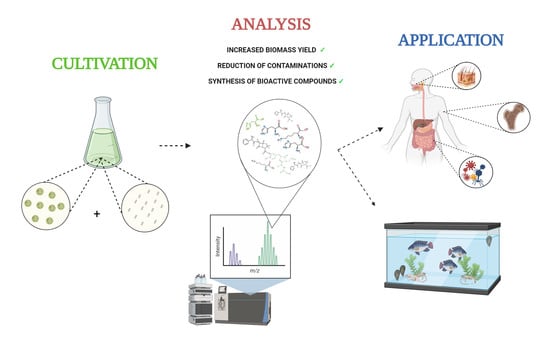
Biotechnological Enhancement of Probiotics through Co-Cultivation with Algae: Future or a Trend?
Abstract
1. Introduction
1.1. Algae as a Source of Bioactive Molecules for Human Well-Being
| Microalgae | Fatty Acids | Pigments | Other | Ref. |
|---|---|---|---|---|
| Spirulina sp. | / | / | Polysaccharides | [41] |
| Aphanizomenon flos-aquae | / | / | Mycosporine-like amino acids (MAA) | [42] |
| Lyngbya majuscula | / | / | Lyngbic acid, malyngamides, grenadadiene, debromogrenadiene, grenadamide | [43,44] |
| Arthrospira platensis | Monounsaturated (oleic acid) and polyunsaturated (γ-linolenic acid, DHA) | Zea, Ast, β-Car, Lut, Cantha | / | [45,46,47] |
| Limnospira maxima | Polyunsaturated (γ-linolenic acid) | C-PC, β-Car | α-tocopherol | [48,49,50] |
| Limnospira fusiformis | / | C-PC, β-Car | α-tocopherol, α-lipoic acid | [51,52] |
| Porphyridium purpureum | Saturated (palmitic acid), monounsaturated (palmitoleic acid), and polyunsaturated (EPA, arachidonic acid) | β-Car, Chl a, Zea, Chlide a, Cry, Phe a, Pheide a, PE, PBPs | / | [53,54,55] |
| Euglena gracilis | Polyunsaturated (EPA and DHA) | β-Car, Zea, Diato, Diadino, Neo | Paramylon, α-tocopherol | [56,57,58] |
| Isochrysis galbana | Polyunsaturated (EPA and DHA) | Fuco, Chl a | Amino acids (Arg, Met, Lys, Thr, Phe, His, Ile, Leu, Val, Trp) | [59,60,61] |
| Diacronema vlkianum | Saturated (myristic and palmitic acid), monounsaturated (palmitoleic acid), and polyunsaturated (stearidonic acid, EPA, and DHA) | Fuco, Lut, Zea, β-Car, Chl a, Chl c, Ast | α-tocopherol, p-sitosterol, stigmasterol | [62,63,64] |
| Crypthecodinium cohnii | Polyunsaturated (DHA) | / | / | [65] |
| Schizochytrium sp. * | Polyunsaturated (DHA) | Ast, Cantha, β-Car, Ech | / | [66,67] |
| Nannochloropsis gaditana | Polyunsaturated (EPA) | Ast, Cantha, Chl a | / | [68,69,70] |
| Nannochloropsis oculata | Polyunsaturated (EPA) | / | α-tocopherol | [71,72] |
| Odontella aurita | Polyunsaturated (EPA) | Chl a, Fuco | / | [73,74] |
| Phaeodactylum tricornutum | Polyunsaturated (EPA) | / | / | [75] |
| Tetraselmis suecica | Polyunsaturated (EPA) | Chl a, Chl b, α-Car, γ-Car, Lut, Lo, Viola, Neo, Ax | / | [76,77] |
| Auxenochlorella protothecoides | Saturated (palmitic and stearic acid), monounsaturated (oleic acid), and polyunsaturated (linoleic and linolenic acid) | Lut | / | [78,79] |
| Chlorella vulgaris | Saturated (palmitic and stearic acid), monounsaturated (oleic acid), and polyunsaturated (linolenic acid) | Ast, β-Car, Lut, Cantha, Lyco | / | [80,81] |
| Scenedesmus sp. “almeriensis” | Saturated (stearic, palmitic, and lauric acid), monounsaturated (oleic acid), polyunsaturated (linoleic and α-linoleic acid) | Lut, Ast, β-Car, Chl a, b, c | Haemagglutinin, MAA, amino acids (Ile, Leu, Met, Lys, Ala, Val, Arg, Cys and others), vitamin B, C, E | [42,82] |
| Haematococcus lacustris | / | Ast | / | [83] |
| Chlamydomonas reinhardtii | Saturated (palmitic acid), monounsaturated (oleic acid), polyunsaturated (α-linoleic and linoleic acid) | Chl a, b, Lut, β-Car, | / | [84,85,86] |
| Dunaliella salina | / | β-Car, α-Car, Zea, Lut | Sterols (7-dehydroporiferasterol, ergosterol) | [87,88] |
1.2. Probiotics as Health Supporters
2. Lessons from Natural Co-Culture Systems
3. Microalgae-Bacteria Consortia in Biotechnology
4. Methods in Biotechnological Co-Cultivation of Algae and Bacteria
4.1. Experimental Setup for the Co-Culture of Algae and Bacteria
| Microalgae | Probiotic Microorganism | T [°C] | pH | Inoculum Ratio | Agitation | Aim of Study | Ref. |
|---|---|---|---|---|---|---|---|
| Isochrysis galbana | Carnobacterium piscicola Lactobacillus brevis Lactobacillus casei ssp. casei, Lactobacillus helveticus Lactococcus lactis spp. Lactis Leuconostoc mesenteroides spp. mesenteroides Pediococcus acidilactici | 22 ± 1 | No data | No data | Manually shaken twice daily | Effect on the growth rate of microalgae | [139] |
| Chlorella sorokiniana | Bifidobacterium longum Lactobacillus plantarum | 4 | No data | 1:1000 1:1 | Without | Evaluate microbial effects (antiviral) on rotavirus | [108] |
| Nannochloropsis oceanica | “Probiotic” bacterial strain isolates | 25 | 8.5 | 6:1 30:1 60:1 | 100 rpm on an orbital shaker | Enhancing eicosapentaenoic acid (EPA) production | [140] |
| Botryococcus braunii | Rhizobium sp. | 20 25 ± 2 | No data | No data | No data | Enhancing growth rate | [141] |
| Isochrysis Galbana, Chaetoceros calcitrans | Bacillus licheniformis Bacillus subtilis | 28 ± 1 | 8 | 4.5:1 | Air-flow | In vitro growth of co-cultured microalgae and bacteria and their effect on oyster C. sikamae | [142] |
| Chlorella sorokiniana | Azospirillum brasilanse | 28 | 7.2 | 1:1 | Air-flow (with CO2) and stir bar | Investigation of oxidative stress in microalgae | [143] |
| Spirulina platensis | Lactobacillus casei Lactobacillus acidophilus Streptococcus thermophilus | 37 | 6.8 6.2 | / | No data | Stimulation of Lactic Acid bacteria growth with spirulina powder and their antibacterial activity | [144] |
| Arthrospira platensis | Lacticaseibacillus casei Lacticaseibacillus rhamnosus | 37 | No data | No data | Without | Evaluation of solid-state fermentation of A. platensis on two species of lactic acid bacteria | [145] |
| Spirulina platensis | Lactobacillus acidophilus | 42 | Endpoint pH = 4.6–4.7 | No data | No data | Formulation of probiotic yogurts enriched with Spirulina biomass | [146] |
| Planktochlorella sp. | Lactobacillus rhamnosus | 37 | No data | / | No data | Prebiotic effect of algal extracts on growth of probiotic species | [147] |

4.2. Downstream Processing and Analysis of Algae and Bacteria Co-Culture
5. Microalgae and Bacteria Consortia for Nutraceuticals

6. Overview of Algae-Probiotics Co-Culture in Aquaculture
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Demoulin, C.F.; Lara, Y.J.; Cornet, L.; François, C.; Baurain, D.; Wilmotte, A.; Javaux, E.J. Cyanobacteria evolution: Insight from the fossil record. Free Radic. Biol. Med. 2019, 140, 206–223. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Plaza, M.; Herrero, M.; Alejandro Cifuentes, A.; Ibáñez, E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Biology of Microalgae; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current status of the algae production industry in Europe: An emerging sector of the blue bioeconomy. Front. Mar. Sci. 2021, 7, 1247. [Google Scholar] [CrossRef]
- Cheng, K.C.; Ogden, K.L. Algal biofuels: The research. Chem. Eng. Prog. 2011, 107, 42–47. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible microalgae and their bioactive compounds in the prevention and treatment of metabolic alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Cerón-García, M.C.; González-López, C.V.; Camacho-Rodríguez, J.; López-Rosales, L.; García-Camacho, F.; Molina-Grima, E. Maximizing carotenoid extraction from microalgae used as food additives and determined by liquid chromatography (HPLC). Food Chem. 2018, 257, 316–324. [Google Scholar] [CrossRef]
- Lima, F.A.V.; Joventino, I.P.; Joventino, F.P.; de Almeida, A.C.; Neves, K.R.T.; do Carmo, M.R.; Leal, L.K.A.M.; de Andrade, G.M.; de Barros Viana, G.S. Neuroprotective activities of Spirulina platensis in the 6-OHDA model of parkinson’s disease are related to its anti-inflammatory effects. Neurochem. Res. 2017, 42, 3390–3400. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.R.; Khalifa, H.A.; Abdel-Motal, S.M.; Mohammed, H.H.; Elewa, Y.H.A.; Mahmoud, H.A. Spirulina platensis attenuates the associated neurobehavioral and inflammatory response impairments in rats exposed to lead acetate. Ecotoxicol. Environ. Saf. 2018, 157, 255–265. [Google Scholar] [CrossRef]
- Moradi-Kor, N.; Ghanbari, A.; Rashidipour, H.; Bandegi, A.R.; Yousefi, B.; Barati, M.; Kokhaei, P.; Rashidy-Pour, A. Therapeutic effects of Spirulina platensis against adolescent stress-induced oxidative stress, brain-derived neurotrophic factor alterations and morphological remodeling in the amygdala of adult female rats. J. Exp. Pharmacol. 2020, 12, 75–85. [Google Scholar] [CrossRef]
- Sorrenti, V.; Castagna, D.A.; Fortinguerra, S.; Buriani, A.; Scapagnini, G.; Willcox, D.C. Spirulina microalgae and brain health: A scoping review of experimental and clinical evidence. Mar. Drugs 2021, 19, 293. [Google Scholar] [CrossRef]
- Marco Antonio Hernández-Lepe, A.W.-M.; López-Díaz, J.A.; Juárez-Oropeza, M.A.; Luqueño-Bocardo, O.I.; Hernández-Torres, R.P.; Ramos-Jiménez, A. Hypolipidemic effect of Arthrospira (Spirulina) maxima supplementation and a systematic physical exercise program in overweight and obese men: A double-blind, randomized, and crossover controlled trial. Mar. Drugs 2019, 17, 270. [Google Scholar] [CrossRef]
- Kang, C.D.; Lee, J.S.; Park, T.H.; Sim, S.J. Complementary limiting factors of astaxanthin synthesis during photoautotrophic induction of haematococcus pluvialis: C/N ratio and light intensity. Appl. Microbiol. Biotechnol. 2007, 74, 987–994. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef]
- Kılınç, B.; Cirik, S.; Turan, G. Organic Agriculture Towards Sustainability; IntechOpen: London, UK, 2014. [Google Scholar] [CrossRef]
- Alghazwi, M.; Kan, Y.Q.; Zhang, W.; Gai, W.P.; Garson, M.J.; Smid, S. Neuroprotective activities of natural products from marine macroalgae during 1999–2015. J. Appl. Phycol. 2016, 28, 3599–3616. [Google Scholar] [CrossRef]
- Cikoš, A.M.; Jurin, M.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Update on monoterpenes from red macroalgae: Isolation, analysis, and bioactivity. Mar. Drugs 2019, 17, 537. [Google Scholar] [CrossRef]
- Hannan, M.A.; Sohag, A.A.M.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, D.F.; Hossain, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [CrossRef]
- Jerković, I.; Cikoš, A.M.; Babić, S.; Čižmek, L.; Bojanić, K.; Aladić, K.; Ul’yanovskii, N.V.; Kosyakov, D.S.; Lebedev, A.T.; Čož-Rakovac, R.; et al. Bioprospecting of less-polar constituents from endemic brown macroalga Fucus virsoides J. Agardh from the adriatic sea and targeted antioxidant effects in vitro and in vivo (zebrafish model). Mar. Drugs 2021, 19, 235. [Google Scholar] [CrossRef] [PubMed]
- Radman, S.; Cikoš, A.-M.; Flanjak, I.; Babić, S.; Čižmek, L.; Šubarić, D.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Less polar compounds and targeted antioxidant potential (in vitro and in vivo) of Codium adhaerens C. Agardh 1822. Pharmaceuticals 2021, 14, 944. [Google Scholar] [CrossRef] [PubMed]
- Radman, S.; Čižmek, L.; Babić, S.; Cikoš, A.-M.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Bioprospecting of less-polar fractions of Ericaria crinita and Ericaria amentacea: Developmental Toxicity and antioxidant activity. Mar. Drugs 2022, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Fields, F.J.; Lejzerowicz, F.; Schroeder, D.; Ngoi, S.M.; Tran, M.; McDonald, D.; Jiang, L.; Chang, J.T.; Knight, R.; Mayfield, S. Effects of the microalgae chlamydomonas on gastrointestinal health. J. Funct. Foods 2020, 65, 103738. [Google Scholar] [CrossRef]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; Mcsorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef]
- Tong, T.; Li, J.; Ko, D.O.; Kim, B.S.; Zhang, C.; Ham, K.S.; Kang, S.G. In vitro antioxidant potential and inhibitory effect of seaweed on enzymes relevant for hyperglycemia. Food Sci. Biotechnol. 2014, 23, 2037–2044. [Google Scholar] [CrossRef]
- Messyasz, B.; Michalak, I.; Łęska, B.; Schroeder, G.; Górka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.; Rój, E.; Wilk, R.; et al. Valuable natural products from marine and freshwater macroalgae obtained from supercritical fluid extracts. J. Appl. Phycol. 2018, 30, 591–603. [Google Scholar] [CrossRef]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and red macroalgae as potential sources of antioxidants and UV radiation-absorbing compounds for cosmeceutical applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef]
- Stolz, P.; Obermayer, B. Manufacturing microalgae for skin care. Cosmet. Toilet. 2005, 120, 99–106. [Google Scholar]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Ariede, M.B.; Morocho-Jácome, A.L.; Candido, T.M.; Lourenço, F.R.; Kato, E.T.M.; Lima, F.V.; Rosado, C.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Is the botryococcus braunii dry biomass an adjuvant for anti-UVB topical formulations? Sci. Pharm. 2020, 88, 22. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [PubMed]
- Schüler, L.M.; Gangadhar, K.N.; Duarte, P.; Placines, C.; Molina-Márquez, A.M.; Léon-Bañares, R.; Sousa, V.S.; Varela, J.; Barreira, L. Improvement of carotenoid extraction from a recently isolated, robust microalga, Tetraselmis sp. CTP4 (Chlorophyta). Bioprocess Biosyst. Eng. 2020, 43, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Kalasariya, H.S.; Yadav, V.K.; Yadav, K.K.; Tirth, V.; Algahtani, A.; Islam, S.; Gupta, N.; Jeon, B.H. Seaweed-based molecules and their potential biological activities: An eco-sustainable cosmetics. Molecules 2021, 26, 5313. [Google Scholar] [CrossRef]
- Lian, J.; Wijffels, R.H.; Smidt, H.; Sipkema, D. The effect of the algal microbiome on industrial production of microalgae. Microb. Biotechnol. 2018, 11, 806–818. [Google Scholar] [CrossRef]
- Lee, S.M.; Ryu, C.M. Algae as new kids in the beneficial plant microbiome. Front. Plant Sci. 2021, 12, 599742. [Google Scholar] [CrossRef]
- Makut, B.B.; Das, D.; Goswami, G. Production of microbial biomass feedstock via co-cultivation of microalgae-bacteria consortium coupled with effective wastewater treatment: A sustainable approach. Algal Res. 2019, 37, 228–239. [Google Scholar] [CrossRef]
- Leyland, B.; Leu, S.; Boussiba, S. Are thraustochytrids algae? Fungal Biol. 2017, 121, 835–840. [Google Scholar] [CrossRef]
- Amaro, H.M.; Barros, R.; Guedes, A.C.; Sousa-Pinto, I.; Malcata, F.X. Microalgal compounds modulate carcinogenesis in the gastrointestinal tract. Trends Biotechnol. 2013, 31, 92–98. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Sitachitta, N.; Gerwick, W.H. Grenadadiene and grenadamide, cyclopropyl-containing fatty acid metabolites from the marine cyanobacterium lyngbya majuscula. J. Nat. Prod. 1998, 61, 681–684. [Google Scholar] [CrossRef]
- Nogle, L.M.; Gerwick, W.H. Diverse secondary metabolites from a puerto rican collection of lyngbya majuscula. J. Nat. Prod. 2003, 66, 217–220. [Google Scholar] [CrossRef]
- Mendes, R.L.; Nobre, B.P.; Cardoso, M.T.; Pereira, A.P.; Palavra, A.F. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorg. Chim. Acta 2003, 356, 328–334. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Jaime, L.; Santoyo, S.; Reglero, G.; Cifuentes, A.; Ibañez, E.; Señoráns, F.J. Screening of functional compounds in supercritical fluid extracts from Spirulina platensis. Food Chem. 2007, 102, 1357–1367. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Anish Zacharia, J.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.; Bisen, P. Spirulina in health care management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef]
- Ponce-Canchihuamán, J.C.; Pérez-Méndez, O.; Hernández-Mũoz, R.; Torres-Durán, P.V.; Juárez-Oropeza, M.A. Protective effects of Spirulina maxima on hyperlipidemia and oxidative-stress induced by lead acetate in the liver and kidney. Lipids Health Dis. 2010, 9, 35. [Google Scholar] [CrossRef]
- Kumar, D.; Dhar, D.W.; Pabbi, S.; Kumar, N.; Walia, S. Extraction and purification of C-phycocyanin from Spirulina platensis (CCC540). Indian J. Plant Physiol. 2014, 19, 184–188. [Google Scholar] [CrossRef]
- Minkova, K.M.; Tchernov, A.A.; Tchorbadjieva, M.I.; Fournadjieva, S.T.; Antova, R.E.; Busheva, M.C. Purification of C-phycocyanin from Spirulina (Arthrospira) fusiformis. J. Biotechnol. 2003, 102, 55–59. [Google Scholar] [CrossRef]
- Upasani, C.D.; Balaraman, R. Protective effect of Spirulina on lead induced deleterious changes in the lipid peroxidation and endogenous antioxidants in rats. Phyther. Res. 2003, 17, 330–334. [Google Scholar] [CrossRef]
- Juin, C.; Bonnet, A.; Nicolau, E.; Bérard, J.B.; Devillers, R.; Thiéry, V.; Cadoret, J.P.; Picot, L. UPLC-MSE profiling of phytoplankton metabolites: Application to the identification of pigments and structural analysis of metabolites in porphyridium purpureum. Mar. Drugs 2015, 13, 2541–2558. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, M.D.; Kathiresan, S.; Bhattacharya, S.; Sarada, R. Culture media optimization of porphyridium purpureum: Production potential of biomass, total lipids, arachidonic and eicosapentaenoic acid. J. Food Sci. Technol. 2016, 53, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, S.; Nie, S.; Li, J. A study on the synthesis and accumulation of phycoerythrin in porphyridium purpureum. AIP Conf. Proc. 2019, 2110, 020028. [Google Scholar] [CrossRef]
- Šantek, B.; Felski, M.; Friehs, K.; Lotz, M.; Flaschel, E. Production of paramylon, a β-1,3-glucan, by heterotrophic cultivation of euglena gracilis on a synthetic medium. Eng. Life Sci. 2009, 9, 23–28. [Google Scholar] [CrossRef]
- Schwarzhans, J.P.; Cholewa, D.; Grimm, P.; Beshay, U.; Risse, J.M.; Friehs, K.; Flaschel, E. Dependency of the fatty acid composition of euglena gracilis on growth phase and culture conditions. J. Appl. Phycol. 2015, 27, 1389–1399. [Google Scholar] [CrossRef]
- Tanno, Y.; Kato, S.; Takahashi, S.; Tamaki, S.; Takaichi, S.; Kodama, Y.; Sonoike, K.; Shinomura, T. Light dependent accumulation of β-carotene enhances photo-acclimation of euglena gracilis. J. Photochem. Photobiol. B Biol. 2020, 209, 111950. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Mendiola, J.A.; Fontecha, J.; Van Den Broek, L.A.M.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Downstream processing of isochrysis galbana: A step towards microalgal biorefinery. Green Chem. 2015, 17, 4599–4609. [Google Scholar] [CrossRef]
- Bonfanti, C.; Cardoso, C.; Afonso, C.; Matos, J.; Garcia, T.; Tanni, S.; Bandarra, N.M. Potential of microalga isochrysis galbana: Bioactivity and bioaccessibility. Algal Res. 2018, 29, 242–248. [Google Scholar] [CrossRef]
- Mishra, N.; Mishra, N. Exploring the biologically active metabolites of isochrysis galbana in pharmaceutical interest: An overview. Int. J. Pharm. Sci. Res. 2018, 9, 2162–2174. [Google Scholar] [CrossRef]
- Donato, M.; Vilela, M.H.; Bandarra, N.M. Fatty acids, sterols, α-tocopherol and total carotenoids composition of diacronema vlkianum. J. Food Lipids 2003, 10, 267–276. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- De Mello-Sampayo, C.; Paterna, A.; Polizzi, A.; Duarte, D.; Batista, I.; Pinto, R.; Gonçalves, P.; Raymundo, A.; Batista, A.P.; Gouveia, L.; et al. Evaluation of marine microalga diacronema vlkianum biomass fatty acid assimilation in wistar rats. Molecules 2017, 22, 1097. [Google Scholar] [CrossRef]
- Liu, L.; Wang, F.; Pei, G.; Cui, J.; Diao, J.; Lv, M.; Chen, L.; Zhang, W. Repeated Fed-Batch Strategy and Metabolomic Analysis to Achieve High Docosahexaenoic Acid Productivity in Crypthecodinium Cohnii. Microb. Cell Fact. 2020, 19, 91. [Google Scholar] [CrossRef]
- Park, H.; Kwak, M.; Seo, J.W.; Ju, J.H.; Heo, S.Y.; Park, S.M.; Hong, W.K. Enhanced production of carotenoids using a thraustochytrid microalgal strain containing high levels of docosahexaenoic acid-rich oil. Bioprocess Biosyst. Eng. 2018, 41, 1355–1370. [Google Scholar] [CrossRef]
- Allen, K.M.; Habte-Tsion, H.M.; Thompson, K.R.; Filer, K.; Tidwell, J.H.; Kumar, V. Freshwater microalgae (Schizochytrium sp.) as a Substitute to Fish Oil for Shrimp Feed. Sci. Rep. 2019, 9, 6178. [Google Scholar] [CrossRef]
- Lubián, L.M.; Montero, O.; Moreno-Garrido, I.; Huertas, I.E.; Sobrino, C.; González-Del Valle, M.; Parés, G. Nannochloropsis (Eustigmatophyceae) as source of commercially valuable pigments. J. Appl. Phycol. 2000, 12, 249–255. [Google Scholar] [CrossRef]
- Menegol, T.; Romero-Villegas, G.I.; López-Rodríguez, M.; Navarro-López, E.; López-Rosales, L.; Chisti, Y.; Cerón-García, M.C.; Molina-Grima, E. Mixotrophic production of polyunsaturated fatty acids and carotenoids by the microalga nannochloropsis gaditana. J. Appl. Phycol. 2019, 31, 2823–2832. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Mantell, C.; Rodríguez, M.; Martínez De La Ossa, E.; Lubián, L.M.; Montero, O. Supercritical fluid extraction of carotenoids and chlorophyll a from nannochloropsis gaditana. J. Food Eng. 2005, 66, 245–251. [Google Scholar] [CrossRef]
- Durmaz, Y.; Vitamin, E. (α-Tocopherol) production by the marine microalgae Nannochloropsis oculata (Eustigmatophyceae) in nitrogen limitation. Aquaculture 2007, 272, 717–722. [Google Scholar] [CrossRef]
- Pieber, S.; Schober, S.; Mittelbach, M. Pressurized fluid extraction of polyunsaturated fatty acids from the microalga Nannochloropsis oculata. Biomass Bioenergy 2012, 47, 474–482. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Fouqueray, M.; Mimouni, V.; Ulmann, L.; Jacquette, B.; Tremblin, G. Effect of UV stress on the fatty acid and lipid class composition in two marine microalgae pavlova lutheri (Pavlovophyceae) and Odontella aurita (Bacillariophyceae). J. Appl. Phycol. 2010, 22, 629–638. [Google Scholar] [CrossRef]
- Haimeur, A.; Ulmann, L.; Mimouni, V.; Guéno, F.; Pineau-Vincent, F.; Meskini, N.; Tremblin, G. The role of Odontella aurita, a marine diatom rich in EPA, as a dietary supplement in dyslipidemia, platelet function and oxidative stress in high-fat fed rats. Lipids Health Dis. 2012, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Mearns-Spragg, A.; Smith, V.J. A fatty acid from the diatom phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant staphylococcus aureus (MRSA). Mar. Biotechnol. 2009, 11, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, H.M.; de la Jara Valido, A.; Duarte, L.C.; Presmanes, K.F. Estimate by means of flow cytometry of variation in composition of fatty acids from Tetraselmis suecica in response to culture conditions. Aquac. Int. 2010, 18, 189–199. [Google Scholar] [CrossRef]
- Sansone, C.; Galasso, C.; Orefice, I.; Nuzzo, G.; Luongo, E.; Cutignano, A.; Romano, G.; Brunet, C.; Fontana, A.; Esposito, F.; et al. The green microalga Tetraselmis suecica reduces oxidative stress and induces repairing mechanisms in human cells. Sci. Rep. 2017, 7, 41215. [Google Scholar] [CrossRef]
- Xiao, Y.; He, X.; Ma, Q.; Lu, Y.; Bai, F.; Dai, J.; Wu, Q. Photosynthetic accumulation of lutein in auxenochlorella protothecoides after heterotrophic growth. Mar. Drugs 2018, 16, 283. [Google Scholar] [CrossRef]
- Krzemińska, I.; Oleszek, M.; Wiacek, D. Liquid anaerobic digestate as a source of nutrients for lipid and fatty acid accumulation by auxenochlorella protothecoides. Molecules 2019, 24, 3582. [Google Scholar] [CrossRef]
- Jay, M.I.; Kawaroe, M.; Effendi, H. Lipid and fatty acid composition microalgae chlorella vulgaris using photobioreactor and open pond. IOP Conf. Ser. Earth Environ. Sci. 2018, 141, 012015. [Google Scholar] [CrossRef]
- Gouveia, L.; Choubert, G.; Pereira, N.; Santinha, J.; Empis, J.; Gomes, E. Pigmentation of gilthead seabream, sparus aurata (L. 1875), using chlorella vulgaris (Chlorophyta, Volvocales) microalga. Aquac. Res. 2002, 33, 987–993. [Google Scholar] [CrossRef]
- Ishaq, A.G.; Matias-Peralta, H.M.; Basri, H. Bioactive compounds from green microalga Scenedesmus and its potential applications: A brief review. Pertanika J. Trop. Agric. Sci. 2016, 39, 1–15. [Google Scholar]
- Han, S.I.; Yao, J.; Lee, C.; Park, J.; Choi, Y.E. A novel approach to enhance astaxanthin production in haematococcus lacustris using a microstructure-based culture platform. Algal Res. 2019, 39, 101464. [Google Scholar] [CrossRef]
- Siaut, M.; Cuiné, S.; Cagnon, C.; Fessler, B.; Nguyen, M.; Carrier, P.; Beyly, A.; Beisson, F.; Triantaphylidès, C.; Li-Beisson, Y.; et al. Oil accumulation in the model green alga chlamydomonas reinhardtii: Characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol. 2011, 11, 7. [Google Scholar] [CrossRef]
- Gille, A.; Trautmann, A.; Posten, C.; Briviba, K. Bioaccessibility of carotenoids from chlorella vulgaris and chlamydomonas reinhardtii. Int. J. Food Sci. Nutr. 2016, 67, 507–513. [Google Scholar] [CrossRef]
- Darwish, R.; Gedi, M.A.; Akepach, P.; Assaye, H.; Zaky, A.S.; Gray, D.A. Chlamydomonas reinhardtii is a potential food supplement with the capacity to outperform chlorella and Spirulina. Appl. Sci. 2020, 10, 6736. [Google Scholar] [CrossRef]
- Hu, C.C.; Lin, J.T.; Lu, F.J.; Chou, F.P.; Yang, D.J. Determination of carotenoids in dunaliella salina cultivated in taiwan and antioxidant capacity of the algal carotenoid extract. Food Chem. 2008, 109, 439–446. [Google Scholar] [CrossRef]
- Francavilla, M.; Trotta, P.; Luque, R. Phytosterols from dunaliella tertiolecta and dunaliella salina: A potentially novel industrial application. Bioresour. Technol. 2010, 101, 4144–4150. [Google Scholar] [CrossRef]
- Lilly, D.M.; Stillwell, R.H. Probiotics: Growth-promoting factors produced by microorganisms. Science 1965, 147, 747–748. [Google Scholar] [CrossRef]
- Martín, R.; Langella, P. Emerging health concepts in the probiotics field: Streamlining the definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef]
- Schrezenmeir, J.; de Vrese, M. Probiotics, prebiotics, and synbiotics—Approaching a definition. Am. J. Clin. Nutr. 2001, 73, 361S–364S. [Google Scholar] [CrossRef]
- Cuello-Garcia, C.A.; Brozek, J.L.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Terracciano, L.; Gandhi, S.; Agarwal, A.; Zhang, Y.; Schünemann, H.J. Probiotics for the Prevention of Allergy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Allergy Clin. Immunol. 2015, 136, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.; Merrifield, C.A.; Hutkins, R. Probiotics for human use. In Nutrition Bulletin; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2018; pp. 212–225. [Google Scholar] [CrossRef]
- Versalovic, J. The human microbiome and probiotics: Implications for pediatrics. Ann. Nutr. Metab. 2013, 63, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication modulation of signaling pathways in the intestine. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Gourbeyre, P.; Denery, S.; Bodinier, M. Probiotics, prebiotics, and synbiotics: Impact on the gut immune system and allergic reactions. J. Leukoc. Biol. 2011, 89, 685–695. [Google Scholar] [CrossRef]
- Lew, L.C.; Liong, M.T. Bioactives from probiotics for dermal health: Functions and benefits. J. Appl. Microbiol. 2013, 114, 1241–1253. [Google Scholar] [CrossRef]
- Puebla-Barragan, S.; Reid, G. Probiotics in cosmetic and personal care products: Trends and challenges. Molecules 2021, 26, 1249. [Google Scholar] [CrossRef]
- Di Marzio, L.; Cinque, B.; Cupelli, F.; De Simone, C.; Cifone, M.G.; Giuliani, M. Increase of skin-ceramide levels in aged subjects following a short-term topical application of bacterial sphingomyelinase from Streptococcus thermophilus. Int. J. Immunopathol. Pharmacol. 2008, 21, 137–143. [Google Scholar] [CrossRef]
- Gueniche, A.; Benyacoub, J.; Blum, S.; Breton, L.; Castiel, I. Probiotics for skin benefits. In Nutritional Cosmetics; Elsevier: Amsterdam, The Netherlands, 2009; pp. 421–439. [Google Scholar] [CrossRef]
- Van Der Aa, L.B.; Heymans, H.S.A.; Van Aalderen, W.M.C.; Sprikkelman, A.B. Probiotics and prebiotics in atopic dermatitis: Review of the theoretical background and clinical evidence: Review article. Pediatr. Allergy Immunol. 2010, 21, e355–e367. [Google Scholar] [CrossRef]
- Guéniche, A.; Bastien, P.; Ovigne, J.M.; Kermici, M.; Courchay, G.; Chevalier, V.; Breton, L.; Castiel-Higounenc, I. Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp. Dermatol. 2010, 19, e1–e8. [Google Scholar] [CrossRef]
- Levkovich, T.; Poutahidis, T.; Smillie, C.; Varian, B.J.; Ibrahim, Y.M.; Lakritz, J.R.; Alm, E.J.; Erdman, S.E. Probiotic bacteria induce a “glow of health”. PLoS ONE 2013, 8, e53867. [Google Scholar] [CrossRef]
- Arck, P.; Handjiski, B.; Hagen, E.; Pincus, M.; Bruenahl, C.; Bienenstock, J.; Paus, R. Is there a “gut-brain-skin axis”? Exp. Dermatol. 2010, 19, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Cantú-Bernal, S.; Domínguez-Gámez, M.; Medina-Peraza, I.; Aros-Uzarraga, E.; Ontiveros, N.; Flores-Mendoza, L.; Gomez-Flores, R.; Tamez-Guerra, P.; González-Ochoa, G. Enhanced Viability and Anti-Rotavirus Effect of Bifidobacterium longum and Lactobacillus plantarum in combination with Chlorella sorokiniana in a dairy product. Front. Microbiol. 2020, 11, 875. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Canon, F.; Nidelet, T.; Guédon, E.; Thierry, A.; Gagnaire, V. Understanding the mechanisms of positive microbial interactions that benefit lactic acid bacteria co-cultures. Front. Microbiol. 2020, 11, 2088. [Google Scholar] [CrossRef]
- Bell, W.; Mitchell, R. Chemotactic and growth responses of marine bacteria to algal extracellular products. Biol. Bull. 1972, 143, 265–277. [Google Scholar] [CrossRef]
- Kouzuma, A.; Watanabe, K. Exploring the potential of algae/bacteria interactions. Curr. Opin. Biotechnol. 2015, 33, 125–129. [Google Scholar] [CrossRef]
- Zhou, J.; Lyu, Y.; Richlen, M.L.; Anderson, D.M.; Cai, Z. Quorum sensing is a language of chemical signals and plays an ecological role in algal-bacterial interactions. Crit. Rev. Plant Sci. 2016, 35, 81–105. [Google Scholar] [CrossRef]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Moran, M.A. Evolutionary ecology of the marine roseobacter clade. Microbiol. Mol. Biol. Rev. 2014, 78, 573–587. [Google Scholar] [CrossRef]
- Durham, B.P.; Sharma, S.; Luo, H.; Smith, C.B.; Amin, S.A.; Bender, S.J.; Dearth, S.P.; Van Mooy, B.A.S.; Campagna, S.R.; Kujawinski, E.B.; et al. Cryptic carbon and sulfur cycling between surface ocean plankton. Proc. Natl. Acad. Sci. USA 2015, 112, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.A.; Green, D.H.; Hart, M.C.; Kü, F.C.; Sunda, W.G.; Carrano, C.J.; Karl, D.M. Photolysis of iron-siderophore chelates promotes bacterial-algal mutualism. Proc. Natl. Acad. Sci. USA 2009, 106, 17071–17076. [Google Scholar] [CrossRef]
- Villa, J.A.; Ray, E.E.; Barney, B.M. Azotobacter vinelandii siderophore can provide nitrogen to support the culture of the green algae Neochloris oleoabundans and Scenedesmus sp. BA032. FEMS Microbiol. Lett. 2014, 351, 70–77. [Google Scholar] [CrossRef]
- Dao, G.H.; Wu, G.X.; Wang, X.X.; Zhuang, L.L.; Zhang, T.Y.; Hu, H.Y. Enhanced growth and fatty acid accumulation of microalgae Scenedesmus sp. LX1 by Two types of auxin. Bioresour. Technol. 2018, 247, 561–567. [Google Scholar] [CrossRef]
- Zuroff, T.R.; Xiques, S.B.; Curtis, W.R. Consortia-mediated bioprocessing of cellulose to ethanol with a symbiotic clostridium phytofermentans/yeast co-culture. Biotechnol. Biofuels 2013, 6, 59. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, S.H.; Cheng, C.L.; Guo, W.Q.; Nagarajan, D.; Ren, N.Q.; Lee, D.J.; Chang, J.S. Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour. Technol. 2016, 222, 485–497. [Google Scholar] [CrossRef]
- García, D.; Alcántara, C.; Blanco, S.; Pérez, R.; Bolado, S.; Muñoz, R. Enhanced carbon, nitrogen and phosphorus removal from domestic wastewater in a novel anoxic-aerobic photobioreactor coupled with biogas upgrading. Chem. Eng. J. 2017, 313, 424–434. [Google Scholar] [CrossRef]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environ. Int. 2013, 51, 59–72. [Google Scholar] [CrossRef]
- Muñoz, R.; Guieysse, B. Algal-bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Ramanan, R.; Heo, J.; Lee, J.; Kim, B.H.; Oh, H.M.; Kim, H.S. Enhancing microalgal biomass productivity by engineering a microalgal-bacterial community. Bioresour. Technol. 2015, 175, 578–585. [Google Scholar] [CrossRef]
- Perečinec, M.G.; Babić, S.; Čižmek, L.; Selmani, A.; Popović, N.T.; Sikirić, M.D.; Strunjak-Perović, I.; Čož-Rakovac, R. Selenite as a lipid inductor in marine microalga dunaliella tertiolecta: Comparison of one-stage and two-stage cultivation strategies. Appl. Biochem. Biotechnol. 2021, 194, 930–949. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- Kim, B.H.; Ramanan, R.; Cho, D.H.; Oh, H.M.; Kim, H.S. Role of rhizobium, a plant growth promoting bacterium, in enhancing algal biomass through mutualistic interaction. Biomass Bioenergy 2014, 69, 95–105. [Google Scholar] [CrossRef]
- Nishio, K.; Hashimoto, K.; Watanabe, K. Light/electricity conversion by defined cocultures of chlamydomonas and geobacter. J. Biosci. Bioeng. 2013, 115, 412–417. [Google Scholar] [CrossRef]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef]
- Horácio, E.H.; Zucareli, C.; Gavilanes, F.Z.; Yunes, J.S.; dos Santos Sanzovo, A.W.; Andrade, D.S. Co-inoculation of rhizobia, azospirilla and cyanobacteria for increasing common bean production. Semin. Agrar. 2020, 41, 2015–2028. [Google Scholar] [CrossRef]
- Gavilanes, F.Z.; Souza Andrade, D.; Zucareli, C.; Horácio, E.H.; Sarkis Yunes, J.; Barbosa, A.P.; Alves, L.A.R.; Cruzatty, L.G.; Maddela, N.R.; de Fátima Guimarães, M. Co-inoculation of anabaena cylindrica with azospirillum brasilense increases grain yield of maize hybrids. Rhizosphere 2020, 15, 100224. [Google Scholar] [CrossRef]
- Geries, L.S.M.; Elsadany, A.Y. Maximizing growth and productivity of onion (Allium cepa L.) by Spirulina platensis extract and nitrogen-fixing endophyte pseudomonas stutzeri. Arch. Microbiol. 2021, 203, 169–181. [Google Scholar] [CrossRef]
- Kopta, T.; Pavlíková, M.; Sȩkara, A.; Pokluda, R.; Maršálek, B. Effect of bacterial-algal biostimulant on the yield and internal quality of lettuce (Lactuca sativa L.) produced for spring and summer crop. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 615–621. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.J.; Del Rio, L.F.; Orsat, V. Producing PHAs in the bioeconomy—Towards a sustainable bioplastic. Sustain. Prod. Consum. 2017, 9, 58–70. [Google Scholar] [CrossRef]
- Padmaperuma, G.; Kapoore, R.V.; Gilmour, D.J.; Vaidyanathan, S. Microbial consortia: A critical look at microalgae co-cultures for enhanced biomanufacturing. Crit. Rev. Biotechnol. 2018, 38, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef]
- Planas, M.; Vázquez, J.A.; Novoa, B. Stimulative effect of lactic acid bacteria in the growth of the microalgae isochrysis galbana. J. Coast. Life Med. 2015, 3, 925–930. [Google Scholar] [CrossRef]
- Liu, B.; Eltanahy, E.E.; Liu, H.; Chua, E.T.; Thomas-Hall, S.R.; Wass, T.J.; Pan, K.; Schenk, P.M. Growth-promoting bacteria double eicosapentaenoic acid yield in microalgae. Bioresour. Technol. 2020, 316, 123916. [Google Scholar] [CrossRef]
- Rivas, M.O.; Vargas, P.; Riquelme, C.E. Interactions of botryococcus braunii cultures with bacterial biofilms. Microb. Ecol. 2010, 60, 628–635. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, A.C.; Mazón-Suástegui, J.M.; Flores-Miranda, M.d.C.; Luna-González, A.; Ochoa, N.; Melgar-Valdés, C.E.; Campa-Córdova, Á.I. Probiotic bacterium and microalga interaction on rearing kumamoto oyster crassostrea sikamea spat. Curr. Microbiol. 2020, 77, 2758–2765. [Google Scholar] [CrossRef]
- Peng, H.; de-Bashan, L.E.; Higgins, B.T. Azospirillum brasilense reduces oxidative stress in the green microalgae Chlorella sorokiniana under different stressors. J. Biotechnol. 2021, 325, 179–185. [Google Scholar] [CrossRef]
- Bhowmik, D.; Dubey, J.; Mehra, S. Probiotic efficiency of spirulina platensis-stimulating growth of lactic acid bacteria. World J. Dairy Food Sci. 2009, 4, 160–163. [Google Scholar]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Solid-state fermentation of Arthrospira platensis to implement new food products: Evaluation of stabilization treatments and bacterial growth on the volatile fraction. Foods 2021, 10, 67. [Google Scholar] [CrossRef]
- Patel, P.; Jethani, H.; Radha, C.; Vijayendra, S.V.N.; Mudliar, S.N.; Sarada, R.; Chauhan, V.S. Development of a carotenoid enriched probiotic yogurt from fresh biomass of Spirulina and its characterization. J. Food Sci. Technol. 2019, 56, 3721–3731. [Google Scholar] [CrossRef]
- Potocki, L.; Oklejewicz, B.; Kuna, E.; Szpyrka, E.; Duda, M.; Zuczek, J. Application of green algal planktochlorella nurekis biomasses to modulate growth of selected microbial species. Molecules 2021, 26, 4038. [Google Scholar] [CrossRef]
- Kim, H.; Kimbrel, J.A.; Vaiana, C.A.; Wollard, J.R.; Mayali, X.; Buie, C.R. Bacterial response to spatial gradients of algal-derived nutrients in a porous microplate. ISME J. 2021. [Google Scholar] [CrossRef]
- Santos, C.A.; Ferreira, M.E.; Lopes Da Silva, T.; Gouveia, L.; Novais, J.M.; Reis, A. A symbiotic gas exchange between bioreactors enhances microalgal biomass and lipid productivities: Taking advantage of complementary nutritional modes. J. Ind. Microbiol. Biotechnol. 2011, 38, 909–917. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, C.; Zhao, L.; Zhang, Y.; Yang, J.; Song, L.; Yang, S. Bioflocculant produced by chlamydomonas reinhardtii. J. Appl. Phycol. 2012, 24, 1245–1251. [Google Scholar] [CrossRef]
- Demuez, M.; González-Fernández, C.; Ballesteros, M. Algicidal microorganisms and secreted algicides: New tools to induce microalgal cell disruption. Biotechnol. Adv. 2015, 33, 1615–1625. [Google Scholar] [CrossRef]
- Lü, F.; Ji, J.; Shao, L.; He, P. Bacterial bioaugmentation for improving methane and hydrogen production from microalgae. Biotechnol. Biofuels 2013, 6, 92. [Google Scholar] [CrossRef]
- Rosero-Chasoy, G.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Buitrón, G.; Chairez, I.; Ruiz, H.A. Microbial co-culturing strategies for the production high value compounds, a reliable framework towards sustainable biorefinery implementation—An overview. Bioresour. Technol. 2021, 321, 124458. [Google Scholar] [CrossRef]
- Cooper, M.B.; Smith, A.G. Exploring mutualistic interactions between microalgae and bacteria in the omics age. Curr. Opin. Plant Biol. 2015, 26, 147–153. [Google Scholar] [CrossRef]
- Lohbeck, K.T.; Riebesell, U.; Reusch, T.B.H. Gene expression changes in the coccolithophore emiliania huxleyi after 500 generations of selection to ocean acidification. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140003. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, C.; Fu, L.; Xu, L.; Cui, X.; Li, Q.; Crittenden, J.C. Responses of the microalga Chlorophyta sp. to bacterial quorum sensing molecules (N-acylhomoserine lactones): Aromatic protein-induced self-aggregation. Environ. Sci. Technol. 2017, 51, 3490–3498. [Google Scholar] [CrossRef]
- Kazamia, E.; Aldridge, D.C.; Smith, A.G. Synthetic ecology—A way forward for sustainable algal biofuel production? J. Biotechnol. 2012, 162, 163–169. [Google Scholar] [CrossRef]
- Adav, S.S.; Ravindran, A.; Cheow, E.S.H.; Sze, S.K. Quantitative proteomic analysis of secretome of microbial consortium during saw dust utilization. J. Proteom. 2012, 75, 5590–5603. [Google Scholar] [CrossRef]
- Guarnieri, M.T.; Nag, A.; Yang, S.; Pienkos, P.T. Proteomic analysis of chlorella vulgaris: Potential targets for enhanced lipid accumulation. J. Proteom. 2013, 93, 245–253. [Google Scholar] [CrossRef]
- Karlsson, R.; Karlsson, A.; Bäckman, O.; Johansson, B.R.; Hulth, S. Identification of key proteins involved in the anammox reaction. FEMS Microbiol. Lett. 2009, 297, 87–94. [Google Scholar] [CrossRef][Green Version]
- Hao, J.; Liebeke, M.; Astle, W.; De Iorio, M.; Bundy, J.G.; Ebbels, T.M.D. Bayesian deconvolution and quantification of metabolites in complex 1D NMR spectra using BATMAN. Nat. Protoc. 2014, 9, 1416–1427. [Google Scholar] [CrossRef]
- Podevin, M.; Fotidis, I.A.; Angelidaki, I. Microalgal process-monitoring based on high-selectivity spectroscopy tools: Status and future perspectives. Crit. Rev. Biotechnol. 2018, 38, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.B.; Duranty, E.; McConico, M.; Vogt, F. Fourier transform infrared (FT-IR) spectroscopy and improved principal component regression (PCR) for quantification of solid analytes in microalgae and bacteria. Appl. Spectrosc. 2011, 65, 442–453. [Google Scholar] [CrossRef]
- Mahapatra, D.M.; Chanakya, H.N.; Ramachandra, T.V. Bioremediation and lipid synthesis through mixotrophic algal consortia in municipal wastewater. Bioresour. Technol. 2014, 168, 142–150. [Google Scholar] [CrossRef]
- Paul, C.; Mausz, M.A.; Pohnert, G. A co-culturing/metabolomics approach to investigate chemically mediated interactions of planktonic organisms reveals influence of bacteria on diatom metabolism. Metabolomics 2013, 9, 349–359. [Google Scholar] [CrossRef]
- Coumans, A.E.; Hensen, E.J.M. A model compound (methyl oleate, oleic acid, triolein) study of triglycerides hydrodeoxygenation over alumina-supported nimo sulfide. Appl. Catal. B Environ. 2017, 201, 290–301. [Google Scholar] [CrossRef]
- Kind, T.; Meissen, J.K.; Yang, D.; Nocito, F.; Vaniya, A.; Cheng, Y.S.; VanderGheynst, J.S.; Fiehn, O. Qualitative analysis of algal secretions with multiple mass spectrometric platforms. J. Chromatogr. A 2012, 1244, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chen, D.; Xu, J.; Zhou, C. Profiles of photosynthetic glycerolipids in three strains of skeletonema determined by UPLC-Q-TOF-MS. J. Appl. Phycol. 2011, 23, 271–282. [Google Scholar] [CrossRef]
- He, H.; Rodgers, R.P.; Marshall, A.G.; Hsu, C.S. Algae polar lipids characterized by online liquid chromatography coupled with hybrid linear quadrupole ion trap/fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2011, 25, 4770–4775. [Google Scholar] [CrossRef]
- Higgins, B.T.; Gennity, I.; Samra, S.; Kind, T.; Fiehn, O.; VanderGheynst, J.S. Cofactor symbiosis for enhanced algal growth, biofuel production, and wastewater treatment. Algal Res. 2016, 17, 308–315. [Google Scholar] [CrossRef]
- Niccolai, A.; Bažec, K.; Rodolfi, L.; Biondi, N.; Zlatić, E.; Jamnik, P.; Tredici, M.R. Lactic acid fermentation of Arthrospira platensis (Spirulina) in a vegetal soybean drink for developing new functional lactose-free beverages. Front. Microbiol. 2020, 11, 560684. [Google Scholar] [CrossRef]
- Gotteland, M.; Riveros, K.; Gasaly, N.; Carcamo, C.; Magne, F.; Liabeuf, G.; Beattie, A.; Rosenfeld, S. The pros and cons of using algal polysaccharides as prebiotics. Front. Nutr. 2020, 7, 163. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Awasthi, M.K.; Varjani, S.; Bhatia, S.K.; Tsai, M.-L.; Hsieh, S.-L.; Chen, C.-W.; Dong, C.-D. Emerging prospects of macro- and microalgae as prebiotic. Microb. Cell Factories 2021, 20, 112. [Google Scholar] [CrossRef]
- Hua, P.; Xiong, Y.; Yu, Z.; Liu, B.; Zhao, L. Effect of chlorella pyrenoidosa protein hydrolysate-calcium chelate on calcium absorption metabolism and gut microbiota composition in low-calcium diet-fed rats. Mar. Drugs 2019, 17, 348. [Google Scholar] [CrossRef]
- Emad, M.; Elbialy, Z. The algal biomass as a mechanical carrier for the lactobacillus bacteria and its uses in the food supplementation. Alex. J. Vet. Sci. 2019, 63, 127. [Google Scholar] [CrossRef]
- Natrah, F.M.I.; Bossier, P.; Sorgeloos, P.; Yusoff, F.M.; Defoirdt, T. Significance of microalgal–bacterial interactions for aquaculture. Rev. Aquac. 2014, 6, 48–61. [Google Scholar] [CrossRef]
- Toi, H.T.; Boeckx, P.; Sorgeloos, P.; Bossier, P.; Van Stappen, G. Co-feeding of microalgae and bacteria may result in increased N assimilation in artemia as compared to mono-diets, as demonstrated by a 15N isotope uptake laboratory study. Aquaculture 2014, 422–423, 109–114. [Google Scholar] [CrossRef]
- Natrah, F.M.I.; Defoirdt, T.; Sorgeloos, P.; Bossier, P. Disruption of bacterial cell-to-cell communication by marine organisms and its relevance to aquaculture. Mar. Biotechnol. 2011, 13, 109–126. [Google Scholar] [CrossRef]
- Pacheco-Vega, J.M.; Cadena-Roa, M.A.; Leyva-Flores, J.A.; Zavala-Leal, O.I.; Pérez-Bravo, E.; Ruiz-Velazco, J.M.J. Effect of isolated bacteria and microalgae on the biofloc characteristics in the pacific white shrimp culture. Aquac. Rep. 2018, 11, 24–30. [Google Scholar] [CrossRef]
- Souza, F.P.; de Lima, E.C.S.; Urrea-Rojas, A.M.; Suphoronski, S.A.; Facimoto, C.T.; Bezerra Júnior, J.d.S.; de Oliveira, T.E.S.; Pereira, U.d.P.; Di Santis, G.W.; de Oliveira, C.A.L.; et al. Effects of dietary supplementation with a microalga (Schizochytrium sp.) on the hemato-immunological, and intestinal histological parameters and gut microbiota of nile tilapia in net cages. PLoS ONE 2020, 15, e0226977. [Google Scholar] [CrossRef]
- Silva-Aciares, F.R.; Carvajal, P.O.; Mejías, C.A.; Riquelme, C.E. Use of macroalgae supplemented with probiotics in the haliotis rufescens (Swainson, 1822) culture in Northern Chile. Aquac. Res. 2011, 42, 953–961. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perković, L.; Djedović, E.; Vujović, T.; Baković, M.; Paradžik, T.; Čož-Rakovac, R. Biotechnological Enhancement of Probiotics through Co-Cultivation with Algae: Future or a Trend? Mar. Drugs 2022, 20, 142. https://doi.org/10.3390/md20020142
Perković L, Djedović E, Vujović T, Baković M, Paradžik T, Čož-Rakovac R. Biotechnological Enhancement of Probiotics through Co-Cultivation with Algae: Future or a Trend? Marine Drugs. 2022; 20(2):142. https://doi.org/10.3390/md20020142
Chicago/Turabian StylePerković, Lucija, Elvis Djedović, Tamara Vujović, Marija Baković, Tina Paradžik, and Rozelindra Čož-Rakovac. 2022. "Biotechnological Enhancement of Probiotics through Co-Cultivation with Algae: Future or a Trend?" Marine Drugs 20, no. 2: 142. https://doi.org/10.3390/md20020142
APA StylePerković, L., Djedović, E., Vujović, T., Baković, M., Paradžik, T., & Čož-Rakovac, R. (2022). Biotechnological Enhancement of Probiotics through Co-Cultivation with Algae: Future or a Trend? Marine Drugs, 20(2), 142. https://doi.org/10.3390/md20020142