Design and Synthesis of Novel Phenylahistin Derivatives Based on Co-Crystal Structures as Potent Microtubule Inhibitors for Anti-Cancer Therapy
Abstract
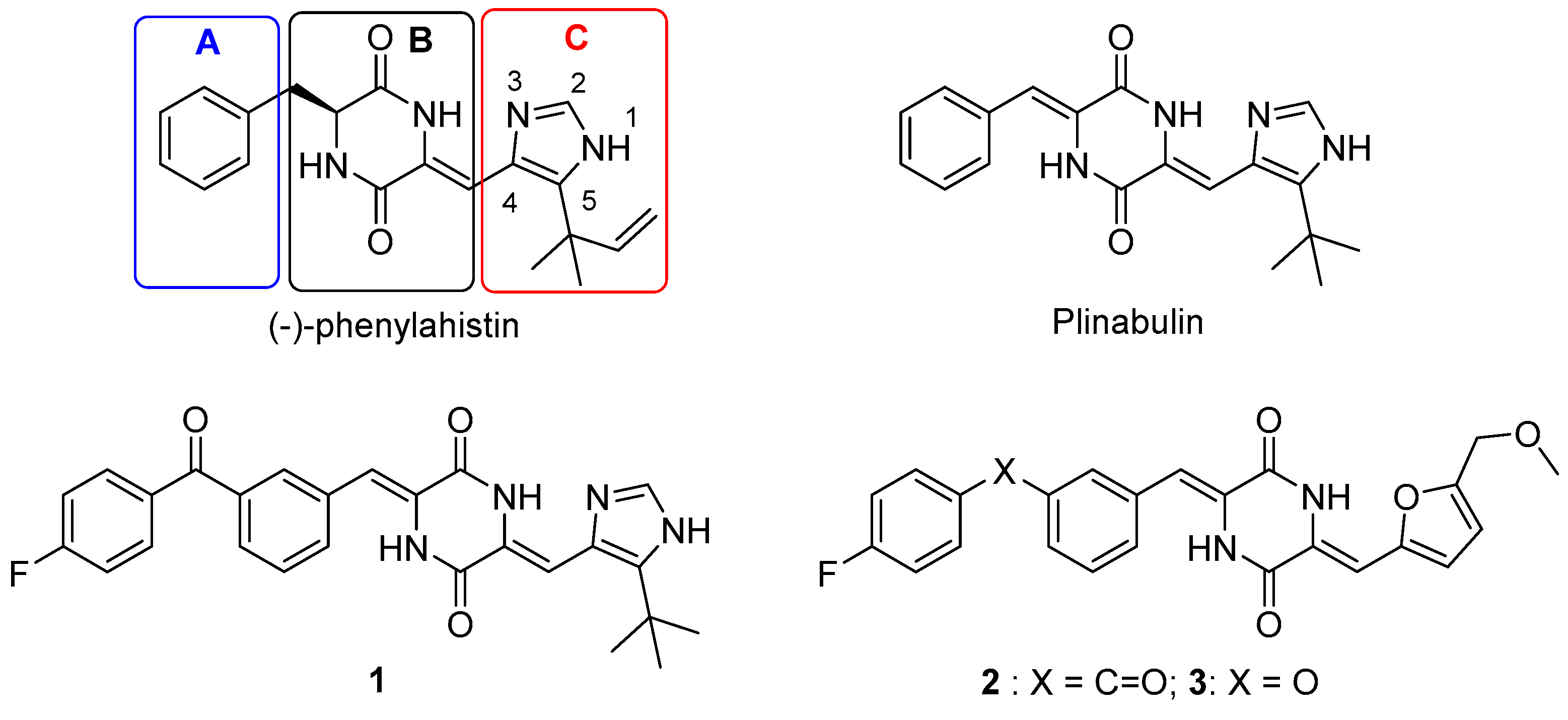
1. Introduction
2. Results and Discussion
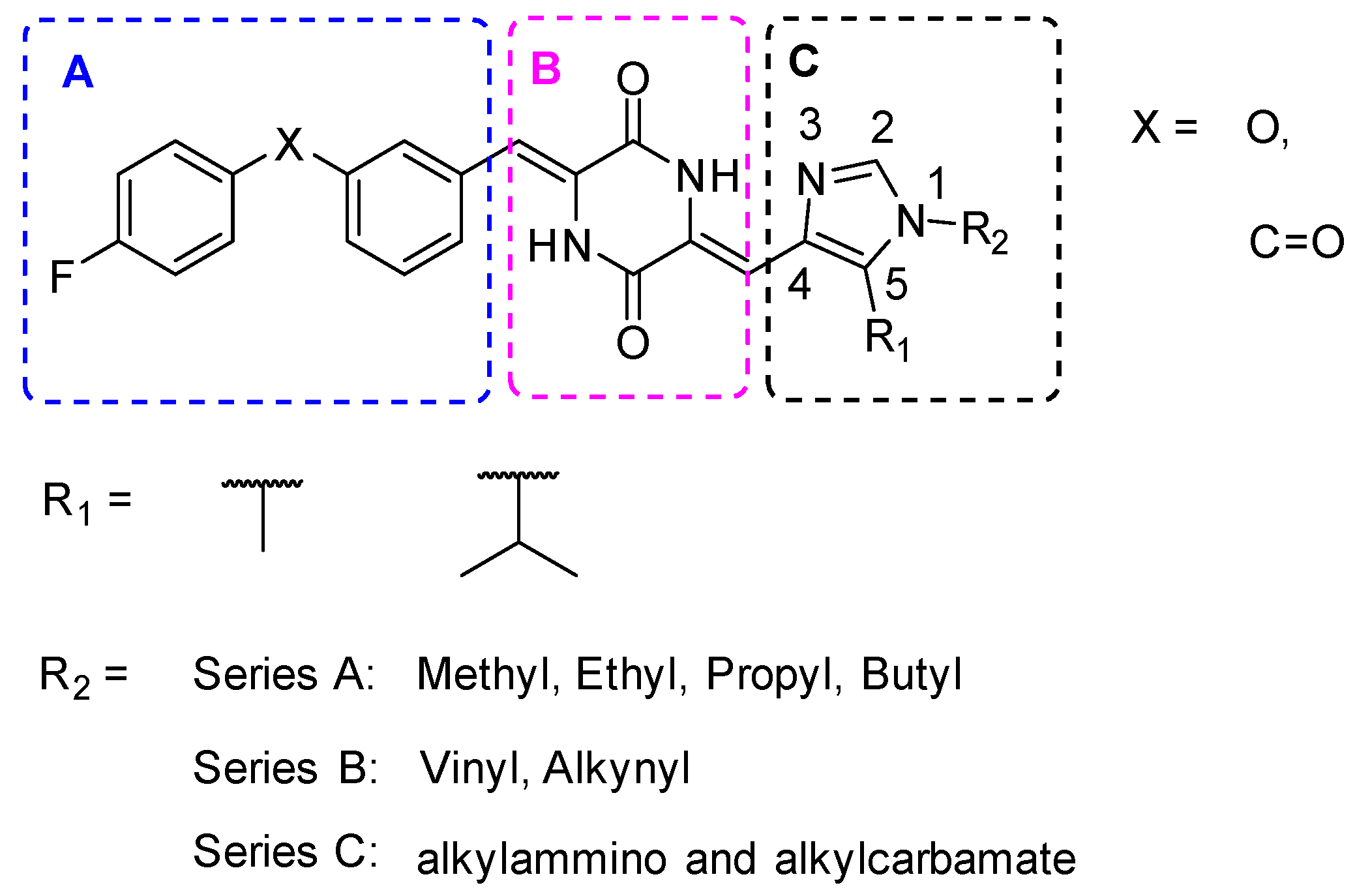
2.1. Design Strategy
2.2. Chemistry
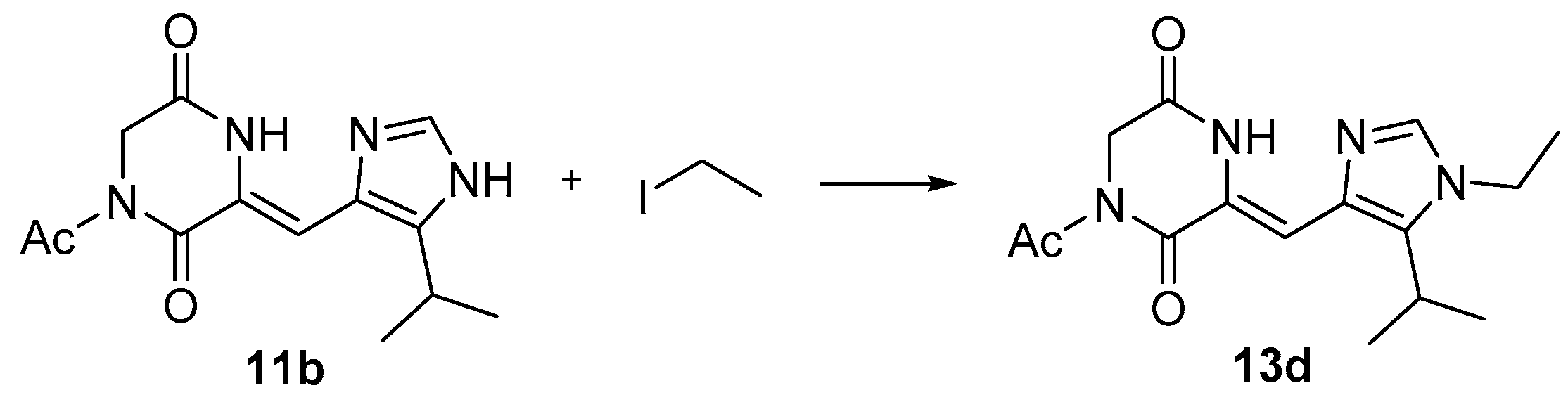
2.2.1. Synthesis of Intermediates 11a–11c
2.2.2. Synthesis of Intermediates 13a–13m
2.2.3. Synthesis of Phenylahistin Derivatives 15a–15q and 16a–16d
2.3. Cytotoxic Activity
2.3.1. Biological Activities of The Synthesized Phenylahistin Derivatives 15a–15q and 16a–16d
2.3.2. Biological Activities of Phenylahistin and Its Derivatives in Various Cancer Cell Lines
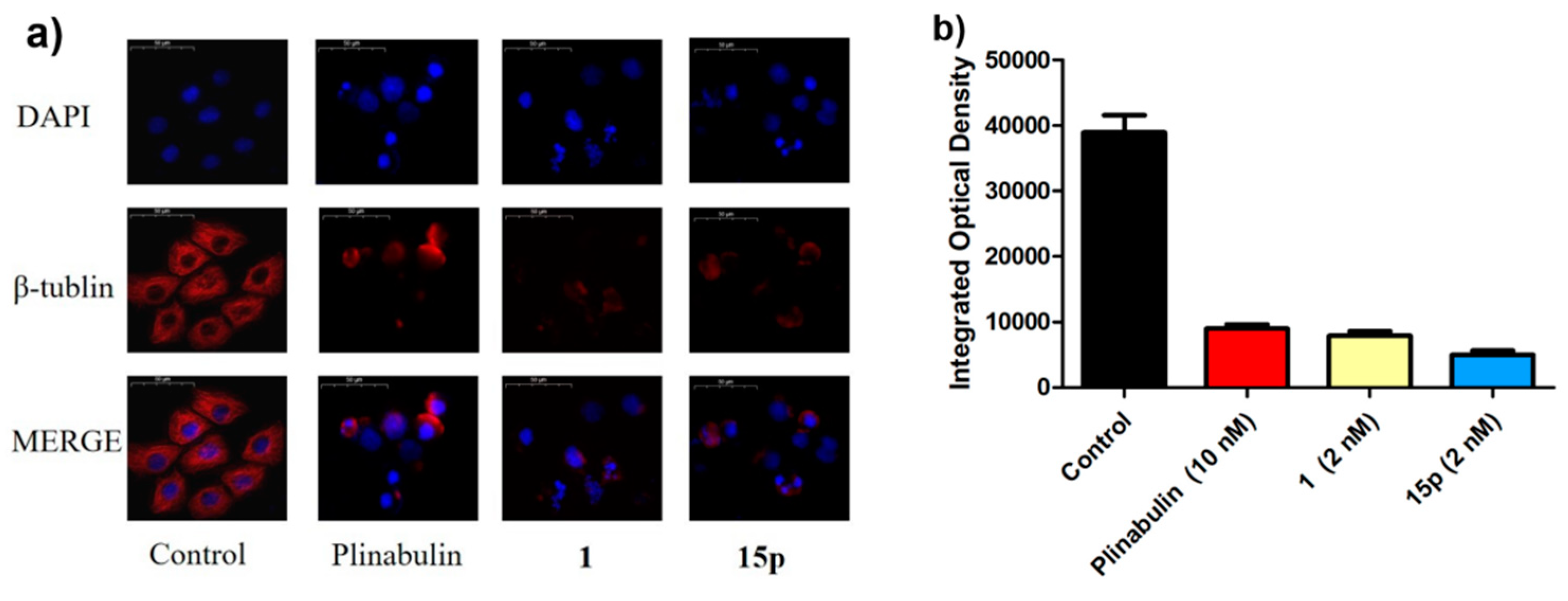
2.4. Immunofluorescece Assay
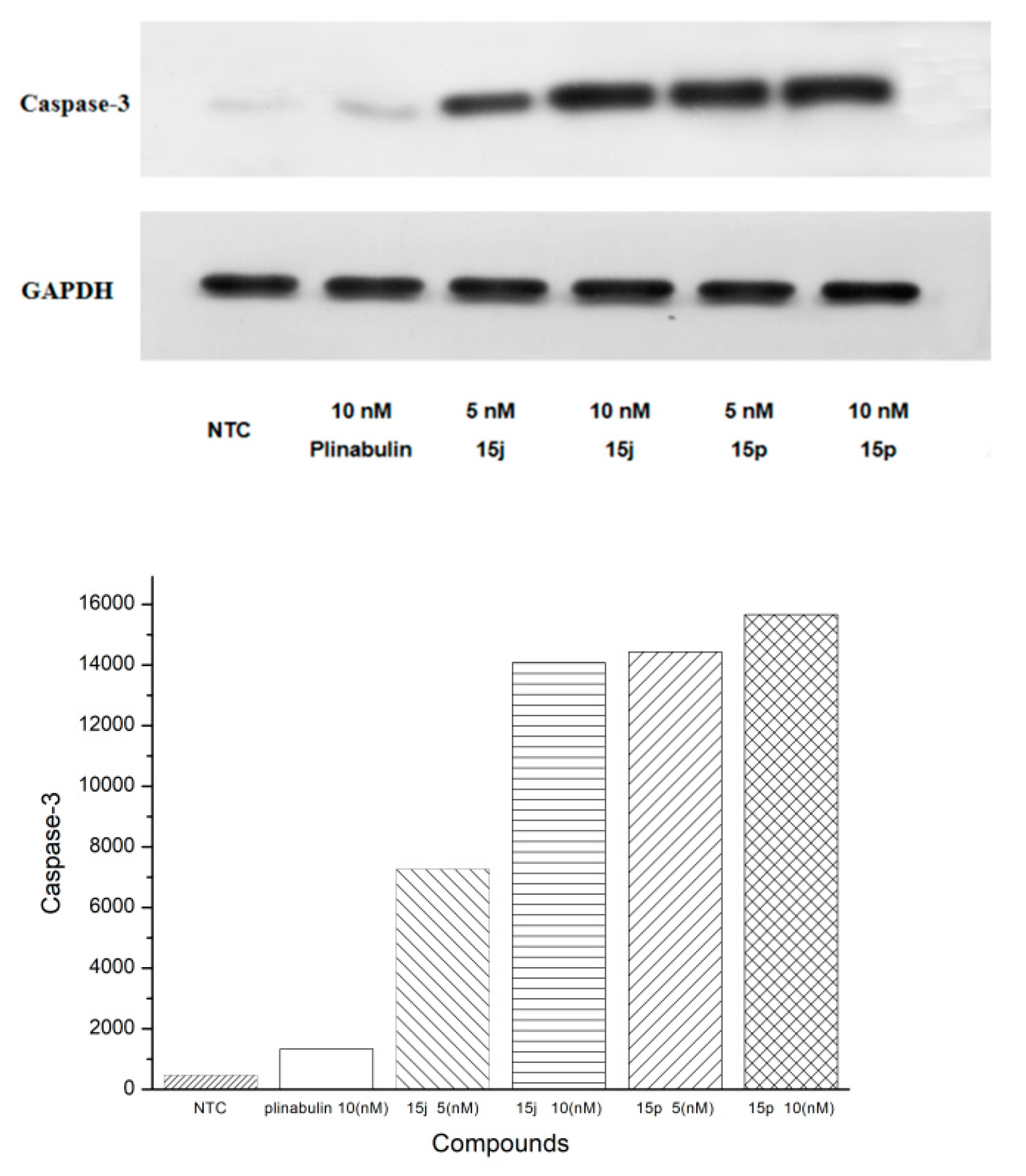
2.5. Western Blotting Test
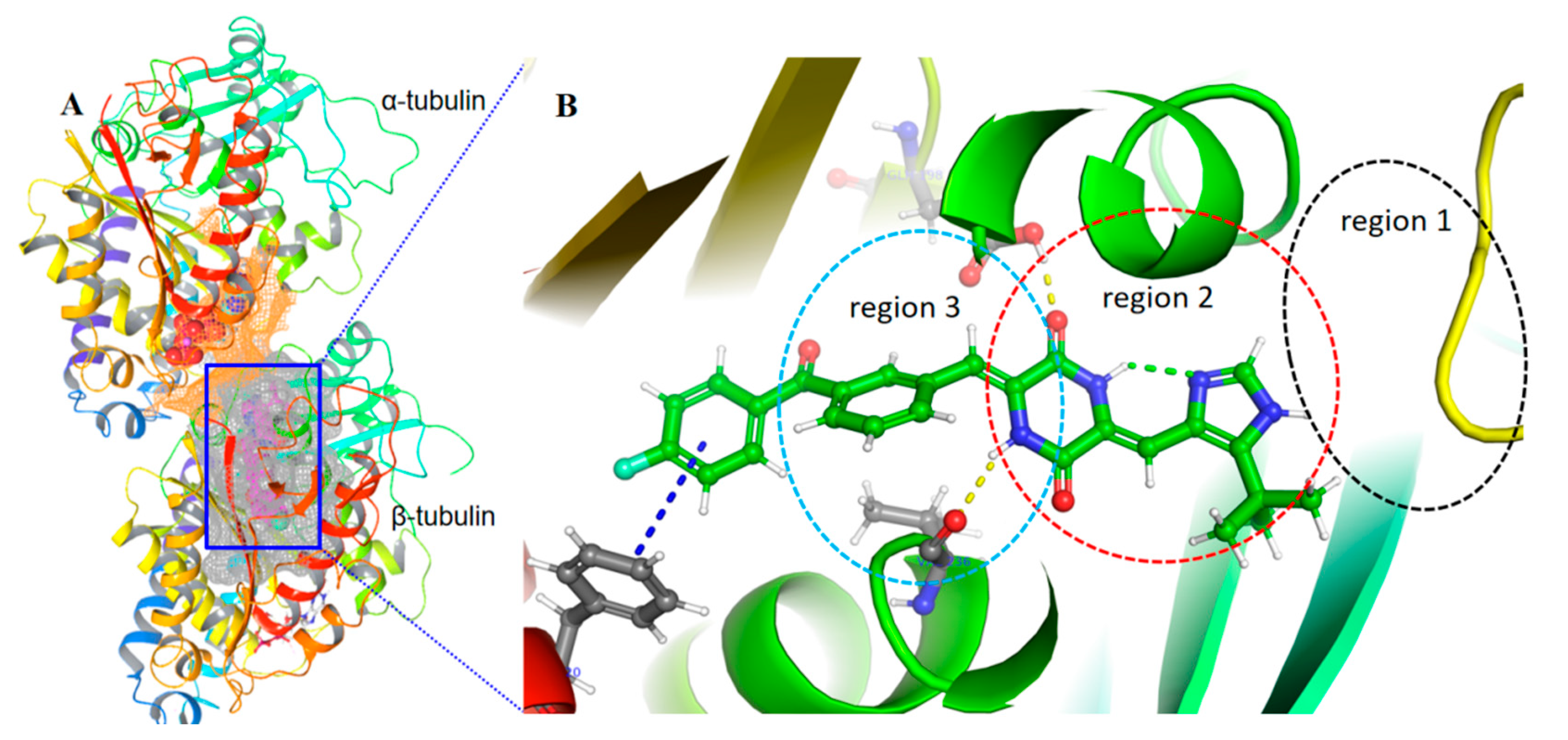
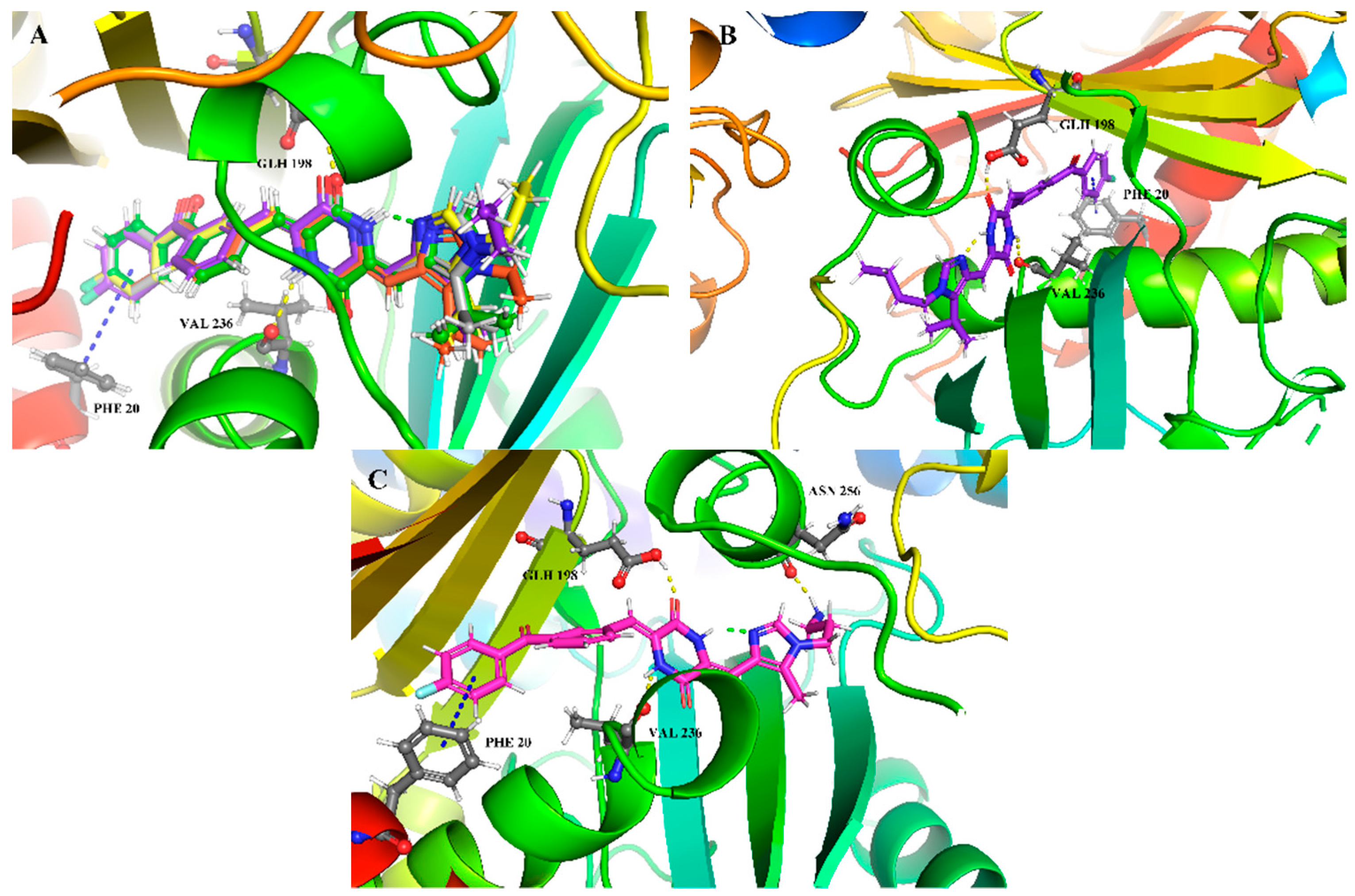
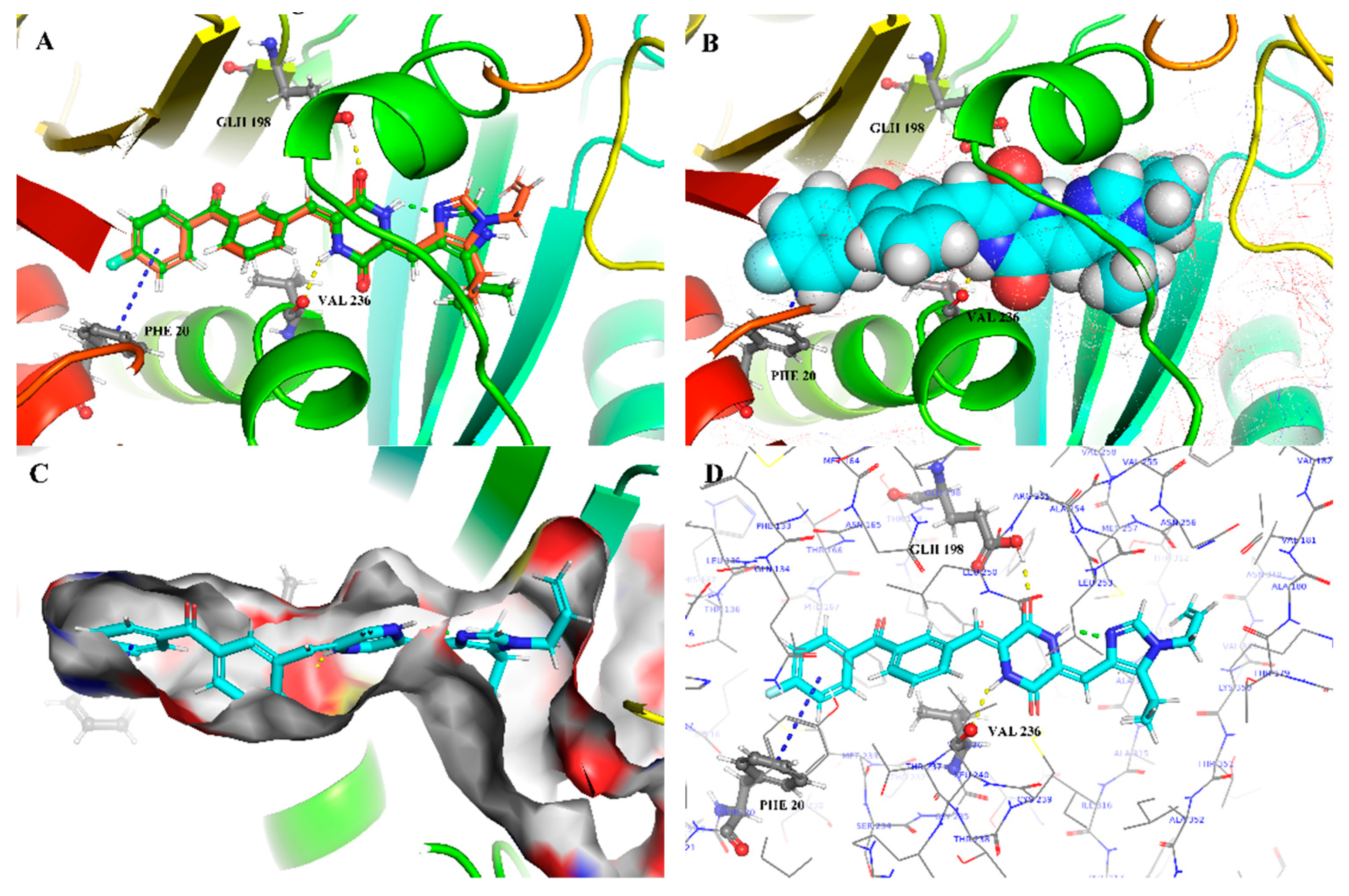
2.6. Theoretical Calculations and Molecular Docking
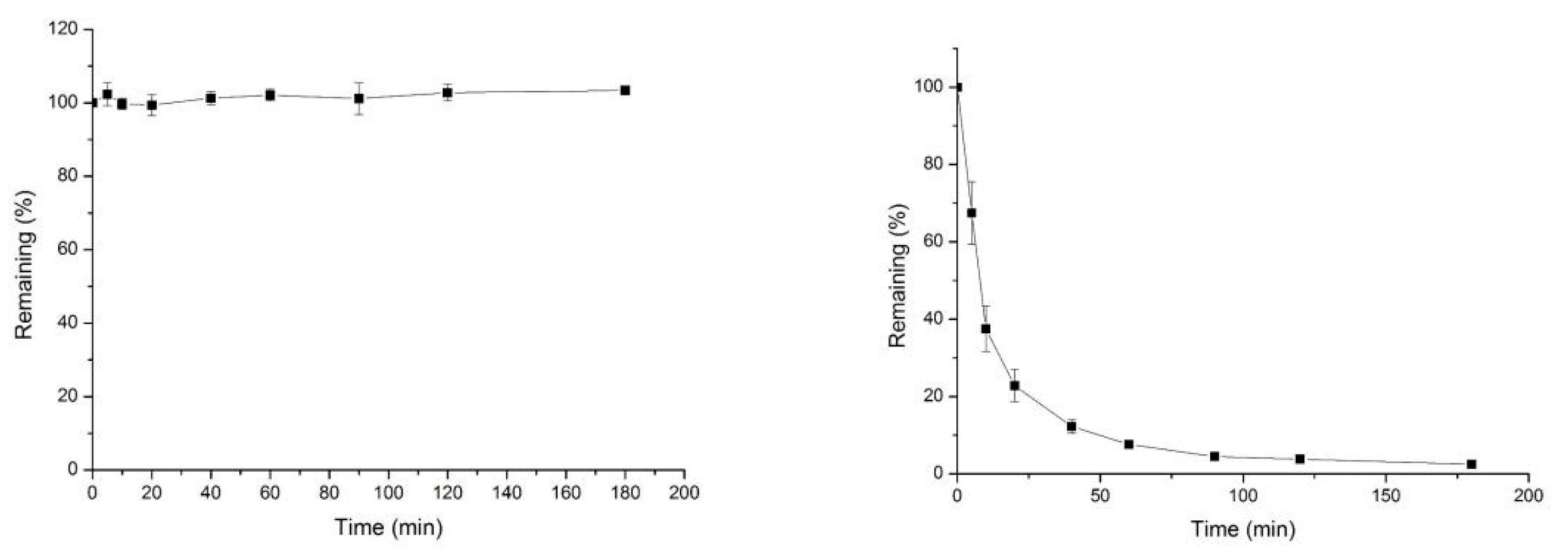
2.7. In Vitro Pharmacokinetic Evaluation of Compound 15p
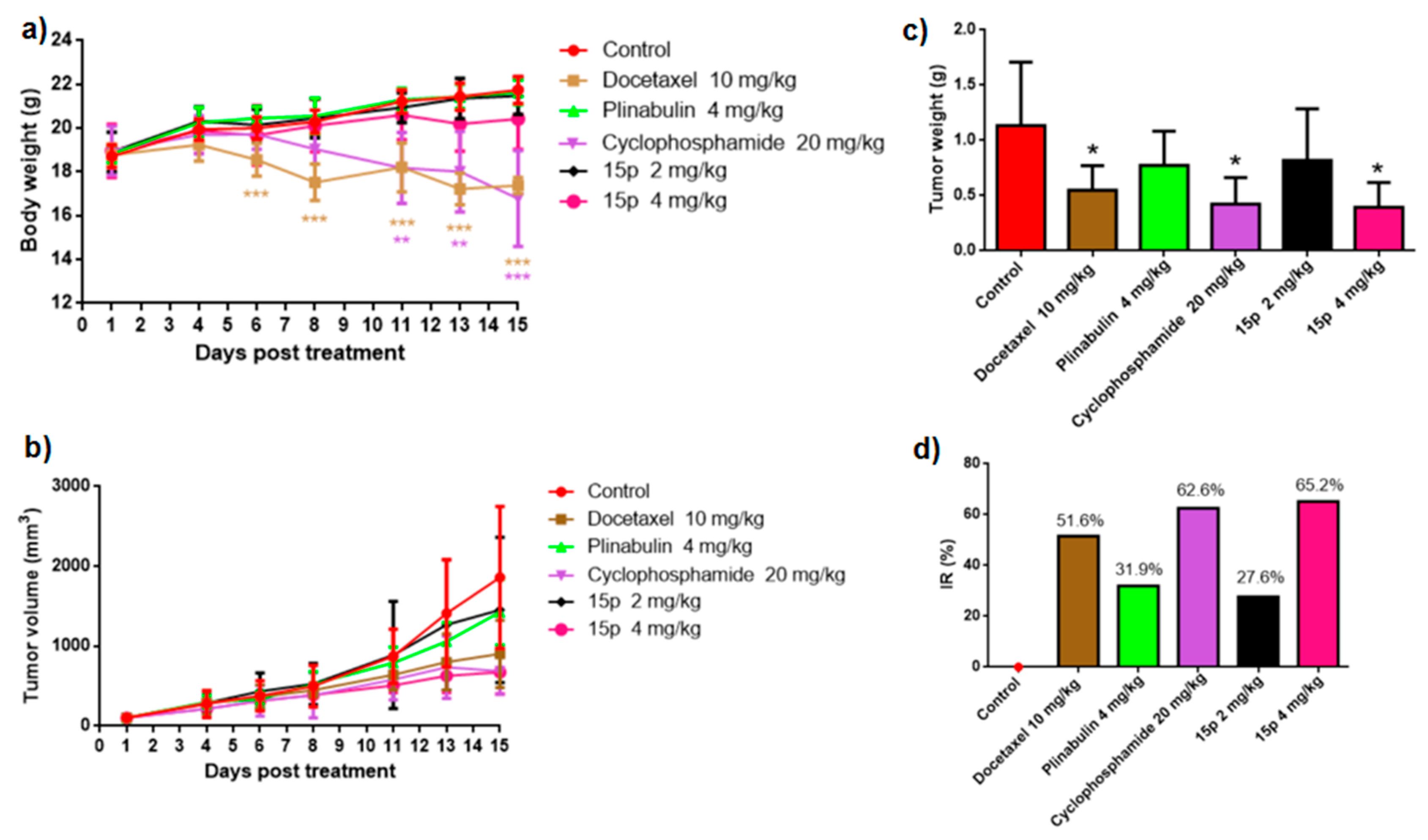
2.8. In Vivo Pharmacodynamic Evaluation of Compound 15p
3. Materials and Methods
3.1. General
3.2. Synthesis
3.2.1. Preparation of Ethyl 5-(isopropyl) Oxazole-4-carboxylate (6)
3.2.2. Preparation of Ethyl 5-(isopropyl)-1H-imidazole-4-carboxylate (7)
3.2.3. Preparation of (5-(isopropyl)-1H-imidazol-4-yl) Methanol (8)
3.2.4. Preparation of 5-(isopropyl)-1H-imidazole-4-carbaldehyde (9b)
3.2.5. Preparation of (Z)-1-acetyl-3-((1H-imidazol-4-yl) methylene) Piperazine-2, 5-dione (11a)
3.2.6. Preparation of (Z)-1-acetyl-3-((5-(isopropyl)-1H-imidazol-4-yl) methylene) Piperazine-2, 5-dione (11b)
3.2.7. Preparation of (Z)-1-acetyl-3-((5-methyl-1-methyl-imidazol-4-yl) Methylene) Piperazine-2, 5-dione (13a)
3.2.8. Preparation of (Z)-1-acetyl-3-((5-isopropyl-1-methyl-imidazol-4-yl) methylene) Piperazine-2, 5-dione (13b)
3.2.9. Preparation of (Z)-1-acetyl-3-((5-methyl-1-ethyl-imidazol-4-yl) methylene) Piperazine-2, 5-dione (13c)
3.2.10. Preparation of (Z)-1-acetyl-3-((5-isopropyl-1-ethyl-imidazol-4-yl) methylene) Piperazine-2, 5-dione (13d)
3.2.11. Preparation of (Z)-1-acetyl-3-((5-methyl-1-(n-propyl)-imidazol-4-yl) Methylene) piperazine-2, 5-dione (13e)
3.2.12. Preparation of (Z)-1-acetyl-3-((5-isopropyl-1-(n-propyl)-imidazol-4-yl) Methylene) piperazine-2, 5-dione (13f)
3.2.13. Preparation of (Z)-1-acetyl-3-((5-isopropyl-1-(n-butyl)-imidazol-4-yl) Methylene) piperazine-2, 5-dione (13g)
3.2.14. Preparation of (Z)-1-acetyl-3-((5-methyl-1-(N-boc-amino- ethyl)-imidazol-4-yl) methylene) Piperazine-2, 5-dione (13h)
3.2.15. Preparation of (Z)-1-acetyl-3-((5-methyl-1-(N-boc-amino- propyl)-imidazol-4-yl) methylene) Piperazine-2, 5-dione (13j)
3.2.16. Preparation of (Z)-1-acetyl-3-((5-isopropyl-1-(N-boc-amino- propyl)-imidazol-4-yl) methylene) Piperazine-2, 5-dione (13k)
3.2.17. Preparation of (Z)-1-acetyl-3-((5-isopropyl-1-allyl-imidazol-4-yl) methylene) Piperazine-2, 5-dione (13l)
3.2.18. Preparation of (Z)-1-acetyl-3-((5-isopropyl-1-(propargyl)-imidazol-4-yl) Methylene) piperazine-2, 5-dione (13m)
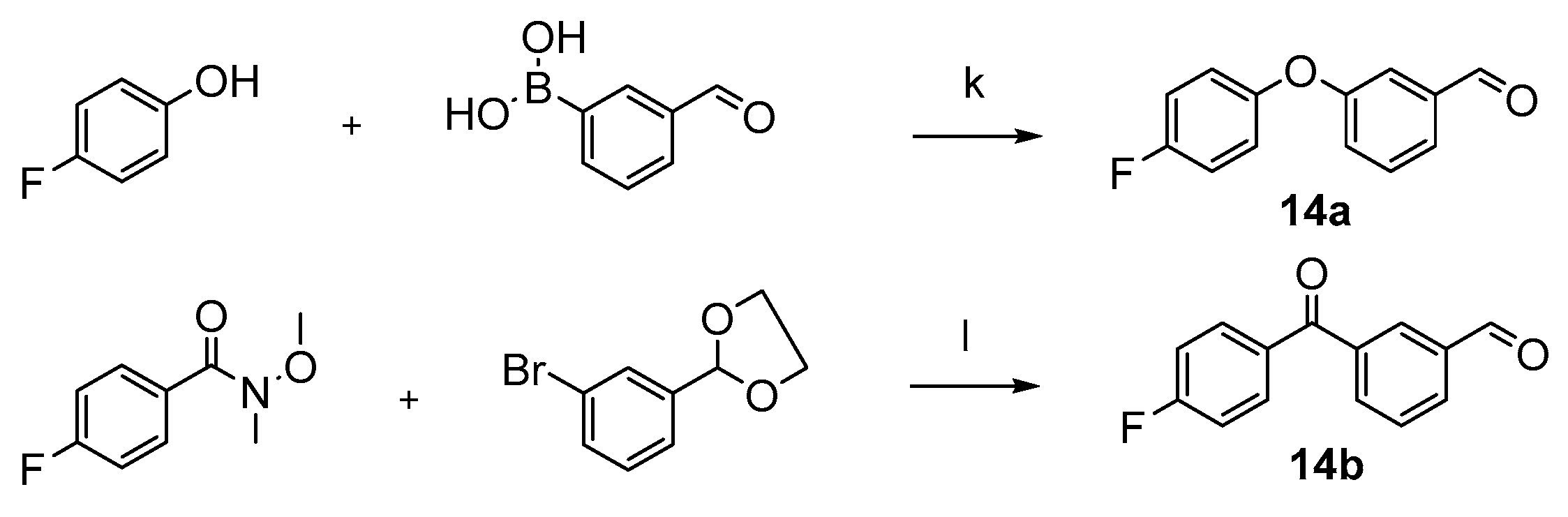
3.2.19. Preparation of 3-(4-fluorophenoxy)benzaldehyde (14a)
3.2.20. Preparation of 3-(4-fluorobenzoyl)benzaldehyde (14b)
3.2.21. (3. Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6-((5-methyl-1-methyl- imidazol-4-yl)methylene)piperazine-2, 5-dione (15a)
3.2.22. (3. Z, 6Z)-3-(4-fluorophenoxy)benzylidene)-6-((5-isopropyl-1-methyl- imidazol-4-yl)methylene) piperazine-2, 5-dione (15b)
3.2.23. (3. Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6-((5-isopropyl-1-methyl- imidazol-4-yl)methylene) piperazine-2, 5-dione (15c)
3.2.24. (3. Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6-((5-methyl-1-ethyl- imidazol-4-yl)methylene)- piperazine-2, 5-dione (15d)
3.2.25. (3. Z, 6Z)-3-(4-fluorophenoxy)benzylidene)-6-((5-isopropyl-1-ethyl- imidazol-4-yl)methylene)- piperazine-2, 5-dione (15e)
3.2.26. (3. Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6-((5-isopropyl-1-ethyl- imidazol-4-yl)methylene)- piperazine-2, 5-dione (15f)
3.2.27. (3. Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6-((5-methyl-1-(n-propyl)-imidazol-4- yl)methylene)- piperazine-2, 5-dione (15g)
3.2.28. (3. Z, 6Z)-3-(4-fluorophenoxy)benzylidene)-6-((5-isopropyl-1-(n- propyl)-imidazol-4-yl)methylene)- piperazine-2, 5-dione (15h)
3.2.29. (3. Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6-((5-isopropyl-1-(n- propyl)-imidazol-4-yl)methylene)- piperazine-2, 5-dione (15i)
3.2.30. (3. Z, 6Z)-3-(4-fluorophenoxy)benzylidene)-6-((5-isopropyl-1-(n- butyl)-imidazol-4-yl)methylene)- piperazine-2, 5-dione (15g)
3.2.31. (3. Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6-((5-isopropyl-1-(n- butyl)-imidazol-4-yl)methylene)- piperazine-2, 5-dione (15k)
3.2.32. Preparation of (3Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6- ((5-methyl-1-(N-boc-amino-ethyl)-imidazol-4-yl) methylene) piperazine-2, 5-dione (15l)
3.2.33. Preparation of (3Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6- ((5-isopropyl-1-(N-boc-amino-ethyl)-imidazol-4-yl) methylene) piperazine-2, 5-dione (15m)
3.2.34. Preparation of (3Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6-((5-methyl-1-(N-boc- amino-propyl)-imidazol-4-yl) methylene) piperazine-2, 5-dione (15n)
3.2.35. Preparation of (3Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6- ((5-isopropyl-1-(N- boc-amino-propyl)-imidazol-4-yl) methylene) piperazine-2, 5-dione (15o)
3.2.36. Preparation of (3Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6- ((5-isopropyl-1-allyl-imidazol-4-yl) methylene) piperazine-2, 5-dione (15p)
3.2.37. Preparation of (3Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6- ((5-isopropyl-1-(propargyl)-imidazol-4-yl) methylene) Piperazine-2, 5-dione (15q)
3.2.38. Preparation of (3Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6- ((5-methyl-1-(amino-ethyl)-imidazol-4-yl) methylene) Piperazine-2, 5-dione (16a)
3.2.39. Preparation of (3Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6- ((5-isopropyl-1-(amino-ethyl)-imidazol-4-yl) methylene) Piperazine-2, 5-dione (16b)
3.2.40. Preparation of (3Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6- ((5-methyl-1-(amino-propyl)-imidazol-4-yl) methylene) piperazine-2, 5-dione (16c)
3.2.41. Preparation of (3Z, 6Z)-3-(4-fluorobenzoyl)benzylidene)-6- ((5-isopropyl-1-(amino-propyl)-imidazol-4-yl) methylene) piperazine-2, 5-dione (16d)
3.3. Biology
3.3.1. Anticancer Activities
3.3.2. Immunofluorescence Assay
3.3.3. Western Blotting Test
3.4. Molecular Modeling
3.5. In Vitro Pharmacokinetics Study
Stability of Liver Microsomal Metabolism
3.6. In Vivo Pharmacodynmic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HRMS | high resolution mass spectrometer |
| 1HNMR | 1H-nuclear magnetic resonance |
| 13CNMR | 13C-nuclear magnetic resonance |
| HMBC | 1H detected heteronuclear multiple bond correlation |
| HMQC | heteronuclear Multiple Quantum Coherence |
| DKP | diketopiperazine |
| DBU | 1,8-diazabicyclo[5.4.0]undec-7-ene |
| TEA | Triethylamine |
| DMF | N,N-dimethylformamide |
| HT-29 | human colon cancer cell |
| BxPC-3 | biopsy xenograft of pancreatic cell |
| NCI-H460 | human lung cancer cell |
| H22 | mouse liver cancer cells |
| Mp | melting point |
References
- AACR Cancer Progress Report. Available online: https://cancerprogressreport.aacr.org/progress/ (accessed on 14 October 2021).
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, M.O.; Prota, A.E. Microtubule-targeting agents: Strategies to hijack the cytoskeleton. Trends Cell Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Shuai, W.; Wang, G.; Zhang, Y.; Bu, F.; Zhang, S.; Miller, D.D.; Li, W.; Ouyang, L.; Wang, Y. Recent Progress on Tubulin Inhibitors with Dual Targeting Capabilities for Cancer Therapy. J. Med. Chem. 2021, 64, 7963–7990. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, E.C.; O’Boyle, N.M. Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review. Pharmaceuticals 2020, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, K.; Kohno, S.; Asari, T.; Harada, T.; Katada, J.; Muramatsu, M.; Kawashima, H.; Sekiya, H.; Uno, I. (−)-Phenylahistin: A new mammalian cell cycle inhibitor produced by aspergillus ustus. Bioorganic Med. Chem. Lett. 1997, 7, 2847–2852. [Google Scholar] [CrossRef]
- Kanoh, K.; Kohno, S.; Katada, J.; Takahashi, J.; Uno, I. (-)-Phenylahistin Arrests Cells in Mitosis by Inhibiting Tubulin Polymerization. J. Antibiot. 1999, 52, 134–141. [Google Scholar] [CrossRef]
- Nicholson, B.; Lloyd, G.K.; Miller, B.R.; Palladino, M.A.; Kiso, Y.; Hayashi, Y.; Neuteboom, S.T. NPI-2358 is a tubulin-depolymerizing agent: In-vitro evidence for activity as a tumor vascular-disrupting agent. Anti Cancer Drugs 2006, 17, 25–31. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Tanaka, K.; Nicholson, B.; Deyanat-Yazdi, G.; Potts, B.; Yoshida, T.; Oda, A.; Kitagawa, T.; Orikasa, S.; Kiso, Y.; et al. Synthesis and Structure–Activity Relationship Study of Antimicrotubule Agents Phenylahistin Derivatives with a Didehydropiperazine-2,5-dione Structure. J. Med. Chem. 2012, 55, 1056–1071. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yamazaki-Nakamura, Y.; Yakushiji, F. Medicinal Chemistry and Chemical Biology of Diketopiperazine-Type Antimicrotubule and Vascular-Disrupting Agents. Chem. Pharm. Bull. 2013, 61, 889–901. [Google Scholar] [CrossRef]
- Kashyap, A.S.; Fernandez-Rodriguez, L.; Zhao, Y.; Monaco, G.; Trefny, M.P.; Yoshida, N.; Martin, K.; Sharma, A.; Olieric, N.; Shah, P.; et al. GEF-H1 Signaling upon Microtubule Destabilization Is Required for Dendritic Cell Activation and Specific Anti-tumor Responses. Cell Rep. 2019, 28, 3367–3380.e8. [Google Scholar] [CrossRef]
- La Sala, G.; Olieric, N.; Sharma, A.; Viti, F.; Perez, F.D.A.B.; Huang, L.; Tonra, J.R.; Lloyd, G.K.; Decherchi, S.; Díaz, J.F.; et al. Structure, Thermodynamics, and Kinetics of Plinabulin Binding to Two Tubulin Isotypes. Chem 2019, 5, 2969–2986. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Gigant, B.; Yu, Y.; Wu, Y.; Chen, X.; Lai, Q.; Yang, Z.; Chen, Q.; Yang, J. Structures of a diverse set of colchicine binding site inhibitors in complex with tubulin provide a rationale for drug discovery. FEBS J. 2015, 283, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Hou, Y.; Ji, C.; Ma, M.; Tian, Z.; Deng, M.; Zhong, L.; Chu, Y.; Li, W. Design, synthesis and biological evaluation of anti-pancreatic cancer activity of plinabulin derivatives based on the co-crystal structure. Bioorganic Med. Chem. 2018, 26, 2061–2072. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Ding, Z.; Wang, S.; Ma, L.; Wang, Y.; Zhong, L.; Li, Z.; Yang, J.; Li, W. Polymorphs, co-crystal structure and pharmacodynamics study of MBRI-001, a deuterium-substituted plinabulin derivative as a tubulin polymerization inhibitor. Bioorganic Med. Chem. 2019, 27, 1836–1844. [Google Scholar] [CrossRef]
- Ding, Z.; Ma, M.; Zhong, C.; Wang, S.; Fu, Z.; Hou, Y.; Liu, Y.; Zhong, L.; Chu, Y.; Li, F.; et al. Development of novel phenoxy-diketopiperazine-type plinabulin derivatives as potent antimicrotubule agents based on the co-crystal structure. Bioorganic Med. Chem. 2019, 28, 115186. [Google Scholar] [CrossRef]
- Ding, Z.; Li, F.; Zhong, C.; Li, F.; Liu, Y.; Wang, S.; Zhao, J.; Li, W. Structure-based design and synthesis of novel furan-diketopiperazine-type derivatives as potent microtubule inhibitors for treating cancer. Bioorganic Med. Chem. 2020, 28, 115435. [Google Scholar] [CrossRef]
- Kanoh, K.; Kohno, S.; Katada, J.; Takahashi, J.; Uno, I.; Hayashi, Y. Synthesis and biological activities of phenylahistin derivatives. Bioorganic Med. Chem. 1999, 7, 1451–1457. [Google Scholar] [CrossRef]
- Munir, I.; Zahoor, A.F.; Rasool, N.; Naqvi, S.A.R.; Zia, K.M.; Ahmad, R. Synthetic applications and methodology development of Chan–Lam coupling: A review. Mol. Divers. 2018, 23, 215–259. [Google Scholar] [CrossRef]
- Ding, Z.; Cheng, H.; Wang, S.; Hou, Y.; Zhao, J.; Guan, H.; Li, W. Development of MBRI-001, a deuterium-substituted plinabulin derivative as a potent anti-cancer agent. Bioorganic Med. Chem. Lett. 2017, 27, 1416–1419. [Google Scholar] [CrossRef]
- Wang, J.; Sareddy, G.R.; Lu, Y.; Pratap, U.P.; Tang, F.; Greene, K.M.; Meyre, P.L.; Tekmal, R.R.; Vadlamudi, R.K.; Brann, D.W. Astrocyte-Derived Estrogen Regulates Reactive Astrogliosis and is Neuroprotective following Ischemic Brain Injury. J. Neurosci. 2020, 40, 9751–9771. [Google Scholar] [CrossRef]
- Deng, M.; Li, L.; Zhao, J.; Yuan, S.; Li, W. Antitumor activity of the microtubule inhibitor MBRI-001 against human hepatocellular carcinoma as monotherapy or in combination with sorafenib. Cancer Chemother. Pharmacol. 2018, 81, 853–862. [Google Scholar] [CrossRef] [PubMed]



| Entry | Temp. | Base | Solv. | Remaining of 11b | Yield |
|---|---|---|---|---|---|
| 1 | 0 °C | NaH | DMF | 62% | - |
| 2 | rt | NaH | DMF | 59% | - |
| 3 | rt | DIPEA | DMF | 82% | - |
| 4 | rt | TEA | DMF | 91% | - |
| 5 | rt | Cs2CO3 | DMF | 0% | 68% |
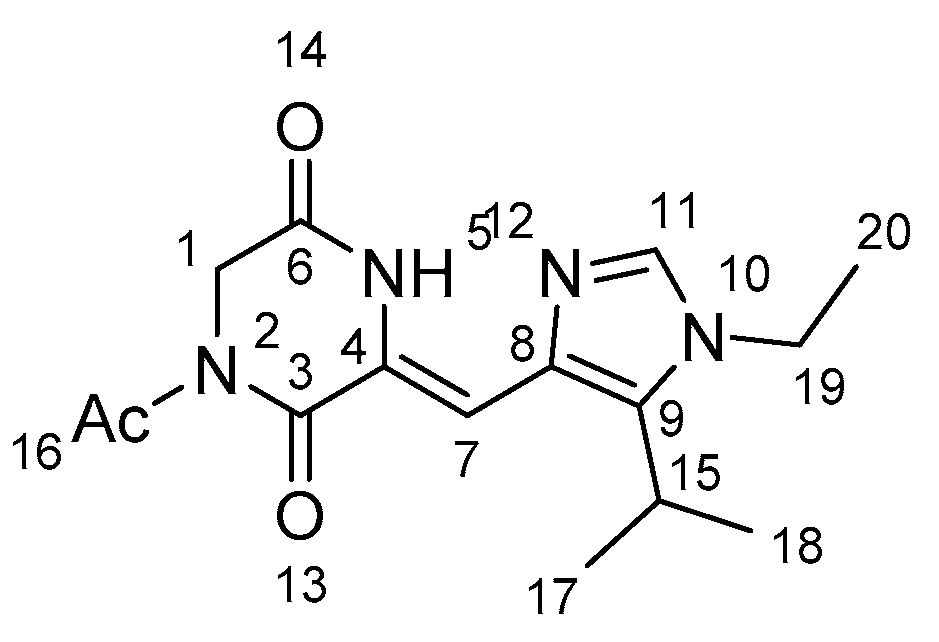
| Hydrogen Type | Chemical Shifts (ppm) | Peak Type | H Number | Number of Protons |
|---|---|---|---|---|
| N-H | 12.02 | s | NH-5 | 1 |
| C-H | 7.45 | s | H-11 | 1 |
| C-H | 7.05 | s | H-7 | 1 |
| C-H | 4.46 | s | H-1 | 2 |
| C-H | 3.99 | q | H-19 | 2 |
| C-H | 3.16 | septet | H-15 | 1 |
| C-H | 2.64 | s | H-16 | 3 |
| C-H | 1.45 | t | H-20 | 3 |
| C-H | 1.41 | d | H-17,18 | 6 |
| C Number | Chemical Shifts (ppm) |
|---|---|
| C-8 | 133.28 |
| C-9 | 139.34 |
| C-11 | 135.72 |
 | ||||||
|---|---|---|---|---|---|---|
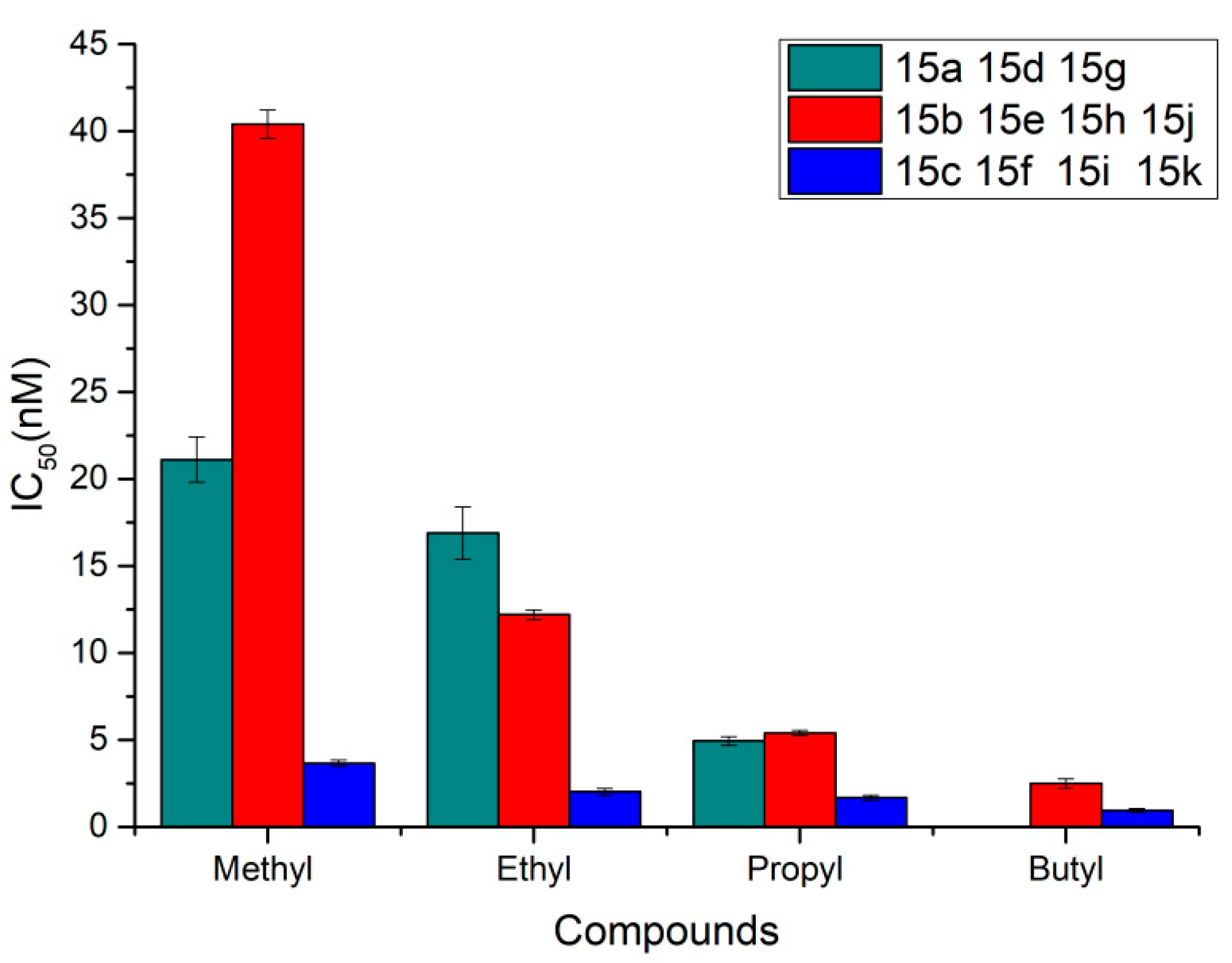
| Compds | X | R1 | R2 | IC50 (nM) a NCI-H460 | IC50 a (nM)/BxPC-3 | IC50 (nM)/HT-29 |
| Plinabulin | 26.2 ± 3.2 | 5.8 ± 0.2 | 6.6 ± 1.6 | |||
| 15a | C=O | Methyl | Methyl | 21.1 ± 1.30 | - | - |
| 15b | O | Isopropyl | Methyl | 40.40 ± 0.82 | - | - |
| 15c | C=O | Isopropyl | Methyl | 3.65 ± 0.18 | - | - |
| 15d | C=O | Methyl | Ethyl | 16.90 ± 1.50 | - | - |
| 15e | O | Isopropyl | Ethyl | 12.20 ± 0.27 | - | - |
| 15f | C=O | Isopropyl | Ethyl | 2.01 ± 0.19 | - | - |
| 15g | C=O | Methyl | n-Propyl | 4.93 ± 0.25 | - | - |
| 15h | O | Isopropyl | n-Propyl | 5.40 ± 0.15 | - | - |
| 15i | C=O | Isopropyl | n-Propyl | 1.67 ± 0.15 | - | - |
| 15j | O | Isopropyl | n-Butyl | 2.49 ± 0.28 | 1.13 ± 0.35 | 2.11 ± 0.09 |
| 15k | C=O | Isopropyl | n-Butyl | 0.94 ± 0.11 | - | - |
| 15l | C=O | Methyl |  | 104.78 ± 11.72 | - | - |
| 16a | C=O | Methyl |  | 33.4 ± 9.59 | - | - |
| 15m | C=O | Isopropyl | 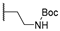 | 27.26 ± 1.32 | - | - |
| 16b | C=O | Isopropyl | 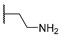 | 11.32 ± 3.45 | 3.27 ± 0.09 | 5.92 ± 0.39 |
| 15n | C=O | Methyl | 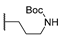 | 145.72 ± 0.27 | - | - |
| 16c | C=O | Methyl | 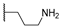 | 102.76 ± 11.67 | - | - |
| 15o | C=O | Isopropyl |  | 12.70 ± 0.71 | - | - |
| 16d | C=O | Isopropyl | 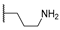 | 5.38 ± 1.46 | 2.35 ± 1.75 | 2.45 ± 0.42 |
| 15p | C=O | Isopropyl | 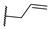 | 1.03 ± 0.18 | 0.81 ± 0.02 | 0.67 ± 0.064 |
| 15q | C=O | Isopropyl | 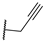 | 1.49 ± 0.31 | 1.15 ± 0.042 | 0.67 ± 0.035 |
| Compds | LogPo/w | PCaco | Docking Score | Compds | LogPo/w | PCaco | Docking Score |
|---|---|---|---|---|---|---|---|
| Plinabulin | 2.48 | 377.79 | 11.68 | 15k | 4.93 | 197.58 | −14.16 |
| 15a | 3.25 | 335.39 | −14.11 | 15l | 4.59 | 57.67 | −10.16 |
| 15b | 4.61 | 571.11 | −14.46 | 16a | 2.44 | 20.93 | −14.56 |
| 15c | 3.34 | 137.94 | −14.55 | 15m | 4.88 | 115.99 | - |
| 15d | 3.57 | 336.58 | −14.17 | 16b | 2.71 | 28.92 | - |
| 15e | 4.85 | 721.47 | −13.91 | 15n | 4.34 | 58.49 | - |
| 15f | 4.20 | 194.15 | −14.30 | 16c | 2.13 | 25.87 | −15.01 |
| 15g | 3.89 | 190.86 | −14.66 | 15o | 5.22 | 64.30 | - |
| 15h | 5.22 | 599.60 | −14.11 | 16d | 3.06 | 19.89 | - |
| 15i | 4.58 | 201.73 | −14.72 | 15p | 4.51 | 200.27 | −14.88 |
| 15j | 5.55 | 506.41 | −13.53 | 15q | 4.35 | 200.28 | −15.34 |
| Parameters | 15p |
|---|---|
| t1/2 (min) | 7.07 |
| CLint (mL/min/kg) | 771.91 |
| CL (mL/min/kg) | 80.60 |
| Group | Dose (mg/kg) | Tumor Volume (D1, mm3) | Tumor Volume (D15, mm3) | Tumor Weight (g) | IR (%) |
|---|---|---|---|---|---|
| Control | -- | 103.3 ± 32.23 | 1861.4 ± 890.1 | 1.13 ± 0.58 | -- |
| Docetaxel | 10 | 103.6 ± 30.75 | 903.6 ± 421.7* | 0.55 ± 0.22* | 51.6 |
| Plinabulin | 4 | 104.7 ± 26.66 | 1425.3 ± 411.2 | 0.77 ± 0.31 | 31.9 |
| Cyclophosphamide | 20 | 105.1 ± 30.26 | 687.2 ± 288.6* | 0.42 ± 0.24* | 62.6 |
| Compound 15p | 2 | 103.7 ± 29.99 | 1453.6 ± 909.5 | 0.82 ± 0.47 | 27.6 |
| Compound 15p | 4 | 102.2 ± 28.62 | 674.5 ± 274.0* | 0.39 ± 0.22* | 65.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Z.; Li, F.; Xie, L.; Gu, M.; Li, C.; Liu, C.; Peng, C.; Li, W. Design and Synthesis of Novel Phenylahistin Derivatives Based on Co-Crystal Structures as Potent Microtubule Inhibitors for Anti-Cancer Therapy. Mar. Drugs 2022, 20, 752. https://doi.org/10.3390/md20120752
Ding Z, Li F, Xie L, Gu M, Li C, Liu C, Peng C, Li W. Design and Synthesis of Novel Phenylahistin Derivatives Based on Co-Crystal Structures as Potent Microtubule Inhibitors for Anti-Cancer Therapy. Marine Drugs. 2022; 20(12):752. https://doi.org/10.3390/md20120752
Chicago/Turabian StyleDing, Zhongpeng, Feifei Li, Lianghui Xie, Minqing Gu, Chunlei Li, Chang Liu, Chao Peng, and Wenbao Li. 2022. "Design and Synthesis of Novel Phenylahistin Derivatives Based on Co-Crystal Structures as Potent Microtubule Inhibitors for Anti-Cancer Therapy" Marine Drugs 20, no. 12: 752. https://doi.org/10.3390/md20120752
APA StyleDing, Z., Li, F., Xie, L., Gu, M., Li, C., Liu, C., Peng, C., & Li, W. (2022). Design and Synthesis of Novel Phenylahistin Derivatives Based on Co-Crystal Structures as Potent Microtubule Inhibitors for Anti-Cancer Therapy. Marine Drugs, 20(12), 752. https://doi.org/10.3390/md20120752






