Characterization of the Dual Functions of LvCrustinVII from Litopenaeus vannamei as Antimicrobial Peptide and Opsonin
Abstract
:1. Introduction
2. Results
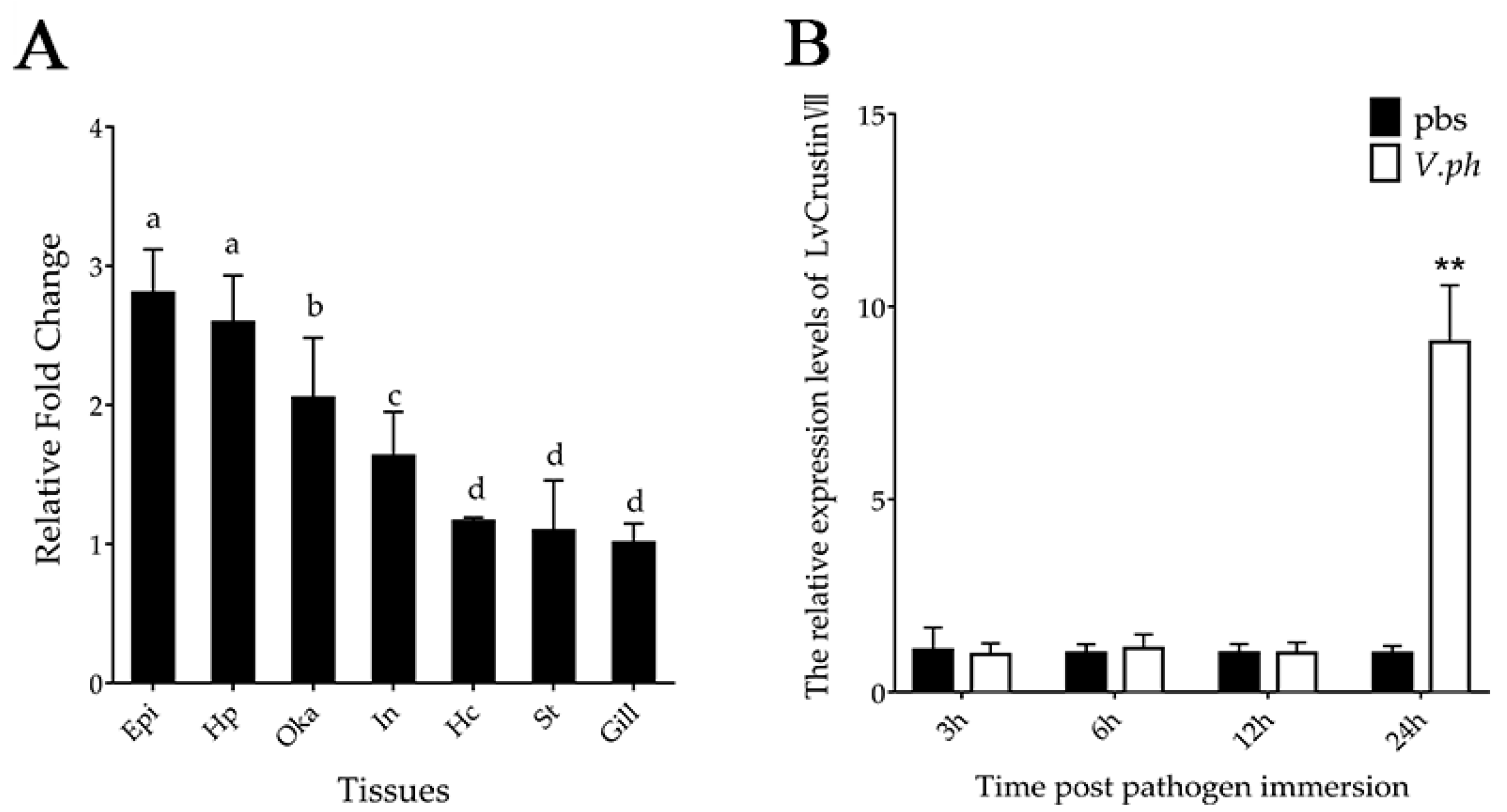
2.1. Tissue Distribution and Immune Responses of LvCrustinVII Transcripts
2.2. Expression and Purification of the Recombinant LvCrustinVII
2.3. Antimicrobial Activity of rLvCrustinVII
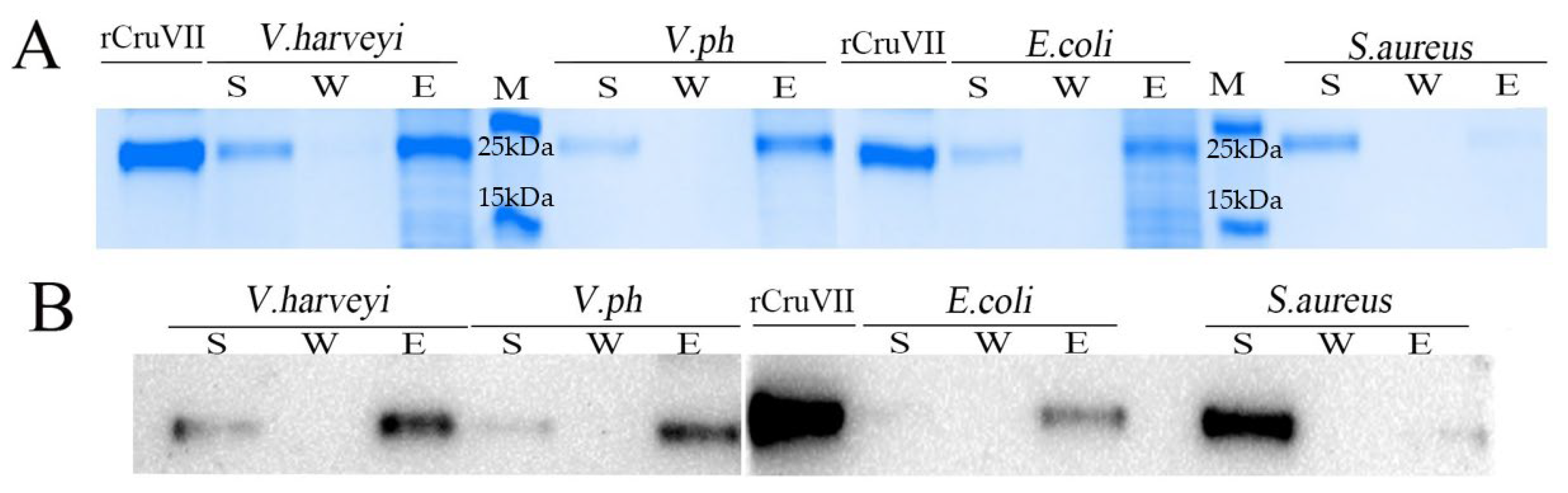
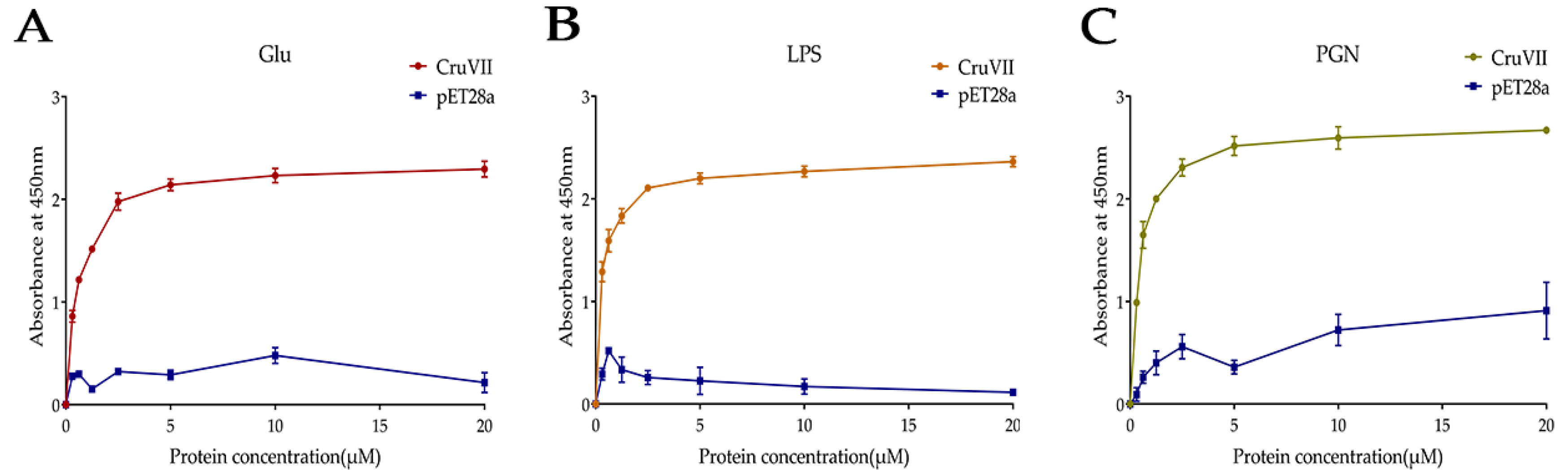
2.4. Microorganism and Polysaccharides Binding Activity of rLvCrustinVII
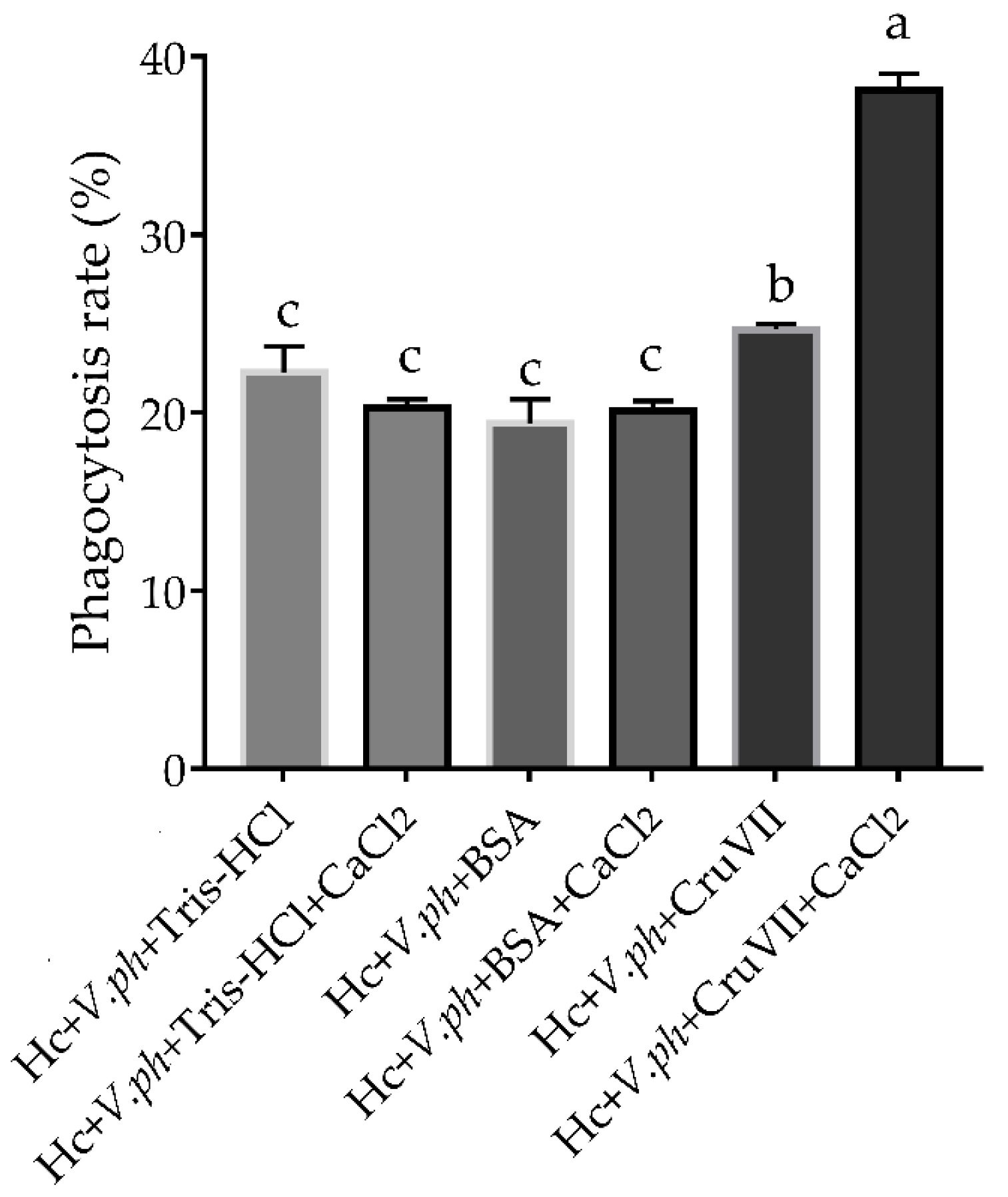
2.5. Agglutinating and Phagocytosis-Enhancing Activitiy of rLvCrustinVII
3. Discussion
4. Materials and Methods
4.1. Animals and Tissues Preparation
4.2. Total RNA Extraction and cDNA Synthesis
4.3. Real-Time Quantitative PCR (qRT-PCR)
4.4. Recombinant Protein Expression and Purification
4.5. Minimal Inhibitory Concentration (MIC) Assay
4.6. Microorganism Binding Assay
4.7. The Polysaccharides Binding Assay
4.8. Microorganism Agglutination Assay
4.9. Phagocytosis Assay
4.10. Ethical Statement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Chung, C.-R.; Jhong, J.-H.; Wang, Z.; Chen, S.; Wan, Y.; Horng, J.-T.; Lee, T.-Y. Characterization and Identification of Natural Antimicrobial Peptides on Different Organisms. Int. J. Mol. Sci. 2020, 21, 986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabere, M.N.; Noble, W.S. Empirical comparison of web-based antimicrobial peptide prediction tools. Bioinformatics 2017, 33, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punginelli, D.; Schillaci, D.; Mauro, M.; Deidun, A.; Barone, G.; Arizza, V.; Vazzana, M. The potential of antimicrobial peptides isolated from freshwater crayfish species in new drug development: A review. Dev. Comp. Immunol. 2021, 126, 104258. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T.G. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, A.; Makarova, O.; Rolff, J. Antimicrobials, Stress and Mutagenesis. PLoS Pathog. 2014, 10, e1004445. [Google Scholar] [CrossRef] [Green Version]
- Hoang, V.L.T.; Kim, S.-K. Antimicrobial peptides from marine sources. Curr. Protein Pept. Sci. 2013, 14, 205–211. [Google Scholar] [CrossRef]
- Moravej, H.; Moravej, Z.; Yazdanparast, M.; Heiat, M.; Mirhosseini, A.; Moghaddam, M.M.; Mirnejad, R. Antimicrobial Peptides: Features, Action, and Their Resistance Mechanisms in Bacteria. Microb. Drug Resist. 2018, 24, 747–767. [Google Scholar] [CrossRef]
- Hadley, E.B.; Hancock, R.E. Strategies for the discovery and advancement of novel cationic antimicrobial peptides. Curr. Top Med. Chem. 2010, 10, 1872–1881. [Google Scholar] [CrossRef]
- Hughes, C.C.; Fenical, W. Antibacterials from the sea. Chemistry 2010, 16, 12512–12525. [Google Scholar] [CrossRef] [Green Version]
- Baxter, R.H.G.; Contet, A.; Krueger, K. Arthropod Innate Immune Systems and Vector-Borne Diseases. Biochemistry 2017, 56, 907–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The Human Cathelicidin Antimicrobial Peptide LL-37 and Mimics are Potential Anticancer Drugs. Front. Oncol. 2015, 5, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvy, J.-P.; Yu, Y.; Dostalova, A.; Kondo, S.; Kurjan, A.; Bulet, P.; Lemaître, B.; Vidal, M.; Cordero, J.B. The antimicrobial peptide defensin cooperates with tumour necrosis factor to drive tumour cell death in Drosophila. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Shirdel, I.; Kalbassi, M.R.; Hosseinkhani, S.; Paknejad, H.; Wink, M. Cloning, characterization and tissue-specific expression of the antimicrobial peptide hepcidin from caspian trout (Salmo caspius) and the antibacterial activity of the synthetic peptide. Fish Shellfish. Immunol. 2019, 90, 288–296. [Google Scholar] [CrossRef]
- Sierra, J.M.; Fusté, E.; Rabanal, F.; Vinuesa, T.; Viñas, M. An overview of antimicrobial peptides and the latest advances in their development. Expert Opin. Biol. Ther. 2017, 17, 663–676. [Google Scholar] [CrossRef]
- Lv, X.; Li, S.; Yu, Y.; Xiang, J.; Li, F. The immune function of a novel crustin with an atypical WAP domain in regulating intestinal microbiota homeostasis in Litopenaeus vannamei. Dev. Comp. Immunol. 2020, 111, 103756. [Google Scholar] [CrossRef]
- Matos, G.M.; Rosa, R.D. On the silver jubilee of crustacean antimicrobial peptides. Rev. Aquac. 2021, 1–19. [Google Scholar] [CrossRef]
- Smith, V.J.; Dyrynda, E.A. Antimicrobial proteins: From old proteins, new tricks. Mol. Immunol. 2015, 68, 383–398. [Google Scholar] [CrossRef] [Green Version]
- Destoumieux, D.; Bulet, P.; Loew, D.; Van Dorsselaer, A.; Rodriguez, J.; Bachère, E. Penaeidins, a New Family of Antimicrobial Peptides Isolated from the Shrimp Penaeus vannamei (Decapoda). J. Biol. Chem. 1997, 272, 28398–28406. [Google Scholar] [CrossRef] [Green Version]
- Sotelo-Mundo, R.R.; Islas-Osuna, A.M.; De-La-Re-Vega, E.; Hernández-López, J.; Vargas-Albores, F.; Yepiz-Plascencia, G. cDNA cloning of the lysozyme of the white shrimp Penaeus vannamei. Fish Shellfish Immunol. 2003, 15, 325–331. [Google Scholar] [CrossRef]
- Gross, P.; Bartlett, T.; Browdy, C.; Chapman, R.; Warr, G. Immune gene discovery by expressed sequence tag analysis of hemocytes and hepatopancreas in the Pacific White Shrimp, Litopenaeus vannamei, and the Atlantic White Shrimp, L. setiferus. Dev. Comp. Immunol. 2001, 25, 565–577. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, R.; Fan, Z.-X.; Zhao, X.-F.; Wang, X.-W.; Wang, J.-X. Characterization of a type-I crustin with broad-spectrum antimicrobial activity from red swamp crayfish Procambarus clarkii. Dev. Comp. Immunol. 2016, 61, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-S.; Zhang, Q.; Zhao, Y.-R.; Jia, W.-M.; Zhao, X.-F.; Wang, J.-X. A new group of anti-lipopolysaccharide factors from Marsupenaeus japonicus functions in antibacterial response. Dev. Comp. Immunol. 2015, 48, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, S.; Li, F.; Lv, X.; Xiang, J. Recombinant expression and functional analysis of an isoform of anti-lipopolysaccharide factors (FcALF5) from Chinese shrimp Fenneropenaeus chinensis. Dev. Comp. Immunol. 2015, 53, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Lian, Y.-Y.; He, H.-H.; Yuan, K.; Zhang, C.-Z.; Yue, G.H.; He, J.-G. Functional characterization of an ER-stress responding Crustin gene in Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 84, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Barreto, C.; Coelho, J.D.R.; Yuan, J.; Xiang, J.; Perazzolo, L.M.; Rosa, R.D. Specific Molecular Signatures for Type II Crustins in Penaeid Shrimp Uncovered by the Identification of Crustin-Like Antimicrobial Peptides in Litopenaeus vannamei. Mar. Drugs 2018, 16, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Z.-Q.; Li, B.; Shen, X.-L.; Wang, K.; Du, J.; Yu, X.-D.; Yuan, J.-J. A new antimicrobial peptide isoform, Pc-crustin 4 involved in antibacterial innate immune response in fresh water crayfish, Procambarus clarkii. Fish Shellfish Immunol. 2019, 94, 861–870. [Google Scholar] [CrossRef]
- Du, Z.-Q.; Wang, Y.; Ma, H.-Y.; Shen, X.-L.; Wang, K.; Du, J.; Yu, X.-D.; Fang, W.-H.; Li, X.-C. A new crustin homologue (SpCrus6) involved in the antimicrobial and antiviral innate immunity in mud crab, Scylla paramamosain. Fish Shellfish Immunol. 2018, 84, 733–743. [Google Scholar] [CrossRef]
- Li, S.; Lv, X.; Yu, Y.; Zhang, X.; Li, F. Molecular and Functional Diversity of Crustin-Like Genes in the Shrimp Litopenaeus vannamei. Mar. Drugs 2020, 18, 361. [Google Scholar] [CrossRef]
- Tassanakajon, A.; Somboonwiwat, K.; Amparyup, P. Sequence diversity and evolution of antimicrobial peptides in invertebrates. Dev. Comp. Immunol. 2015, 48, 324–341. [Google Scholar] [CrossRef]
- Mu, C.; Zheng, P.; Zhao, J.; Wang, L.; Zhang, H.; Qiu, L.; Gai, Y.; Song, L. Molecular characterization and expression of a crustin-like gene from Chinese mitten crab, Eriocheir sinensis. Dev. Comp. Immunol. 2010, 34, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, S.; Yu, Y.; Zhang, X.; Li, F. Characterization of a gill-abundant crustin with microbiota modulating function in Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 105, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Donpudsa, S.; Visetnan, S.; Supungul, P.; Tang, S.; Tassanakajon, A.; Rimphanitchayakit, V. Type I and type II crustins from Penaeus monodon, genetic variation and antimicrobial activity of the most abundant crustinPm4. Dev. Comp. Immunol. 2014, 47, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; He, N.; Xu, X. Mj-DWD, a double WAP domain-containing protein with antiviral relevance in Marsupenaeus japonicus. Fish Shellfish Immunol. 2008, 25, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Visetnan, S.; Supungul, P.; Tassanakajon, A.; Donpudsa, S.; Rimphanitchayakit, V. A single WAP domain-containing protein from Litopenaeus vannamei possesses antiproteinase activity against subtilisin and antimicrobial activity against AHPND-inducing Vibrio parahaemolyticus. Fish Shellfish Immunol. 2017, 68, 341–348. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, S. Comparative genomics analysis of five families of antimicrobial peptide-like genes in seven ant species. Dev. Comp. Immunol. 2012, 38, 262–274. [Google Scholar] [CrossRef]
- Relf, J.M.; Chisholm, J.R.S.; Kemp, G.D.; Smith, V.J. Purification and characterization of a cysteine-rich 11.5-kDa antibacterial protein from the granular haemocytes of the shore crab, Carcinus maenas. JBIC J. Biol. Inorg. Chem. 1999, 264, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Arockiaraj, J.; Gnanam, A.J.; Muthukrishnan, D.; Gudimella, R.; Milton, J.; Singh, A.; Muthupandian, S.; Kasi, M.; Bhassu, S. Crustin, a WAP domain containing antimicrobial peptide from freshwater prawn Macrobrachium rosenbergii: Immune characterization. Fish Shellfish Immunol. 2013, 34, 109–118. [Google Scholar] [CrossRef]
- Battison, A.L.; Summerfield, R.; Patrzykat, A. Isolation and characterisation of two antimicrobial peptides from haemocytes of the American lobster Homarus americanus. Fish Shellfish Immunol. 2008, 25, 181–187. [Google Scholar] [CrossRef]
- Cui, Z.; Song, C.; Liu, Y.; Wang, S.; Li, Q.; Li, X. Crustins from eyestalk cDNA library of swimming crab Portunus trituberculatus: Molecular characterization, genomic organization and expression analysis. Fish Shellfish Immunol. 2012, 33, 937–945. [Google Scholar] [CrossRef]
- Tassanakajon, A.; Amparyup, P.; Somboonwiwat, K.; Supungul, P. Cationic antimicrobial peptides in penaeid shrimp. Mar. Biotechnol. 2010, 12, 487–505. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.J.; Fernandes, J.M.; Kemp, G.D.; Hauton, C. Crustins: Enigmatic WAP domain-containing antibacterial proteins from crustaceans. Dev. Comp. Immunol. 2008, 32, 758–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Wang, K.; Zhang, R.; Zhang, C.; Cao, X.; Huang, X.; Zhang, Y.; Ren, Q. Identification of two carcinin isoforms (MnCarc1 and MnCarc2) and their function in the antimicrobial immunity of Macrobrachium nipponense. Fish Shellfish Immunol. 2020, 106, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Amparyup, P.; Donpudsa, S.; Tassanakajon, A. Shrimp single WAP domain (SWD)-containing protein exhibits proteinase inhibitory and antimicrobial activities. Dev. Comp. Immunol. 2008, 32, 1497–1509. [Google Scholar] [CrossRef]
- Krusong, K.; Poolpipat, P.; Supungul, P.; Tassanakajon, A. A comparative study of antimicrobial properties of crustinPm1 and crustinPm7 from the black tiger shrimp Penaeus monodon. Dev. Comp. Immunol. 2012, 36, 208–215. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.; Qiu, L.; Zhang, H.; Gai, Y.; Song, L. A double WAP domain-containing protein from Chinese mitten crab Eriocheir sinensis with antimicrobial activities against Gram-negative bacteria and yeast. Dev. Comp. Immunol. 2012, 36, 183–190. [Google Scholar] [CrossRef]
- Li, S.; Jin, X.-K.; Guo, X.-N.; Yu, A.-Q.; Wu, M.-H.; Tan, S.-J.; Zhu, Y.-T.; Li, W.-W.; Wang, Q. A Double WAP Domain-Containing Protein Es-DWD1 from Eriocheir sinensis Exhibits Antimicrobial and Proteinase Inhibitory Activities. PLoS ONE 2013, 8, e73563. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.-J.; Sun, Q.-L.; Jiang, S.; Zhang, J.; Sun, L. First characterization of an anti-lipopolysaccharide factor (ALF) from hydrothermal vent shrimp: Insights into the immune function of deep-sea crustacean ALF. Dev. Comp. Immunol. 2018, 84, 382–395. [Google Scholar] [CrossRef]
- Li, M.; Ma, C.; Zhu, P.; Yang, Y.; Lei, A.; Chen, X.; Liang, W.; Chen, M.; Xiong, J.; Li, C. A new crustin is involved in the innate immune response of shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 94, 398–406. [Google Scholar] [CrossRef]
- Ganz, T.; Lehrer, I.R. Antibiotic peptides from higher eukaryotes: Biology and applications. Mol. Med. Today 1999, 5, 292–297. [Google Scholar] [CrossRef]
- Bulet, P.; Stocklin, R.; Menin, L. Anti-microbial peptides: From invertebrates to vertebrates. Immunol. Rev. 2004, 198, 169–184. [Google Scholar] [CrossRef]
- Liu, N.; Lan, J.-F.; Sun, J.-J.; Jia, W.-M.; Zhao, X.-F.; Wang, J.-X. A novel crustin from Marsupenaeus japonicus promotes hemocyte phagocytosis. Dev. Comp. Immunol. 2015, 49, 313–322. [Google Scholar] [CrossRef]
- Sun, B.; Wang, Z.; Zhu, F. The crustin-like peptide plays opposite role in shrimp immune response to Vibrio alginolyticus and white spot syndrome virus (WSSV) infection. Fish Shellfish Immunol. 2017, 66, 487–496. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schmitt, P.; Rosa, R.D.; Destoumieux-Garzón, D. An intimate link between antimicrobial peptide sequence diversity and binding to essential components of bacterial membranes. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2016, 1858, 958–970. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.-X.; Wang, Y.; Fang, W.-H.; Wang, Y.; Zhou, J.-F.; Zhao, S.; Li, X.-C. Newly identified type II crustin (SpCrus2) in Scylla paramamosain contains a distinct cysteine distribution pattern exhibiting broad antimicrobial activity. Dev. Comp. Immunol. 2018, 84, 1–13. [Google Scholar] [CrossRef]
- Zhu, L.; Lan, J.-F.; Huang, Y.-Q.; Zhang, C.; Zhou, J.-F.; Fang, W.-H.; Yao, X.-J.; Wang, H.; Li, X.-C. SpALF4: A newly identified anti-lipopolysaccharide factor from the mud crab Scylla paramamosain with broad spectrum antimicrobial activity. Fish Shellfish Immunol. 2013, 36, 172–180. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; Gao, F.; Cui, Z. A novel C-type lectin with a YPD motif from Portunus trituberculatus (PtCLec1) mediating pathogen recognition and opsonization. Dev. Comp. Immunol. 2020, 106, 103609. [Google Scholar] [CrossRef]


| Microorganism | Minimal Inhibitory Concentrations (μM) |
|---|---|
| V. harveyi | 2.5 |
| V. parahaemolyticus | 2.5 |
| E. coli | >20 |
| S. aureus | >20 |
| Primer Name | Primer Sequence (5′-3′) | Annealing Temperature (°C) |
|---|---|---|
| 18S-qF | TATACGCTAGTGGAGCTGGAA | 55 |
| 18S-qR | GGGGAGGTAGTGACGAAAAAT | |
| LvCrustinVII-qF | CGTCCTCATCGGGCTCCTT | 60 |
| LvCrustinVII-qR | CGGCAATGTAGGCTTGGTGG | |
| rLvCrustinVII-F | GGATCCGAAGAATCGGAGGAAAACACGCGT | 60 |
| rLvCrustinVII-R | AAGCTTCTAGGAGGAATAGCACGGTTGCGC | |
| T7-F | TAATACGACTCACTATAGGG | 55 |
| T7-R | GCTAGTTATTGCTCAGCGGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Li, S.; Lv, Q.; Miao, M.; Li, X.; Li, F. Characterization of the Dual Functions of LvCrustinVII from Litopenaeus vannamei as Antimicrobial Peptide and Opsonin. Mar. Drugs 2022, 20, 157. https://doi.org/10.3390/md20030157
Hu J, Li S, Lv Q, Miao M, Li X, Li F. Characterization of the Dual Functions of LvCrustinVII from Litopenaeus vannamei as Antimicrobial Peptide and Opsonin. Marine Drugs. 2022; 20(3):157. https://doi.org/10.3390/md20030157
Chicago/Turabian StyleHu, Jie, Shihao Li, Qian Lv, Miao Miao, Xuechun Li, and Fuhua Li. 2022. "Characterization of the Dual Functions of LvCrustinVII from Litopenaeus vannamei as Antimicrobial Peptide and Opsonin" Marine Drugs 20, no. 3: 157. https://doi.org/10.3390/md20030157
APA StyleHu, J., Li, S., Lv, Q., Miao, M., Li, X., & Li, F. (2022). Characterization of the Dual Functions of LvCrustinVII from Litopenaeus vannamei as Antimicrobial Peptide and Opsonin. Marine Drugs, 20(3), 157. https://doi.org/10.3390/md20030157






