Abstract
Five novel tyrosine-decahydrofluorene analogues, xenoacremones D–H (1–5), each bearing a fused 6/5/6 tricarbocyclic core and a 13-membered para-cyclophane ring system, were isolated from the endophytic fungus Xenoacremonium sinensis. Compound 1 was a novel polyketide synthase–nonribosomal peptide synthetase (PKS–NRPS) tyrosine-decahydrofluorene hybrid containing a 6/5/6/6/5 ring system. Their structures were elucidated from comprehensive spectroscopic analysis and electronic circular dichroism (ECD) calculations. All compounds were evaluated for their inhibitory activities on LPS-induced NO production in macrophages and their cytotoxicities against the NB4 and U937 cell lines. Compounds 3 and 5 exhibited potent anti-inflammatory activities in vitro. Compounds 1 and 3–5 displayed significant antiproliferative activity against the tumor cell lines (IC50 < 20 µM).
1. Introduction
Endophytic fungi from medicinal plants are known for their ability to produce a variety of bioactive natural products with novel skeletons [1,2,3,4]. Their secondary metabolites, such as alkaloids, terpenoids and polyketides, possess antibacterial, cytotoxic, anti-inflammatory, antidiabetic and other biological activities [5,6,7]. The genus Xenoacremonium belongs to the family Nectriaceae, which is rarely reported [8]. X. sinensis was first isolated by our team and identified as a new species in 2019 (Figure S1). Filamentous fungi such as Nectriaceae can produce a variety of polyketide–nonribosomal peptide (PK–NRP) hybrids. The members of the unusual family of tyrosine-decahydrofluorenes are rare polyketide synthase–nonribosomal peptide synthetase (PKS-NRPS) secondary metabolites in nature; they include hirsutellones A–F [9,10], pyrrocidines A, B and their analogues [11,12,13], GKK1032A2 [14], penicipyrrodiether [15] and trichogamide A [16]. These PKS-NRPS hybrids were isolated from various fungal species, some of which were from the family Nectriaceae, such as Hirsutella spp. [9,17], Neonectria ramulariae [12,18] and Acremonium zeae [19]. They share a structural skeleton that contains a fused [6,5,6] tricarbocyclic decahydrofluorene, a γ-lactam and a 13-membered para-cyclophane ether ring system, and they possess antitumor, antifungal, antibacterial and antitubercular activities [20]. The unique PKS-NRPS skeletons have attracted considerable attention from chemists, and hirsutellones have been successively synthesized using different methods [21,22,23,24,25]. In our previous research on antitumor secondary metabolites from endophytic fungi, we obtained three novel tyrosine-decahydrofluorene derivatives, xenoacremones A–C, from X. sinensis, isolated from twigs of the mangrove plant Ceriops tagal. The biosynthetic pathway of these compounds was clarified by gene deletion in X. sinensis and heterologous expression investigation [26]. Subsequent studies on the bioactive analogues in X. sinensis (ML-31) have obtained five new tyrosine-decahydrofluorene derivatives, xenoacremones D–H (1–5) (Figure 1). Herein, we describe the isolation, structural elucidation and biological activities of compounds 1–5.
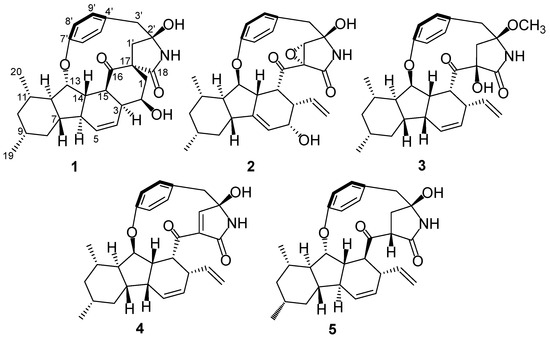
Figure 1.
Structures of compounds 1–5.
2. Results and Discussion
2.1. Structure Elucidation
Xenoacremone D (1) was obtained as a white solid, and its molecular formula was determined to be C29H35NO5 by high-resolution electrospray ionization mass spectrometry (HRESIMS) data (Figure 1), requiring 13 degrees of unsaturation. The 1H NMR data of 1 (Table 1) revealed a 1,4-substituted benzene ring with axial chirality (δH 6.96, 6.95, 6.94 and 6.70), two olefinic protons (δH 6.02 and 5.60), two oxygenated methines (δH 5.16 and 4.75) and two doublet methyls (δH 1.11 and 0.96). The 13C NMR and HSQC spectra (Table 1) showed one ketone and one amide carbonyl group, ten quaternary carbons, ten methines, five methylenes and two methyls.

Table 1.
1H NMR (600 MHz) and 13C NMR data of compounds 1–5 in CD3OD.
Analysis of its 2D NMR data confirmed the whole structure, which had a tyrosine-decahydrofluorene skeleton and resembled that of hirsutellone B and pyrrospirone A [9,13]. The proton spin systems from H-1 to H-15 observed in the 1H-1H COSY spectrum, as well as the HMBC correlations from H-7 to C-6, C-11 and C-13 and from H-14 to C-3, C-5, C-6 and C-15, indicated the presence of a decahydrofluorene moiety. Furthermore, the 1H-1H COSY cross-peaks of H-1/H-2/H-3 and the HMBC correlations from H-1 to C-3, C-16, C-17 and C-18 and from H-1’ to C-1, C-16 and C-17 revealed the presence of a methylene (C-1), an oxygenated methine (C-2) and a quaternary carbon (C-17) in 1, which were different from those of hirsutellone B (Figure 2). The HMBC correlations and the degrees of unsaturation indicated that the methylene at C-1 was linked at C-17 to form a cyclohexane moiety, and C-17 was the connectivity of a spiro center between the cyclohexane and γ-lactam ring. Additional HMBC correlations from H-1’ and H-3’ to a quaternary carbon C-2’ (δC 83.5) led to the assignment of C-2’ for the γ-position of the lactam ring, and its up-field shift revealed the attachment of a hydroxyl group. In addition, the HMBC correlations from H-1’ to C-3’, C-16 and C-18 and from H-3’ to C-5’ and C-9’ completed the linkages of the phenyl and 6/5/6/6/5 pentacarbocyclic moieties to form the 13-membered macrocyclic ether of 1. Consequently, its planar structure containing a spiro-ring system was determined.
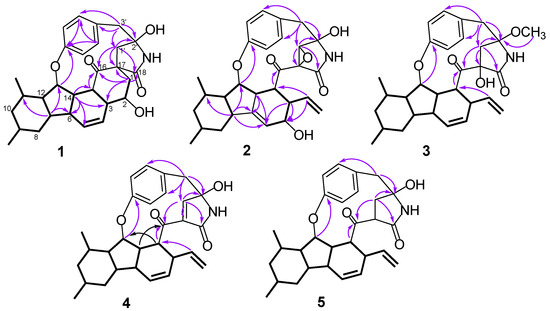
Figure 2.
Key HMBC and 1H-1H COSY correlations of compounds 1–5.
The relative configuration of 1 was ascertained by the NOESY experiment. The NOE cross-peaks of H-7/H-11, H-7/H-9, H-12/H-8a and H-12/H-10a indicated these protons were axial positions. Additional NOE cross-peaks of H-7/H-14, and H-13/H-6’ placed these protons in the β-orientation (Figure 3). However, the NOE cross-peaks of H-6/H-12 and H-15/H-3 indicated that they were co-facial. Further NOE cross-peaks of H-1/H-1’β and H-1α/H-3 revealed that the methylene at C-1 was β-oriented and C-18 of the γ-lactam was α-oriented. The vicinal coupling constant (J12,13 = 8.3 Hz) between H-12 and H-13 implied their trans-configuration, and H-13 was axial. Moreover, the Δ4,5 geometry was assigned as Z by its coupling constant (J = 9.1 Hz). To determine the absolute stereochemistry of 1, the theoretically calculated electronic circular dichroism (ECD) spectra were obtained by time-dependent density functional theory (TDDFT). The conformations of 1a and 1b (1b was the enantiomer of 1a) were compared using ECD calculations at the B3LYP level. The Cotton effects were identical with the calculated curve of the enantiomer 1a (Figure 4), which confirmed its absolute configuration as 2R,3S,6S,7S,9R,11S,12R,13S,14S,15S,17S,2’R.
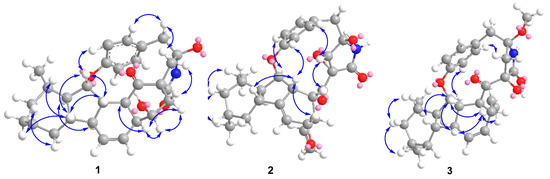
Figure 3.
Key NOE correlations of compounds 1–3.
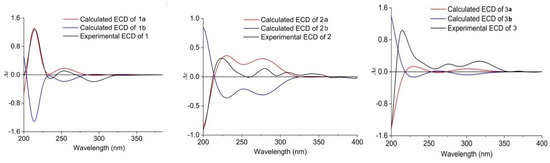
Figure 4.
Experimental and calculated ECD of compounds 1–3 (in MeOH).
Xenoacremone E (2) was shown via HRESIMS to have a molecular formula of C29H33NO6. The 1H and 13C NMR data (Table 1) of 2 were also similar to those of hirsutellone B. The position of the double bond in 2 was deduced by the 1H-1H COSY cross-peaks between H-3 and H-5 and key HMBC correlations from H-7, H-14 and H-5 to C-6 and from H-7, H-14 and H-3 to C-5 (Figure 2). Further HMBC correlations from H-3 to C-4 and C-5 and from H-2 to C-4, as well as the chemical shifts of H-4 (δH 3.84, dd, J = 4.2, 1.2 Hz) and C-4 (δC 67.3), indicated the presence of a hydroxyl group at C-4. Additional HMBC correlations from H-3’ to C-1’ (δC 63.5) and from H-1’ (δH 3.6, s) to the quaternary carbons C-17 (δC 58.7), C-18 and C-2’, combined with the degree of unsaturation of 2, indicated the presence of an epoxide moiety at C-1’ and C-17 in the γ-lactam ring. The NOE correlations (Figure 3) of H-14 to H-3/H-7, H-15 to H-1’ and H-1’ to H-9’ indicated that these hydrogens were co-facial and β-oriented, while the correlations of H-13 to H-6’, H-10α to H3-19/H3-20 hinted that these hydrogens were α-oriented. The ECD spectrum of 2 was determined and the Cotton effects were identical with the calculated curve of the enantiomer 2a (Figure 4). Thus, the absolute configuration of 2 was assigned (3S,4S,7R,9R,11S,12R,13R,14R,15R,17S,1’S,2’R) by comparison of the experimental and calculated ECD spectra (Figure 4).
Xenoacremone F (3) was determined to be C30H37NO5 on the basis of its HR ESIMS and NMR spectra (Table 1). Comparison of its NMR data with those of 2 revealed that 3 had one more methoxyl group (δC 49.7, δH 3.24) and one less epoxide group than 2. The HMBC correlations from –OCH3 to C-2’ confirmed the assignment of the methoxyl group at C-2’ (Figure 2). Further HMBC correlations from H-1’ to C-17, C-18 and C-2’ indicated that the hydroxyl group was located at C-17 in the γ-lactam ring. The NOE correlations (Figure S3) of H-6 to H-7/H-14, H-7 to H-9/H-11/H-14, H-15 to H-1’ and H-3’β to H-9’ indicated that these hydrogens were in the β-orientation, similar to those of 2. The NOE correlations of H-13 to H-6’, H-9’ to H-1’α and H-3’α, as well as H-10α to H3-19/H3-20/H-12 indicated their α-orientation. The ECD spectrum was determined to elucidate the absolute configuration, which was compared to the experimental ECD curve. The ECD spectrum of 3 generated for the tyrosine-decahydrofluorene rings resembled that of compound 2, which was consistent with the experimental data of 3a (Figure S4). Therefore, the absolute configuration of 3 was established as depicted in Figure 1.
Xenoacremone G (4) has the molecular formula C29H33NO4 with 14 degrees of unsaturation, as determined by HR ESIMS data. The 1H and 13C NMR spectroscopic data of 4 (Table 1) were similar to those of 3, and the differences were the absence of the methoxyl group at C-2’ and the presence of an extra double bond (δC 153.7, δH 6.44; δC 134.8) in 4. Further analysis of the 2D NMR data, particularly the HMBC correlations (Figure 2) from H-1’ to C-17 and C-2’ and from H-3’ to C-1’, confirmed the location of the double bond at C-1’ and C-17. The relative configuration of 4 was assigned as depicted by key NOE correlations (Figure S3) of H-6 to H-14, H-14 to H-3, H-3’β to H-9’, H-13 to H-6’ and H-15 to H-1’. The absolute configuration of 4 was identified by comparing its experimental and calculated ECD data (Figure S3). Therefore, 4 was confirmed as 3R,6R,7S,9R,11S,12R,13R,14S,15R,2’R, and it had ECD effects similar to those of 2 (Figure S4).
The HR ESIMS data of xenoacremone H (5) suggested that it had the molecular formula of C29H35NO4. Comprehensive analysis of NMR data for the two compounds indicated that 5 possessed the similar planar structure as 4, where a pair of double bond in γ-lactam ring disappeared (Table 1). The NOESY correlations (Figure S3) of H-12 to H-6 and H-15 to H-6 placed these hydrogens in the α-orientation, while the correlations of H-7 to H-14, H-13 to H-11/H-8’ and H-3 to H-14/H-17 indicated that these hydrogens were in the β-orientation, indicating that 5 had a similar stereochemistry than 1. The vicinal coupling constant (J12,13 = 7.7 Hz) between H-12 and H-13 implied their trans-configuration, which was consistent with that of compound 1. The absolute configuration of 5 was determined by ECD calculation (Figure S4). Its ECD curve was similar to that of 1 (Figure 4). The experimental ECD spectrum of 5 was in accordance with the calculated ECD spectrum for 5a. Therefore, the absolute configuration of 5 was established as 3R,6S,7S,9R,11S,12R,13S,14S,15S,17S,2’S.
2.2. Biological Assay
Compounds 1–5 were evaluated for their anti-inflammatory activities based on the inhibition of nitric oxide (NO) production in LPS-induced RAW264.7 macrophages. The results showed that compounds 3 and 5 exhibited significant inhibitory activities against the production of NO, with IC50 values in the vicinity of 12.8 and 6.7 μM, respectively (Table 2). Compound 5 showed the most potent inhibitory activity, which was stronger than the positive control resveratrol and compounds such as caffeic acid phenthyl ester (9.3 μM) and aspirin (43.2 μM). All the compounds were also examined for their cytotoxicity against the NB4 and U937 cell lines. Compounds 1 and 3–5 displayed cytotoxicities with IC50 values less than 20 µM (Table 3).

Table 2.
Inhibitory effects of compounds 1–5 on NO production in LPS-induced RAW264.7 cells a.

Table 3.
Cytotoxicities of compounds 1–5 (IC50 values: μM) a.
3. Materials and Methods
3.1. General Experimental Procedures
NMR spectra were measured on a Bruker ARX-600 spectrometer (600 MHz, Bruker Co., Ltd., Karlsruhe, Germany). The 1H and 13C NMR chemical shifts of 1–4 were recorded relative to the solvent peaks of CD3OD (δH 3.31 and δC 49.00), and the 1H and 13C NMR chemical shifts of 5 were recorded relative to the solvent peaks of CDCl3 (δH 7.26 and δC 77.16). Optical rotations were recorded with a PerkinElmer 241 polarimeter. ECD spectra were obtained on a JASCO J-815 spectrometer (Tokyo, Japan). HRESIMS data were recorded on a Waters Vion QTOF/MS spectrometer (Waters Micromass, Manchester, UK) in positive electrospray ionization mode. High-performance liquid chromatography (HPLC) was carried out on an Agilent 1260 quaternary system with a UV detector (Agilent, Technologies Co., Ltd., Palo Alto, CA, USA) combined with analytical, semipreparative or preparative Cosmosil C18-MSII columns (250 mm × 4.6 mm or 250 mm × 10 mm). A UPLC reversed-phase C18 analytical column (35 °C, 2.1 mm × 100 mm, 1.7 μm, BEH, Waters) was adopted. Thin-layer chromatography (TLC) was performed on a silica gel plate GF254 (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China). Column chromatography (CC) was performed on Sephadex LH-20 (Pharmacia Fine Chemical Co., Ltd., Uppsala, Sweden), ODS (50 μm, YMC, Tokyo, Japan) and silica gel (200–300 mesh, Qingdao Haiyang Chemical Ltd., Qingdao, China) columns. Human carcinoma cell lines U937, NB4 and A549 were obtained from the Chinese National Infrastructure of Cell Line Resource (NICR). PBS was purchased from HyClone (Solarbio, Beijing, China). FBS was purchased from Every Green (TIANHANG, China). CCK-8 was purchased from Dojindo (Beijing, China).
3.2. Fungal Material
The endophytic fungus ML-31 was isolated from twigs of the mangrove plant Ceriops tagal collected in Hainan Province, China, in July 2013. The plant species was identified by Yi Sun, and the fungus was identified as X. sinensis on the basis of its rRNA gene sequence. The accession number for the biosynthetic cluster (xen) is MT876600 in the GenBank database at NCBI. The strain was deposited at the Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences.
3.3. Extraction and Isolation
The fungus ML-31 was cultured on rice medium for 7 days. Then, fermented rice substrate was cut into small fragments, which were ultrasonically extracted with EtOAc three times. The solvent was then removed under reduced pressure (under vacuum) to yield the total extract (5.3 g). The crude extract was fractionated by ODS flash CC (5 × 30 cm) and eluted with 2 L each of MeOH-H2O (20:80, 40:60, 60:40, 80:20, 100:0). The fraction eluted with 80% MeOH was subjected to Sephadex LH-20 chromatography (CH2Cl2:MeOH 1:1) to obtain six subfractions (A–F), and subfraction B was subsequently purified by HPLC (Kromasil Eternity XT-5-C18 column, 250 mm × 10 mm i.d., 5 μm, 2 mL min−1), with gradient elution from 70% to 80% MeOH in H2O with 0.2% AcOH to afford compounds 1 (tR = 15.0 min, 3.2 mg) and 5 (tR = 27.2 min, 2.5 mg). The fraction eluted with MeOH-H2O (60:40) was subjected to Sephadex LH-20 chromatography (CH2Cl2-MeOH, 1:1) to yield five subfractions (A–E). Fraction C was then isolated by silica gel CC (200−300 mesh) and eluted with a CH2Cl2-acetone gradient system (50:1, 20:1, 10:1, 6:1, 3:1, 2:1, 1:1) to yield five subfractions. The five fractions were analyzed by HPLC. Fraction 4.1 was further purified by HPLC (ACN/H2O, 45%/55%) to obtain compounds 2 (2.4 mg) and 3 (3.6 mg), and fraction 4.3 was subjected to HPLC with 55% MeCN in H2O to afford compound 4 (4.5 mg).
Compound 1: white solid; [α]25D –16.7 (c 0.10, MeOH); CD (MeOH) 215 (Δε +8.87) nm, 235 (Δε −0.70) nm, 254 (Δε +0.77) nm, 293 (Δε −1.32) nm. 1H NMR (600 MHz, CD3OD) and 13C NMR (150 MHz, CD3OD) data (Table 1). HR-ESIMS m/z 478.2606 [M + H]+, calcd for C29H36NO5, 478.2588.
Compound 2: white solid; [α]25D +25.7 (c 0.10, MeOH); CD (MeOH) 224 (Δε +0.32) nm, 278 (Δε −0.16) nm, 315 (Δε +0.07) nm. 1H NMR (600 MHz, CD3OD) and 13C NMR (150 MHz, CD3OD) data (Table 1). HR-ESIMS m/z 492.2395 [M + H]+, calcd for C29H34NO6, 492.2381.
Compound 3: white solid; [α]25D +3.8 (c 0.10, MeOH); CD (MeOH) 215 (Δε +1.04) nm, 276 (Δε +0.19) nm, 318 (Δε +0.26) nm. 1H NMR (600 MHz, CD3OD) and 13C NMR (150 MHz, CD3OD) data (Table 1). HR-ESIMS m/z 492.2766 [M + H]+, calcd for C30H38NO5, 492.2745.
Compound 4: white solid; [α]25D +11.2 (c 0.10, MeOH); CD (MeOH) 222 (Δε +3.25) nm, 285 (Δε +1.15) nm, 331 (Δε +0.80) nm. 1H NMR (600 MHz, CD3OD) and 13C NMR (150 MHz, CD3OD) data (Table 1). HR-ESIMS m/z 460.2486 [M + H]+, calcd for C29H34NO4, 460.2482.
Compound 5: white solid; [α]25D −7.2 (c 0.10, MeOH); CD (MeOH) 214 (Δε +8.72) nm, 237 (Δε −0.51) nm, 255 (Δε +0.29) nm, 300 (Δε −1.18) nm. 1H NMR (600 MHz, CD3OD) and 13C NMR (150 MHz, CD3OD) data (Table 1). HR-ESIMS m/z 462.2443 [M + H]+, calcd for C29H36NO4, 462.2439.
3.4. Computational of ECD
A conformational search was carried out in the MMFF94 molecular mechanics force field using the MOE (Molecular Operating Environment) software package [27], and all the conformers within an energy window of 10 kcal/mol were regarded as the initial conformations. Monte Carlo protocols were used in the experiment. The geometry optimization and frequency calculations were performed with Gaussian16 RevB.01 [28], using the ωB97XD or B3LYP functional at the 6-311G (d,p) level of theory to verify the stability and obtain the energies at 298.15 K and 1 atm pressure. The Boltzmann distribution was calculated according to their Gibbs free energies. ECD calculations were conducted by using the Cam-B3LYP functional at the TZVP level of theory. The solvation model based on density (SMD) was used as the solvation model [29]. The Boltzmann-averaged ECD spectra were obtained by using SpecDis 1.71 software [30]. Methanol was used for structural optimization.
3.5. Cytotoxicity Assays
The cytotoxicities of 1–5 against human carcinoma cells of lines U937 and NB4 were tested using the CCK-8 method. The cells were sustained in RPMI-1640 supplemented with 10% (v/v) fetal bovine serum (FBS) and 0.5% (v/v) penicillin–streptomycin solution (10,000 units/mL penicillin and 10,000 μg/mL streptomycin, 100×) in a humidified atmosphere containing 5% CO2 at 37 °C. The cells were digested by trypsinization and then diluted to a concentration of 1 × 104 cells/mL. The diluted cell suspensions were then placed into 96-well microtiter plates and incubated with the test samples for 72 h. The control contained 2 μL of MeOH. After incubation, CCK-8 solution was added to each well, and the plates were incubated for 4 h. The absorption was measured at a wavelength of 450 nm.
3.6. Assay of the Inhibition of NO Production in RAW264.7 Murine Macrophages
The nitrite concentration in the medium was measured as an indicator of NO production according to the Griess reaction. RAW 264.7 macrophages were seeded in three replicates at a density of 1 × 105 cells/well and incubated overnight at 37 °C with 5% CO2. The cells were then treated with the range (3.125~50 μg/mL) of compounds 1–5 in the presence of 1 μg/mL LPS for 30 min. The culture supernatant was aspirated, and the cells were further incubated at the same LPS concentration for 24 h. After incubation, 100 mL of cell-free supernatant was mixed with 100 μL of Griess reagent containing equal volumes of 2% (w/v) sulfanilamide in 5% (w/v) phosphoric acid and 0.2% (w/v) N-(1-naphthyl) ethylenediamine solution to determine nitrite production. Absorbance was measured in a microplate reader at 540 nm against a calibration curve with NaNO2 standards. Data are expressed as the mean ± SD of three independent experiments.
4. Conclusions
In summary, we isolated and fully identified five novel tyrosine-decahydrofluorene analogues bearing a rare fused 6/5/6 tricarbocyclic core and a 13-membered para-cyclophane ring system from the endophytic fungus X. sinensis ML-31. Compound 1 had a 6/5/6/6/5 pentacarbocyclic skeleton with a [5,6]-spiro ring and a para-cyclophane ring system. Compounds 1, 3 and 5 showed significant cytotoxic activities against the NB4 and U937 cell lines (IC50 < 20 μM). Compounds 3 and 5 also exhibited potent inhibitory activities against the production of NO (IC50, 12.8 and 6.7 μM, respectively). Our findings expand the knowledge of tyrosine-decahydrofluorene derivatives and can further facilitate biosynthesis investigation.
Supplementary Materials
The following are available online at www.mdpi.com/article/10.3390/md20060375/s1, Figures S1–S44: The HRESIMS, 1 D, 2D NMR and ECD spectra of compounds 1–5.
Author Contributions
Y.S. conceived and designed the experiments. Z.L. and L.L. (Li Liu) carried out the chemical experiments. A.W. and S.Z. carried out the bioactivity assay. L.L. (Li Li) performed the ECD calculations. Y.W. determined the NMR spectra. Y.S. and L.L. (Li Liu) wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grants from the CACMS Innovation Fund (CI2021A04514).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ortega, H.E.; Torres-Mendoza, D.; Caballero, Z.E.; Cubilla-Rios, L. Structurally uncommon secondary metabolites derived from endophytic fungi. J. Fungi 2021, 7, 570. [Google Scholar] [CrossRef] [PubMed]
- El-Bondkly, E.A.M.; El-Bondkly, A.A.M.; El-Bondkly, A.A.M. Marine endophytic fungal metabolites: A whole new world of pharmaceutical therapy exploration. Heliyon 2021, 7, e06362. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Babalola, O.O. Pharmacological potential of fungal endophytes associated with medicinal plants: A review. J. Fungi 2021, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Li, X.Q.; Zhao, D.L.; Zhang, P. Antifungal secondary metabolites produced by the fungal endophytes: Chemical diversity and potential use in the development of biopesticides. Front. Microbiol. 2021, 12, 689527. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chaturvedi, P.; Kulkarni, M.G.; Staden, J.V. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol. Adv. 2020, 39, 107462. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Li, S.; Zhang, X.; Zhao, C. Biological ativities of some new secondary metabolites isolated from endophytic fungi: A review study. Int. J. Mol. Sci. 2021, 22, 959. [Google Scholar] [CrossRef] [PubMed]
- Manganyi, M.C.; Ateba, C.N. Untapped potentials of endophytic fungi: A review of novel bioactive compounds with biological applications. Microorganisms 2020, 8, 1934. [Google Scholar] [CrossRef]
- Lombard, L.; van der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Generic concepts in Nectriaceae. Stud. Mycol. 2015, 80, 189–245. [Google Scholar] [CrossRef] [Green Version]
- Isaka, M.; Rugseree, N.; Maithip, P.; Kongsaeree, P.; Prabpai, S.; Thebtaranonth, Y. Hirsutellones A-E, antimycobacterial alkaloids from the insect pathogenic fungus Hirsutella nivea BCC 2594. Tetrahedron 2005, 61, 5577–5583. [Google Scholar] [CrossRef]
- Isaka, M.; Prathumpai, W.; Wongsa, P.; Tanticharoen, M. Hirsutellone F, a dimer of antitubercular alkaloids from the seed fungus Trichoderma species BCC 7579. Org. Lett. 2006, 8, 2815–2817. [Google Scholar] [CrossRef]
- He, H.; Yang, H.Y.; Bigelis, R.; Eric, H.; Greenstein, S.M.; Carter, G.T. Pyrrocidines A and B, new antibiotics produced by a filamentous fungus. Tetrahedron Lett. 2002, 43, 1633–1636. [Google Scholar] [CrossRef]
- Shiono, Y.; Kosukegawa, A.; Koseki, T.; Murayama, T.; Kwon, E.; Uesugi, S.; Kimura, K. A dimeric pyrrocidine from Neonectria ramulariae is an inhibitor of prolyl oligopeptidase. Phytochem. Lett. 2012, 5, 91–95. [Google Scholar] [CrossRef]
- Shiono, Y.; Shimanuki, K.; Hiramatsu, F.; Koseki, T.; Murayama, T.; Fujisawa, N.; Kimura, K. Pyrrospirones A and B, apoptosis inducers in HL-60 cells, from an endophytic fungus, Neonectria Ramulariae Wollenw KS-246. Bioorg. Med. Chem. Lett. 2008, 23, 6050–6053. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Liermann, J.C.; Opatz, T.; Anke, H.; Thines, E. GKK1032A2, a secondary metabolite from Penicillium sp. IBWF-029-96, inhibits conidial germination in the rice blast fungus Magnaporthe oryzae. J. Antibiot. 2012, 65, 99–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, T.; Chen, M.; Ge, Z.; Chai, W.; Li, X.; Zhang, Z.; Lian, X. Bioactive penicipyrrodiether A, an adduct of GKK1032 analogue and phenol A derivative, from a marine-sourced fungus Penicillium sp. ZZ380. J. Org. Chem. 2018, 83, 13395–13401. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shen, H.; Zhang, P.; Cheng, H.; Dai, X.; Liu, L. Anti-glioma trichobamide A with an unprecedented tetrahydro-5H-furo[2,3-b]pyrrol-5-one functionality form ascidian-derived fungus Trichobotrys effuse 4729. Chem. Commun. 2019, 55, 1438–1441. [Google Scholar] [CrossRef] [PubMed]
- Madla, S.; Isaka, M.; Wongsa, P. Modification of culture conditions for production of the anti–tubercular hirsutellones by the insect pathogenic fungus Hirsutella nivea BCC 2594. Lett. Appl. Microbiol. 2008, 47, 74–78. [Google Scholar] [CrossRef]
- Shiono, Y.; Furukawa, M.; Koseki, T.; Kwon, E.; Kurniawan, A.H.; Sato, S.; Harneti, D.; Maharani, R.; Supratman, U.; Uesugi, S.; et al. A pyrrocidine derivative produced by fungus Neonectria ramulariae In–2 isolated from a Beetle Holotrichia picea. Phytochem. Lett. 2018, 26, 120–124. [Google Scholar] [CrossRef]
- Wicklow, D.T.; Poling, S.M.; Summerbell, R.C. Occurrence of pyrrocidine and dihydroresorcylide production among Acremonium zeae populations from maize grown in different regions. Can. J. Plant Pathol. 2008, 30, 425–433. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, S. Recent advances of synthesis of fluorenone and fluorene containing natural products. Tetrahedron 2016, 72, 1717–1735. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Sarlah, D.; Wu, T.R.; Zhan, W. Total synthesis of hirsutellone B. Angew. Chem. Int. Ed. 2009, 48, 6870–6874. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Sun, Y.P.; Sarlah, D.; Zhan, W.; Wu, T.R. Bioinspired synthesis of hirsutellones A, B, and C. Org. Lett. 2011, 13, 5708–5710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchiro, H.; Kato, R.; Arai, Y.; Hasegawa, M.; Kobayakawa, Y. Total synthesis of hirsutellone B via UIImann-type direct 13-membered macrocyclization. Org. Lett. 2011, 13, 6268–6271. [Google Scholar] [CrossRef] [PubMed]
- Reber, K.P.; Tilley, S.D.; Carson, C.A.; Sorensen, E.J. Toward a synthesis of hirsutellone B by the concept of double cyclization. J. Org. Chem. 2013, 78, 9584–9607. [Google Scholar] [CrossRef] [Green Version]
- Sugata, H.; Inagaki, K.; Ode, T.; Hayakawa, T.; Karoji, Y.; Baba, M.; Kato, R.; Hasegawa, D.; Tsubogo, T.; Uchiro, H. Total synthesis of GKK1032A2 via direct 13-membered macrocyclization using a nucleophilic aromatic substitution of an (η6-arene) Chromium complex. Chem. Asian J. 2017, 12, 628–632. [Google Scholar] [CrossRef]
- Liu, Z.G.; Li, W.; Zhang, P.; Fan, J.; Zhang, F.B.; Wang, C.X.; Li, S.M.; Sun, Y.; Chen, S.L.; Yin, W.B. Tricarbocyclic core formation of tyrosine decahydrofluorenes implies a three-enzyme cascade with XenF-mediated sigmatropic rearrangement as a prerequisite. Acta Pharm. Sin. B 2021, 11, 3655–3664. [Google Scholar] [CrossRef]
- MOE2009.10. Chemical Computing Group Inc. Available online: https://www.chemcomp.com/Products.htm (accessed on 31 July 2021).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Haghdani, S.; Hoff, B.H.; Koch, H.; Åstrand, P.O. Optical rotation calculations for fluorinated alcohols, amines, amides, and esters. J. Phys. Chem. A 2016, 120, 7973–7986. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Pescitelli, G. SpecDis Version 1.71. 2017. Available online: https:/specdis-software.jimdo.com (accessed on 12 July 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).