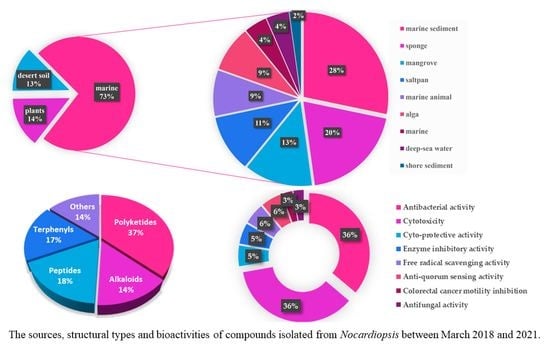
Genus Nocardiopsis: A Prolific Producer of Natural Products
Abstract
:1. Introduction
2. Polyketides
3. Alkaloids
4. Peptides
5. Terphenyls
6. Others
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jose, P.A.; Maharshi, A.; Jha, B. Actinobacteria in natural products research: Progress and prospects. Microbiol. Res. 2021, 246, 126708. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Clement, C.; Meier-Kolthoff, J.P.; Klenk, H.-P.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Demain, A.L.; Sanchez, S. Microbial drug discovery: 80 years of progress. J. Antibiot. 2009, 62, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, G.B.; Balachandran, L. Antibacterial agents from actinomycetes—A review. Front. Biosci. Elite Ed. 2012, E4, 240–253. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; Desoukey, S.Y.; Fouad, M.A.; Abdelmohsen, U.R.; Kamel, M.S.; Gulder, T.A.M. Natural product potential of the genus Nocardiopsis. Mar. Drugs 2018, 16, 147. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manivasagan, P.; Kang, K.-H.; Sivakumar, K.; Li-Chan, E.C.Y.; Oh, H.-M.; Kim, S.-K. Marine actinobacteria: An important source of bioactive natural products. Environ. Toxicol. Pharmacol. 2014, 38, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014, 169, 262–278. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Abuelsoud, W.; Hassan, Y.M.; Alkhalifah, D.H.M.; Hozzein, W.N.; Zrieq, R.; Beemster, G.T.; Schoenaers, S. An actinomycete strain of Nocardiopsis lucentensis reduces arsenic toxicity in barley and maize. J. Hazard. Mater. 2021, 417, 126055. [Google Scholar] [CrossRef] [PubMed]
- Adenan, N.H.; Lim, Y.Y.; Ting, A.S.Y. Nocardiopsis sp. for the removal of triphenylmethane dyes: Decolorization and optimization studies. Water Air Soil Pollut. 2021, 232, 414. [Google Scholar] [CrossRef]
- Patel, G.B.; Rakholiya, P.; Shindhal, T.; Varjani, S.; Tabhani, N.; Shah, K.R. Lipolytic Nocardiopsis for reduction of pollution load in textile industry effluent and SWISS model for structural study of lipase. Bioresour. Technol. 2021, 341, 125673. [Google Scholar] [CrossRef] [PubMed]
- Bennur, T.; Ravi Kumar, A.; Zinjarde, S.S.; Javdekar, V. Nocardiopsis species: A potential source of bioactive compounds. J. Appl. Microbiol. 2016, 120, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, L.; Zhou, Y.; Han, B. Natural products from actinomycetes associated with marine organisms. Mar. Drugs 2021, 19, 629. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Solanki, D.S.; Parihar, K.; Tak, A.; Gehlot, P.; Pathak, R.; Singh, S.K. Actinomycetes isolates of arid zone of Indian Thar Desert and efficacy of their bioactive compounds against human pathogenic bacteria. Biol. Futur. 2021, 72, 431–440. [Google Scholar] [CrossRef]
- Pinto-Almeida, A.; Bauermeister, A.; Luppino, L.; Grilo, I.R.; Oliveira, J.; Sousa, J.R.; Petras, D.; Rodrigues, C.F.; Prieto-Davo, A.; Tasdemir, D.; et al. The diversity, metabolomics profiling, and the pharmacological potential of actinomycetes isolated from the Estremadura Spur Pockmarks (Portugal). Mar. Drugs 2022, 20, 21. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Du, S.; Wang, G. Antimicrobial activity and functional genes of actinobacteria from coastal wetland. Curr. Microbiol. 2021, 78, 3058–3067. [Google Scholar] [CrossRef]
- Tatar, D. Isolation, phylogenetic analysis and antimicrobial activity of halophilic actinomycetes from different saline environments located near Corum province. Biologia 2021, 76, 773–780. [Google Scholar] [CrossRef]
- Li, H.-W.; Zhi, X.-Y.; Yao, J.-C.; Zhou, Y.; Tang, S.-K.; Klenk, H.-P.; Zhao, J.; Li, W.-J. Comparative genomic analysis of the genus Nocardiopsis provides new insights into its genetic mechanisms of environmental adaptability. PLoS ONE 2013, 8, e61528. [Google Scholar]
- Bennur, T.; Kumar, A.R.; Zinjarde, S.; Javdekar, V. Nocardiopsis species as potential sources of diverse and novel extracellular enzymes. Appl. Microbiol. Biotechnol. 2014, 98, 9173–9185. [Google Scholar]
- Bennur, T.; Kumar, A.R.; Zinjarde, S.; Javdekar, V. Nocardiopsis species: Incidence, ecological roles and adaptations. Microbiol. Res. 2015, 174, 33–47. [Google Scholar]
- Shady, N.H.; Tawfike, A.F.; Yahia, R.; Fouad, M.A.; Brachmann, A.O.; Piel, J.; Abdelmohsen, U.R.; Kamel, M.S. Cytotoxic activity of actinomycetes Nocardia sp. and Nocardiopsis sp. associated with marine sponge Amphimedon sp. Nat. Prod. Res. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Siddharth, S.; Aswathanarayan, J.B.; Kuruburu, M.G.; Madhunapantula, S.R.V.; Vittal, R.R. Diketopiperazine derivative from marine actinomycetes Nocardiopsis sp. SCA30 with antimicrobial activity against MRSA. Arch. Microbiol. 2021, 203, 6173–6181. [Google Scholar] [CrossRef]
- Trivedi, N.; Thumar, J. Chemical profiling of antimicrobial metabolites from halophilic actinomycete Nocardiopsis sp. Al-H10-1 (KF384482) isolated from Alang, Gulf of Khambhat, India. bioRxiv 2021. [Google Scholar] [CrossRef]
- Goel, N.; Fatima, S.W.; Kumar, S.; Sinha, R.; Khare, S.K. Antimicrobial resistance in biofilms: Exploring marine actinobacteria as a potential source of antibiotics and biofilm inhibitors. Biotechnol. Rep. 2021, 30, e00613. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Kim, G.J.; Choi, H.; Choi, I.-W.; Lee, D.-S. Anti-inflammatory and anti-fibrotic activities of Nocardiopsis sp. 13G027 in lipopolysaccharides-induced RAW 264.7 macrophages and transforming growth factor beta-1-stimulated nasal polyp-derived fibroblasts. Microbiol. Biotechnol. Lett. 2021, 49, 543–551. [Google Scholar] [CrossRef]
- Sarmiento-Vizcaíno, A.; Martín, J.; Reyes, F.; García, L.A.; Blanco, G. Bioactive natural products in actinobacteria isolated in rainwater from storm clouds transported by western winds in Spain. Front. Microbiol. 2021, 12, 773095. [Google Scholar] [CrossRef]
- Gamaleldin, N.M.; Bakeer, W.; El-Gendy, A.O.; Sayed, A.M.; Shamikh, Y.I.; Hassan, H.M.; Horn, H.; Abdelmohsen, U.R.; Hozzein, W.N. Exploration of chemical diversity and antitrypanosomal activity of some Red Sea-derived actinomycetes using the OSMAC approach supported by LC-MS-based metabolomics and molecular modelling. Antibiotics 2020, 9, 629. [Google Scholar] [CrossRef]
- Widada, J.; Damayanti, E.; Alhakim, M.R.; Yuwono, T.; Mustofa, M. Two strains of airborne Nocardiopsis alba producing different volatile organic compounds (VOCs) as biofungicide for Ganoderma boninense. FEMS Microbiol. Lett. 2021, 368, fnab138. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, P.; Liu, P.; Wang, P.; Hou, J.; Li, W.; Zhu, W. New pyran-2-ones from alkalophilic actinomycete, Nocardiopsis alkaliphila sp. nov. YIM-80379. Chem. Biodivers. 2013, 10, 281–287. [Google Scholar] [CrossRef]
- Lu, C.; Li, Y.; Wang, H.; Wang, B.; Shen, Y. A new phenoxazine derivative isolated from marine sediment actinomycetes, Nocardiopsis sp. 236. Drug Discov. Ther. 2013, 7, 101–104. [Google Scholar]
- Tian, S.; Yang, Y.; Liu, K.; Xiong, Z.; Xu, L.; Zhao, L. Antimicrobial metabolites from a novel halophilic actinomycete Nocardiopsis terrae YIM 90022. Nat. Prod. Res. 2014, 28, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Imoto, M.; Isshiki, K.; Sawa, T.; Naganawa, H.; Kurasawa, S.; Zhu, B.; Umezawa, K. Isolation of a new indole alkaloid, pendolmycin, from Nocardiopsis. J. Nat. Prod. 1988, 51, 1184–1187. [Google Scholar] [CrossRef]
- Lin, Z.; Torres, J.P.; Ammon, M.A.; Marett, L.; Teichert, R.W.; Reilly, C.A.; Kwan, J.C.; Hughen, R.W.; Flores, M.; Tianero, M.D.; et al. A Bacterial source for mollusk pyrone polyketides. Chem. Biol. 2013, 20, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Lu, Y.; Xing, Y.; Ma, Y.; Lu, J.; Bao, W.; Wang, Y.; Xi, T. A novel anticancer and antifungus phenazine derivative from a marine actinomycete BM-17. Microbiol. Res. 2012, 167, 616–622. [Google Scholar] [CrossRef]
- Kase, H.; Iwahashi, K.; Matsuda, Y. K-252a, a potent inhibitor of protein kinase C from microbial origin. J. Antibiot. 1986, 39, 1059–1065. [Google Scholar] [CrossRef] [Green Version]
- Raju, R.; Piggott, A.M.; Huang, X.-C.; Capon, R.J. Nocardioazines: A novel bridged diketopiperazine scaffold from a marine-derived bacterium inhibits P-glycoprotein. Org. Lett. 2011, 13, 2770–2773. [Google Scholar] [CrossRef]
- Kim, M.C.; Kwon, O.-W.; Park, J.-S.; Kim, S.Y.; Kwon, H.C. Nocapyrones H–J, 3,6-disubstituted α-pyrones from the marine actinomycete Nocardiopsis sp. KMF-001. Chem. Pharm. Bull. 2013, 61, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Xie, L.; Xia, G.; Zhang, J.; Nie, Y.; Hu, J.; Wang, S.; Zhang, R. A new tetrodotoxin-producing actinomycete, Nocardiopsis dassonvillei, isolated from the ovaries of puffer fish Fugu rubripes. Toxicon 2005, 45, 851–859. [Google Scholar] [CrossRef]
- Peltola, J.S.P.; Andersson, M.A.; Kampfer, P.; Auling, G.; Kroppenstedt, R.M.; Busse, H.-J.; Salkinoja-Salonen, M.S.; Rainey, F.A. Isolation of toxigenic Nocardiopsis strains from indoor environments and description of two new Nocardiopsis species, N. exhalans sp. nov. and N. umidischolae sp. nov. Appl. Environ. Microbiol. 2001, 67, 4293–4304. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Ogura, H.; Akasaka, K.; Oikawa, T.; Matsuura, N.; Imada, C.; Yasuda, H.; Igarashi, Y. Nocapyrones: α- and γ-pyrones from a marine-derived Nocardiopsis sp. Mar. Drugs 2014, 12, 4110–4125. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.-W.; Guo, Z.-X.; Lu, C.-H. Two new polyketides from Nocardiopsis lucentensis DSM 44048. Nat. Prod. Res. 2016, 30, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Saurav, K.; Yu, Z.; Mandi, A.; Kurtan, T.; Li, J.; Tian, X.; Zhang, Q.; Zhang, W.; Zhang, C. α-Pyrones with diverse hydroxy substitutions from three marine-derived Nocardiopsis strains. J. Nat. Prod. 2016, 79, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, D.; Kim, S.-H.; Park, W.; Shin, Y.; Kim, W.K.; Lee, S.K.; Oh, K.-B.; Shin, J.; Oh, D.-C. Borrelidins C.–E: New antibacterial macrolides from a saltern-derived halophilic Nocardiopsis sp. Mar. Drugs 2017, 15, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; He, H.; Ma, R.; Ji, Z.; Wei, Q.; Dai, H.; Zhang, L.; Song, F. Madurastatin B3, a rare aziridine derivative from actinomycete Nocardiopsis sp. LS150010 with potent anti-tuberculosis activity. J. Ind. Microbiol. Biotechnol. 2017, 44, 589–594. [Google Scholar] [CrossRef]
- Eliwa, E.M.; Abdel-Razek, A.S.; Frese, M.; Wibberg, D.; Halawa, A.H.; El-Agrody, A.M.; Bedair, A.H.; Kalinowski, J.; Sewald, N.; Shaaban, M. New bioactive compounds from the marine-derived actinomycete Nocardiopsis lucentensis sp. ASMR2. Z. Naturforsch. B J. Chem. Sci. 2017, 72, 351–360. [Google Scholar] [CrossRef]
- Sun, M.-W.; Zhang, X.-M.; Bi, H.-L.; Li, W.-J.; Lu, C.-H. Two new sesquiterpenoids produced by halophilic Nocardiopsis chromatogenes YIM 90109. Nat. Prod. Res. 2017, 31, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Abdel-Razek, A.S.; Frese, M.; Stammler, H.G.; El-Haddad, A.F.; Ibrahim, T.M.A.; Sewald, N.; Shaaban, M. Terretonin N: A new meroterpenoid from Nocardiopsis sp. Molecules 2018, 23, 299. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Nepal, K.K.; Harmody, D.; McCarthy, P.J.; Wright, A.E.; Wang, G.; Chen, J.; Zhu, H. Nocardiopsistins A–C: New angucyclines with anti-MRSA activity isolated from a marine sponge-derived Nocardiopsis sp. HB-J378. Synth. Syst. Biotechnol. 2018, 3, 246–251. [Google Scholar] [CrossRef]
- Lombo, F.; Brana, A.F.; Salas, J.A.; Mendez, C. Genetic organization of the biosynthetic gene cluster for the antitumor angucycline oviedomycin in Streptomyces antibioticus ATCC 11891. ChemBioChem 2004, 5, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Kharel, M.K.; Pahari, P.; Shepherd, M.D.; Tibrewal, N.; Nybo, S.E.; Shaaban, K.A.; Rohr, J. Angucyclines: Biosynthesis, mode-of-action, new natural products, and synthesis. Nat. Prod. Rep. 2012, 29, 264–325. [Google Scholar] [CrossRef]
- Messaoudi, O.; Sudarman, E.; Bendahou, M.; Jansen, R.; Stadler, M.; Wink, J. Kenalactams A–E, polyene macrolactams isolated from Nocardiopsis CG3. J. Nat. Prod. 2019, 82, 1081–1088. [Google Scholar] [CrossRef]
- Zhao, T.; Chang, Y.; Zhu, T.; Li, J.; Gu, Q.; Li, D.; Che, Q.; Zhang, G. α-Pyrone derivatives with cyto-protective activity from two Takla Makan desert soil derived actinomycete Nocardiopsis strains recovered in seawater based medium. Nat. Prod. Res. 2019, 33, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; Parkin, K.L. Limited contribution of isoflavones to hepatocellular phase II enzyme-inducing activity of soybean (Glycine max) extracts. Food Chem. 2009, 113, 1069–1075. [Google Scholar] [CrossRef]
- Park, J.S.; Jung, J.S.; Jeong, Y.H.; Hyun, J.W.; Le, T.K.V.; Kim, D.H.; Choi, E.C.; Kim, H.S. Antioxidant mechanism of isoflavone metabolites in hydrogen peroxide-stimulated rat primary astrocytes: Critical role of hemeoxygenase-1 and NQO1 expression. J. Neurochem. 2011, 119, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-X.; Sun, C.-X.; Shah, M.; Zhang, G.-J.; Gu, Q.-Q.; Zhu, T.-J.; Che, Q.; Li, D.-H. New metabolites from a Mariana Trench-derived actinomycete Nocardiopsis sp. HDN 17-237. J. Asian Nat. Prod. Res. 2020, 22, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, X.; Sun, C.; Chang, Y.; Huang, X.; Zhu, T.; Zhang, G.; Che, Q.; Li, D. Saliniquinone derivatives, saliniquinones G−I and heraclemycin E, from the marine animal-derived Nocardiopsis aegyptia HDN19-252. Mar. Drugs 2021, 19, 575. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Yang, S.-H.; Du, Y.E.; Park, K.; Kim, D.; Shin, D.; Kim, J.; Kim, S.-H.; Kim, Y.K.; Shin, J. Borrelidin from saltern-derived halophilic Nocardiopsis sp. dissociates amyloid-β and tau fibrils. J. Alzheimer’s Dis. Rep. 2021, 5, 7–13. [Google Scholar] [CrossRef]
- Castro-Falcon, G.; Millan-Aguinaga, N.; Roullier, C.; Jensen, P.R.; Hughes, C.C. Nitrosopyridine probe to detect polyketide natural products with conjugated alkenes: Discovery of novodaryamide and nocarditriene. ACS Chem. Biol. 2018, 13, 3097–3106. [Google Scholar] [CrossRef]
- Kim, T.; Lee, S.-A.; Noh, T.; Choi, P.; Choi, S.-J.; Song, B.G.; Kim, Y.; Park, Y.-T.; Huh, G.; Kim, Y.-J.; et al. Synthesis, structure revision, and cytotoxicity of nocarbenzoxazole G. J. Nat. Prod. 2019, 82, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, X.; Hao, H.; Li, W.; Lu, C. Nocarbenzoxazoles A–G, benzoxazoles produced by halophilic Nocardiopsis lucentensis DSM 44048. J. Nat. Prod. 2015, 78, 2123–2127. [Google Scholar] [CrossRef]
- Yang, C.L.; Zhang, B.; Xue, W.W.; Li, W.; Xu, Z.F.; Shi, J.; Shen, Y.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Discovery, biosynthesis, and heterologous production of loonamycin, a potent anticancer indolocarbazole alkaloid. Org. Lett. 2020, 22, 4665–4669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, S.; Zhang, L.; Zhang, Q.; Zhang, W.; Chen, Y.; Zhang, W.; Zhang, H.; Zhang, C. Dassonmycins A and B, polycyclic thioalkaloids from a marine sponge-derived Nocardiopsis dassonvillei SCSIO 40065. Org. Lett. 2021, 23, 2858–2862. [Google Scholar] [CrossRef]
- Miao, L.; Qian, S.; Qi, S.; Jiang, W.; Dong, K. Culture medium optimization and active compounds investigation of an anti-quorum sensing marine actinobacterium Nocardiopsis dassonvillei JS106. Microbiology 2021, 90, 112–123. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; Attia, E.Z.; Desoukey, S.Y.; Fouad, M.A.; Abdelmohsen, U.R.; Hajjar, D.; Anany, M.A.; Wajant, H.; Kamel, M.S.; Gulder, T.A.M. New cytotoxic cyclic peptide from the marine sponge-associated Nocardiopsis sp. UR67. Mar. Drugs 2018, 16, 290. [Google Scholar] [CrossRef] [Green Version]
- Muhajir, M.I.; Hardianto, A.; Al-Anshori, J.; Sumiarsa, D.; Mayanti, T.; Harneti, D.; Hidayat, A.T.; Supratman, U.; Maharani, R. Total synthesis of nocardiotide A by using a combination of solid-and solution-phase methods. ChemistrySelect 2021, 6, 12941–12946. [Google Scholar] [CrossRef]
- Chen, H.; Wan, C.; Zhang, L. A new diketopiperazine isolated from a Nocardiopsis strain TRM20105 guided by bioassay against Candida albicans. Nat. Prod. Res. 2019, 33, 3421–3425. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Gamage, C.D.B.; Kim, G.J.; Hillman, P.F.; Lee, C.; Lee, E.Y.; Choi, H.; Kim, H.; Nam, S.-J.; Fenical, W. Androsamide, a cyclic tetrapeptide from a marine Nocardiopsis sp. suppresses motility of colorectal cancer cells. J. Nat. Prod. 2020, 83, 3166–3172. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.-X.; Liu, Q.; Li, X.-M.; Lu, C.-H.; Shen, Y.-M. Four pairs of proline-containing cyclic dipeptides from Nocardiopsis sp. HT88, an endophytic bacterium of Mallotus nudiflorus L. Nat. Prod. Res. 2020, 34, 2219–2224. [Google Scholar] [CrossRef]
- Storr, S.J.; Carragher, N.O.; Frame, M.C.; Parr, T.; Martin, S.G. The calpain system and cancer. Nat. Rev. Cancer 2011, 11, 364–374. [Google Scholar] [CrossRef]
- Joshi, S.; Singh, A.R.; Zulcic, M.; Bao, L.; Messer, K.; Ideker, T.; Dutkowski, J.; Durden, D.L. Rac2 controls tumor growth, metastasis and M1-M2 macrophage differentiation in vivo. PLoS ONE 2014, 9, e95893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wertheimer, E.; Gutierrez-Uzquiza, A.; Rosemblit, C.; Lopez-Haber, C.; Sosa, M.S.; Kazanietz, M.G. Rac signaling in breast cancer: A tale of GEFs and GAPs. Cell. Signal. 2012, 24, 353–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnison, P.G.; Bibb, M.J.; Bierbaum, G.; Bowers, A.A.; Bugni, T.S.; Bulaj, G.; Camarero, J.A.; Campopiano, D.J.; Challis, G.L.; Clardy, J. Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2013, 30, 108–160. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; O’Brien, R.V.; Khosla, C. Nonproteinogenic amino acid building blocks for nonribosomal peptide and hybrid polyketide scaffolds. Angew. Chem. Int. Ed. 2013, 52, 7098–7124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, C.T.; Tang, Y. Natural Product Biosynthesis; The Royal Society of Chemistry: London, UK, 2017. [Google Scholar]
- Mishra, A.K.; Choi, J.; Choi, S.-J.; Baek, K.-H. Cyclodipeptides: An overview of their biosynthesis and biological activity. Molecules 2017, 22, 1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giessen, T.W.; Marahiel, M.A. The tRNA-dependent biosynthesis of modified cyclic dipeptides. Int. J. Mol. Sci. 2014, 15, 14610–14631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Wang, Y.; Ouyang, Y.; Fu, P.; Zhu, W. Cytotoxic p-terphenyls from a marine-derived Nocardiopsis species. J. Nat. Prod. 2019, 82, 3504–3508. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Che, Q.; Xing, L.; Ma, C.; Han, Y.; Zhu, T.; Pfeifer, B.A.; Peng, J.; Zhang, G.; Li, D. Antibacterial p-terphenyl with a rare 2,2′-bithiazole substructure and related compounds isolated from the marine-derived actinomycete Nocardiopsis sp. HDN154086. J. Nat. Prod. 2021, 84, 1226–1231. [Google Scholar] [CrossRef]
- Eliwa, E.M.; Abdel-Razek, A.S.; Frese, M.; Halawa, A.H.; El-Agrody, A.M.; Bedair, A.H.; Sewald, N.; Shaaban, M. New naturally occurring phenolic derivatives from marine Nocardiopsis sp. AS23C: Structural elucidation and in silico computational studies. Vietnam J. Chem. 2019, 57, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Siddharth, S.; Rai, V.R. Isolation and characterization of bioactive compounds with antibacterial, antioxidant and enzyme inhibitory activities from marine-derived rare actinobacteria, Nocardiopsis sp. SCA21. Microb. Pathog. 2019, 137, 103775. [Google Scholar] [CrossRef]
- Sunish, K.S.; Sreedharan, P.; Daniel, S.; Biji, M.; Rosamma, P.; Sukumaran, V.; Mohandas, A.; Singh, I.S.B. A novel substituted derivative of sterol from marine actinomycetes Nocardiopsis alba MCCB 110 antagonistic to the aquaculture pathogen Vibrio harveyi. Microb. Pathog. 2021, 157, 104967. [Google Scholar] [CrossRef]

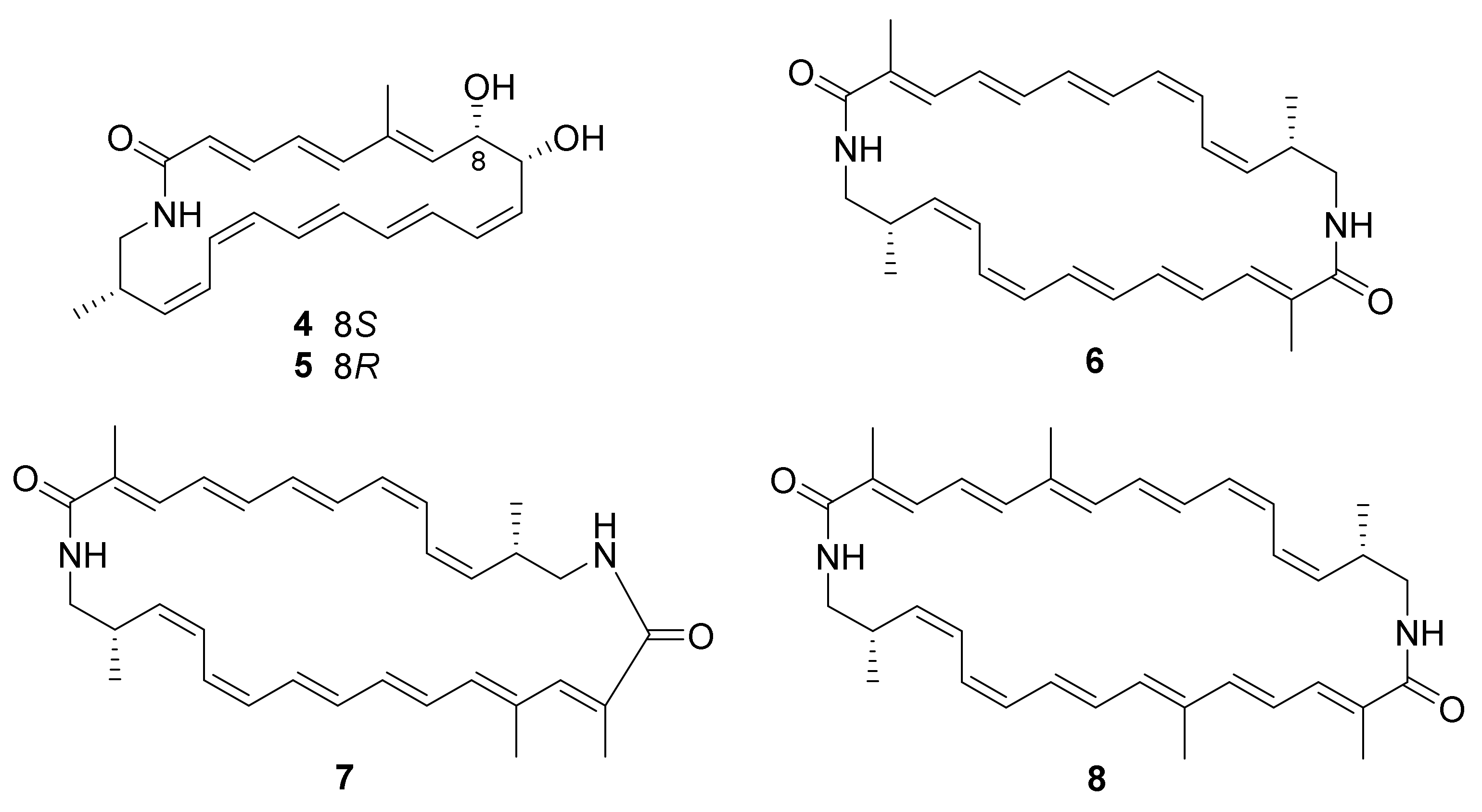
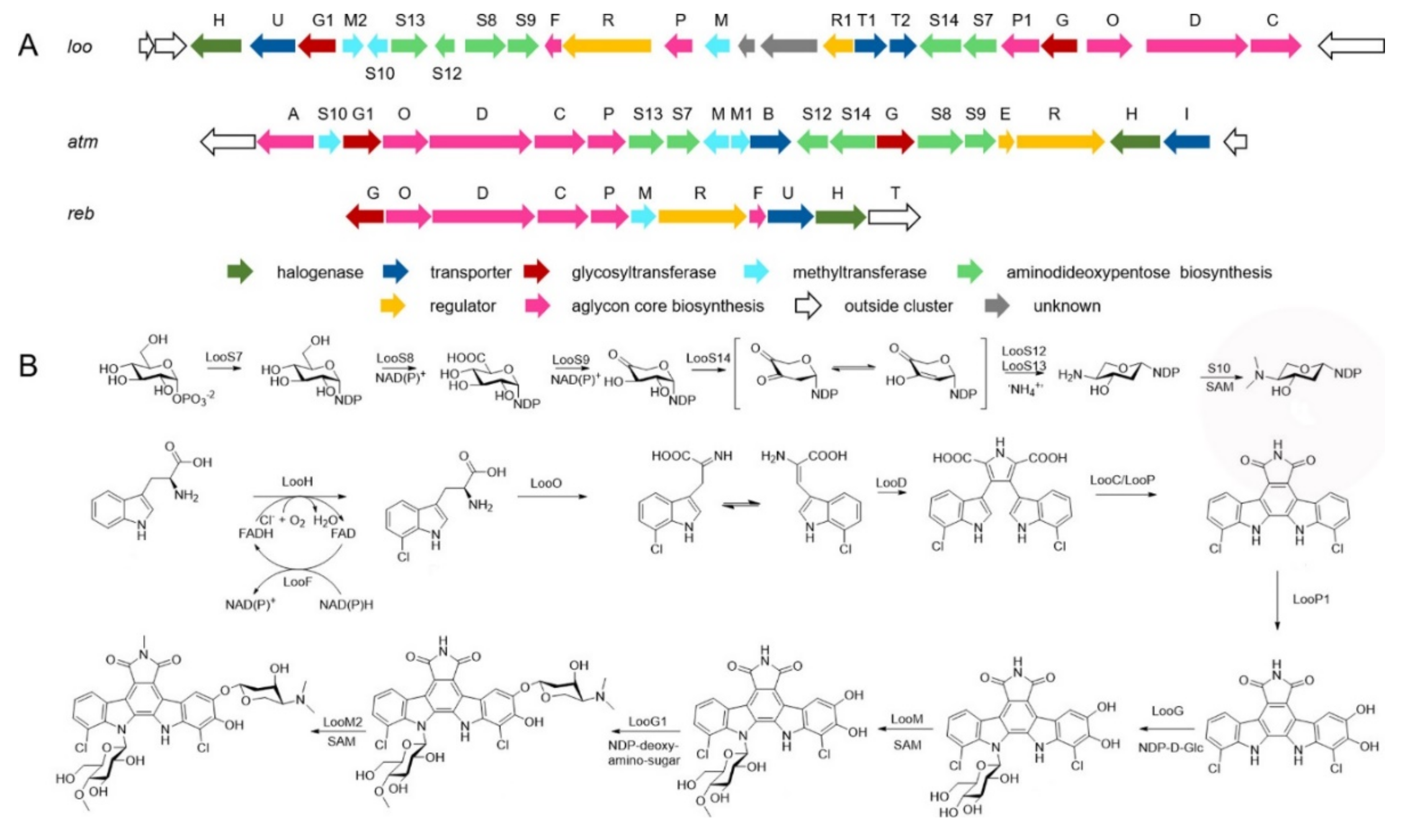
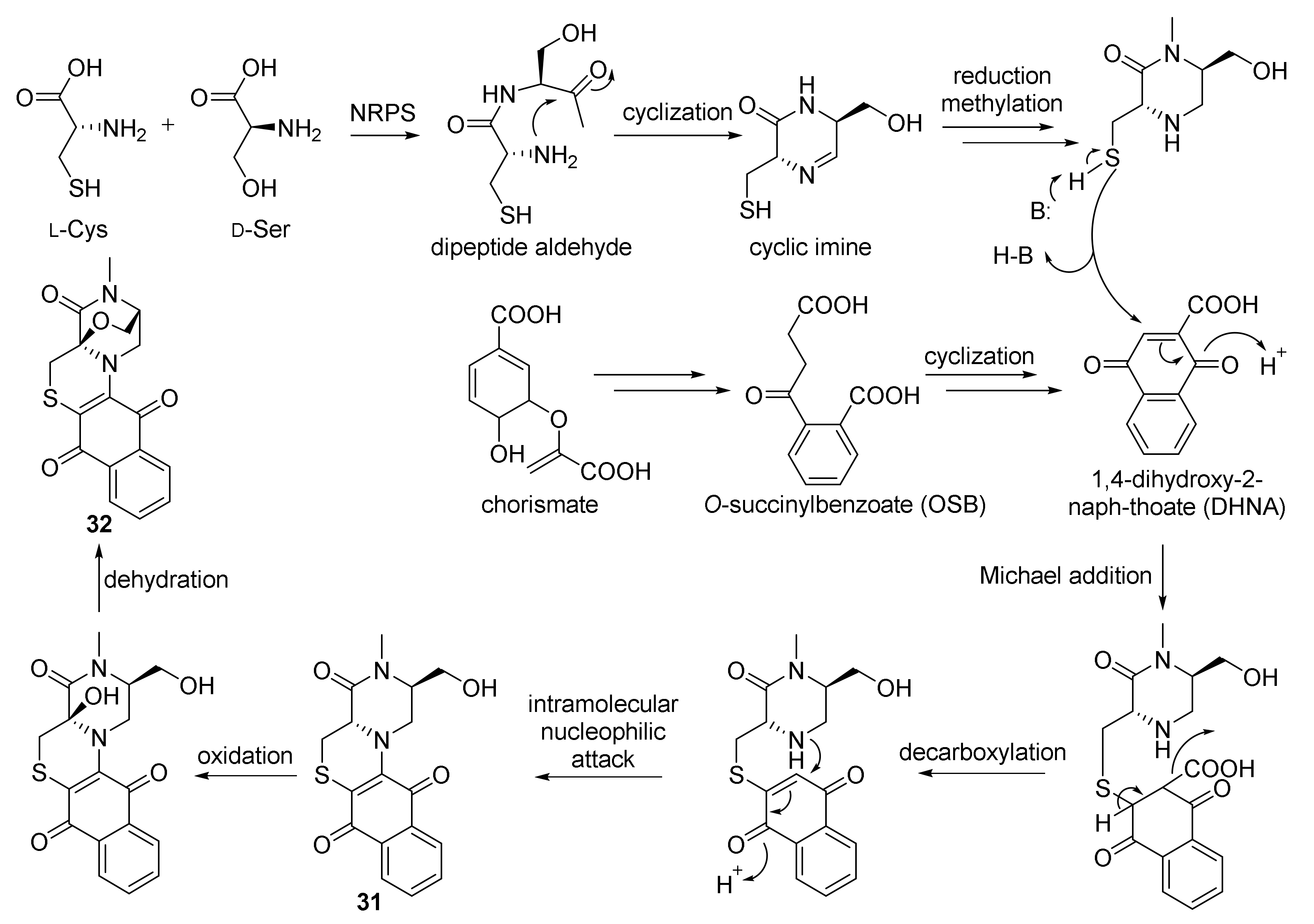

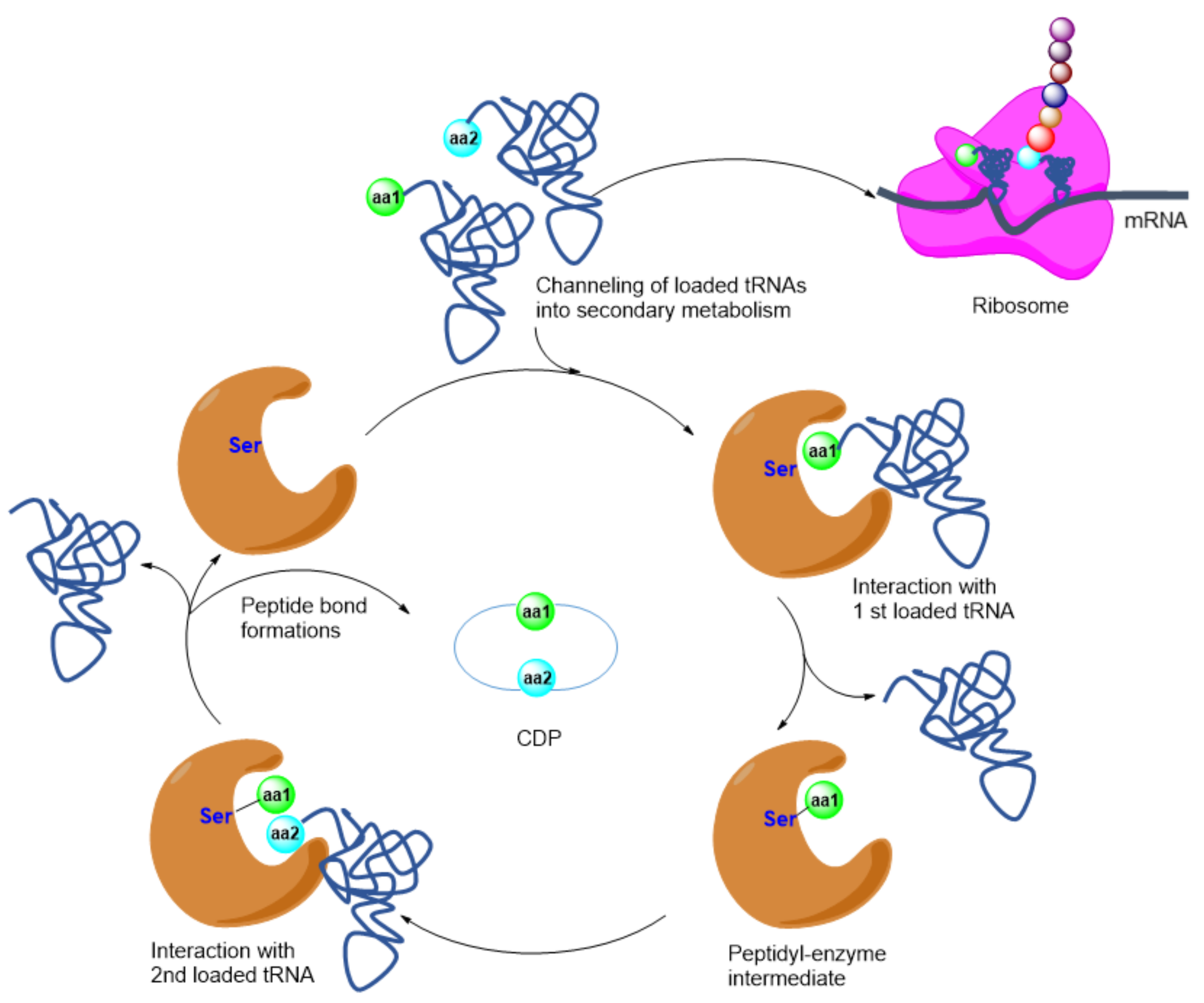

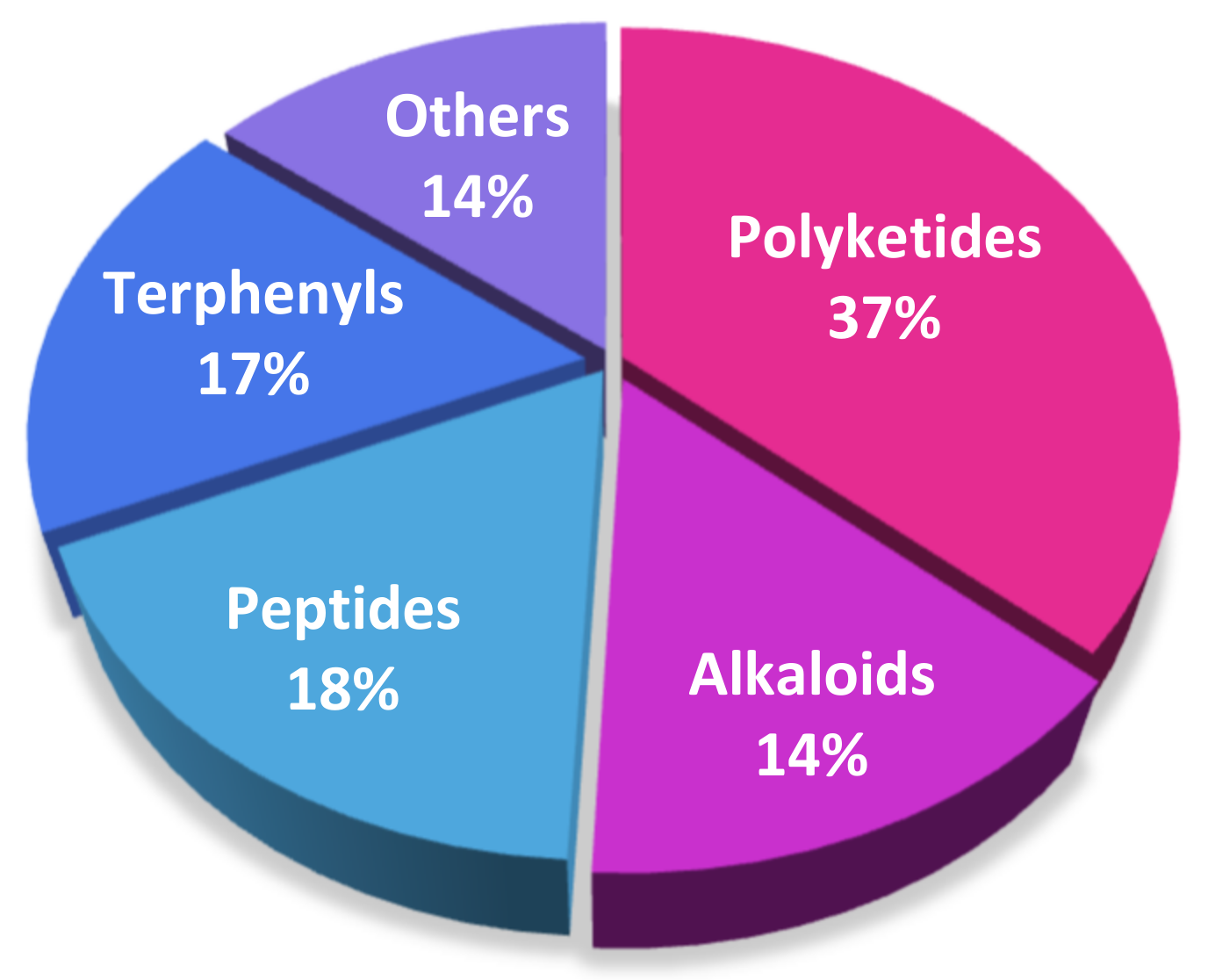

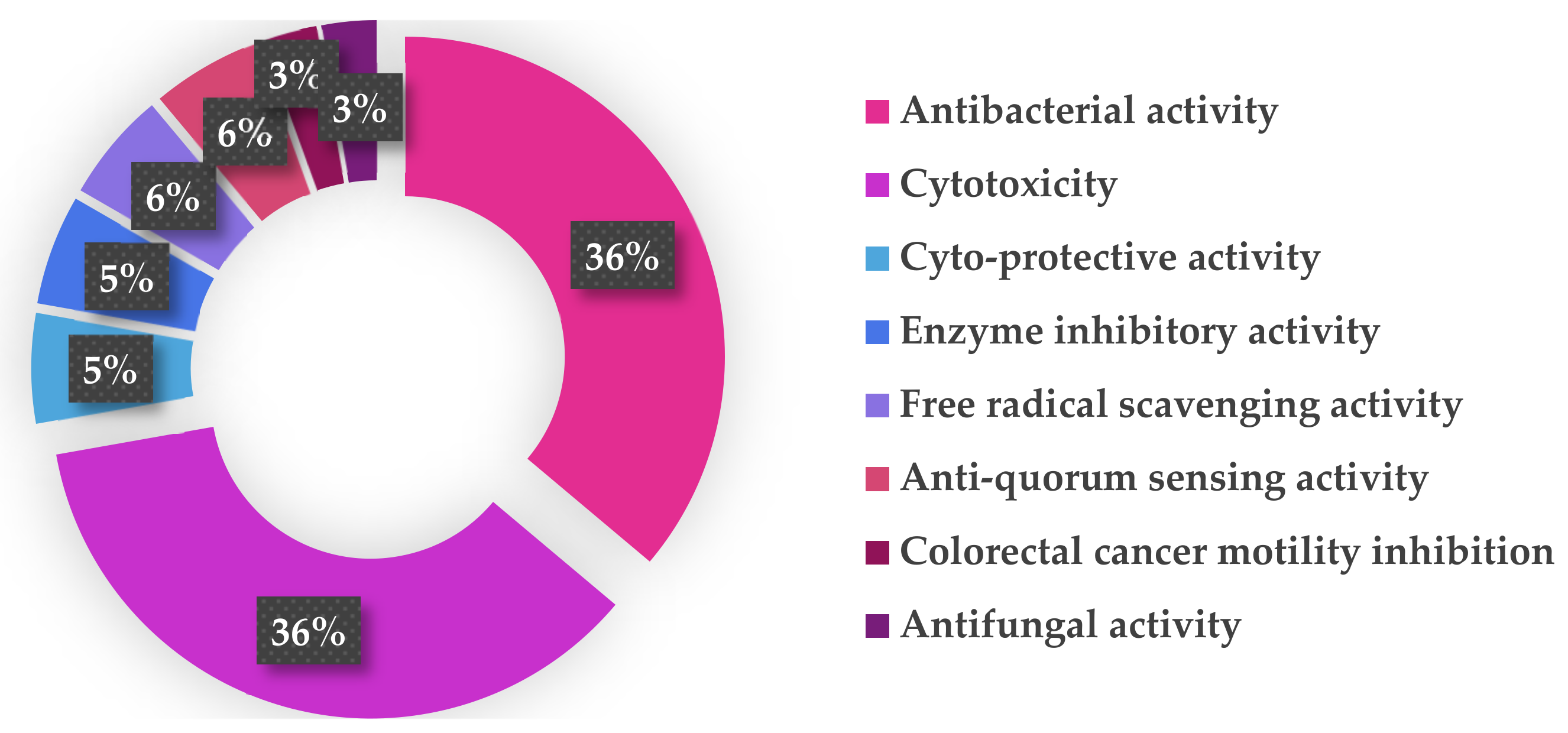
| Types | Comps. | Sources | Distribution | Bioactivities | Years | Refs |
|---|---|---|---|---|---|---|
| Polyketides | 1–3 | Sponge Theonella sp. derived Nocardiopsis sp. HB-J378 (GenBank No. MH779065) | Antibacterial activity | 2018 | [48] | |
| 4, 5 | Saltpan-derived Nocardiopsis sp. CG3 (GenBank No. MG972881) | Kenadsa, Algeria | 2019 | [51] | ||
| 6−8 | Saltpan-derived Nocardiopsis sp. CG3 (GenBank No. MG972881) | Kenadsa, Algeria | Cytotoxicity | 2019 | [51] | |
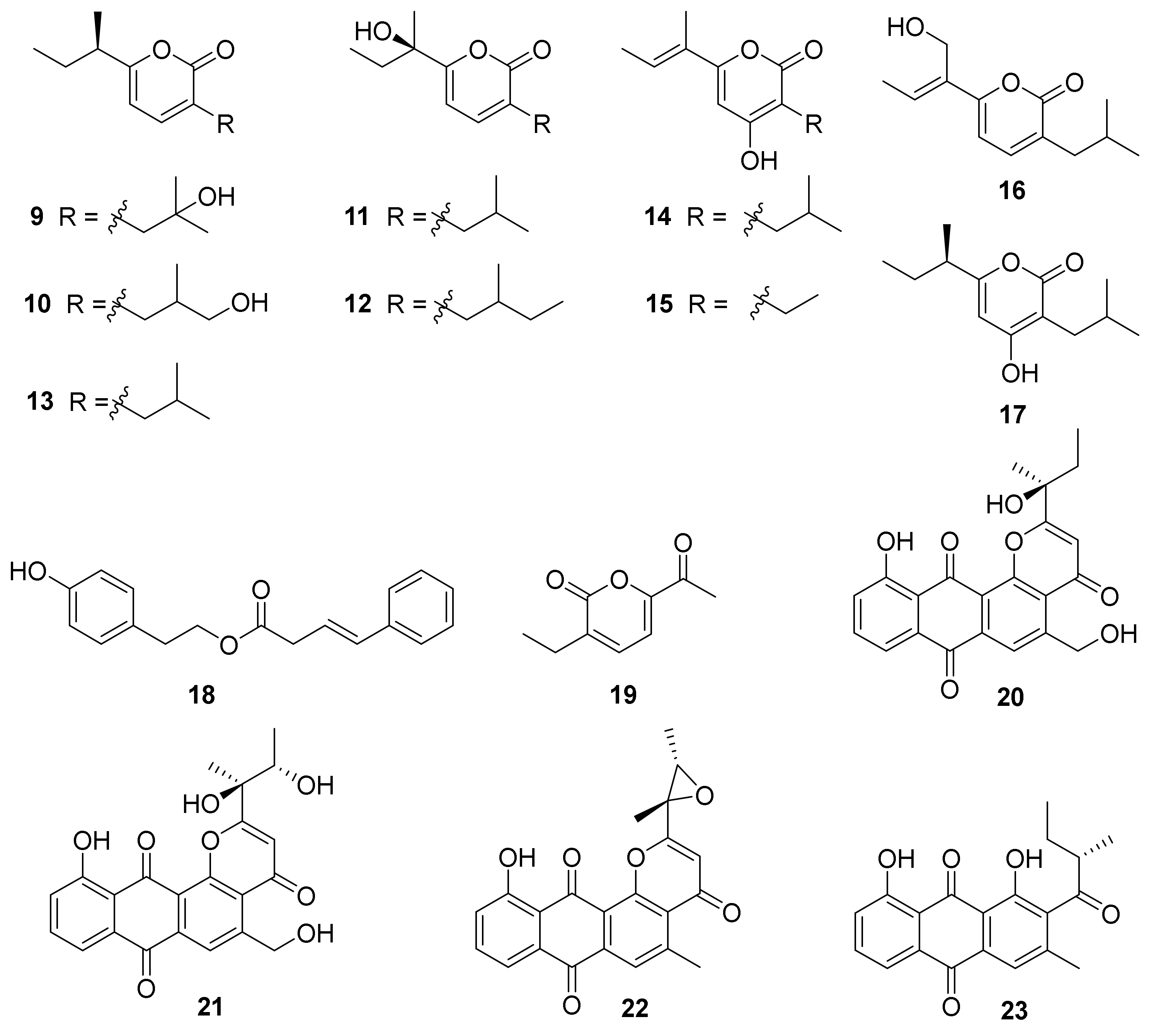
| 9−12, 14, 15, 17 | Desert soil-derived Nocardiopsis spp. HDN154-146 (Genbank no. KY794927) and HDN154-168 (Genbank No. MF952649) | Xinjiang, China | 2019 | [52] | ||
| 13, 16 | Desert soil-derived Nocardiopsis spp. HDN154-146 (Genbank no. KY794927) and HDN154-168 (Genbank No. MF952649) | Xinjiang, China | Cytoprotective activity | 2019 | [52] | |
| 18, 19 | Deep-sea water-derived N. dassonvillei subsp. albirubida HDN 17-237 (Genbank No. MN416280) | Mariana Trench | 2020 | [55] | ||
| 20, 21 | Marine animal-derived N. aegyptia HDN19-252 (GenBank No. MN822699) | Antarctic | Antibacterial activity | 2021 | [56] | |
| 22, 23 | Marine animal derived strain N. aegyptia HDN19-252 (GenBank No. MN822699) | Antarctic | 2021 | [56] | ||
| Alkaloids | 24 | Marine-derived Nocardiopsis sp. CNY-503 | 2018 | [58] | ||
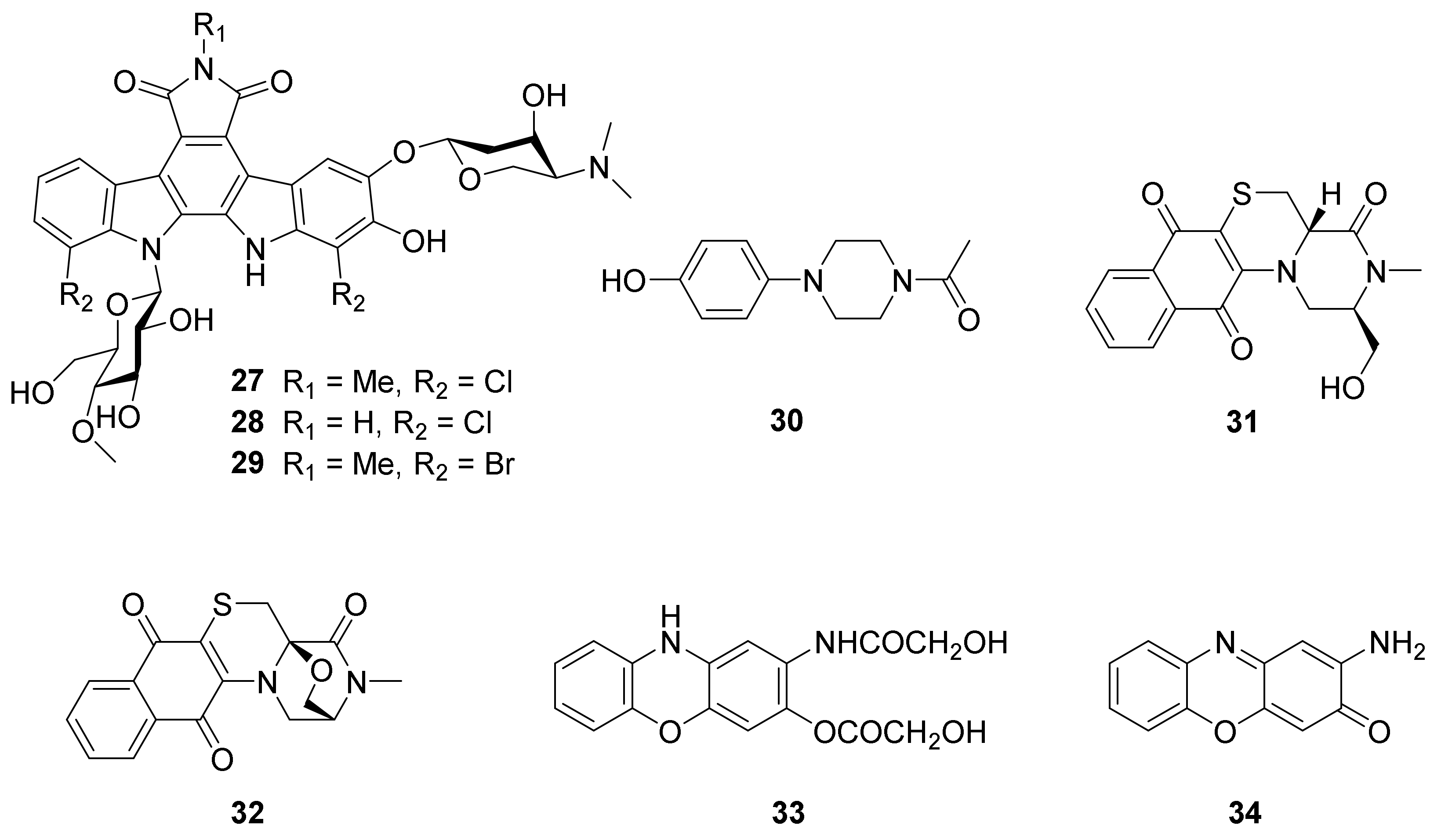
| 27 | Marine sediment-derived N. flavescens NA01583 (GenBank No. MT371575) | Hainan China | Cytotoxicity | 2020 | [61] | |
| 28, 29 | Marine sediment-derived N. flavescens NA01583 (GenBank No. MT371575) | Hainan China | 2020 | [61] | ||
| 30 | Marine sediment-derived Nocardiopsis sp. SCA30 (GenBank No. MT573349) | Havelock Island | Antibacterial and anticancer activities | 2021 | [22] | |
| 31, 32 | Sponge Petrosia sp.-derived N. dassonvillei SCSIO 40065 (GenBank No. MW492395) | South China Sea | Antibacterial and cytotoxic activities | 2021 | [62] | |
| 33, 34 | Marine sediment-derived N. dassonvillei JS106 (GenBank No. MN416229) | Lianyungang, China, | Antiquorum sensing activity | 2021 | [63] | |
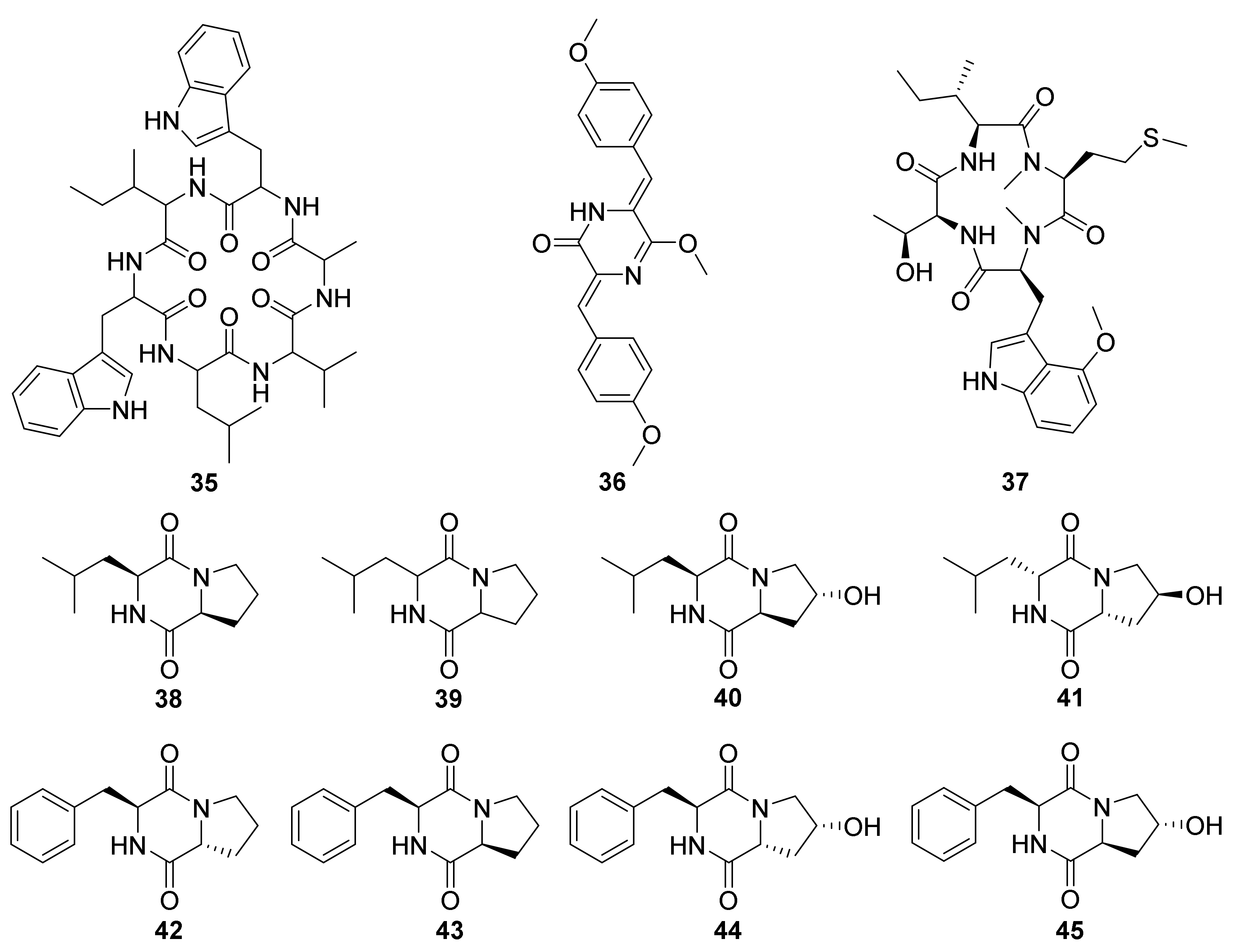
| Peptides | 35 | Sponge Callyspongia sp.-derived Nocardiopsis sp. UR67 | Red Sea | Cytotoxicity | 2018 | [64] |
| 36 | Cotton field-derived Nocardiopsis sp. TRM20105 | Tarim Basin | Antifungal activity | 2019 | [66] | |
| 37 | Shore sediment-derived Nocardiopsis sp. CNT-189 (GenBank No. KY111725.1) | Bahamas | Cytotoxicity and colorectal cancer motility inhibitor | 2020 | [67] | |
| 38−45 | Stems of Mallotus nudiflorus L-derived Nocardiopsis sp. HT88 (Genbank No. MH817156) | 2020 | [68] | |||
| Terphenyls | 46, 49−51 | Mangrove-derived Nocardiopsis sp. OUCMDZ-4936 (Genbank No. MK129184) | Hainan China | Cytotoxicity | 2019 | [77] |
| 47, 48 | Mangrove-derived Nocardiopsis sp. OUCMDZ-4936 (Genbank No. MK129184) | Hainan China | 2019 | [77] | ||
| 52, 53 | Marine sediment-derived Nocardiopsis sp. HDN154086 (GenBank No. MK129184) | South China Sea | Antibacterial activity | 2021 | [78] | |
| 54−56 | Marine sediment-derived Nocardiopsis sp. HDN154086 (GenBank No. MK129184) | South China Sea | 2021 | [78] | ||
| Others | 57−59 | Sponge Callyspongia sp.-derived Nocardiopsis sp. UR67 | Red Sea | 2018 | [64] | |
| 60−62 | Alga Sargassum arnaudianum-derived Nocardiopsis sp. AS23C (GenBank No. MH144210) | Red Sea | 2019 | [79] | ||
| 63, 64 | Marine sediment-derived Nocardiopsis sp. SCA21 (GenBank No. MH105056) | Havelock Island | Enzyme inhibitory, antibacterial, and free radical scavenging activities | 2019 | [80] | |
| 65 | Marine-derived N. alba MCCB110 (GenBank No. EU008081) | Antibacterial activity | 2021 | [81] |
| Strains | Comps. | Values (MIC) | Pros | Cons |
|---|---|---|---|---|
| MRSA | 1, 3 | 12.5 μg/mL | Specific inhibition of MRSA | Moderate activity [48] |
| 2 | 3.12 μg/mL | Strong activity; Specific inhibition of MRSA | [48] | |
| MRCNS | 20/21 (μM) | 6.2/6.2 | Broad-spectrum antibacterial activity; Strong activity against MRCNS compared with positive control | Moderate activity against B. subtilis and Proteus sp. compared with positive control [56] |
| B. subtilis | 6.2/6.2 | |||
| Proteus sp. | 12.5/6.2 | |||
| B. cereus | 6.2/6.2 | |||
| E. coli | 6.2/6.2 | |||
| M. Phlei | 6.2/3.1 | |||
| MRSA ATCC NR-46071 | 30 (μg/mL) | 15.6 | Strong activity | [22] |
| MRSA ATCC NR-46171 | 7.8 | |||
| B. subtilis 1064 | 31/32 (μg/mL) | 8/16 | Broad-spectrum antibacterial activity | Weak activity [62] |
| M. Luteus SCSIO ML01 | 16/32 | |||
| S. aureus ATCC 29213 | 64/64 | |||
| MRSA shhsA1 | 32/32 | |||
| E. faecalis ATCC 29212 | 32/- | |||
| V. alginolyticus 13214 | 32/64 | |||
| Proteus sp. | 52/53 (μM) | 3.1/12 | 52 showed strong and broad-spectrum activity, and 53 displayed strong activity of MRSA | 53 displayed moderate activity against Proteus sp. and B. subtilis [78] |
| B. cereus | 1.5/- | |||
| M. phlei | 6.2/- | |||
| B. subtilis | 3.1/12 | |||
| MRSA | -/6.2 | |||
| V. parahemolyticus | 6.2/- | |||
| E. coli | 3.1/- | |||
| K. pneumoniae ATCC 13883 | 63/64 (μg/mL) | 125/250 | Broad-spectrum antibacterial activity | Weak activity [80] |
| Listeria cytogens ATCC 13932 | 62.5/- | |||
| S. aureus ATCC 12600 | 62.5/125 | |||
| B. subtilis ATCC 6633 | 7.81/7.81 | |||
| MRSA ATCC NR-46171 | 15.62/7.81 | |||
| MRSA ATCC NR-46071 | 125/15.62 | |||
| V. harveyi MCCB 111 | 65 | 20 mm (zone of inhibition) | Not toxic against VERO cell line and shrimp hemocytes up to 1000 ppm | [81] |
| Bioactivities | Cells/Stains/Enzyme | Comps. | Values | Pros | Cons |
|---|---|---|---|---|---|
| Cytotoxicity (IC50) | Hela cells KB3.1 | 6/7/8 (μM) | 6.8/5.4/2.4 | Compound 8 with broad-spectrum cytotoxicity | Moderate activity [51] |
| PC-3 | 6.3/5.0/2.1 | ||||
| A549 | -/-/6.5 | ||||
| SKOV-3 | -/10.0/5.5 | ||||
| SH-SY5Y | 27 (nM) | 283.6 | Extremely potent and broad-spectrum cytotoxicity | [61] | |
| Sum1315 | 121.3 | ||||
| HT29 | 81.3 | ||||
| SW620 | 90.5 | ||||
| HCT116 | 31.4 | ||||
| HeLa | 100.1 | ||||
| SW872 | 92.3 | ||||
| HCC78 | 41.5 | ||||
| 30 | Broad-spectrum cytotoxicity | IC50 untested [22] | |||
| SF-268 | 31/32 (μM) | 17.0/11.9 | Broad-spectrum cytotoxicity | Moderate activity [62] | |
| MCF-7 | 25.7/20.7 | ||||
| HepG2 | 31.2/12.0 | ||||
| A549 | 34.4/13.5 | ||||
| MM. 1S | 35 (μM) | 8 | Broad-spectrum cytotoxicity | Moderate activity [64] | |
| HeLa | 11 | ||||
| CT26 | 12 | ||||
| AGS | 37 (μM) | 13 | Broad-spectrum cytotoxicity | Moderate activity [67] | |
| Caco2 | 18 | ||||
| HCT116 | 21 | ||||
| L-02 | 49 (μM) | 17 | Strong and broad-spectrum cytotoxicity | [77] | |
| A549 | 5.1 | ||||
| K562 | 0.77 | ||||
| MCF-7 | 6.0 | ||||
| P6C | 9.4 | ||||
| N87 | 46/50/51 (μM) | -/1.0/- | |||
| A673 | -/0.76/8.9 | ||||
| MV4-11 | 4.0/0.16/0.77 | ||||
| K562 | 9.0/4.8/8.9 | ||||
| A549 | 7.8/0.48/9.7 | ||||
| BT474 | 6.0/3.6/- | ||||
| H1229 | -/0.72/- | ||||
| HUCCT1 | -/0.20/- | ||||
| B16F10 | -/0.76/- | ||||
| MDA-MB-468 | 2.8/1.1/0.67 | ||||
| H1975 | -/3.1/4.4 | ||||
| HL60 | 0.38/0.17/5.0 | ||||
| A431 | 4.6/-/- | ||||
| U251 | -/4.7/- | ||||
| HCC1954 | 0.10/0.48/2.0 | ||||
| MCF-7 | 18/-/17 | ||||
| MKN-45 | -/0.49/12 | ||||
| DU-145 | -/0.52/1.0 | ||||
| SPC-A1 | -/2.0/9.8 | ||||
| HCT116 | -/1.9/- | ||||
| 143B | 5.5/5.0/7.7 | ||||
| H2228 | 1.7/0.94/5.0 | ||||
| MDA-MB-231 | -/2.0/2.0 | ||||
| Cytoprotective activity | HaCaT cells | 13, 16 | [52] | ||
| Antiquorum sensing activity (IC50) | C. violaceum 12472 | 33/34 (μg/mL) | 23.59/6.82 | [63] | |
| Antifungal activity (MIC) | C. albicans | 36 | 3.16 mM | Weak activity [66] | |
| Colorectal cancer motility inhibition | Caco2 | 37 | Strong activity | [67] | |
| Enzyme-inhibitory activity (IC50) | α-glucosidase | 63/64 | 94.61/202.33 | strong activity against α-glucosidase | [80] |
| α-amylase | 103.23/- | ||||
| Free radical scavenging activity | 63/64 | Strong activity | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Wang, Y.-F.; Wang, H.; Wang, B. Genus Nocardiopsis: A Prolific Producer of Natural Products. Mar. Drugs 2022, 20, 374. https://doi.org/10.3390/md20060374
Shi T, Wang Y-F, Wang H, Wang B. Genus Nocardiopsis: A Prolific Producer of Natural Products. Marine Drugs. 2022; 20(6):374. https://doi.org/10.3390/md20060374
Chicago/Turabian StyleShi, Ting, Yi-Fei Wang, Han Wang, and Bo Wang. 2022. "Genus Nocardiopsis: A Prolific Producer of Natural Products" Marine Drugs 20, no. 6: 374. https://doi.org/10.3390/md20060374
APA StyleShi, T., Wang, Y.-F., Wang, H., & Wang, B. (2022). Genus Nocardiopsis: A Prolific Producer of Natural Products. Marine Drugs, 20(6), 374. https://doi.org/10.3390/md20060374