Fucoxanthin Is a Potential Therapeutic Agent for the Treatment of Breast Cancer
Abstract
1. Introduction
1.1. Breast Cancer Prevalence
1.2. Genetic Mutations & Clinical Outcomes
1.3. Clinical Challenges
2. Fucoxanthin
2.1. Sources
2.2. Chemical Structures
2.3. Absorption
2.4. Safety
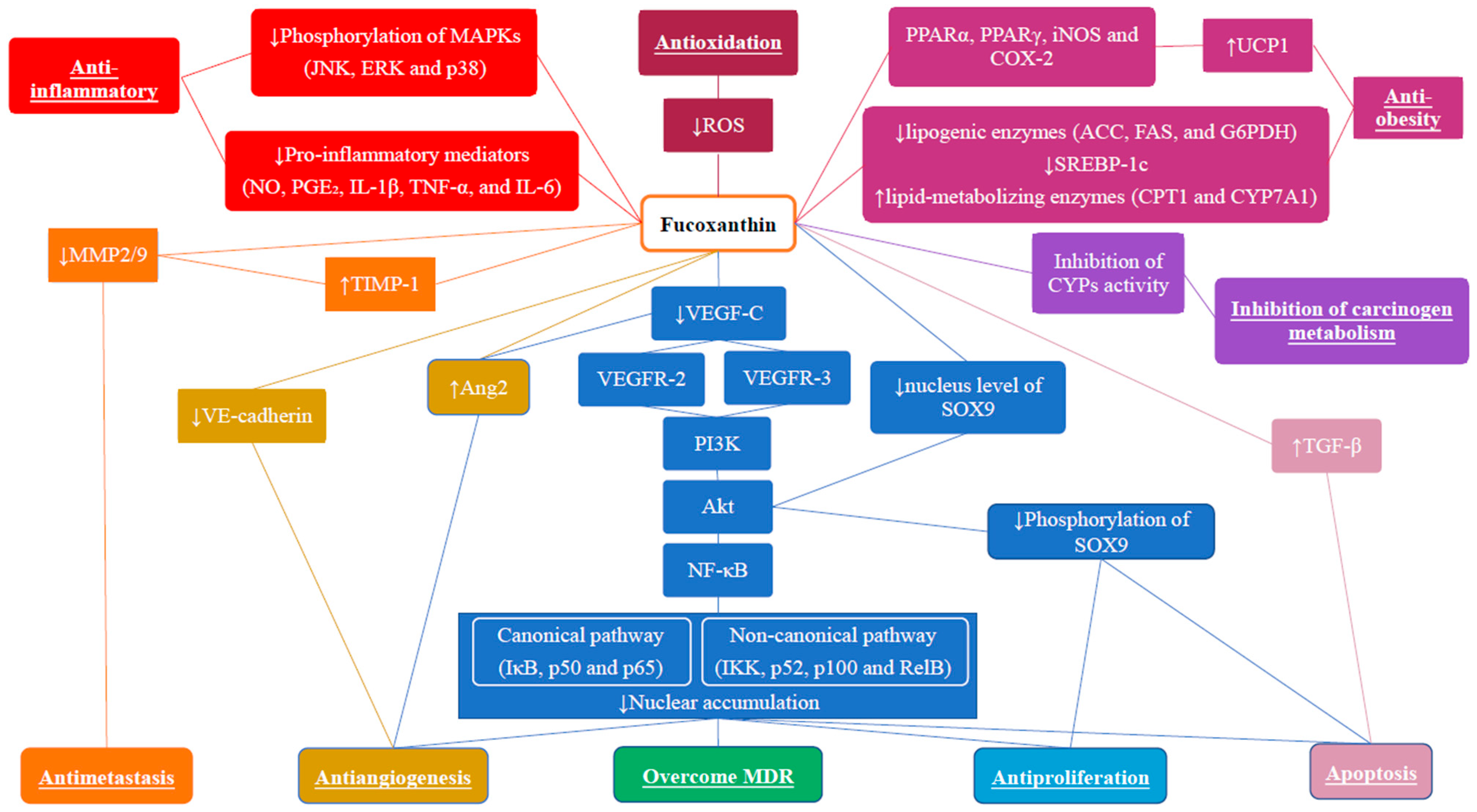
3. Anti-Breast Cancer Effects of Fucoxanthin
3.1. Anti-Proliferative Effect
3.2. Apoptotic Effect
3.3. Anti-Metastatic Effects
3.4. Anti-Angiogenic Effects
3.5. Modulation of Tumor Microenvironment
3.6. Modulation of Carcinogen Metabolism
3.7. Overcome Multidrug Resistance
3.8. Anti-Oxidative Effects and Cancer Prevention
3.9. Anti-Obesity Effect and Cancer Prevention
4. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, L.; Bonaccio, M.; de Gaetano, G.; Donati, M.B. Epidemiology of breast cancer, a paradigm of the “common soil” hypothesis. Semin. Cancer Biol. 2021, 72, 4–10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Estimated number of New Cases from 2020 to 2040, Both Sexes, Age [0–85+]. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=20&single_unit=100000&types=0 (accessed on 4 March 2022).
- World Health Organization (WHO). Estimated Number of Deaths from 2020 to 2040, Both Sexes, Age [0–85+]. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=20&single_unit=100000&types=1 (accessed on 4 March 2022).
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H. Molecular Pathology of Breast Cancer. Am. J. Clin. Pathol. 2012, 138, 770–780. [Google Scholar] [CrossRef]
- Prat, A.; Perou, C.M. Deconstructing the molecular portraits of breast cancer. Mol. Oncol. 2010, 5, 5–23. [Google Scholar] [CrossRef]
- Prat, A.; Ellis, M.J.; Perou, C.M. Practical implications of gene-expression-based assays for breast oncologists. Nat. Rev. Clin. Oncol. 2011, 9, 48–57. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Dittmer, J. Breast cancer stem cells: Features, key drivers and treatment options. Semin. Cancer Biol. 2018, 53, 59–74. [Google Scholar] [CrossRef]
- Sotiriou, C.; Neo, S.-Y.; McShane, L.M.; Korn, E.L.; Long, P.M.; Jazaeri, A.; Martiat, P.; Fox, S.B.; Harris, A.L.; Liu, E.T. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc. Natl. Acad. Sci. USA 2003, 100, 10393–10398. [Google Scholar] [CrossRef]
- Balmaña, J.; Diez, O.; Rubio, I.T.; Cardoso, F. BRCA in breast cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2011, 22, vi31–vi34. [Google Scholar] [CrossRef] [PubMed]
- Paluch-Shimon, S.; Cardoso, F.; Sessa, C.; Balmaña, J.; Cardoso, M.J.; Gilbert, F.; Senkus, E. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 2016, 27, v103–v110. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D. Germline BRCA1 Mutations and a Basal Epithelial Phenotype in Breast Cancer. Cancer Spectrum Knowl. Environ. 2003, 95, 1482–1485. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Reis-Filho, J.S. Basal-like breast cancer and the BRCA1 phenotype. Oncogene 2006, 25, 5846–5853. [Google Scholar] [CrossRef]
- Turner, N.C.; Reis-Filho, J.S.; Russell, A.M.; Springall, R.J.; Ryder, K.; Steele, D.; Savage, K.; Gillett, C.E.; Schmitt, F.C.; Ashworth, A.; et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene 2006, 26, 2126–2132. [Google Scholar] [CrossRef]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, Breast Cancer Subtypes, and Survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492. [Google Scholar] [CrossRef]
- Morris, G.J.; Naidu, S.; Topham, A.K.; Guiles, F.; Xu, Y.; McCue, P.; Schwartz, G.F.; Park, P.K.; Rosenberg, A.L.; Brill, K.; et al. Differences in breast carcinoma characteristics in newly diagnosed African–American and Caucasian patients. Cancer 2007, 110, 876–884. [Google Scholar] [CrossRef]
- Bane, A.L.; Beck, J.C.; Bleiweiss, I.; Buys, S.S.; Catalano, E.; Daly, M.B.; Giles, G.; Godwin, A.K.; Hibshoosh, H.; Hopper, J.L.; et al. BRCA2 Mutation-associated Breast Cancers Exhibit a Distinguishing Phenotype Based on Morphology and Molecular Profiles from Tissue Microarrays. Am. J. Surg. Pathol. 2007, 31, 121–128. [Google Scholar] [CrossRef]
- Deng, C.-X. BRCA1: Cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res. 2006, 34, 1416–1426. [Google Scholar] [CrossRef]
- Dine, J.; Deng, C.-X. Mouse models of BRCA1 and their application to breast cancer research. Cancer Metastasis Rev. 2012, 32, 25–37. [Google Scholar] [CrossRef]
- Davis, N.M.; Sokolosky, M.; Stadelman, K.; Abrams, S.L.; Libra, M.; Candido, S.; Nicoletti, F.; Polesel, J.; Maestro, R.; D’Assoro, A.; et al. Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: Possibilities for therapeutic intervention. Oncotarget 2014, 5, 4603–4650. [Google Scholar] [CrossRef] [PubMed]
- Elizalde, P.V.; Russo, R.I.C.; Chervo, M.F.; Schillaci, R. ErbB-2 nuclear function in breast cancer growth, metastasis and resistance to therapy. Endocr. Relat. Cancer 2016, 23, T243–T257. [Google Scholar] [CrossRef] [PubMed]
- Appert-Collin, A.; Hubert, P.; Crémel, G.; Bennasroune, A. Role of ErbB Receptors in Cancer Cell Migration and Invasion. Front. Pharmacol. 2015, 6, 283. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Wendt, M.K. The paradoxical functions of EGFR during breast cancer progression. Signal Transduct. Target. Ther. 2017, 2, 16042. [Google Scholar] [CrossRef]
- Zhang, D.; LaFortune, T.A.; Krishnamurthy, S.; Esteva, F.; Cristofanilli, M.; Liu, P.; Lucci, A.; Singh, B.; Hung, M.-C.; Hortobagyi, G.N.; et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Reverses Mesenchymal to Epithelial Phenotype and Inhibits Metastasis in Inflammatory Breast Cancer. Clin. Cancer Res. 2009, 15, 6639–6648. [Google Scholar] [CrossRef]
- Alanazi, I.O.; Khan, Z. Understanding EGFR Signaling in Breast Cancer and Breast Cancer Stem Cells: Overexpression and Therapeutic Implications. Asian Pac. J. Cancer Prev. 2016, 17, 445–453. [Google Scholar] [CrossRef]
- Kim, A.; Jang, M.H.; Lee, S.J.; Bae, Y.K. Mutations of the Epidermal Growth Factor Receptor Gene in Triple-Negative Breast Cancer. J. Breast Cancer 2017, 20, 150. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Olopade, O.I. MYC and Breast Cancer. Genes Cancer 2010, 1, 629–640. [Google Scholar] [CrossRef]
- Chen, Y.; Olopade, O.I. MYC in breast tumor progression. Expert Rev. Anticancer Ther. 2008, 8, 1689–1698. [Google Scholar] [CrossRef]
- Jung, M.; Russell, A.J.; Liu, B.; George, J.; Liu, P.Y.; Liu, T.; DeFazio, A.; Bowtell, D.; Oberthuer, A.; London, W.B.; et al. A Myc Activity Signature Predicts Poor Clinical Outcomes in Myc-Associated Cancers. Cancer Res. 2016, 77, 971–981. [Google Scholar] [CrossRef]
- Fernandez-Medarde, A.; Santos, E. Ras in Cancer and Developmental Diseases. Genes Cancer 2011, 2, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Ray, B.K. Induction of Ras by SAF -1/ MAZ through a feed-forward loop promotes angiogenesis in breast cancer. Cancer Med. 2014, 4, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, S.; Kim, S.-J.; Han, H.-J.; Kim, S.H.; Zheng, J.; Lee, H.-B.; Han, W.; Noh, D.-Y.; Na, H.-K.; Surh, Y.-J. H-Ras induces Nrf2-Pin1 interaction: Implications for breast cancer progression. Toxicol. Appl. Pharmacol. 2020, 402, 115121. [Google Scholar] [CrossRef] [PubMed]
- Banys-Paluchowski, M.; Milde-Langosch, K.; Fehm, T.; Witzel, I.; Oliveira-Ferrer, L.; Schmalfeldt, B.; Müller, V. Clinical relevance of H-RAS, K-RAS, and N-RAS mRNA expression in primary breast cancer patients. Breast Cancer Res. Treat. 2019, 179, 403–414. [Google Scholar] [CrossRef]
- Esteva, F.J.; Valero, V.; Pusztai, L.; Boehnke-Michaud, L.; Buzdar, A.U.; Hortobagyi, G.N. Chemotherapy of Metastatic Breast Cancer: What to Expect in 2001 and beyond. Oncologist 2001, 6, 133–146. [Google Scholar] [CrossRef]
- Fisusi, F.A.; Akala, E.O. Drug Combinations in Breast Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 3–23. [Google Scholar] [CrossRef]
- Tanabe, M. Combination Chemotherapy of Mitomycin C and Methotrexate Was Effective on Metastatic Breast Cancer Resistant to Eribulin, Vinorelbine, and Bevacizumab after Anthracycline, Taxane, and Capecitabine. Case Rep. Oncol. 2016, 9, 422–426. [Google Scholar] [CrossRef]
- Panasci, L.; Shenouda, G.; Begin, L.; Pollak, M.; Reinke, A.; Margolese, R. Mitomycin C and mitoxantrone chemotherapy for advanced breast cancer: Efficacy with minimal gastrointestinal toxicity and alopecia. Cancer Chemother. Pharmacol. 1990, 26, 457–460. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Pernas, S.; Tolaney, S.M.; Winer, E.P.; Goel, S. CDK4/6 inhibition in breast cancer: Current practice and future directions. Ther. Adv. Med. Oncol. 2018, 10, 175883591878645. [Google Scholar] [CrossRef]
- Seidman, A.D.; Fornier, M.N.; Esteva, F.J.; Tan, L.; Kaptain, S.; Bach, A.; Panageas, K.S.; Arroyo, C.; Valero, V.; Currie, V.; et al. Weekly Trastuzumab and Paclitaxel Therapy for Metastatic Breast Cancer with Analysis of Efficacy by HER2 Immunophenotype and Gene Amplification. J. Clin. Oncol. 2001, 19, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, K.L.; Burstein, H.J.; Storniolo, A.M.; Rugo, H.S.; Sledge, G.; Aktan, G.; Ellis, C.; Florance, A.; Vukelja, S.; Bischoff, J.; et al. Overall Survival Benefit with Lapatinib in Combination with Trastuzumab for Patients with Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer: Final Results from the EGF104900 Study. J. Clin. Oncol. 2012, 30, 2585–2592. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.; Garcia-Saenz, J.A.; Xu, B.; Harb, W.; Moroose, R.; Pluard, T.; Cortés, J.; Kiger, C.; Germa, C.; Wang, K.; et al. Safety and Efficacy of Neratinib in Combination with Capecitabine in Patients with Metastatic Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer. J. Clin. Oncol. 2014, 32, 3626–3633. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef]
- Ngamcherdtrakul, W.; Yantasee, W. siRNA therapeutics for breast cancer: Recent efforts in targeting metastasis, drug resistance, and immune evasion. Transl. Res. 2019, 214, 105–120. [Google Scholar] [CrossRef]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.U.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic Behavior of Breast Cancer Subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Martin, L.J. Fucoxanthin and Its Metabolite Fucoxanthinol in Cancer Prevention and Treatment. Mar. Drugs 2015, 13, 4784–4798. [Google Scholar] [CrossRef]
- Abu-Ghannam, N.; Shannon, E. Seaweed carotenoid, fucoxanthin, as functional food. In Microbial Functional Foods and Nutraceuticals; Gupta, V.K., Treichel, H., Shapaval, V.O., de Oliveira, L.A., Tuohy, M.G., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 39–64. [Google Scholar]
- Martin, M.; Hartley, A.V.; Jin, J.; Sun, M.; Lu, T. Phosphorylation of NF-κB in cancer. In Adenosine Triphosphate in Health and Disease; Mozsik, G., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A Promising Medicinal and Nutritional Ingredient. Evid.-Based Complement. Altern. Med. 2015, 2015, 723515. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Sashima, T.; Hosokawa, M.; Miyashita, K. Comparative evaluation of growth inhibitory effect of stereoisomers of fucoxanthin in human cancer cell lines. J. Funct. Foods 2009, 1, 88–97. [Google Scholar] [CrossRef]
- Kim, S.; Chojnacka, K. Marine Algae Extracts: Processes, Products, and Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical Scavenging and Singlet Oxygen Quenching Activity of Marine Carotenoid Fucoxanthin and Its Metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, R.K.; Bhaskar, N.; Baskaran, V. Comparative effects of β-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol. Cell. Biochem. 2009, 331, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T. Carotenoids: Physical, Chemical, and Biological Functions and Properties; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Dai, Y.L.; Jiang, Y.F.; Lu, Y.A.; Yu, J.B.; Kang, M.C.; Jeon, Y.J. Fucoxanthin-rich fraction from Sargassum fusiformis alleviates particulate matter-induced inflammation in vitro and in vivo. Toxicol. Rep. 2021, 8, 349–358. [Google Scholar] [CrossRef]
- Grasa-López, A.; Miliar-García, Á.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Reyes-Maldonado, E.; Jaramillo-Flores, M.-E. Undaria pinnatifida and Fucoxanthin Ameliorate Lipogenesis and Markers of Both Inflammation and Cardiovascular Dysfunction in an Animal Model of Diet-Induced Obesity. Mar. Drugs 2016, 14, 148. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Dietary Combination of Fucoxanthin and Fish Oil Attenuates the Weight Gain of White Adipose Tissue and Decreases Blood Glucose in Obese/Diabetic KK-Ay Mice. J. Agric. Food Chem. 2007, 55, 7701–7706. [Google Scholar] [CrossRef]
- Mikami, N.; Hosokawa, M.; Miyashita, K.; Sohma, H.; Ito, Y.M.; Kokai, Y. Reduction of HbA1c levels by fucoxanthin-enriched akamoku oil possibly involves the thrifty allele of uncoupling protein 1 (UCP1): A randomised controlled trial in normal-weight and obese Japanese adults. J. Nutr. Sci. 2017, 6, e5. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Lee, S.G.; Nam, J.-O. Therapeutic Effect of Seaweed Derived Xanthophyl Carotenoid on Obesity Management; Overview of the Last Decade. Int. J. Mol. Sci. 2020, 21, 2502. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Zhang, X.; Chen, Q.; Chen, H.; Sun, L.; Liu, G. Protective Effect of Fucoxanthin Isolated from Laminaria japonica against Visible Light-Induced Retinal Damage Both in Vitro and in Vivo. J. Agric. Food Chem. 2016, 64, 416–424. [Google Scholar] [CrossRef]
- Chen, S.-J.; Lee, C.-J.; Lin, T.-B.; Peng, H.-Y.; Liu, H.-J.; Chen, Y.-S.; Tseng, K.-W. Protective Effects of Fucoxanthin on Ultraviolet B-Induced Corneal Denervation and Inflammatory Pain in a Rat Model. Mar. Drugs 2019, 17, 152. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Kawee-Ai, A.; Kuntiya, A.; Kim, S.M. Anticholinesterase and Antioxidant Activities of Fucoxanthin Purified from the Microalga Phaeodactylum Tricornutum. Nat. Prod. Commun. 2013, 8, 1934578X1300801. [Google Scholar] [CrossRef]
- Rodrigues, E.; Mariutti, L.R.B.; Mercadante, A.Z. Scavenging Capacity of Marine Carotenoids against Reactive Oxygen and Nitrogen Species in a Membrane-Mimicking System. Mar. Drugs 2012, 10, 1784–1798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Fan, Y.; Gao, Y.; Li, X.; Hu, Z.; Ding, K.; Wang, Y.; Wang, X. Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE and Nrf2-autophagy pathways. Sci. Rep. 2017, 7, 46763. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An Antibacterial Carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef]
- Nishino, H.; Murakoshi, M.; Tokuda, H.; Satomi, Y. Cancer prevention by carotenoids. Arch. Biochem. Biophys. 2009, 483, 165–168. [Google Scholar] [CrossRef]
- Le Goff, M.; Le Ferrec, E.; Mayer, C.; Mimouni, V.; Lagadic-Gossmann, D.; Schoefs, B.; Ulmann, L. Microalgal carotenoids and phytosterols regulate biochemical mechanisms involved in human health and disease prevention. Biochimie 2019, 167, 106–118. [Google Scholar] [CrossRef]
- Sugawara, T.; Matsubara, K.; Akagi, R.; Mori, M.; Hirata, T. Antiangiogenic Activity of Brown Algae Fucoxanthin and Its Deacetylated Product, Fucoxanthinol. J. Agric. Food Chem. 2006, 54, 9805–9810. [Google Scholar] [CrossRef]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown Algae Fucoxanthin Is Hydrolyzed to Fucoxanthinol during Absorption by Caco-2 Human Intestinal Cells and Mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [CrossRef]
- Beppu, F.; Niwano, Y.; Sato, E.; Kohno, M.; Tsukui, T.; Hosokawa, M.; Miyashita, K. In vitro and in vivo evaluation of mutagenicity of fucoxanthin (FX) and its metabolite fucoxanthinol (FXOH). J. Toxicol. Sci. 2009, 34, 693–698. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2012, 1821, 70–77. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Dawson, H.D.; Harrison, E.H. Carotenoid Transport Is Decreased and Expression of the Lipid Transporters SR-BI, NPC1L1, and ABCA1 Is Downregulated in Caco-2 Cells Treated with Ezetimibe. J. Nutr. 2005, 135, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Yamashita, K.; Asai, A.; Nagao, A.; Shiraishi, T.; Imai, I.; Hirata, T. Esterification of xanthophylls by human intestinal Caco-2 cells. Arch. Biochem. Biophys. 2009, 483, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, M.B.; Park, Y.K.; Lee, J.Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef]
- Asai, A.; Sugawara, T.; Ono, H.; Nagao, A. Biotransformation of Fucoxanthinol into Amarouciaxanthin A in Mice and HEPG2 cells: Formation and Cytotoxicity of Fucoxanthin Metabolites. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ozaki, Y.; Mizuno, M.; Yoshida, M.; Nishitani, Y.; Azuma, T.; Komoto, A.; Maoka, T.; Tanino, Y.; Kanazawa, K. Pharmacokinetics of fucoxanthinol in human plasma after the oral administration of kombu extract. Br. J. Nutr. 2011, 107, 1566–1569. [Google Scholar] [CrossRef]
- Yonekura, L.; Kobayashi, M.; Terasaki, M.; Nagao, A. Keto-Carotenoids Are the Major Metabolites of Dietary Lutein and Fucoxanthin in Mouse Tissues. J. Nutr. 2010, 140, 1824–1831. [Google Scholar] [CrossRef]
- Matsuno, T.; Ookubo, M.; Komori, T. Carotenoids of Tunicates, III. The Structural Elucidation of Two New Marine Carotenoids, Amarouciaxanthin A and B. J. Nat. Prod. 1985, 48, 606–613. [Google Scholar] [CrossRef]
- Yamano, Y.; Chary, M.V.; Wada, A. Stereocontrolled First Total Syntheses of Amarouciaxanthin A and B. Org. Lett. 2013, 15, 5310–5313. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Randell, R. Fat burners: Nutrition supplements that increase fat metabolism. Obes. Rev. 2011, 12, 841–851. [Google Scholar] [CrossRef]
- Iio, K.; Okada, Y.; Ishikura, M. Single and 13-Week Oral Toxicity Study of Fucoxanthin Oil from Microalgae in Rats. Food Hyg. Saf. Sci. 2011, 52, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Hitoe, S.; Shimoda, H. Seaweed Fucoxanthin Supplementation Improves Obesity Parameters in Mild Obese Japanese Subjects. Funct. Foods Health Dis. 2017, 7, 246. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Nagao, A. Absorption and Metabolism of Xanthophylls. In Marine Carotenoids; Sugawara, T., Maoka, T., Eds.; MDPI AG: Basel, Switzerland, 2021. [Google Scholar]
- Eid, S.Y.; Althubiti, M.A.; Abdallah, M.E.; Wink, M.; El-Readi, M.Z. The carotenoid fucoxanthin can sensitize multidrug resistant cancer cells to doxorubicin via induction of apoptosis, inhibition of multidrug resistance proteins and metabolic enzymes. Phytomedicine 2020, 77, 153280. [Google Scholar] [CrossRef] [PubMed]
- Konishi, I.; Hosokawa, M.; Sashima, T.; Kobayashi, H.; Miyashita, K. Halocynthiaxanthin and fucoxanthinol isolated from Halocynthia roretzi induce apoptosis in human leukemia, breast and colon cancer cells. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 142, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Ayyad, S.E.; Basaif, S.; Badria, A.; Ezmirly, S.; Alarif, W.; Badria, F. Antioxidant, cytotoxic, antitumor, and protective DNA damage metabolites from the red sea brown alga Sargassum sp. Pharmacogn. Res. 2011, 3, 160. [Google Scholar] [CrossRef] [PubMed]
- Rwigemera, A.; Mamelona, J.; Martin, L.J. Inhibitory effects of fucoxanthinol on the viability of human breast cancer cell lines MCF-7 and MDA-MB-231 are correlated with modulation of the NF-κB pathway. Cell Biol. Toxicol. 2014, 30, 157–167. [Google Scholar] [CrossRef]
- Rwigemera, A.; Mamelona, J.; Martin, L.J. Comparative effects between fucoxanthinol and its precursor fucoxanthin on viability and apoptosis of breast cancer cell lines MCF-7 and MDA-MB-231. Anticancer Res. 2015, 35, 207–219. [Google Scholar]
- Wang, J.; Ma, Y.; Yang, J.; Jin, L.; Gao, Z.; Xue, L.; Hou, L.; Sui, L.; Liu, J.; Zou, X. Fucoxanthin inhibits tumour-related lymphangiogenesis and growth of breast cancer. J. Cell. Mol. Med. 2019, 23, 2219–2229. [Google Scholar] [CrossRef]
- Malhão, F.; Macedo, A.; Costa, C.; Rocha, E.; Ramos, A. Fucoxanthin Holds Potential to Become a Drug Adjuvant in Breast Cancer Treatment: Evidence from 2D and 3D Cell Cultures. Molecules 2021, 26, 4288. [Google Scholar] [CrossRef]
- Jang, H.; Choi, J.; Park, J.-K.; Won, G.; Seol, J.W. Fucoxanthin Exerts Anti-Tumor Activity on Canine Mammary Tumor Cells via Tumor Cell Apoptosis Induction and Angiogenesis Inhibition. Animals 2021, 11, 1512. [Google Scholar] [CrossRef]
- Tanemura, Y.; Yamanaka-Okumura, H.; Sakuma, M.; Nii, Y.; Taketani, Y.; Takeda, E. Effects of the intake of Undaria pinnatifida (Wakame) and its sporophylls (Mekabu) on postprandial glucose and insulin metabolism. J. Med. Investig. 2014, 61, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, H.; Imai, T.; Mase, T.; Sekiya, M.; Yokoi, K.; Hayashi, H.; Shibata, A.; Hayashi, T.; Nishikawa, M.; Suda, N.; et al. Seaweed Prevents Breast Cancer? Jpn. J. Cancer Res. 2001, 92, 483–487. [Google Scholar] [CrossRef] [PubMed]
- De la Mare, J.-A.; Sterrenberg, J.N.; Sukhthankar, M.G.; Chiwakata, M.T.; Beukes, D.R.; Blatch, G.L.; Edkins, A.L. Assessment of potential anti-cancer stem cell activity of marine algal compounds using an in vitro mammosphere assay. Cancer Cell Int. 2013, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, H.; Imai, T.; Tanaka, Y.; Tsukamura, K.; Hayakawa, Y.; Kikumori, T.; Mase, T.; Itoh, T.; Nishikawa, M.; Hayashi, H.; et al. Wakame Seaweed Suppresses the Proliferation of 7,12-Dimethylbenz(a)-anthracene-induced Mammary Tumors in Rats. Jpn. J. Cancer Res. 1999, 90, 922–927. [Google Scholar] [CrossRef]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef]
- Knabbe, C.; Lippman, M.E.; Wakefield, L.M.; Flanders, K.C.; Kasid, A.; Derynck, R.; Dickson, R.B. Evidence that transforming growth factor-β is a hormonally regulated negative growth factor in human breast cancer cells. Cell 1987, 48, 417–428. [Google Scholar] [CrossRef]
- Kesari, A.L.; Chellam, V.G.; Mathew, B.S.; Nair, M.K.; Pillai, M.R. Transforming growth factor beta related to extent of tumor angiogenesis but not apoptosis or proliferation in breast carcinoma. Breast Cancer 1999, 6, 29–36. [Google Scholar] [CrossRef]
- Visvader, J.E. Cells of origin in cancer. Nature 2011, 469, 314–322. [Google Scholar] [CrossRef]
- Lawson, J.C.; Blatch, G.L.; Edkins, A.L. Cancer stem cells in breast cancer and metastasis. Breast Cancer Res. Treat. 2009, 118, 241–254. [Google Scholar] [CrossRef]
- Peitzsch, C.; Tyutyunnykova, A.; Pantel, K.; Dubrovska, A. Cancer stem cells: The root of tumor recurrence and metastases. Semin. Cancer Biol. 2017, 44, 10–24. [Google Scholar] [CrossRef]
- Chang, J.C. Cancer stem cells. Medicine 2016, 95, S20–S25. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Ponti, D.; Costa, A.; Zaffaroni, N.; Pratesi, G.; Petrangolini, G.; Coradini, D.; Pilotti, S.; Pierotti, M.A.; Daidone, M.G. Isolation and in vitro Propagation of Tumorigenic Breast Cancer Cells with Stem/Progenitor Cell Properties. Cancer Res. 2005, 65, 5506–5511. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, C.; Tafuku, S.; Kadekaru, T.; Sawada, S.; Tomita, M.; Okudaira, T.; Nakazato, T.; Toda, T.; Uchihara, J.-N.; Taira, N.; et al. Antiadult T-cell leukemia effects of brown algae fucoxanthin and its deacetylated product, fucoxanthinol. Int. J. Cancer 2008, 123, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ishikawa, C.; Katano, H.; Yasumoto, T.; Mori, N. Fucoxanthin and its deacetylated product, fucoxanthinol, induce apoptosis of primary effusion lymphomas. Cancer Lett. 2011, 300, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.A.; Gabra, H.; Leonard, R.C. Continuous 5-fluorouracil in the treatment of breast cancer. Br. J. Cancer 1994, 70, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Nakshatri, H.; Bhat-Nakshatri, P.; Martin, D.A.; Goulet, R.J.; Sledge, G.W. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol. Cell. Biol. 1997, 17, 3629–3639. [Google Scholar] [CrossRef]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef]
- Jana, S.; Krishna, B.M.; Singhal, J.; Horne, D.; Awasthi, S.; Salgia, R.; Singhal, S.S. SOX9: The master regulator of cell fate in breast cancer. Biochem. Pharmacol. 2020, 174, 113789. [Google Scholar] [CrossRef]
- Afonja, O.; Raaka, B.M.; Huang, A.; Das, S.; Zhao, X.; Helmer, E.; Juste, D.; Samuels, H.H. RAR agonists stimulate SOX9 gene expression in breast cancer cell lines: Evidence for a role in retinoid-mediated growth inhibition. Oncogene 2002, 21, 7850–7860. [Google Scholar] [CrossRef]
- Chakravarty, G.; Rider, B.; Mondal, D. Cytoplasmic compartmentalization of SOX9 abrogates the growth arrest response of breast cancer cells that can be rescued by trichostatin A treatment. Cancer Biol. Ther. 2011, 11, 71–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chakravarty, G.; Moroz, K.; Makridakis, N.M.; Lloyd, S.A.; Galvez, S.E.; Canavello, P.R.; Lacey, M.R.; Agrawal, K.; Mondal, D. Prognostic significance of cytoplasmic SOX9 in invasive ductal carcinoma and metastatic breast cancer. Exp. Biol. Med. 2011, 236, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, K.N.; Abou-Hamad, J.; Pascoal, J.; Labrèche, C.; Garland, B.; Sabourin, L.A. AKT-mediated phosphorylation of Sox9 induces Sox10 transcription in a murine model of HER2-positive breast cancer. Breast Cancer Res. 2021, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef]
- Morales, J.; Li, L.; Fattah, F.J.; Dong, Y.; Bey, E.A.; Patel, M.; Gao, J.; Boothman, D.A. Review of Poly (ADP-ribose) Polymerase (PARP) Mechanisms of Action and Rationale for Targeting in Cancer and Other Diseases. Crit. Rev. Eukaryot. Gene Expr. 2014, 24, 15–28. [Google Scholar] [CrossRef]
- Meza-Sosa, K.F.; Miao, R.; Navarro, F.; Zhang, Z.; Zhang, Y.; Hu, J.J.; Hartford, C.C.R.; Li, X.L.; Pedraza-Alva, G.; Pérez-Martínez, L.; et al. SPARCLE, a p53-induced lncRNA, controls apoptosis after genotoxic stress by promoting PARP-1 cleavage. Mol. Cell 2022, 82, 785–802.e10. [Google Scholar] [CrossRef]
- Hapach, L.A.; Mosier, J.A.; Wang, W.; Reinhart-King, C.A. Engineered models to parse apart the metastatic cascade. npj Precis. Oncol. 2019, 3, 20. [Google Scholar] [CrossRef]
- Cancer.Net. Stages of Cancer. Available online: https://www.cancer.net/navigating-cancer-care/diagnosing-cancer/stages-cancer (accessed on 21 March 2022).
- Yoshimatsu, Y.; Miyazaki, H.; Watabe, T. Roles of signaling and transcriptional networks in pathological lymphangiogenesis. Adv. Drug Deliv. Rev. 2016, 99 Pt B, 161–171. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Z.; Liu, X.; Teng, H.; Zhang, C.; Hou, L.; Zou, X. Anti-Metastasis Effect of Fucoidan from Undaria pinnatifida Sporophylls in Mouse Hepatocarcinoma Hca-F Cells. PLoS ONE 2014, 9, e106071. [Google Scholar] [CrossRef]
- Lin, S.-W.; Gao, Z.-X.; Lin, L.-R.; Luo, X.; Liu, L.-L.; Yang, T.-C. Treponema pallidum enhances human monocyte migration and invasion by dysregulating the MMP/TIMP balance. Int. Immunopharmacol. 2019, 75, 105744. [Google Scholar] [CrossRef]
- Guo, W.; Gao, X.; Zhan, R.; Zhao, Z.; Xu, K.; Tang, B. Tricolor imaging of MMPs to investigate the promoting roles of inflammation on invasion and migration of tumor cells. Talanta 2021, 222, 121525. [Google Scholar] [CrossRef] [PubMed]
- Zetter, B.R. Angiogenesis and Tumor Metastasis. Annu. Rev. Med. 1998, 49, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Badodekar, N.; Sharma, A.; Patil, V.; Telang, G.; Sharma, R.; Patil, S.; Vyas, N.; Somasundaram, I. Angiogenesis induction in breast cancer: A paracrine paradigm. Cell Biochem. Funct. 2021, 39, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Wilkus, K.; Brodaczewska, K.; Kajdasz, A.; Kieda, C. Distinctive Properties of Endothelial Cells from Tumor and Normal Tissue in Human Breast Cancer. Int. J. Mol. Sci. 2021, 22, 8862. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M.; Claesson-Welsh, L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 2006, 312, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef]
- Dobrucki, L.W.; Tsutsumi, Y.; Kalinowski, L.; Dean, J.; Gavin, M.; Sen, S.; Mendizabal, M.; Sinusas, A.J.; Aikawa, R. Analysis of angiogenesis induced by local IGF-1 expression after myocardial infarction using microSPECT-CT imaging. J. Mol. Cell. Cardiol. 2010, 48, 1071–1079. [Google Scholar] [CrossRef]
- Fagiani, E.; Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 2013, 328, 18–26. [Google Scholar] [CrossRef]
- Lobov, I.B.; Brooks, P.C.; Lang, R.A. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11205–11210. [Google Scholar] [CrossRef]
- Giannotta, M.; Trani, M.; Dejana, E. VE-Cadherin and Endothelial Adherens Junctions: Active Guardians of Vascular Integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef]
- Liu, B.; Sun, L.; Liu, Q.; Gong, C.; Yao, Y.; Lv, X.; Lin, L.; Yao, H.; Su, F.; Li, D.; et al. A Cytoplasmic NF-κB Interacting Long Noncoding RNA Blocks IκB Phosphorylation and Suppresses Breast Cancer Metastasis. Cancer Cell 2015, 27, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Duan, J.; Jiang, Y.; Wang, L.; Huang, N.; Lin, L.; Liao, Y.; Liao, W. Metastasis-associated in colon cancer-1 upregulates vascular endothelial growth factor-C/D to promote lymphangiogenesis in human gastric cancer. Cancer Lett. 2015, 357, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Shono, T.; Ono, M.; Izumi, H.; Jimi, S.I.; Matsushima, K.; Okamoto, T.; Kohno, K.; Kuwano, M. Involvement of the transcription factor NF-kappaB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol. Cell. Biol. 1996, 16, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Ohzeki, Y.; Shimizu, S.; Naito, S.; Ohtsuru, A.; Yamamoto, T.; Kuroiwa, Y. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with Ets-1 in endothelial cells. Life Sci. 1998, 64, 249–258. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Waaijer, S.J.; Zwager, M.C.; de Vries, E.G.; van der Vegt, B.; Schröder, C.P. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat. Rev. 2018, 70, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.W. Macrophages define the invasive microenvironment in breast cancer. J. Leukoc. Biol. 2008, 84, 623–630. [Google Scholar] [CrossRef]
- Choi, J.; Gyamfi, J.; Jang, H.; Koo, J.S. The role of tumor-associated macrophage in breast cancer biology. Histol. Histopathol. 2018, 33, 133–145. [Google Scholar]
- Nandi, B.; Shapiro, M.; Samur, M.K.; Pai, C.; Frank, N.Y.; Yoon, C.; Prabhala, R.H.; Munshi, N.C.; Gold, J.S. Stromal CCR6 drives tumor growth in a murine transplantable colon cancer through recruitment of tumor-promoting macrophages. OncoImmunology 2016, 5, e1189052. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Lee, C.H.; Wu, C.L.; Shiau, A.L. Toll-like Receptor 4 Signaling Promotes Tumor Growth. J. Immunother. 2010, 33, 73–82. [Google Scholar] [CrossRef]
- Mehta, A.K.; Kadel, S.; Townsend, M.G.; Oliwa, M.; Guerriero, J.L. Macrophage Biology and Mechanisms of Immune Suppression in Breast Cancer. Front. Immunol. 2021, 12, 643771. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, P. Regulation of Immune Responses by Prostaglandin E2. J. Immunol. 2011, 188, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.A.; Liao, W.; Sarkar, A.; Kim, M.V.; Bivona, M.R.; Liu, K.; Pamer, E.G.; Li, M.O. The cellular and molecular origin of tumor-associated macrophages. Science 2014, 344, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional Profiling of the Human Monocyte-to-Macrophage Differentiation and Polarization: New Molecules and Patterns of Gene Expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.Y.; Cho, S.W.; Kim, Y.A.; Kim, D.; Oh, B.C.; Park, D.J.; Park, Y.J. Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates. J. Pathol. Transl. Med. 2015, 49, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Berger, A. Science commentary: Th1 and Th2 responses: What are they? BMJ 2000, 321, 424. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef]
- Kim, K.N.; Heo, S.J.; Yoon, W.J.; Kang, S.M.; Ahn, G.; Yi, T.H.; Jeon, Y.J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, U.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, A.L.; Millis, L.; Vapenik, J.; Sigola, L. Lipopolysaccharide-mediated enhancement of zymosan phagocytosis by RAW 264.7 macrophages is independent of opsonins, laminarin, mannan, and complement receptor 3. J. Surg. Res. 2014, 189, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Satomi, Y.; Nishino, H. Inhibition of the enzyme activity of cytochrome P450 1A1, 1A2 and 3A4 by fucoxanthin, a marine carotenoid. Oncol. Lett. 2013, 6, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Desta, Z.; Kreutz, Y.; Nguyen, A.T.; Li, L.; Skaar, T.; Kamdem, L.K.; Henry, N.L.; Hayes, D.F.; Storniolo, A.M.; Stearns, V.; et al. Plasma Letrozole Concentrations in Postmenopausal Women with Breast Cancer Are Associated with CYP2A6 Genetic Variants, Body Mass Index, and Age. Clin. Pharmacol. Ther. 2011, 90, 693–700. [Google Scholar] [CrossRef]
- Gonzalez, F.J.; Gelboin, H.V. Role of Human Cytochromes P450 in the Metabolic Activation of Chemical Carcinogens and Toxins. Drug Metab. Rev. 1994, 26, 165–183. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef]
- Shimada, T. Xenobiotic-Metabolizing Enzymes Involved in Activation and Detoxification of Carcinogenic Polycyclic Aromatic Hydrocarbons. Drug Metab. Pharmacokinet. 2006, 21, 257–276. [Google Scholar] [CrossRef]
- Ding, X.; Kaminsky, L.S. Human Extrahepatic Cytochromes P450: Function in Xenobiotic Metabolism and Tissue-Selective Chemical Toxicity in the Respiratory and Gastrointestinal Tracts. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 149–173. [Google Scholar] [CrossRef]
- Schneider, E.; Clark, D.S. Cytochrome P450 (CYP) enzymes and the development of CYP biosensors. Biosens. Bioelectron. 2013, 39, 1–13. [Google Scholar] [CrossRef]
- Murray, G.I. The role of cytochrome P450 in tumour development and progression and its potential in therapy. J. Pathol. 2000, 192, 419–426. [Google Scholar] [CrossRef]
- Luo, B.; Yan, D.; Yan, H.; Yuan, J. Cytochrome P450: Implications for human breast cancer (Review). Oncol. Lett. 2021, 22, 548. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, D.O.; Thurber, T.; Lewis-Traylor, A.; Berry, C.; Barber, W.H.; Zhou, X.; Bigler, S.; Vance, R. Differential Association of Cytochrome P450 3A4 Genotypes with Onsets of Breast Tumors in African American Versus Caucasian Patients. J. Investig. Med. 2011, 59, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Dudbridge, F.; Orr, N.; Gibson, L.; Jones, M.E.; Schoemaker, M.J.; Folkerd, E.J.; Haynes, B.P.; Hopper, J.L.; Southey, M.C.; et al. Genetic variation at CYP3A is associated with age at menarche and breast cancer risk: A case-control study. Breast Cancer Res. 2014, 16, R51. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Walker, K.; Gibson, L.J.; Orr, N.; Folkerd, E.; Haynes, B.; Palles, C.; Coupland, B.; Schoemaker, M.; Jones, M.; et al. CYP3A Variation, Premenopausal Estrone Levels, and Breast Cancer Risk. JNCI J. Natl. Cancer Inst. 2012, 104, 657–669. [Google Scholar] [CrossRef]
- Sangrajrang, S.; Sato, Y.; Sakamoto, H.; Ohnami, S.; Laird, N.M.; Khuhaprema, T.; Brennan, P.; Boffetta, P.; Yoshida, T. Genetic polymorphisms of estrogen metabolizing enzyme and breast cancer risk in Thai women. Int. J. Cancer 2009, 125, 837–843. [Google Scholar] [CrossRef]
- Bai, X.; Xie, J.; Sun, S.; Zhang, X.; Jiang, Y.; Pang, D. The associations of genetic polymorphisms in CYP1A2 and CYP3A4 with clinical outcomes of breast cancer patients in northern China. Oncotarget 2017, 8, 38367–38377. [Google Scholar] [CrossRef]
- Yim, S.K.; Kim, K.; Chun, S.; Oh, T.; Jung, W.; Jung, K.; Yun, C.H. Screening of Human CYP1A2 and CYP3A4 Inhibitors from Seaweed in Silico and In Vitro. Mar. Drugs 2020, 18, 603. [Google Scholar] [CrossRef]
- Nebert, D.W.; Wikvall, K.; Miller, W.L. Human cytochromes P450 in health and disease. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120431. [Google Scholar] [CrossRef]
- Tan, B.S.; Tiong, K.H.; Muruhadas, A.; Randhawa, N.; Choo, H.L.; Bradshaw, T.D.; Stevens, M.F.; Leong, C.O. CYP2S1 and CYP2W1 Mediate 2-(3,4-Dimethoxyphenyl)-5-Fluorobenzothiazole (GW-610, NSC 721648) Sensitivity in Breast and Colorectal Cancer Cells. Mol. Cancer Ther. 2011, 10, 1982–1992. [Google Scholar] [CrossRef]
- Cardenas-Rodriguez, N.; Lara-Padilla, E.; Bandala, C.; Lopez-Cruz, J.; Uscanga-Carmona, C.; Lucio-Monter, P.; Floriano-Sanchez, E. CYP2W1, CYP4F11 and CYP8A1 Polymorphisms and Interaction of CYP2W1 Genotypes with Risk Factors in Mexican Women with Breast Cancer. Asian Pac. J. Cancer Prev. 2012, 13, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic Aromatic Hydrocarbons: From Metabolism to Lung Cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Goth-Goldstein, R. Interindividual variation in CYP1A1 expression in breast tissue and the role of genetic polymorphism. Carcinogenesis 2000, 21, 2119–2122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nilsson, R.; Antić, R.; Berni, A.; Dallner, G.; Dettbarn, G.; Gromadzinska, J.; Joksić, G.; Lundin, C.; Palitti, F.; Prochazka, G.; et al. Exposure to polycyclic aromatic hydrocarbons in women from Poland, Serbia and Italy—Relation between PAH metabolite excretion, DNA damage, diet and genotype (the EU DIEPHY project). Biomarkers 2013, 18, 165–173. [Google Scholar] [CrossRef]
- Al-Dhfyan, A.; Alhoshani, A.; Korashy, H.M. Aryl hydrocarbon receptor/cytochrome P450 1A1 pathway mediates breast cancer stem cells expansion through PTEN inhibition and β-Catenin and Akt activation. Mol. Cancer 2017, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H. CYP Induction-Mediated Drug Interactions: In Vitro Assessment and Clinical Implications. Pharm. Res. 2006, 23, 1089–1116. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.H.; Chen, Z.S. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updates 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Dean, M. The Human ATP-Binding Cassette (ABC) Transporter Superfamily. Genome Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef]
- Hollenstein, K.; Dawson, R.J.; Locher, K.P. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 2007, 17, 412–418. [Google Scholar] [CrossRef]
- Ween, M.; Armstrong, M.; Oehler, M.; Ricciardelli, C. The role of ABC transporters in ovarian cancer progression and chemoresistance. Crit. Rev. Oncol. Hematol. 2015, 96, 220–256. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, S.V.; Kimchi-Sarfaty, C.; Sauna, Z.E.; Gottesman, M.M. P-glycoprotein: From genomics to mechanism. Oncogene 2003, 22, 7468–7485. [Google Scholar] [CrossRef] [PubMed]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2017, 61, 5108–5121. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Fortunati, E.; Liu, D.X.; Li, Y. Pleiotropic Roles of ABC Transporters in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 3199. [Google Scholar] [CrossRef]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Carotenoids reverse multidrug resistance in cancer cells by interfering with ABC-transporters. Phytomedicine 2012, 19, 977–987. [Google Scholar] [CrossRef]
- Shin, J.; Song, M.H.; Oh, J.W.; Keum, Y.S.; Saini, R.K. Pro-oxidant Actions of Carotenoids in Triggering Apoptosis of Cancer Cells: A Review of Emerging Evidence. Antioxidants 2020, 9, 532. [Google Scholar] [CrossRef]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Vijay, K.; Sowmya PR, R.; Arathi, B.P.; Shilpa, S.; Shwetha, H.J.; Raju, M.; Baskaran, V.; Lakshminarayana, R. Low-dose doxorubicin with carotenoids selectively alters redox status and upregulates oxidative stress-mediated apoptosis in breast cancer cells. Food Chem. Toxicol. 2018, 118, 675–690. [Google Scholar] [CrossRef]
- Muftah, A.A.; Aleskandarany, M.A.; Al-kaabi, M.M.; Sonbul, S.N.; Diez-Rodriguez, M.; Nolan, C.C.; Caldas, C.; Ellis, I.O.; Rakha, E.A.; Green, A.R. Ki67 expression in invasive breast cancer: The use of tissue microarrays compared with whole tissue sections. Breast Cancer Res. Treat. 2017, 164, 341–348. [Google Scholar] [CrossRef]
- Nahed, A.S.; Shaimaa, M.Y. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol. Med. 2016, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Schulze-Osthoff, K. Apoptosis-based therapies and drug targets. Cell Death Differ. 2005, 12, 942–961. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.L.; Engle, J.T.; Griffin, E.A.; Miller, J.P.; Chu, W.; Zhou, D.; Mach, R.H. Imaging Caspase-3 Activation as a Marker of Apoptosis-Targeted Treatment Response in Cancer. Mol. Imaging Biol. 2014, 17, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, E.; Sahin, A.A.; Chen, J.S.; Krishnamurthy, R.R.; Aggarwal, N.; Brun, A.M.; Sapino, A.; Zhang, F.; Sharma, D.; Yang, X.H.; et al. Down-regulation of caspase 3 in breast cancer: A possible mechanism for chemoresistance. Oncogene 2002, 21, 8843–8851. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzidis, D.; Gilmore, T.D. Transcription factor cross-talk: The estrogen receptor and NF-κB. Trends Endocrinol. Metab. 2005, 16, 46–52. [Google Scholar] [CrossRef]
- Frasor, J.; Weaver, A.; Pradhan, M.; Dai, Y.; Miller, L.D.; Lin, C.Y.; Stanculescu, A. Positive Cross-Talk between Estrogen Receptor and NF-κB in Breast Cancer. Cancer Res. 2009, 69, 8918–8925. [Google Scholar] [CrossRef]
- Zubair, A.; Frieri, M. Role of Nuclear Factor-κB in Breast and Colorectal Cancer. Current Allergy Asthma Rep. 2012, 13, 44–49. [Google Scholar] [CrossRef]
- Cogswell, P.C.; Guttridge, D.C.; Funkhouser, W.K.; Baldwin, A.S. Selective activation of NF-κB subunits in human breast cancer: Potential roles for NF-κB2/p52 and for Bcl-3. Oncogene 2000, 19, 1123–1131. [Google Scholar] [CrossRef]
- Guo, J.; Verma, U.N.; Gaynor, R.B.; Frenkel, E.P.; Becerra, C.R. Enhanced Chemosensitivity to Irinotecan by RNA Interference-Mediated Down-Regulation of the Nuclear Factor-κB p65 Subunit. Clin. Cancer Res. 2004, 10, 3333–3341. [Google Scholar] [CrossRef]
- Pan, X.; Arumugam, T.; Yamamoto, T.; Levin, P.A.; Ramachandran, V.; Ji, B.; Lopez-Berestein, G.; Vivas-Mejia, P.E.; Sood, A.K.; McConkey, D.J.; et al. Nuclear Factor- B p65/relA Silencing Induces Apoptosis and Increases Gemcitabine Effectiveness in a Subset of Pancreatic Cancer Cells. Clin. Cancer Res. 2008, 14, 8143–8151. [Google Scholar] [CrossRef]
- Wang, B.; Wei, H.; Prabhu, L.; Zhao, W.; Martin, M.; Hartley, A.V.; Lu, T. Role of Novel Serine 316 Phosphorylation of the p65 Subunit of NF-κB in Differential Gene Regulation. J. Biol. Chem. 2015, 290, 20336–20347. [Google Scholar] [CrossRef] [PubMed]
- Hongisto, V.; Jernström, S.; Fey, V.; Mpindi, J.P.; Kleivi Sahlberg, K.; Kallioniemi, O.; Perälä, M. High-Throughput 3D Screening Reveals Differences in Drug Sensitivities between Culture Models of JIMT1 Breast Cancer Cells. PLoS ONE 2013, 8, e77232. [Google Scholar] [CrossRef] [PubMed]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer 2018, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Commoner, B.; Townsend, J.; Pake, G.E. Free Radicals in Biological Materials. Nature 1954, 174, 689–691. [Google Scholar] [CrossRef]
- Simic, M.G.; Bergtold, D.S.; Karam, L.R. Generation of oxy radicals in biosystems. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 1989, 214, 3–12. [Google Scholar] [CrossRef]
- Darley-Usmar, V.; Halliwell, B. Blood radicals: Reactive nitrogen species, reactive oxygen species, transition metal ions, and the vascular system. Pharm. Res. 1996, 13, 649–662. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; FitzGerald, R.J.; Soler-Vila, A.; Brunton, N. Antioxidant activity and phenolic content of pressurised liquid and solid-liquid extracts from four Irish origin macroalgae. Int. J. Food Sci. Technol. 2014, 49, 1765–1772. [Google Scholar] [CrossRef]
- Nomura, T.; Kikuchi, M.; Kubodera, A.; Kawakami, Y. Proton-donative antioxidant activity of fucoxanthin with 1,1-Diphenyl-2-Picrylhydrazyl (DPPH). IUBMB Life 1997, 42, 361–370. [Google Scholar] [CrossRef]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the Major Antioxidant inHijikia fusiformis, a Common Edible Seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef]
- Zaragozá, M.C.; López, D.; Sáiz, M.P.; Poquet, M.; Pérez, J.; Puig-Parellada, P.; Màrmol, F.; Simonetti, P.; Gardana, C.; Lerat, Y.; et al. Toxicity and Antioxidant Activity in Vitro and in Vivo of Two Fucus vesiculosus Extracts. J. Agric. Food Chem. 2008, 56, 7773–7780. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Mortensen, A.; Skibsted, L.H.; Sampson, J.; Rice-Evans, C.; Everett, S.A. Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett. 1997, 418, 91–97. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.; Varol, A.; Thakral, F.; Yerer, M.; Sak, K.; Varol, M.; Jain, A.; Khan, M.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 17 March 2022).
- Engin, A. Obesity-associated Breast Cancer: Analysis of risk factors. Adv. Exp. Med. Biol. 2017, 960, 571–606. [Google Scholar]
- Barone, I.; Giordano, C.; Bonofiglio, D.; Andò, S.; Catalano, S. The weight of obesity in breast cancer progression and metastasis: Clinical and molecular perspectives. Semin. Cancer Biol. 2020, 60, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; McGuire, V. Evidence of association between plasma high-density lipoprotein cholesterol and risk factors for breast cancer. J. Natl. Cancer Inst. 1990, 82, 460–468. [Google Scholar] [CrossRef]
- McDonnell, D.P.; Chang, C.-Y.; Nelson, E.R. The estrogen receptor as a mediator of the pathological actions of cholesterol in breast cancer. Climacteric J. Int. Menopause Soc. 2014, 17 (Suppl. S2), 60–65. [Google Scholar] [CrossRef]
- Garcia-Estevez, L.; Moreno-Bueno, G. Updating the role of obesity and cholesterol in breast cancer. Breast Cancer Res. BCR 2019, 21, 35. [Google Scholar] [CrossRef]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncol. 2017, 18, e457–e471. [Google Scholar] [CrossRef]
- Yang, X.R.; Chang-Claude, J.; Goode, E.L.; Couch, F.J.; Nevanlinna, H.; Milne, R.L.; Gaudet, M.; Schmidt, M.K.; Broeks, A.; Cox, A.; et al. Associations of breast cancer risk factors with tumor subtypes: A pooled analysis from the Breast Cancer Association Consortium studies. J. Natl. Cancer Inst. 2011, 103, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Munsell, M.F.; Sprague, B.L.; Berry, D.A.; Chisholm, G.; Trentham-Dietz, A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol. Rev. 2014, 36, 114–136. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Aragaki, A.K.; Prentice, R.L.; Manson, J.E.; Chlebowski, R.; Carty, C.L.; Ochs-Balcom, H.M.; Thomson, C.A.; Caan, B.J.; Tinker, L.F.; et al. Overweight, Obesity, and Postmenopausal Invasive Breast Cancer Risk: A Secondary Analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015, 1, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Nagrani, R.; Mhatre, S.; Rajaraman, P.; Soerjomataram, I.; Boffetta, P.; Gupta, S.; Parmar, V.; Badwe, R.; Dikshit, R. Central obesity increases risk of breast cancer irrespective of menopausal and hormonal receptor status in women of South Asian Ethnicity. Eur. J. Cancer 2016, 66, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Ritte, R.; Lukanova, A.; Berrino, F.; Dossus, L.; Tjønneland, A.; Olsen, A.; Overvad, T.F.; Overvad, K.; Clavel-Chapelon, F.; Fournier, A.; et al. Adiposity, hormone replacement therapy use and breast cancer risk by age and hormone receptor status: A large prospective cohort study. Breast Cancer Res. 2012, 14, R76. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Fortner, R.T.; Katzke, V.; Kühn, T.; Kaaks, R. Obesity and Breast Cancer. Recent results in cancer research. Obesity Cancer 2016, 208, 43–65. [Google Scholar]
- Miyashita, K.; Nishikawa, S.; Beppu, F.; Tsukui, T.; Abe, M.; Hosokawa, M. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J. Sci. Food Agric. 2011, 91, 1166–1174. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.; Ye, L.; Choi, J.; Giang, A.H.; Khandekar, M.; Virtanen, K.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Gammone, M.; D’Orazio, N. Anti-Obesity Activity of the Marine Carotenoid Fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef]
- Pangestuti, R.; Siahaan, E.A. Seaweed-derived carotenoids. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 95–107. [Google Scholar]
- Ikeda, K.; Yamada, T. UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes. Front. Endocrinol. 2020, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.; Pedersen, L.M.; Lillefosse, H.H.; Fjære, E.; Bronstad, I.; Hao, Q.; Petersen, R.K.; Hallenborg, P.; Ma, T.; de Matteis, R.; et al. UCP1 Induction during Recruitment of Brown Adipocytes in White Adipose Tissue Is Dependent on Cyclooxygenase Activity. PLoS ONE 2010, 5, e11391. [Google Scholar] [CrossRef] [PubMed]
- Vegiopoulos, A.; Müller-Decker, K.; Strzoda, D.; Schmitt, I.; Chichelnitskiy, E.; Ostertag, A.; Diaz, M.B.; Rozman, J.; Hrabe De Angelis, M.; Nüsing, R.M.; et al. Cyclooxygenase-2 Controls Energy Homeostasis in Mice by de Novo Recruitment of Brown Adipocytes. Science 2010, 328, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch. Biochem. Biophys. 2010, 504, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.I.; Shin, H.S.; Kim, H.M.; Yoon, S.A.; Kang, S.W.; Kim, J.H.; Ko, H.C.; Kim, S.J. Petalonia binghamiae Extract and Its Constituent Fucoxanthin Ameliorate High-Fat Diet-Induced Obesity by Activating AMP-Activated Protein Kinase. J. Agric. Food Chem. 2012, 60, 3389–3395. [Google Scholar] [CrossRef]
- Woo, M.N.; Jeon, S.M.; Kim, H.J.; Lee, M.K.; Shin, S.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef]
- Ha, A.W.; Kim, W.K. The effect of fucoxanthin rich power on the lipid metabolism in rats with a high fat diet. Nutr. Res. Pract. 2013, 7, 287–293. [Google Scholar] [CrossRef]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef]
- Jeon, S.M.; Kim, H.J.; Woo, M.N.; Lee, M.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin-rich seaweed extract suppresses body weight gain and improves lipid metabolism in high-fat-fed C57BL/6J mice. Biotechnol. J. 2010, 5, 961–969. [Google Scholar] [CrossRef]
- Cheng, J.; Fujita, A.; Ohsaki, Y.; Suzuki, M.; Shinohara, Y.; Fujimoto, T. Quantitative electron microscopy shows uniform incorporation of triglycerides into existing lipid droplets. Histochem. Cell Biol. 2009, 132, 281–291. [Google Scholar] [CrossRef]
- Zhang, S.; Hunter, D.J.; Forman, M.R.; Rosner, B.A.; Speizer, F.E.; Colditz, G.A.; Manson, J.E.; Hankinson, S.E.; Willett, W.C. Dietary Carotenoids and Vitamins A, C, and E and Risk of Breast Cancer. JNCI J. Natl. Cancer Inst. 1999, 91, 547–556. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A. Dietary prevention of breast cancer in high-risk women: Role of carotenoids. Am. J. Clin. Nutr. 2021, 113, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic Syndrome. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Rupnick, M.A.; Panigrahy, D.; Zhang, C.Y.; Dallabrida, S.M.; Lowell, B.B.; Langer, R.; Folkman, M.J. Adipose tissue mass can be regulated through the vasculature. Proc. Natl. Acad. Sci. USA 2002, 99, 10730–10735. [Google Scholar] [CrossRef]
| Algal Extract or Compound | Cell Type(s) | Study Design | Anti-Proliferation Effects | Reference |
|---|---|---|---|---|
| Organic extract of Halocynthia roretzi, Fx | MCF-7 | Fx/Fxol were dissolved in ethanol adjusted to less than 0.5% in volume. Viable MCF-7 cell number was measured colorimetrically with WST-1 reagent. | Cell viability: ~90% after 48 h at 25 μM ~60% after 72 h at 25 μM | [91] |
| Organic extract of Halocynthia roretzi, Fxol | MCF-7 | Cell viability: ~30% after 48 h at 25 μM ~15% after 72 h at 25 μM | ||
| Organic extract of Halocynthia roretzi, Fx | MCF-7 | Fx/Fxol were dissolved in ethanol adjusted to less than 0.5% in the culture medium. The DNA fragments were stained with ethidium bromide and visualized. | DNA fragmentation level: 2-fold of ctrl after 48 h at 12.5 μM 6-fold of ctrl after 48 h at 25 μM | [91] |
| Organic extract of Halocynthia roretzi, Fxol | MCF-7 | DNA fragmentation level: 7-fold of ctrl after 48 h at 12.5 μM 12-fold of ctrl after 48 h at 25 μM | ||
| Methanol extract of Sargassum, Fucoxanthin (60 mg, 0.017% dry wt.) | MCF-7 | The viability of the cells was examined by microscopical examination using hemocytometer and trypan blue stain. | IC50 = 11.5 μM Cell viability: ~60% after 24 h at 20 μM ~30% after 48 h at 20 μM | [92] |
| Methanol extract of Sargassum, Fucoxanthin (60 mg, 0.017% dry wt.) | MCF-7 | Bleomycin-dependent DNA damage assay with absorbance measured at 532 nm. | DNA fragmentation level: 39-fold of ctrl after 24h at 20 μM 42-fold of ctrl after 48h at 20 μM | [92] |
| Fx | MCF-7 | Treatments of BC cells with 20, 30, or 40 μM of Fx/Fxol in a time-dependent (12, 24, or 48 h) manner, cell-titer blue cell viability assay was done, the amount of reduced resorufin was measured as fluorescence signal at 560Ex/590Em | IC50 = 121.89 μM Cell viability: ~70% after 24h at 30 μM ~50% after 24 h at 40 μM ~20% after 48 h at 30 μM ~10% after 48 h at 40 μM | [93] |
| MDA-MB-237 | IC50 = 141.54 μMCell viability: ~50% after 24 h at 30 μM~30% after 24 h at 40 μM~20% after 48h at 30 μM~10% after 48 h at 40 μM | |||
| Fxol | MCF-7 | IC50 = 39.63 μM Cell viability: ~60% after 12 h at 40 μM ~40% after 24 h at 40 μM ~10% after 48h at 40 μM | ||
| MDA-MB-237 | IC50 = 33.59 μM Cell viability: ~40% after 12 h at 40 μM ~20% after 24 h at 40 μM >10% after 48 h at 40 μM | |||
| Fx | MCF-7 | Treatments of BC cells with 10 and 20 μM of Fx/Fxol in a time-dependent (6, 12, 24 or 48 h) manner, cell-Titer blue cell viability assay was done, the amount of reduced resorufin was measured by its fluorescence signal at 560Ex/590Em | Non-significant | [94] |
| MDA-MB-237 | Cell viability: ~90% after 48 h at 20 μM | |||
| Fxol | MCF-7 | Cell viability: ~40% after 48 h at 20 μM | ||
| MDA-MB-237 | Cell viability: ~80% after 24 h at 20 μM ~50% after 48 h at 20 μM | |||
| Fx | MDA-MB-237 | Treatments of MDA-MB-231 cells with Fx (25, 50, 100 μmol/L) for 12, 24 or 48 h. MTT assay with absorbance was measured at 492 nm. | Cell viability: ~90% after 24 h at 100 μM ~70% after 48 h at 100 μM | [95] |
| Fx | MDA-MB-231 xenograft model | Five days after BC cell inoculation, Fx (100, 500 μmol/L; 100 μL/mouse) was injected into the tumour peripheral every day for 26 days. Tumour size was measured every 4 days. | Tumor volume: 100 μmol/L group is ~20% smaller after 26 days 500 μmol/L group is ~60% smaller after 26 days | [95] |
| Tumor weight: 100 μmol/L group is ~30% smaller after 26 days 500 μmol/L group is ~60% smaller after 26 days | ||||
| Fx | MCF-7 | Treatments of BC cells with 10, 20 and 50 μM of Fx. MTT assay was done with absorbance measured at 570 nm. | Cell viability: ~70% after 72 h at 10 μM ~40% after 72 h at 20 μM ~20% after 72 h at 50 μM | [96] |
| SKBR3 | Cell viability: ~80% after 72 h at 10 μM ~40% after 72 h at 20 μM ~10% after 72 h at 50 μM | |||
| MDA-MB-237 | Cell viability: ~70% after 72 h at 10 μM ~20% after 72 h at 20 μM ~10% after 72 h at 50 μM | |||
| Fx | CMT-U27 | Treatments of BC cells with 0, 5, 10, and 20 μM of Fx. Crystal violet staining with absorbance was measured at 550 nm. | Cell viability: ~60% after 24 h at 10 μM ~45% after 24 h at 20 μM | [97] |
| Wakame | Female Sprague-Dawley (SD) rats | Rats in control group (I-A) and group (I-B and I-C) were given wakame seaweed of 1.0% and 5.0% of their body weight, respectively, for 8 weeks. Changes in the body weight and tumor size were measured. | Tumor size: I-B is ~60% smaller in week 8 I-C is ~80% smaller in week 8 and no significant increase in tumor size since week 0 | [98] |
| Mekabu, ~6.7 mg/mL | MCF7 | 1.0 g of powdered mekabu was dissolved in 150 mL of distilled water and 1 mL of mekabu solution was added to culture medium. DNA fragmentation was analyzed by apoptosis ladder detection kit. | DNA fragmentation level: 2.5-fold of ctrl after 96 h | [99] |
| T-47D | DNA fragmentation level: 2-fold of ctrl after 96 h | |||
| Mekabu solution | Female Sprague-Dawley (SD) rats | Powdered mekabu 1.5 g was mixed with 1000 mL of distilled water and was filtered as mekabu solution. Weekly changes in body weight, incidence and the number of mammary tumors in each rat were observed for 32 weeks. | Tumor size of mekabu group is ~95% smaller after 32 weeks | [99] |
| Fx | MCF-7 | Treatments of MCF-7 cells with Fx (0, 5, 10, 15 μM) and growth of the mammospheres for 8 days. WST-1 assay was done with absorbance measured at 450 nm. | Cell viability: ~90% after 24 h at 5 μM ~80% after 48 h at 10 μM | [100] |
| Algal Extract or Compound | Cell Type(s) | Study Design | Apoptosis % | Reference |
|---|---|---|---|---|
| Fx | MCF-7 | Treatments of BC cells with 20 μM Fx/Fxol followed by staining with FITC-Annexin V, ethidium homodimer III and Hoechst 33342. Fluorescence was assessed using an Axio Observer A1 inverted fluorescence microscope with FITC, rhodamine and DAPI filters | <10% after 12 h at 20 μM | [93] |
| MDA-MB-231 | ~50% after 12 h at 20 μM | |||
| Fxol | MCF-7 | ~40% after 12 h at 20 μM | ||
| MDA-MB-231 | ~40% after 12 h at 20 μM | |||
| Fx | MCF-7 | Treatments of BC cells with 10 μM Fx/Fxol followed by staining with FITC-Annexin V, ethidium homodimer III and Hoechst 33342. Fluorescence was assessed using an Axio Observer A1 inverted fluorescence microscope with FITC, rhodamine and DAPI filters | ~40% after 24 h at 10 μM | [94] |
| MDA-MB-231 | ~60% after 24 h at 10 μM | |||
| Fxol | MCF-7 | ~70% after 24 h at 10 μM | ||
| MDA-MB-231 | ~60% after 24 h at 10 μM | |||
| Fx | CMT-U27 | Treatments of BC cells with 20 μM Fx. Flow cytometric annexin V assay was used, and fluorescence was measured at 488 nm (excitation) and 525 nm (emission) | ~60% after 24 h at 20 μM | [97] |
| mekabu | MCF7 | Treatments of BC cells with 1.0 g mekabu solution followed by staining with propidium iodide (PI). Fluorescence Intensity for PI was measured by flow cytometry gated by FSC vs. SSC. | ~30% after 24 h at 15 μM ~60% after 72 h at 15 μM | [99] |
| MDA-MB-237 | ~70% after 24 h at 15 μM ~70% in 72 h at 15 μM | |||
| T-47D | ~60% after 24 h at 15 μM ~70% after 72 h at 15 μM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, T.-Y.; Kwan, H.-Y. Fucoxanthin Is a Potential Therapeutic Agent for the Treatment of Breast Cancer. Mar. Drugs 2022, 20, 370. https://doi.org/10.3390/md20060370
Lau T-Y, Kwan H-Y. Fucoxanthin Is a Potential Therapeutic Agent for the Treatment of Breast Cancer. Marine Drugs. 2022; 20(6):370. https://doi.org/10.3390/md20060370
Chicago/Turabian StyleLau, Tsz-Ying, and Hiu-Yee Kwan. 2022. "Fucoxanthin Is a Potential Therapeutic Agent for the Treatment of Breast Cancer" Marine Drugs 20, no. 6: 370. https://doi.org/10.3390/md20060370
APA StyleLau, T.-Y., & Kwan, H.-Y. (2022). Fucoxanthin Is a Potential Therapeutic Agent for the Treatment of Breast Cancer. Marine Drugs, 20(6), 370. https://doi.org/10.3390/md20060370






