Abstract
The cytotoxicity-bioassay-guided fractionation of the ethanol extract from the marine sponge Guitarra abbotti, whose 1-O-alkyl-sn-glycerol ethers (AGEs) have not been investigated so far, led to the isolation of a complex lipid fraction containing, along with previously known compounds, six new lipids of the AGE type. The composition of the AGE fraction as well as the structures of 6 new and 22 previously known compounds were established using 1H and 13C NMR, GC/MS, and chemical conversion methods. The new AGEs were identified as: 1-O-(Z-docos-15-enyl)-sn-glycerol (1), 1-O-(Z-docos-17-enyl)-sn-glycerol (2), 1-O-(Z-tricos-15-enyl)-sn-glycerol (3), 1-O-(Z-tricos-16-enyl)-sn-glycerol (4), 1-O-(Z-tricos-17-enyl)-sn-glycerol (5), and 1-O-(Z-tetracos-15-enyl)-sn-glycerol (6). The isolated AGEs show weak cytotoxic activity in THP-1, HL-60, HeLa, DLD-1, SNU C4, SK-MEL-28, and MDA-MB-231 human cancer cells. A further cytotoxicity analysis in JB6 P+ Cl41 cells bearing mutated MAP kinase genes revealed that ERK2 and JNK1 play a cytoprotective role in the cellular response to the AGE-induced cytotoxic effects.
1. Introduction
It is well known that many marine invertebrates, including sponges [1,2,3,4,5,6,7,8,9,10,11,12,13], corals [14,15,16,17,18,19], mollusks [14,20,21,22,23,24,25], starfish [26], holothurians [27,28], crabs [21], and ascidians [29] as well as some marine algae [30] are well-established sources of a variety of natural 1-O-alkyl-sn-glycerol ethers (AGEs); for a review, see [31,32]. AGE molecules consist of a long-chain alkyl moiety linked to the glycerol by an ether bond at the sn-1 position. Previously, it was established that all the natural AGEs are enantiomerically pure, with an S configuration of the asymmetric carbon in the glycerol moiety [33,34]. The AGEs metabolism is controlled by the activity of alkylglycerol monooxygenase (AGMO), which is capable of cleavage of the ether bond of AGEs [35,36]. In marine invertebrates, AGEs mostly present as complex inseparable mixtures of ethers containing different alkyl radicals of various lengths and levels of unsaturation [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. It is highly likely that AGEs are used by invertebrates as a part of their chemical defense against predators. Some reports showed the toxicity of AGEs against fish [10] as well as an antifeedant effect against starfish [14,20]. Both natural and synthetic AGEs possess various biological activities; for a review, see [37]. Among others, there are such useful properties as anticancer [2,3,17,18,21,29,38,39,40,41,42,43,44,45], anti-influenza [46], antibacterial [16,30,47,48], antifungal [49], and antifouling [50] activities found in these metabolites. AGEs have been described to reduce cardiovascular and rheumatoid arthritis risk factors [51], the side effects of radiotherapy [52,53,54], obesity [55,56,57,58], microglial activation, and a neuropathic pain [22,59]. Additionally, AGEs are effective adjuvants [60,61], which modulate endothelial cell permeability [62], the immune response in vitro and in vivo [63,64,65,66], open the blood–brain barrier [67,68], are able to penetrate the skin [69], and improve sperm motility [70]. The search for new structural variants of these lipids and the study of their diversity and biological activities represent an interesting aspect of the marine natural products research field.
In a continuation of the studies on cytotoxic marine natural products and their synthetic analogues [71,72,73,74], we have examined the ethanol extract of the cold-water marine sponge Guitarra abbotti, which exhibited a cytotoxic activity against human leukemia THP-1 cells in a screening assay. The AGEs of this sponge have never been studied before. A bioassay-guided fractionation of the crude extract led to the isolation of an AGE mixture containing 6 new (1–6, Figure 1) and 22 known (7–28, Table 1 and Table 2) compounds of this class. The structures of the AGEs and the composition of the mixture as well as its cytotoxic properties and the partial molecular mechanism of its cytotoxic action are reported.
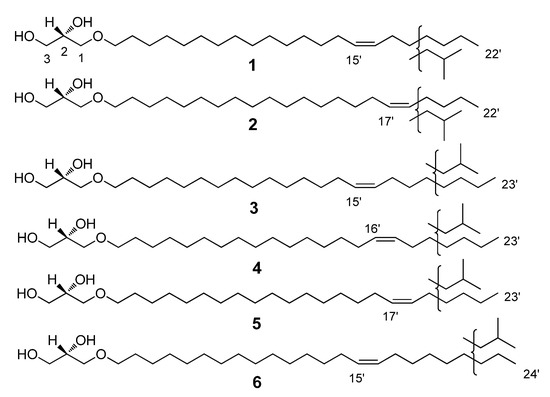
Figure 1.
Structures of the new compounds, 1–6: 1-O-(Z-docos-15-enyl)-sn-glycerol (1), 1-O-(Z-docos-17-enyl)-sn-glycerol (2), 1-O-(Z-tricos-15-enyl)-sn-glycerol (3), 1-O-(Z-tricos-16-enyl)-sn-glycerol (4), 1-O-(Z-tricos-17-enyl)-sn-glycerol (5), and 1-O-(Z-tetracos-15-enyl)-sn-glycerol (6).

Table 1.
GC data and characteristic MS fragmentation of TMS derivatives of AGEs.

Table 2.
GC data and characteristic MS fragmentation of DMDS derivatives of acetylated AGEs.
2. Results
2.1. Composition of the Isolated AGE Fraction
The isolated AGE mixture exhibited the characteristic signals in the 1H and 13C NMR spectra, similar to those previously published [5,6,9,10,12] (see Materials and Methods).
To establish the composition and the structures of the isolated AGEs, including the percentages of the compounds, the AGEs were converted to their corresponding TMS ethers and further analyzed with GC/MS. The GC data and characteristic MS fragmentation of the TMS derivatives of AGEs are shown in Table 1. The data analysis revealed that 22 of the identified AGEs (compounds 7–28) exhibit very similar fragmentations to compounds isolated earlier from other biological sources [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. This fragmentation, in the majority of cases, was related to the loss of a methyl group, trimethylsilanol, (trimethylsilanoxy)methylene, and tetramethylene groups. Nevertheless, six previously unknown compounds (Figure 1) required a more detailed analysis of their structures.
Next, to determine the exact double bond position in the unsaturated AGEs, correspondent acetates and then their dimethyldisulfide derivatives were synthesized and further investigated with GC/MS. The fragmentation scheme is represented in Figure 2, and the generated GC data and characteristic MS fragmentations are shown in Table 2. The S configuration of the stereogenic center at C-2 of the glycerol moiety of AGEs is the same as was determined for the structural analogues previously isolated from marine invertebrates [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Thus, 18 unsaturated substances were identified, 6 of which, with carbon chain lengths of C22-C24, were identified as new compounds: 1-O-(Z-docos-15’-enyl)-sn-glycerol (1); 1-O-(Z-docos-17’-enyl)-sn-glycerol (2); 1-O-(Z-tricos-15’-enyl)-sn-glycerol (3); 1-O-(Z-tricos-16’-enyl)-sn-glycerol (4); 1-O-(Z-tricos-17’-enyl)-sn-glycerol (5); and 1-O-(Z-tetracos-15’-enyl)-sn-glycerol (6) (Figure 1, Table 2).
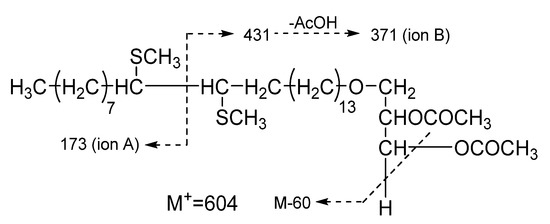
Figure 2.
Characteristic MS fragmentation of DMDS derivative of acetylated 1-O-(Z-tetracos-15’-enyl)-sn-glycerol (6).
Apart from the 6 new compounds, 1–6, the 22 previously known AGEs (10 saturated and 12 unsaturated) were identified in the isolated mixture: 1-O-(tetradecanyl)-sn-glycerol (7); 1-O-(pentadecanyl)-sn-glycerol (TR 16.58 of TMS derivarive (TMS-d), Table 1) (8); 1-O-(pentadecanyl)-sn-glycerol (TR 16.87 of TMS-d, Table 1) (9); 1-O-(hexadecanyl)-sn-glycerol (TR 17.35 of TMS-d, Table 1) (10); 1-O-(hexadecanyl)-sn-glycerol (TR 17.64 of TMS-d, Table 1) (11); 1-O-(heptadecanyl)-sn-glycerol (TR 18.10 of TMS-d, Table 1) (12); 1-O-(heptadecanyl)-sn-glycerol (TR 18.18 of TMS-d, Table 1) (13); 1-O-(heptadecanyl)-sn-glycerol (TR 18.38 of TMS-d, Table 1) (14); 1-O-(octadecanyl)-sn-glycerol (TR 18.80 of TMS-d, Table 1) (15); 1-O-(octadecanyl)-sn-glycerol (TR 19.10 of TMS-d, Table 1) (16); 1-O-(Z-hexadec-7’-enyl)-sn-glycerol (TR 44.69 of DMDS-d, Table 2) (17); 1-O-(Z-hexadec-9’-enyl)-sn-glycerol (TR 45.00 of DMDS-d, Table 2) (18); 1-O-(Z-hexadec-11’-enyl)-sn-glycerol (TR 45.69 of DMDS-d, Table 2) (19); 1-O-(Z-hexadec-13’-enyl)-sn-glycerol (TR 47.69 of DMDS-d, Table 2) (20); 1-O-(Z-octadec-9-enyl)-sn-glycerol (TR 51.10 of DMDS-d, Table 2) (21); 1-O-(Z-octadec-11-enyl)-sn-glycerol (TR 51.40 of DMDS-d, Table 2) (22); 1-O-(Z-octadec-12-enyl)-sn-glycerol (TR 51.62 of DMDS-d, Table 2) (23); 1-O-(Z-octadec-13-enyl)-sn-glycerol (TR 52.13 of DMDS-d, Table 2) (24); 1-O-(Z-cos-11’-enyl)-sn-glycerol (TR 58.48 of DMDS-d, Table 2) (25); 1-O-(Z-cos-13’-enyl)-sn-glycerol (TR 59.39 of DMDS-d, Table 2) (26); 1-O-(Z-cos-15’-enyl)-sn-glycerol (TR 60.29 of DMDS-d, Table 2) (27); and 1-O-(Z-tetracos-17’-enyl)-sn-glycerol (TR 87.30 of DMDS-d, Table 2) (28) [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32].
2.2. Anticancer Effects of the Isolated AGE Fraction
The cytotoxicity of the isolated AGE mixture was evaluated using the MTS viability assay in seven human cancer cell lines [75]. The calculated IC50 values are shown in Table 3.

Table 3.
Cytotoxic activity of the isolated AGE mixture against human cancer cell lines. Cisplatin was used as a positive control.
We used dominant negative mutant (DNM) JB6 Cl41 cells to elucidate the roles of the three major MAP kinases (MAPKs) in the cytotoxic effects of the isolated AGE mixture. The cytotoxic activity of the isolated AGE fraction against the normal mouse epidermal cell line JB6 Cl41 and its stable transfectants JB6 Cl41 DN-JNK1, JB6 Cl41 DN-p38, and JB6 Cl41 DN-ERK2 cells is shown in Figure 3. The DN cell lines contain a mutation in the kinase coding gene, which leads to the inactivation of the corresponding kinase in the cells. An examination of the drug effect in the cells bearing inactivated kinase may help to reveal a role of the kinase in the therapeutic effect of the drug. Our experiments indicated that an inactivation of ERK2 and JNK1 results in higher cytotoxicity of the tested AGE fraction. These data suggest that ERK2 and JNK1 (but not p38) play a cytoprotective role in the cellular response to the AGE treatment.
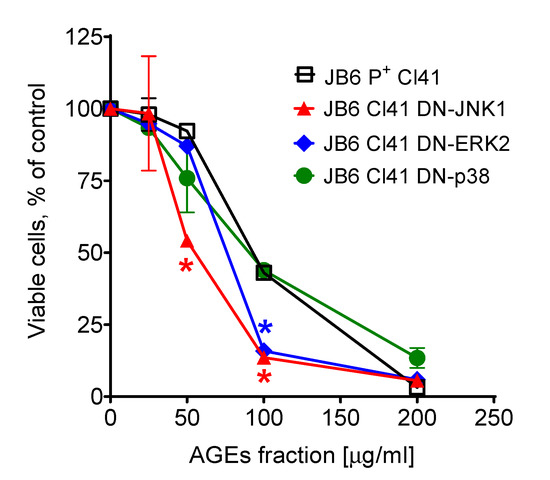
Figure 3.
Cytotoxic effect of the isolated AGE mixture in JB6 P+ Cl41 cells and its stable transfectants, JB6 Cl41 DN-JNK1, JB6 Cl41 DN-p38, and JB6 Cl41 DN-ERK2 cells. * Significant different (p < 0.05, Student’s t-test) in the viability of the cells bearing the mutant kinase compared to the viability measured in JB6 P+ Cl41 cells (wild-type) exposed to the same concentration of the AGE mixture.
3. Discussion
The mixture of the native AGEs was isolated from the extracts of the sponge G. abbotti without any prior derivatization or hydrolysis. A comparison with the literature data [5,6,9,10,12] for the 1H NMR spectrum of the AGE mixture (see Materials and Methods) made it possible to correlate the signals at δ 3.55–3.86 ppm with the protons of the glyceride group, those at δ 3.46 ppm with the protons at C1’ of the alkyl chains, those at δ 1.57 ppm with the protons at C2’ of the alkyl chains, those at δ 1.22–1.38 ppm with the protons of other CH2 groups in the alkyl chains, those at δ 5.34 ppm with the protons at double bonds, those at δ 2.01 ppm with the allylic protons, and those at 0.85–0.88 ppm with protons of the terminal methyl groups of the alkyl chain. Thus, the analysis of the NMR spectra showed that the mixture consists of saturated and unsaturated AGEs, the side chains of which may contain n- and iso- terminal methyl groups (Figures S1–S3, Supplementary Materials). The Z-geometry of the double bonds in the unsaturated lipids was determined by the small coupling constant, J = 4.6 Hz, of the olefinic proton signal in the 1H spectrum (according to 4.5–6.3 Hz for the Z-configuration in [6,9]) as well as by the shielded chemical shifts of the allylic (δ 26.1 ppm) and olefinic (δ 129.9 ppm) carbon atoms in the 13C NMR spectrum of the AGE mixture [6,76]. The S configuration at the asymmetric carbon of the glycerol moiety was previously established for all the natural AGEs [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Therefore, we assume the same configuration for our isolates. To establish the chemical structures of the lipids of the isolated AGE fraction, first their TMS ethers were obtained and analyzed by the GC/MS method (Figures S4 and S5). The GC data and characteristic MS fragmentation of the TMS derivatives of AGEs (see Table 1) revealed the structures of the 10 previously known saturated AGEs that are part of the isolated alkylglycerol mixture [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. The GC/MS data not only confirmed the presence of both saturated and unsaturated AGEs in the mixture but also indicate their approximately equal ratio. Thus, the two main saturated AGEs, containing side chains with 16 and 17 carbon atoms, accounted for 21% and 13% of the total mixture, while the two main unsaturated AGEs with 22 and 24 carbon atoms in the side chains accounted for 16% and 18%, respectively (Table 1). The positions of the double bonds in the unsaturated AGEs were determined based on the analysis of the characteristic MS fragmentation of the DMDS derivatives of the acetylated AGEs (Table 2, Figure 2, Figures S6 and S7). As a result, after analyzing the mass spectra of the TMS and DMDS derivatives of the unsaturated lipids that are part of the AGE mixture, 18 unsaturated substances were structurally identified, 6 of which, with carbon chain lengths of C22-C24, are new compounds. Their chemical structures were established as follows: 1-O-(Z-docos-15’-enyl)-sn-glycerol (1), 1-O-(Z-docos-17’-enyl)-sn-glycerol (2), 1-O-(Z-tricos-15’-enyl)-sn-glycerol (3), 1-O-(Z-tricos-16’-enyl)-sn-glycerol (4), 1-O-(Z-tricos-17’-enyl)-sn-glycerol (5), and 1-O-(Z-tetracos-15’-enyl)-sn-glycerol (6) (Figure 1).
Different studies suggest that in a living organism AGEs serve as precursors and can be enzymatically converted into various types of biologically active ether lipid compounds [77]. At the same time, enzymatically unmodified AGEs also exhibit biological activities. Ether lipids execute various biological functions, including the modulation of several important signaling pathways [78,79]. Thus, AGEs can be used to generate plasmalogens or platelet-activating factor (PAF) in various biological systems in vitro and in vivo [80,81]. Plasmalogens are important membrane constituents as well as regulators of cholesterol biosynthesis and transport; they play a role in intercellular communication, cell migration, and signal transduction [77,80]. PAF, in turn, is involved in a wide range of membrane-dependent processes and has potent biological activities towards various cell types and systems of an organism, including inflammation, circulation, reproduction, and development; for a review, see [82,83]. A deficiency of plasmalogens can impair some membrane-associated signaling, such as AKT/PKB, leading to a myelination defect [84], while supplementation with plasmalogens modulates important signaling pathways associated with ERK, AKT, p38, and JNK [85,86,87]. On the other hand, there are some studies which highlight the bioactivity of non-modified AGEs. For example, AGEs were found to accumulate in adipocytes upon differentiation and regulate adipogenesis [58], induce calcium influx in human lymphocytes [88], and inhibit PKC in vitro and in vivo [89].
MAP kinases play roles in various biological processes [85,86,87]. Due to the broad spectrum of biological targets and processes affected by AGEs, we have evaluated the importance of MAPK-dependent pathways for the cytotoxic effect of the isolated AGE fraction. In our study, using mouse epidermal JB6 Cl41 cells we demonstrated that ERK2- and JNK1- (but not p38) related pathways may play a cytoprotective role in the cytotoxic response to AGE exposure (Figure 3). Thus, the cells bearing knocked-out ERK2 and JNK1 genes are more sensitive to the cytotoxic effect of the isolated AGE fraction compared to the original JB6 Cl41 cells expressing nonmutant MAPK genes. Further experiments, including those examining an effect on MAPK activation, would be necessary to validate this speculation. Within the current study, the further examination of biological activity could not be performed due to the limited amount of the isolated AGE fraction.
The inhibitory effect of AGEs on protein kinase C [89] suggests their potent action against proliferative diseases. Indeed, it was shown that the treatment with AGEs prevents tumor growth in vivo due to the inhibition of angiogenesis [38,39,41,44]. Interestingly, unsaturated AGEs in some assays are significantly more active when compared to saturated molecules [38,39].
We demonstrated here that the AGE mixture inhibits the viability of seven human cancer cell lines representing different cancer entities (Table 3). In all the cell lines, apart from HL-60 and SK-MEL-28, the cytotoxic effects of AGEs from marine invertebrates were reported for the very first time. The highest cytotoxic activity, with IC50 = 35.9 µg/mL (Table 3), was shown for THP-1 cells, highlighting the potential of these and related compounds for the treatment of human leukemia. However, it should be noted that the cytotoxic activity of the isolated AGE fraction was overall rather weak.
4. Materials and Methods
4.1. General Procedures
The 1H and 13C NMR spectra were recorded on a Bruker DRX-300 spectrometer (Bruker GmbH, Bremen, Germany) at 300 and 75 MHz, respectively, in CDCl3 with tetramethylsilane as an internal standard.
The GC/MS data for the TMS derivatives were obtained using a Hewlett Packard GC HP6890 instrument (Agilent Technologies Inc., Santa Clara, CA, USA) with an HP5973 mass-selective detector. The injector and transfer line temperatures were 270 °C. A Hewlett Packard HP-5MS capillary column (Agilent Technologies Inc., Santa Clara, CA, USA), 30 m × 0.25 mm, phase layer 0.25 µm, was used at 100 °C with a 2 °C/min ramp to 270 °C, which was held for 30 min. The column contained 5% phenylmethylsiloxane and it was used as the mobile phases at a flow rate of 1 mL/min. The sample was dissolved in chloroform at a concentration of 10 mg/mL. The injection volume was 0.2 μL, and the split ratio was 15:1. The mass spectra were recorded at 70 eV.
The GC/MS data for the DMDS-derived AGEs were obtained using a Shimadzu GCMS-QP5050 instrument (Shimadzu, Kyoto, Japan). An MDN-5S capillary column (Shimadzu, Kyoto, Japan), 30 m × 0.25 mm, phase layer 0.25 µm, was used at 200 °C with a 2 °C/min ramp to 300 °C, which was held for 45 min. The split ratio was 15:1, and the flow rate was 1 mL/min. The injector temperature was 270 °C. The mass spectra were recorded at 70 eV.
Low-pressure column liquid chromatography was performed using KSK silica gel (50–100 μm, Sorbpolymer, Krasnodar, Russia). Sorbfil silica gel plates (4.5 × 6.0 cm, 5–17 μm, Sorbpolymer, Krasnodar, Russia) were used for TLC. HPLC was performed using an Agilent 1100 instrument (Agilent Technologies Inc, Santa Clara, CA, USA) equipped with a differential refractometer on a Diasorb-60-Silicagel (250 × 4.6 mm) column (BioChemMak, Moscow, Russia). Cells were counted using an Olympus inverted research microscope (Olympus, Tokyo, Japan). The absorption of MTS/farmazan was measured spectrophotometrically using the μQuant microplate reader (Bio-Tek Instruments Inc., Winooski, VT, USA).
4.2. Reagents
Minimum essential medium (MEM), DMEM, and RPMI medium were purchased from BioloT (Sankt-Peterburg, Russian Federation); fetal bovine serum (FBS) was purchased from Thermo Fisher Scientific (Cramlington, Northumberland, UK); penicillin/streptomycin was purchased from Bio-Whittaker (Walkersville, MD, USA); and L-glutamine was purchased from Mediatech Inc. (Herndon, VA, USA). The Cell Titer 96 Aqueous One Solution Reagent (MTS) kit for the cell viability assay was purchased from Promega (Madison, WI, USA).
4.3. Animal Material
The marine sponge Guitarra abbotti (family Guitarridae, order Poecilosclerida) was collected by dredging at the depth of 109 m at 48°00′08′′ N, 153°20′07′′ E, Kuril Islands, the Sea of Okhotsk, Pacific Ocean. A voucher specimen is kept in the collection of the G.B. Elyakov Pacific Institute of Bioorganic Chemistry. The taxonomic identification was performed by V.B. Krasokhin.
4.4. Extraction and Isolation
Animal materials (1050 g, wet weight) were extracted with EtOH (2 L) immediately after collection. After evaporation in vacuo, the ethanol extract was re-dissolved in 200 mL of EtOH/H2O (5:1, v/v) and extracted with 3 × 200 mL of n-hexane. The aqueous-ethanolic and combined n-hexane fractions after evaporation were subjected to an evaluation of cytotoxic activity against human leukemia THP-1 cells by the MTS method and showed IC50 = 0.6 and 0.5 mg/mL, respectively. The n-hexane fraction was selected for the further isolation of anticancer compounds. This fraction (3.525 g) was evaporated and subjected to column chromatography on a silica gel column (diameter/length = 6:14 cm) using an n-hexane/AcOEt gradient as an eluent with n-hexane to AcOEt ratios of 19:1, 9:1, 4:1, 3:2, 1:1, 2:3, 1:4, 1:9, and 1:19 (v/v), then with 100% EtOAc and then with 100% EtOH. A 200 mL volume of eluent was used for the elution of each fraction. A subsequent evaluation of the cytotoxic activity revealed that the fraction eluted with n-hexane/AcOEt (2:3, v/v) possessed the highest cytotoxicity towards THP-1 cells, IC50 = 125 μg/mL. This fraction (299 mg) was further subjected to the same silica gel column chromatography using 330 mL of n-hexane/AcOEt (3:2, v/v) and 400 mL of n-hexane/AcOEt (1:1, v/v) as eluents. The collected fractions (10 mL each) were examined using TLC (SiO2 and n-hexane/AcOEt (1:1, v/v)) as a chromatographic system, with the further detection of spots on the TLC chromatograms using H2SO4/EtOH (1:9, v/v). Following the TLC analysis, three fractions were obtained, i.e., (i) fraction #1 (one grey spot on the TLC, Rf = 0.5); (ii) fraction #2 (one violet spot on the TLC with Rf = 0.2); and (iii) fraction #3 (two spots on the TLC, Rf = 0.5 and Rf = 0.2). The examination of the cytotoxic activity against human THP-1 cells revealed fraction #2 to be the most active, with IC50 = 62.5 μg/mL. Further, fraction #2 (185 mg, dry weight) was additionally purified by HPLC using a Diasorb-60-Silica gel column and n-hexane/AcOEt (3:2, v/v) as the eluent. As a result, the more active mixture (IC50 = 35.9 μg/mL in THP-1 cells) of the purified 1-O-alkylglycerol ethers (AGEs) was obtained (160 mg, dry weight).
4.5. Characterization of the Purified 1-O-Alkylglycerol Ether (AGE) Mixture
The chemical nature of the isolated purified fraction was established using their NMR spectra. Generally, the next spectroscopic information was obtained.
1H NMR (CDCl3, 300 MHz) δ 0.85 d, J = 6.6 Hz, iso-CH3; 0.88 t, J = 6.7 Hz, n-CH3; 1.22-1.38, m, (CH2)n, aliphatic chain; 1.57, quint, J = 6.9 Hz, 2H-2’; 2.01, q, J = 6.3 Hz, 2H allylic protons; 3.46, td, J = 6.6 Hz, J = 1.6 Hz, 2H-1’; 3.55, dd, J = 9.2 Hz, J = 3.5 Hz, 2H-1; 3.64 dd, J = 11.4 Hz, J = 5.2 Hz, 1H-3a; 3.72 dd, J = 11.4 Hz, J = 3.8 Hz, 1H-3b; 3.86, m, 1H-2; 5.34, t, J = 4.6 Hz, 2H, olefinic protons.
13C NMR (CDCl3, 75 MHz) δ 14.10 (CH3); 22.64 (CH2); 26.05 (2CH2, allylic carbons); 27.19 (CH2); 28.97 (CH2); 29.07 (CH2); 29.30 (CH2); 29.34 (CH2); 29.44 (CH2); 29.54 (CH2); 29.66 (CH2); 31.76 (CH2); 31.90 (CH2); 64.24 (CH2-3); 70.40 (CH-2); 71.84 (CH2-1); 72.48 (CH2-1’); 129.88 (CH=CH).
These data indicate the AGE nature of the compounds in the isolated mixture. These compounds contain a glycerol moiety linked by an ether bond with a fatty alcohol residue. In the analyzed compounds, the fatty alcohol residues contain either normal or iso-ends, and some of them have an additional disubstituted double bond. The majority of the identified metabolite compounds have previously been isolated from different biological sources. However, six of them (1–6) were new natural products (see below).
4.6. Preparation of Trimethylsilyl Derivatives (TMS-d) and Their GC/MS Analysis
First, 0.1 mg of dried AGE mixture was treated with 0.1 mL of BSTFA (Supelco, Bellefonte, PA, USA) at 60 °C for 1 h to convert the AGEs to their trimethylsilyl derivatives (TMS-d). The obtained TMS-d were analyzed by the GC/MS method (Figures S4 and S5, Supplementary Materials). The mass spectra of TMS-d of the new and previously known AGEs, although the exact position of the double bond could not be precisely determined in some cases, provide valuable information about their structures.
The mass spectra data of the TMS-d for the previously known compounds (7–28) are given below.
TMS-d of 1-O-(tetradecanyl)-sn-glycerol (7), TR 16.07, m/z 417, 285, 205, 147, 117, 103, 73; 57; TMS-d of 1-O-(pentadecanyl)-sn-glycerol (8), TR 16.58, m/z 431, 356, 299, 205, 147, 117, 103, 73; 57; TMS-d of 1-O-(pentadecanyl)-sn-glycerol (9), TR 16.87, m/z 431, 356, 299, 205, 147, 117, 103, 73; 57; TMS-d of 1-O-(hexadecanyl)-sn-glycerol (10), TR 17.35, m/z 445, 370, 313, 205, 147, 117, 103, 73, 57; TMS-d of 1-O-(Z-hexadec-7’ or 9’ or 11’ or 13’-enyl)-sn-glycerol (17 or 18 or 19 or 20), TR 17.51, m/z 458, 443, 355, 311, 265, 205, 147, 117, 103, 73, 55; TMS-d of 1-O-(Z-hexadec-7’ or 9’ or 11’ or 13’-enyl)-sn-glycerol (17 or 18 or 19 or 20), TR 17.59, m/z 458, 355, 311, 265, 205, 147, 117, 103, 73, 55; TMS-d of 1-O-(hexadecanyl)-sn-glycerol (11), TR 17.64, m/z 445, 370, 313, 205, 147, 117, 103, 73, 57; TMS-d of 1-O-(heptadecanyl)-sn-glycerol (12), TR 18.10, m/z 459, 384, 327, 205, 147, 117, 103, 73, 57; TMS-d of 1-O-(heptadecanyl)-sn-glycerol (13), TR 18.18, m/z 459, 384, 327, 205, 147, 117, 103, 73, 57; TMS-d of 1-O-(heptadecanyl)-sn-glycerol (14), TR 18.38, m/z 459, 384, 327, 205, 147, 117, 103, 73, 57; TMS-d of 1-O-(octadecanyl)-sn-glycerol (15), TR 18.80, m/z 473, 398, 341, 205, 147, 117, 103, 73, 57; TMS-d of 1-O-(Z-octadec-9’ or 11’ or 12’ or 13’-enyl)-sn-glycerol (21 or 22 or 23 or 24), TR 18.93, m/z 486, 471, 293, 205, 147, 117, 103, 73, 55; TMS-d of 1-O-(Z-octadec-9’ or 11’ or 12’ or 13’-enyl)-sn-glycerol (21 or 22 or 23 or 24), TR 18.99, m/z 486, 471, 293, 205, 147, 117, 103, 73, 55.; TMS-d of 1-O-(octadecanyl)-sn-glycerol (16), TR 19.10, m/z 473, 398, 341, 205, 147, 117, 103, 73, 57; TMS-d of 1-O-(Z-cos-11’ or 13’ or 15’-enyl)-sn-glycerol (25 or 26 or 27), TR 20.38, m/z 514, 321, 205, 147, 117, 103, 73, 55; TMS-d of 1-O-(Z-tetracos-17’-enyl)-sn-glycerol (28), TR 23.43, m/z 480, 467, 390, 377, 205, 147, 117, 103, 73, 55.
For the six new identified compounds (1–6) (Figure 1), the mass spectra of their TMS-d are given below.
TMS-d of 1-O-(Z-docos-15’-enyl)-sn-glycerol (1), retention time (TR) 21.74, m/z 542, 527, 395, 349, 205, 147, 117, 103, 73, 55; TMS-d of 1-O-(Z-docos-17’-enyl)-sn-glycerol (2), TR 21.81, m/z 542, 527, 395, 349, 205, 147, 117, 103, 73, 55; TMS-d of 1-O-(Z-tricos-15’-enyl)-sn-glycerol (3) or 1-O-(Z-tricos-16’-enyl)-sn-glycerol (4), or 1-O-(Z-tricos-17’-enyl)-sn-glycerol (5), TR 22.56, m/z 363, 205, 147, 117, 103, 73, 55; TMS-d of 1-O-(Z-tetracos-15’-enyl)-sn-glycerol (6), TR 23.35, m/z 480, 467, 390, 377, 205, 147, 117, 103, 73, 55.
4.7. Preparation of Acetate Derivatives
First, 1.3 mg of the dried AGE mixture was treated with 0.4 mL of Ac2O/Py (1:1, v/v) at RT overnight to convert the AGEs to their diacetate derivatives. Then, 2 mL of EtOH was added, and the sample was dried in vacuo.
4.8. Preparation of Dimethyldisulfide Acetate Derivatives (DMDS-d) of AGEs and Their GC/MS Analysis
First, 1.2 mg of dried AGE acetate mixture (see above) was mixed with 0.2 mL of dimethyldisulfide (DMDS) and 0.05 mL of iodine solution in Et2O (60 mg/mL) and incubated at RT overnight. Then, 5 mL of n-hexane was added, and the mixture was washed with 5 mL of an aqueous 5% solution of Na2S2O3 × 5H2O until the color of iodine disappeared. The n-hexane fraction was separated, and the reaction products were extracted one more time from the polar fraction using n-hexane (1 mL). The combined n-hexane fractions were dried over sodium sulfate, evaporated in vacuo, and re-dissolved in hexane for further analysis.
Mass spectra of the DMDS-d of the new AGEs, 1–6:
DMDS-d of 1-O-(Z-docos-15’-enyl)-sn-glycerol (1), TR 70.67, m/z 576, 529, 431, 371, 311, 285, 263, 255, 206, 159, 145, 97, 83, 69, 55; DMDS-d of 1-O-(Z-docos-17’-enyl)-sn-glycerol (2), TR 72.07, m/z 576, 529, 459, 399, 339, 313, 291, 283, 234, 159, 117, 97, 83, 69, 55; DMDS-d of 1-O-(Z-tricos-15’-enyl)-sn-glycerol (3), TR 77.50, m/z 590, 436, 371, 341, 311, 173, 159, 145, 117, 97, 83, 69, 55; DMDS-d of 1-O-(Z-tricos-16’-enyl)-sn-glycerol (4), TR 77.90, m/z 590, 436, 385, 341, 297, 159, 145, 117, 97, 83, 69, 55; DMDS-d of 1-O-(Z-tricos-17’-enyl)-sn-glycerol (5), TR 78.67, m/z 590, 436, 399, 341, 283, 159, 145, 131, 117, 97, 83, 69, 55; DMDS-d of 1-O-(Z-tetracos-15’-enyl)-sn-glycerol (6), TR 86.27, m/z 604, 450, 371, 311, 255, 207, 173, 159, 109, 97, 83, 69, 55.
Mass spectra of the DMDS-d of the previously known AGEs:
DMDS-d of 1-O-(Z-hexadec-7’-enyl)-sn-glycerol (17), TR 44.69, m/z 492, 319, 259, 173, 159, 143, 95, 89, 81, 69, 61, 55; DMDS-d of 1-O-(Z-hexadec-9’-enyl)-sn-glycerol (18), TR 45.00, m/z 492, 347, 287, 171, 159, 145, 95, 89, 81, 69, 61, 55; DMDS-d of 1-O-(Z-hexadec-11’-enyl)-sn-glycerol (19), TR 45.69, m/z 492, 375, 315, 199, 159, 117, 95, 89, 81, 69, 61, 55; DMDS-d of 1-O-(Z-hexadec-13’-enyl)-sn-glycerol (20), TR 47.69, m/z 492, 403, 343, 227, 159, 95, 89, 81, 69, 61, 55; DMDS-d of 1-O-(Z-octadec-9’-enyl)-sn-glycerol (21), TR 51.10, m/z 520, 347, 287, 227, 173, 159, 95, 81, 69, 55; DMDS-d of 1-O-(Z-octadec-11’-enyl)-sn-glycerol (22), TR 51.40, m/z 520, 375, 315, 255, 145, 159, 95, 81, 69, 55; DMDS-d of 1-O-(Z-octadec-12’-enyl)-sn-glycerol (23), TR 51.62, m/z 520, 389, 329, 269, 131, 159, 95, 81, 69, 55; DMDS-d of 1-O-(Z-octadec-13’-enyl)-sn-glycerol (24), TR 52.13, m/z 520, 403, 343, 283, 117, 159, 95, 81, 69, 55; DMDS-d of 1-O-(Z-cos-11’-enyl)-sn-glycerol (25), TR 58.48, m/z 548, 375, 315, 255, 199, 159, 117, 109, 95, 83, 69, 55; DMDS-d of 1-O-(Z-cos-13’-enyl)-sn-glycerol (26), TR 59.39, m/z 548, 403, 343, 227, 159, 117, 109, 95, 83, 69, 55; DMDS-d of 1-O-(Z-cos-15’-enyl)-sn-glycerol (27), TR 60.29, m/z 548, 431, 371, 255, 199, 159, 117, 109, 95, 83, 69, 55; DMDS-d of 1-O-(Z-tetracos-17’-enyl)-sn-glycerol (28), TR 87.30, m/z 604, 459, 399, 339, 283, 159, 145, 97, 83, 69, 55.
4.9. Cell Culture
The JB6 P+ Cl41 mouse epidermal cell line and its stable transfectants JB6 Cl 41 DN-JNK1, JB6 Cl 41 DN-p38, and JB6 Cl 41 DN-ERK2, which have the knockout JNK1 p38 and ERK2 genes, respectively, were cultured as monolayers at 37 °C and 5% CO2 in MEM containing 5% fetal bovine serum (FBS), 2 mM L-glutamine, 100 units/mL penicillin, and 100 mg/mL streptomycin. The human cancer cell lines HL-60 (promyelocytic leukemia), THP-1 (monocytic leukemia), HeLa (cervix carcinoma), SNU C4 (colon cancer), DLD-1 (colon cancer), MDA-MB-231 (breast adenocarcinoma), and SK-MEL-28 (melanoma) were obtained from the American Type Culture Collection (Rockville, MD, USA). The HL-60, THP-1, HeLa, DLD-1, SNU C4, and SK-MEL-28 cancer cell lines were cultured at 37 °C and 5% CO2 in RPMI medium containing 10% FBS, 2 mM L-glutamine, 100 units/mL penicillin, and 100 mg/mL streptomycin. The MDA-MB-231 cancer cell line was cultured at 37 °C and 5% CO2 in DMEM containing 10% FBS, 2mM L-glutamine, 100 units/mL penicillin, and 100 mg/mL streptomycin. HL-60 and THP-1 cells were cultured as suspensions, and the other cell lines were cultured as monolayers. Information regarding the genetic background of these cell lines is available online at the ATCC website.
4.10. Cell Viability Test
The effect of the obtained AGE mixture on the viability of the THP-1, HL-60, HeLa, DLD-1, SNU C4, SK-MEL-28, MDA-MB-231, JB6 P+ Cl41, JB6 Cl 41 DN-JNK1, JB6 Cl 41 DN-p38, and JB6 Cl 41 DN-ERK2 cell lines was evaluated using the MTS assay [75]. Briefly, corresponding cells were seeded in 96-well plates (6000 cells per well) and incubated overnight in 100 μL of medium per well for adherent cells, or 50 μL/well for non-adherent cells (THP-1, HL-60). For adherent cells, the media were then replaced with fresh media containing the AGE mixture at various concentrations in a total volume of 0.1 mL per well, and the cells were incubated for 22 h. For suspension cells, 50 μL /well of fresh medium containing the AGE mixture was added, and the cells were incubated for 22 h. Then, 10 mL of the MTS reagent was added into each well, and the MTS reduction was measured spectrophotometrically 2 h later at 492 nm and 690 nm (background) using the µQuant microplate reader.
4.11. Statistical Analysis
The statistical analyses were performed using Statistica 6.0 (StatSoft, Inc., Tulsa, OK, USA) or GraphPad Prism v.9.1.1 software (GraphPad Software, San Diego, CA, USA). The results of two independent experiments, each performed in triplicate, were used for the analyses. Significant differences from control were calculated using Student’s t-test. The method of regressions was used to calculate the IC50 values.
5. Conclusions
In conclusion, 6 new and 22 previously known 1-O-alkylglycerol ethers were identified in the AGE fraction isolated from the marine sponge Guitarra abbotti. Their structures were established using 1H and 13C NMR spectroscopy and a GC/MS analysis of their TMS and DMDS derivatives as well as a comparison with the literature data. The isolated AGE fraction consisted of both saturated and unsaturated AGEs, which presented at a nearly equimolar ratio. The isolated AGEs exhibited a rather weak cytotoxic activity towards seven human cancer cell lines. Moreover, the active MAP kinases ERK2 and JNK1 were shown to play a cytoprotective role in the cellular response to the AGE-induced cytotoxic effects.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/md20070409/s1, Figure S1: 1H NMR spectrum of AGE mixture in CDCl3 (300 MHz); Figure S2: 13C NMR spectrum of AGE mixture in CDCl3 (75 MHz); Figure S3: DEPT spectrum of AGE mixture in CDCl3; Figure S4: GC data for the TMS-derivatives of AGEs; Figure S5: GC/MS data for the TMS-derivatives of AGEs; Figure S6: GC data for the DMDS-derivatized AGEs; Figure S7: GC/MS data for the DMDS-derivatized AGEs.
Author Contributions
Conception and design, S.A.D., S.N.F., T.N.M., G.v.A. and V.A.S.; Development of methodology, T.N.M., S.A.D., S.N.F., and V.A.S.; Acquisition of data, V.I.S., L.K.S., A.G.G., A.I.K., O.P.M., S.N.F. and S.A.D.; Data analysis, all authors; Data interpretation, all authors; Taxonomic identification of the animal material, V.B.K.; Anticancer activity examination, S.A.D. and S.N.F.; Writing—original draft preparation, S.A.D. and S.N.F.; Writing—review and editing, all authors; Review and/or revision of the final version of the manuscript, all authors; Artwork, S.A.D. and S.N.F.; Fundraising, V.A.S. and G.v.A.; Study supervision, S.N.F. and S.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
The chemical part of this study was supported by the Ministry of Science and Higher Education of the Russian Federation (grant 13.1902.21.0012; contract No. 075-15-2020-796).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original data are available from the correspondent authors on request.
Acknowledgments
We devote this article to the 80th anniversary of the birth of an outstanding Russian scientist, Academician of the Russian Academy of Sciences, Professor Valentin A. Stonik, who made a great contribution to the study of various natural products. For many years Professor Stonik has been leading a scientific school known worldwide working on marine and terrestrial natural compounds from diverse chemical classes such as steroids, terpenoids, alkaloids, glycosides, peptides, and lipids as well as products of mixed biosynthesis. The authors are grateful to Z. Dong (Hormel Institute, Austin, Minnesota, USA), who kindly provided JB6 cells used in this work. The study was carried out using the equipment of the Collective Facilities Center “The Far Eastern Center for Structural Molecular Research (NMR/MS) (CSMR PIBOC FEB RAS)”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Y.; Jung, J.H.; Ji, H.; Zhang, S. Glicerolipids from a Sarcotragus species sponge. Molecules 2006, 11, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Zhao, Q.; Choi, K.; Hong, J.; Lee, D.S.; Lee, C.-O.; Jung, J.H. A new glycerol ether from a marine sponge Stelleta species. Nat. Prod. Sci. 2003, 9, 232–234. [Google Scholar]
- Seo, Y.; Cho, K.W.; Lee, H.-S.; Rho, J.-R.; Shin, J. New acetylenic enol ethers of glycerol from the sponge Petrosia sp. J. Nat. Prod. 1999, 62, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.D.; Wahidullah, S.; D’Souza, L.D.; Kamat, S.Y. Steroids from marine sponges Suberites vestigium and Chrotella australiensis. Indian J. Chem. 1997, 36B, 719–721. [Google Scholar]
- Quijano, L.; Cruz, F.; Navarrete, I.; Gomez, P.; Rios, T. Alkyl glycerol monoethers in the marine sponge Desmapsamma anchorata. Lipids 1994, 29, 731–734. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Aknin, M.; Fall, A.; Samb, A.; Miralles, J. An unusual ether glycolipid from the senegalese sponge Trikentrion loeve Carter. Tetrahedron 1993, 49, 2711–2716. [Google Scholar] [CrossRef]
- Prinsep, M.R.; Blunt, J.W.; Munro, M.H.G. A new sterol sulfate from the marine sponge Stylopus australis. J. Nat. Prod. 1989, 52, 657–659. [Google Scholar] [CrossRef]
- Smith, G.M.; Djerassi, C. Phospholipid studies of marine organisms: 14. Ether lipids of the sponge Tethya aurantia. Lipids 1987, 22, 236–240. [Google Scholar] [CrossRef]
- Guella, G.; Mancini, I.; Pietra, F. (+)-Raspailyne-A, a novel, acid-sensitive acetylenic enol ether glyceride from the marine sponge Raspailia pumila. J. Chem. Soc. Chem. Commun. 1986, 1, 77–78. [Google Scholar] [CrossRef]
- Myers, B.L.; Crews, P. Chiral ether glycerides from a marine sponge. J. Org. Chem. 1983, 48, 3583–3585. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Vanderah, D.J.; Hollenbeak, K.H.; Enwall, C.E.L.; Gopichand, Y.; SenGupta, P.K.; Hossain, M.B.; van der Helm, D. Metabolites from the marine sponge Tedania ignis. A new atisanediol and several known diketopiperazines. J. Org. Chem. 1983, 48, 3941–3945. [Google Scholar] [CrossRef]
- Cardellina, J.H.; Graden, C.J.; Greer, B.J. 17Z-Tetracosenyl 1-glycerol ether from the sponges Cinachyra alloclada and Ulosa ruetzleri. Lipids 1983, 18, 107–110. [Google Scholar] [CrossRef]
- Do, M.N.; Erickson, K.L. Branched chain mono-glycerol ethers from a Taiwanese marine sponge of the genus Aaptos. Tetrahedron Lett. 1983, 24, 5699–5702. [Google Scholar] [CrossRef]
- McClintock, J.B.; Baker, B.J.; Slattery, M.; Neine, J.N.; Bryan, P.J.; Yoshida, W.; Davies-Coleman, M.T.; Faulkner, D.J. Chemical defence of common Antarctic shallow-water nudibranch Tritoniella belli Eliot (Mollusca: Tritonidae) and its prey, Clavularia frankliniana Rouel (Cnidaria: Octocorallia). J. Chem. Ecol. 1994, 20, 3361–3372. [Google Scholar] [CrossRef]
- Imbs, A.B.; Demidkova, D.A.; Dautova, T.N. Lipids and fatty acids of cold-water soft corals and hydrocorals: A comparison with tropical species and implications for coral nutrition. Mar. Biol. 2016, 163, 202. [Google Scholar] [CrossRef]
- Diaz, Y.M.; Laverde, G.V.; Gamba, L.R.; Wandurraga, H.M.; Arevalo-Ferro, C.; Rodriguez, F.R.; Bertran, C.D.; Hernandez, L.C. Biofilm inhibition activity of compounds isolated from two Eunicea species collected at the Caribbean Sea. Rev. Bras. Farm. 2015, 25, 605–611. [Google Scholar] [CrossRef][Green Version]
- Han, A.-R.; Song, J.-I.; Jang, D.S.; Min, H.-Y.; Lee, S.K.; Seo, E.-K. Cytotoxic constituents of the octocoral Dendronephthya gidantea. Arch. Pharm. Res. 2005, 28, 290–293. [Google Scholar] [CrossRef]
- Pettit, G.R.; Fujii, Y. Antineoplastic agents. 81. The glycerol ethers of Palythoa liscia. J. Nat. Prod. 1982, 45, 640–643. [Google Scholar] [CrossRef]
- Kind, C.A.; Bergmann, W. Contributions to the study of marine products. XI. The occurence of octadecyl alcohol, batyl alcohol, and cetyl palmitate in gorgonias. J. Org. Chem. 1942, 7, 424–427. [Google Scholar] [CrossRef]
- Gustafson, K.; Andersen, R.J. Chemical studies of British Columbia nudibranchs. Tetrahedron 1985, 41, 1101–1108. [Google Scholar] [CrossRef]
- Latyshev, N.A.; Ermakova, S.P.; Ermolenko, E.V.; Imbs, A.B.; Kasyanov, S.P.; Sultanov, R.M. 1-O-alkylglycerols from the hepatopancreas of the crab Paralithodes camtschaticus, liver of the squid Berryteuthis magister, and liver of the skate Bathyraja parmifera, and their anticancer activity on human melanoma cells. J. Food Biochem. 2019, 43, e12828. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.A.; Manzhulo, I.V.; Sultanov, R.M.; Ermolenko, E.V. Adult hippocampal neurogenesis in neuropathic pain and alkyl glycerol ethers treatment. Acta Histochem. 2017, 119, 812–821. [Google Scholar] [CrossRef]
- Hayashi, K.; Okawa, Y.; Kawasaki, K. Liver lipids of gonatid squid Berryteuthis magister: A rich source of alkyi glyceryl ethers. Bull. Jap. Soc. Sci. Fish. 1985, 51, 1523–1526. [Google Scholar] [CrossRef]
- Phleger, C.F.; Nichols, P.D.; Virtue, P. Lipids and buoyancy in Southern ocean pteropods. Lipids 1997, 32, 1093–1100. [Google Scholar] [CrossRef]
- Thompson, G.A.; Lee, P. Studies of the α-glyceryl ether lipids occurring in molluscan tissues. Biochim. Biophys. Acta 1965, 98, 151–159. [Google Scholar] [CrossRef]
- Hayashi, K. Content and composition of glyceryl ethers in the pyloric ceca and ovaries of the starfish Distolasterias nippon, Asterina pectinifera, and Lysastrosoma anthosticta. Fish. Sci. 1998, 64, 852–853. [Google Scholar] [CrossRef][Green Version]
- Rybin, V.; Pavel, K.; Mitrofanov, D. 1-O-Alkylglycerol ether lipids in two holothurian species: Cucumaria japonica and C. okhotensis. Nat. Prod. Commun. 2007, 2, 933–936. [Google Scholar] [CrossRef]
- Santos, V.L.C.; Billett, D.S.M.; Wolff, G.A. 1-O-Alkylglyceryl ether lipids of the gut walls and contents of an Abyssal holothurian (Oneirophanta mutabilis). J. Braz. Chem. Soc. 2002, 13, 653–657. [Google Scholar] [CrossRef][Green Version]
- Takeara, R.; Jimenez, P.C.; Wilke, D.V.; de Moraes, M.O.; Pessoa, C.; Lopes, N.P.; Lopes, J.L.C.; da Cruz Lotufo, T.M.; Costa-Lotufo, L.V. Antileukemic effects of Didemnum psammatodes (Tunicata: Ascidiacea) constituents. Comp. Biochem. Physiol. Part A 2008, 151, 363–369. [Google Scholar] [CrossRef]
- Othmani, A.; Bunet, R.; Bonnefont, J.-L.; Briand, J.-F.; Culioli, G. Settlement inhibition of marine biofilm bacteria and barnacle larvae by compounds isolated from the Mediterranean brown alga Taonia atomaria. J. Appl. Phycol. 2016, 28, 1975–1986. [Google Scholar] [CrossRef]
- Magnusson, C.D.; Haraldsson, G.G. Ether lipids. Chem. Phys. Lipids 2011, 164, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Urate, K.; Takaishi, N. Ether lipids based on the glyceryl ether skeleton: Present state, future potential. JAOCS 1996, 73, 819–830. [Google Scholar] [CrossRef]
- Baer, E.; Fisher, H.O.L. Studies on acetone-glyceraldehyde, and optically active glycerides IX. Configuration of the natural batyl, chimil, and selachyl alcohols. J. Biol. Chem. 1941, 140, 397–410. [Google Scholar] [CrossRef]
- IUPAC-IUB Commission on Biochemical Nomenclature. The nomenclature of lipids (Recommendations 1976). J. Lipid Res. 1978, 19, 114–128. [Google Scholar] [CrossRef]
- Taguchi, H.; Armarego, W.L.F. Glyceryl-ether monooxygenase [EC 1.14.16.5]. A microsomal enzyme of ether lipid metabolism. Med. Res. Rev. 1998, 18, 43–89. [Google Scholar] [CrossRef]
- Watschinger, K.; Keller, M.A.; Golderer, G.; Hermann, M.; Maglione, M.; Sarg, B.; Lindner, H.H.; Hermetter, A.; Werner-Felmayer, G.; Konrat, R.; et al. Identification of the gene encoding alkylglycerol monooxygenase defines a third class of tetrahydrobiopterin-dependent enzymes. Proc. Natl. Acad. Sci. USA 2010, 107, 13672–13677. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, T.; Palmieri, B. An update on the therapeutic role of alkylglycerols. Mar. Drugs 2010, 8, 2267–2300. [Google Scholar] [CrossRef] [PubMed]
- Deniau, A.-L.; Mosset, P.; Le Bot, D.; Legrand, A.B. Which alkylglycerols from shark liver oil have anti-tumour activities? Biochimie 2011, 93, 1–3. [Google Scholar] [CrossRef]
- Deniau, A.-L.; Le Bot, D.; Mosset, P.; Legrand, A.B. Activités antitumorale et antimétastasique des alkylglycérols naturels: Relation structure-activité. OCL Ol. Corps Gras Lipides 2010, 17, 236–237. (In French) [Google Scholar] [CrossRef]
- Pedrono, F.; Saıag, B.; Moulinoux, J.-P.; Legrand, A.B. 1-O-Alkylglycerols reduce the stimulating effects of bFGF on endothelial cell proliferation in vitro. Cancer Lett. 2007, 251, 317–322. [Google Scholar] [CrossRef]
- Pedrono, F.; Martin, B.; Leduc, C.; Le Lan, J.; Saiag, B.; Legrand, P.; Moulinoux, J.-P.; Legrand, A.B. Natural alkylglycerols restrain growth and metastasis of grafted tumors in mice. Nutr. Cancer 2004, 48, 64–69. [Google Scholar] [CrossRef]
- Krotkiewski, M.; Przybyszewska, M.; Janik, P. Cytostatic and cytotoxic effects of alkylglycerols (Ecomer). Med. Sci. Monit. 2003, 9, 131–135. [Google Scholar]
- Hernández-Colina, M.; Cermeño, A.M.; García, A.D. Selective cytotoxic effect of 1-O-undecylglycerol in human melanoma cells. J. Pharm. Pharmacog. Res. 2016, 4, 84–94. [Google Scholar]
- Brohult, A.; Brohult, J.; Brohult, S. Regression of tumor growth after administration of alkoxyglycerols. Acta Obstet. Gynecol. Scand. 1978, 57, 79–83. [Google Scholar] [CrossRef]
- Brohult, A.; Brohult, J.; Brohult, S. Biochemical effects of alkylglycerols and their use in cancer therapy. Acta Chem. Scand. 1970, 24, 730–732. [Google Scholar] [CrossRef]
- Di Iannitti, T.; Capone, S.; Palmieri, B. A telephone interview to assess alkylglycerols effectiveness in preventing influenza-like symptoms in Modena, Emilia Romagna, Italy, in the season 2009–2010. Clin. Ter. 2011, 162, e115–e118. [Google Scholar]
- Daza, K.E.C.; Gómez, E.S.; Murillo, B.D.M.; Wandurraga, H.M. Natural and enantiopure alkylglycerols as antibiofilms against clinical bacterial isolates and quorum sensing inhibitors of Chromobacterium violaceum ATCC 12472. Antibiotics 2021, 10, 430. [Google Scholar]
- Montoya, D.J.F.; Jordan, L.A.C.; Murillo, B.M.; Gomez, E.S.; Wandurraga, H.M. Enantiomeric synthesis of natural alkylglycerols and their antibacterial and antibiofilm activities. Nat. Prod. Res. 2021, 35, 2544–2550. [Google Scholar] [CrossRef]
- Hayens, M.P.; Buckley, H.R.; Higgins, M.L.; Pieringer, R.A. Synergism between the antifungal agents amphotericin B and alkyl glycerol ethers. Antimicrob. Agent. Chemother. 1994, 38, 1523–1529. [Google Scholar] [CrossRef]
- Nascimento, T.S.; Monteiro, L.G.; Braga, E.F.; Batista, W.R.; Albert, A.L.M.; Chantre, L.G.F.; de Machado, S.P.; Lopes, R.S.C.; Lopes, C.C. Synthesis of natural ether lipids and 1-O-hexadecylglycero-arylboronates via an epoxide-ring opening approach: Potential antifouling additives to marine paint coatings. Int. J. Adv. Res. Sci. Eng. Technol. 2018, 5, 326–332. [Google Scholar] [CrossRef][Green Version]
- Parri, A.; Fito, M.; Torres, C.F.; Munoz-Aguayo, D.; Schroder, H.; Cano, J.F.; Vazquez, L.; Reglero, G.; Covas, M.-I. Alkylglycerols reduce serum complement and plasma vascular endothelial growth factor in obese individuals. Inflammopharmacology 2016, 24, 127–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brohult, A.; Brohult, J.; Brohult, S.; Joelsson, I. Effect of alkoxyglycerols on the frequency of fistulas following radiation therapy for carcinoma of the uterine cervix. Acta Obstet. Gynecol. Scand. 1979, 58, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Alexander, P.; Connell, D.I.; Brohult, A.; Brohult, S. Reduction of radiation induced shortening of life span by a diet augmented with alkoxy glycerol esters and essential fatty acids. Gerontologia 1959, 3, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Bronhult, A.; Holmberg, J. Alkoxyglycerols in the treatment of leukopenia caused by irradiation. Nature 1954, 174, 1102–1103. [Google Scholar] [CrossRef]
- Yu, H.; Dilbaz, S.; Cobmann, J.; Hoang, A.C.; Diedrich, V.; Herwig, A.; Harauma, A.; Hoshi, Y.; Moriguchi, T.; Landgraf, K.; et al. Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J. Clin. Investig. 2019, 129, 2485–2499. [Google Scholar] [CrossRef]
- Hofer, D.C.; Pessentheiner, A.R.; Pelzmann, H.J.; Schlager, S.; Madreiter-Sokolowski, C.T.; Kolb, D.; Eichmann, T.O.; Rechberger, G.; Bilban, M.; Graier, W.F.; et al. Critical role of the peroxisomal protein PEX16 in white adipocyte development and lipid homeostasis. Biochim. Biophys. Acta 2017, 1862, 358–368. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, S.; Tang, N.; Cai, W.; Qian, L. Oral administration of alkylglycerols differentially modulates high-fat diet-induced obesity and insulin resistance in mice. Evid. Based Complement. Alter. Med. 2013, 2013, 834027. [Google Scholar] [CrossRef]
- Homan, E.A.; Kim, Y.-G.; Cardia, J.P.; Saghatelian, A. Monoalkylglycerol ether lipids promote adipogenesis. J. Am. Chem. Soc. 2011, 133, 5178–5181. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.A.; Manzhulo, I.V.; Kipryushina, Y.; Ermolenko, E.V. Neuroinflammation and adult hippocampal neurogenesis in neuropathic pain and alkyl glycerol ethers treatment in aged mice. Int. J. Mol. Med. 2019, 43, 2153–2163. [Google Scholar] [CrossRef]
- Acevedo, R.; Gil, D.; del Campo, J.; Bracho, G.; Valdes, Y.; Perez, O. The adjuvant potential of synthetic alkylglycerols. Vaccine 2006, 24, S32–S33. [Google Scholar] [CrossRef]
- Ngwenya, B.Z.; Foster, D.M. Enhancement of antibody production by lysophosphatidylcholine and alkylglycerol. Proc. Soc. Exp. Biol. Med. 1991, 196, 69–75. [Google Scholar] [CrossRef]
- Marigny, K.; Pedrono, F.; Martin-Chouly, C.A.E.; Youmine, H.; Saiag, B.; Legrand, A.B. Modulation of endothelial permeability by 1-O-alkylglycerols. Acta Physiol. Scand. 2002, 176, 263–268. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, M.; Wu, S.; Zhong, Y.; Van Tol, E.; Cai, W. Alkylglycerols modulate the proliferation and differentiation of non-specific agonist and specific antigen-stimulated splenic lymphocytes. PLoS ONE 2014, 9, e96207. [Google Scholar] [CrossRef]
- Homma, S.; Yamamoto, N. Activation process of macrophages after in vitro treatment of mouse lymphocytes with dodecylglycerol. Clin. Exp. Immunol. 1990, 79, 307–313. [Google Scholar] [CrossRef]
- Yamamoto, N.; St Claire, D.A., Jr.; Homma, S.; Ngwenya, B.Z. Activation of mouse macrophages by alkylglycerols, inflammation products of cancerous tissues. Cancer Res. 1988, 48, 6044–6049. [Google Scholar]
- Brohult, A.; Brohult, J.; Brohult, S. Effect of irradiation and alkoxyglycerol treatment on the formation of antibodies after Salmonella vaccination. Experientia 1972, 8, 954–955. [Google Scholar] [CrossRef]
- Erdlenbruch, B.; Alipour, M.; Fricker, G.; Miller, D.S.; Kugler, W.; Eibl, H.; Lakomek, M. Alkylglycerol opening of the blood–brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. Br. J. Pharm. 2003, 140, 1201–1210. [Google Scholar] [CrossRef]
- Erdlenbruch, B.; Jendrossek, V.; Eibl, H.; Lakomek, M. Transient and controllable opening of the blood-brain barrier to cytostatic and antibiotic agents by alkylglycerols in rats. Exp. Brain Res. 2000, 135, 417–422. [Google Scholar]
- Bernal-Chávez, S.A.; Pérez-Carreto, L.Y.; Nava-Arzaluz, M.G.; Ganem-Rondero, A. Alkylglycerol derivatives, a new class of skin penetration modulators. Molecules 2017, 22, 185. [Google Scholar] [CrossRef]
- Cheminade, C.; Gautier, V.; Hichami, A.; Allaume, P.; Le Lannou, D.; Legrand, A.B. 1-O-Alkylglycerols improve boar sperm motility and fertility. Biol. Reprod. 2002, 66, 421–428. [Google Scholar] [CrossRef]
- Shubina, L.K.; Makarieva, T.N.; Denisenko, V.A.; Popov, R.S.; Dyshlovoy, S.A.; Grebnev, B.B.; Dmitrenok, P.S.; von Amsberg, G.; Stonik, V.A. Gracilosulfates A–G, monosulfated polyoxygenated steroids from the marine sponge Haliclona gracilis. Mar. Drugs 2020, 18, 454. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Kaune, M.; Kriegs, M.; Hauschild, J.; Busenbender, T.; Shubina, L.K.; Makarieva, T.N.; Hoffer, K.; Bokemeyer, C.; Graefen, M.; et al. Marine alkaloid monanchoxymycalin C: A new specific activator of JNK1/2 kinase with anticancer properties. Sci. Rep. 2020, 10, 13178. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Kuzmich, A.S.; Sabutskii, Y.E.; Guzii, A.G.; Popov, R.S.; Ogurtsov, V.A.; Rakitin, O.A.; Polonik, S.G. Synthesis and studies of acetylthioglycoside conjugates of 4-chloro-1,2-dithiole-3-thione as potential antitumor agents. Russ. Chem. Bull. 2021, 70, 573–579. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Kuzmich, A.S.; Agafonova, I.G.; Sabutskii, Y.E.; Guzii, A.G.; Popov, R.S.; Ogurtsov, V.A.; Rakitin, O.A.; Polonik, S.G. Synthesis and study of thioglycoside conjugates of 4-chloro-1,2-dithiol-3-one as potential cancer-preventive substances in vitro and in vivo. Russ. Chem. Bull. 2022, 71, 489–495. [Google Scholar] [CrossRef]
- Baltrop, J.A.; Owen, T.C.; Cory, A.H.; Cory, J.G. 5-(3-Carboxymethoxyphenyl)-2-(4,5-dimethylthiazolyl)-3-(4-sulfophenyl) tetrazolium, innersalt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators. Bioorg. Med. Chem. Lett. 1991, 1, 611–614. [Google Scholar]
- Gunstone, F.D.; Pollard, M.R.; Scrimgeour, C.M.; Vedanayagam, H.S. Fatty acids. Part 50. 13C Nuclear magnetic resonance studies of olefinic fatty acids and esters. Chem. Phys. Lipids 1977, 18, 115–129. [Google Scholar] [CrossRef]
- Dorninger, F.; Forss-Petter, S.; Wimmer, I.; Berger, J. Plasmalogens, platelet-activating factor and beyond—ether lipids in signaling and neurodegeneration. Neurobiol. Disease 2020, 145, 105061. [Google Scholar] [CrossRef]
- Dorninger, F.; Forss-Petter, S.; Berger, J. From peroxisomal disorders to common neurodegenerative diseases—the role of ether phospholipids in the nervous system. FEBS Lett. 2017, 591, 2761–2788. [Google Scholar] [CrossRef]
- Dean, J.M.; Lodhi, I.J. Structural and functional roles of ether lipids. Protein Cell 2018, 9, 196–206. [Google Scholar] [CrossRef]
- Paul, S.; Rasmiena, A.A.; Huynh, K.; Smith, A.A.T.; Mellett, N.A.; Jandeleit-Dahm, K.; Lancaster, G.I.; Meikle, P.J. Oral supplementation of an alkylglycerol mix comprising different alkyl chains effectively modulates multiple endogenous plasmalogen species in mice. Metabolites 2021, 11, 299. [Google Scholar] [CrossRef]
- Hichami, A.; Duroudier, V.; Leblais, V.; Vernhet, L.; Le Goffic, F.; Ninio, E.; Legrand, A. Modulation of platelet-activating-factor production by incorporation of naturally occurring 1-O-alkylglycerols in phospholipids of human leukemic monocyte-like THP-1 cells. Eur. J. Biochem. 1997, 250, 242–248. [Google Scholar] [CrossRef]
- Koltai, M.; Hosford, D.; Guinot, P.; Esanu, A.; Braquet, P. Platelet activating factor (PAF). A review of its effects, antagonists and possible future clinical implications (Part I). Drugs 1991, 42, 9–29. [Google Scholar] [CrossRef]
- Koltai, M.; Hosford, D.; Guinot, P.; Esanu, A.; Braquet, P. Platelet activating factor (PAF). A review of its effects, antagonists and possible future clinical implications (Part II). Drugs 1991, 42, 174–204. [Google Scholar] [CrossRef]
- da Silva, T.F.; Eira, J.; Lopes, A.T.; Malheiro, A.R.; Sousa, V.; Luoma, A.; Avila, R.L.; Wanders, R.J.A.; Just, W.W.; Kirschner, D.A.; et al. Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination. J. Clin. Investig. 2014, 124, 2560–2570. [Google Scholar] [CrossRef]
- Hossain, M.S.; Mineno, K.; Katafuchi, T. Neuronal orphan G-protein coupled receptor proteins mediate plasmalogens-induced activation of ERK and Akt signaling. PLoS ONE 2016, 11, e0150846. [Google Scholar] [CrossRef]
- Ali, F.; Hossain, M.S.; Sejimo, S.; Akashi, K. Plasmalogens inhibit endocytosis of toll-like receptor 4 to attenuate the inflammatory signal in microglial cells. Mol. Neurobiol. 2019, 56, 3404–3419. [Google Scholar] [CrossRef]
- Youssef, M.; Ibrahim, A.; Akashi, K.; Hossain, M.S. PUFA-plasmalogens attenuate the LPS-induced nitric oxide production by inhibiting the NF-kB, p38 MAPK and JNK pathways in microglial cells. Neuroscience 2019, 397, 18–30. [Google Scholar] [CrossRef]
- Pedrono, F.; Khan, N.A.; Legrand, A.B. Regulation of calcium signaling by 1-O-alkylglycerols in human Jurkat T lymphocytes. Life Sci. 2004, 74, 2793–2801. [Google Scholar] [CrossRef]
- McNeely, T.B.; Rosen, G.; Londner, M.V.; Turco, S.J. Inhibitory effects on protein kinase C activity by lipophosphoglycan fragments and glycosylphosphatidylinositol antigens of the protozoan parasite Leishmania. Biochem. J. 1989, 259, 601–604. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).