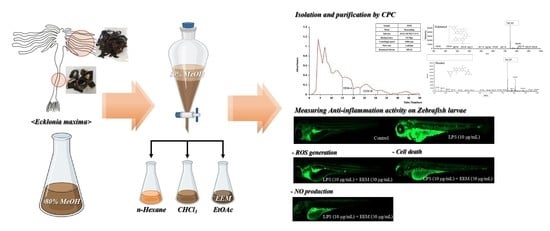
Isolation and Characterization of Efficient Active Compounds Using High-Performance Centrifugal Partition Chromatography (CPC) from Anti-Inflammatory Activity Fraction of Ecklonia maxima in South Africa
Abstract
:1. Introduction
2. Results
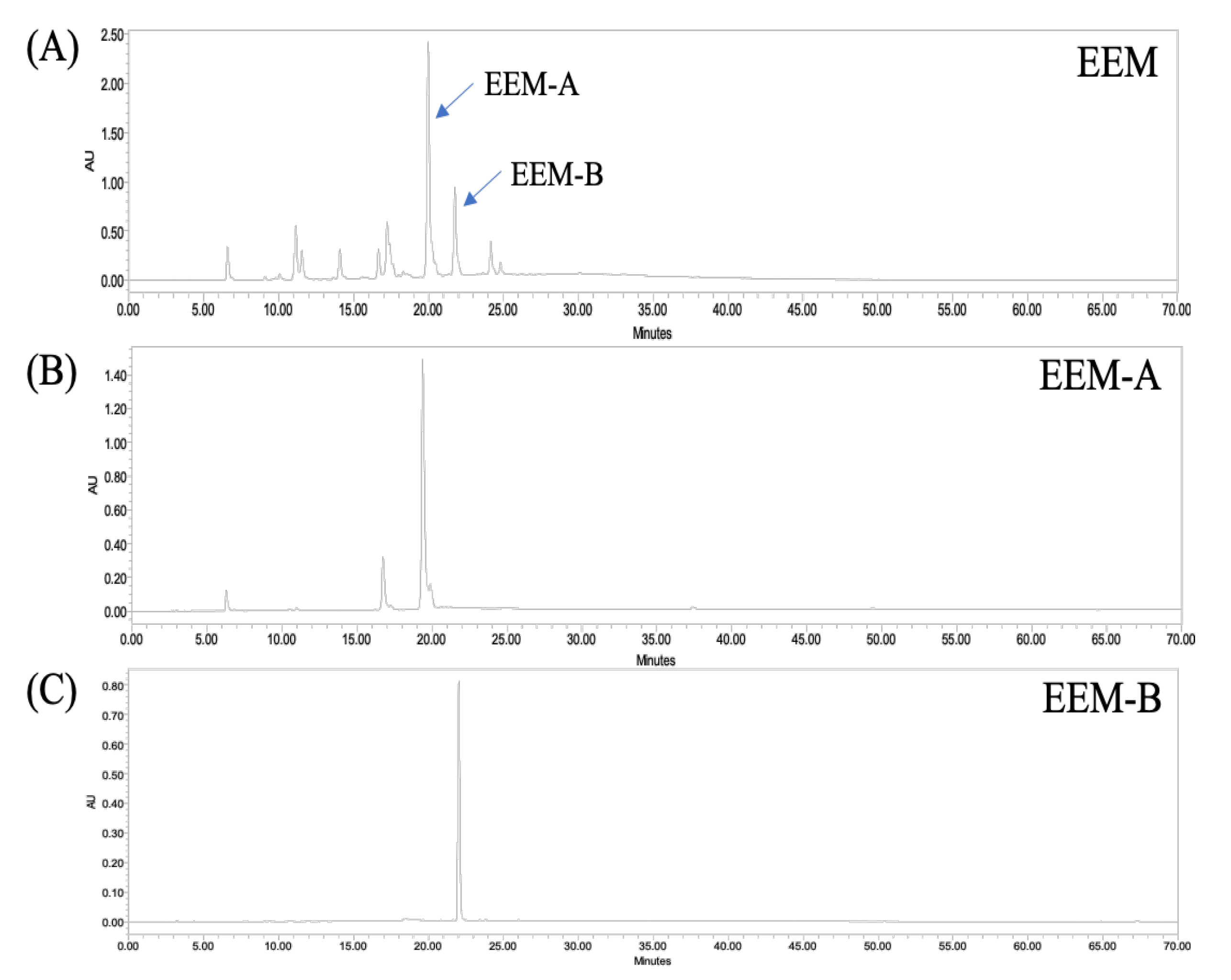
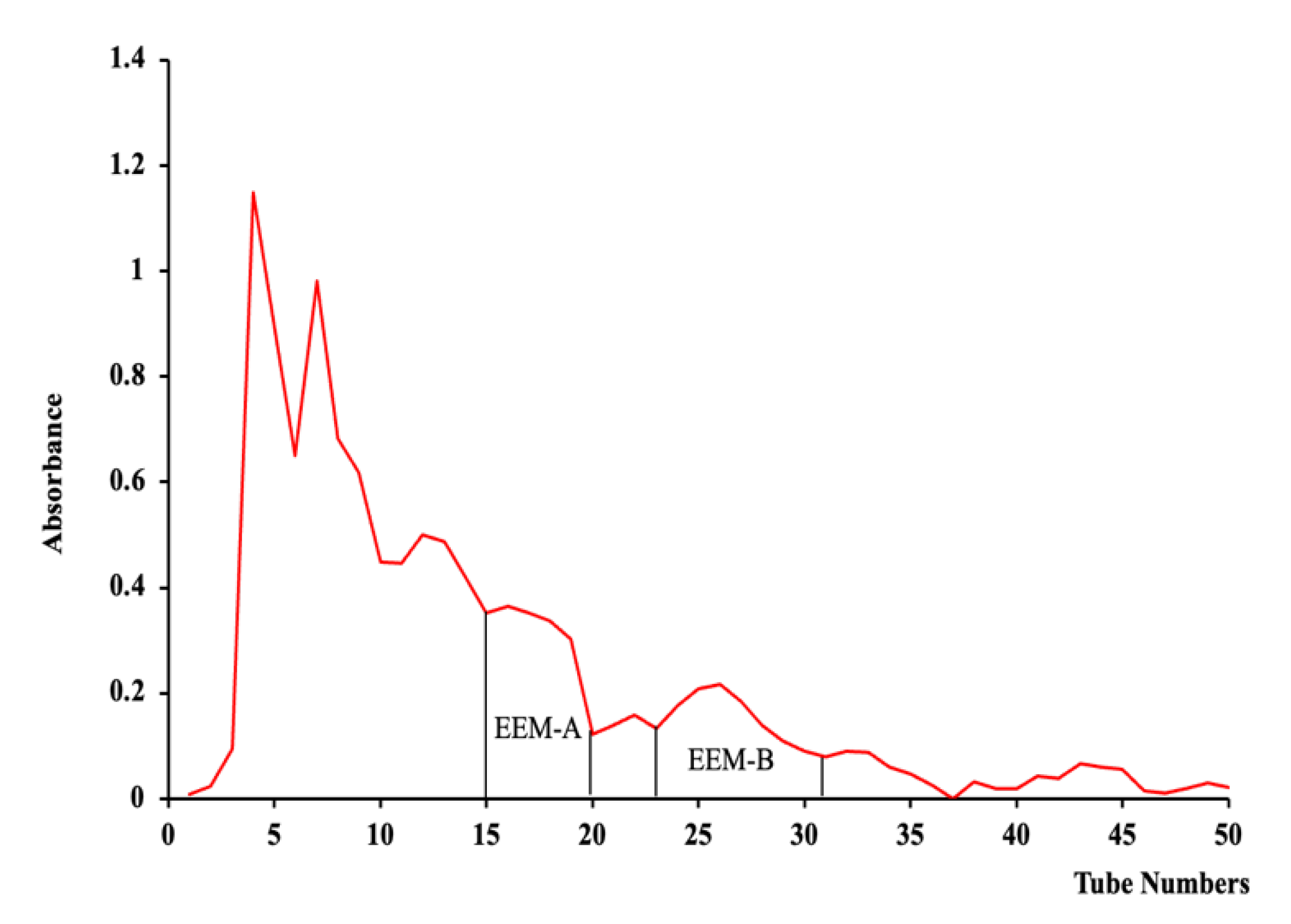
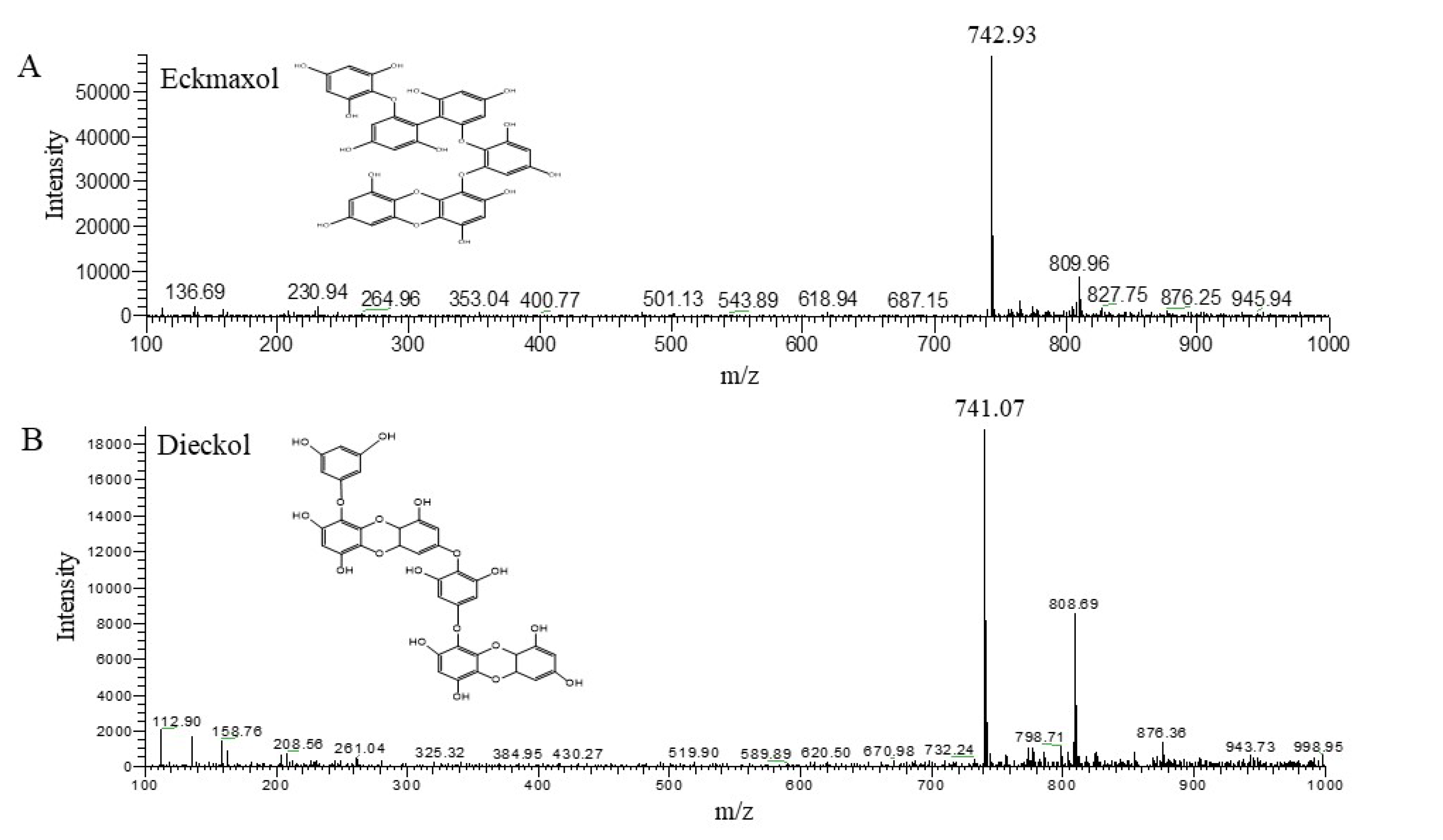
2.1. Isolation and Characterization of Phlorotannins from EEM
2.2. Protective Effect of EEM on Heartbeat Rates and Survival Rates in LPS-Induced Zebrafish Embryos
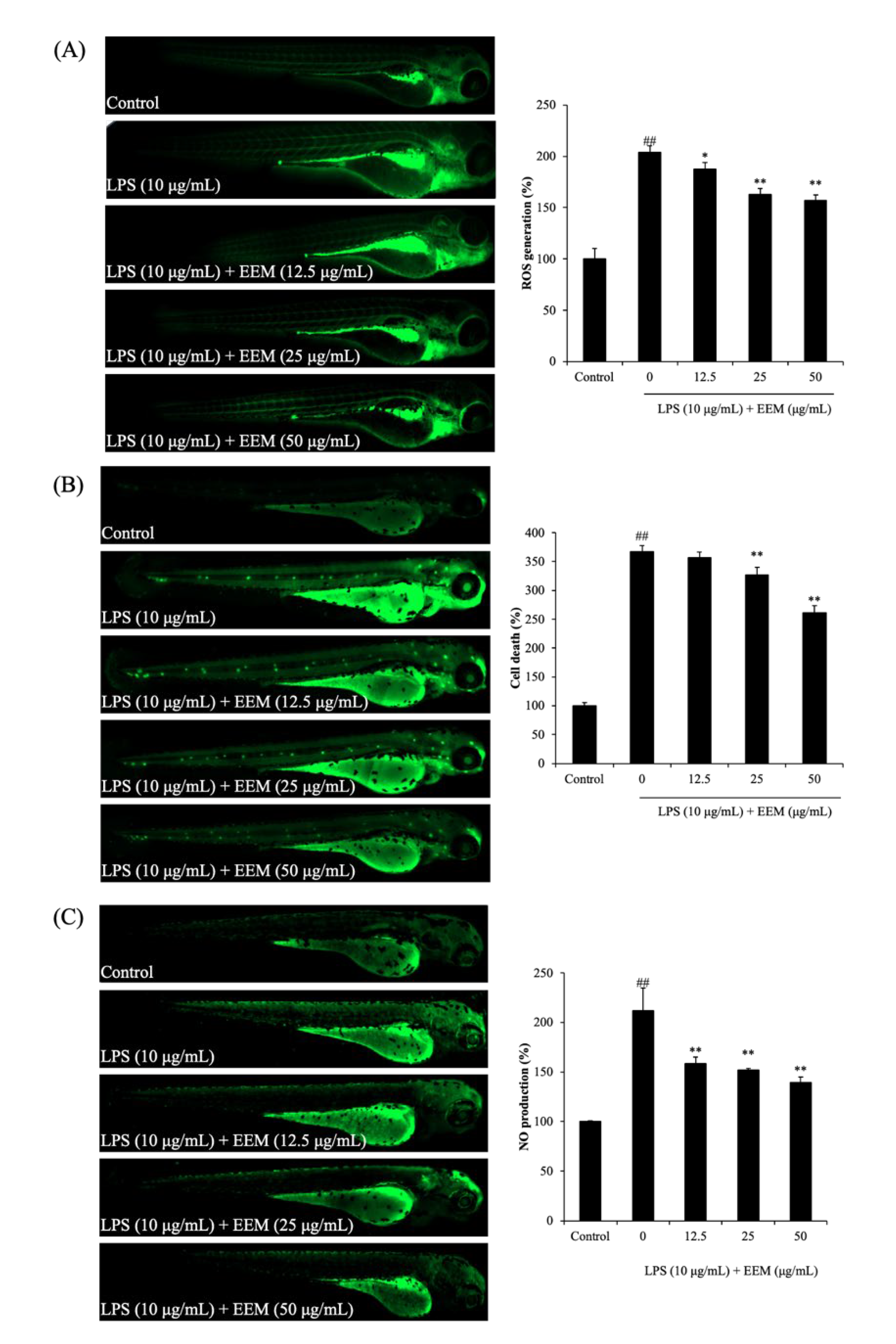
2.3. Inhibitory Effect of EEM on LPS-Induced ROS Generation, NO Production, and Cell Death
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Ethyl Acetate Fraction of E. maxima (EEM) and Isolation of Phlorotannins
4.3. HPLC-PDA-ESI/MS Analysis of Phlorotannins
4.4. In Vivo Zebrafish Embryo Model
4.4.1. Application of EEM and LPS to Zebrafish Embryos
4.4.2. Measurement of Heartbeat and Survival Rate
4.4.3. Measurement of ROS Generation, NO Production, and Cell Death via Image Analysis in Zebrafish Embryos
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, M.-C.; Wijesinghe, W.; Lee, S.-H.; Kang, S.-M.; Ko, S.-C.; Yang, X.; Kang, N.; Jeon, B.-T.; Kim, J.; Lee, D.-H. Dieckol isolated from brown seaweed Ecklonia cava attenuates type II diabetes in db/db mouse model. Food Chem. Toxicol. 2013, 53, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, E.-A.; Ahn, G.; Jee, Y.; Jeon, Y.-J. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells. Nutr. Res. Pract. 2017, 11, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjeewa, K.A.; Lee, J.-S.; Kim, W.-S.; Jeon, Y.-J. The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Shanura Fernando, I.; Asanka Sanjeewa, K.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Ranasinghe, P.; Gunasekara, U.; Jeon, Y.-J. Antioxidant and anti-inflammatory functionality of ten Sri Lankan seaweed extracts obtained by carbohydrase assisted extraction. Food Sci. Biotechnol. 2018, 27, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.-N.; Kim, K.-N.; Cha, S.-H.; Song, C.-B.; Lee, J.; Heo, M.-S.; Yeo, I.-K.; Lee, N.-H.; Jee, Y.-H.; Kim, J.-S. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Kim, E.-K.; Tang, Y.; Kim, Y.-S.; Hwang, J.-W.; Choi, E.-J.; Lee, J.-H.; Lee, S.-H.; Jeon, Y.-J.; Park, P.-J. First evidence that Ecklonia cava-derived dieckol attenuates MCF-7 human breast carcinoma cell migration. Marine Drugs 2015, 13, 1785–1797. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.-C.; Ahn, G.; Yang, X.; Kim, K.-N.; Kang, S.-M.; Lee, S.-H.; Ko, S.-C.; Ko, J.-Y.; Kim, D.; Kim, Y.-T. Hepatoprotective effects of dieckol-rich phlorotannins from Ecklonia cava, a brown seaweed, against ethanol induced liver damage in BALB/c mice. Food Chem. Toxicol. 2012, 50, 1986–1991. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, E.-A.; Kang, M.-C.; Lee, J.-H.; Yang, H.-W.; Lee, J.-S.; Lim, T.I.; Jeon, Y.-J. Polyphenol-rich fraction from Ecklonia cava (a brown alga) processing by-product reduces LPS-induced inflammation in vitro and in vivo in a zebrafish model. Algae 2014, 29, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Park, M.-H.; Heo, S.-J.; Kang, S.-M.; Ko, S.-C.; Han, J.-S.; Jeon, Y.-J. Dieckol isolated from Ecklonia cava inhibits α-glucosidase and α-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem. Toxicol. 2010, 48, 2633–2637. [Google Scholar] [CrossRef]
- Ko, S.-C.; Lee, M.; Lee, J.-H.; Lee, S.-H.; Lim, Y.; Jeon, Y.-J. Dieckol, a phlorotannin isolated from a brown seaweed, Ecklonia cava, inhibits adipogenesis through AMP-activated protein kinase (AMPK) activation in 3T3-L1 preadipocytes. Environ. Toxicol. Pharmacol. 2013, 36, 1253–1260. [Google Scholar] [CrossRef]
- Bolton, J.; Anderson, R.; Smit, A.; Rothman, M. South African kelp moving eastwards: The discovery of Ecklonia maxima (Osbeck) Papenfuss at de Hoop Nature Reserve on the south coast of South Africa. Afr. J. Mar. Sci. 2012, 34, 147–151. [Google Scholar] [CrossRef]
- Troell, M.; Robertson-Andersson, D.; Anderson, R.J.; Bolton, J.J.; Maneveldt, G.; Halling, C.; Probyn, T. Abalone farming in South Africa: An overview with perspectives on kelp resources, abalone feed, potential for on-farm seaweed production and socio-economic importance. Aquaculture 2006, 257, 266–281. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.-H.; Kim, H.-S.; Lee, W.; Han, E.J.; Kim, S.-Y.; Fernando, I.S.; Ahn, G.; Kim, K.-N. Eckol from Ecklonia cava ameliorates TNF-α/IFN-γ-induced inflammatory responses via regulating MAPKs and NF-κB signaling pathway in HaCaT cells. Int. Immunopharmacol. 2020, 82, 106146. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.A.; Jayawardena, T.U.; Kim, S.-Y.; Kim, H.-S.; Ahn, G.; Kim, J.; Jeon, Y.-J. Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-κB and MAPK pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Wang, L.; Kim, H.S.; Oh, J.Y.; Je, J.G.; Jeon, Y.-J.; Ryu, B. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against UVB-induced damage in vitro in human dermal fibroblasts and in vivo in zebrafish. Food Chem. Toxicol. 2020, 136, 110963. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, S.Y.; Fernando, I.S.; Sanjeewa, K.A.; Wang, L.; Lee, S.H.; Ko, S.C.; Kang, M.C.; Jayawardena, T.U.; Jeon, Y.J. Free radical scavenging activity of the peptide from the Alcalase hydrolysate of the edible aquacultural seahorse (Hippocampus abdominalis). J. Food Biochem. 2019, 43, e12833. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-A.; Kim, S.-Y.; Kim, J.; Oh, J.-Y.; Kim, H.-S.; Yoon, W.-J.; Kang, D.-H.; Heo, S.-J. Tuberatolide B isolated from Sargassum macrocarpum inhibited LPS-stimulated inflammatory response via MAPKs and NF-κB signaling pathway in RAW 264.7 cells and zebrafish model. J. Funct. Foods 2019, 52, 109–115. [Google Scholar] [CrossRef]
- Sanjeewa, K.A.; Fernando, I.; Kim, S.-Y.; Kim, H.-S.; Ahn, G.; Jee, Y.; Jeon, Y.-J. In vitro and in vivo anti-inflammatory activities of high molecular weight sulfated polysaccharide; containing fucose separated from Sargassum horneri. Int. J. Biol. Macromol. 2018, 107, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Wang, L.; Jayawardena, T.U.; Kim, E.-A.; Heo, S.-J.; Fernando, I.S.; Lee, J.-H.; Jeon, Y.-J. High-performance centrifugal partition chromatography (HPCPC) for efficient isolation of diphlorethohydroxycarmalol (DPHC) and screening of its antioxidant activity in a zebrafish model. Process Biochem. 2020, 88, 189–196. [Google Scholar] [CrossRef]
- Kim, S.M.; Shang, Y.F.; Um, B.H. A preparative method for isolation of fucoxanthin from Eisenia bicyclis by centrifugal partition chromatography. Phytochem. Anal. 2011, 22, 322–329. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Huang, C.; Zhao, J.; Lin, J.; Zhou, X.; Naman, C.B.; Wang, N.; Gerwick, W.H.; Wang, Q. Eckmaxol, a phlorotannin extracted from Ecklonia maxima, produces anti-β-amyloid oligomer neuroprotective effects possibly via directly acting on glycogen synthase kinase 3β. ACS Chem. Neurosci. 2018, 9, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Kang, M.-S.; Hwang, H.-J.; Eom, S.-H.; Yang, J.-Y.; Lee, M.-S.; Lee, W.-J.; Jeon, Y.-J.; Choi, J.-S.; Kim, Y.-M. Synergistic effect between dieckol from Ecklonia stolonifera and β-lactams against methicillin-resistant Staphylococcus aureus. Biotechnol. Bioprocess Eng. 2008, 13, 758–764. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, M.-H.; Kang, S.-M.; Ko, S.-C.; Kang, M.-C.; Cho, S.; Park, P.-J.; Jeon, B.-T.; Kim, S.-K.; Han, J.-S. Dieckol isolated from Ecklonia cava protects against high-glucose induced damage to rat insulinoma cells by reducing oxidative stress and apoptosis. Biosci. Biotechnol. Biochem. 2012, 76, 1445–1451. [Google Scholar] [CrossRef]
- Eom, S.-H.; Moon, S.-Y.; Lee, D.-S.; Kim, H.-J.; Park, K.; Lee, E.-W.; Kim, T.H.; Chung, Y.-H.; Lee, M.-S.; Kim, Y.-M. In vitro antiviral activity of dieckol and phlorofucofuroeckol-A isolated from edible brown alga Eisenia bicyclis against murine norovirus. Algae 2015, 30, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-H.; Kim, H.-S.; Ko, J.-Y.; Kim, C.-Y.; Lee, J.-H.; Jeon, Y.-J. A single-step isolation of useful antioxidant compounds from Ishige okamurae by using centrifugal partition chromatography. Fish. Aquat. Sci. 2016, 19, 22. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhang, Q.-W.; Li, S.-P. Preparative purification of coniferyl ferulate from Angelica sinensis oil by high performance centrifugal partition chromatography. J. Med. Plants Res. 2011, 5, 104–108. [Google Scholar]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef]
- Lee, J.-H.; Ko, J.-Y.; Oh, J.-Y.; Kim, C.-Y.; Lee, H.-J.; Kim, J.; Jeon, Y.-J. Preparative isolation and purification of phlorotannins from Ecklonia cava using centrifugal partition chromatography by one-step. Food Chem. 2014, 158, 433–437. [Google Scholar] [CrossRef]
- Cholan, P.M.; Han, A.; Woodie, B.R.; Watchon, M.; Kurz, A.R.; Laird, A.S.; Britton, W.J.; Ye, L.; Holmes, Z.C.; McCann, J.R. Conserved anti-inflammatory effects and sensing of butyrate in zebrafish. Gut Microbes 2020, 12, 1824563. [Google Scholar] [CrossRef]
- Rangasamy, B.; Hemalatha, D.; Shobana, C.; Nataraj, B.; Ramesh, M. Developmental toxicity and biological responses of zebrafish (Danio rerio) exposed to anti-inflammatory drug ketoprofen. Chemosphere 2018, 213, 423–433. [Google Scholar] [CrossRef]
- Xu, C.; Niu, L.; Guo, H.; Sun, X.; Chen, L.; Tu, W.; Dai, Q.; Ye, J.; Liu, W.; Liu, J. Long-term exposure to the non-steroidal anti-inflammatory drug (NSAID) naproxen causes thyroid disruption in zebrafish at environmentally relevant concentrations. Sci. Total Environ. 2019, 676, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yi, M.; Ding, L.; He, S.; Yan, X. Isolation and purification of a neuroprotective phlorotannin from the marine algae Ecklonia maxima by size exclusion and high-speed counter-current chromatography. Marine Drugs 2019, 17, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwangi, H.M.; Van Der Westhuizen, J.; Marnewick, J.; Mabusela, W.T.; Kabanda, M.M.; Ebenso, E.E. Isolation, identification and radical scavenging activity of phlorotannin derivatives from brown algae, Ecklonia maxima: An experimental and theoretical study. Free Radic. Antioxid. 2013, 3, S1–S10. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Jayawardena, T.U.; Yang, H.-W.; Lee, H.-G.; Jeon, Y.-J. The potential of sulfated polysaccharides isolated from the brown seaweed Ecklonia maxima in cosmetics: Antioxidant, anti-melanogenesis, and photoprotective activities. Antioxidants 2020, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Daub, C.D.; Mabate, B.; Malgas, S.; Pletschke, B.I. Fucoidan from Ecklonia maxima is a powerful inhibitor of the diabetes-related enzyme, α-glucosidase. Int. J. Biol. Macromol. 2020, 151, 412–420. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Shen, H.; Kreisel, D.; Goldstein, D.R. Processes of sterile inflammation. J. Immunol. 2013, 191, 2857–2863. [Google Scholar] [CrossRef]
- Ryu, B.; Choi, I.-W.; Qian, Z.-J.; Heo, S.-J.; Kang, D.-H.; Oh, C.; Jeon, Y.-J.; Jang, C.H.; Park, W.S.; Kang, K.-H. Anti-inflammatory effect of polyphenol-rich extract from the red alga Callophyllis japonica in lipopolysaccharide-induced RAW 264.7 macrophages. Algae 2014, 29, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-I.; Shin, H.-C.; Kim, S.H.; Park, W.-Y.; Lee, K.-T.; Choi, J.-H. 6,6′-Bieckol, isolated from marine alga Ecklonia cava, suppressed LPS-induced nitric oxide and PGE2 production and inflammatory cytokine expression in macrophages: The inhibition of NFκB. Int. Immunopharmacol. 2012, 12, 510–517. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, S.; Edwards, M.S.; Lee, I.-A. Anti-inflammatory effects of polyphenol extracts from Ulva linza (Ulvophyceae, Chlorophyta). Toxicol. Environ. Health Sci. 2018, 10, 212–219. [Google Scholar] [CrossRef]
- Kim, E.-A.; Lee, S.-H.; Ko, C.-I.; Cha, S.-H.; Kang, M.-C.; Kang, S.-M.; Ko, S.-C.; Lee, W.-W.; Ko, J.-Y.; Lee, J.-H. Protective effect of fucoidan against AAPH-induced oxidative stress in zebrafish model. Carbohydr. Polym. 2014, 102, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Örkényi, R.; Éles, J.; Faigl, F.; Vincze, P.; Prechl, A.; Szakács, Z.; Kóti, J.; Greiner, I. Continuous synthesis and purification by coupling a multistep flow reaction with centrifugal partition chromatography. Angew. Chem. Int. Ed. 2017, 56, 8742–8745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Yang, H.-W.; Ding, Y.; Wang, Y.; Jeon, Y.-J.; Moon, S.-H.; Jeon, B.-T.; Sung, S.-H. Anti-inflammatory effects of enzymatic hydrolysates of velvet antler in Raw 264.7 cells in vitro and zebrafish model. EXCLI J. 2015, 14, 1122. [Google Scholar] [PubMed]
- Lee, S.H.; Han, J.S.; Heo, S.J.; Hwang, J.Y.; Jeon, Y.J. Protective effects of dieckol isolated from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicol. In Vitro 2010, 24, 375–381. [Google Scholar] [CrossRef]

| Solvent Condition | K-Value | ||
|---|---|---|---|
| Eckmaxol | Dieckol | ||
| Hexane:EtOAc:MeOH:Water | 1:9:3:7 | 2.59 | 5.94 |
| 2:7:3:7 | 0.94 | 1.41 | |
| 2:8:3:7 | 1.16 | 2.34 | |
| 2:8:4:6 | 0.35 | 0.37 | |
| 3:7:3:7 | 0.23 | 0.29 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-S.; Je, J.-G.; An, H.; Baek, K.; Lee, J.M.; Yim, M.-J.; Ko, S.-C.; Kim, J.-Y.; Oh, G.-W.; Kang, M.-C.; et al. Isolation and Characterization of Efficient Active Compounds Using High-Performance Centrifugal Partition Chromatography (CPC) from Anti-Inflammatory Activity Fraction of Ecklonia maxima in South Africa. Mar. Drugs 2022, 20, 471. https://doi.org/10.3390/md20080471
Kim H-S, Je J-G, An H, Baek K, Lee JM, Yim M-J, Ko S-C, Kim J-Y, Oh G-W, Kang M-C, et al. Isolation and Characterization of Efficient Active Compounds Using High-Performance Centrifugal Partition Chromatography (CPC) from Anti-Inflammatory Activity Fraction of Ecklonia maxima in South Africa. Marine Drugs. 2022; 20(8):471. https://doi.org/10.3390/md20080471
Chicago/Turabian StyleKim, Hyun-Soo, Jun-Geon Je, Hyesuck An, Kyunghwa Baek, Jeong Min Lee, Mi-Jin Yim, Seok-Chun Ko, Ji-Yul Kim, Gun-Woo Oh, Min-Cheol Kang, and et al. 2022. "Isolation and Characterization of Efficient Active Compounds Using High-Performance Centrifugal Partition Chromatography (CPC) from Anti-Inflammatory Activity Fraction of Ecklonia maxima in South Africa" Marine Drugs 20, no. 8: 471. https://doi.org/10.3390/md20080471
APA StyleKim, H.-S., Je, J.-G., An, H., Baek, K., Lee, J. M., Yim, M.-J., Ko, S.-C., Kim, J.-Y., Oh, G.-W., Kang, M.-C., Ham, Y. M., Jeon, Y.-J., & Lee, D.-S. (2022). Isolation and Characterization of Efficient Active Compounds Using High-Performance Centrifugal Partition Chromatography (CPC) from Anti-Inflammatory Activity Fraction of Ecklonia maxima in South Africa. Marine Drugs, 20(8), 471. https://doi.org/10.3390/md20080471