Sulfated Glucan from the Green Seaweed Caulerpa sertularioides Inhibits Adipogenesis through Suppression of Adipogenic and Lipogenic Key Factors
Abstract
:1. Introduction
2. Results
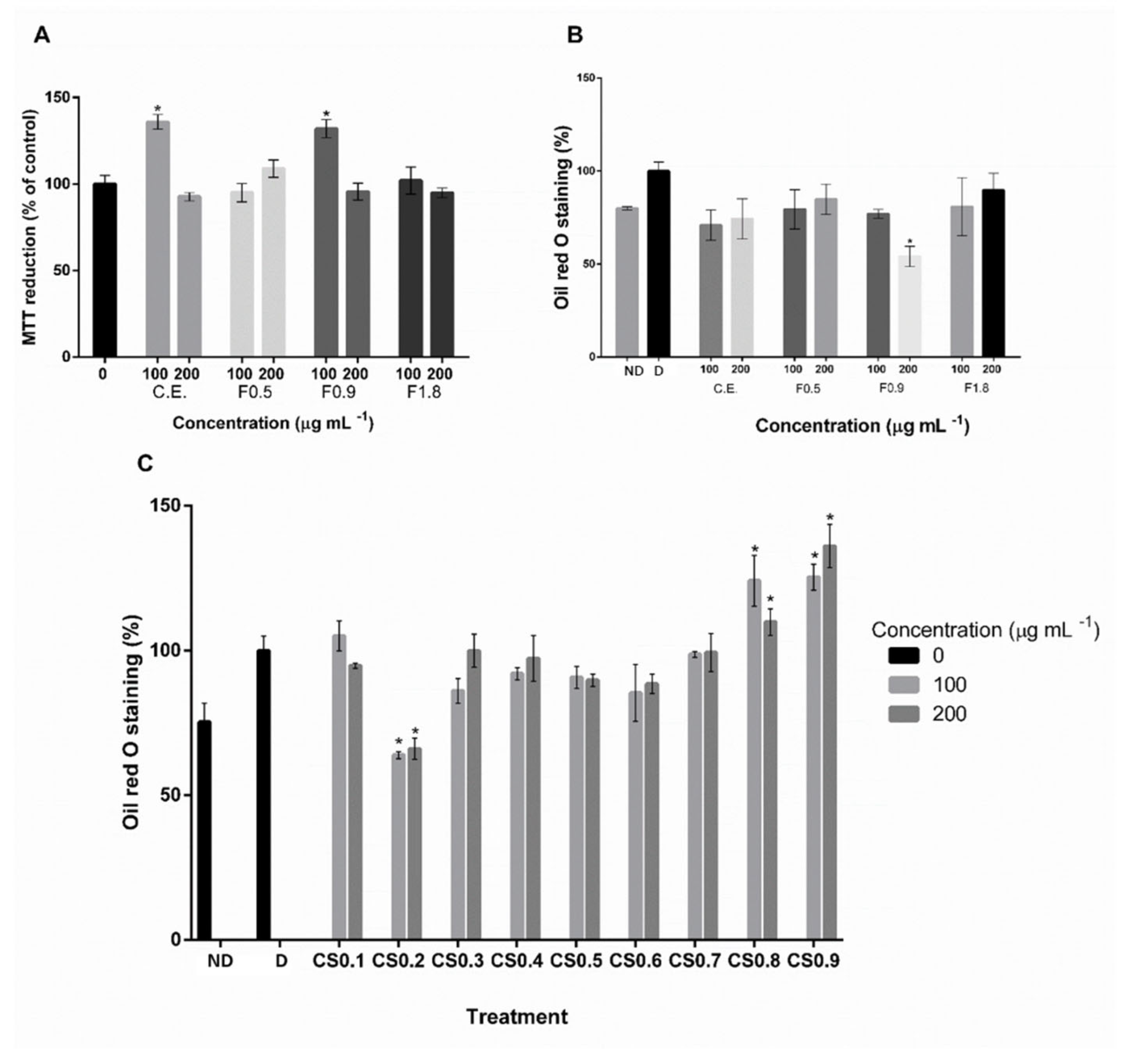
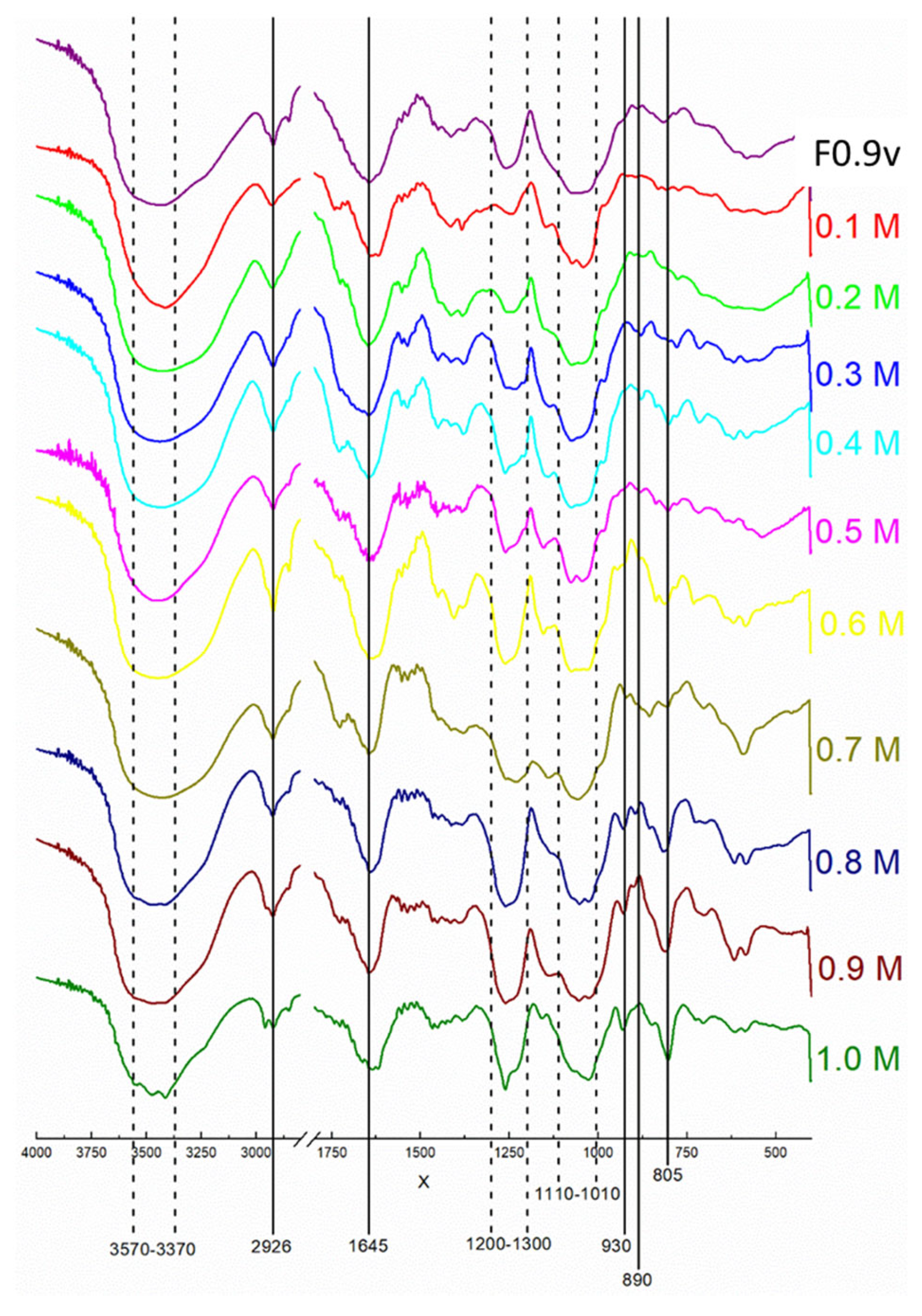
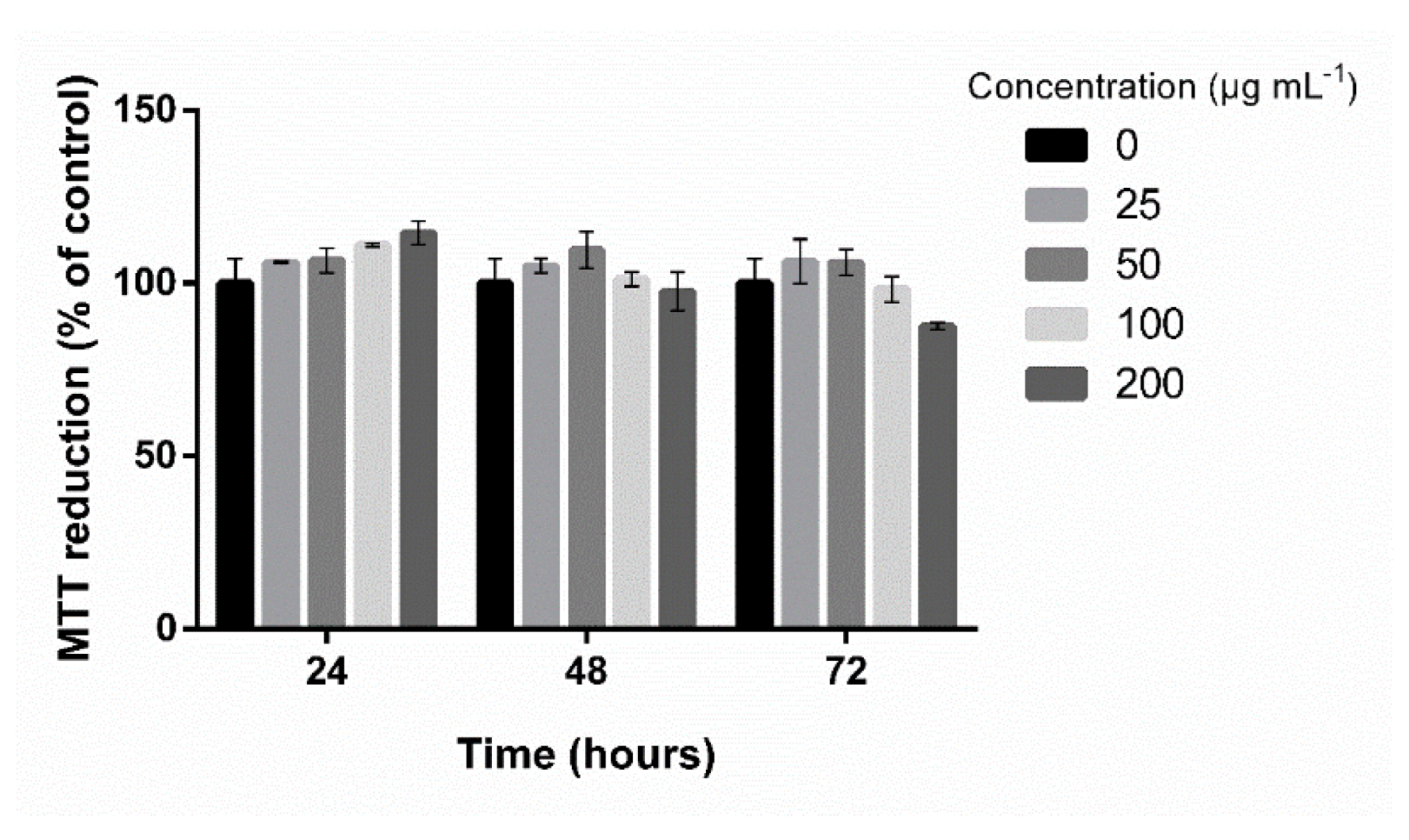
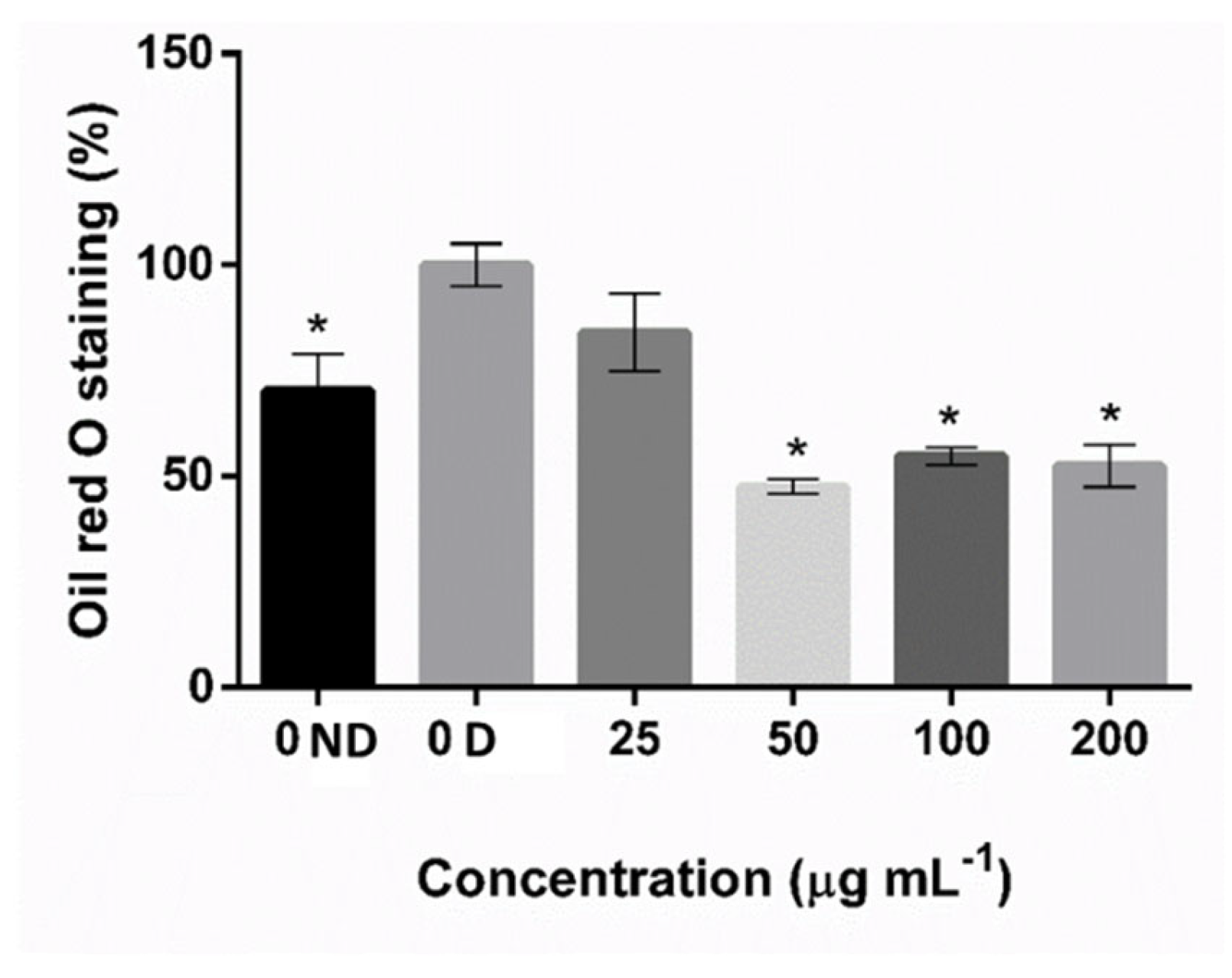
2.1. Extract Characterization and Screening of Anti-Adipogenic Activity
2.2. Effects of SPS from C. sertularioides on Adipocyte Differentiation
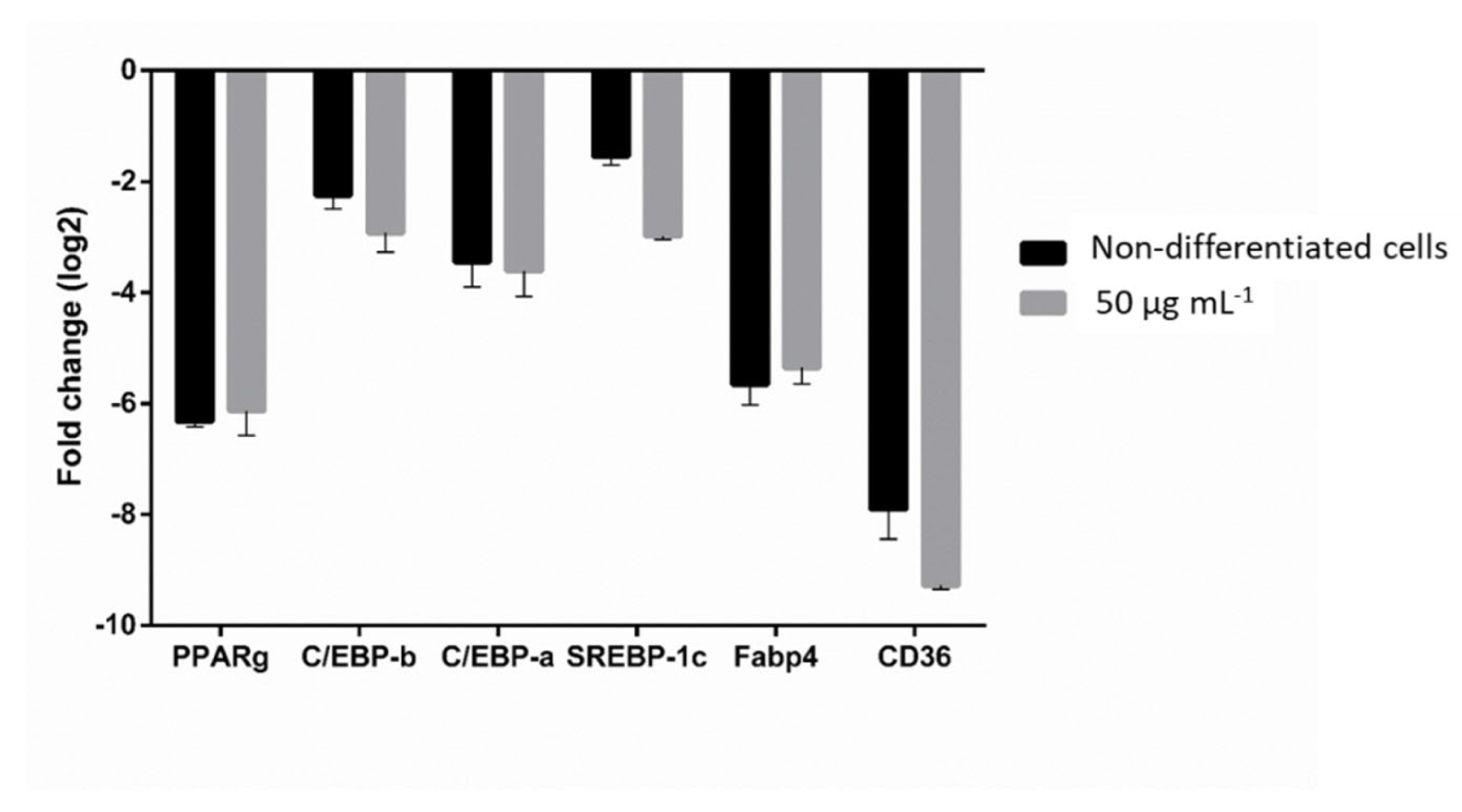
2.3. Effect of SPS from C. sertularioides on the Expression of Adipogenic and Lipogenic Markers
3. Discussion
4. Materials and Methods
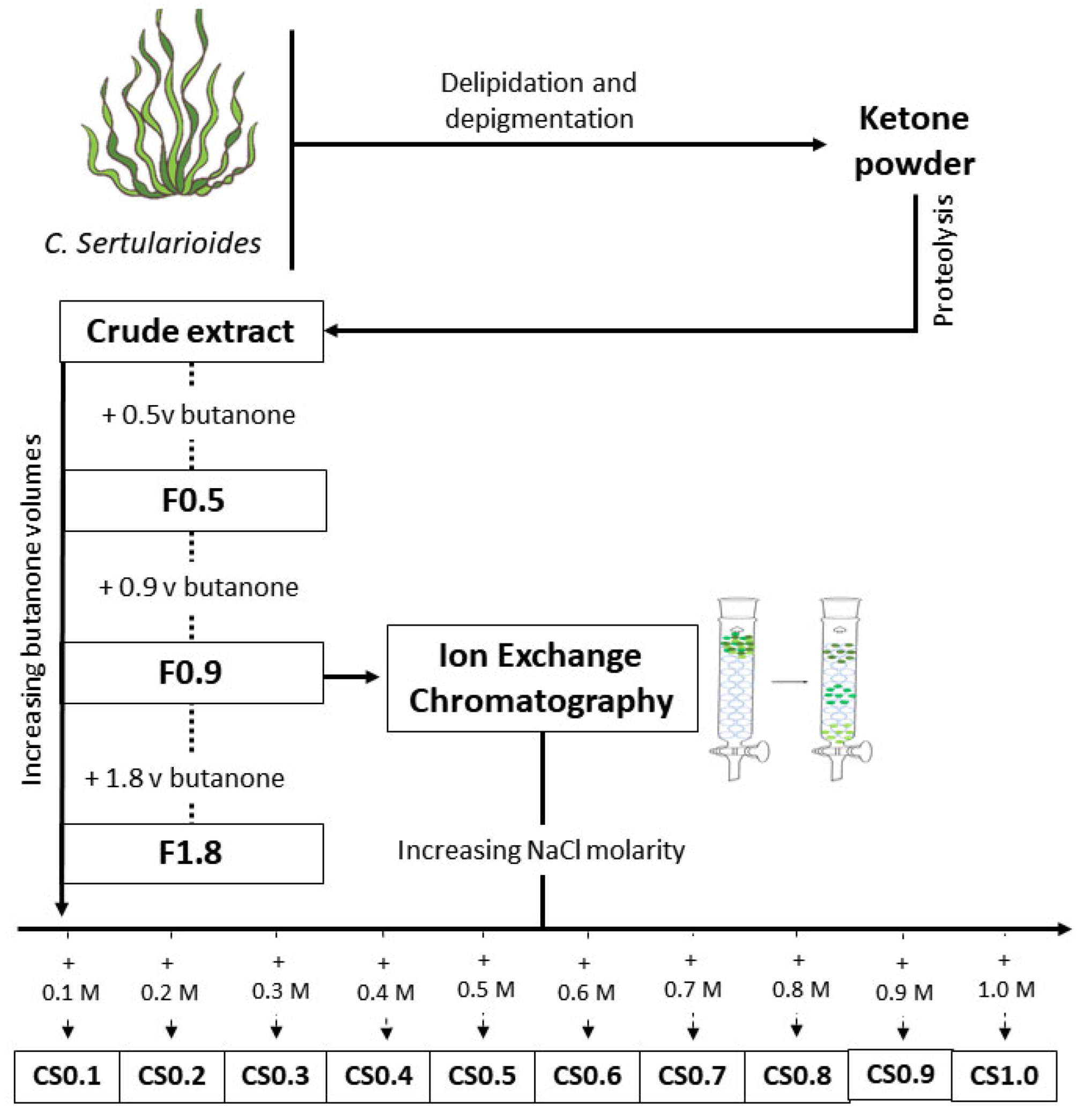
4.1. Extraction and Preparation of SPS-Containing Samples
Ion Exchange Chromatography
4.2. Analyses of Chemical Characterization of SPS-Containing Samples
4.2.1. Chemical Characterization
4.2.2. Fourier Transformed Infrared (FTIR) Spectroscopy Analysis
4.3. Cultivation and Differentiation of 3T3-L1 Cells
4.4. Cytotoxicity
4.5. Oil Red O Staining
4.6. Analysis of Gene Expression
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Son, J.W.; Kim, S. Comprehensive Review of Current and Upcoming Anti-Obesity Drugs. Diabetes Metab. J. 2020, 44, 802–818. [Google Scholar] [CrossRef]
- Stegenga, H.; Haines, A.; Jones, K.; Wilding, J. Identification, assessment, and management of overweight and obesity: Summary of updated NICE guidance. BMJ 2014, 349, g6608. [Google Scholar] [CrossRef] [Green Version]
- Jin, B.R.; Kim, H.J.; Sim, S.A.; Lee, M.; An, H.J. Anti-Obesity Drug Orlistat Alleviates Western-Diet-Driven Colitis-Associated Colon Cancer via Inhibition of STAT3 and NF-κB-Mediated Signaling. Cells 2021, 10, 2060. [Google Scholar] [CrossRef]
- Kültürsay, B.; Keskin, B.; Karagöz, A.Ö.Y.A.; Kaymaz, C. Giant pulmonary artery aneurysm caused by sibutramine-associated pulmonary arterial hypertension: First case in the literature. Anatol. J. Cardiol. 2021, 25, 512–514. [Google Scholar] [CrossRef]
- Siebenhofer, A.; Winterholer, S.; Jeitler, K.; Horvath, K.; Berghold, A.; Krenn, C.; Semlitsch, T. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database Syst. Rev. 2021, 1, CD007654. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; González-Arceo, M.; Trepiana, J.; Eseberri, I.; Fernández-Quintela, A.; Milton-Laskibar, I.; Aguirre, L.; González, M.; Portillo, M.P. Anti-obesity effects of macroalgae. Nutrients 2020, 12, 2378. [Google Scholar] [CrossRef]
- Khan, M.I.; Khan, M.Z.; Shin, J.H.; Shin, T.S.; Lee, Y.B.; Kim, M.Y.; Kim, J.D. Pharmacological Approaches to Attenuate Inflammation and Obesity with Natural Products Formulations by Regulating the Associated Promoting Molecular Signaling Pathways. BioMed Res. Int. 2021, 2021, 2521273. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Jang, M.-S. Anti-obesity effects of Laminaria japonica fermentation on 3T3-L1 adipocytes are mediated by the inhibition of C/EBP-α/β and PPAR-γ. Cell. Mol. Biol. 2018, 64, 71–77. [Google Scholar] [CrossRef]
- Guru, A.; Issac, P.K.; Velayutham, M.; Saraswathi, N.T.; Arshad, A.; Arockiaraj, J. Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds. Mol. Biol. Rep. 2021, 48, 743–761. [Google Scholar] [CrossRef]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Haylett, W.L.; Ferris, W.F. Adipocyte-progenitor cell communication that influences adipogenesis. Cell. Mol. Life Sci. 2020, 77, 115–128. [Google Scholar] [CrossRef]
- Chater, P.I.; Wilcox, M.D.; Houghton, D.; Pearson, J.P. The role of seaweed bioactives in the control of digestion: Implications for obesity treatments. Food Funct. 2015, 6, 3420–3427. [Google Scholar] [CrossRef]
- Jee, W.; Lee, S.-H.; Ko, H.M.; Jung, J.H.; Chung, W.-S.; Jang, H.-J. Anti-Obesity Effect of Polygalin C Isolated from Polygala japonica Houtt. via Suppression of the Adipogenic and Lipogenic Factors in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2021, 22, 10405. [Google Scholar] [CrossRef]
- Park, Y.H.; An, M.; Kim, J.-K.; Lim, Y.-H. Antiobesity effect of ethanolic extract of Ramulus mori in differentiated 3T3-L1 adipocytes and high-fat diet-induced obese mice. J. Ethnopharmacol. 2020, 251, 112542. [Google Scholar] [CrossRef]
- Eddouks, M.; Bidi, A.; El Bouhali, B.; Hajji, L.; Zeggwagh, N.A. Antidiabetic plants improving insulin sensitivity. J. Pharm. Pharmacol. 2014, 66, 1197–1214. [Google Scholar] [CrossRef]
- Lee, H.-G.; Lu, Y.A.; Li, X.; Hyun, J.-M.; Kim, H.-S.; Lee, J.J.; Kim, T.H.; Kim, H.M.; Kang, M.-C.; Jeon, Y.J. Anti-Obesity Effects of Grateloupia elliptica, a Red Seaweed, in Mice with High-Fat Diet-Induced Obesity via Suppression of Adipogenic Factors in White Adipose Tissue and Increased Thermogenic Factors in Brown Adipose Tissue. Nutrients 2020, 12, 308. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Câmara, R.B.; Nobre, L.T.; Costa, M.S.; Almeida-Lima, J.; Farias, E.H.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Filho, G.P.C.; Lima, M.E.G.B.; Rocha, H.A.d.O.; Moreira, S.M.G. Role of sulfated polysaccharides from seaweeds in bone regeneration: A systematic review. Carbohydr. Polym. 2022, 284, 119204. [Google Scholar] [CrossRef]
- Cengiz, S.; Cavas, L.; Yurdakoc, K.; Pohnert, G. The sesquiterpene caulerpenyne from Caulerpa spp. is a lipoxygenase inhibitor. Mar. Biotechnol. 2011, 13, 321–326. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential Role of Seaweed Polyphenols in Cardiovascular-Associated Disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef] [Green Version]
- Barros-Gomes, J.A.C.; Nascimento, D.L.A.; Silveira, A.C.R.; Silva, R.K.; Gomes, D.L.; Melo, K.R.T.; Almeida-Lima, J.; Camara, R.B.G.; Silva, N.B.; Rocha, H.A.O. In Vivo Evaluation of the Antioxidant Activity and Protective Action of the Seaweed Gracilaria birdiae. Oxid. Med. Cell Longev. 2018, 2018, 9354296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-J.; Lee, O.-H.; Lee, B.-Y. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci. 2010, 86, 791–797. [Google Scholar] [CrossRef]
- Oliveira, R.M.; Câmara, R.B.G.; Monte, J.F.S.; Viana, R.L.S.; Melo, K.R.T.; Queiroz, M.F.; Filgueira, L.G.A.; Oyama, L.M.; Rocha, H.A.O. Commercial fucoidans from Fucus vesiculosus can be grouped into antiadipogenic and adipogenic agents. Mar. Drugs 2018, 16, 193. [Google Scholar] [CrossRef] [Green Version]
- Sim, S.Y.; Shin, Y.E.; Kim, H.K. Fucoidan from Undaria pinnatifida has anti-diabetic effects by stimulation of glucose uptake and reduction of basal lipolysis in 3T3-L1 adipocytes. Nutr. Res 2019, 65, 54–62. [Google Scholar] [CrossRef]
- Filho, G.P.C.; Oliveira, R.D.; de Medeiros, S.R.B.; Rocha, H.A.O.; Moreira, S.M.G. Sulfated polysaccharides from green seaweed Caulerpa prolifera suppress fat accumulation. J. Appl. Phycol. 2020, 32, 4299–4307. [Google Scholar] [CrossRef]
- Barbosa, J.D.S.; Costa, M.S.S.P.; Melo, L.F.M.D.; Medeiros, M.J.C.D.; Pontes, D.D.L.; Scortecci, K.C.; Rocha, H.A.O. In vitro immunostimulating activity of sulfated polysaccharides from Caulerpa cupressoides var. flabellata. Mar. Drugs 2019, 17, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, K.; Adhikari, U.; Lerouge, P.; Ray, B. Polysaccharides from Caulerpa racemosa: Purification and structural features. Carbohydr. Polym. 2007, 68, 407–415. [Google Scholar] [CrossRef]
- Meloni, A.; DeYoung, M.; Lowe, C.; Parkes, D. GLP-1 receptor activated insulin secretion from pancreatic β-cells: Mechanism and glucose dependence. Diabetes Obes. Metab. 2013, 15, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Hofbauer, K.G.; Nicholson, J.R.; Boss, O. The obesity epidemic: Current and future pharmacological treatments. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 565–592. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A complex interplay of multiple molecular determinants and pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef]
- Bindhu, J.; Das, A. An edible fungi Pleurotus ostreatus inhibits adipogenesis via suppressing expression of PPAR γ and C/EBP α in 3T3-L1 cells: In vitro validation of gene knock out of RNAs in PPAR γ using CRISPR spcas9. Biomed. Pharmacother. 2019, 116, 109030. [Google Scholar]
- Filho, G.P.C.; de Sousa, A.F.G.; Câmara, R.B.G.; Rocha, H.A.O.; de Medeiros, S.R.B.; Moreira, S.M.G. Genotoxicity and osteogenic potential of sulfated polysaccharides from Caulerpa prolifera seaweed. Int. J. Biol. Macromol. 2018, 114, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Wynne, M.J. A checklist of benthic marine algae of the tropical and subtropical western Atlantic. Can. J. Bot. 1986, 64, 2239–2281. [Google Scholar] [CrossRef]
- Sabry, D.A.; Cordeiro, S.L.; Silva, C.H.F.; Farias, E.H.C.; Sassaki, G.L.; Nader, H.B.; Rocha, H.A.O. Pharmacological prospection and structural characterization of two purified sulfated and pyruvylated homogalactans from green algae Codium isthmocladum. Carbohydr. Polym. 2019, 222, 115010. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef]
- Gomes, D.L.; Melo, K.R.T.; Queiroz, M.F.; Batista, L.A.N.C.; Santos, P.C.; Costa, M.S.S.P.; Almeida-Lima, J.; Camara, R.B.G.; Costa, L.S.; Rocha, H.A.O. In vitro studies reveal antiurolithic effect of antioxidant sulfated polysaccharides from the green seaweed Caulerpa cupressoides var flabellata. Mar. Drugs 2019, 17, 326. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
| Extracts | Yield (%) | Total Sugars (%) | Sulfate (%) | Protein (%) | Phenolic Compounds (%) |
|---|---|---|---|---|---|
| Crude extract | - * | 82.3 ± 0.9 | 25.5 ± 0.6 | N.D | <0.1 |
| F0.5 | 51.2 | 72.7 ± 0.3 | 37.2 ± 0.2 | N.D | <0.1 |
| F0.9 | 20.8 | 59.1 ± 2.2 | 11.2 ± 0.4 | N.D | <0.1 |
| F1.8 | 27.9 | 26.5 ± 0.2 | 20.5 ± 0.6 | N.D | <0.1 |
| Samples | Yield (%) | Total Sugar (%) | Sulfate (%) | Phenolic Compounds (%) | Monosaccharide Composition (Molar Ratio) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glc | Rha | Fuc | Xyl | Man | Gal | |||||
| CS0.1 | 14.7 | 55.2 ± 1.9 | 0.5 ± 0.1 | <0.1 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| CS0.2 | 20.6 | 63.6 ± 1.9 | 2.8 ± 0.4 | <0.1 | 1 | - | - | - | - | |
| CS0.3 | 12.4 | 58.7 ± 0.9 | 9.8 ± 0.6 | <0.1 | 1 | 12 | - | 2.0 | 6 | |
| CS0.4 | 12.7 | 62.4 ± 2.0 | 12.8 ± 0.1 | <0.1 | 1 | - | - | 2.0 | - | |
| CS0.5 | 10.7 | 55.2 ± 3.1 | 13.5 ± 0.1 | <0.1 | 1 | 0.5 | - | 1.2 | 5.7 | - |
| CS0.6 | 8.9 | 64.5 ± 1.5 | 15.7 ± 1.6 | <0.1 | 1 | - | - | 0.1 | 2.2 | - |
| CS0.7 | 7.9 | 68.0 ± 3.5 | 21.6 ± 1.0 | <0.1 | 1 | - | - | 0.2 | 0.6 | - |
| CS0.8 | 7.4 | 48.8 ± 2.3 | 21.6 ± 1.0 | <0.1 | 1 | - | - | 2.0 | 2.6 | 3.8 |
| CS0.9 | 3.0 | 42.7 ± 1.0 | 26.3 ± 1.1 | <0.1 | 1 | - | - | 6.8 | 0.6 | 0.3 |
| CS1.0 | 1.8 | 60.4 ± 1.6 | 17.7 ± 0.4 | <0.1 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Gene Symbol | Primer Sequence (5′-3′) |
|---|---|
| β-actin | F: TGTCCACCTTCCAGCAGATGT |
| R: AGCTCAGTAACAGTCCGCCTAG | |
| PPARγ | F: TGCTGTTATGGGTGAAACTCTG |
| R: CTGTGTCAACCATGGTAATTTCT | |
| C/EBPβ | F: ATCGACTTCAGCCCCTACCT |
| R: TAGTCGTCGGCGAAGAGG | |
| C/EBPα | F: AGCTGCCTGAGAGCTCCTT |
| R: GACCCGAAACCATCCTCTG | |
| SREBP-1c | F: TCAAGCAGGAGAACCTGACC |
| R: TCATGCCCTCCATAGACACA | |
| Fabp4 | F: CAGCCTTTCTCACCTGGAAGA |
| R: TTGTGGCAAAGCCCACTC | |
| CD36 | F: GGCCAAGCTATTGCGACAT |
| R: CAGATCCGAACACAGCGTAGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves Filho, G.P.; Batista, L.A.N.C.; de Medeiros, S.R.B.; Rocha, H.A.O.; Moreira, S.M.G. Sulfated Glucan from the Green Seaweed Caulerpa sertularioides Inhibits Adipogenesis through Suppression of Adipogenic and Lipogenic Key Factors. Mar. Drugs 2022, 20, 470. https://doi.org/10.3390/md20080470
Chaves Filho GP, Batista LANC, de Medeiros SRB, Rocha HAO, Moreira SMG. Sulfated Glucan from the Green Seaweed Caulerpa sertularioides Inhibits Adipogenesis through Suppression of Adipogenic and Lipogenic Key Factors. Marine Drugs. 2022; 20(8):470. https://doi.org/10.3390/md20080470
Chicago/Turabian StyleChaves Filho, Gildacio Pereira, Lucas Alighieri Neves Costa Batista, Silvia Regina Batistuzzo de Medeiros, Hugo Alexandre Oliveira Rocha, and Susana Margarida Gomes Moreira. 2022. "Sulfated Glucan from the Green Seaweed Caulerpa sertularioides Inhibits Adipogenesis through Suppression of Adipogenic and Lipogenic Key Factors" Marine Drugs 20, no. 8: 470. https://doi.org/10.3390/md20080470
APA StyleChaves Filho, G. P., Batista, L. A. N. C., de Medeiros, S. R. B., Rocha, H. A. O., & Moreira, S. M. G. (2022). Sulfated Glucan from the Green Seaweed Caulerpa sertularioides Inhibits Adipogenesis through Suppression of Adipogenic and Lipogenic Key Factors. Marine Drugs, 20(8), 470. https://doi.org/10.3390/md20080470







