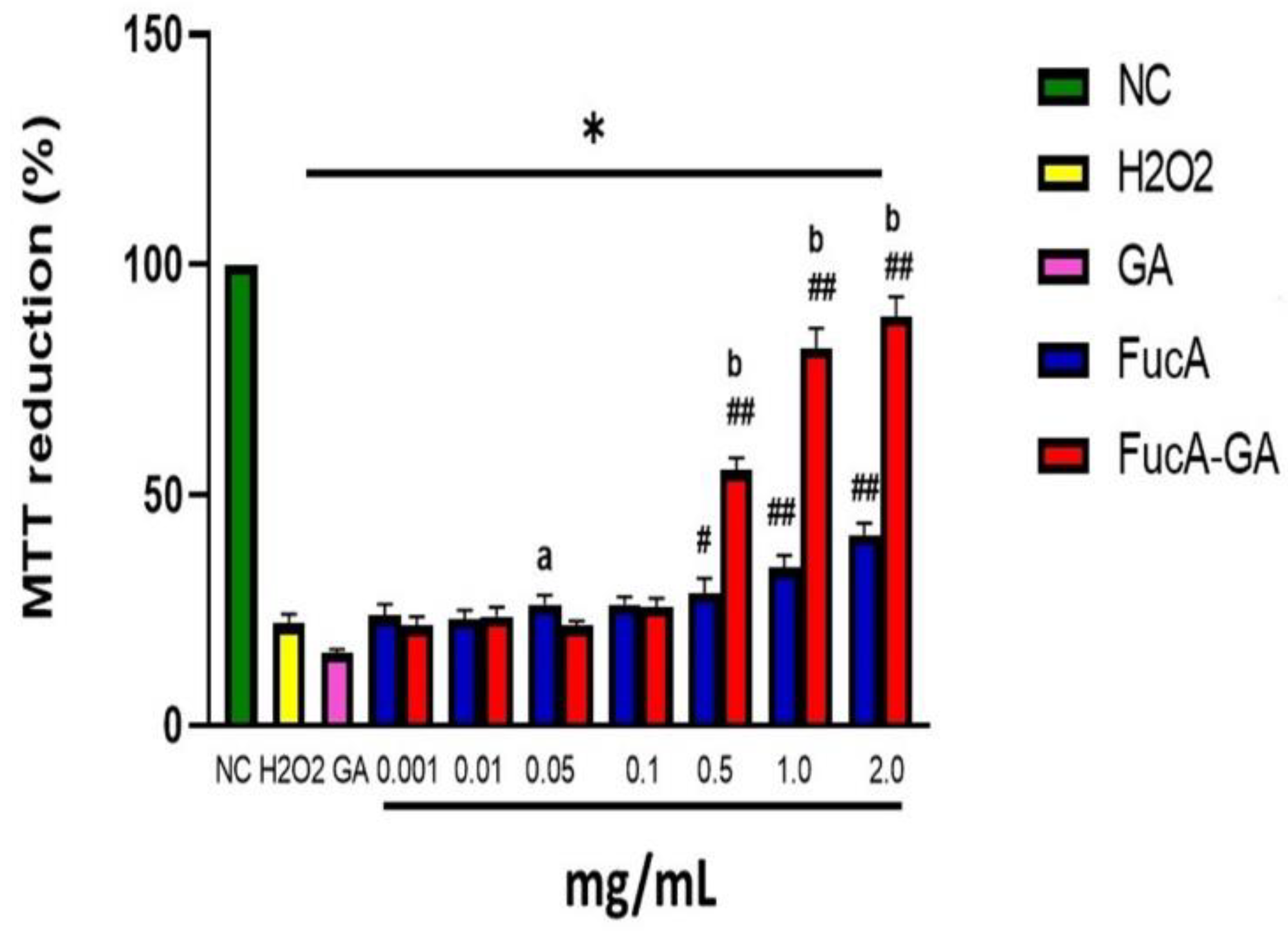
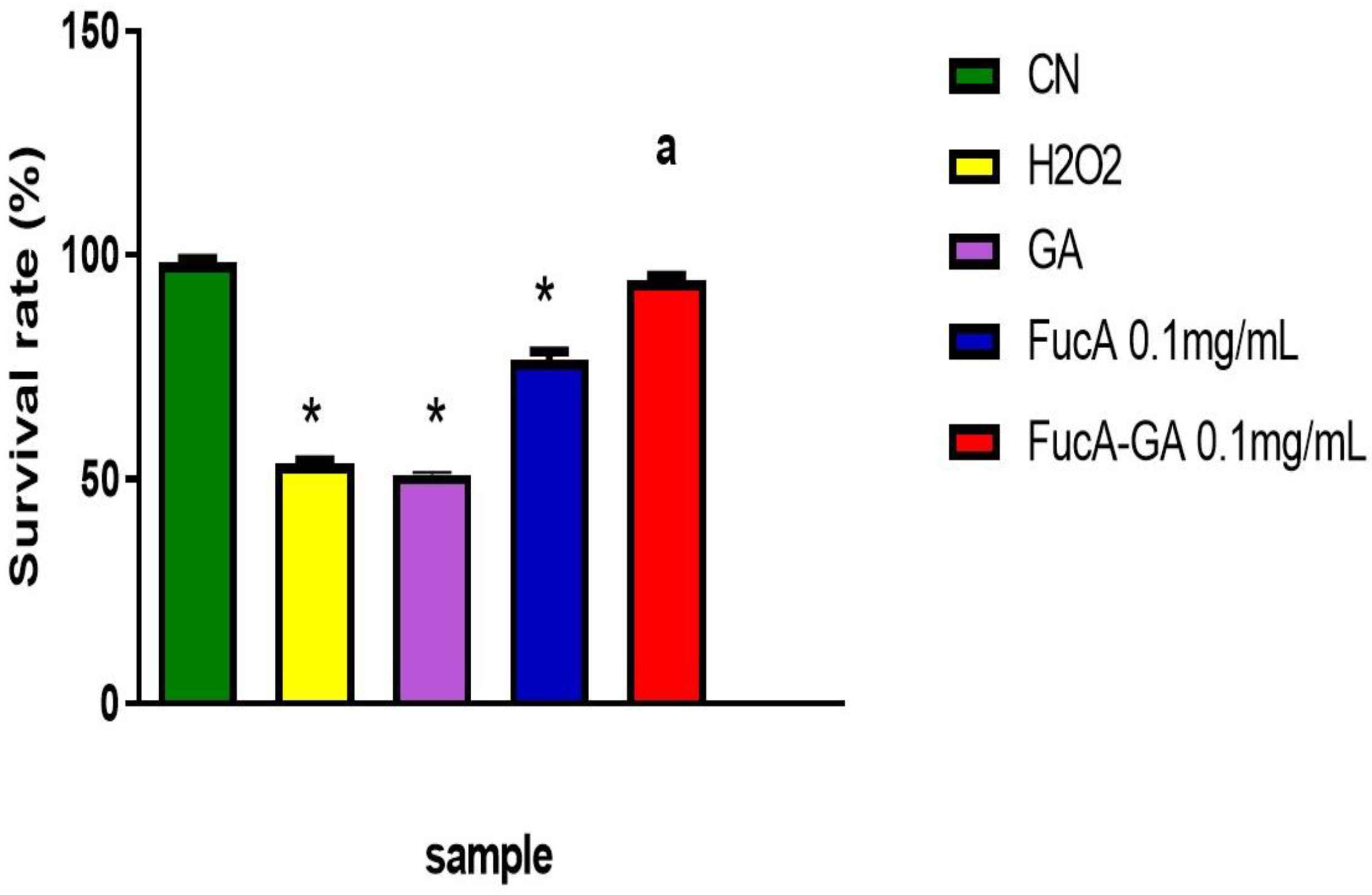
2.3.1. Cytotoxicity Assessment and Cell Death Induction by FucA and FucA-GA on MC3T3 Cells
2.3.2. Induced Oxidative Stress Assay with MC3T3 Cells
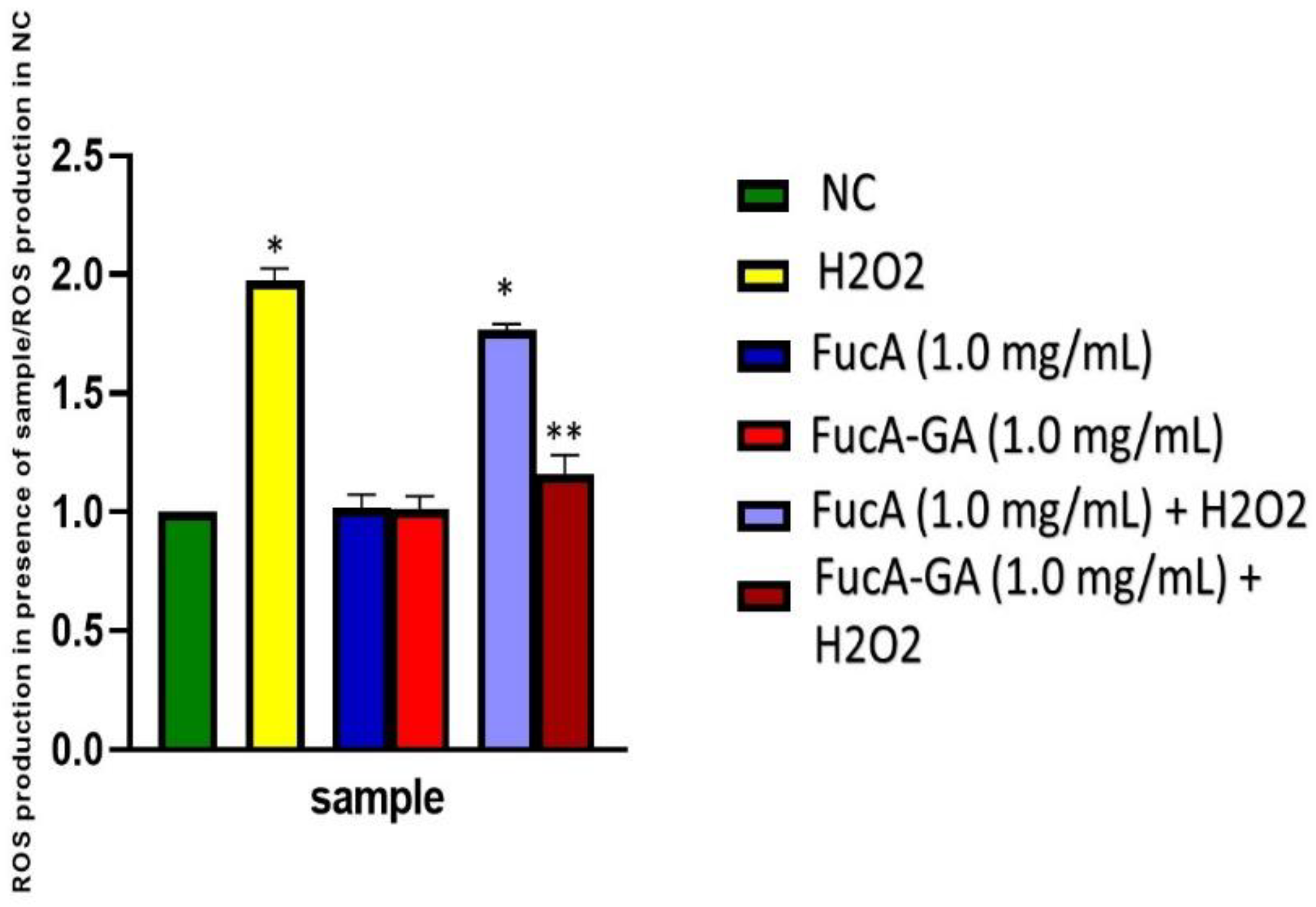
2.3.3. Intracellular Reactive Oxygen Species Production
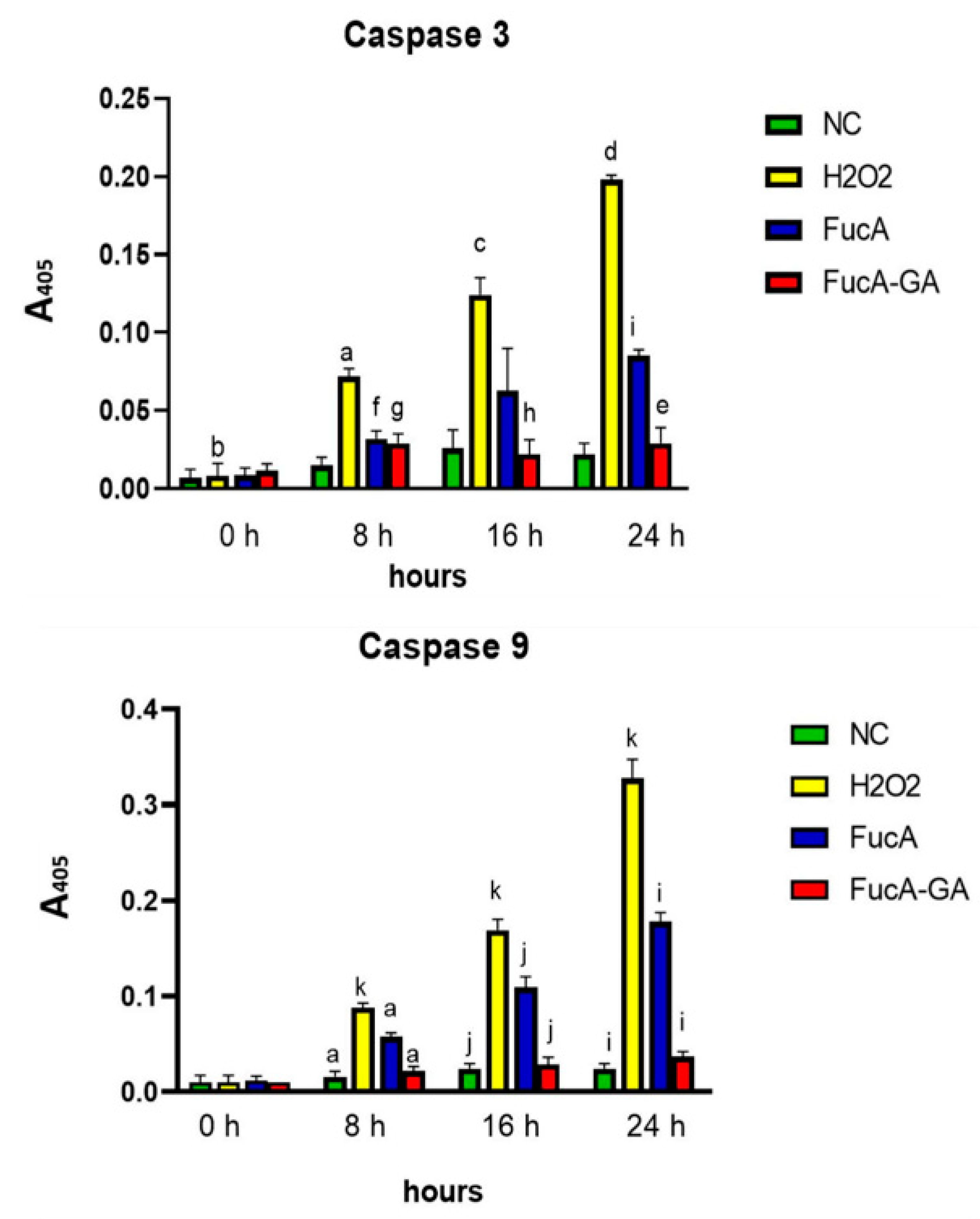
2.3.4. Effect of FucA and FucA-GA on Caspase-3 and Caspase-9 in MC3T3 Cells
2.4. In Vivo Experiments
3. Materials and Methods
3.1. Extraction of FucA
3.2. Conjugation of GA and FucA
3.3. Dosage of Total Phenolic Compounds, Total Sugar, Protein, and Detrmination of Monossacharide Composition and Molecular Weight
3.4. Assessment of Reducing Power
3.5. Evaluation of Hydroxyl (OH) Radical Scavenging
3.6. Ferrous Ion-Chelating Ability
3.7. Copper-Chelating Ability
3.8. Determination of Total Antioxidant Capacity
3.9. MTT Assay
3.10. Induced Oxidative Stress Assay
3.11. Analysis of Cell Proliferation with BrdU Incorporation
3.12. Intracellular ROS Production
3.13. Caspase-3 and -9 Activity Assays
3.14. Zebrafish Embryo Development
3.15. Embryo Death Analysis after H2O2-Induced Oxidative Stress
3.16. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luthuli, S.; Wu, S.; Cheng, Y.; Xiaoli, Z.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydr. Res. 2006, 341, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2018, 212, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Leite, E.L.; Medeiros, M.G.L.; Rocha, H.A.O.; Farias, G.G.M.; Silva, L.F.; Chavante, S.F. Structure of a new fucan from the algae Spatoglossum schröederi. Plant. Sci. 1998, 132, 215–228. [Google Scholar] [CrossRef]
- Almeida-Lima, J.; Costa, L.S.; Silva, N.B.; Silveira, R.F.M.; Silva, F.V.; Felipe, M.B.M.C. Evaluating the possible genotoxic, mutagenic and tumor cell proliferation-inhibition effects of a non-anticoagulant, but antithrombotic algal heterofucan. J. Appl. Toxicol. 2010, 30, 708–715. [Google Scholar] [CrossRef]
- Arokiarajan, M.S.; Thirunavukkarasu, R.; Joseph, J.; Ekaterina, O.; Aruni, W. Advance research in biomedical applications on marine sulfated polysaccharide. Int. J. Biol. Macromol. 2022, 1, 870–881. [Google Scholar] [CrossRef]
- Schaeffer, D.J.; Krylov, V.S. Anti-HIV activity of extracts and compounds from algae and cyanobacteria. Ecotoxicol. Environ. Saf. 2000, 45, 208–227. [Google Scholar] [CrossRef]
- Cao, L.M.; Sun, Z.X.; Makale, E.C.; Du, G.K.; Long, W.F.; Huang, H.R. Antitumor activity of fucoidan: A systematic review and meta-analysis. Transl. Cancer Res. 2021, 10, 5390–5405. [Google Scholar] [CrossRef]
- Grauffel, V.; Kloareg, B.; Mabeu, S.; Durand, P.; Jozefonvicz. New natural polysaccharides with potent antithrombotic activity: Fucans from brown algae. Biomaterials 1989, 10, 363–369. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Nayak, R.; Behera, C.; Dash, S.R.; Nayak, S.; Sahu, B.B.; Bhutia, S.K.; Jena, M. Multifunctional role of fucoidan, sulfated polysaccharides in human health and disease: A journey under the sea in pursuit of potent therapeutic agents. Int. J. Biol. Macromol. 2020, 1, 4263–4278. [Google Scholar] [CrossRef]
- Catarino, M.D.; Amarante, S.J.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Brown Algae Phlorotannins: A Marine Alternative to Break the Oxidative Stress, Inflammation and Cancer Network. Foods 2021, 6, 1478. [Google Scholar] [CrossRef]
- Ito, M.; Koba, K.; Hikihara, R.; Ishimaru, M.; Shibata, T.; Hatate, H.; Tanaka, R. Analysis of functional components and radical scavenging activity of 21 algae species collected from the Japanese coast. Food Chem. 2018, 6, 147–156. [Google Scholar] [CrossRef]
- Kim, S.K.; Himaya, S.W. Medicinal effects of phlorotannins from marine brown algae. Adv. Food Nutr. Res. 2011, 64, 97–109. [Google Scholar] [CrossRef]
- Kim, S.K.; Pangestuti, R. Biological activities and potential health benefits of fucoxanthin derived from marine brown algae. Adv. Food Nutr. Res. 2011, 64, 111–128. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 15, 687–704. [Google Scholar] [CrossRef]
- Athukorala, Y.; Ahn, G.N.; Jee, Y.-H.; Kim, G.-Y.; Kim, S.-H.; Ha, J.-H.; Kang, J.-S.; Lee, K.-W.; Jeon, Y.-J. Antiproliferative activity of sulfated polysaccharide isolated from an enzymatic digest of Ecklonia cava on the U-937 cell line. J. Appl. Phycol. 2009, 21, 307–314. [Google Scholar] [CrossRef]
- Kalita, P.; Ahmed, A.B.; Sen, S.; Chakraborty, R. A comprehensive review on polysaccharides with hypolipidemic activity: Occurrence, chemistry and molecular mechanism. Int. J. Biol. Macromol. 2022, 1, 681–698. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Han, X.; Ma, Y.; Zhang, Z.; Zhao, L.; Guan, F.; Ma, S. Fucoidan: A promising agent for brain injury and neurodegenerative disease intervention. Food Funct. 2021, 11, 3820–3830. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Lim, S.Y. Fucoidans and Bowel Health. Mar. Drugs 2021, 30, 436. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidatives stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Pitocco, D.; Zaccardi, F.; Di Stasio, E.; Romitelli, F.; Santini, S.A.; Zuppi, C.; Ghirlanda, G. Oxidative stress, nitric oxide, and diabetes. Rev. Diabet. Stud. 2010, 7, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Liu, Q.; Tjhioe, W.; Zhao, J.; Lu, A.; Zhang, G.; Tan, R.X.; Zhou, M.; Xu, J.; Feng, H.T. Therapeutic potential and outlook of alternative medicine for osteoporosis. Curr. Drug Targets 2017, 18, 1051–1068. [Google Scholar] [CrossRef]
- Cauley, J.A. Osteoporosis: Fracture epidemiology update 2016. Curr. Opin. Rheumatol. 2017, 29, 150–156. [Google Scholar] [CrossRef]
- Bădilă, A.E.; Rădulescu, D.M.; Ilie, A.; Niculescu, A.G.; Grumezescu, A.M.; Rădulescu, A.R. Bone Regeneration and Oxidative Stress: An Updated Overview. Antioxidants 2022, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Kimball, J.S.; Johnson, J.P.; Carlson, D.A. Oxidative Stress and Osteoporosis. J. Bone Joint Surg. Am. 2021, 4, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Vales, T.P.; Cho, B.K.; Kim, J.K.; Kim, H.J. Development of gallic acid-modified hydrogels using interpenetrating chitosan network and evaluation of their antioxidant activity. Molecules 2017, 22, 1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.S.; Yang, S.S.; You, H.K.; Shin, H.I.; Lee, J. Fucoidan-induced osteogenic differentiation promotes angiogenesis by inducing vascular endothelial growth factor secretion and accelerates bone repair. J. Tissue Eng. Regen. Med. 2018, 12, 1311–1324. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E.H.C.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Almeida-Lima, J.; Dantas-Santos, N.; Gomes, D.L.; Cordeiro, S.L.; Sabry, D.A.; Freitas, M.L.; Silva, N.B.; Moura, C.E.B.; Lemos, T.M.A.M.; Leite, E.L.; et al. Evaluation of acute and subchronic toxicity of a non-anticoagulant, but antithrombotic algal heterofucan from the Spatoglossum schröederi in Wistar rats. Rev. Bras. De Farmacogn. 2011, 21, 674–679. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Lu, Z.; Nie, G.; Belton, P.S.; Tang, H.; Zhao, B. Structure-activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super Sanita 2007, 43, 348–361. [Google Scholar] [CrossRef]
- Ji, H.F.; Zhang, H.Y.; Shen, L. Proton dissociation is important to understanding structure-activity relationships of gallic acid antioxidants. Bioorg. Med. Chem. Lett. 2006, 1, 4095–4098. [Google Scholar] [CrossRef]
- Cazzola, M.; Corazzari, I.; Prenesti, E.; Bertone, E.; Vernè, E.; Ferraris, S. Bioactive glass coupling with natural polyphenols: Surface modification, bioactivity and anti-oxidant ability. Appl. Surf. Sci. 2016, 367, 237–248. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 21, 7313–7352. [Google Scholar] [CrossRef]
- Bozic, M.; Gorgieva, S.; Kokol, V. Laccase-mediated functionalization of chitosanby caffeic and gallic acids for modulating antioxidant and antimicrobial properties. Carbohydr. Polym. 2012, 87, 2388–2398. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.F.; Kan, J.; Jin, C.H. Synthesis of chitosan-GA conjugate: Structure characterization and in vitro anti-diabetic potential. Int. J. Biol. Macromol. 2013, 62, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tian, J.; Li, S.; Wu, T.; Hu, Y.; Chen, S.; Sugawara, T.; Ye, X. Structural properties of films and rheology of film-forming solutions of chitosan gallate for food packaging. Carbohydr. Polym. 2016, 146, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Melo, K.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O.; Costa, L.S. GA-Chitosan Conjugate Inhibits the Formation of Calcium Oxalate Crystals. Molecules 2019, 24, 2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes-Negreiros, M.M.; Batista, L.A.N.C.; Viana, R.L.S.; Sabry, D.A.; Paiva, A.A.O.; Paiva, W.S.; Machado, R.I.A.; Sousa Junior, F.L.; Lima, D.L.; Vitoriano, J.O. Gallic acid-laminarin conjugate is a better antioxidant than sulfated or carboxylated laminarin. Antioxidants 2020, 9, 1192. [Google Scholar] [CrossRef]
- Paiva, W.S.; Queiroz, M.F.; Sabry, D.A.; Santiago, A.L.C.M.A.; Sassaki, G.L.; Batista, A.C.L.; Rocha, H.A.O. Preparation, structural characterization, and property investigation of GA-grafted fungal chitosan conjugate. J. Fungi 2021, 7, 812. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, S.K.; Ahn, C.B.; Je, J.Y. Preparation, characterization, and antioxidant properties of gallic acid-grafted-chitosans. Carbohydr. Polym. 2011, 83, 1617–1622. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O.; Costa, L.S. Gallic acid-dextran conjugate: Green synthesis of a novel antioxidant molecule. Antioxidants 2019, 8, 478. [Google Scholar] [CrossRef] [Green Version]
- Vittorio, O.; Cirillo, G.; Iemma, F.; Turi, G.D.; Jacchetti, E.; Curcio, M.; Barbuti, S.; Funel, N.; Parisi, O.I.; Puoci, F.; et al. Dextran-Catechin Conjugate: A potential treatment against the pancreatic ductal adenocarcinoma. Pharm. Rev. 2012, 29, 2601–2614. [Google Scholar] [CrossRef]
- Apak, R. Current issues in antioxidant measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Yang, D.P.; Tang, G.Y. Multipotent antioxidants: From screening to design. Drug Discov. Today 2006, 11, 749–754. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D.; Tsai, H.L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Pirker, K.F.; Baratto, M.C.; Basosi, R.; Goodman, B.A. Influence of pH on the speciation of copper (II) in reactions with the green tea polyphenols, epigallocatechin gallate and gallic acid. J. Inorg. Biochem. 2012, 112, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W.L. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar] [CrossRef]
- Melo, K.R.; Camara, R.B.; Queiroz, M.F.; Vidal, A.A.; Lima, C.R.; Melo-Silveira, R.F.; Almeida-Lima, J.; Rocha, H.A. Evaluation of sulfated polysaccharides from the brown seaweed Dictyopteris justii as antioxidant agents and as inhibitors of the formation of calcium oxalate crystals. Molecules 2013, 25, 14543–14563. [Google Scholar] [CrossRef] [Green Version]
- Fidelis, G.P.; Silva, C.H.F.; Nobre, L.T.D.B.; Medeiros, V.P.; Rocha, H.A.O.; Costa, L.S. Antioxidant fucoidans obtained from tropical seaweed protect pre-osteoblastic cells from hydrogen peroxide-induced damage. Mar. Drugs 2019, 28, 506. [Google Scholar] [CrossRef] [Green Version]
- Gromadzka, G.; Tarnacka, B.; Flaga, A.; Adamczyk, A. Copper Dyshomeostasis in Neurodegenerative Diseases-Therapeutic Implications. Int. J. Mol. Sci. 2020, 4, 9259. [Google Scholar] [CrossRef]
- Møller, M.; Lenartowicz, M.-T.; Zabot, A.; Josiane, L.; Burglen, C. Clinical expression of Menkes disease in females with normal karyo type Orphanet. J. Rare Dis. 2012, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Shribman, S.; Marjot, T.; Sharif, A.; Vimalesvaran, S.; Ala, A.; Alexander, G.; Dhawan, A.; Dooley, J.; Gillett, G.T.; Kelly, D.; et al. Investigation and management of Wilson’s disease: A practical guide from the British Association for the Study of the Liver. Lancet Gastroenterol. Hepatol. 2022, 7, 560–575. [Google Scholar] [CrossRef]
- Brewer, G.J. Novel therapeutic approaches to the treatment of Wilson’s disease. Expert Opin. Pharmocother. 2006, 7, 317–324. [Google Scholar] [CrossRef]
- Yu, J.; Li, Q.; Wu, J.; Yang, X.; Yang, S.; Zhu, W.; Liu, Y.; Tang, W.; Nie, S.; Hassouna, A.; et al. Fucoidan extracted from sporophyll of Undaria pinnatifida grown in Weihai, China-Chemical composition and comparison of antioxidant activity of different molecular weight fractions. Front. Nutr. 2021, 8, 636930. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. Amst. 2019, 24, 370. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, J.; Jin, W.; Zhang, H.; Zhang, Q. Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydr. Polym. 2012, 87, 87153–87159. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X. A critical review of the abilities, determinants, and possible molecular mechanisms of seaweed polysaccharides antioxidants. Int. J. Mol. Sci. 2020, 21, 7774. [Google Scholar] [CrossRef]
- Wrigth, J.S.; Jonhson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef]
- Curcio, M.; Puoci, F.; Iemma, F.; Parisi, O.I.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Covalent insertion of antioxidant molecules on chitosan by a free radical grafting procedure. J. Agric. Food Chem. 2009, 57, 5933–5938. [Google Scholar] [CrossRef]
- Downey, P.A.; Siegel, M.I. Bone biology and the clinical implications for osteoporosis. Phys. Ther. 2006, 86, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and anti-inflammatory effects of fucoidan: A review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Sumantran, V.N. Cellular chemosensitivity assays: An overview. Methods Mol. Biol. 2011, 731, 219–236. [Google Scholar] [CrossRef]
- Vega-Avila, E.; Pugsley, M.K. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc. West Pharmacol. Soc. 2011, 54, 10–14. [Google Scholar]
- Sakagami, H.; Satoh, K. Prooxidant action of two antioxidants: Ascorbic acid and gallic acid. Anticancer Res. 1997, 17, 221–224. [Google Scholar]
- Liao, C.Y.; Wu, T.C.; Yang, S.F.; Chang, J.T. Effects of NAC and Gallic Acid on the Proliferation Inhibition and Induced Death of Lung Cancer Cells with Different Antioxidant Capacities. Molecules 2021, 27, 75. [Google Scholar] [CrossRef] [PubMed]
- Lima, K.G.; Krause, G.C.; Schuster, A.D.; Catarina, A.V.; Basso, B.S.; De Mesquita, F.C.; Pedrazza, L.; Marczak, E.S.; Martha, B.A.; Nunes, F.B.; et al. Gallic acid reduces cell growth by induction of apoptosis and reduction of IL-8 in HepG2 cells. Biomed. Pharmacother. 2016, 84, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Sohi, K.K.; Mittal, N.; Hundal, M.K.; Khanduja, K.L. Gallic acid, an antioxidant, exhibits antiapoptotic potential in normal human lymphocytes: A Bcl-2 independent mechanism. J. Nutr. Sci. Vitaminol. 2003, 49, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.H.; Liu, T.Z.; Chen, C.H.; Wong, C.H.; Chen, C.H.; Lu, F.J.; Chen, S.C. The efficacy of protective effects of tannic acid, gallic acid, ellagic acid, and propyl gallate against hydrogen peroxide-induced oxidative stress and DNA damages in IMR-90 cells. Mol. Nutr. Food Res. 2007, 51, 962–968. [Google Scholar] [CrossRef]
- Erol-Dayi, Ö.; Arda, N.; Erdem, G. Protective effects of olive oil phenolics and gallic acid on hydrogen peroxide-induced apoptosis. Eur. J. Nutr. 2012, 51, 955–960. [Google Scholar] [CrossRef]
- Kang, M.K.; Kang, N.J.; Jang, Y.J.; Lee, K.W.; Lee, H.J. Gallic acid induces neuronal cell death through activation of c-Jun N-terminal kinase and downregulation of Bcl-2. Ann. N. Y. Acad. Sci. 2009, 1171, 514–520. [Google Scholar] [CrossRef]
- Arteaga, C.; Boix, N.; Teixido, E.; Marizande, F.; Cadena, S.; Bustillos, A. The zebrafish embryo as a model to test protective effects of food antioxidant compounds. Molecules 2021, 24, 5786. [Google Scholar] [CrossRef]
- Singulani, J.L.; Scorzoni, L.; Gomes, P.C.; Nazaré, A.C.; Polaquini, C.R.; Regasini, L.O.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Activity of gallic acid and its ester derivatives in Caenorhabditis elegans and zebrafish (Danio rerio) models. Future Med. Chem. 2017, 9, 1863–1872. [Google Scholar] [CrossRef]
- Jeong, J.W.; Hwang, S.J.; Han, M.H.; Lee, D.-S.; Yoo, J.S.; Choi, I.-W.; Cha, H.J.; Kim, S.; Kim, H.-S.; Kim, G.-Y.; et al. Fucoidan inhibits lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages and zebrafish larvae. Mol. Cell. Toxicol. 2017, 13, 405–417. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Wang, L.; Asanka Sanjeewa, K.K.; In Kang, S.; Lee, J.S.; Jeon, Y.J. Antioxidant potential of sulfated polysaccharides from Padina boryana; protective effect against oxidative stress in in vitro and in vivo zebrafish model. Mar. Drugs 2020, 18, 212. [Google Scholar] [CrossRef] [Green Version]
- Wynne, M.J. A checklist of benthic marine algae of the tropical and subtropical western Atlantic. Can. J. Bot. 1986, 64, 2239–2281. [Google Scholar] [CrossRef]
- Rodrigues-Souza, I.; Pessatti, J.B.K.; da Silva, L.R.; de Lima Bellan, D.; de Souza, I.R.; Cestari, M.M.; de Assis, H.C.S.; Rocha, H.A.O.; Simas, F.F.; da Silva Trindade, E.; et al. Protective potential of sulfated polysaccharides from tropical seaweeds against alkylating- and oxidizing-induced genotoxicity. Int. J. Biol. Macromol. 2022, 30, 524–534. [Google Scholar] [CrossRef]
- Nascimento, A.K.L.; Melo-Silveira, R.F.; Dantas-Santos, N.; Fernandes, J.M.; Zucolotto, S.M.; Rocha, H.A.O.; Scortecci, K.C. Antioxidant and Antiproliferative Activities of Leaf Extracts from Plukenetia volubilis Linneo (Euphorbiaceae). Evid. Based Complement Altern. Med. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209. [Google Scholar] [CrossRef]
- Camara, R.B.G.; Costa, L.S.; Fidelis, G.P.; Nobre, L.T.D.B.; Dantas-Santos, N.; Cordeiro, S.L.; Costa, M.S.S.P.; Alves, L.G.; Rocha, H.A.O. Heterofucans from the Brown Seaweed Canistrocarpus cervicornis with Anticoagulant and Antioxidant Activities. Mar. Drugs 2011, 9, 124–138. [Google Scholar] [CrossRef] [Green Version]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Amorim, M.O.R.; Gomes, D.L.; Dantas, L.A.; Viana, R.L.S.; Chiquetti, S.C.; Almeida-Lima, J.; Costa, L.S.; Rocha, H.A.O. Fucan-coated silver nanoparticles synthesized by a green method induce human renal adenocarcinoma cell death. Int. J. Biol. Macromol. 2016, 93, 57–65. [Google Scholar] [CrossRef]
- Presa, F.B.; Marques, M.L.M.; Viana, R.L.S.; Nobre, L.T.D.B.; Costa, L.S.; Rocha, H.A.O. The Protective Role of Sulfated Polysaccharides from Green Seaweed Udotea flabellum in Cells Exposed to Oxidative Damage. Mar. Drugs 2018, 16, 135. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.A.; Seung-Hong, L.; Chang-IK, K.; Seon-Heui, C.; Min-Cheol, K.; Sung-Myung, K.; Seok-Chun, K.; Won-Woo, L.; Ju-Young, K.; Ji-Hyeok, L.; et al. Protective effect of fucoidan against AAPH-induced oxidative stressin zebrafish model. Carbohydr. Polym. 2014, 102, 185–191. [Google Scholar] [CrossRef]
- Kim, B.S.; Kang, H.J.; Park, J.Y.; Lee, J. Fucoidan promotes osteoblast differentiation via JNK-and ERK-dependent BMP2–Smad 1/5/8 signaling in human mesenchymal stem cells. Exp. Mol. Med. 2015, 47, 128. [Google Scholar] [CrossRef]
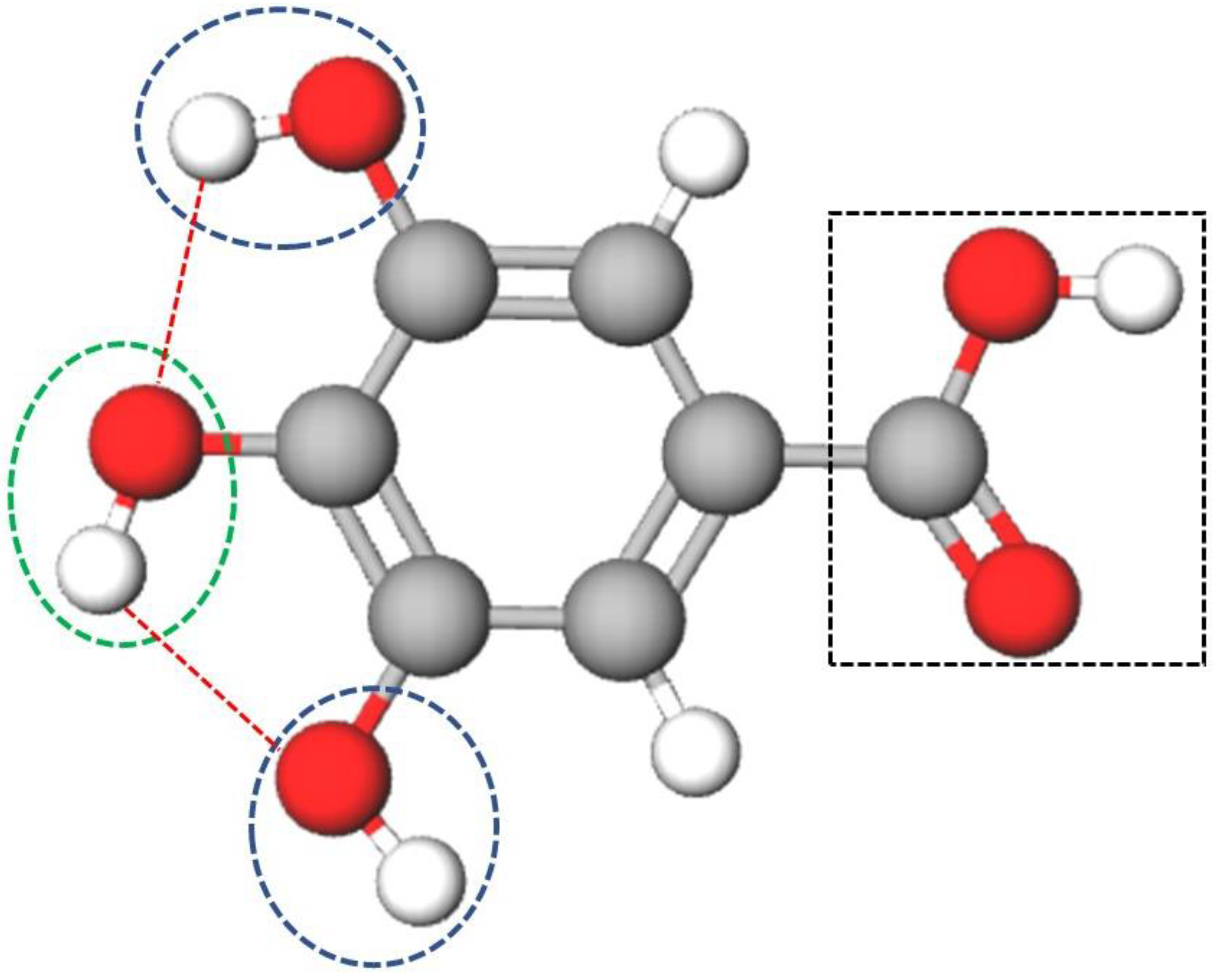
| Samples | Sugar% | Phenolic Compounds % | MW kDa | Molar Ratio | ||||
|---|---|---|---|---|---|---|---|---|
| Fuc | Xil | GlucA | Gal | Sulfate | ||||
| FucA | 77 ± 2 | nd | 21.0 | 1 | 0.32 | 0.60 | nd | 1.52 |
| FucA-GA | 70 ± 3 * | 3.7 ± 0.3 ** | 20.5 | 1 | 0.33 | 0.55 | nd | 1.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Melo, K.D.C.M.; Lisboa, L.d.S.; Queiroz, M.F.; Paiva, W.S.; Luchiari, A.C.; Camara, R.B.G.; Costa, L.S.; Rocha, H.A.O. Antioxidant Activity of Fucoidan Modified with Gallic Acid Using the Redox Method. Mar. Drugs 2022, 20, 490. https://doi.org/10.3390/md20080490
de Melo KDCM, Lisboa LdS, Queiroz MF, Paiva WS, Luchiari AC, Camara RBG, Costa LS, Rocha HAO. Antioxidant Activity of Fucoidan Modified with Gallic Acid Using the Redox Method. Marine Drugs. 2022; 20(8):490. https://doi.org/10.3390/md20080490
Chicago/Turabian Stylede Melo, Keylla Dayanne Coelho Marinho, Lucas dos Santos Lisboa, Moacir Fernandes Queiroz, Weslley Souza Paiva, Ana Carolina Luchiari, Rafael Barros Gomes Camara, Leandro Silva Costa, and Hugo Alexandre Oliveira Rocha. 2022. "Antioxidant Activity of Fucoidan Modified with Gallic Acid Using the Redox Method" Marine Drugs 20, no. 8: 490. https://doi.org/10.3390/md20080490
APA Stylede Melo, K. D. C. M., Lisboa, L. d. S., Queiroz, M. F., Paiva, W. S., Luchiari, A. C., Camara, R. B. G., Costa, L. S., & Rocha, H. A. O. (2022). Antioxidant Activity of Fucoidan Modified with Gallic Acid Using the Redox Method. Marine Drugs, 20(8), 490. https://doi.org/10.3390/md20080490







