Identification of Volatiles of the Dinoflagellate Prorocentrum cordatum
Abstract
:1. Introduction
2. Results
2.1. Volatile Analysis
2.2. Identification of Algal Volatiles
3. Discussion
3.1. Apocarotenoids
3.2. Aromatic Compounds
3.3. Aliphatic Compounds
3.4. Function of the Identified Volatiles
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. Collection of Headspace Volatiles
4.3. Experimental Procedures
4.3.1. General Experimental Procedures
4.3.2. GC/MS Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khanaychenko, A.N.; Telesh, I.V.; Skarlato, S.O. Bloom-forming potentially toxic dinoflagellates Prorocentrum cordatum in marine plankton food webs. Protistology 2019, 13, 95–125. [Google Scholar] [CrossRef]
- Hajdu, S.; Pertola, S.; Kuosa, H. Prorocentrum minimum (Dinophyceae) in the Baltic Sea: Morphology, occurrence—A review. Harmful Algae 2005, 4, 471–480. [Google Scholar] [CrossRef]
- Heil, C.A.; Glibert, P.M.; Fan, C. Prorocentrum minimum (Pavillard) Schiller: A review of a harmful algal bloom species of growing worldwide importance. Harmful Algae 2005, 4, 449–470. [Google Scholar] [CrossRef]
- Johnson, M.D. Inducible Mixotrophy in the Dinoflagellate Prorocentrum minimum. J. Eukaryot. Microbiol. 2015, 62, 431–443. [Google Scholar] [CrossRef]
- Stoecker, D.K.; Li, A.; Coats, D.W.; Gustafson, D.E.; Nannen, M.K. Mixotrophy in the dinoflagellate Prorocentrum minimum. Mar. Ecol. Prog. Ser. 1997, 152, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.S. Les “Red Waters” a la Lagune de Óbidos: Ses causes probables et ses rapports avec la toxicité des bivalves. In Proceedings of the 4th International Seaweed Symposium, Biarritz, France, September 1963; de Virville, A.D., Feldman, J., Eds.; The MacMillan Co.: New York, NY, USA, 1964; pp. 265–275. [Google Scholar]
- Anderson, D.M.; White, A.; Baden, D. Ecological factors related to Prorocentrum minimum blooms in Obidos Lagoon (Portugal). In Toxic dinoflagellates; Silva, E.S., Ed.; Elsevier: New York, NY, USA, 1985; pp. 251–256. [Google Scholar]
- Silva, E.S.; Sousa, I. Experimental work on the dinoflagellate toxin production. Arq. Do Inst. Nac. De Saude 1981, 6, 381–387. [Google Scholar]
- Akiba, T.; Hattori, Y. Food Poisoning caused by eating Asari (Venerupis semidecussata) and Oyster (Ostrea gigas) and Studies on the Toxic Substance, Venerupin. Jpn. J. Exp. Med. 1949, 20, 271–284. [Google Scholar]
- Nakajima, M. Studies on the source of shellfish poison in Lake Hamana. I. Relation of the abundance of a species of dinoflagellate, Prorocentrum sp. to shellfish toxicity. Bull. Jpn. Soc. Sci. Fish. 1965, 31, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, M. Studies on the source of shellfish poison in Lake Hamana. II. Shellfish toxicity during the ‘red-tide’. Bull. Jpn. Soc. Sci. Fish. 1965, 31, 204–207. [Google Scholar] [CrossRef]
- Nakajima, M. Studies on the source of shellfish poison in Lake Hamana. III. Poisonous effects of shellfish feeding on Prorocentrum sp. Bull. Jpn. Soc. Sci. Fish. 1965, 31, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, M. Studies on the source of shellfish poison in Lake Hamana. IV. Identification and collection of the noxious dinoflagellate. Bull. Jpn. Soc. Sci. Fish. 1968, 34, 130–131. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, M.M.; Rehman, A.U.; Harms, C.E. Mass mortality of fishes caused by dinoflagellate bloom in Gwadar Bay, southwestern Pakistan. In Toxic Marine Phytoplankton; Granéli, E., Sundstrom, B., Edler, L., Anderson, D.M., Eds.; Elsevier: New York, NY, USA, 1990; pp. 209–214. [Google Scholar]
- Azanza, R.V.; Fukuyo, Y.; Yap, L.G.; Takayama, H. Prorocentrum minimum bloom and its possible link to a massive fish kill in Bolinao, Pangasinan, Northern Philippines. Harmful Algae 2005, 4, 519–524. [Google Scholar] [CrossRef]
- Moncheva, S.; Petrova-Karadjova, V.; Palasov, A. Harmful algal blooms along the Bulgarian Black Sea coast and possible patterns of fish and zoobenthic mortalities. In Harmful Algal Blooms; Lassus, G.P., Arzul, E., Erard-Le Denn, P., Gentien, C., Marcaillou-Le Baut, Eds.; Lavoisier: Paris, France, 1995; pp. 193–198. [Google Scholar]
- Wang, H.; Tomasch, J.; Jarek, M.; Wagner-Döbler, I. A dual-species co-cultivation system to study the interactions between Roseobacters and dinoflagellates. Front. Microbiol. 2014, 5, 311. [Google Scholar] [CrossRef]
- Wang, H.; Tomasch, J.; Michael, V.; Bhuju, S.; Jarek, M.; Petersen, J.; Wagner-Döbler, I. Identification of Genetic Modules Mediating the Jekyll and Hyde Interaction of Dinoroseobacter shibae with the Dinoflagellate Prorocentrum minimum. Front. Microbiol. 2015, 6, 1262. [Google Scholar] [CrossRef]
- Andersen, R.J.; Le Blanc, M.J.; Sum, F.W. 1-(2,6,6-Trimethyl-4-hydroxycyclohexenyl)-1,3-butanedione, an extracellular metabolite from the dinoflagellate Prorocentrum minimum. J. Org. Chem. 1980, 45, 1169–1170. [Google Scholar] [CrossRef]
- Trick, C.G.; Harrison, P.J.; Andersen, R.J. Extracellular Secondary Metabolite Production by the Marine Dinoflagellate Prorocentrum minimum in Culture. Can. J. Fish. Aquat. Sci. 1981, 38, 864–867. [Google Scholar] [CrossRef]
- Trick, C.G.; Andersen, R.J.; Harrison, P.J. Environmental Factors Influencing the Production of an Antibacterial Metabolite from a Marine Dinoflagellate, Prorocentrum minimum. Can. J. Fish. Aquat. Sci. 1984, 41, 423–432. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Sielicki, M.; Haxo, F.T. Chloroplast pigment patterns in dinoflagellates. J. Phycol. 1975, 11, 374–384. [Google Scholar] [CrossRef]
- Isoe, S.; Be Hyeon, S.; Sakan, T. Photo-oxygenation of carotenoids. I. The formation of dihydroactinidiolide and β-ionone from β-carotene. Tetrahedron Lett. 1969, 10, 279–281. [Google Scholar] [CrossRef]
- Johansen, J.E.; Svec, W.A.; Liaaen-Jensen, S.; Haxo, F.T. Carotenoids of the dinophyceae. Phytochemistry 1974, 13, 2261–2271. [Google Scholar] [CrossRef]
- Weisskopf, L.; Schulz, S.; Garbeva, P. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Microbiol. 2021, 19, 391–404. [Google Scholar] [CrossRef]
- Durham, B.P.; Dearth, S.P.; Sharma, S.; Amin, S.A.; Smith, C.B.; Campagna, S.R.; Armbrust, E.V.; Moran, M.A. Recognition cascade and metabolite transfer in a marine bacteria-phytoplankton model system. Environ. Microbiol. 2017, 19, 3500–3513. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-H.; Ramanan, R.; Kim, B.-H.; Lee, J.; Kim, S.; Yoo, C.; Choi, G.-G.; Oh, H.-M.; Kim, H.-S. Novel approach for the development of axenic microalgal cultures from environmental samples. J. Phycol. 2013, 49, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae-bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohnert, G.; Lumineau, O.; Cueff, A.; Adolph, S.; Cordevant, C.; Lange, M.; Poulet, S. Are volatile unsaturated aldehydes from diatoms the main line of chemical defence against copepods? Mar. Ecol. Prog. Ser. 2002, 245, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Mansky, J.; Wang, H.; Ebert, M.; Härtig, E.; Jahn, D.; Tomasch, J.; Wagner-Döbler, I. The Influence of Genes on the “Killer Plasmid” of Dinoroseobacter shibae on Its Symbiosis with the Dinoflagellate Prorocentrum minimum. Front. Microbiol. 2022, 12, 804767. [Google Scholar] [CrossRef]
- Park, B.S.; Guo, R.; Lim, W.-A.; Ki, J.-S. Pyrosequencing reveals specific associations of bacterial clades Roseobacter and Flavobacterium with the harmful dinoflagellate Cochlodinium polykrikoides growing in culture. Mar. Ecol. 2017, 38, e12474. [Google Scholar] [CrossRef]
- Dickschat, J.S. Capturing volatile natural products by mass spectrometry. Nat. Prod. Rep. 2014, 31, 838–861. [Google Scholar] [CrossRef]
- Schulz, S.; Fuhlendorff, J.; Reichenbach, H. Identification and synthesis of volatiles released by the myxobacterium Chondromyces crocatus. Tetrahedron 2004, 60, 3863–3872. [Google Scholar] [CrossRef]
- Moran, L.; Bou, G.; Aldai, N.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Barron, L.; Lafarga, T. Characterisation of the volatile profile of microalgae and cyanobacteria using solid-phase microextraction followed by gas chromatography coupled to mass spectrometry. Sci. Rep. 2022, 12, 3661. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Shkrob, I.; Dor, I. Separation and identification of hydrocarbons and other volatile compounds from cultured blue-green algae Nostoc sp. by gas chromatography–mass spectrometry using serially coupled capillary columns with consecutive nonpolar and semipolar stationary phases. J. Chromatogr. A 1999, 862, 221–229. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 21 April 2021).
- Subbaraju, G.V.; Manhas, M.S.; Bose, A.K. A Convenient Synthesis of 2-Hydroxy-2,6,6-trimethylcyclohexanone: A Versatile Intermediate. Synthesis 1992, 1992, 816–818. [Google Scholar] [CrossRef]
- Constantino, M.G.; Donate, P.M.; Petragnani, N. An efficient synthesis of (±)-abscisic acid. J. Org. Chem. 1986, 51, 253–254. [Google Scholar] [CrossRef]
- Babler, J.H.; Malek, N.C.; Coghlan, M.J. Selective hydrolysis of α,β- and β,γ-unsaturated ketals: A method for deconjugation of β,β-disubstituted α,β-unsaturated ketones. J. Org. Chem. 1978, 43, 1821–1823. [Google Scholar] [CrossRef]
- Rosini, G.; Ballini, R.; Zanotti, V. Cycloaddition of dichloroketene with functionalized cycloalkenes, synthesis of bicyclo[4.2.0]octanone-3-yl derivatives and of 3,4-dicarbomethoxy-1-methylbicyclo[4,2,0]octan-7-one. Tetrahedron 1983, 39, 1085–1090. [Google Scholar] [CrossRef]
- Tomas, M.C. Aspects of Thionitrites and Nitric Oxide in Chemistry and Biology. Ph.D. Thesis, University of London, London, UK, 1999. [Google Scholar]
- Schobert, R.; Barnickel, B. A Regioselective Tsuji-Trost Pentadienylation of 3-Allyltetronic Acid. Synthesis 2009, 2009, 2778–2784. [Google Scholar] [CrossRef]
- Surmont, R.; Verniest, G.; de Kimpe, N. Short synthesis of the seed germination inhibitor 3,4,5-trimethyl-2(5H)-furanone. J. Org. Chem. 2010, 75, 5750–5753. [Google Scholar] [CrossRef]
- Sharma, V.; Kelly, G.T.; Watanabe, C.M.H. Exploration of the molecular origin of the azinomycin epoxide: Timing of the biosynthesis revealed. Org. Lett. 2008, 10, 4815–4818. [Google Scholar] [CrossRef]
- Felemban, A.; Braguy, J.; Zurbriggen, M.D.; Al-Babili, S. Apocarotenoids Involved in Plant Development and Stress Response. Front. Plant Sci. 2019, 10, 1168. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Z. Why Algae Release Volatile Organic Compounds-The Emission and Roles. Front. Microbiol. 2019, 10, 491. [Google Scholar] [CrossRef] [Green Version]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Langhoff, S. Carotinoid Abbauende Enzymaktivitäten aus Mikroorganismen. Ph.D. Thesis, Leibniz Universität Hannover, Hannover, Germany, 2002. [Google Scholar]
- Krammer, G.E.; Werkhoff, P.; Sommer, H.; Schmidt, C.O.; Gatfield, I.; Bertram, H.-J. Carotenoid Degradation Products in Paprika Powder. In Carotenoid-Derived Aroma Compounds; Winterhalter, P., Rouseff, R.L., Eds.; American Chemical Society: Washington, DC, USA, 2001; pp. 206–219. ISBN 9780841237292. [Google Scholar]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Hornero-Méndez, D.; Vicario, I.M. Structures, Nomenclature and General Chemistry of Carotenoids and Their Esters. In Carotenoid Esters in Foods: Physical, Chemical and Biological Properties; Mercadante, A.Z., Ed.; Royal Society of Chemistry: Cambridge, MA, USA, 2019; pp. 1–50. ISBN 978-1-78801-242-3. [Google Scholar]
- Reese, K.L.; Fisher, C.L.; Lane, P.D.; Jaryenneh, J.D.; Moorman, M.W.; Jones, A.D.; Frank, M.; Lane, T.W. Chemical Profiling of Volatile Organic Compounds in the Headspace of Algal Cultures as Early Biomarkers of Algal Pond Crashes. Sci. Rep. 2019, 9, 13866. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.-M.; Chung, G.-H.; Shin, T.-S. Volatile compounds of the green alga, Capsosiphon fulvescens. J. Appl. Phycol. 2012, 24, 1003–1013. [Google Scholar] [CrossRef]
- El Amrani Zerrifi, S.; El Khalloufi, F.; Mugani, R.; El Mahdi, R.; Kasrati, A.; Soulaimani, B.; Barros, L.; Ferreira, I.C.F.R.; Amaral, J.S.; Finimundy, T.C.; et al. Seaweed Essential Oils as a New Source of Bioactive Compounds for Cyanobacteria Growth Control: Innovative Ecological Biocontrol Approach. Toxins 2020, 12, 527. [Google Scholar] [CrossRef]
- Cotsaris, E.; Bruchet, A.; Mallevialle, J.; Bursill, D.B. The identification of odorous metabolites produced from algal monocultures. Water Sci. Technol. 1995, 31, 251–258. [Google Scholar] [CrossRef]
- Höckelmann, C.; Moens, T.; Jüttner, F. Odor compounds from cyanobacterial biofilms acting as attractants and repellents for free-living nematodes. Limnol. Oceanogr. 2004, 49, 1809–1819. [Google Scholar] [CrossRef] [Green Version]
- Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef]
- Cozzani, S.; Muselli, A.; Desjobert, J.-M.; Bernardini, A.-F.; Tomi, F.; Casanova, J. Chemical composition of essential oil of Teucrium polium subsp. capitatum (L.) from Corsica. Flavour Fragr. J. 2005, 20, 436–441. [Google Scholar] [CrossRef]
- Moronkola, D.O.; Ogunwande, I.A.; Oyewole, I.O.; Başer, K.H.C.; Ozek, T.; Ozek, G. Studies on the Volatile Oils of Momordica charantia L. (Cucurbitaceae) and Phyllanthus amarus Sch. et Thonn (Euphorbiaceae). J. Essent. Oil Res. 2009, 21, 393–399. [Google Scholar] [CrossRef]
- Sefidkon, F.; Jalili, A.; Mirhaji, T. Essential oil composition of three Artemisia spp. from Iran. Flavour Fragr. J. 2002, 17, 150–152. [Google Scholar] [CrossRef]
- Tarantilis, P.A.; Polissiou, M.G. Isolation and Identification of the Aroma Components from Saffron (Crocus sativus). J. Agric. Food Chem. 1997, 45, 459–462. [Google Scholar] [CrossRef]
- Radman, S.; Cikoš, A.-M.; Flanjak, I.; Babić, S.; Čižmek, L.; Šubarić, D.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Less Polar Compounds and Targeted Antioxidant Potential (In Vitro and In Vivo) of Codium adhaerens C. Agardh 1822. Pharmaceuticals 2021, 14, 944. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Baldermann, S.; Yoshikawa, K.; Fujita, A.; Mase, N.; Watanabe, N. Determination of Volatile Compounds in Four Commercial Samples of Japanese Green Algae Using Solid Phase Microextraction Gas Chromatography Mass Spectrometry. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, M.; Evgenidou, E.; Lambropoulou, D.; Konstantinou, I. A review on advanced oxidation processes for the removal of taste and odor compounds from aqueous media. Water Res. 2014, 53, 215–234. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Xu, B.; Kumirska, J.; Qi, F. Occurrence of earthy–musty taste and odors in the Taihu Lake, China: Spatial and seasonal patterns. RSC Adv. 2016, 6, 79723–79733. [Google Scholar] [CrossRef] [Green Version]
- Harada, K.; Ozaki, K.; Tsuzuki, S.; Kato, H.; Hasegawa, M.; Kuroda, E.K.; Arii, S.; Tsuji, K. Blue color formation of cyanobacteria with β-cyclocitral. J. Chem. Ecol. 2009, 35, 1295–1301. [Google Scholar] [CrossRef]
- Arii, S.; Tsuji, K.; Tomita, K.; Hasegawa, M.; Bober, B.; Harada, K. Cyanobacterial blue color formation during lysis under natural conditions. Appl. Environ. Microbiol. 2015, 81, 2667–2675. [Google Scholar] [CrossRef] [Green Version]
- Ikawa, M.; Sasner, J.J.; Haney, J.F. Activity of cyanobacterial and algal odor compounds found in lake waters on green algae Chlorella pyrenoidosa growth. Hydrobiologia 2001, 443, 19–22. [Google Scholar] [CrossRef]
- Chang, D.-W.; Hsieh, M.-L.; Chen, Y.-M.; Lin, T.-F.; Chang, J.-S. Kinetics of cell lysis for Microcystis aeruginosa and Nitzschia palea in the exposure to β-cyclocitral. J. Hazard. Mater. 2011, 185, 1214–1220. [Google Scholar] [CrossRef]
- Baldermann, S.; Yamamoto, M.; Yang, Z.; Kawahashi, T.; Kuwano, K.; Watanabe, N. C13-Apocarotenoids: More than Flavor Compounds? In Carotenoid Cleavage Products; Winterhalter, P., Ebeler, S.E., Eds.; American Chemical Soc: Washington, DC, USA, 2013; pp. 73–80. ISBN 978-0-8412-2778-1. [Google Scholar]
- Jüttner, F. Dynamics of the volatile organic substances associated with cyanobacteria and algae in a eutrophic shallow lake. Appl. Environ. Microbiol. 1984, 47, 814–820. [Google Scholar] [CrossRef] [Green Version]
- Ellouze, I.; Abderrabba, M.; Sabaou, N.; Mathieu, F.; Lebrihi, A.; Bouajila, J. Season’s variation impact on Citrus aurantium leaves essential oil: Chemical composition and biological activities. J. Food Sci. 2012, 77, T173–T180. [Google Scholar] [CrossRef]
- Tajabadi, F.; Khalighi-Sigaroodi, F.; Rezazadeh, S. Improving Gas Chromatography–Mass Spectrometry Analysis of Essential Oils by Multivariate Curve Resolution: Full Identification of Co-eluting Compounds of Dracocephalum moldavica L. Chromatographia 2017, 80, 1069–1077. [Google Scholar] [CrossRef]
- Manzo, A.; Musso, L.; Panseri, S.; Iriti, M.; Dallavalle, S.; Catalano, E.; Scarì, G.; Giorgi, A. Screening of the chemical composition and bioactivity of Waldheimia glabra (Decne.) Regel essential oil. J. Sci. Food Agric. 2016, 96, 3195–3201. [Google Scholar] [CrossRef]
- Ngan, L.T.M.; Moon, J.-K.; Kim, J.-H.; Shibamoto, T.; Ahn, Y.-J. Growth-inhibiting effects of Paeonia lactiflora root steam distillate constituents and structurally related compounds on human intestinal bacteria. World J. Microbiol. Biotechnol. 2012, 28, 1575–1583. [Google Scholar] [CrossRef]
- Intisar, A.; Zhang, L.; Luo, H.; Zhang, R.; Wu, Z.; Zhang, W. Difference in Essential Oil Composition of Rhizome of Polygonum bistorta L. from Different Asian Regions and Evaluation of its Antibacterial Activity. J. Essent. Oil Bear. Plants 2012, 15, 964–971. [Google Scholar] [CrossRef]
- Höckelmann, C.; Jüttner, F. Volatile organic compound (VOC) analysis and sources of limonene, cyclohexanone and straight chain aldehydes in axenic cultures of Calothrix and Plectonema. Water Sci. Technol. 2004, 49, 47–54. [Google Scholar] [CrossRef]
- Höckelmann, C.; Jüttner, F. Off-flavours in water: Hydroxyketones and β-ionone derivatives as new odour compounds of freshwater cyanobacteria. Flavour Frag. J. 2005, 20, 387–394. [Google Scholar] [CrossRef]
- Kaiser, R. Carotenoid-Derived Aroma Compounds in Flower Scents. In Carotenoid-Derived Aroma Compounds; Winterhalter, P., Rouseff, R.L., Eds.; American Chemical Society: Washington, DC, USA, 2001; pp. 160–182. ISBN 9780841237292. [Google Scholar]
- Kawakami, M.; Kobayashi, A. Carotenoid-Derived Aroma Compounds in Tea. In Carotenoid-Derived Aroma Compounds; Winterhalter, P., Rouseff, R.L., Eds.; American Chemical Society: Washington, DC, USA, 2001; pp. 145–159. ISBN 9780841237292. [Google Scholar]
- Straubinger, M.; Bau, B.; Eckstein, S.; Fink, M.; Winterhalter, P. Identification of Novel Glycosidic Aroma Precursors in Saffron (Crocus sativus L.). J. Agric. Food Chem. 1998, 46, 3238–3243. [Google Scholar] [CrossRef]
- Rödel, W.; Petrzika, M. Analysis of the volatile components of saffron. J. High Resol. Chromatogr. 1991, 14, 771–774. [Google Scholar] [CrossRef]
- Riad, N.; Zahi, M.R.; Trovato, E.; Bouzidi, N.; Daghbouche, Y.; Utczás, M.; Mondello, L.; El Hattab, M. Chemical screening and antibacterial activity of essential oil and volatile fraction of Dictyopteris polypodioides. Microchem. J. 2020, 152, 104415. [Google Scholar] [CrossRef]
- Moularat, S.; Robine, E.; Ramalho, O.; Oturan, M.A. Detection of fungal development in closed spaces through the determination of specific chemical targets. Chemosphere 2008, 72, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Chen, Y.; Han, X.; Zhan, Z.; Tian, S.; Cui, Q.; Wang, Y. Chemical composition of essential oils of Litsea cubeba harvested from its distribution areas in China. Molecules 2012, 17, 7057–7066. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, L.; Zheng, C.; Yang, Y.; Wang, X.; Hui, J.; Zhou, Q. Key Odorant Differences in Fragrant Brassica napus and Brassica juncea Oils Revealed by Gas Chromatography-Olfactometry, Odor Activity Values, and Aroma Recombination. J. Agric. Food Chem. 2020, 68, 14950–14960. [Google Scholar] [CrossRef]
- Hu, C.-D.; Liang, Y.-Z.; Li, X.-R.; Guo, F.-Q.; Zeng, M.-M.; Zhang, L.-X.; Li, H.-D. Essential Oil Composition of Osmanthus fragrans Varieties by GC-MS and Heuristic Evolving Latent Projections. Chromatographia 2009, 70, 1163–1169. [Google Scholar] [CrossRef]
- Zucko, J.; Dunlap, W.C.; Shick, J.M.; Cullum, J.; Cercelet, F.; Amin, B.; Hammen, L.; Lau, T.; Williams, J.; Hranueli, D.; et al. Global genome analysis of the shikimic acid pathway reveals greater gene loss in host-associated than in free-living bacteria. BMC Genomics 2010, 11, 628. [Google Scholar] [CrossRef] [Green Version]
- Portwich, A.; Garcia-Pichel, F. Biosynthetic pathway of mycosporines (mycosporine-like amino acids) in the cyanobacterium Chlorogloeopsis sp. strain PCC 6912. Phycologia 2003, 42, 384–392. [Google Scholar] [CrossRef]
- Pope, M.A.; Spence, E.; Seralvo, V.; Gacesa, R.; Heidelberger, S.; Weston, A.J.; Dunlap, W.C.; Shick, J.M.; Long, P.F. O-Methyltransferase is shared between the pentose phosphate and shikimate pathways and is essential for mycosporine-like amino acid biosynthesis in Anabaena variabilis ATCC 29413. ChemBioChem 2015, 16, 320–327. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.-H.; Yang, D.J.; Kulkarni, A.; Moh, S.H.; Kim, K.W. Mycosporine-Like Amino Acids Promote Wound Healing through Focal Adhesion Kinase (FAK) and Mitogen-Activated Protein Kinases (MAP Kinases) Signaling Pathway in Keratinocytes. Mar. Drugs 2015, 13, 7055–7066. [Google Scholar] [CrossRef] [Green Version]
- Klisch, M.; Sinha, R.P.; Richter, P.R.; Häder, D.-P. Mycosporine-like amino acids (MAAs) protect against UV-B-induced damage in Gyrodinium dorsum Kofoid. J. Plant Physiol. 2001, 158, 1449–1454. [Google Scholar] [CrossRef]
- Peinado, N.K.; Abdala Díaz, R.T.; Figueroa, F.L.; Helbling, E.W. Ammonium and UV radiation stimulate the accumulation of mycosporine-like amino acids in Porphyra columbina (Rhodophyta) from Patagonia, Argentina. J. Phycol. 2004, 40, 248–259. [Google Scholar] [CrossRef] [Green Version]
- Egesa, D.; Chuck, C.J.; Plucinski, P. Multifunctional Role of Magnetic Nanoparticles in Efficient Microalgae Separation and Catalytic Hydrothermal Liquefaction. ACS Sustain. Chem. Eng. 2018, 6, 991–999. [Google Scholar] [CrossRef]
- Kamenarska, Z.; Gasic, M.J.; Zlatovic, M.; Rasovic, A.; Sladic, D.; Kljajic, Z.; Stefanov, K.; Seizova, K.; Najdenski, H.; Kujumgiev, A.; et al. Chemical Composition of the Brown Algae Padina pavonia (L.) Gaill. from the Adriatic Sea. Bot. Mar. 2002, 45, 339–345. [Google Scholar] [CrossRef]
- Reemtsma, T.; Fiehn, O.; Kalnowski, G.; Jekel, M. Microbial transformations and biological effects of fungicide-derived benzothiazoles determined in industrial wastewater. Environ. Sci. Technol. 1995, 29, 478–485. [Google Scholar] [CrossRef]
- Yajima, I.; Nakamura, M.; Sakakibara, H.; Ide, J.; Yanai, T.; Hayashi, K. Volatile Flavor Components of Dried Bonito (Katsuobushi) II. From Neutral Fraction. Agr. Biol. Chem. 1983, 47, 1755–1760. [Google Scholar] [CrossRef] [Green Version]
- Hannemann, K.; Puchta, V.; Simon, E.; Ziegler, H.; Ziegler, G.; Spiteller, G. The Common Occurrence of Furan Fatty Acids in Plants. Lipids 1989, 24, 296–298. [Google Scholar] [CrossRef]
- Schulz, S.; Hötling, S. The use of the lactone motif in chemical communication. Nat. Prod. Rep. 2015, 32, 1042–1066. [Google Scholar] [CrossRef] [Green Version]
- Rempt, M.; Weinberger, F.; Grosser, K.; Pohnert, G. Conserved and species-specific oxylipin pathways in the wound-activated chemical defense of the noninvasive red algae Gracilaria chilensis and the invasive Gracilaria vermiculophylla. Beilstein J. Org. Chem. 2012, 8, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Horinouchi, S.; Beppu, T. A-factor as a microbial hormone that controls cellular differentiation and secondary metabolism in Streptomyces griseus. Mol. Microbiol. 1994, 12, 859–864. [Google Scholar] [CrossRef]
- Riclea, R.; Gleitzmann, J.; Bruns, H.; Junker, C.; Schulz, B.; Dickschat, J.S. Algicidal lactones from the marine Roseobacter clade bacterium Ruegeria pomeroyi. Beilstein J. Org. Chem. 2012, 8, 941–950. [Google Scholar] [CrossRef] [Green Version]
- Creelman, R.A.; Mulpuri, R. The Oxylipin Pathway in Arabidopsis. Arabidopsis Book 2002, 1, e0012. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; von Dahl, C.C.; Preston, C.A. Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef] [Green Version]
- Wichard, T.; Gerecht, A.; Boersma, M.; Poulet, S.A.; Wiltshire, K.; Pohnert, G. Lipid and fatty acid composition of diatoms revisited: Rapid wound-activated change of food quality parameters influences herbivorous copepod reproductive success. Chembiochem 2007, 8, 1146–1153. [Google Scholar] [CrossRef] [Green Version]
- Alsufyani, T.; Engelen, A.H.; Diekmann, O.E.; Kuegler, S.; Wichard, T. Prevalence and mechanism of polyunsaturated aldehydes production in the green tide forming macroalgal genus Ulva (Ulvales, Chlorophyta). Chem. Phys. Lipids 2014, 183, 100–109. [Google Scholar] [CrossRef]
- Boonprab, K.; Matsui, K.; Akakabe, Y.; Yotsukura, N.; Kajiwara, T. Hydroperoxy-arachidonic acid mediated n-hexanal and (Z)-3- and (E)-2-nonenal formation in Laminaria angustata. Phytochemistry 2003, 63, 669–678. [Google Scholar] [CrossRef]
- Boonprab, K.; Matsui, K.; Akakabe, Y.; Yoshida, M.; Yotsukura, N.; Chirapart, A.; Kajiwara, T. Formation of Aldehyde Flavor (n-hexanal, 3Z-nonenal and 2E-nonenal) in the Brown Alga, Laminaria Angustata. J. Appl. Phycol. 2006, 18, 409–412. [Google Scholar] [CrossRef]
- Kaminski, E.; Stawicki, S.; Wasowicz, E. Volatile Flavor Compounds Produced by Molds of Aspergillus, Penicillium, and Fungi imperfecti. Appl. Microbiol. 1974, 27, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Schnürer, J.; Olsson, J.; Börjesson, T. Fungal volatiles as indicators of food and feeds spoilage. Fungal Genet. Biol. 1999, 27, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhu, Z.; Chen, J.-J.; Yang, R.; Luo, Q.; Xu, J.; Shan, H.; Yan, X.-J. A multifunctional lipoxygenase from Pyropia haitanensis—The cloned and functioned complex eukaryotic algae oxylipin pathway enzyme. Algal Res. 2015, 12, 316–327. [Google Scholar] [CrossRef]
- Chen, H.; Yang, R.; Chen, J.; Luo, Q.; Cui, X.; Yan, X.; Gerwick, W.H. 1-Octen-3-ol, a self-stimulating oxylipin messenger, can prime and induce defense of marine alga. BMC Plant Biol. 2019, 19, 37. [Google Scholar] [CrossRef]
- Kamenarska, Z.; Ivanova, A.; Stancheva, R.; Stoyneva, M.; Stefanov, K.; Dimitrova-Konaklieva, S.; Popov, S. Volatile compounds from some Black Sea red algae and their chemotaxonomic application. Botanica Marina 2006, 49, 47–56. [Google Scholar] [CrossRef]
- Fink, P. Ecological functions of volatile organic compounds in aquatic systems. Mar. Freshwater Behav. Physiol. 2007, 40, 155–168. [Google Scholar] [CrossRef]
- Schulz, S.; Biwer, P.; Harig, T.; Koteska, D.; Schlawis, C. Chemical Ecology of Bacterial Volatiles. In Comprehensive Natural Products III, 3rd ed.; Begley, T., Ed.; Elsevier: San Diego, CA, USA, 2020; pp. 161–178. ISBN 9780081026915. [Google Scholar]
- Yao, S.; Lyu, S.; An, Y.; Lu, J.; Gjermansen, C.; Schramm, A. Microalgae-bacteria symbiosis in microalgal growth and biofuel production: A review. J. Appl. Microbiol. 2019, 126, 359–368. [Google Scholar] [CrossRef]
- Buchan, A.; LeCleir, G.R.; Gulvik, C.A.; González, J.M. Master recyclers: Features and functions of bacteria associated with phytoplankton blooms. Nat. Rev. Microbiol. 2014, 12, 686–698. [Google Scholar] [CrossRef]
- Johnson, I.; Girijan, S.; Tripathy, B.K.; Ali, M.A.S.; Kumar, M. Algal–bacterial symbiosis and its application in wastewater treatment. In Emerging Technologies in Environmental Bioremediation; Shah, M., Rodriguez-Couto, S., Şengör, C., Sevinç, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 341–372. ISBN 9780128198605. [Google Scholar]
- Moran, M.A.; Kujawinski, E.B.; Schroer, W.F.; Amin, S.A.; Bates, N.R.; Bertrand, E.M.; Braakman, R.; Brown, C.T.; Covert, M.W.; Doney, S.C.; et al. Microbial metabolites in the marine carbon cycle. Nat. Microbiol. 2022, 7, 508–523. [Google Scholar] [CrossRef]
- Cole, J.J. Interactions between bacteria and algae in aquatic ecosystems. Ann. Rev. Ecol. Syst. 1982, 13, 291–314. [Google Scholar] [CrossRef]
- Johnston, A.; Crombie, A.T.; El Khawand, M.; Sims, L.; Whited, G.M.; McGenity, T.J.; Colin Murrell, J. Identification and characterisation of isoprene-degrading bacteria in an estuarine environment. Environ. Microbiol. 2017, 19, 3526–3537. [Google Scholar] [CrossRef] [Green Version]
- Moore, E.R.; Weaver, A.J.; Davis, E.W.; Giovannoni, S.J.; Halsey, K.H. Metabolism of key atmospheric volatile organic compounds by the marine heterotrophic bacterium Pelagibacter HTCC1062 (SAR11). Environ. Microbiol. 2022, 24, 212–222. [Google Scholar] [CrossRef]
- Pohnert, G. Diatom/copepod interactions in plankton: The indirect chemical defense of unicellular algae. ChemBioChem 2005, 6, 946–959. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Sunda, W.G.; Price, N.M.; Morel, F.M.M. Trace metal ion buffers and their use in culture studies. In Algal Culturing Techniques, 1st ed.; Andersen, R.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Bardili, B.; Marschall-Weyerstahl, H.; Weyerstahl, P. Bildung und Reaktivität von hydroxysubstituierten γ- und δ-Lactonen. Liebigs Ann. Chem. 1985, 1985, 275–300. [Google Scholar] [CrossRef]
- Schulz, S.; Möllerke, A. MACE—Mass Spectra for Chemical Ecology (Release 2). Available online: https://doi.org/10.24355/DBBS.084-202203110733-0 (accessed on 15 March 2022).
- Schulz, S.; Möllerke, A. MACE—Mass Spectra for Chemical Ecology. Available online: http://www.oc.tu-bs.de/schulz/html/MACE.html (accessed on 15 February 2022).
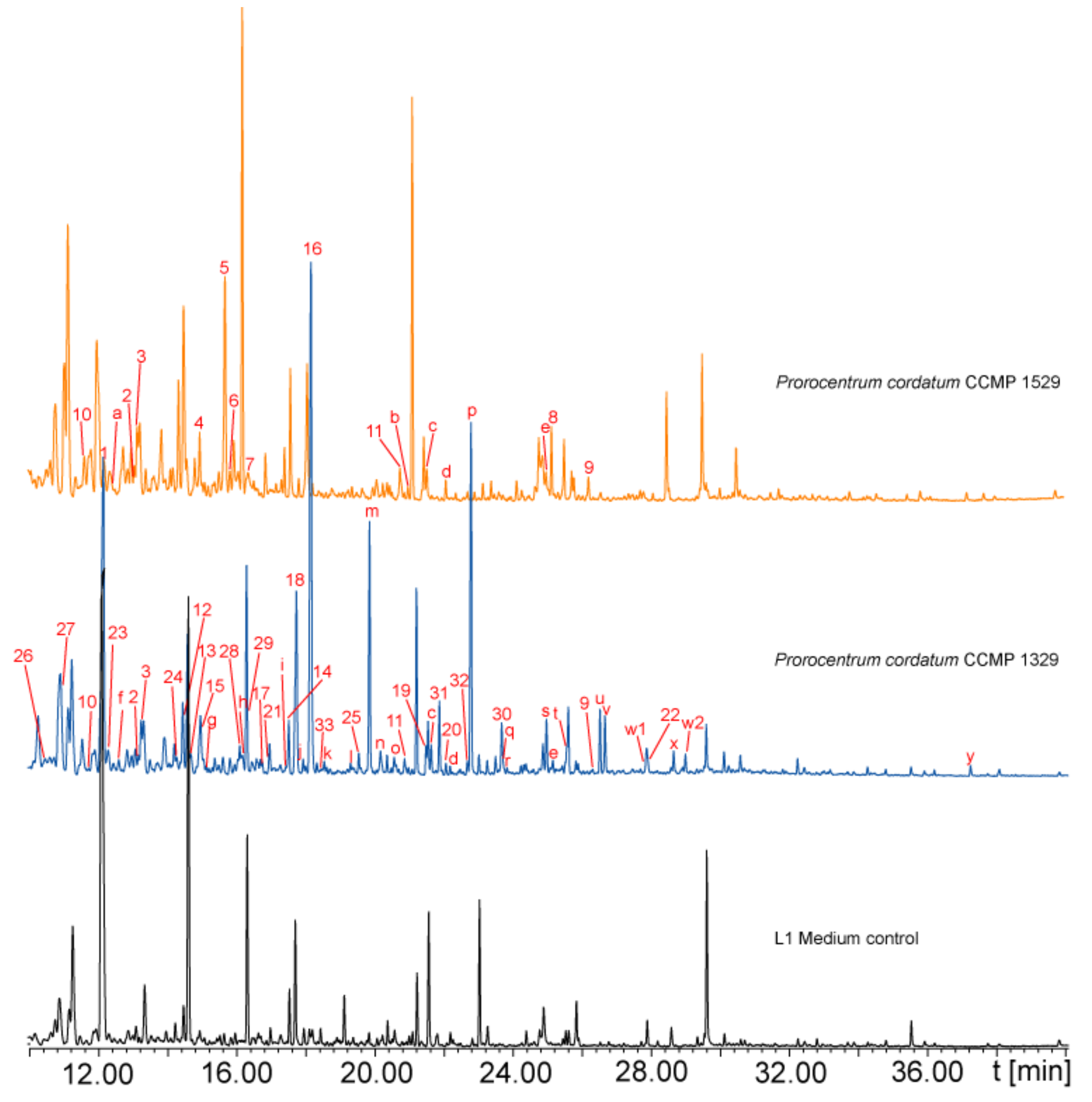
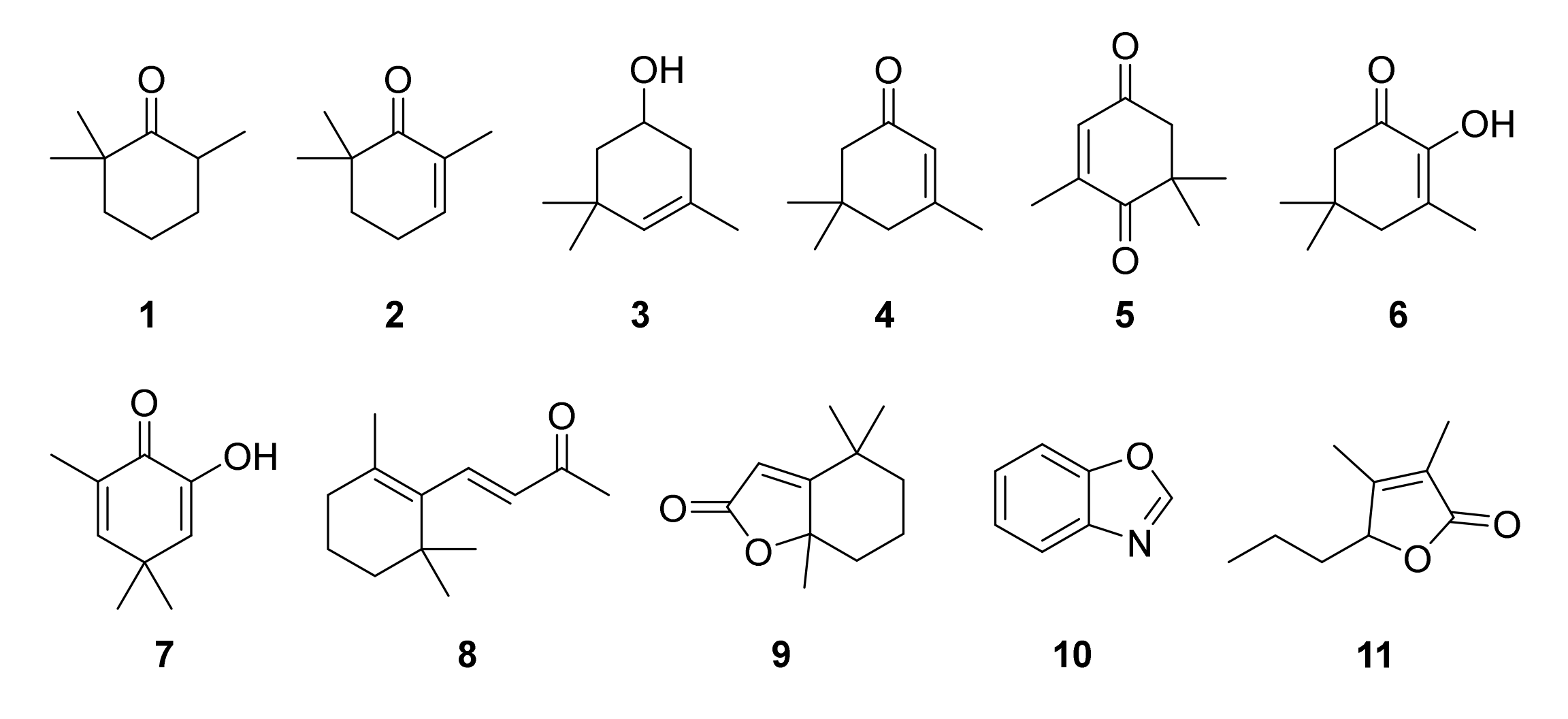
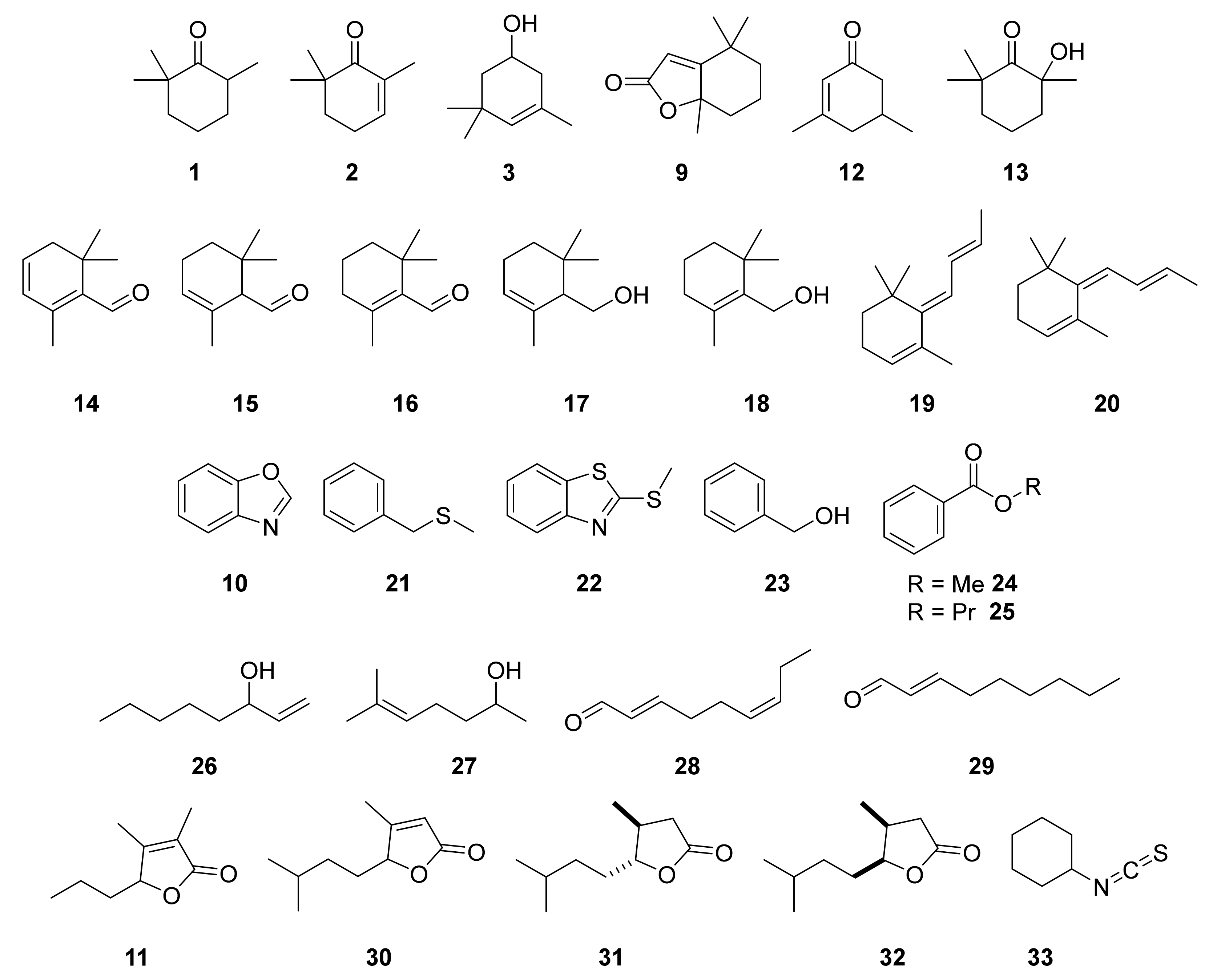
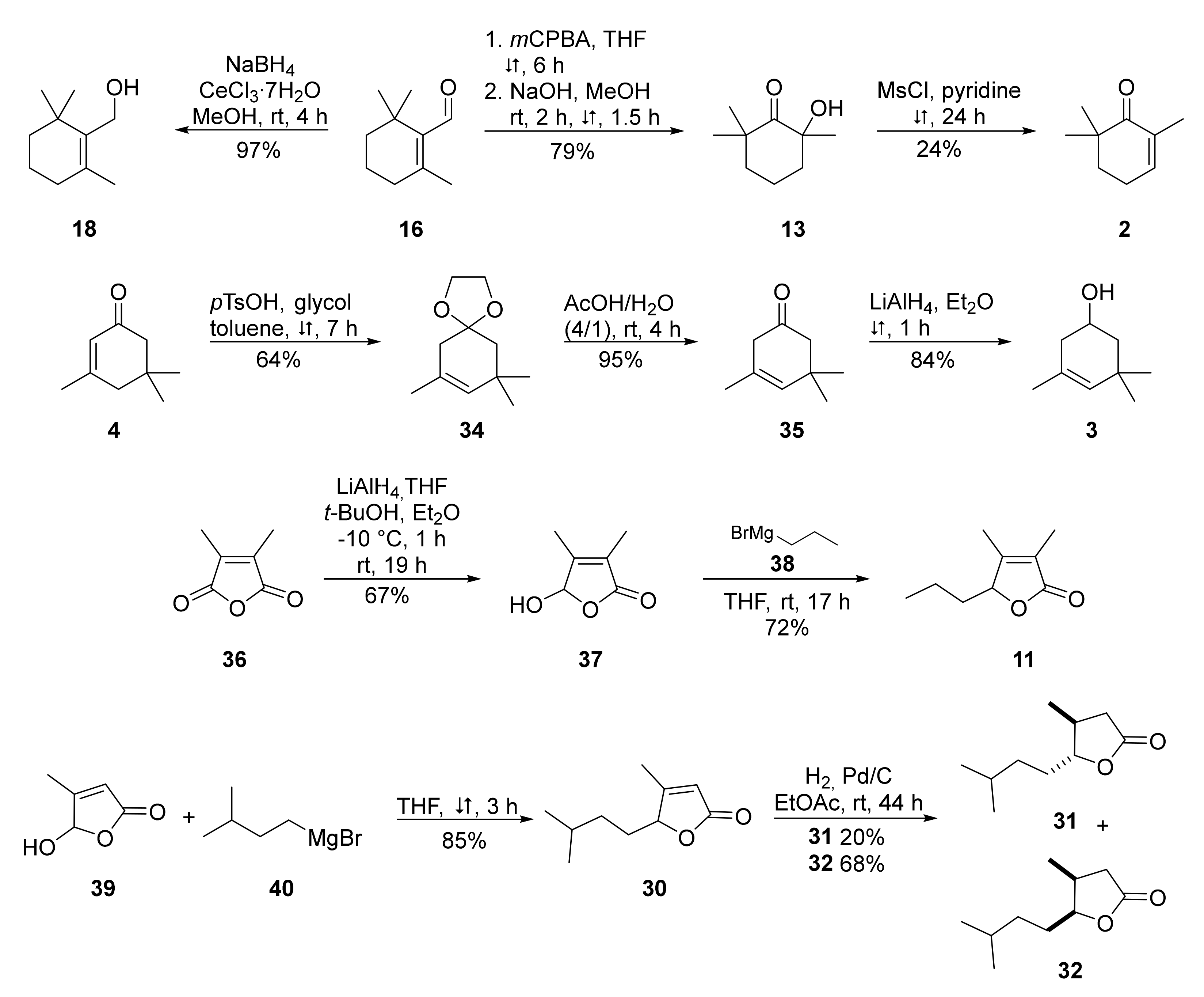
| Compound | I (exp) a | I (lit) b | Identification c | Rep 1 | Rep 2 | Rep 3 |
|---|---|---|---|---|---|---|
| Benzoxazole (10) | 1019 | 1067 | ms, ri | x | x | x |
| 2,2,6-Trimethylcyclohexan-1-one (1) | 1035 | 1035 | ms, ri | x | x | |
| Unknown compound M 138 or M 154 (a) | 1042 | x | x | x | ||
| 2,6,6-Trimethylcyclohex-2-en-1-one (2) | 1060 | 1060 | ms, ri, syn | x | x | x |
| 3,5,5-Trimethylcyclohex-3-en-1-ol (3) | 1064 | 1064 | ms, ri, syn | x | x | x |
| α-Isophorone (4) | 1120 | 1120 | ms, ri | x | x | x |
| 4-Oxoisophorone (5) | 1144 | 1142 | ms, ri | x | x | x |
| 2-Hydroxyisophorone (6) | 1148 | 1150 | ms, ri | x | x | |
| 2-Hydroxy-4,4,6-trimethylcyclohexa-2,5-dienone (7) | 1163 | 1165 | ms, ri | x | x | |
| 2,3-Dimethyl-2-hepten-4-olide (11) | 1318 | 1322 | ms, ri, syn | x | x | |
| Unknown compound M 152 or M 180 (b) | 1327 | x | x | |||
| Unknown compound M 166 (c) | 1347 | x | x | x | ||
| Unknown compound (d) | 1368 | x | x | x | ||
| Unknown compound M 210 (e) | 1482 | x | x | x | ||
| trans-β-Ionone (8) | 1488 | 1486 | ms, ri | x | x | x |
| Dihydroactinidiolide (9) | 1533 | 1532 | ms, ri | x | x |
| Compound | I (exp) a | I (lit) b | Identification c | Rep 1 | Rep 2 | Rep 3 |
|---|---|---|---|---|---|---|
| 1-Octen-3-ol (26) | 982 | 980 | ms, ri | x | x | x |
| 6-Methyl-5-hepten-2-ol (27) | 995 | 994 | ms, ri | x | x | x |
| Benzoxazole (10) | 1019 | 1067 | ms, ri | x | x | x |
| 2,2,6-Trimethylcyclohexan-1-one (1) | 1035 | 1035 | ms, ri | x | x | |
| Benzyl alcohol (23) | 1036 | 1036 | ms, ri | x | x | |
| Unknown compound M 150(f) | 1045 | ms, ri | x | x | x | |
| 2,6,6-Trimethylcyclohex-2-en-1-one (2) | 1060 | 1060 | ms, ri, syn | x | x | x |
| 3,5,5-Trimethylcyclohex-3-en-1-ol (3) | 1064 | 1065 | ms, ri, syn | x | x | x |
| Methyl benzoate (24) | 1095 | 1095 | ms, ri | x | x | x |
| 3,5-Dimethylcyclohex-2-en-1-one (12) | 1101 | 1099 | ms, ri, syn | x | x | x |
| 2-Hydroxy-2,6,6-trimethylcyclohexan-1-one (13) | 1108 | 1109 | ms, ri, syn | x | x | x |
| α-Cyclocitral (15) | 1117 | 1116 | ms, ri | x | x | x |
| Unknown compound M 98 (g) | 1122 | x | x | x | ||
| (2E,6Z)-Nonadienal (28) | 1154 | 1154 | ms, ri, syn | x | x | |
| Unknown compound M 147 (h) | 1156 | x | x | x | ||
| (E)-2-Nonenal (29) | 1160 | 1160 | ms, ri | x | x | x |
| α-Cyclogeraniol (17) | 1175 | 1184 | ms, ri | x | x | x |
| Benzyl(methyl)sulfane (21) | 1181 | 1183 | ms, ri | x | x | |
| Unknown compound M 173 (i) | 1198 | x | x | x | ||
| Safranal (14) | 1200 | 1201 | ms, ri | x | x | |
| β-Cyclogeraniol (18) | 1207 | 1209 | ms, ri, syn | x | x | x |
| Unknown compound (j) | 1217 | x | x | x | ||
| β-Cyclocitral (16) | 1222 | 1222 | ms, ri | x | x | x |
| Cyclohexyl isothiocyanate (33) | 1233 | 1232 | ms, ri, syn | x | x | x |
| Unknown compound M 175 (k) | 1236 | x | x | x | ||
| Unknown compound M 176 (l) | 1262 | x | x | x | ||
| Propyl benzoate (25) | 1271 | 1272 | ms, ri | x | x | x |
| Unknown compound M 161 (m) | 1282 | x | x | x | ||
| Unknown compound (n) | 1293 | x | x | x | ||
| Unknown compound M 166 (o) | 1308 | x | x | x | ||
| 2,3-Dimethyl-2-hepten-4-olide (11) | 1319 | 1322 | ms, ri, syn | x | x | x |
| (6E,8E)-Megastigma-4,6,8-triene (19) | 1342 | x | x | x | ||
| Unknown compound M 166 (c) | 1348 | x | x | x | ||
| trans-3,7-Dimethyl-4-octanolide (31) | 1356 | 1358 | ms, ri, syn | x | x | x |
| (6Z,8E)-Megastigma-4,6,8-triene (20) | 1363 | 1358 | x | x | x | |
| Unknown compound (d) | 1368 | x | x | |||
| cis-3,7-Dimethyl-4-octanolide (32) | 1387 | 1389 | ms, ri, syn | x | x | x |
| Unknown compound M 189 (p) | 1391 | x | x | x | ||
| 3,7-Dimethyl-2-octen-4-olide (30) | 1426 | 1429 | ms, ri, syn | x | x | x |
| Unknown compound M 154 (q) | 1427 | x | x | x | ||
| Unknown compound M 204 (r) | 1432 | x | x | x | ||
| Unknown compound M 177 (s) | 1478 | x | x | x | ||
| Unknown compound M 210 (e) | 1482 | x | x | |||
| Unknown compound M 198 (t) | 1501 | x | x | x | ||
| Dihydroactinidiolide (9) | 1533 | 1532 | ms, ri | x | x | x |
| Unknown compound M 177 (u) | 1542 | x | x | |||
| Unknown compound M 253 (v) | 1548 | x | x | x | ||
| Unknown compound M 201 (w1) | 1598 | x | x | x | ||
| 2-(Methylthio)benzo[d]thiazole (22) | 1601 | 1589 | ms, ri | x | x | x |
| Unknown compound M 205 (x) | 1634 | x | x | |||
| Unknown compound M 201 (w2) | 1649 | x | x | x | ||
| Unknown compound (y) | 2056 | x | x | x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koteska, D.; Sanchez Garcia, S.; Wagner-Döbler, I.; Schulz, S. Identification of Volatiles of the Dinoflagellate Prorocentrum cordatum. Mar. Drugs 2022, 20, 371. https://doi.org/10.3390/md20060371
Koteska D, Sanchez Garcia S, Wagner-Döbler I, Schulz S. Identification of Volatiles of the Dinoflagellate Prorocentrum cordatum. Marine Drugs. 2022; 20(6):371. https://doi.org/10.3390/md20060371
Chicago/Turabian StyleKoteska, Diana, Selene Sanchez Garcia, Irene Wagner-Döbler, and Stefan Schulz. 2022. "Identification of Volatiles of the Dinoflagellate Prorocentrum cordatum" Marine Drugs 20, no. 6: 371. https://doi.org/10.3390/md20060371
APA StyleKoteska, D., Sanchez Garcia, S., Wagner-Döbler, I., & Schulz, S. (2022). Identification of Volatiles of the Dinoflagellate Prorocentrum cordatum. Marine Drugs, 20(6), 371. https://doi.org/10.3390/md20060371







