Abstract
Filter-feeding bivalves can accumulate paralytic shellfish toxins (PST) produced by toxic microalgae, which may induce oxidative stress and lipid peroxidation. Peroxisomal acyl-coenzyme A oxidases (ACOXs) are key enzymes functioning in maintaining redox and lipid homeostasis, but their roles in PST response in bivalves are less understood. Herein, a total of six and six ACOXs were identified in the Chlamys farreri and Patinopecten yessoensis genome, respectively, and the expansion of ACOX1s was observed. Gene expression analysis revealed an organ/tissue-specific expression pattern in both scallops, with all ACOXs being predominantly expressed in the two most toxic organs, digestive glands and kidneys. The regulation patterns of scallop ACOXs after exposure to different PST-producing algaes Alexandrium catenella (ACDH) and A. minutum (AM-1) were revealed. After ACDH exposure, more differentially expressed genes (DEGs) were identified in C. farreri digestive glands (three) and kidneys (five) than that in P. yessoensis (two), but the up-regulated DEGs showed similar expression patterns in both species. In C. farreri, three DEGs were found in both digestive glands and kidneys after AM-1 exposure, with two same CfACOX1s being acutely and chronically induced, respectively. Notably, these two CfACOX1s also showed different expression patterns in kidneys between ACDH (acute response) and AM-1 (chronic response) exposure. Moreover, inductive expression of CfACOXs after AM-1 exposure was observed in gills and mantles, and all DEGs in both tissues were up-regulated and their common DEGs exhibited both acute and chronic induction. These results indicate the involvement of scallop ACOXs in PST response, and their plasticity expression patterns between scallop species, among tissues, and between the exposure of different PST analogs.
1. Introduction
Organisms are constantly affected by various stresses from the environment, which can trigger a series of cellular and systemic events that affect their survival and adaptation [1]. Oxidative stress induced by the generation of excess reactive oxygen species (ROS) has emerged as a critical factor for organisms to respond to environmental stresses. The most typical responses to oxidative stress involve distinct organelles, including mitochondria, lysosomes, and peroxisome [2,3,4], among which peroxisome are primarily known for their role in cellular lipid metabolism, and are also increasingly recognized as potential regulators of oxidative stress-related signaling pathways as many peroxisomal enzymes catalyze redox reactions as part of their normal function [4].
Peroxisomal acyl-coenzyme A oxidases (ACOXs) are the rate-limiting enzymes that catalyze the initial step of the β-oxidation system in the peroxisome and are always used as biomarkers for peroxisome proliferation [5]. They can catalyze the α,β-dehydrogenation of acyl-CoA by transferring electrons in the form of H− from their prosthetic group FADH2 to O2, thereby generating H2O2, which is detoxified by catalase [6]. ACOXs are generally classified into three subtypes: ACOX1, ACOX2, and ACOX3. The difference among these three enzyme types is that ACOX1 can only desaturate straight-chain acyl-CoA; ACOX2 mainly acts on branched-chain acyl-CoA; ACOX3 recognizes different acyl-CoAs with or without a 2-methyl branch [7]. Studies have shown that ACOXs are not only essential for fatty acid oxidation and redox homeostasis but also play an important role in the response to oxidative stress caused by environmental xenobiotics. For example, in Chinese toad, cadmium exposure triggered oxidative stress and down-regulated the expression of ACOX in the liver [8]. The up-regulation of ACOX protein was observed in sea cucumber after a diet with tussah immunoreactive substances (TIS) [9]. Furthermore, bisphenol A (BPA) exposure caused an imbalance in the redox state in the liver of common carp and up-regulated the expression of lipid metabolism-related genes, including ACOX1, in the liver [10].
For aquatic organisms, increased toxic stresses in habitats have a direct impact on the suitability and survival of their populations. Harmful algal blooms (HABs) are an important source of toxic stress in aquatic habitats [11]. During HABs, filter-feeding bivalves can accumulate paralytic shellfish toxins (PST) produced by marine microalgae, especially the dinoflagellates of the genus Alexandrium [12]. PST are acute neurotoxins and include at least 57 derivatives of saxitoxin (STX) [13], which can reversibly bind voltage-gated Na+ channels (NaV) of excitable cell membranes, thus blocking the conduction of nerve signals and causing neuromuscular disorders [14,15]. Although bivalves could tolerate high concentrations of PST due to possessing toxin-resistant amino acids in NaV protein [14,16], PST accumulation also induces oxidative stress, resulting in an imbalance between the production of ROS and the antioxidant system [17,18,19]. Studies on the mechanisms of oxidative stress caused by PST in bivalves are extensive but mainly focus on the antioxidant-related genes, such as the genes encoding superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione S-transferase (GST), which were found to be regulated in response to PST exposure [20,21,22]. However, whether the peroxisomal β-oxidation pathway, which is essential for redox homeostasis, is involved in PST response remains poorly studied.
Among bivalves, scallops have an excellent ability to accumulate a higher level of PST, and can retain these toxins for a longer time than other members [23], making them to be an ideal species to study the toxin stress and tolerance of bivalves. To investigate the effect of PST exposure on the peroxisomal β-oxidation system, we systematically identified the ACOX genes in Chlamys farreri and Patinopecten yessoensis, the main commercial species of scallops cultured in China [16,24]. We also analyzed the expression responses of ACOX genes after exposure to two different PST-producing Alexandrium (A. catenella and A. minutum). Our results revealed the expansion of scallop ACOX genes, and their toxin-, tissue- and species-specific expression pattern during toxic algae challenges. Our findings will expand the understanding of ACOX functions in scallops and can guide future studies focusing on the role of peroxisomal β-oxidation and its interactions with PST.
2. Results and Discussion
2.1. Identification of ACOXs in Scallop Genome
A total of six and six ACOX genes were identified in C. farreri (CfACOX) and P. yessoensis (PyACOX) genomes, respectively (Table S1). Basic information regarding their genome position, CDS length, protein length, ACOX domain region, isoelectric point (pI), and molecular weight (MW) were summarized in Table 1. The coding sequences of CfACOXs ranged from 1896 to 2187 bp and encoded proteins from 631 to 728 amino acids (aa), whereas PyACOXs varied from 1989 to 2187 bp encoding 662 to 728 aa. The predicted MW of ACOX proteins ranged from 70.84 kDa to 81.47 kDa with the pIs ranging from 5.74 to 8.89 in C. farreri, and 74.51 kDa to 81.36 kDa with pIs from 5.81 to 8.86 in P. yessoensis.

Table 1.
Basic information of scallop ACOX genes.
In C. farreri, we identified four ACOX1s, one ACOX2, and one ACOX3, and also obtained four ACOX1s, one ACOX2, and one ACOX3 in P. yessoensis (Table 2). Notably, the numbers of ACOX1s in scallops and oyster (three copies) genomes were more than that in vertebrates (one copy), and owl limpet (one copy), implying the expansion of ACOX1s in bivalves. We also found the gene expansion event in Caenorhabditis elegans, but the expanded genes in this species were annotated as ACOX1/2. In addition, ACOX2 was not found in zebrafish, while ACOX3 was absent from fruit fly, which was possibly a result of species-specific losses.

Table 2.
Gene number comparison of ACOX genes among selected vertebrates, Drosophila melanogaster, Caenorhabditis elegans, and mollusk genomes.
2.2. Conserved Structures of ACOX Genes in C. farreri and P. yessoensis
Sequence alignment of scallop ACOX proteins with their homologs in other selected species revealed the presence of conserved structural domains (Figure 1A and Figure S1), including the fatty acyl CoA oxidase (ACOX) domain (K-W/F-W-I/V/P-G-G/N/D), FAD-binding motif (CGGHGY), and peroxisomal targeting signal (PTS) [7]. Near the N-terminal of scallop ACOXs, a conserved stretch of six amino acids (K-Y/W/I-W-P/S-G/A-N/H/S) is present, proposed as an ACOX motif. The ACOX domain of ACOX1 is more similar to ACOX2 than ACOX3, but all of them contain conserved Trp (W), which contribute to the stabilization of the isoalloxazine ring of FAD through hydrogen bonds [25]. Like all other ACOXs, FAD-binding motif was highly conserved in scallops, as well as among different ACOX members. Behind the FAD-binding motif, a conserved glutamate, which constitutes the catalytic site involved in the α-proton abstraction from the substrate [26], is also found in scallop ACOXs. In addition, the tripeptide SKLs or a related sequence (S/A/V/P-K/R/H/Q/N-L/I/M) at its carboxy-terminal end was shown to be the targeting signal for many peroxisomal matrix proteins [27]. All scallop ACOX proteins contained the PTS, with ACOX2 and ACOX3 containing SKL, and ACOX1 members containing SKL or several variants (S/A-K/R-L).
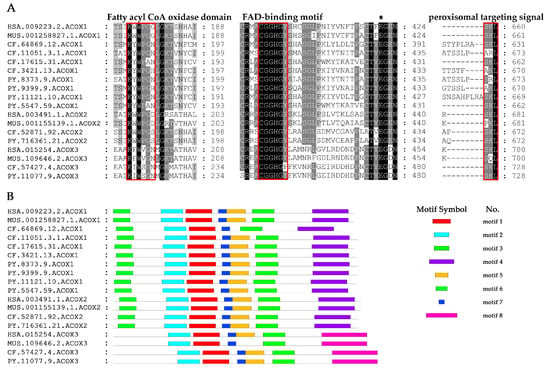
Figure 1.
Analysis of conserved protein structures presented in scallop ACOX proteins compared with their vertebrate homologs. (A) Alignments of conserved structural domains of CfACOXs and PyACOXs. The fatty acyl CoA oxidase (ACOX) domain, FAD-binding motif, and peroxisomal targeting signal (PTS) are labeled with red frames, while the conserved glutamate is indicated with asterisk (*). (B) The conserved motifs of CfACOXs and PyACOXs. Each colored box represents a motif in the protein. (HSA: Homo sapiens, MUS: Mus musculus, CF: Chlamys farreri, PY: Patinopecten yessoensis).
We also found the motifs of scallop ACOX proteins were highly conserved (Figure 1B). Almost all CfACOX1 and PyACOX1 members contained seven conserved motifs, which showed high similarity in terms of the type, order, and the number of motifs with their vertebrate homologs. Similar results were also observed in ACOX2 and ACOX3. In addition, the motifs of scallop ACOX1 and ACOX2 members were more similar than that of ACOX3. ACOX1 and ACOX2 shared all seven motifs, while only five motifs were shared with ACOX3. These results indicate that scallop ACOX members probably have analogous functions to other vertebrate counterparts.
2.3. Phylogenetic Relationship of ACOXs between Bivalves and Other Organisms
Phylogenetic analysis of ACOX amino acid sequences from 11 selected species was conducted. As shown in Figure 2, the scallop ACOX members can be classified into two major branches. One branch contained vertebrate ACOX1 and ACOX2 members, as well as their corresponding ACOX orthologous in mollusks, fruit fly, and nematode. Each member of ACOX1 and ACOX2 in scallops clustered into well-supported separate clades with its orthologues of other species. Several C. elegans ACOX1/2 and a Drosophila melanogaster ACOX1/2 formed another sub-clade and were rooted at the clade of ACOX1 and ACOX2, indicating they shared the same ancestor. This finding also implies that ACOX1 and ACOX2 were produced before the speciation of mollusks. Notably, species-specific expansion of ACOX1s was observed in bivalves. In the bivalve specific group, several pairs of C. farreri and P. yessoensis ACOX1s were closely associated and clustered with C. gigas. Given that lineage-specific gene expansion is often associated with the emergence of new biological functions and a response to diverse environmental pressures [28], the high number of bivalve ACOX1s derived from bivalve-specific expansion suggests that these genes may play a major role in bivalve’s adaptation to the stressful marine environment. The other branch was dominated by ACOX3 members, and scallop ACOX3s were well distributed with their corresponding ACOX3 orthologous from other species, suggesting that they are evolutionarily conserved.
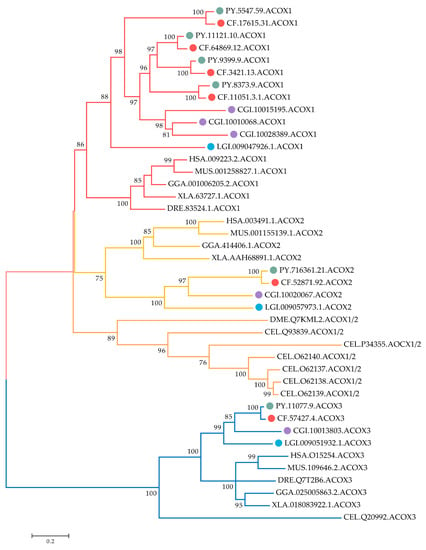
Figure 2.
Phylogenetic tree of ACOX proteins from C. farreri, P. yessoensis, and other selected organisms. The tree was constructed using the maximum-likelihood (ML) method with LG + G + I module. Numbers at the branch point of the node represent the value resulting from 1000 replications. CfACOX, PyACOX, Crassostrea gigas ACOX, and Lottia gigantean ACOX proteins are marked with red, green, purple, and blue dots, respectively. Branches of ACOX1, ACOX2, and ACOX3 proteins are highlighted in red, yellow, and blue, respectively. HAS: H. sapiens, MUS: M. musculus, GGA: Gallus gallus, XLA: Xenopus laevis, DRE: Danio rerio, DME: D. melanogaster, CEL: C. elegans, LGI: L. gigantean, CGI: C. gigas, CF: C. farreri, PY: P. yessoensis. The accession numbers of ACOXs used in the phylogenetic analysis are listed in Table S2.
2.4. Expression Profiles of Scallops ACOXs in Developmental Stages and Adult Organs/Tissues
According to the RNA-Seq data, we found all CfACOX and PyACOX genes were expressed (RPKM > 1) in at least one of the selected developmental stages or organs/tissues (Figure 3, Table S3). During the development stages of scallops, most ACOXs, including several ACOX1s (CF.17615.31 and CF.64869.12 in C. farreri, PY.5547.59, PY.8373.9 and PY.11121.10 in P. yessoensis), one ACOX2 and one ACOX3 in both scallops, showed widespread expression from zygote to larvae and juvenile stages, indicating their maternal origin played an important role in maintaining the fatty acid metabolic balance during development. The other two CfACOX1s (CF.3421.13 and CF.11051.3.1) and one PyACOX1 (PY.9399.9) started to be abundantly expressed from the blastula or D stage veliger, and sustained their expression in the following umbo larvae and juvenile stages, implying the involvement of these ACOX1s during scallop metamorphosis and post-larval development.
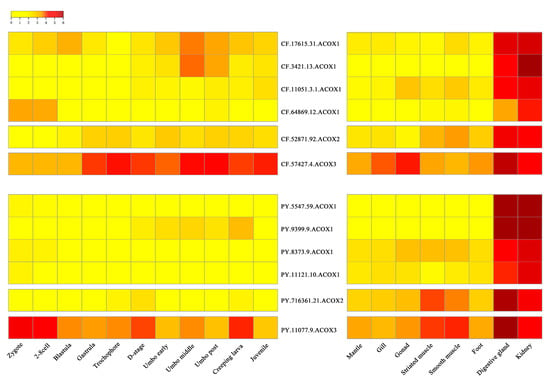
Figure 3.
Heatmap of ACOX gene expression profiles of C. farreri and P. yessoensis during developmental stages and in adult organs/tissues. The expression levels, as represented by log2 (RPKM + 1) values, are shown in the gradient heat map with colors ranging from yellow (low expression) to red (high expression).
In adult organs/tissues, the organ/tissue-specific expression patterns of ACOXs were found in both scallops and all CfACOXs and PyACOXs were predominantly expressed in both digestive glands and kidneys (Figure 3, Table S3). For expanded ACOX1s including four CfACOX1s and four PyACOX1s, the highest expression level was observed in the kidneys followed by the digestive glands, while ACOX2 and ACOX3 genes were the most abundantly expressed in the digestive glands, followed by the kidneys, and showed relatively lower expression in other organs/tissues. Abundant ACOX transcripts in kidneys and digestive glands have also been found in other species [29]. The kidneys and digestive glands were reported to be the crucial metabolic and defense organs against oxidative stress, the dominant ACOXs expression in these two tissues suggested that scallop ACOXs might play crucial roles in the molluscan metabolism and defense system.
2.5. Expression Regulation of Scallops ACOXs after Toxic Dinoflagellates Exposure
To evaluate the possible involvement of scallop ACOXs in response to PST-producing algae challenge, we examined their expression changes after exposure to toxic dinoflagellates Alexandrium (Figure 4, Table S4). Dinoflagellates of the genus Alexandrium are the major PST producers in scallop farming, but the toxicities and harmful mechanisms of different Alexandrium species are varied [30]. We first analyzed the expression profile of ACOXs in the digestive glands and kidneys of two scallop species (P. yessoensis and C. farreri) after exposure to A. catenella (strain ACDH), which mainly produces N-sulphocarbamoyl derivatives (C1/2) [30]. In scallops, the digestive glands and kidneys showed different roles in toxin metabolism, with the former primarily responsible for absorbing PST directly from algae, and the latter mainly responsible for transforming toxins into higher toxic analogs [16]. In P. yessoensis, the two same PyACOX1s (PY.5547.59 and PY.9399.9) were significantly up-regulated (|log2Fold Change (FC)| > 1 and p < 0.05) in both digestive glands and kidneys (Figure 4A). These two differentially expressed genes (DEGs) exhibited similar expression patterns, with PY.5547.59 showing both acute and chronic induction, and PY.9399.9 showing acute response in both tissues. By contrast, more ACOXs were regulated in C. farreri after ACDH challenge. In digestive glands, two CfACOX1s (CF.3421.13 and CF.11051.3.1) and one CfACOX2 showed significant up-regulation, while all the CfACOXs except CfACOX2 showed significant alteration in kidneys with two CfACOX1s (CF.17615.31 and CF.3421.13) and CfACOX3 being up-regulated and the other two CfACOX1s (CF.11051.3.1 and CF.64869.12) being down-regulated (Figure 4A). The DEGs in both tissues showed similar expression patterns, with all up-regulated DEGs being acutely induced on day 1. Although different types of PST were contained in digestive glands (mainly C1/C2) and kidneys (mainly transformed type NeoSTX) after ACDH exposure [31], the similar expression patterns of CfACOXs indicate that the toxic effects produced by C1/C2 and NeoSTX might have similar effects on the β-oxidation pathway. For down-regulated DEGs in kidneys, the chronic response was observed on days 5, 10, and 15 post exposure.
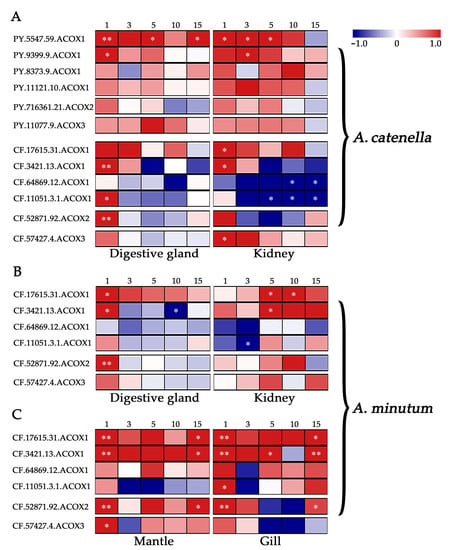
Figure 4.
Temporal expressions of PyACOXs and CfACOXs in digestive glands and kidneys after exposure to (A) Alexandrium catenella (ACDH) and (B) A. minutum (AM-1), and (C) that in mantles and gills after exposure to A. minutum (AM-1). The heatmap was based on log2FC values. The exposure time (1, 3, 5, 10, or 15 days) is displayed above the heatmap. * represents significant regulation with |log2FC| > 1 and p-Value < 0.05, ** represents very significant regulation with |log2FC| > 1 and FDR < 0.05.
As the strong response in C. farreri to ACDH, we further investigated the transcriptional responses of ACOXs in this species after exposure to the other toxic dinoflagellates, A. minutum (strain AM-1) (Figure 4B), which mainly produces PST analogs of gonyautoxins (GTXs, mainly GTX1-4) with higher toxicity [30]. After AM-1 exposure, two CfACOX1s (CF.17615.31 and CF.3421.13) and one CfACOX2 were identified to be differentially expressed in digestive glands, while three CfACOX1s (CF.17615.31, CF.3421.13, and CF.11051.3.1) were significantly regulated in kidneys (|log2FC| > 1 and p < 0.05). Two of these DEGs (CF.17615.31 and CF.3421.13) were regulated in both tissues, but they exhibited different expression patterns, being acutely up-regulated on day 1 in digestive glands, while showing chronic induction on day 5 or day 10 in kidneys. Considering the difference in toxin profiles between digestive glands (mainly GTX1-4) and kidneys (containing transformed type STX) after AM-1 exposure [16,31], the organ/tissue-specific pattern of CfACOXs might be due to the different response mechanisms to GTXs and STX challenges. Compared to the results of ACDH exposure, we found CfACOXs expression patterns were diverse between AM-1 and ACDH challenges in the kidneys. All up-regulated DEGs were acutely induced after ACDH exposure, whereas they showed chronic induction after AM-1 exposure (Figure 4A, B). This time-specific expression pattern might be related to the toxicity levels of PST in kidneys. By contrast, CfACOXs exhibited similar acute responses in digestive glands with both diets.
Furthermore, we also observed the inductive expression of CfACOXs after AM-1 exposure in mantles and gills (Figure 4C), the two organs having relatively large contact areas with the surrounding water and playing crucial roles in water filtering and toxin absorption [32,33,34]. All DEGs in both tissues were up-regulated after AM-1 challenge, with two CfACOX1s (CF.17615.31 and CF.3421.13), one CfACOX2, and one CfACOX3 showing significant alteration in mantles, while three CfACOX1s (CF.17615.31, CF.3421.13, and CF.11051.3.1) and one CfACOX2 being significantly up-regulated in gills (|log2FC| > 1 and p < 0.05). Notably, three DEGs (CF.17615.31, CF.3421.13, and CF.52871.92) were found in both tissues, and they exhibited similar expression patterns with exhibiting both acute (day 1) and chronic responses (day 15) to AM-1, which was different from the results in digestive glands and kidneys. It is worth noting that one ACOX1 member (CF.3421.13) in C. farreri was up-regulated in all tissues after both Alexandrium exposure, which could be a useful indicator of the peroxisomal β-oxidation pathway under algal toxin stress in bivalves.
Overall, the expression changes in ACOXs were widely observed in four tissues of scallops after exposure to different toxic algae, suggesting the involvement of the peroxisomal β-oxidation pathway in PST response. Most ACOXs, especially the expanded ACOX1s, were up-regulated after toxic algae challenge, which also indicates the activation of the β-oxidation pathway. In addition, we found diversified responsive patterns of scallop ACOX genes between different scallop species, between the exposure of different dinoflagellates, as well as among tissues, which implies the expression plasticity of scallop ACOXs in response to the stress caused by PST-producing algae. Our findings will provide comprehensive information for understanding the adaptive evolution and functional diversity of ACOXs in scallops.
3. Materials and Methods
3.1. Identification and Sequence Analysis of ACOX Genes in C. farreri and P. yessoensis
Firstly, we used the available sequences of ACOXs from invertebrates (D. melanogaster and C. elegans) and vertebrates (H. sapiens, M. musculus, G. gallus, X. laevis, and D. rerio) as queries to search against the transcriptomes of two scallops (P. yessoensis and C. farreri) to obtain candidate ACOX sequences [16,24]. The candidate ACOXs sequences were translated through the ORF finder (https://www.ncbi.nlm.nih.gov/orffinder/, accessed on 15 March 2022). Then, the predicted ACOX proteins were aligned to the public databases including KEGG (https://www.kegg.jp/, accessed on 18 March 2022), UniProt (http://www.uniprot.org/, accessed on 18 March 2022), and the NCBI non-redundant (Nr) protein sequence database with BLASTP (e-value set: 1E-05). The sequences with significant blast hit to known ACOX proteins were obtained as candidates and further verified, whether or not they contained ACOX domain, by the Conserved Domains Database (https://www.ncbi.nlm.nih.gov/cdd, accessed on 25 March 2022) and SMART tools (http://smart.embl-heidelberg.de/, accessed on 25 March 2022). Similar methods were used to identify ACOX genes in C. gigas and L. gigantean [35,36]. The compute pI/MW tool (https://web.expasy.org/compute_pi/, accessed on 5 April 2022) was used to predict the pI value and MW (kDa). “Multiple EM for Motif Elicitation” (MEME) version 5.4.1 (https://meme-suite.org, accessed on 15 April 2022) was employed to compare conserved motifs among scallop ACOX proteins with other organisms.
3.2. Multiple Alignment and Phylogenetic Analysis
The ACOX protein sequences from four mollusks and other representative species were employed for phylogenetic analysis. Multiple alignments of ACOX proteins were performed using Clustal Muscle [37]. The maximum likelihood (ML) phylogenetic tree was constructed using MEGA 6.0 with the best fit model LG + G + I model (LG model and Gamma distribution with Invariant sites) and 1000 bootstrap pseudo-replicates [38].
3.3. Expression Analysis of ACOX Genes during Developmental Stages and in Adult Organs/Tissues
The spatiotemporal expression profiles of C. farreri and P. yessoensis ACOX genes in different developmental stages and adult organs/tissues (at least three biological replicates in each sample) were obtained from published RNA-seq data [16,24]. Developmental stage samples include zygotes, 2–8 cells, blastula, gastrula, trochophore, d-stage larvae, umbo early larva, umbo middle larva, umbo post larva, creeping larva, and juvenile scallop. Samples of adult organs/tissues include mantle, gill, gonad, striated muscle, smooth muscle, foot, digestive gland, and kidney. The expression of all ACOX genes was normalized and represented in the form of reads per kilobase of exon model per million mapped reads (RPKM). A custom R script was used to generate a heatmap with the log2(RPKM+1) value.
3.4. Expression Analysis of ACOX Genes Exposed to Toxic Dinoflagellates
In order to analyze the effect of PST-producing Alexandrium on the expression of ACOX genes in scallops, we challenged the C. farreri and P. yessoensis through exposure to the A. catenella (strain ACDH) and/or A. minutum (strain AM-1). These two toxic strains of Alexandrium were cultured independently in F/2 medium under a light–dark cycle of 14h:10h and were collected when the cell density reached 3 × 105 cells/mL in the exponential growth phase [39,40]. Before the feeding experiment, the PST profiles of two toxic strains were measured by high-performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS) analysis following the methods described in our previous study [41]. The main types of PST in ACDH and AM-1 were N-sulphocarbamoyl derivatives (C1/C2) and gonyautoxins (GTXs, mainly GTX1-4), respectively, and no other noxious metabolites were previously reported in these two strains [41]. C. farreri and P. yessoensis were acclimated in filtered and aerated seawater at 12.5 ± 0.5 °C for three weeks and then maintained independently with aeration during the exposure experiments. Each scallop was fed 3 L of Alexandrium with a density of 2500 cells/mL once a day. Three individuals were randomly collected on days 0, 1, 3 (acute response), 5, 10, and 15 (chronic response) of exposure. The digestive glands, kidneys, gills, and mantles of these scallops were dissected, washed with sterile seawater, and then stored in a −80 °C ultra-low temperature environment.
Total RNA was extracted using the conventional guanidinium isothiocyanate method [42]. RNA-seq libraries were constructed using the NEB Next mRNA Library Prep Kit and subjected to PE125 sequencing on the Illumina HiSeq2000 platform. RNA-seq reads were mapped to C. farreri and P. yessoensis genomes using Tophat 2.0.9 [43]. The expression of all ACOX genes was normalized and represented in the form of RPKM. Fold change (FC) for each test time point was calculated as log2FC between toxin-exposed and control groups. Significantly DEGs were identified using the edgeR package [44] with statistically significant cutoff of |log2FC| > 1 and p-Value < 0.05, and the very significantly DEGs with cutoff of |log2FC| > 1 and corrected FDR value < 0.05. A heatmap was generated with the log2FC values using Multiple Experiment Viewer 4.9.0 software (https://sourceforge.net/projects/mev-tm4/files/mev-tm4/MeV%204.9.0/, accessed on 26 April 2022).
4. Conclusions
In the present study, we performed the first systematic analysis of ACOX genes in bivalve mollusks. Our data revealed the expansion of ACOXs in bivalve genomes, and their considerable structural diversity, conserved domains and motifs, as well as conserved evolutionary relationships. The organ/tissue-specific expression pattern of scallop ACOXs was also revealed, which was predominantly expressed in digestive glands and kidneys. Furthermore, we found that most of ACOXs, especially the expanded ACOX1s, showed significant alteration after toxic algae challenge, and the regulatory patterns of ACOXs presented scallop species-, dinoflagellate strain- and tissue-dependent expression patterns.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md20080472/s1, Figure S1: Multiple sequences alignments of the deduced amino acid sequence of scallop ACOXs and their vertebrate homologs; Table S1: Sequences of ACOX proteins in C. farreri, P. yessoensis, C. gigas, and L. gigantea; Table S2: The accession numbers of ACOXs used in phylogenetic analysis.; Table S3: The average RPKM of ACOXs in developmental stages and adult organs/tissues; Table S4: The log2(FC), p and FDR values of scallop ACOXs after toxic A. catenella and A. miutum exposure at different time points.
Author Contributions
Conceptualization, X.H. and H.W.; methodology, H.W. and M.L.; formal analysis, H.W. and M.L.; investigation, M.L., Y.W. and Z.T.; writing—original draft preparation, H.W. and M.L.; writing—review and editing, H.W. and X.H.; supervision, J.H. and Z.B.; funding acquisition, X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key Research and Development Project (2019YFC1605704), Key R&D Project of Shandong Province (2020ZLYS10), Sanya Yazhou Bay Science and Technology City (Grant No. SKJC-KJ-2019KY01), Taishan Industry Leading Talent Project (LJNY201816).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Zoneco Group Co., Ltd (Liaoning, China) and Xunshan Group Co., Ltd. (Shandong, China) for providing the scallops used in this study. The authors also thank Rencheng Yu, Fanzhou Kong and Yang Liu (Institute of Oceanology, Chinese Academy of Sciences) for providing algaes A. catenella and A. minutum.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, G.; Li, L.; Meng, J.; Qi, H.; Qu, T.; Xu, F.; Zhang, L. Molecular basis for adaptation of oysters to stressful marine intertidal environments. Annu. Rev. Anim. Biosci. 2016, 4, 357–381. [Google Scholar] [CrossRef] [PubMed]
- Repnik, U.; Turk, B. Lysosomal-mitochondrial cross-talk during cell death. Mitochondrion 2010, 10, 662–669. [Google Scholar] [CrossRef]
- Kurz, T.; Eaton, J.W.; Brunk, U.T. Redox activity within the lysosomal compartment: Implications for aging and apoptosis. Antioxid. Redox Signal. 2010, 13, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, M.; Fransen, M. Peroxisomal metabolism and oxidative stress. Biochimie 2014, 98, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, J.K.; Hashimoto, T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: An adaptive metabolic system. Annu. Rev. Nutr. 2001, 21, 193–230. [Google Scholar] [CrossRef]
- Camões, F.; Islinger, M.; Guimarães, S.C.; Kilaru, S.; Schuster, M.; Godinho, L.F.; Steinberg, G.; Schrader, M. New insights into the peroxisomal protein inventory: Acyl-CoA oxidases and -dehydrogenases are an ancient feature of peroxisomes. Biochim. Biophys. Acta 2015, 1853, 111–125. [Google Scholar] [CrossRef] [Green Version]
- Van Veldhoven, P.P. Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J. Lipid Res. 2010, 51, 2863–2895. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Zhang, Y.; Chai, L.; Wang, H. Histological changes, lipid metabolism and oxidative stress in the liver of Bufo gargarizans exposed to cadmium concentrations. Chemosphere 2017, 179, 337–346. [Google Scholar] [CrossRef]
- Mi, R.; Sun, Y.; Li, J.; Ma, S.; Wen, Z.; Li, X.; Meng, N.; Li, Y.; Du, X.; Li, S. Immune-related proteins detected through iTRAQ-based proteomics analysis of intestines from Apostichopus japonicus in response to tussah immunoreactive substances. Fish Shellfish Immunol. 2018, 74, 436–443. [Google Scholar] [CrossRef]
- Gu, Z.; Jia, R.; He, Q.; Cao, L.; Du, J.; Feng, W.; Jeney, G.; Xu, P.; Yin, G. Alteration of lipid metabolism, autophagy, apoptosis and immune response in the liver of common carp (Cyprinus carpio) after long-term exposure to bisphenol A. Ecotoxicol. Environ. Saf. 2021, 211, 111923. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, S.; Zhang, H.; Lin, S. Omics study of harmful algal blooms in China: Current status, challenges, and future perspectives. Harmful Algae 2021, 107, 102079. [Google Scholar] [CrossRef] [PubMed]
- Deeds, J.R.; Landsberg, J.H.; Etheridge, S.M.; Pitcher, G.C.; Longan, S.W. Non-traditional vectors for paralytic shellfish poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bricelj, V.M.; Connell, L.; Konoki, K.; Macquarrie, S.P.; Scheuer, T.; Catterall, W.A.; Trainer, V.L. Sodium channel mutation leading to saxitoxin resistance in clams increases risk of PSP. Nature 2005, 434, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, L.E. Saxitoxin, a toxic marine natural product that targets a multitude of receptors. Nat. Prod. Rep. 2006, 23, 200–222. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Hu, X.; Xun, X.; Zhang, J.; Guo, X.; Jiao, W.; Zhang, L.; Liu, W.; Wang, J.; et al. Scallop genome reveals molecular adaptations to semi-sessile life and neurotoxins. Nat. Commun. 2017, 8, 1721. [Google Scholar] [CrossRef]
- Fabioux, C.; Sulistiyani, Y.; Haberkorn, H.; Hégaret, H.; Amzil, Z.; Soudant, P. Exposure to toxic Alexandrium minutum activates the detoxifying and antioxidant systems in gills of the oyster Crassostrea gigas. Harmful Algae 2015, 48, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Ma, F.; Fan, H.; Li, A. Effects of feeding Alexandrium tamarense, a paralytic shellfish toxin producer, on antioxidant enzymes in scallops (Patinopecten yessoensis) and mussels (Mytilus galloprovincialis). Aquaculture 2013, 396, 76–81. [Google Scholar] [CrossRef]
- Cao, R.; Wang, D.; Wei, Q.; Wang, Q.; Yang, D.; Liu, H.; Dong, Z.; Zhang, X.; Zhang, Q.; Zhao, J. Integrative biomarker assessment of the influence of saxitoxin on marine bivalves: A comparative study of the two bivalve species oysters, Crassostrea gigas, and scallops, Chlamys farreri. Front. Physiol. 2018, 9, 1173. [Google Scholar] [CrossRef] [Green Version]
- Lian, S.; Zhao, L.; Xun, X.; Lou, J.; Li, M.; Li, X.; Wang, S.; Zhang, L.; Hu, X.; Bao, Z. Genome-wide identification and characterization of SODs in Zhikong scallop reveals gene expansion and regulation divergence after toxic dinoflagellate exposure. Mar. Drugs 2019, 17, 700. [Google Scholar] [CrossRef] [Green Version]
- Hlaing, S.M.M.; Lou, J.; Cheng, J.; Xun, X.; Li, M.; Lu, W.; Hu, X.; Bao, Z. Tissue-biased and species-specific regulation of glutathione peroxidase (GPx) genes in scallops exposed to toxic dinoflagellates. Toxins 2020, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Cheng, J.; Xun, X.; Li, X.; Li, M.; Zhang, X.; Li, T.; Bao, Z.; Hu, X. Glutathione S-transferase genes in scallops and their diverse expression patterns after exposure to PST-producing dinoflagellates. Mar. Life Sci. Technol. 2020, 2, 252–261. [Google Scholar] [CrossRef]
- Tan, K.S.; Ransangan, J. Factors influencing the toxicity, detoxification and biotransformation of paralytic shellfish toxins. Rev. Environ. Contam. Toxicol. 2015, 235, 1–25. [Google Scholar] [PubMed]
- Wang, S.; Zhang, J.; Jiao, W.; Li, J.; Xun, X.; Sun, Y.; Guo, X.; Huan, P.; Dong, B.; Zhang, L.; et al. Scallop genome provides insights into evolution of bilaterian karyotype and development. Nat. Ecol. Evol. 2017, 1, 120. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.J. Structural insight into the substrate specificity of acyl-CoA oxidase1 from Yarrowia lipolytica for short-chain dicarboxylyl-CoAs. Biochem. Biophys. Res. Commun. 2018, 495, 1628–1634. [Google Scholar] [CrossRef]
- Tokuoka, K.; Nakajima, Y.; Hirotsu, K.; Miyahara, I.; Nishina, Y.; Shiga, K.; Tamaoki, H.; Setoyama, C.; Tojo, H.; Miura, R. Three-dimensional structure of rat-liver acyl-CoA oxidase in complex with a fatty acid: Insights into substrate-recognition and reactivity toward molecular oxygen. J. Biochem. 2006, 139, 789–795. [Google Scholar] [CrossRef]
- Brocard, C.; Hartig, A. Peroxisome targeting signal 1: Is it really a simple tripeptide? Biochim. Biophys. Acta 2006, 1763, 1565–1573. [Google Scholar] [CrossRef] [Green Version]
- Lespinet, O.; Wolf, Y.I.; Koonin, E.V.; Aravind, L. The role of lineage-specific gene family expansion in the evolution of eukaryotes. Genome Res. 2002, 12, 1048–1059. [Google Scholar] [CrossRef] [Green Version]
- He, A.Y.; Liu, C.Z.; Chen, L.Q.; Ning, L.J.; Zhang, M.L.; Li, E.C.; Du, Z.Y. Identification, characterization and nutritional regulation of two isoforms of acyl-coenzyme A oxidase 1 gene in Nile tilapia (Oreochromis niloticus). Gene 2014, 545, 30–35. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, T.; Tan, Z.J.; Wang, L.P.; Zhou, M.J. Toxicity of dinoflagellate Alexandrium species. Oceanol. Limnol. Sinica 2007, 38, 55–56. [Google Scholar]
- Hu, B.; Li, M.; Yu, X.; Xun, X.; Lu, W.; Li, X.; Li, Y.; Lou, J.; Wang, S.; Zhang, L.; et al. Diverse expression regulation of Hsp70 genes in scallops after exposure to toxic Alexandrium dinoflagellates. Chemosphere 2019, 234, 62–69. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira David, J.A.; Salaroli, R.B.; Fontanetti, C.S. Fine structure of Mytella falcata (Bivalvia) gill filaments. Micron 2008, 39, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhang, W.; Zhang, X.; Tian, Y.; Wang, X.; Hao, Z.; Chang, Y. Transcriptional changes in the Japanese scallop (Mizuhopecten yessoensis) shellinfested by Polydora provide insights into the molecular mechanism of shell formation and immunomodulation. Sci. Rep. 2018, 8, 17664. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Kong, F.Z.; Xun, X.G.; Dai, L.; Geng, H.X.; Hu, X.L.; Yu, R.C.; Bao, Z.M.; Zhou, M.J. Biokinetics and biotransformation of paralytic shellfish toxins in different tissues of Yesso scallops, Patinopecten yessoensis. Chemosphere 2020, 261, 128063. [Google Scholar] [CrossRef] [PubMed]
- Simakov, O.; Marletaz, F.; Cho, S.J.; Edsinger-Gonzales, E.; Havlak, P.; Hellsten, U.; Kuo, D.H.; Larsson, T.; Lv, J.; Arendt, D.; et al. Insights into bilaterian evolution from three spiralian genomes. Nature 2013, 493, 526–531. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Yang, P.; Zhang, L.; Wang, X.; Qi, H.; et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Navarro, J.M.; Muñoz, M.G.; Contreras, A.M. Temperature as a factor regulating growth and toxin content in the dinoflagellate Alexandrium catenella. Harmful Algae 2006, 5, 762–769. [Google Scholar] [CrossRef]
- Hwang, D.F.; Lu, Y.H. Influence of environmental and nutritional factors on growth, toxicity, and toxin profile of dinoflagellate Alexandrium minutum. Toxicon 2000, 38, 1491–1503. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Xun, X.; Li, M.; Lou, J.; Zhang, Y.; Shi, J.; Hu, J.; Bao, Z.; Hu, X. Toxin- and species-dependent regulation of ATP-binding cassette (ABC) transporters in scallops after exposure to paralytic shellfish toxin-producing dinoflagellates. Aquat. Toxicol. 2021, 230, 105697. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Bao, Z.; Hu, J.; Shao, M.; Zhang, L.; Bi, K.; Zhan, A.; Huang, X. Cloning and characterization of tryptophan 2,3-dioxygenase gene of Zhikong scallop Chlamys farreri (Jones and Preston 1904). Aquac. Res. 2006, 37, 1187–1194. [Google Scholar] [CrossRef]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).