Chitosan/Alginate Nanoparticles for the Enhanced Oral Antithrombotic Activity of Clam Heparinoid from the Clam Coelomactra antiquata
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Properties of DG1
2.2. Optimisation of Clam Heparinoid Nanoparticles
2.2.1. Effect of SA Concentration on NPs
2.2.2. Effect of CaCl2 Concentration on NPs
2.2.3. Effect of CTS Concentration on NPs
2.3. Effect of DG1 Concentration
2.4. Effect of Cur Concentration
2.5. Characterisation of Clam Heparinoid Nanoparticles
2.5.1. SEM Analysis
2.5.2. FITR Analysis
2.5.3. DSC Analysis
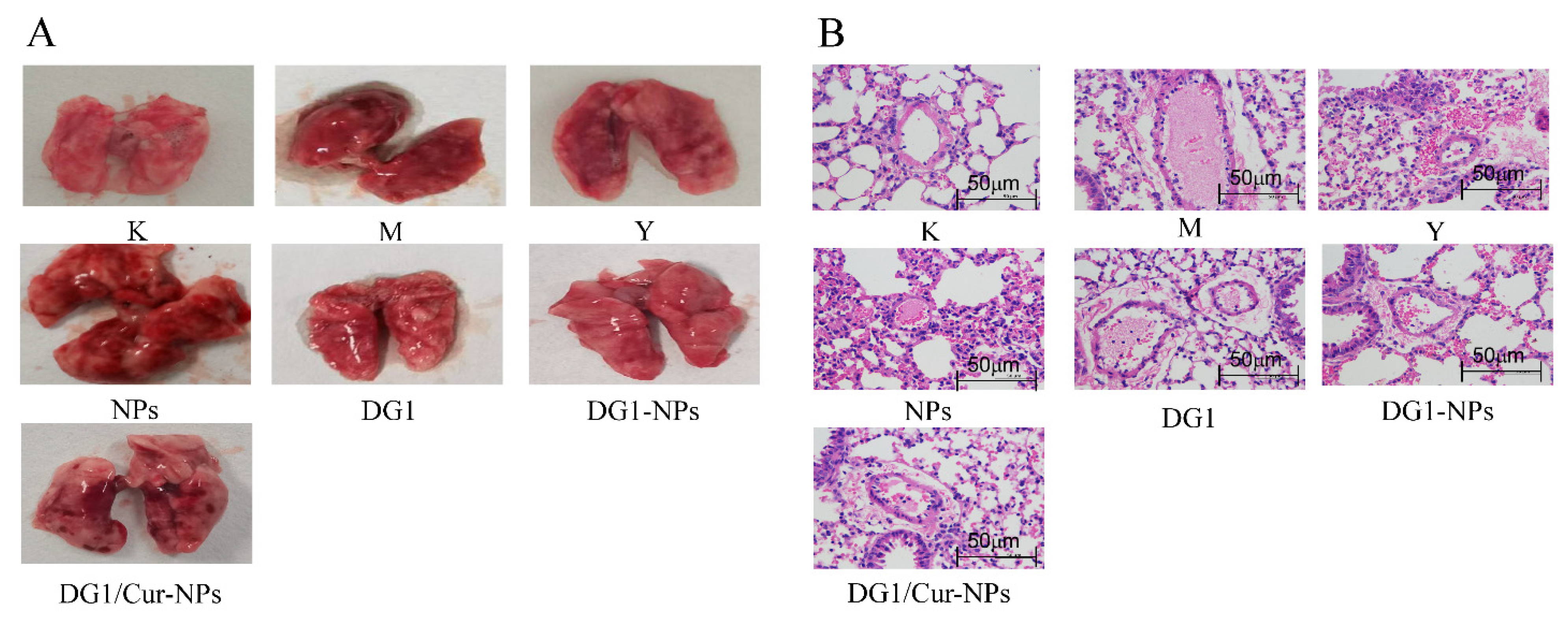
2.6. Effect of DG1-NPs and DG1/Cur-NPs on Collagen plus Epinephrine-Induced Acute Pulmo-nary Thrombosis in Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Determination of Physicochemical Properties of DG1
4.3. Preparation and Optimisation of Clam Heparinoid Nanoparticles
4.4. Characterisation of Clam Heparinoid Nanoparticles
4.5. Effect of DG1-NPs and DG1/Cur-NPs on Collagen Plus Epinephrine-induced Acute Pulmonary Thrombosis in Mice
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Osinbowale, O.; Ali, L.; Chi, Y.W. Venous thromboembolism: A clinical review. Postgrad. Med. 2010, 122, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.-V.; McCumber, M.; et al. Thrombosis: A major contributor to global disease burden. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2363–2371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Nisio, M.; van Es, N.; Büller, H.R. Deep vein thrombosis and pulmonary embolism. Lancet 2016, 388, 3060–3073. [Google Scholar] [CrossRef]
- Fang, G.; Tang, B. Advanced delivery strategies facilitating oral absorption of heparins. Asian J. Pharm. Sci. 2020, 15, 449–460. [Google Scholar] [CrossRef]
- Neves, A.-R.; Correia-da-Silva, M.; Sousa, E.; Pinto, M. Strategies to Overcome Heparins’ Low Oral Bioavailability. Pharmaceuticals 2016, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Nakamura, S.; Sato, Y.; Takayama, T.; Fukuda, K.; Fujita, M.; Murakami, K.; Yokoe, H. Heparinoid Complex-Based Heparin-Binding Cytokines and Cell Delivery Carriers. Molecules 2019, 24, 4630. [Google Scholar] [CrossRef] [Green Version]
- Oduah, E.I.; Linhardt, R.J.; Sharfstein, S.T. Heparin: Past, Present, and Future. Pharmaceuticals 2016, 9, 38. [Google Scholar] [CrossRef]
- Du, Z.; Jia, X.; Chen, J.; Zhou, S.; Chen, J.; Liu, X.; Cao, X.; Zhong, S.; Hong, P. Isolation and Characterization of a Heparin-Like Compound with Potent Anticoagulant and Fibrinolytic Activity from the Clam Coelomactra antiquata. Mar. Drugs 2019, 18, 6. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zeng, R.; Wang, X.; Cai, H.; Chen, J.; Zhong, Y.; Zhong, S.; Jia, X. Antithrombotic Activity of Heparinoid G2 and Its Derivatives from the Clam Coelomactra antiquata. Mar. Drugs 2022, 20, 50. [Google Scholar] [CrossRef]
- Shah, B.-H.; Nawaz, Z.; Pertani, S.-A.; Roomi, A.; Mahmood, H.; Saeed, S.-A.; Gilani, A.-H. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem. Pharmacol. 1999, 58, 1167–1172. [Google Scholar] [CrossRef]
- Keihanian, F.; Saeidinia, A.; Bagheri, R.-K.; Johnston, T.-P.; Sahebkar, A. Curcumin, hemostasis, thrombosis, and coagulation. J. Cell. Physiol. 2018, 233, 4497–4511. [Google Scholar] [CrossRef] [PubMed]
- Rukoyatkina, N.; Shpakova, V.; Bogoutdinova, A.; Kharazova, A.; Mindukshev, I.; Gambaryan, S. Curcumin by activation of adenosine A2A receptor stimulates protein kinase a and potentiates inhibitory effect of cangrelor on platelets. Biochem. Biophys. Res. Commun. 2022, 586, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Bagre, A.-P.; Jain, K.; Jain, N.-K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int. J. Pharm. 2013, 456, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Qian, Y.; Fang, G. Development of Lipid-Polymer Hybrid Nanoparticles for Improving Oral Absorption of Enoxaparin. Pharmaceutics 2020, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.T.; Jeon, O.C.; Byun, Y.; Kim, Y.J.; Lee, Y.-K. Evaluation of the oral absorption of heparin conjugated with sodium deoxycholate as a facilitating agent in GI tract. Macromol. Res. 2009, 17, 79–83. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, Y.; Wang, P.; Zhang, R.; Huo, C.; Gao, T.; Song, C.; Xing, J.; Dong, Y. Mucoadhesive nanoparticles-based oral drug delivery systems enhance ameliorative effects of low molecular weight heparin on experimental colitis. Carbohydr. Polym. 2020, 246, 116660. [Google Scholar] [CrossRef] [PubMed]
- Hoffart, V.; Lamprecht, A.; Maincent, P.; Lecompte, T.; Vigneron, C.; Ubrich, N. Oral bioavailability of a low molecular weight heparin using a polymeric delivery system. J. Control. Release 2006, 113, 38–42. [Google Scholar] [CrossRef]
- Duttagupta, D.S.; Jadhav, V.M.; Kadam, V.J. Chitosan: A propitious biopolymer for drug delivery. Curr. Drug Deliv. 2015, 12, 369–381. [Google Scholar] [CrossRef]
- Kothale, D.; Verma, U.; Dewangan, N.; Jana, P.; Jain, A.; Jain, D. Alginate as Promising Natural Polymer for Pharmaceutical, Food, and Biomedical Applications. Curr. Drug Deliv. 2020, 17, 755–775. [Google Scholar] [CrossRef]
- Iacob, A.T.; Lupascu, F.G.; Apotrosoaei, M.; Vasincu, I.M.; Tauser, R.G.; Lupascu, D.; Giusca, S.E.; Caruntu, I.-D.; Profire, L. Recent Biomedical Approaches for Chitosan Based Materials as Drug Delivery Nanocarriers. Pharmaceutics 2021, 13, 587. [Google Scholar] [CrossRef]
- Fan, B.; Xing, Y.; Zheng, Y.; Sun, C.; Liang, G. pH-responsive thiolated chitosan nanoparticles for oral low-molecular weight heparin delivery: In vitro and in vivo evaluation. Drug Deliv. 2016, 23, 238–247. [Google Scholar] [CrossRef]
- Yin, X.B.; Yu, Q.Z.; Li, B.W.; Zhang, C.D. Preparation and Characterization of Sodium Alginate/Chitosan Composite Nanoparticles Loaded with Chondroitin Sulfate. Adv. Mater. Sci. Eng. 2021, 2021, 6665488. [Google Scholar] [CrossRef]
- Treenate, P.; Monvisade, P. In vitro drug release profiles of pH-sensitive hydroxyethylacryl chitosan/sodium alginate hydrogels using paracetamol as a soluble model drug. Int. J. Biol. Macromol. 2017, 99, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dai, Y.-N.; Zhang, J.-P.; Wang, A.-Q.; Wei, Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar] [PubMed]
- Thai, H.; Thuy Nguyen, C.; Thi Thach, L.; Thi Tran, M.; Duc Mai, H.; Thi Thu Nguyen, T.; Duc Le, G.; Van Can, M.; Dai Tran, L.; Bach, G.L.; et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhu, M.; Wang, D.; Tao, W.; Liu, D.; Zhang, F.; Linhardt, R.J.; Ye, X.; Chen, S. Oral Administration of Fucosylated Chondroitin Sulfate Oligomers in Gastro-Resistant Microcapsules Exhibits a Safe Antithrombotic Activity. Thromb. Haemost. 2021, 121, 15–26. [Google Scholar] [CrossRef]
- Bhunchu, S.; Rojsitthisak, P.; Rojsitthisak, P. Effects of preparation parameters on the characteristics of chitosan–alginate nanoparticles containing curcumin diethyl disuccinate. J. Drug Deliv. Sci. Technol. 2015, 28, 64–72. [Google Scholar] [CrossRef]
- Paluck, S.J.; Nguyen, T.H.; Maynard, H.D. Heparin-Mimicking Polymers: Synthesis and Biological Applications. Biomacromolecules 2016, 17, 3417–3440. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Chapter 10—Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 369–400. [Google Scholar]
- Yuan, Y.; Zhang, S.; Ma, M.; Wang, D.; Xu, Y. Encapsulation and delivery of curcumin in cellulose nanocrystals nanoparticles using pH-driven method. LWT 2022, 155, 112863. [Google Scholar] [CrossRef]
- Sushko, N.; Firsov, S.; Zhbankov, R.; Tsarenkov, V.; Marchewka, M.; Ratajczak, C. Vibrational spectra of heparins. J. Appl. Spectrosc. 1994, 61, 704–707. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Swarnakar, N.K.; Mohamed, D.F.; Attia, M.A.; Pauletti, G.M. Permeation-Enhancing Nanoparticle Formulation to Enable Oral Absorption of Enoxaparin. AAPS PharmSciTech 2020, 21, 88. [Google Scholar] [CrossRef]
- Xu, G.; Li, L.; Bao, X.; Yao, P. Curcumin, casein and soy polysaccharide ternary complex nanoparticles for enhanced dispersibility, stability and oral bioavailability of curcumin—ScienceDirect. Food Biosci. 2020, 35, 100569. [Google Scholar] [CrossRef]
- D’Souza, A.A.; Devarajan, P.V. Bioenhanced oral curcumin nanoparticles: Role of carbohydrates. Carbohydr. Polym. 2016, 136, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Corcione, C.E.; Frigione, M. Characterization of Nanocomposites by Thermal Analysis. Materials 2012, 5, 2960–2980. [Google Scholar] [CrossRef] [Green Version]
- de Moura, A.; Gaglieri, C.; da Silva-Filho, L.C.; Caires, F.J. Mechanochemical synthesis, characterization and thermoanalytical study of a new curcumin derivative. J. Therm. Anal. Calorim. 2021, 146, 587–594. [Google Scholar] [CrossRef]
- Pantwalawalkar, J.; More, H.; Bhange, D.; Patil, U.; Jadhav, N. Novel curcumin ascorbic acid cocrystal for improved solubility. J. Drug Deliv. Sci. Technol. 2021, 61, 102233. [Google Scholar] [CrossRef]
- Sadeghi, F.; Ashofteh, M.; Homayouni, A.; Abbaspour, M.; Nokhodchi, A.; Garekani, H.A. Antisolvent precipitation technique: A very promising approach to crystallize curcumin in presence of polyvinyl pyrrolidon for solubility and dissolution enhancement. Colloids Surf. B Biointerfaces 2016, 147, 258–264. [Google Scholar] [CrossRef]
- Miao, R.; Liu, J.; Wang, J. Overview of mouse pulmonary embolism models. Drug Discov. Today Dis. Models 2010, 7, 77–82. [Google Scholar] [CrossRef]
- Beviglia, L.; Poggi, A.; Rossi, C.; McLane, M.A.; Calabrese, R.; Scanziani, E.; Cook, J.J.; Niewiarowski, S. Mouse antithrombotic assay. Inhibition of platelet thromboembolism by disintegrins. Thromb. Res. 1993, 71, 301–315. [Google Scholar] [CrossRef]
- Shi, J.; Wu, S. The effect of curcumin on platelet aggregation and thrombosis in rats. Acad. J. Chin. PLA Med. Sch. 1996, 1, 31–33. [Google Scholar]
- Azevedo, M.A.; Bourbon, A.I.; Vicente, A.A.; Cerqueira, M.A. Alginate/chitosan nanoparticles for encapsulation and controlled release of vitamin B2. Int. J. Biol. Macromol. 2014, 71, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernabé-Pineda, M.; Ramírez-Silva, M.T.; Romero-Romo, M.; González-Vergara, E.; Rojas-Hernández, A. Determination of acidity constants of curcumin in aqueous solution and apparent rate constant of its decomposition. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 1091–1097. [Google Scholar] [CrossRef]
- Gupta, A.K.; Chopra, B.S.; Vaid, B.; Sagar, A.; Raut, S.; Badmalia, M.D.; Ashish; Khatri, N.A.-O. Protective effects of gelsolin in acute pulmonary thromboembolism and thrombosis in the carotid artery of mice. PLoS ONE 2019, 14, e0215717. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, K.A. Antithrombotic activity of methanolic extract of Umbilicaria esculenta. J. Ethnopharmacol. 2006, 105, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, S.; Kim, S.J. Spirulan from blue-green algae inhibits fibrin and blood clots: Its potent antithrombotic effects. J. Biochem. Mol. Toxicol. 2015, 29, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ren, M.; Dai, X.; Qu, D.; Yang, M.; Zhang, T.; Jiang, B. Lysimachia christinae Hance regresses preestablished cholesterol gallstone in mice. J. Ethnopharmacol. 2015, 166, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.S.; Yang, Y.J.; Liu, X.W.; Kong, Z.; Li, J.Y. Protective effect of aspirin eugenol ester on mice with acute pulmonary embolism. Chin. J. Vet. Med. 2019, 39, 1836–1839. [Google Scholar]
- Alexakis, T.; Boadi, D.K.; Quong, D.; Groboillot, A.; O’Neill, I.; Poncelet, D.; Neufeld, R.J. Microencapsulation of DNA within alginate microspheres and crosslinked chitosan membranes for in vivo application. Appl. Biochem. Biotechnol. 1995, 50, 93–106. [Google Scholar] [CrossRef]
- Lopes, M.; Shrestha, N.; Correia, A.; Shahbazi, M.A.; Sarmento, B.; Hirvonen, J.; Veiga, F.; Seiça, R.; Ribeiro, A.; Santos, H.A. Dual chitosan/albumin-coated alginate/dextran sulfate nanoparticles for enhanced oral delivery of insulin. J. Control. Release Off. J. Control. Release Soc. 2016, 232, 29–41. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Chen, J.; Li, R.; Jia, X.; Liu, X.; Song, B.; Zhong, S. Study on structure, anticoagulant and fibrinolytic activities of different molecular weights of heparin from Clam Coelomactra antiquata. Food Ferment. Ind. 2021, 47, 119–125. [Google Scholar]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulfate content of sulfated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gundry, S.R.; Klein, M.D.; Drongowski, R.A.; Kirsh, M.M. Clinical evaluation of a new rapid heparin assay using the dye azure A. Am. J. Surg. 1984, 148, 191–194. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, S.; Liao, W.; Zhang, L.; Liu, J.; Gao, Y. Formation, Physicochemical Stability, and Redispersibility of Curcumin-Loaded Rhamnolipid Nanoparticles Using the pH-Driven Method. J. Agric. Food Chem. 2020, 68, 7103–7111. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.; Ravi, P.R.; Kathuria, H.; Vats, R. Self-assembled lecithin-chitosan nanoparticles improve the oral bioavailability and alter the pharmacokinetics of raloxifene. Int. J. Pharm. 2020, 588, 119731. [Google Scholar] [CrossRef] [PubMed]
- Men, Z.; Lu, X.; He, T.; Wu, M.; Su, T.; Shen, T. Microneedle patch-assisted transdermal administration of recombinant hirudin for the treatment of thrombotic diseases. Int. J. Pharm. 2022, 612, 121332. [Google Scholar] [CrossRef]
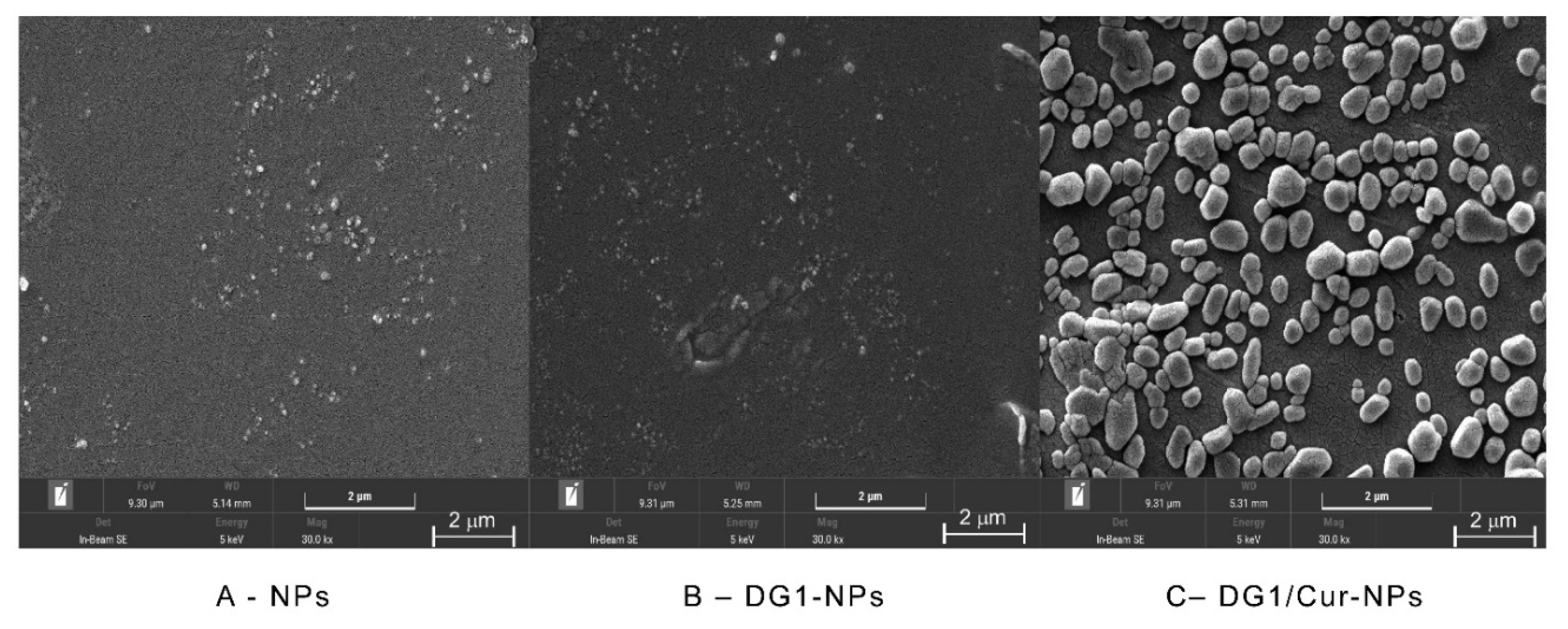
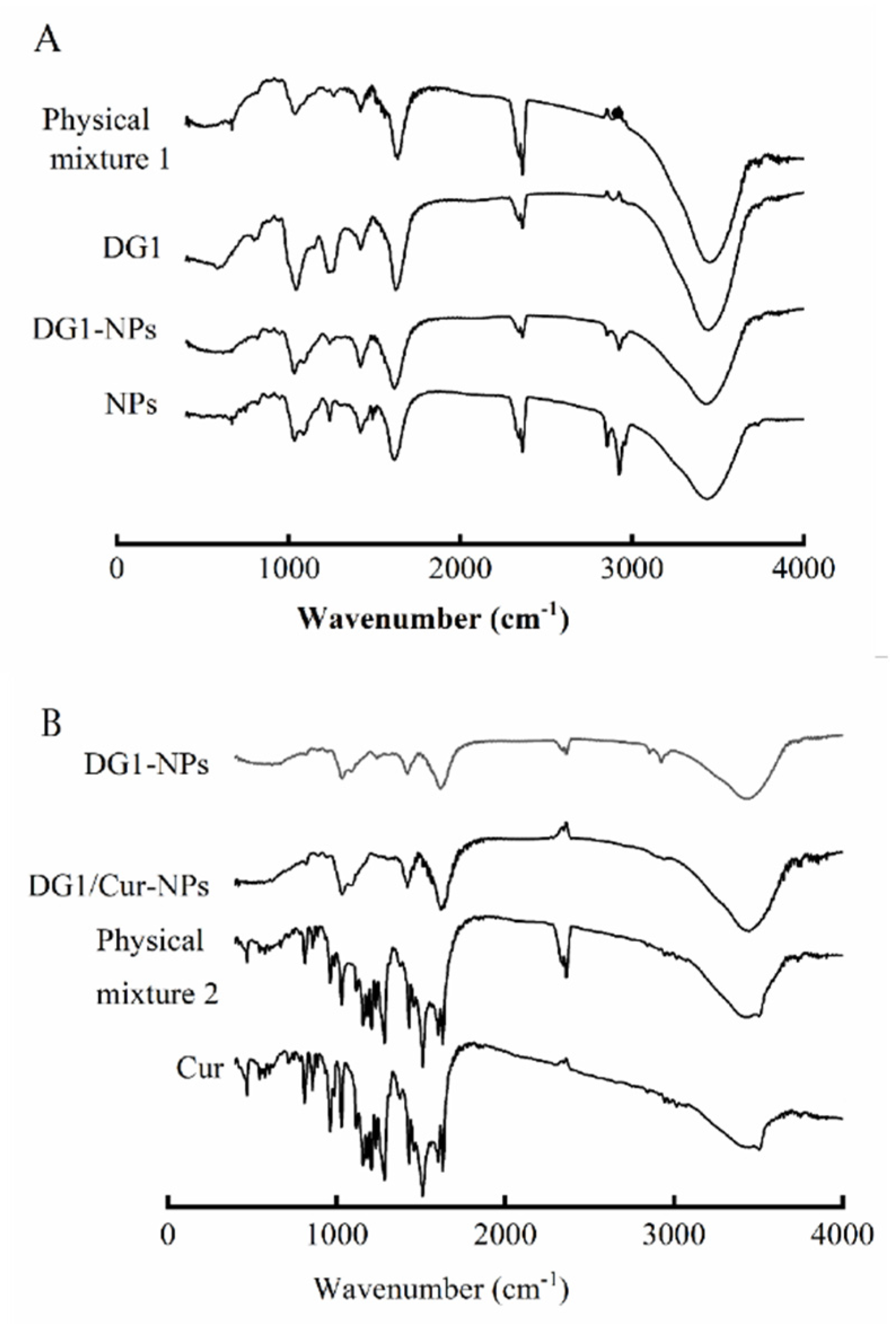
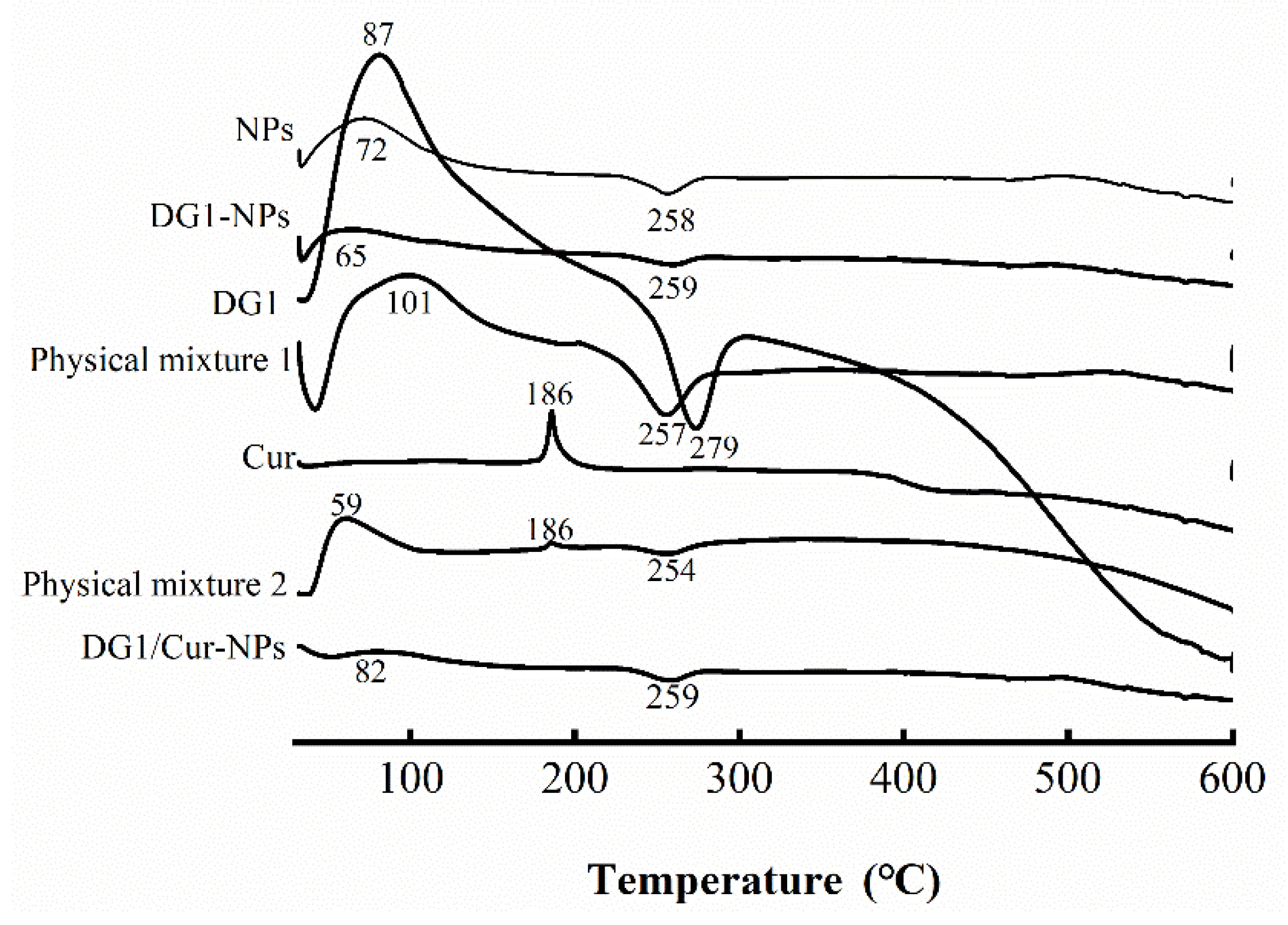
| Name of Sample | Content of Sulfate Radical (%) | Content of Uronic Acid (%) | Zeta Potential (mV) | Particle Size (nm) | Maximum Initial Degradation Temperature (°C) |
|---|---|---|---|---|---|
| DG1 | 24.74 ± 0.64 | 22.38 ± 0.93 | −25.1 ± 1.0 | 183.9 ± 6.2 | 246 |
| SA Concentration (mg/mL) | Particles Size (nm) | PDI a | Zeta Potential (mV) |
|---|---|---|---|
| 0.2 | 180.0 ± 2.7 | 0.23 ± 0.01 | 20.5 ± 4.8 |
| 0.4 | 186.0 ± 2.7 | 0.40 ± 0.06 | −9.5 ± 0.3 |
| 0.6 | 236.5 ± 6.6 | 0.52 ± 0.03 | −26.4 ± 1.1 |
| 0.8 | 261.9 ± 46.2 | 0.71 ± 0.11 | −34.1 ± 1.3 |
| 1.0 | 293.6 ± 14.9 | 0.83 ± 0.17 | −37.2 ± 1.3 |
| CaCl2 Concentration (mg/mL) | Particles Size (nm) | PDI a | Zeta Potential (mV) |
|---|---|---|---|
| 0.06 | 185.5 ± 4.1 | 0.57 ± 0.04 | −38.7 ± 1.3 |
| 0.165 | 184.2 ± 4.0 | 0.49 ± 0.03 | −32.2 ± 1.4 |
| 0.33 | 171.9 ± 1.1 | 0.50 ± 0.00 | −27.9 ± 1.9 |
| 0.66 | 246.2 ± 4.0 | 0.60 ± 0.07 | −27.2 ± 0.1 |
| 1.32 | 352.8 ± 5.8 | 0.40 ± 0.01 | −20.9 ± 0.9 |
| CTS Concentration (mg/mL) | Particles Size (nm) | PDI a | Zeta Potential (mV) |
|---|---|---|---|
| 0.15 | 147.3 ± 3.8 | 0.60 ± 0.04 | −32.3 ± 0.3 |
| 0.3 | 181.8 ± 4.1 | 0.54 ± 0.04 | −30.7 ± 1.5 |
| 0.45 | 196.8 ± 4.0 | 0.59 ± 0.01 | −30.6 ± 0.5 |
| 0.6 | 191.8 ± 3.2 | 0.34 ± 0.00 | −29.9 ± 0.5 |
| 0.75 | 175.9 ± 3.3 | 0.48 ± 0.03 | −29.9 ± 0.4 |
| DG1 Concentration (mg/mL) | Particles Size (nm) | PDI a | Zeta Potential (mV) | Encapsulation Efficiency (%) | Loading Capacity (U/mg) |
|---|---|---|---|---|---|
| 2.4 | 210.2 ± 0.7 | 0.47 ± 0.01 | −31.8 ± 1.1 | 91.37 ± 0.00 | 6.06 ± 0.00 |
| 4.8 | 248.3 ± 8.5 | 0.50 ± 0.01 | −31.2 ± 0.8 | 92.37 ± 1.62 | 10.51 ± 0.11 |
| 7.2 | 252.4 ± 0.9 | 0.49 ± 0.03 | −33.8 ± 1.0 | 94.52 ± 1.24 | 13.90 ± 0.17 |
| 9.6 | 366.6 ± 21.1 | 0.66 ± 0.07 | −35.2 ± 1.5 | 98.09 ± 0.47 | 18.01 ± 0.08 |
| 12 | 225.5 ± 4.4 | 0.51 ± 0.03 | −35.2 ± 1.6 | 90.09 ± 0.37 | 18.89 ± 0.07 |
| Cur Concentration (mg/mL) | Particles Size (nm) | PDI a | Zeta Potential (mV) | Cur Encapsulation Efficiency (%) | Cur Loading Capacity (%) |
|---|---|---|---|---|---|
| 0.06 | 465.3 ± 32.7 | 0.59 ± 0.04 | −33.7 ± 0.3 | 27.90 ± 3.33 | 0.10 ± 0.01 |
| 0.12 | 354.9 ± 33.4 | 0.62 ± 0.06 | −28.5 ± 2.0 | 23.81 ± 1.91 | 0.17 ± 0.01 |
| 0.24 | 339.7 ± 11.2 | 0.51 ± 0.04 | −28.4 ± 1.3 | 21.04 ± 2.61 | 0.30 ± 0.04 |
| 0.48 | 1840.3 ± 223.4 | 1.00 ± 0.00 | −27.9 ± 0.6 | 8.24 ± 0.38 | 0.23 ± 0.01 |
| 0.72 | 2079.0 ± 162.8 | 1.00 ± 0.00 | 27.1 ± 0.6 | 5.57 ± 0.77 | 0.23 ± 0.03 |
| Group | Protection Effect (%) | Iung Index |
|---|---|---|
| K b | - | 0.62 ± 0.08 |
| M b | 0 | 1.06 ± 0.30 ## a |
| Y b | 40 | 0.91 ± 0.22 |
| NPs | 10 | 0.95 ± 0.24 |
| DG1 | 40 | 0.80 ± 0.26 ** a |
| DG1-NPs | 50 | 0.77 ± 0.15 ** a |
| DG1/Cur-NPs | 50 | 0.79 ± 0.18 ** a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.-L.; Cai, H.-Y.; Chen, J.-P.; Li, R.; Zhong, S.-Y.; Jia, X.-J.; Liu, X.-F.; Song, B.-B. Chitosan/Alginate Nanoparticles for the Enhanced Oral Antithrombotic Activity of Clam Heparinoid from the Clam Coelomactra antiquata. Mar. Drugs 2022, 20, 136. https://doi.org/10.3390/md20020136
Chen G-L, Cai H-Y, Chen J-P, Li R, Zhong S-Y, Jia X-J, Liu X-F, Song B-B. Chitosan/Alginate Nanoparticles for the Enhanced Oral Antithrombotic Activity of Clam Heparinoid from the Clam Coelomactra antiquata. Marine Drugs. 2022; 20(2):136. https://doi.org/10.3390/md20020136
Chicago/Turabian StyleChen, Guan-Lan, Hong-Ying Cai, Jian-Ping Chen, Rui Li, Sai-Yi Zhong, Xue-Jing Jia, Xiao-Fei Liu, and Bing-Bing Song. 2022. "Chitosan/Alginate Nanoparticles for the Enhanced Oral Antithrombotic Activity of Clam Heparinoid from the Clam Coelomactra antiquata" Marine Drugs 20, no. 2: 136. https://doi.org/10.3390/md20020136
APA StyleChen, G.-L., Cai, H.-Y., Chen, J.-P., Li, R., Zhong, S.-Y., Jia, X.-J., Liu, X.-F., & Song, B.-B. (2022). Chitosan/Alginate Nanoparticles for the Enhanced Oral Antithrombotic Activity of Clam Heparinoid from the Clam Coelomactra antiquata. Marine Drugs, 20(2), 136. https://doi.org/10.3390/md20020136








