Abstract
In this paper, eight new galaxamide analogues (Z-1~Z-8) were synthesized and evaluated for their cytotoxic activities against five cancer cell lines, MCF-7, MD-MBA-231, HepG2, Hela, and A549, using MTT assays. The modified analogue Z-6 displayed broad spectrum cytotoxic activity toward each tested cell line with IC50 values of 1.65 ± 0.30 (MCF-7), 2.91 ± 0.17 (HepG2), 4.59 ± 0.27 (MD-MBA-231), 5.69 ± 0.37 (Hela), and 5.96 ± 0.41 (A549) μg/mL, respectively. The galaxamides Z-3 and Z-6 induced concentration-dependent apoptosis of the MCF-7 cells after 72 h as evaluated by the flow cytometry experiment. The results showed that these compounds could induce MCF-7 cell apoptosis by arresting the G0/G1 phase of the cell cycle and finally achieving the effect of inhibiting the proliferation of MCF-7 cells.
1. Introduction
Cyclopeptides mainly isolated from various marine species, which exhibited antitumor, [1,2,3] antiviral, [4] anti-inflammatory [5,6] and antibacterial [7,8,9] activities. Cyclopeptides usually exhibited superior biological activities due to their conformational rigidity and resistance to proteolytic degradation. [10] Therefore, cyclopeptides had attracted great attention and become one of the important components in the development of antitumor drugs. [11] To enhance the antitumor activity of cyclopeptides, they were always modified by changing the configurations of amino acid residues. In addition, the fragment of proline was widely existing in natural cyclopeptides, [12] which might be the active group for antitumor and antibacterial activities.
In our group, previous phytochemical investigation on the alga of Galaxaura filamentosa had led to the isolation of a novel cyclic pentapeptide, galaxamide (Figure 1). It was composed of five L-leucines including two N-methylated ones, which showed significant antitumor activities against GRC-1 and HepG2 cell lines. [13] In the preliminary work, the galaxamide analogues had been synthesized by replacing one of the leucines with penylalanine and changing the configuration of amino acids, which also showed potential antitumor activities. [14,15,16]
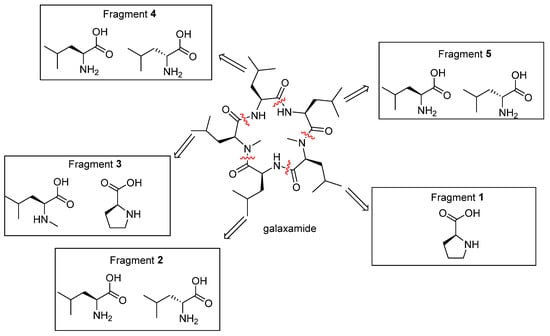
Figure 1.
Design in the series of galaxamide analogues.
In this paper, eight new galaxamide analogues (Z-1~Z-8) were synthesized by replacing one or two N-Me-L-leucines in the galaxamide with L-proline or changing the stereo configuration of leucine. In vitro antitumor activities of these new compounds against MCF-7, HepG2, Hela, MD-MBA-231, A549, and HUVEC cell lines were also evaluated by MTT assay. On the one hand, a great majority of the synthesized compounds showed strong antitumor activities. On the other hand, they showed weak inhibitory effects on the non-neoplastic cells. Particularly, the IC50 values of Z-3 and Z-6 on all of the tested cancer cell lines were smaller than those of galaxamide. Furthermore, we described the effects as well as the antitumor mechanisms of Z-3, Z-6, and galaxamide as they induced apoptosis of MCF-7 cells.
2. Results and Discussion
2.1. Chemistry
On the basis of galaxamide, eight new galaxamide derivatives were designed and synthesized by replacing one or two N-Me-L-leucine with L-proline or changing the stereo configuration of leucine from L to D (Figure 1 and Figure 2).
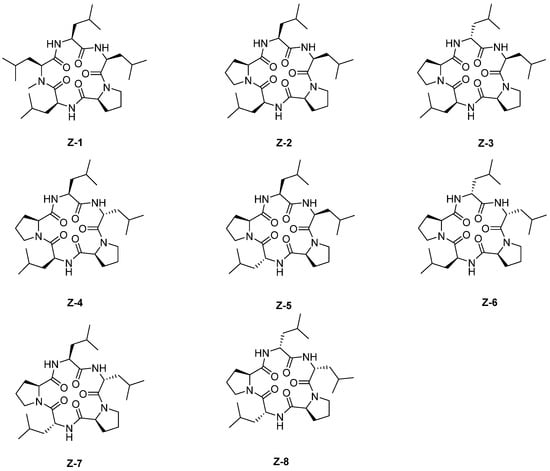
Figure 2.
The structures of Z-1~Z-8.
First, the linear pentapeptide was synthesized by using Fmoc peptide synthetic strategy with 2-chloro trityl resin. [17] Furthermore, the cyclic peptide was compounded by liquid-phase cyclization. The cyclization site of the linear pentapeptide was selected between two consecutive leucines. The detailed schematic illustration of the synthetic route was shown in Scheme 1 (taking Z-1 as an example). Among them, Fmoc-N-Me-Leu-OH was synthesized by the oxazolidinone intermediate method (Scheme 2). [18]
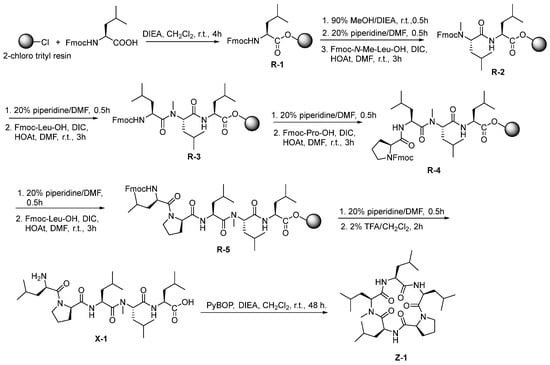
Scheme 1.
Synthesis scheme of Z-1.
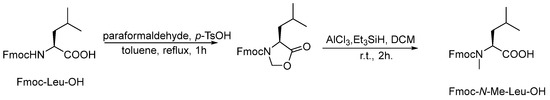
Scheme 2.
Synthesis scheme of Fmoc-N-Me-Leu-OH.
2.2. Biological Activity
2.2.1. Inhibitory Activities of Galaxamide and Its Analogues
The cytotoxicity of the synthesized compounds against five different human cancer cell lines (MCF-7, MD-MBA-231, HepG2, Hela and A459) and one type of normal human cell line (HUVEC) was investigated by MTT assay, the IC50 values of which are shown in Table 1. The results indicated that Z-3 and Z-6 exhibited stronger cytotoxic activities against each cell line than galaxamide, but that they showed weaker inhibitory effects on HUVEC. Particularly, Z-6 had the strongest inhibition rate.

Table 1.
The inhibitory effects of galaxamide and its analogues against several cell lines.
2.2.2. Effects of Analogues on the Cell Cycle of MCF-7
Compounds Z-3 and Z-6 exhibited better inhibition effects on the growth of all cancer cell lines than the others, especially on that of MCF-7. Therefore, propidium iodide (PI) staining method was used to further investigate the changes in the cell cycle distribution of MCF-7. In particular, MCF-7 cells were exposed to galaxamide, Z-3, and Z-6 with concentrations of 0, 2.5, 5, and 10 µg/mL for 72 h. Generally, the main effects of galaxamide, Z-3, and Z-6 on MCF-7 cells were in the G0/G1 phase. There was no obvious sub diploid peak in flow cytometry. However, as the concentration increased, the number of cells in the G0/G1 phase also gradually increased. Hence, the effects of galaxamide, Z-3, and Z-6 on MCF-7 cells mainly blocked the growth of cells in the G0/G1 phase (Figure 3).
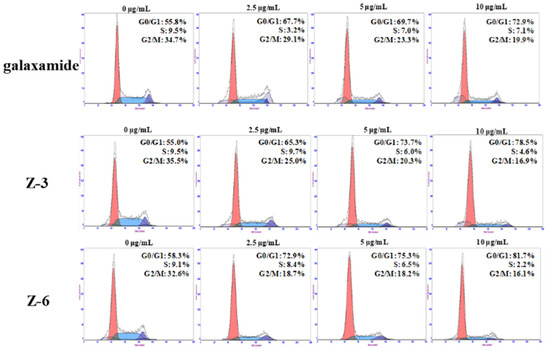
Figure 3.
Cell cycle phase in MCF-7 induced by galaxamide, Z-3, and Z-6 when exposed for 72 h.
2.2.3. Apoptotic Mechanism Study of Galaxamide and Its Analogues
The Annexin V-FITC/PI double staining method was used to analyze the changes in the apoptosis of MCF-7 cells after 72 h of galaxamide, Z-3, and Z-6 exposure. The results showed that the apoptosis rate of MCF-7 increased with the increasing of the concentration after 72 h of incubation. At low concentrations, the effects of all the tested compounds were not obvious. When the concentration reached 10 μg/mL, the apoptotic effects of galaxamide, Z-3, and Z-6 on MCF-7 cells were more obvious in the late apoptotic state. Particularly, the apoptotic effects of Z-3 and Z-6 on MCF-7 cells were significantly higher than those of galaxamide (Figure 4).
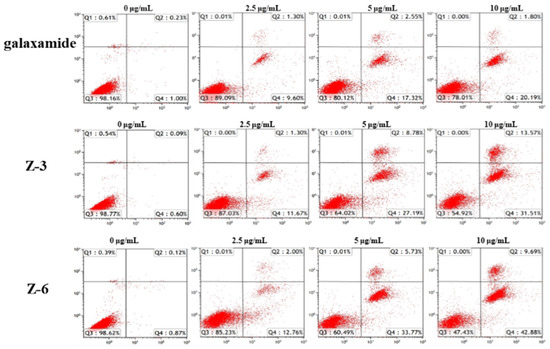
Figure 4.
Monomers used in the synthesis of galaxamide analogues.
2.2.4. Effects of Galaxamide and Its Analogues on Cell Nuclear Integrity
The morphological alteration of MCF-7 cells was analyzed by the method of Hoechst 33,342 staining (Figure 5). After the MCF-7 cell line was treated with galaxamide, Z-3, and Z-6 for 72 h, the morphology of the nuclei was significantly changed. The pyknotic nuclei increased with the increasing concentration of these compounds. At the same time, karyorrhexis and karyolysis were observed, indicating that these compounds could cause obvious cell apoptosis.
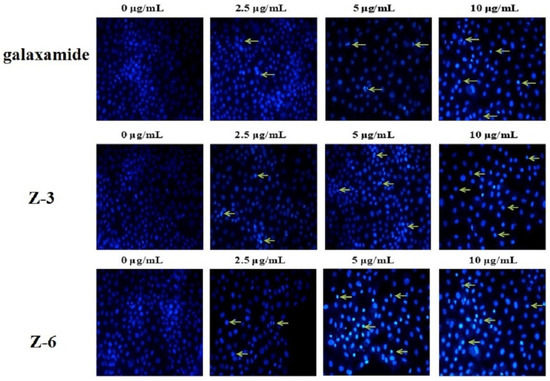
Figure 5.
Morphological observation of galaxamide and its analogues inducing apoptosis in MCF-7 cells as indicated by Hoechst 33,342 staining.
3. Materials and Methods
3.1. General
NMR spectra were measured on a Bruker Avance 300 spectrometer (Bruker, Billerica, MA, USA) in CDCl3. Chemical shifts are reported as δ values in parts per million (ppm) relative to tetramethyl silane (TMS) and J values are expressed in Hertz. The ESI mass spectra were obtained on a LCQ DECA XP LC-MS mass spectrometer. Silica gel (200–300 mesh) for column chromatography and silica GF254 for TLC were produced by the Qingdao Marine Chemical Company (Qingdao, China). All reactions avoiding water and air were carried out under nitrogen atmosphere conditions. The starting materials and reagents used in reactions were obtained commercially from Acros, Aldrich, and GL Biochem, and used without purification, unless otherwise indicated.
3.2. Compounds
3.2.1. Synthesis of Fmoc-N-Me-Leu-OH
A solution of Fmoc-Leu-OH (7.07 g, 2 mmol), paraformaldehyde (4.00 g), and p-toluenesulphonic acid (0.20 g, catalytic amount) in toluene (100 mL) were suspended in a 250 mL three neck RBF. The mixture was stirred and refluxed for 30 min in a Dean-Stark setup and monitored by TLC. Following this, the solution was allowed to be cooled and poured into saturated aqueous NaHCO3. The organic layer was dried over anhydrous sodium sulfate and concentrated, the residue was purified by column chromatography on silica gel (eluant ratio of Vpetroleum ether/Vethylacetate = 8:1) to obtain 7.15 g of white powder with a yield of 97.9%.
Then, the white powder (7.00 g, 2 mmol), AlCl3 (4.00 g, 3 mmol), triethylsilane (5 mL), and dry DCM (150 mL) solution were added to a 250 mL round-bottom flask. The reaction was stirred at ambient temperature until the TLC showed an absence of the starting material. The organic phase was washed with 1M HCl. Subsequently, the organic layer was dried and filtered concentrated with anhydrous sodium sulfate. It was then dried once more under a vacuum and purified using silica-gel column chromatography (eluant ratio of Vpetroleum ether/Vethyl acetate = 8:1). Finally, 6.54 g of white powder was obtained with a yield of 92.7%. 1H NMR (300 MHz, DMSO-d6), δH: 12.77 (s, 1H), 7.90 (t, J = 7.5 Hz, 2H), 7.65 (td, J = 7.8, 3.8 Hz, 2H), 7.49–7.38 (m, 2H), 7.38–7.28 (m, 2H), 4.69–4.45 (m, 1H), 4.46–4.13 (m, 3H), 2.72 (s, 3H), 1.80–1.43 (m, 2H), 1.37 (d, J = 12.2 Hz, 1H), 1.00–0.60 (m, 6H); ESI-MS m/z: 368.2 [M + H]+, 385.1 [M + NH4]+, 390.1 [M + Na]+.
3.2.2. Synthesis of Linear Pentapeptide HO-Leu-N-Me-Leu-Leu-Pro-Leu-H (X-1)
Synthesis of Fmoc-Leu-Resin (R-1). The linear peptide was synthesized by using 2-chlorotrityl chloride (CTC) resin following the standard Fmoc strategy. Firstly, using 15 mL of dichloromethane dissolved Fmoc-Leu-OH (1.06 g, 3 mmol), and then adding 0.5 mL of DIEA. Then, the liquid was added to the peptide synthesis tube containing the CTC resin (1.00 g, load: 0.985 mmol/g). The reaction tube was placed on a shaker at room temperature for reaction 4 h. After the reaction, the resin was washed with DCM (3 × 5 mL), DMF (3 × 5 mL), and MeOH (3 × 5 mL). After filtration, the remaining unreacted sites of the resin were capped with a solution of DCM/MeOH/DIEA (4:5:1, 10 mL) for 30 min. Deprotection of Fmoc group was carried out using 20% piperidine in DMF for (2 × 10 min) at room temperature.
Synthesis of Fmoc-N-Me-Leu-Leu-Resin (R-2). Fmoc-N-Me-Leu-OH (1.10 g, 3 mmol), HOAt (0.41 g, 3 mmol), and DIC (0.47 mL, 3 mmol) were dissolved in 15 mL of DMF and reacted at room temperature for 5 min. Following this, the reaction solution was added to the peptide synthesis tube which contained the previously synthesized R-1, and was placed on a shaker to react for 3 h at room temperature. After filtration, the resin was washed with DCM (3 × 5 mL), DMF (3 × 5 mL), and MeOH (3 × 5 mL). The reaction was monitored using the Kaiser test for the primary amine and the chloranil test for the secondary amine.
Synthesis of Fmoc-Leu-N-Me-Leu-Leu-Resin (R-3). After deprotection of the Fmoc group, the method of synthesizing R-3 was the same as that of R-2. Finally, the Fmoc-Leu-OH was connected to R-2 to synthesize R-3.
Synthesis of Fmoc-Pro-Leu-N-Me-Leu-Leu-Resin (R-4). After deprotection of the Fmoc group, the method of synthesizing R-4 was the same as that of R-2. Finally, the Fmoc-Pro-OH was connected to R-3 to synthesize R-4.
Synthesis of Fmoc-Leu-Pro-Leu-N-Me-Leu-Leu-Resin (R-5). After deprotection of the Fmoc group, the method of synthesizing R-5 was the same as that of R-2. Finally, the Fmoc-Leu-OH was connected to R-4 to synthesize R-5.
Cleavage of resin peptides. Cleavage was performed using 1% TFA in DCM for (2 × 30 min) at rt. After cleavage, the filtrate was immediately neutralized with 1% pyridine in MeOH and concentrated under reduced pressure. The crude peptide was isolated via precipitation from diethyl ether (20 mL) and collected through centrifuge as a white solid, which can be directly used for a macrocyclization reaction without further purification.
3.2.3. Synthesis of Linear Pentapeptide HO-Leu-Pro-Leu-Pro-Leu-H (X-2)
The method of synthesizing X-2 was the same as that of X-1.
3.2.4. Synthesis of Linear Pentapeptide HO-D-Leu-Pro-Leu-Pro-Leu-H (X-3)
The method of synthesizing X-3 was the same as that of X-1.
3.2.5. Synthesis of Linear Pentapeptide HO-Leu-Pro-Leu-Pro-D-Leu-H (X-4)
The method of synthesizing X-4 was the same as that of X-1.
3.2.6. Synthesis of Linear Pentapeptide HO-Leu-Pro-D-Leu-Pro-Leu-H (X-5)
The method of synthesizing X-5 was the same as that of X-1.
3.2.7. Synthesis of Linear Pentapeptide HO-D-Leu-Pro-Leu-Pro-D-Leu-H (X-6)
The method of synthesizing X-6 was the same as that of X-1.
3.2.8. Synthesis of Linear Pentapeptide HO-Leu-Pro-D-Leu-Pro-D-Leu-H (X-7)
The method of synthesizing X-7 was the same as that of X-1.
3.2.9. Synthesis of Linear Pentapeptide HO-D-Leu-Pro-D-Leu-Pro-D-Leu-H (X-8)
The method of synthesizing X-8 was the same as that of X-1.
3.2.10. Synthesis of Cyclo (Leu-N-Me-Leu-Leu-Pro-Leu) (Z-1)
Firstly, X-1 (0.10 g, 0.15 mmol) and PyBOP (0.27 g, 0.52 mmol) were dissolved in dichloromethane (400 mL) using a 500-mL round-bottom flask. Subsequently, this mixture reacted at room temperature for 48 h with the addition of DIEA to adjust the pH around 7~8. After that, saturated NaHCO3 and H2O were used to wash this reaction solution separately. The resultant organic phase was dried with anhydrous Na2SO4 and concentrated. Then, the obtained solid was purified by RP-HPLC (Agilent Eclipse ZORBAX SB-C18, 5 µm, 9.4 × 250 nm) to obtain 39.4 mg of white powder with a yield of 40.7%. 1H NMR (300 MHz, Chloroform-d), δH 7.16 (d, J = 8.8 Hz, 1H), 6.81 (d, J = 8.2 Hz, 1H), 6.67 (d, J = 7.6 Hz, 1H), 4.85 (td, J = 8.5, 5.1 Hz, 1H), 4.55–4.45 (m, 1H), 4.34 (dd, J = 7.2, 3.5 Hz, 1H), 4.25 (td, J = 10.0, 4.0 Hz, 1H), 3.76 (dd, J = 11.7, 8.9 Hz, 1H), 3.61–3.45 (m, 4H), 3.27 (s, 3H), 2.06–1.37 (m, 14H), 0.85–1.05 (m, 24H); 13C NMR (75 MHz, Chloroform-d), δC 174.2, 172.6, 172.5, 171.7, 171.0, 77.2, 69.0, 61.2, 54.1, 50.8, 48.8, 47.9, 42.0, 41.5, 39.5, 38.2, 37.7, 32.3, 25.8, 25.1, 25.0, 24.7, 24.4, 23.5, 23.3, 23.2, 23.0, 22.0 (2C), 21.5; HR-ESI-MS m/z: 564.4 [M + H]+, 580.7 [M + NH4]+, 586.6 [M + Na]+.
3.2.11. Synthesis of Cyclo (Leu-Pro-Leu-Pro-Leu) (Z-2)
The method of synthesizing Z-2 was followed as per that described for Z-1. Finally, 34.3 mg of white powder with a yield of 35.5% were obtained. 1H NMR (300 MHz, Chloroform-d), δH 7.44–7.33 (m, 2H), 7.17 (d, J = 8.1 Hz, 1H), 4.80 (ddd, J = 22.2, 11.0, 6.4 Hz, 1H), 4.63 (dd, J = 11.7, 7.3 Hz, 2H), 4.29 (t, J = 9.3 Hz, 1H), 3.72 (dd, J = 14.8, 7.8 Hz, 1H), 3.56 (dd, J = 10.5, 5.7 Hz, 2H), 2.48–1.37 (m, 19H), 1.13–0.79 (m, 18H); 13C NMR (75 MHz, Chloroform-d), δC 173.4, 172.6, 171.9, 171.0, 170.8, 61.0, 60.4, 53.2, 49.2, 48.8, 47.7, 47.2, 42.4, 42.3, 40.7, 27.4, 25.2, 25.1, 25.0 (2C), 24.9, 23.5, 23.3 (2C), 22.1, 21.9, 21.3, 21.1; HR-ESI-MS m/z: 534.4 [M + H]+, 550.7 [M + NH4]+, 556.8 [M + Na]+.
3.2.12. Synthesis of Cyclo (Leu-Pro-Leu-Pro-Leu) (Z-3)
The method of synthesizing Z-3 was followed as per described for Z-1. Finally, 52.1 mg of white powder with a yield of 53.9% were obtained. 1H NMR (300 MHz, Chloroform-d), δH 7.62 (d, J = 8.7 Hz, 1H), 7.47 (d, J = 8.4 Hz, 1H), 6.87 (d, J = 9.0 Hz, 1H), 4.80 (t, J = 7.7 Hz, 2H), 4.69 (d, J = 7.8 Hz, 1H), 4.51 (td, J = 10.0, 4.1 Hz, 1H), 4.25 (t, J = 6.6 Hz, 1H), 3.89–3.51 (m, 4H), 2.39 (t, J = 8.8 Hz, 1H), 2.29–1.82 (m, 8H), 1.78–1.36 (m, 8H), 0.93 (d, J = 6.6 Hz, 18H); 13C NMR (75 MHz, Chloroform-d), δC 172.6, 172.0, 171.9, 171.9, 171.0, 61.0, 59.9, 51.5, 49.3, 49.1, 47.6, 47.4, 41.0, 40.9, 40.4, 29.2, 27.0, 25.3, 25.1, 25.0, 24.7, 24.6, 23.5, 23.1, 23.0, 22.4, 21.7, 21.4; HR-ESI-MS m/z: 534.7 [M + H]+, 550.7 [M + NH4]+, 556.8 [M + Na]+.
3.2.13. Synthesis of Cyclo (Leu-Pro-D-Leu-Pro-Leu) (Z-4)
The method of synthesizing Z-4 was followed as per that described for Z-1. Finally, 35.2 mg of white powder with a yield of 36.4% were obtained. 1H NMR (300 MHz, Chloroform-d), δH 8.07 (d, J = 8.0 Hz, 1H), 7.16 (d, J = 9.1 Hz, 1H), 6.57 (d, J = 8.8 Hz, 1H), 4.86 (d, J = 7.6 Hz, 1H), 4.79 (t, J = 7.9 Hz, 1H), 4.72–4.60 (m, 1H), 4.51 (td, J = 9.1, 5.0 Hz, 1H), 4.37 (dd, J = 8.5, 3.9 Hz, 1H), 3.98 (dt, J = 11.1, 6.0 Hz, 1H), 3.61 (td, J = 9.3, 4.1 Hz, 1H), 3.56–3.42 (m, 2H), 2.29 (qt, J = 9.2, 4.6 Hz, 2H), 2.16 (dt, J = 15.5, 8.0 Hz, 1H), 2.10–1.84 (m, 6H), 1.81 -1.43 (m, 7H), 1.32 (dt, J = 12.0, 6.7 Hz, 1H), 1.05–0.83 (m, 18H); 13C NMR (75 MHz, Chloroform-d), δC 172.9, 172.2, 171.4, 171.3, 171.0, 77.3, 62.0, 58.7, 51.5, 50.3, 49.5, 47.3, 40.2, 40.0, 39.7, 29.7, 25.8, 25.2, 24.9 (2C), 24.7 (2C), 23.3, 23.2, 22.8, 22.5, 21.6, 21.5; HR-ESI-MS m/z: 534.4 [M + H]+, 550.7 [M + NH4]+, 556.8 [M + Na]+.
3.2.14. Synthesis of Cyclo (Leu-Pro-Leu-Pro-D-Leu) (Z-5)
The method of synthesizing Z-5 was followed as per that described for Z-1. Finally, 57.0 mg of white powder with a yield of 58.9% were obtained. 1H NMR (300 MHz, Chloroform-d), δH 8.62 (d, J = 9.0 Hz, 1H), 7.12 (d, J = 8.7 Hz, 1H), 6.93 (d, J = 6.7 Hz, 1H), 4.77 (q, J = 7.9 Hz, 1H), 4.66–4.50 (m, 3H), 4.40 (dt, J = 8.6, 6.1 Hz, 1H), 4.28–4.02 (m, 2H), 3.68 (td, J = 9.2, 3.9 Hz, 1H), 3.47 (td, J = 9.5, 6.7 Hz, 1H), 2.41–2.17 (m, 1H), 2.17–1.81 (m, 8H), 1.81–1.50 (m, 6H), 1.51–1.35 (m, 2H), 1.02–0.84 (m, 18H); 13C NMR (75 MHz, Chloroform-d), δC 174.0, 172.7, 171.7, 171.0, 170.9, 60.6, 59.2, 50.8, 49.9, 48.9, 47.5, 46.8, 41.3, 39.5, 37.8, 29.9, 28.1, 25.1, 24.8 (3C), 24.0, 23.3, 23.0 (2C), 22.5, 22.4, 22.0; HR-ESI-MS m/z: 534.4 [M + H]+, 550.7 [M + NH4]+, 556.8 [M + Na]+.
3.2.15. Synthesis of Cyclo (D-Leu-Pro-Leu-Pro-D-Leu) (Z-6)
The method of synthesizing Z-6 was followed as per that described for Z-1. Finally, 33.4 mg of white powder with a yield of 34.5% were obtained. 1H NMR (300 MHz, Chloroform-d), δC 8.00 (d, J = 7.3 Hz, 1H), 7.59 (d, J = 6.8 Hz, 1H), 7.27 (d, J = 9.2 Hz, 1H), 4.77 (td, J = 9.5, 4.2 Hz, 1H), 4.64 (d, J = 7.6 Hz, 1H), 4.55 (d, J = 7.5 Hz, 2H), 4.24–4.12 (m, 1H), 4.07 (dt, J = 11.5, 6.3 Hz, 1H), 3.91 (q, J = 8.5 Hz, 1H), 3.63–3.41 (m, 2H), 2.48–1.29 (m, 18H), 1.11–0.76 (m, 18H); 13C NMR (75 MHz, Chloroform-d), δC 174.4, 172.4, 172.1, 171.8, 171.3, 60.8, 59.8, 55.8, 50.2, 49.1, 47.4, 47.0, 41.0, 39.8, 39.1, 29.7, 26.7, 25.2, 25.1, 24.9, 24.8, 24.3, 23.3, 23.2, 23.0, 22.0, 21.7 (2C); HR-ESI-MS m/z: 534.4 [M + H]+, 550.7 [M + NH4]+, 556.8 [M + Na]+.
3.2.16. Synthesis of Cyclo (Leu-Pro-D-Leu-Pro-D-Leu) (Z-7)
The method of synthesizing Z-7 was followed as per that described for Z-1. Finally, 52.8 mg of white powder with a yield of 54.6% were obtained. 1H NMR (300 MHz, Chloroform-d), δH 8.03 (d, J = 8.3 Hz, 1H), 7.17 (d, J = 9.0 Hz, 1H), 6.60 (d, J = 8.8 Hz, 1H), 4.79–4.86 (m, 1H), 4.74 (d, J = 7.9 Hz, 1H), 4.69–4.60 (m, 1H), 4.46 (td, J = 9.3, 4.7 Hz, 1H), 4.31 (dd, J = 9.0, 3.9 Hz, 1H), 3.97 (dd, J = 10.1, 5.4 Hz, 1H), 3.40–3.62 (m, 3H), 2.39–2.18 (m, 2H), 2.18–2.08 (m, 1H), 2.08–1.79 (m, 6H), 1.75–1.42 (m, 7H), 1.29 (ddd, J = 13.6, 9.7, 3.8 Hz, 1H), 1.03–0.81 (m, 18H); 13C NMR (75 MHz, Chloroform-d), δC 172.6, 172.3, 171.4, 171.3, 171.2, 62.0, 58.6, 51.5, 50.1, 49.5, 47.2, 46.4, 40.3, 40.0, 39.7, 29.6, 25.6, 25.2, 25.0, 24.9, 24.7, 24.7, 23.3, 23.2, 22.8, 22.5, 21.6, 21.5; HR-ESI-MS m/z: 534.4 [M + H]+, 550.7 [M + NH4]+, 556.8 [M + Na]+.
3.2.17. Synthesis of Cyclo (D-Leu-Pro-D-Leu-Pro-D-Leu) (Z-8)
The method of synthesizing Z-8 was followed as per that described for Z-1. Finally, 44.7 mg of white powder with a yield of 46.2% were obtained. 1H NMR (300 MHz, Chloroform-d), δH 7.94 (d, J = 8.4 Hz, 1H), 7.17 (d, J = 9.1 Hz, 1H), 6.53 (d, J = 8.8 Hz, 1H), 4.85 (d, J = 7.5 Hz, 1H), 4.78 (d, J = 8.1 Hz, 1H), 4.68 (d, J = 8.3 Hz, 1H), 4.50 (td, J = 9.4, 4.6 Hz, 1H), 4.34 (dd, J = 8.8, 4.0 Hz, 1H), 3.97 (dt, J = 11.9, 6.2 Hz, 1H), 3.60–3.43 (m, 3H), 2.31 (td, J = 12.9, 5.2 Hz, 2H), 2.16 (dt, J = 11.8, 8.1 Hz, 1H), 2.11–1.82 (m, 6H), 1.80–1.42 (m, 7H), 1.32 (ddd, J = 13.6, 9.8, 3.8 Hz, 1H), 1.25–0.85 (m, 18H); 13C NMR (75 MHz, Chloroform-d), δC 172.7, 172.1, 171.4, 171.3, 171.1, 62.0, 58.5, 51.4, 50.1, 49.5, 47.3, 46.5, 40.4, 40.0, 39.8, 29.7, 25.4, 25.2, 25.0, 24.9, 24.7, 24.7, 23.3, 23.2, 22.8, 22.5, 21.5 (2C); HR-ESI-MS m/z: 534.4 [M + H]+, 550.7 [M + NH4]+, 556.8 [M + Na]+.
3.3. In Vitro Cytotoxic Assays
Cell culture: MCF-7/MD-MAB-231 (human breast cancer cell line ATCC HTB-22/HTB-26), Hep G2 (human liver cancer cell line ATCC HB-8065), Hela (human cervical carcinoma cell line ATCC CCL-2), A459 (human lung cancer cell line ATCC CCL-185), and HUVEC (primary umbilical vein endothelial cell line ATCC PCS-100-010) were obtained from the Jinan Department of Pathology, University School of Medicine (Jinan University, Guangzhou, China). The cells were cultured in a Modified Eagle’s Medium (DMEM) including 10% fetal bovine serum, 1% penicillin-streptomycin, and antifungal agent, and cultured under standard conditions (5% CO2 atmosphere and temperature at 37 °C).
3.4. MTT Assay
The MTT method was used to evaluate the cytotoxicities of galaxamide and its analogues on the five cancerous cells. In this experiment, the number of regulated cells was 2 × 103 cells/well. Then, the cells, as well as galaxamide and its analogues of different concentrations (0, 2.5, 5, and 10 μg/mL), were seeded on a 96-well cell culture plate and cultured in a 37 °C, 5% CO2 incubator for 72 h. After that, 20 µL/well of MTT solution (5 mg/mL phosphate-buffered saline) was added and incubated for 5 h. The medium was discarded and replaced with 100 µL/well of DMSO to dissolve the formazan salt. The color intensity of the cell culture at 570 nm was measured using a microplate spectrophotometer (SpectroAma™ 250, Winooski, VT, USA).
3.5. Flow Cytometry Experiments
The concentration of 1 × 104 cells per well of MCF-7 cells were seeded in 96-well plates. The cell cultures were added to the compounds with a concentration of 0, 2.5, 5, and 10 µg/mL, and incubated for 72 h. After that, 5 µL of Annexin V-fluorescein isothiocyanate (FITC) and 5 µL of PI were added for dual staining of the cells for analysis by FACS flow cytometry. Cell cycles and apoptotic cells were determined by counting the FITC-positive and PI-negative fractions.
3.6. Cell Apoptotic Analysis
The apoptotic cells were quantified according to the instructions provided in an Annexin V-FITC (Sigma-Aldrich, St. Louis, MO, USA) cell apoptosis assay kit. Briefly, about 1.5 × 105 cells were plated in 96-well plates and treated with galaxamide and its representative analogues (0, 2.5, 5, and 10 µg/mL) for 48 h. The cells were then resuspended in a 200-mL binding buffer. Afterward, 5 mL of annexin V-fluorescein isothiocyanate (FITC) was added, followed by incubation in darkness at room temperature for 10 min. The cells were again resuspended in 200 mL binding buffer and stained with 5 mL PI. The prepared cells were then analyzed using a flow cytometry (Coulter Epics Elite, Miami, FL, USA). The cells in the FITC-positive and PI-negative fractions were regarded as apoptotic cells.
3.7. Hoechst 33,342 Staining
Test compounds at 0, 2.5, 5, and 10 µg/mL were added to the cell cultures which included the exponentially growing MCF-7 cells (1 × 105), and incubated for 48 h. After that, the cell culture was washed with 1 mL of PBS and then added with 3 mL of 4% paraformaldehyde solution for 15 min. Then, 1 mL of the prepared Hoechst 33,342 staining solution was added. The resulting mixture was incubated at room temperature for 15 min in the dark. Ultimately, cell staining was observed with a fluorescence microscope.
4. Conclusions
In this paper, eight new cyclic pentapeptides (Z-1~Z-8) were synthesized, and were designed by replacing one or two N-Me-L-leucine with L-proline or changing the stereo configuration of leucine. The anticancer activities of these new compounds were also tested against five human cancer cell lines. The results showed that the inhibitory effects of most analogues were much better than that of galaxamide. Moreover, all tested compounds exhibited low cytotoxic activity against the normal liver cell line HUVEC. Compound Z-6 displayed the strongest inhibitory effects against HepG2, MCF-7, and MD-MAB-231 cells with IC50 values of 2.91 ± 0.17, 1.65 ± 0.30, 4.59 ± 0.27 μg/mL, respectively. These galaxamide analogues arrested the cell cycle in the G0/G1 phase and induced apoptosis, which was later confirmed by the representative compounds Z-3 and Z-6. In our preliminary work, a series of galaxamide analogues had been synthesized by replacing one of the amino acids to phenylalanine, the cytotoxic activities of which were also tested against several cancerous cell lines. [14,16] The results showed that most of these newly synthesized compounds exhibited stronger inhibitory activities against MCF-7 cells than the previous ones, which suggests that proline might be the active group of these analogues against MCF-7 cells. Th structure-activity relationship needs further investigation.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/md20030158/s1, Figures S1–S24: The analyzed data of HR-ESI-MS and NMR spectra of compounds Z-1~Z-8.
Author Contributions
D.L. and X.L. synthesized compounds; D.L. wrote this paper; S.Z. performed biological experiments; and B.Z. and S.X. conceived and designed the experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Natural Resources of the Guangdong Province (Nos. GDNRC [2020] 037 and GDNRC [2021] 48); the National Key Research and Development Plan of China (Nos. 2018YFC0310900 and 2019YFD0901905); the National Natural Science Foundation of China (Nos. 41876145, 81874292 and 21672084); and the Science and Technology Planning Project of Guangzhou (Nos. 202102010066 and 201704030042).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hamann, M.T.; Otto, C.S.; Scheuer, P.J. Bioactive peptides from a marine mollusk elysiarufescens and its algal diet Bryopsis sp. J. Org. Chem. 1996, 61, 6594–6600. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.F.; Cichacz, Z.; Barkoczy, J.; Dorsaz, A.; Herald, D.L.; Williams, M.D.; Doubek, D.L.; Schmidt, J.M.; Tackett, L.P.; Brune, D.C. Isolation and structure of the marine sponge cell growth inhibitory cyclic peptide phakellistin. J. Nat. Prod. 1993, 56, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, A.S.; Bugni, T.S.; Feng, X.D.; Harper, M.K.; Skalicky, J.J.; Mohammed, K.A.; Andjelic, C.D.; Barrows, L.R.; Ireland, C.M. Theopapuamide, a cyclic depsipeptide from a papua new guinea lithistid sponge Theonella swinhoei. J. Nat. Prod. 2006, 69, 1582–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zampella, A.; D’Auria, M.V.; Paloma, L.G.; Casapullo, A.; Minale, L.; Debitus, C.; Henin, Y.; Callipeltin, A. An anti-HIV cyclic depsipeptide from the new caledonian lithistida sponge Callipelta sp. J. Am. Chem. Soc. 1996, 118, 6202–6209. [Google Scholar] [CrossRef]
- Kashinath, K.; Vasudevan, N.; Reddy, D.S. Studies toward the synthesis of potent anti-inflammatory peptides solomonamides A and B: Synthesis of a macrocyclic skeleton and key fragment 4-amino-6-(2′-amino-4′-hydroxyphenyl)-3-hydroxy-2-methyl-6-oxohexanoic acid (AHMOA). Org. Lett. 2012, 14, 6222–6225. [Google Scholar] [CrossRef] [PubMed]
- Festa, C.; De Marino, S.; Sepe, V.; Monti, M.C.; Luciano, P.; Valeria D’Auria, M.; Debitus, C.; Bucci, M.; Vellecco, V.; Zampella, A. Perthamides C and D, two new potent anti-inflammatory cyclopeptides from a solomon lithistid sponge Theonella swinhoei. Tetrahedron 2009, 65, 10424–10429. [Google Scholar] [CrossRef]
- Bewley, C.A.; Detritus, C.; Faulkner, D.J. Microsclerodermins A and B. antifungal cyclic peptides from the lithistid sponge Microscleroderma sp. J. Am. Chem. Soc. 1994, 116, 7631–7636. [Google Scholar] [CrossRef]
- Schmidt, E.W.; Faulkner, D.J. Microsclerodermins C-E, sntifungal cyclic peptides from the lithistid marine sponges Theonella sp. and Microscleroderma sp. Tetrahedron 1998, 54, 3043–3056. [Google Scholar] [CrossRef]
- Qureshi, A.; Colin, P.L.; Faulkner, D.J. Microsclerodermins F-I, antitumor and antifungal cyclic peptides from the lithistid sponge Microscleroderma sp. Tetrahedron 2000, 56, 3679–3685. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Harper, J.B.; Hunter, L. Incrementally increasing the length of a peptide backbone: Effect on macrocyclization efficiency. Org. Biomol. Chem. 2014, 12, 4598–4601. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Jimenez, G.M.; Burgos-Hernandez, A.; Ezquerra-Brauer, J.M. Bioactivep peptides and depsipeptides with anticancer potential: Sources from marine animals. Mar. Drugs 2012, 10, 963–986. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.Y.; Dahiya, R.; Qin, H.L.; Mourya, R.; Maharaj, S. Natural proline-rich cyclopolypeptides from marine organisms: Chemistry, synthetic methodologies and biological status. Mar. Drugs 2016, 14, 194–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.J.; Liao, X.J.; Xu, S.H.; Diao, J.Z.; Du, B.; Zhou, X.L.; Pan, S.S. Isolation, structure determination, and synthesis of galaxamide, a rare cytotoxic cyclic pentapeptide from a marine algae Galaxaura filamentosa. Org. Lett. 2008, 10, 4569–4572. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liao, X.J.; Qiu, S.L.; Liu, Z.H.; Du, B.; Xu, S.H. Paper synthesis, cytotoxicity and apoptosis induction in human tumor cells by galaxamide and its analogues. Mar. Drugs 2014, 12, 4521–4538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunagariya, J.; Zhong, S.H.; Chen, J.; Bai, D.; Bhadja, P.; Long, W.; Liao, X.; Tang, X.; Xu, S. Design and aynthesis of analogues of marine natural product galaxamide, an N-methylated cyclic pentapeptide, as potential anti-tumor agent in vitro. Mar. Drugs 2016, 14, 161–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, D.F.; Yu, S.M.; Zhong, S.H.; Zhao, B.X.; Qiu, S.L.; Chen, J.W.; Lunagariya, J.; Liao, X.J.; Xu, S.H. D-amino acid position influences the antiCancer activity of galaxamide analogs: An apoptotic mechanism study. Int. J. Mol. Sci. 2017, 18, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redman, J.E.; Ghadiri, M.R. Synthesis of photoactive p-azidotetrafluorophenylalanine containing peptide by solid-phase fmoc methodology. Org. Lett. 2002, 4, 4467–4469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Govender, T.; Norstrom, T.; Arvidsson, P.I. An improved synthesis of fmoc-N-methyl-α-amino acids. J. Org. Chem. 2005, 70, 6918–6920. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).