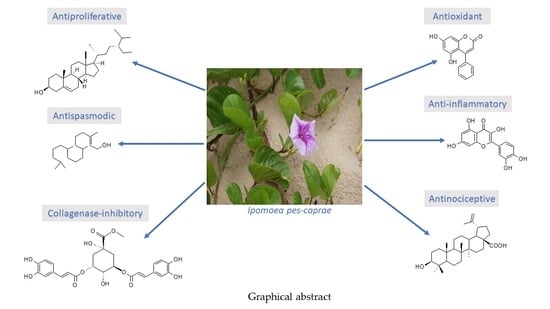
A Medicinal Halophyte Ipomoea pes-caprae (Linn.) R. Br.: A Review of Its Botany, Traditional Uses, Phytochemistry, and Bioactivity
Abstract
:1. Introduction
2. Botany
3. Establishment of I. pes-caprae in New Habitats
4. Traditional Uses
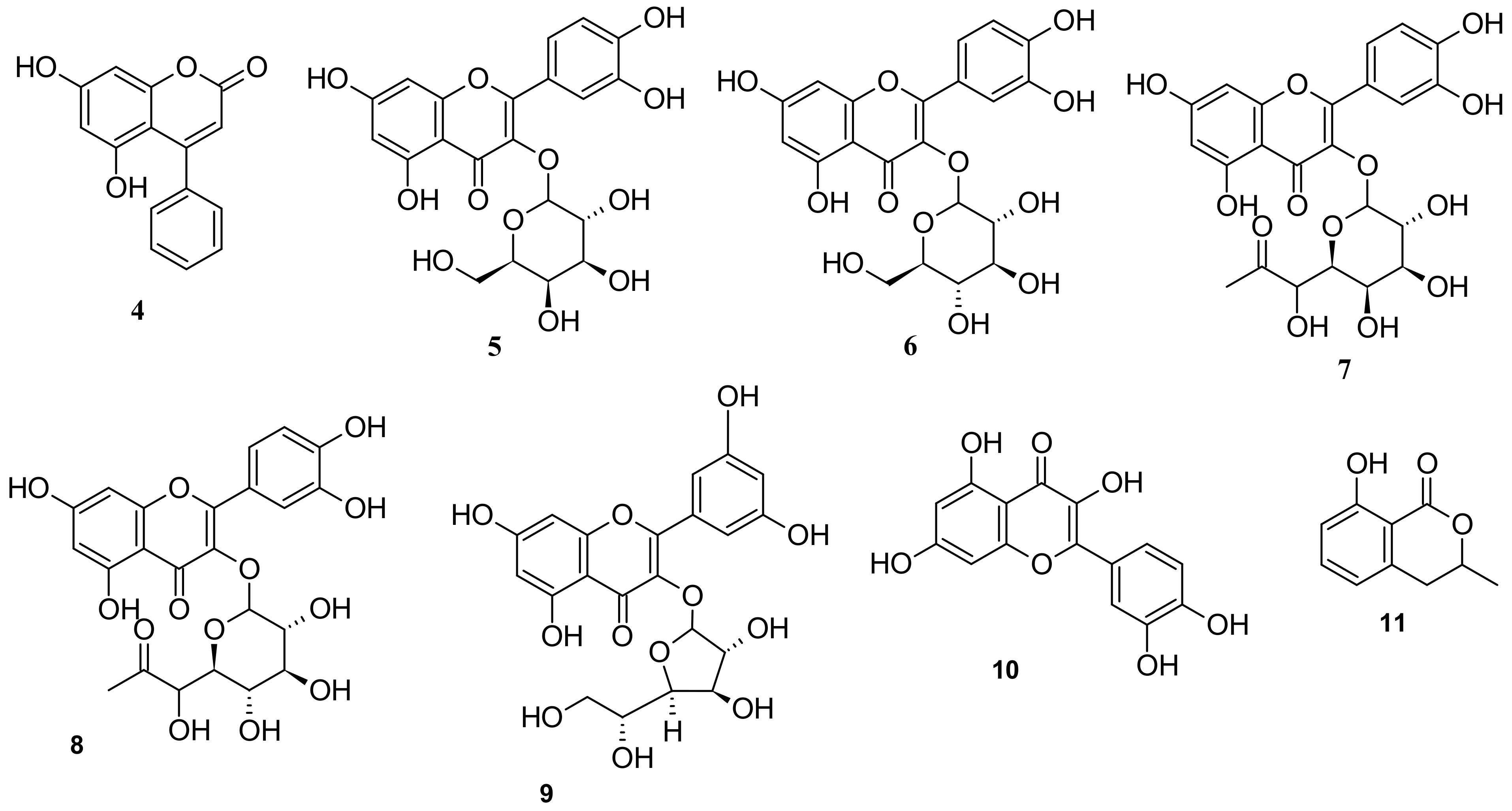
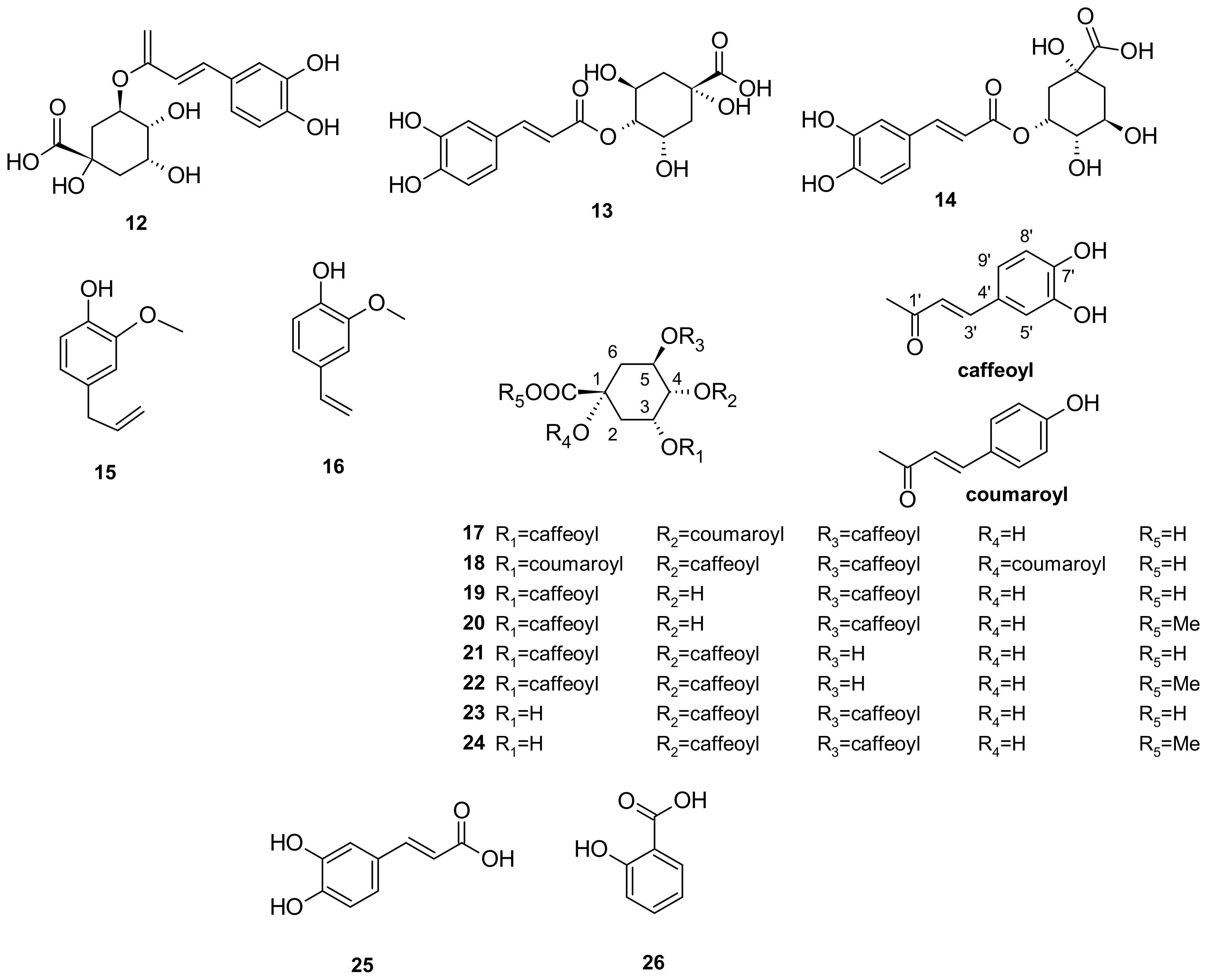
5. Phytochemistry of I. pes-caprae
5.1. Alkaloid
5.2. Norisoprenoids
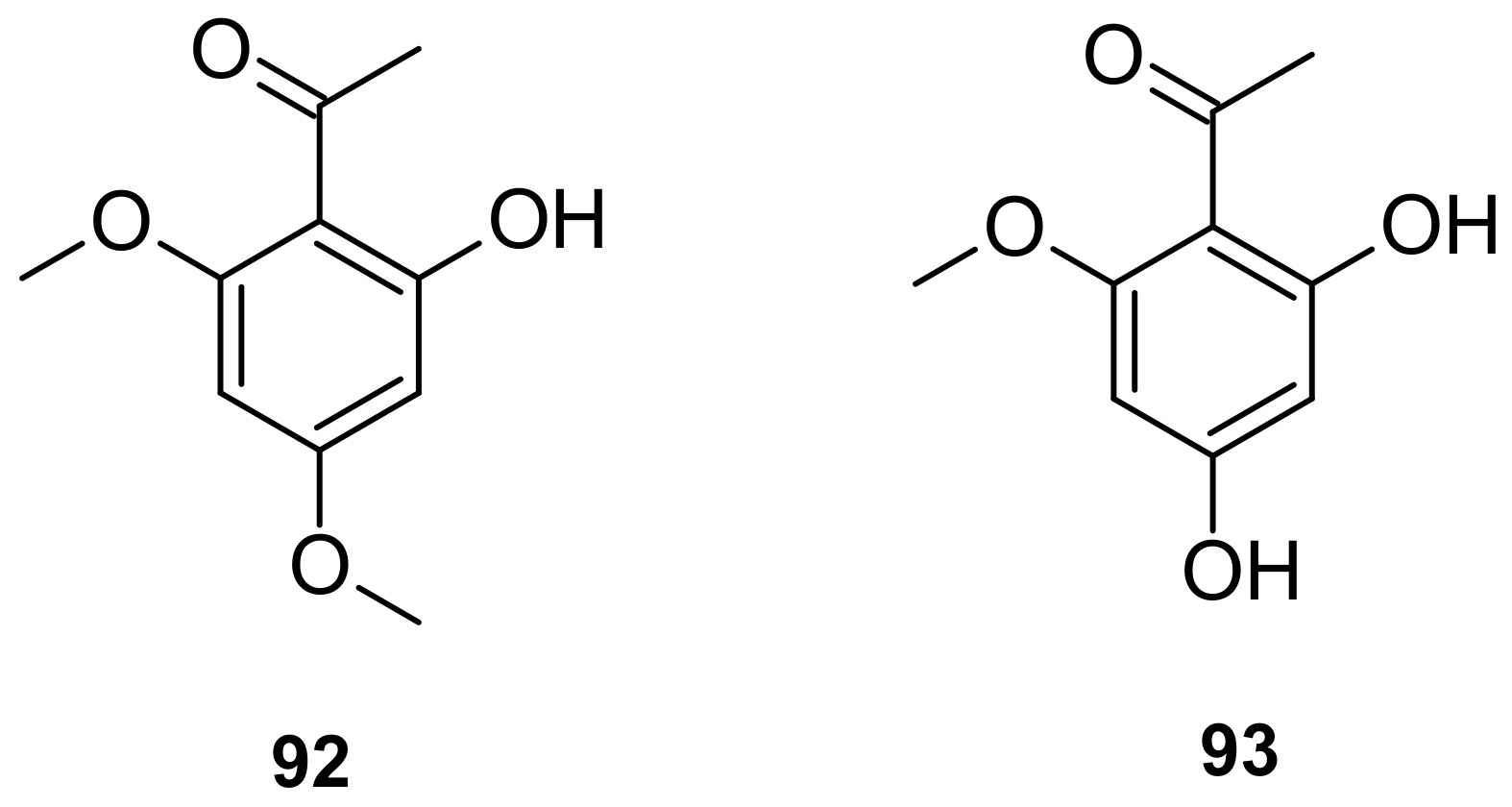
5.3. Phenols
5.4. Terpenoids
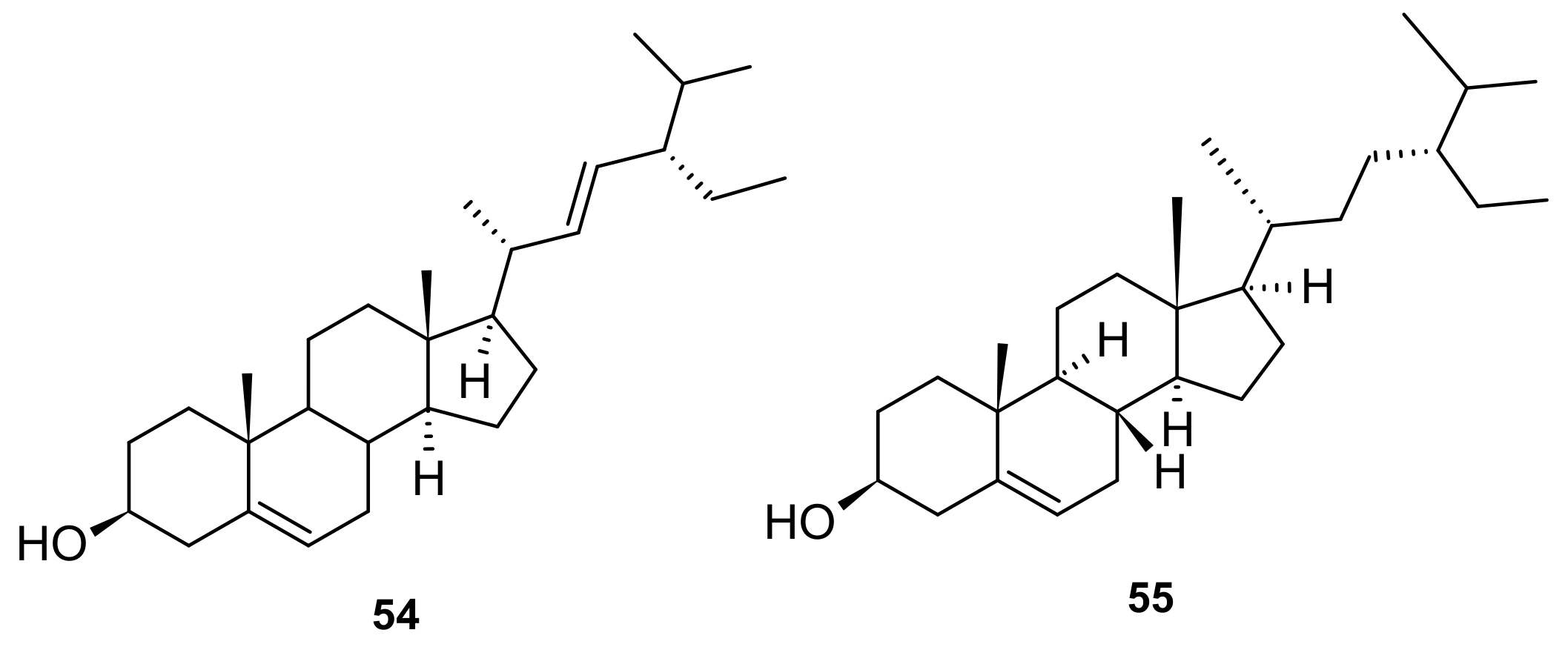
5.5. Steroids
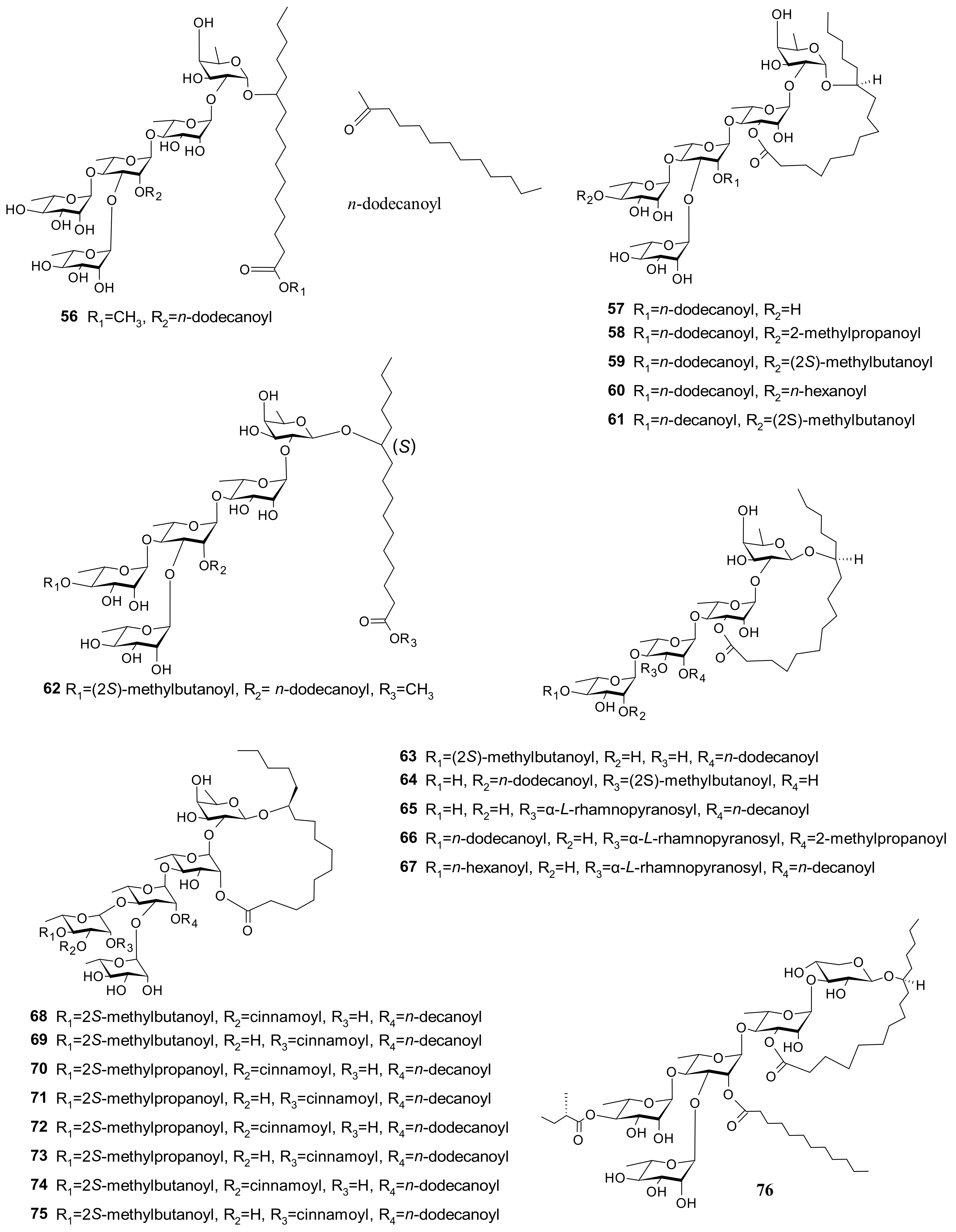
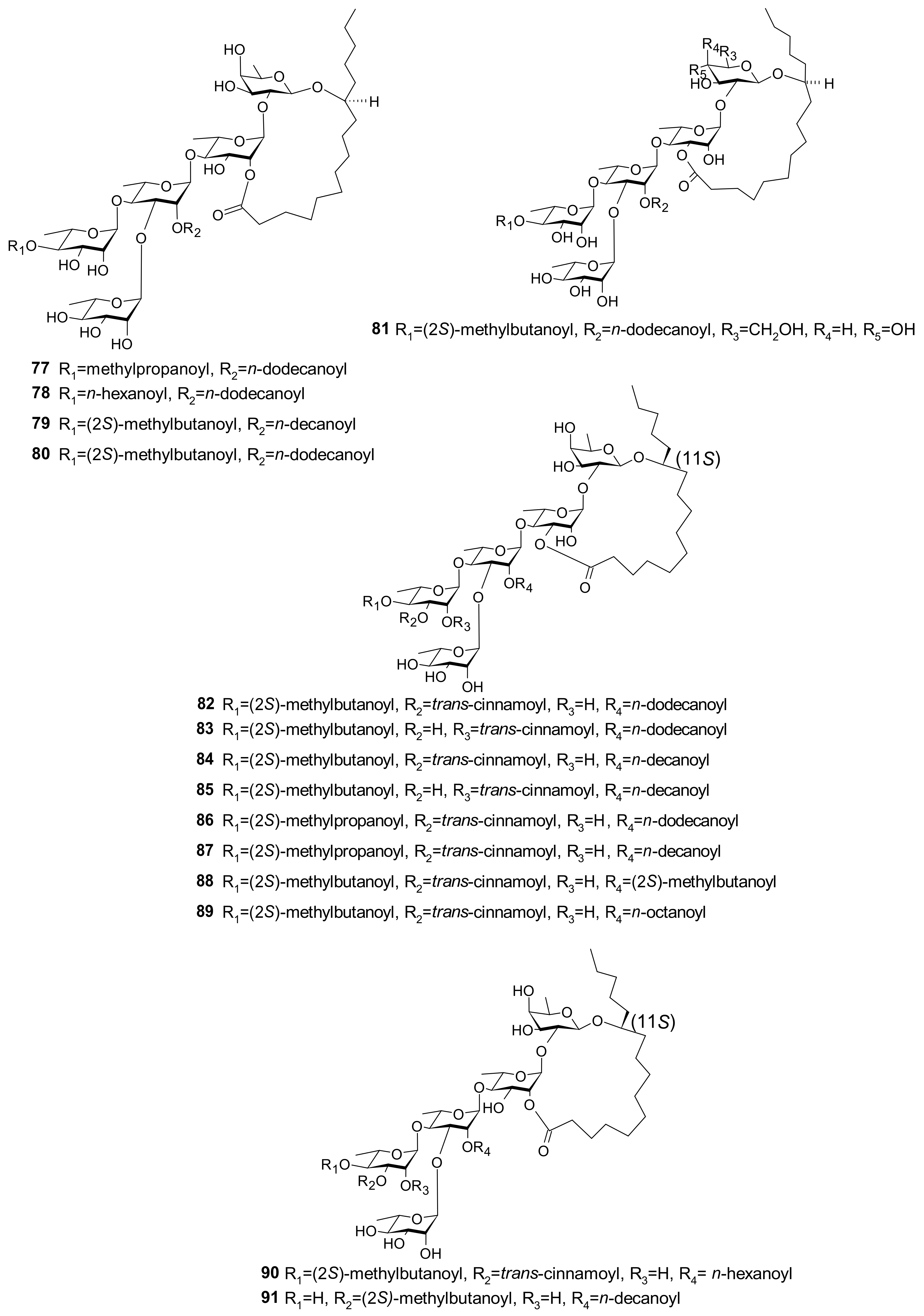
5.6. Glycosides
5.7. Other Constituents
6. Bioactivities of I. pes-caprae
6.1. Antioxidant Activity
| Compound | Pharmacological Activities | Reference |
|---|---|---|
| 4 | DPPH * scavenging (IC50 ** = 0.032 μM) Hydroxyl radical scavenging (IC50 = 0.055 μM) | [32] |
| 6 | DPPH assay (RC50 *** = 0.048 µM) | [50] |
| 10 | DPPH scavenging (IC50 = 0.028 µM) | [43] |
| 14 | DPPH scavenging (IC50 = 10.45 µM) | [51] |
| 21 | DPPH scavenging (IC50 = 3.29 µM) | [51] |
| 23 | DPPH scavenging (IC50 = 3.79 µM) | [51] |
| 25 (Caffeic acid) | DPPH scavenging (IC50 = 0.033 µM) | [52] |
| 36 (β-Amyrin) | Superoxide radical scavenging (IC50 = 0.190 μM) | [44] |
| 46 (β-Caryophyllene) | DPPH scavenging (IC50 = 1.25 μM) FRAP **** scavenging (IC50 = 3.23 μM) | [55] |
| 55 (β-Sitosterol) | DPPH scavenging (IC50 = 0.338 µM) ABTS ***** scavenging (IC50 = 0.289 µM) H2O2 scavenging (IC50 = 0.675 µM) | [45] |
6.2. Anti-Inflammatory Activity
| Compound | Pharmacological Activities | Reference |
|---|---|---|
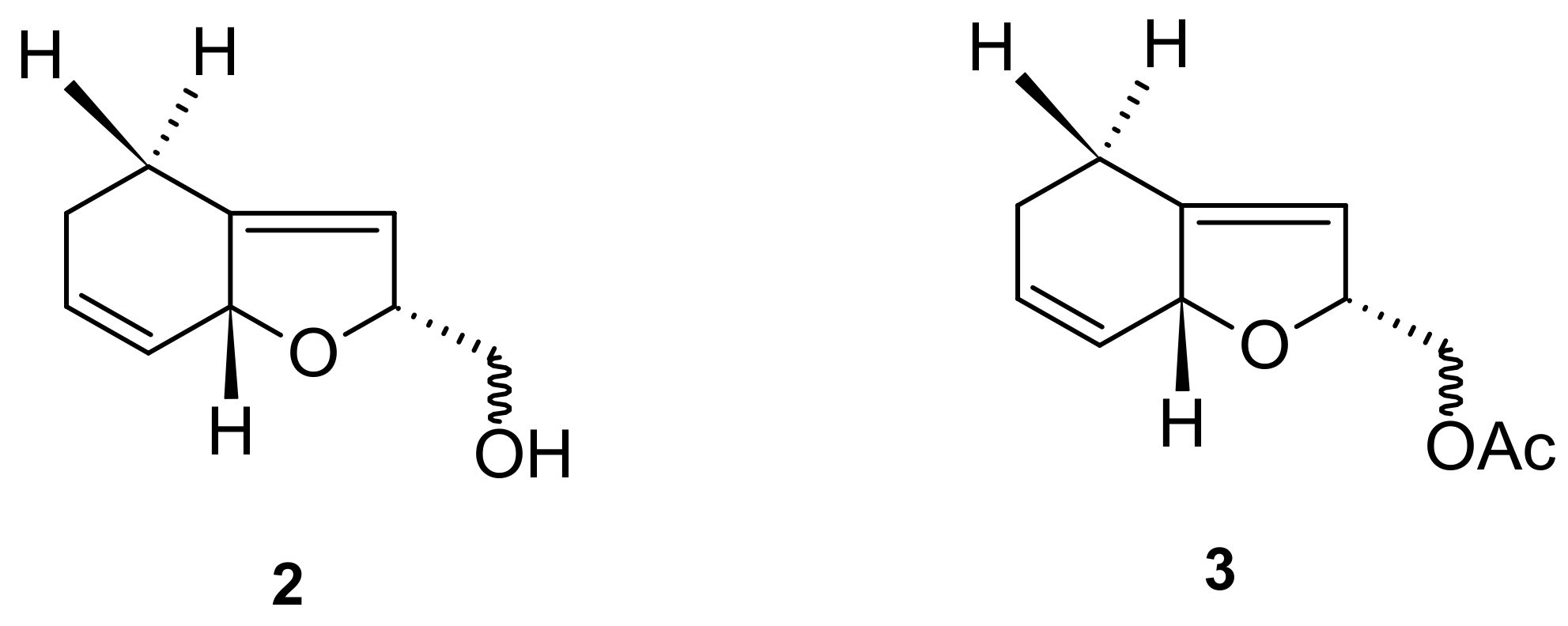
| 2 (Actinidol la) 3 (Actinidol lb) | 1.0 mg/ear produced 35% inhibition of oedema formation | [31] |
| 10 | Inhibitory effect on NO * production IC50 ** = 37.1 µM Inhibitory effect on TNF-α *** production with IC50 = 4.14 µM | [65] |
| 11 [(-) Mellein] | Inhibition of prostaglandin synthesis IC50 = 340 µM 1.0 mg/ear produced 37% inhibition of oedema formation | [31,34] |
| 15 (Eugenol) | 1.0 mg/ear produced 38% inhibition of oedema formation. Inhibition of prostaglandin synthesis IC50 = 9.2 µM | [31,34] |
| 16 | Inhibition of prostaglandin synthesis IC50 = 18 µM | [34] |
| 31 | 0.6 mg/ear produced 30% inhibition of oedema formation. Inhibition of prostaglandin synthesis IC50 = 230 µM | [31,34] |
| 32 (E-phytol) | 1.0 mg/ear produced 47% inhibition of oedema formation | [31] |
| 33 (Caryophyllene oxide) | Inhibited writhing response by 75.19% at 25 mg/kg body weight | [66] |
| 37 (α-Amyrin acetate) | 4 mg/100g i.p. **** produced 19.1% inhibition | [68] |
| 38 (β -Amyrin acetate) | 4 mg/100g i.p. produced 43.6% inhibition | [68] |
6.3. Antinociceptive Activity
| Compound | Pharmacological Activities | Reference |
|---|---|---|
| 6 | 10 mg/kg i.p. * inhibited constriction by 34.5% | [24] |
| 37 (α-Amyrin acetate) 38 (β-Amyrin acetate) | 10 mg/kg i.p. inhibited constriction by 54.4% | |
| 39 (Betulinic acid) | 10 mg/kg i.p. inhibited constriction by 88.1% | |
| 40 (Glochidone) | 10 mg/kg i.p. inhibited constriction by 75.5% |
6.4. Antimicrobial Activity
| Compound | Pharmacological Activities | Reference |
|---|---|---|
| 14 (Chlorogenic acid) | MIC * value of 0.057 µM against Shigella dysenteriae and MIC value of 0.113 µM against Staphylococcus aureus (Antibacterial) | [78] |
| 25 (Caffeic acid) | MIC value of 1.421 μM against S. aureus ATCC 25293 (antibacterial) | [79] |
| 32 (E-Phytol) | MIC50 ** value of 0.219 µM against Escherichia coli and MIC value of 3.37 µM against S. aureus (antibacterial) MIC50 value of 0.219 µM against Candida albicans and Aspergillus niger (antifungal) | [76] |
| 46 (β-Caryophyllene) | MIC value of 3 μM against S. Aureus (antibacterial) | [55] |
| 53 (Sericic acid) | MIC value of 0.135 µM against C. albicans and Cryptococcus neoformans (antifungal) | [84] |
| 55 (β-Sitosterol) | 0.048 μM produced inhibition zones of 14 mm (E. coli), 13 mm (S. aureus), 11 mm (Pseudomonas aeruginosa), and 10 mm (Klebsiella pneumoniae) (antibacterial) | [77] |
| 92 (Xanthoxyline) | MIC value of 0.255 µM against C. neoformans and MIC value of 0.382 µM against Aspergillus fumigatus (antifungal) | [86] |
6.5. Collagenase Inhibitory Activity
| Compound | Collagenase Inhibitory Activity IC50 * Value (µM) | Reference |
|---|---|---|
| 17 | 19.1 | [35] |
| 18 | 14.2 | |
| 19 (Isochlorogenic acid A) | 23.6 | |
| 20 | 5.8 | |
| 21 (Isochlorogenic acid B) | 31.7 | |
| 22 | 16.2 | |
| 23 (Isochlorogenic acid C) | 37.2 | |
| 24 | 26.6 | |
| 25 (Caffeic acid) | 82.7 |
6.6. Antispasmodic Activity
| Compound Name | Pharmacological Activities | Reference |
|---|---|---|
| 32 (E-Phytol) | 0.105 µM produced 41% inhibition on submaximal contractions of guinea-pig ileal smooth muscle | [36] |
| 50 (β-Damascenone) | 0.163 µM produced 45% inhibitions respectively of submaximal contractions of guinea-pig ileal smooth muscle | |
| 92 (Xanthoxyline) | Inhibition of acetylcholine-induced contraction in guinea-pig ileum (IC50 * = 47 µM) | [90] |
6.7. Anticancer, Antitumor, and Antiproliferative Activities
| Compound | Pharmacological Activities | Reference |
|---|---|---|
| 4 | Decreased HCT116 * cell viability up to 30% after 24 h of treatment (IC50 ** = 0.055 μM) | [32] |
| 10 | Inhibition of the growth of HL-60 *** cells (IC50 = 7.7 μM) | [92] |
| 27 (Limonene) | Inhibition of the proliferation of A549 **** cells (IC50 = 0.162 μM) | [98] |
| 28 (α-Terpineol) | Inhibition of the proliferation of A549 cells (IC50 = 0.333 μM) | [98] |
| 34 (α-Pinene) | Inhibition of the proliferation of A549 cells (IC50 = 0.162 µM) | [98] |
| 35 (α-Amyrin) | Inhibition of the proliferation of A549 (IC50 = 0.022) and A2780 ***** cell lines (IC50 = 0.052 μM) | [95] |
| 44 (Linalool) | Inhibition of the proliferation of A549 cells (IC50 = 0.919 μM) | [98] |
| 46 (β-Caryophyllene) | Selective anti-proliferative effect against HCT116 (IC50 = 19 µM) and PANC-1 ****** (IC50 = 27 µM) | [55] |
| 54 (Stigmasterol) | Inhibition of proliferation and colony formation of gastric cancer SNU-1 cells ******* (IC50 = 15 µM) 30 μM increased the percentage of apoptotic cells in gastric cancer SNU-1 cells from 1.75 to 43.66% | [94] |
| 55 (β-Sitosterol) | Inhibition of the proliferation of HepG2 ******** (IC50 = 0.017 μM) and Huh7 ********* cells (IC50 = 0.021μM) | [96] |
| 56 (Pescaproside A) 57 (Pescaprein I) 58 (Pescaprein Ⅱ) 59 (Pescaprein III) 60 (Pescaprein IV) 61 (Stoloniferin III) | Weak cytotoxicity against nasopharyngeal, colon, squamous cell cervical, and ovarian carcinomas (ED50 ********** = 5–20 µg/mL) | [3] |
| 93 | 45.2% inhibition of the growth of human lung cancer cell A549 at 0.275 µM | [100] |
6.8. Multidrug-Resistance Efflux-Inhibiting Activity
| Compound | Pharmacological Activities | Reference |
|---|---|---|
| 58 (Pescaprein II) 59 (Pescaprein III) 61 (Stoloniferin III) 76 (Pescaprein XVIII) 77 (Pescaprein ⅩⅨ) 78 (Pescaprein XX) 79 (Stoloniferin IX) 80 (Stoloniferin X) 81 (Murucoidin VI) | Multidrug-resistance inhibition against Staphylococcus aureus SA-1199. A total of 25 μg/mL of each compound potentiated the norfloxacin effect by 4-fold (MIC * from 32 μg/mL to 8 μg/mL) | [39] |
| 82 (Pescaprein XXI) 83 (Pescaprein XXII) 84 (Pescaprein XXIII) 85 (Pescaprein XXIV) 86 (Pescaprein XXV) 87 (Pescaprein XXVI) 88 (Pescaprein XXVII) 89 (Pescaprein XXVIII) 90 (Pescaprein XXIX) 91 (Pescaprein XXX) | Multidrug-resistance inhibitory effect against MCF-7/ADR ** cells. A total of 5 μg/mL of each compound potentiated the doxorubicin effect by 1.5–3.7-fold, producing IC50 *** values of 1.76, 3.98, 2.00, 3.20, 2.83, 1.58, 3.12, 2.57, 1.82, and 2.60 μg/mL for compounds 82–91, respectively | [40] |
6.9. Miscellaneous Uses
| Compound | Pharmacological Activities | Reference |
|---|---|---|
| 1 (Calystegine B2) | Potent inhibitory activity toward rat lysosomal β-glucosidase (IC50 * = 0.75 µM) | [105] |
| 5 | Inhibitory activity against ACE ** (IC50 = 180 μM) | [102] |
| 6 (Isoquercetin) | Inhibitory activity against ACE (IC50 = 71 μM) | |
| 10 (Quercetin) | Inhibitory activity against ACE (IC50 = 151 μM) 25 μM concentration inhibited TNF-α ***, IL-6 ****, and IL-12 ***** production at 60%, 55%, and 70%, respectively | [102,103] |
| 93 | Anti-angiogenic activities (IC50 = 0.083 μM) | [100] |
7. Materials and Methods
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Devall, M.S. The Biological Flora of Coastal Dunes and Wetlands. 2. Ipomoea pes-caprae (L.) Roth. J. Coast. Res. 1992, 8, 442–456. [Google Scholar]
- Manigaunha, A.; Ganesh, N.; Kharya, M.D. Morning Glory: A New Thirst In-Search of De-Novo Therapeutic Approach. Int. J. Phytomed. 2010, 2, 18–21. [Google Scholar] [CrossRef]
- Pereda-Miranda, R.; Escalante-Sánchez, E.; Escobedo-Martínez, C. Characterization of Lipophilic Pentasaccharides from Beach Morning Glory (Ipomoea pes-caprae). J. Nat. Prod. 2005, 68, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Emendörfer, F.; Emendörfer, F.; Bellato, F.; Noldin, V.F.; Niero, R.; Cechinel-Filho, V.; Cardozo, A.M. Evaluation of the Relaxant Action of Some Brazilian Medicinal Plants in Isolated Guinea-Pig Ileum and Rat Duodenum. J. Pharm. Pharmaceut. Sci. 2005, 8, 63–68. [Google Scholar]
- Pothula, V.V.S.; Kanikaram, S. In Vitro Antiplasmodial Efficacy of Mangrove Plant, Ipomoea pes-caprae against Plasmodium falciparum (3D7 Strain). Asian Pac. J. Trop. Dis. 2015, 5, 947–956. [Google Scholar] [CrossRef]
- Brown, S.H.; Frank, M.S. Railroad Vine (Ipomoea pes-caprae): Identification and Uses. Edis 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Nilam, R.; Jyoti, P.; Sumitra, C. Pharmacognostic and Phytochemical Studies of Ipomoea pes-caprae, an Halophyte from Gujarat. J. Pharmacogn. Phytochem. 2018, 7, 11–18. [Google Scholar]
- Miryeganeh, M.; Takayama, K.; Tateishi, Y.; Kajita, T. Long-Distance Dispersal by Sea-Drifted Seeds Has Maintained the Global Distribution of Ipomoea pes-caprae Subsp. Brasiliensis (Convolvulaceae). PLoS ONE 2014, 9, e91836. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, P.Y.; Liu, N.; Zhang, W.W.; Wang, J.; Jian, S.G. Biological and Ecophysiological Characteristics of a Beach Plant Ipomoea pes-caprae. J. Hunan Univ. Sci. Technol. (Nat. Sci. Ed.) 2011, 26, 117–121. [Google Scholar]
- Okui, T.; Nohara, S.; Furukawa, A. The Role of Adventitious Roots in Supplying Water to Ipomoea pes-caprae. Tropics 2003, 12, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Kamakura, M.; Furukawa, A. Compensatory Function for Water Transport by Adventitious Roots of Ipomoea pes-caprae. J. Plant Res. 2009, 122, 327–333. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Zheng, J.X.; Mo, H.; Xia, K.F.; Jian, S.G. Functional Identification of Salt-Stress-Related Genes Using the Fox Hunting System from Ipomoea pes-caprae. Int. J. Mol. Sci. 2018, 19, 3446. [Google Scholar] [CrossRef] [Green Version]
- Suarez, N. Comparative Leaf Anatomy and Pressure-Volume Analysis in Plants of Ipomoea pes-caprae Experimenting Saline and/or Drought Stress. Int. J. Bot. 2011, 7, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Ipomoea pes-caprae (L.) R. Br. Available online: https://www.gbif.org/species/7999386 (accessed on 27 December 2021).
- Devall, M.S.; Thien, L.B. Inland Occurrence of the Strand Plant Ipomoea pes-caprae (Convolvulaceae) around Lake Nicaragua. Southwest. Nat. 2005, 50, 380–384. [Google Scholar] [CrossRef]
- St. John, H. Classification and Distribution of the Ipomoea pes-caprae Group (Convolvulaceae). Bot. Jahrebucher 1970, 89, 563–583. [Google Scholar]
- Ridley, H.N. The Dispersal and Plants throughout the World; Reeve: Ashford, UK, 1930. [Google Scholar]
- Pongprayoon, U.; Bohlin, L.; Sandberg, F. Inhibitory Effect of Extract of Ipomoea pes-caprae on Guinea-Pig Ileal Smooth Muscle. Acta Pharm. Nord. 1989, 1, 41–44. [Google Scholar]
- Chan, E.W.C.; Baba, S.; Chan, H.T.; Kainuma, M.; Tangah, J. Medicinal Plants of Sandy Shores: A Short Review on Vitex trifolia L. and Ipomoea pes-caprae (L.) R. Br. Indian J. Nat. Prod. Resour. 2016, 7, 107–115. [Google Scholar]
- Marie, D.E.P.; Dejan, B.; Quetin-Leclercq, J. GC-MS Analysis of the Leaf Essential Oil of Ipomoea pes-caprae, a Traditional Herbal Medicine in Mauritius. Nat. Prod. Commun. 2007, 2, 1225–1228. [Google Scholar] [CrossRef] [Green Version]
- Iwu, M.M.; Anyanwu, B.N. Phytotherapeutic Profile of Nigerian Herbs. I: Anti-Inflammatory and Anti-Arthritic Agents. J. Ethnopharmacol. 1982, 6, 263–274. [Google Scholar] [CrossRef]
- Teerakulkittipong, N.; Phosri, S.; Chetkhetkran, M. Effect of Extraction Methods on Yield, Total Phenolic Content and Antioxidant Activity of Ipomoea pes-caprae (L.) R. Br. Leaves. Proc. RSU Int. Res. Conf. 2020, 701–708. [Google Scholar]
- Zhao, K.; Feng, L. Resource of Halophytic Vegetation in China; China Science Press: Beijing, China, 2001. [Google Scholar]
- Krogh, R.; Kroth, R.; Berti, C.; Madeira, A.O.; Souza, M.M.; Cechinel-Filho, V.; Delle-Monache, F.; Yunes, R.A. Isolation and Identification of Compounds with Antinociceptive Action from Ipomoea pes-caprae (L.) R. Br. Pharmazie 1999, 54, 464–466. [Google Scholar] [PubMed]
- Salguero, C.P. A Compendium of Traditional Thai Herbal Medicine. In A Thai Herbal: Traditional Recipes for Health and Harmony; Findhorn Press: Forress, UK, 2003; pp. 125–246. [Google Scholar]
- Lorenzi, H.; Matos, F.J.A. Plantas Medicinais No Brasil: Nativas e Exóticas. In Instituto Plantarum Nova Odessa; Plantarum Institute for Flora Studies: Nova Odessa, Brazil, 2002; p. 182. [Google Scholar]
- Vivek, P.; Jayakumari, D.; Jayasree, P. Hypoglycaemic Effect of Vriddhadaru [Argyreia nervosa (Burm. F.) Boj.] in Alloxan Induced Diabetic Rabbits. Int. J. Adv. Ayurveda Yoga Unani Siddha Homeopath. 2016, 5, 322–329. [Google Scholar]
- Manigauha, A.; Kharya, M.D.; Ganesh, N. In Vivo Antitumor Potential of Ipomoea pes-caprae on Melanoma Cancer. Pharmacogn. Mag. 2015, 11, 426–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schimming, T.; Jenett-Siems, K.; Mann, P.; Tofern-Reblin, B.; Milson, J.; Johnson, R.W.; Deroin, T.; Austin, D.F.; Eich, E. Calystegines as Chemotaxonomic Markers in the Convolvulaceae. Phytochemistry 2005, 66, 469–480. [Google Scholar] [CrossRef]
- Asano, N.; Yokoyama, K.; Sakurai, M.; Ikeda, K.; Kizu, H.; Kato, A.; Arisawa, M.; Höke, D.; Dräger, B.; Watson, A.A.; et al. Dihydroxynortropane Alkaloids from Calystegine-Producing Plants. Phytochemistry 2001, 57, 721–726. [Google Scholar] [CrossRef]
- Pongprayoon, U.; Bohlin, L.; Baeckstrom, P.; Jacobson, U.; Lindstrom, M. Inhibition of Ethyl Phenylpropiolate-Induced Rat Ear Oedema by Compounds Isolated from Ipomoea pes-caprae (L.) R. Br. Phytother. Res. 1992, 6, 104–107. [Google Scholar] [CrossRef]
- Alagesan, V.; Ramalingam, S.; Kim, M.; Venugopal, S. Antioxidant Activity Guided Isolation of a Coumarin Compound from Ipomoea pes-caprae (Convolvulaceae) Leaves Acetone Extract and Its Biological and Molecular Docking Studies. Eur. J. Integr. Med. 2019, 32, 100984. [Google Scholar] [CrossRef]
- Gonçalves, F.M.B.; Ramos, A.C.; da Silva Mathias, M.; de Souza Sales, Q.; Ramos, C.C.; Antunes, F.; de Oliveira, R.R. Phytochemical Analysis and Hypotensive Activity of Ipomoea pes-caprae on Blood Pressure of Normotensive Rats. Rodriguesia 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Pongprayoon, U.; Baeckström, P.; Jacobsson, U.; Lindström, M.; Bohlin, L. Compounds Inhibiting Prostaglandin Synthesis Isolated from Ipomoea pes-caprae. Planta Med. 1991, 57, 515–518. [Google Scholar] [CrossRef]
- Teramachi, F.; Koyano, T.; Kowithayakorn, T.; Hayashi, M.; Komiyama, K.; Ishibashi, M. Collagenase Inhibitory Quinic Acid Esters from Ipomoea pes-caprae. J. Nat. Prod. 2005, 68, 794–796. [Google Scholar] [CrossRef]
- Pongprayoon, U.; Baeckstrom, P.; Jacobsson, U.; Lindstrom, M.; Bohlin, L. Antispasmodic Activity of β-Damascenone and E-Phytol Isolated from Ipomoea pes-caprae. Planta Med. 1992, 58, 19–21. [Google Scholar] [CrossRef]
- Escobedo-Martínez, C.; Pereda-Miranda, R. Resin Glycosides from Ipomoea pes-caprae. J. Nat. Prod. 2007, 70, 974–978. [Google Scholar] [CrossRef]
- Tao, H.; Hao, X.; Liu, J.; Ding, J.; Fang, Y.; Gu, Q.; Zhu, W. Resin Glycoside Constituents of Ipomoea pes-caprae (Beach Morning Glory). J. Nat. Prod. 2008, 71, 1998–2003. [Google Scholar] [CrossRef]
- Escobedo-Martínez, C.; Cruz-Morales, S.; Fragoso-Serrano, M.; Mukhlesur Rahman, M.; Gibbons, S.; Pereda-Miranda, R. Characterization of a Xylose Containing Oligosaccharide, an Inhibitor of Multidrug Resistance in Staphylococcus aureus, from Ipomoea pes-aprae. Phytochemistry 2010, 71, 1796–1801. [Google Scholar] [CrossRef]
- Yu, B.W.; Luo, J.G.; Wang, J.S.; Zhang, D.M.; Yu, S.S.; Kong, L.Y. Pentasaccharide Resin Glycosides from Ipomoea pes-caprae. J. Nat. Prod. 2011, 74, 620–628. [Google Scholar] [CrossRef]
- Qasim, M.; Abideen, Z.; Adnan, M.Y.; Gulzar, S.; Gul, B.; Rasheed, M.; Khan, M.A. Antioxidant Properties, Phenolic Composition, Bioactive Compounds and Nutritive Value of Medicinal Halophytes Commonly Used as Herbal Teas. S. Afr. J. Bot. 2017, 110, 240–250. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Majewska, M.; Skrzycki, M.; Podsiad, M.; Czeczot, H. Evaluation of Antioxidant Potential of Flavonoids: An In Vitro Study. Acta Pol. Pharm.-Drug Res. 2011, 68, 611–615. [Google Scholar]
- Sunil, C.; Irudayaraj, S.S.; Duraipandiyan, V.; Al-Dhabi, N.A.; Agastian, P.; Ignacimuthu, S. Antioxidant and Free Radical Scavenging Effects of β-Amyrin Isolated from S. cochinchinensis Moore. Leaves. Ind. Crops Prod. 2014, 61, 510–516. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ullah, F.; Subhan, F.; Sadiq, A.; Ali, G.; Ovais, M.; Shahid, M.; Ahmad, A.; Wadood, A.; et al. Anti-Alzheimer’s Studies on β-Sitosterol Isolated from Polygonum hydropiper L. Front. Pharmacol. 2017, 8, 697. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, J.Z.; Oliveira, C.; Incerpi, S.; Kumar, V.; Fiore, A.M.; de Vito, P.; Prasad, A.K.; Malhotra, S.; Parmar, V.S.; Saso, L. Antioxidant Activity of 4-Methylcoumarins. J. Pharm. Pharmacol. 2010, 59, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kim, J.K.; Kim, J.; Jung, S.H.; Lee, K. Characterization of Caffeoylquinic Acids from Lepisorus Thunbergianus and Their Melanogenesis Inhibitory Activity. ACS Omega 2020, 5, 30946–30955. [Google Scholar] [CrossRef]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic Acid: A Review of Its Potential Use in Medications and Cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, L.; Wang, X.; Lan, R.; Wang, M.; Du, G.; Guan, W.; Liu, J.; Brennan, M.; Guo, H.; et al. Antioxidant Activity Evaluation of Dietary Flavonoid Hyperoside Using Saccharomyces cerevisiae as a Model. Molecules 2019, 24, 788. [Google Scholar] [CrossRef] [Green Version]
- Razavi, S.M.; Zahri, S.; Zarrini, G.; Nazemiyeh, H.; Mohammadi, S. Biological Activity of Quercetin-3-O-Glucoside, a Known Plant Flavonoid. Russ. J. Bioorg. Chem. 2009, 35, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.M.; Chuang, D.Y.; Wang, S.Y.; Kuo, Y.H.; Tsai, P.W.; Shyur, L.F. Metabolite Profiling and Chemopreventive Bioactivity of Plant Extracts from Bidens pilosa. J. Ethnopharmacol. 2004, 95, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Rivero-cruz, J.F.; Granados-pineda, J.; Pedraza-chaverri, J.; Rivero-cruz, B.E. Phytochemical Constituents, Antioxidant, Cytotoxic, and Antimicrobial Activities of the Ethanolic Extract of Mexican Brown Propolis. Antioxidants 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randjelović, P.; Veljković, S.; Stojiljković, N.; Sokolović, D.; Ilić, I.; Laketić, D.; Randjelović, D.; Randjelović, N. The Beneficial Biological Properties of Salicylic Acid. Acta Fac. Med. Naissensis 2015, 32, 259–265. [Google Scholar] [CrossRef]
- Li, Z.H.; Cai, M.; Liu, Y.S.; Sun, P.L.; Luo, S.L. Antibacterial Activity and Mechanisms of Essential Oil from Citrus medica L. Var. Sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef] [Green Version]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Türkez, H.; Çelik, K.; Toğar, B. Effects of Copaene, a Tricyclic Sesquiterpene, on Human Lymphocytes Cells In Vitro. Cytotechnology 2014, 66, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a Natural Monoterpene: A Review of Its Biological Properties. Open Chem. 2018, 1, 91–102. [Google Scholar] [CrossRef]
- Tepe, B.; Akpulat, H.A.; Sokmen, M. Evaluation of the Chemical Composition and Antioxidant Activity of the Essential Oils of Peucedanum longifolium (Waldst. & Kit.) and P. palimbioides (Boiss.). Rec. Nat. Prod. 2011, 5, 108–116. [Google Scholar]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal Plants with Anti-Inflammatory Activities. Nat. Prod. Res. 2016, 30, 1343–1352. [Google Scholar] [CrossRef]
- Pongprayoon, U.; Bohlin, L.; Soonthornsaratune, P.; Wasuwat, S. Antiinflammatory Activity of Ipomoea pes-caprae (L.) R. Br. Phytother. Res. 1991, 5, 63–66. [Google Scholar] [CrossRef]
- Davis, D.L.; Stevens, K.L.; Jurd, L. Chemistry of Tobacco Constituents. Oxidation of α-Ionone and the Acid-Catalyzed Rearrangement of 5-Keto-α-Ionone. J. Agric. Food Chem. 1976, 24, 187–189. [Google Scholar] [CrossRef]
- Magalhães, C.B.; Riva, D.R.; Depaula, L.J.; Brando-Lima, A.; Koatz, V.L.G.; Leal-Cardoso, J.H.; Zin, W.A.; Faffe, D.S. In Vivo Anti-Inflammatory Action of Eugenol on Lipopolysaccharide-Induced Lung Injury. J. Appl. Physiol. 2010, 108, 845–851. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Ruangnoo, S.; Jaiaree, N.; Makchuchit, S.; Panthong, S.; Thongdeeying, P.; Itharat, A. An In Vitro Inhibitory Effect on RAW 264.7 Cells by Antiinflammatory Compounds from Smilax corbularia Kunth. Asian Pac. J. Allergy Immunol. 2012, 30, 268–274. [Google Scholar]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and Anti-Inflammatory Activity of Caryophyllene Oxide from Annona squamosa L. Bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Ghavam, M.; Manca, M.L.; Manconi, M.; Bacchetta, G. Chemical Composition and Antimicrobial Activity of Essential Oils Obtained from Leaves and Flowers of Salvia hydrangea DC. Ex Benth. Sci. Rep. 2020, 10, 15647. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.B.; Bhalla, T.N.; Tangri, K.K.; Bhargava, K.P. Biochemical Study of the Anti-Inflammatory Activity of α and β-Amyrin Acetate. Biochem. Pharmacol. 1971, 20, 401–405. [Google Scholar] [CrossRef]
- Safayhi, H.; Sailer, E.R. Anti-Inflammatory Actions of Pentacyclic Triterpenes. Planta Med. 1997, 63, 487–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velázquez-González, C.; Cariño-Cortés, R.; Gayosso de Lucio, J.A.; Ortiz, M.I.; de la O Arciniega, M.; Altamirano-Báez, D.A.; Ángeles, L.J.; Bautista-Ávila, M. Antinociceptive and Anti-Inflammatory Activities of Geranium bellum and Its Isolated Compounds. BMC Complement. Altern. Med. 2014, 14, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Maria De Souza, M.; Madeira, A.; Berti, C.; Krogh, R.; Yunes, R.A.; Cechinel-Filho, V. Antinociceptive Properties of the Methanolic Extract Obtained from Ipomoea pes-caprae (L.) R. Br. J. Ethnopharmacol. 2000, 69, 85–90. [Google Scholar] [CrossRef]
- Otuki, M.F.; Ferreira, J.; Lima, F.; Meyre-Silva, C.; Malheiros, Â.; Muller, L.A.; Cani, G.S.; Santos, A.R.S.; Yunes, R.A.; Calixto, J.B. Antinociceptive Properties of Mixture of α-Amyrin and β-Amyrin Triterpenes: Evidence for Participation of Protein Kinase C and Protein Kinase A Pathways. J. Pharmacol. Exp. Ther. 2005, 313, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari Moghaddam, M.; Ahmad, F.B.H.; Samzadeh-Kermani, A. Biological Activity of Betulinic Acid: A Review. Pharmacol. Amp. Pharm. 2012, 3, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Quintans-Júnior, L.; Moreira, J.C.F.; Pasquali, M.A.B.; Rabie, S.M.S.; Pires, A.S.; Schröder, R.; Rabelo, T.K.; Santos, J.P.A.; Lima, P.S.S.; Cavalcanti, S.C.H.; et al. Antinociceptive Activity and Redox Profile of the Monoterpenes (+)-Camphene, p -Cymene, and Geranyl Acetate in Experimental Models. ISRN Toxicol. 2013, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nagababu, P.; Umamaheswara Rao, V. Pharmacological Potential of Ipomea pes-caprae (L.) R. Br. Whole Plant Extracts. Pelagia Res. Libr. Pharm. Sin. 2015, 6, 52–60. [Google Scholar]
- Ghaneian, M.T.; Ehrampoush, M.H.; Jebali, A.; Hekmatimoghaddam, S.; Mahmoudi, M. Antimicrobial Activity, Toxicity and Stability of Phytol as a Novel Surface Disinfectant. Environ. Health Eng. Manag. J. 2015, 2, 13–16. [Google Scholar]
- Sen, A.; Dhavan, P.; Shukla, K.K.; Singh, S.; Tejovathi, G. Analysis of IR, NMR and Antimicrobial Activity of β-Sitosterol Isolated from Momordica charantia. Sci. Secur. J. Biotech. 2012, 1, 9–13. [Google Scholar]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef] [PubMed]
- Kȩpa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wasik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus Clinical Strains. BioMed Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyama, D.O.; Ferreira, M.J.P.; Romoff, P.; Fávero, O.A.; Gaeta, H.H.; Toyama, M.H. Effect of Chlorogenic Acid (5-Caffeoylquinic Acid) Isolated from Baccharis oxyodonta on the Structure and Pharmacological Activities of Secretory Phospholipase A2 from Crotalus durissus terrificus. BioMed Res. Int. 2014, 2014, 726585. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Huang, H.; Yang, T.; Ye, Y.; Shan, J.; Yin, Z.; Luo, L. Chlorogenic Acid Protects Mice against Lipopolysaccharide-Induced Acute Lung Injury. Injury 2010, 41, 746–752. [Google Scholar] [CrossRef]
- Cheng, S.S.; Wu, C.L.; Chang, H.T.; Kao, Y.T.; Chang, S.T. Antitermitic and Antifungal Activities of Essential Oil of Calocedrus formosana Leaf and Its Composition. J. Chem. Ecol. 2004, 30, 1957–1967. [Google Scholar] [CrossRef]
- Garcia, M.C.F.; Soares, D.C.; Santana, R.C.; Saraiva, E.M.; Siani, A.C.; Ramos, M.F.S.; Danelli, M.D.G.M.; Souto-Padron, T.C.; Pinto-Da-Silva, L.H. The In Vitro Antileishmanial Activity of Essential Oil from Aloysia gratissima and Guaiol, Its Major Sesquiterpene against Leishmania Amazonensis. Parasitology 2018, 145, 1219–1227. [Google Scholar] [CrossRef]
- Mbunde, M.V.N.; Innocent, E.; Mabiki, F.; Andersson, P.G. In Vitro Study for Antifungal Compounds from Parinari curatellifolia (Chrysobalanaceae) and Terminalia sericea (Combretaceae). Int. J. Biol. Chem. Sci. 2021, 15, 367–378. [Google Scholar] [CrossRef]
- Sieniawska, E.; Swatko-Ossor, M.; Sawicki, R.; Skalicka-Woźniak, K.; Ginalska, G. Natural Terpenes Influence the Activity of Antibiotics against Isolated Mycobacterium tuberculosis. Med. Princ. Pract. 2017, 26, 108–112. [Google Scholar] [CrossRef]
- Pinheiro, T.R.; Yunes, R.A.; López, S.N.; Santecchia, C.B.; Zacchino, S.A.S.; Cechinel Filho, V. In Vitro Antifungal Evaluation and Studies on the Mode of Action of Xanthoxyline Derivatives. Arzneim.-Forsch./Drug Res. 1999, 49, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, A.B.; Salwinski, A.; Simonsen, H.T. Collagenase and Tyrosinase Inhibitory Effect of Isolated Constituents from the Moss Polytrichum formosum. Plants 2021, 10, 1271. [Google Scholar] [CrossRef]
- Zoofishan, Z.; Kúsz, N.; Csorba, A.; Tóth, G.; Hajagos-Tóth, J.; Kothencz, A.; Gáspár, R.; Hunyadi, A. Antispasmodic Activity of Prenylated Phenolic Compounds from the Root Bark of Morus nigra. Molecules 2019, 24, 2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meira, M.; Pereira Da Silva, E.; David, J.M.; David, J.P. Review of the Genus Ipomoea: Traditional Uses, Chemistry and Biological Activities. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2012, 22, 682–713. [Google Scholar] [CrossRef] [Green Version]
- Cechinel Filho, V.; Gomes Miguel, O.; José Nunes, R.; Batista Calixto, J.; Augusto Yunes, R. Antispasmodic Activity of Xanthoxyline Derivatives: Structure-Activity Relationships. J. Pharm. Sci. 1995, 84, 473–475. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer Activity of Natural Compounds from Plant and Marine Environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.B.; Liang, N.C. Studies on the Inhibitory Effects of Quercetin on the HL-60 Leukemia Cells. Biochem. Pharmacol. 1997, 54, 1013–1018. [Google Scholar] [CrossRef]
- Chou, C.C.; Yang, J.S.; Lu, H.F.; Ip, S.W.; Lo, C.; Wu, C.C.; Lin, J.P.; Tang, N.Y.; Chung, J.G.; Chou, M.J.; et al. Quercetin-Mediated Cell Cycle Arrest and Apoptosis Involving Activation of a Caspase Cascade through the Mitochondrial Pathway in Human Breast Cancer MCF-7 Cells. Arch. Pharm. Res. 2010, 33, 1181–1191. [Google Scholar] [CrossRef]
- Li, K.; Yuan, D.; Yan, R.; Meng, L.; Zhang, Y.; Zhu, K. Stigmasterol Exhibits Potent Antitumor Effects in Human Gastric Cancer Cells Mediated via Inhibition of Cell Migration, Cell Cycle Arrest, Mitochondrial Mediated Apoptosis and Inhibition of JAK/STAT Signalling Pathway. J. BUON 2018, 23, 1420–1425. [Google Scholar]
- Dinku, W.; Isaksson, J.; Rylandsholm, F.G.; Bouř, P.; Brichtová, E.; Choi, S.U.; Lee, S.H.; Jung, Y.S.; No, Z.S.; Svendsen, J.S.M.; et al. Anti-Proliferative Activity of a Novel Tricyclic Triterpenoid Acid from Commiphora africana Resin against Four Human Cancer Cell Lines. Appl. Biol. Chem. 2020, 63, 16. [Google Scholar] [CrossRef]
- Vo, T.K.; Ta, Q.T.H.; Chu, Q.T.; Nguyen, T.T.; Vo, V.G. Anti-Hepatocellular-Cancer Activity Exerted by β-Sitosterol and β-Sitosterol-Glucoside from Indigofera zollingeriana Miq. Molecules 2020, 25, 3021. [Google Scholar] [CrossRef]
- Chan, W.K.; Tan, L.T.H.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Chen, H.; Chen, H.; Zhong, B.; Luo, X.; Chun, J. Antioxidant and Anticancer Activities of Essential Oil from Gannan Navel Orange Peel. Molecules 2017, 22, 1391. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.; Chen, Y. Betulinic Acid and the Pharmacological Effects of Tumor Suppression (Review). Mol. Med. Rep. 2016, 14, 4489–4495. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; He, Q.; Yang, Y.; Liu, K.; Li, X. Chemical Constituents of Euphorbia tibetica and Their Biological Activities. Chin. J. Nat. Med. 2014, 12, 38–42. [Google Scholar] [CrossRef]
- Lira-Ricárdez, J.; Pereda-Miranda, R. Reversal of Multidrug Resistance by Amphiphilic Morning Glory Resin Glycosides in Bacterial Pathogens and Human Cancer Cells. Phytochem. Rev. 2020, 19, 1211–1229. [Google Scholar] [CrossRef]
- Balasuriya, N.; Rupasinghe, H.P.V. Antihypertensive Properties of Flavonoid-Rich Apple Peel Extract. Food Chem. 2012, 135, 2320–2325. [Google Scholar] [CrossRef]
- Ho, S.T.; Tung, Y.T.; Wu, Y.J.; Lin, C.C.; Wu, J.H. Immune-Regulatory Activity of Methanolic Extract of Acacia confusa Heartwood and Melanoxetin Isolated from the Extract. Holzforschung 2015, 69, 645–652. [Google Scholar] [CrossRef]
- Borges de Melo, E.; da Silveira Gomes, A.; Carvalho, I. α- and β-Glucosidase Inhibitors: Chemical Structure and Biological Activity. Tetrahedron 2006, 62, 10277–10302. [Google Scholar] [CrossRef]
- Haraguchi, M.; Gorniak, S.L.; Ikeda, K.; Minami, Y.; Kato, A.; Watson, A.A.; Nash, R.J.; Molyneux, R.J.; Asano, N. Alkaloidal Components in the Poisonous Plant, Ipomoea carnea (Convolvulaceae). J. Agric. Food Chem. 2003, 51, 4995–5000. [Google Scholar] [CrossRef]
- Pereda-Miranda, R.; Bah, M. Biodynamic Constituents in the Mexican Morning Glories: Purgative Remedies Transcending Boundaries. Curr. Top. Med. Chem. 2003, 3, 111–131. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinniyi, G.; Lee, J.; Kim, H.; Lee, J.-G.; Yang, I. A Medicinal Halophyte Ipomoea pes-caprae (Linn.) R. Br.: A Review of Its Botany, Traditional Uses, Phytochemistry, and Bioactivity. Mar. Drugs 2022, 20, 329. https://doi.org/10.3390/md20050329
Akinniyi G, Lee J, Kim H, Lee J-G, Yang I. A Medicinal Halophyte Ipomoea pes-caprae (Linn.) R. Br.: A Review of Its Botany, Traditional Uses, Phytochemistry, and Bioactivity. Marine Drugs. 2022; 20(5):329. https://doi.org/10.3390/md20050329
Chicago/Turabian StyleAkinniyi, Ganiyu, Jeonghee Lee, Hiyoung Kim, Joon-Goo Lee, and Inho Yang. 2022. "A Medicinal Halophyte Ipomoea pes-caprae (Linn.) R. Br.: A Review of Its Botany, Traditional Uses, Phytochemistry, and Bioactivity" Marine Drugs 20, no. 5: 329. https://doi.org/10.3390/md20050329
APA StyleAkinniyi, G., Lee, J., Kim, H., Lee, J.-G., & Yang, I. (2022). A Medicinal Halophyte Ipomoea pes-caprae (Linn.) R. Br.: A Review of Its Botany, Traditional Uses, Phytochemistry, and Bioactivity. Marine Drugs, 20(5), 329. https://doi.org/10.3390/md20050329