Polyketide Derivatives, Guhypoxylonols A–D from a Mangrove Endophytic Fungus Aspergillus sp. GXNU-Y45 That Inhibit Nitric Oxide Production
Abstract
:1. Introduction
2. Results and Discussion
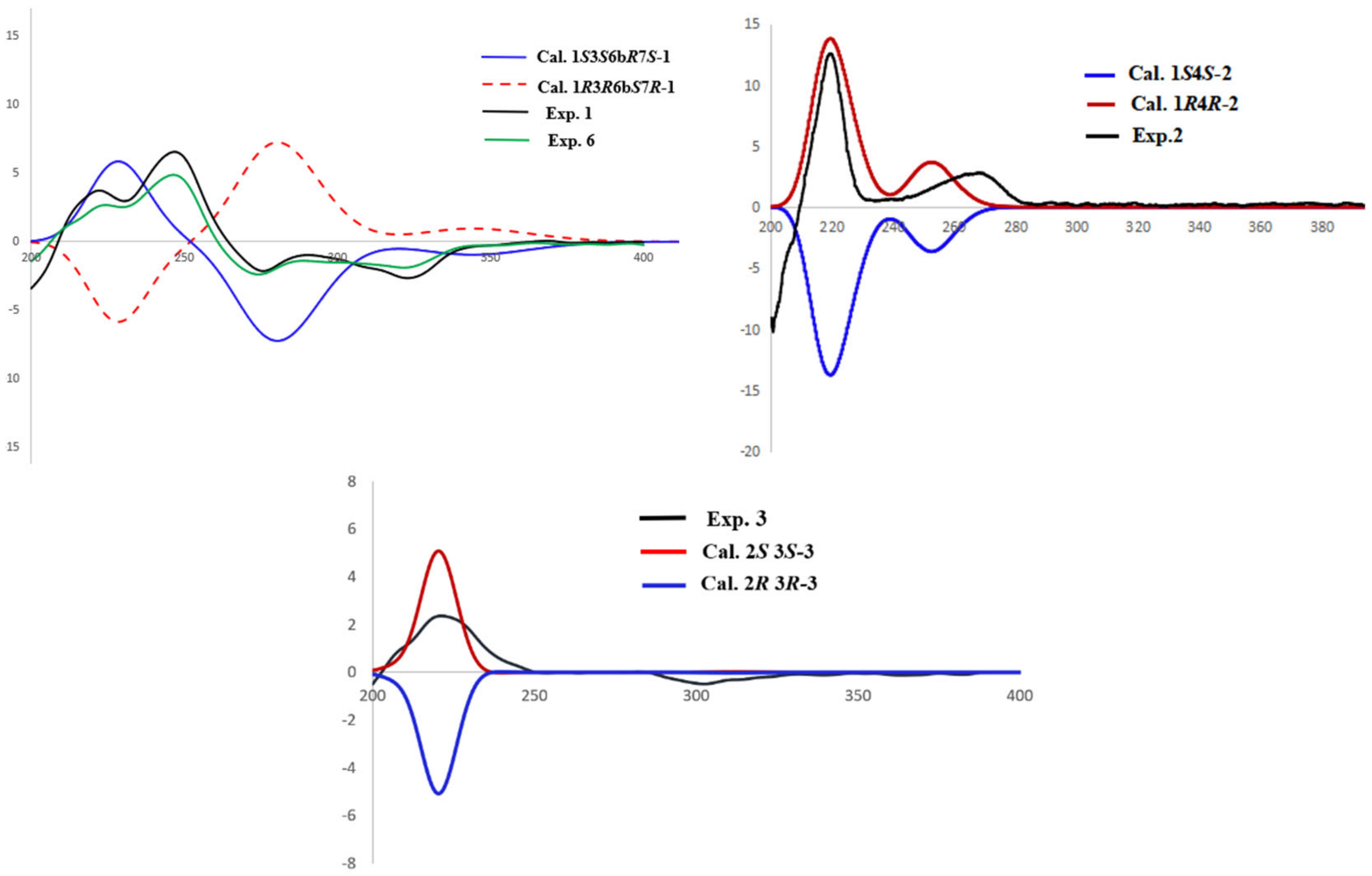
2.1. Structure Elucidation of the Compounds
2.2. Anti-Inflammatory Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
3.4. Extraction and Isolation
3.5. Anti-Inflammatory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carroll, A.; Copp, B.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Chen, S.; Yuan, J. Statistical Research on the Bioactivity of New Marine Natural Products Discovered during the 28 Years from 1985 to 2012. Mar. Drugs 2015, 13, 202–221. [Google Scholar] [CrossRef]
- Cao, F.; Meng, Z.-H.; Wang, P.; Luo, D.-Q.; Zhu, H.-J. Dipleosporalones A and B, Dimeric Azaphilones from a Marine-Derived Pleosporales sp. Fungus. J. Nat. Prod. 2020, 83, 1283–1287. [Google Scholar] [CrossRef]
- Carroll, A.; Copp, B.; Davis, R.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 112–173. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.-T.; Yang, L.; Kong, F.-D.; Ma, Q.-Y.; Xie, Q.-Y.; Dai, H.-F.; Yu, Z.-F.; Zhao, Y.-X. Cytotoxic Indole-Diterpenoids from the Marine-Derived Fungus Penicillium sp. KFD28. Mar. Drugs 2021, 19, 613. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.-J.; Hillman, P.F.; Lee, J.; Hwang, S.; Lee, E.-Y.; Cha, S.-S.; Yang, I.; Oh, D.-C.; Nam, S.-J.; Fenical, W. Antibacterial Meroterpenoids, Merochlorins G–J from the Marine Bacterium Streptomyces sp. Mar. Drugs 2021, 19, 618. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Lin, Y.; Luo, M.; Lu, Y.; Huang, X.; She, Z. Diaporisoindoles A–C: Three Isoprenylisoindole Alkaloid Derivatives from the Mangrove Endophytic Fungus Diaporthe sp. SYSU-HQ3. Org. Lett. 2017, 19, 5621–5624. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, Y.; Li, J.; Huang, X.; Yan, T.; Cao, W.; Liu, H.; Long, Y.; She, Z. Diaporindenes A–D: Four Unusual 2,3-Dihydro-1H-indene Analogues with Anti-inflammatory Activities from the Mangrove Endophytic Fungus Diaporthe sp. SYSU-HQ3. J. Org. Chem. 2018, 83, 11804–11813. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ding, M.; Liu, W.; Huang, X.; Liu, Z.; Lu, Y.; Liu, H.; She, Z. Anti-inflammatory meroterpenoids from the mangrove endophytic fungus Talaromyces amestolkiae YX1. Phytochemistry 2018, 146, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, Y.; Nie, Y.; Liu, Z.; Chen, S.; Zhang, Z.; Lu, Y.; He, L.; Huang, X.; She, Z. Polyketides from the Mangrove-Derived Endophytic Fungus Nectria sp. HN001 and Their α-Glucosidase Inhibitory Activity. Mar. Drugs 2016, 14, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukai, M.; Tsukada, M.; Miki, K.; Suzuki, T.; Sugita, T.; Kinoshita, K.; Takahashi, K.; Shiro, M.; Koyama, K. Hypoxylonols C–F, Benzo[j]fluoranthenes from Hypoxylon truncatum. J. Nat. Prod. 2011, 75, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Kamisuki, S.; Ishimaru, C.; Onoda, K.; Kuriyama, I.; Ida, N.; Sugawara, F.; Yoshidab, H.; Mizushina, Y. Nodulisporol and nodulisporone, novel specific inhibitors of human DNA polymerase k from a fungus, Nodulisporium sp. Bioorg. Med. Chem. 2007, 15, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Ge, H.M.; Song, Y.C.; Ding, H.; Zhu, H.L.; Zhao, A.X.A.; Tan, R.X. Cytotoxic Benzo[j]fluoranthene Metabolites from Hypoxylon truncatum IFB-18, an Endophyte of Artemisia annua. J. Nat. Prod. 2007, 70, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.N.; Ma, Q.Y.; Kong, F.D.; Xie, Q.Y.; Dai, H.F.; Zhou, L.M.; Yu, Z.F.; Zhao, Y.X. Napthrene Compounds from Mycelial Fermentation Products of Marasmius berteroi. Molecules 2020, 25, 3898. [Google Scholar] [CrossRef] [PubMed]
- Sone, Y.; Nakamura, S.; Sasaki, M.; Hasebe, F.; Kim, S.-Y.; Funa, N. Bacterial Enzymes Catalyzing the Synthesis of 1,8-Dihydroxynaphthalene, a Key Precursor of Dihydroxynaphthalene Melanin, from Sorangium cellulosum. Appl. Environ. Microbiol. 2018, 84, e00258-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Deng, S.; Zhou, D.; Huang, Y.; Li, C.; Hao, L.; Zhang, G.; Su, S.; Xu, X.; Yang, R.-Y.; et al. 3,4-seco-Dammarane Triterpenoid Saponins with Anti-Inflammatory Activity Isolated from the Leaves of Cyclocarya paliurus. J. Agric. Food Chem. 2020, 68, 2041–2053. [Google Scholar] [CrossRef] [PubMed]

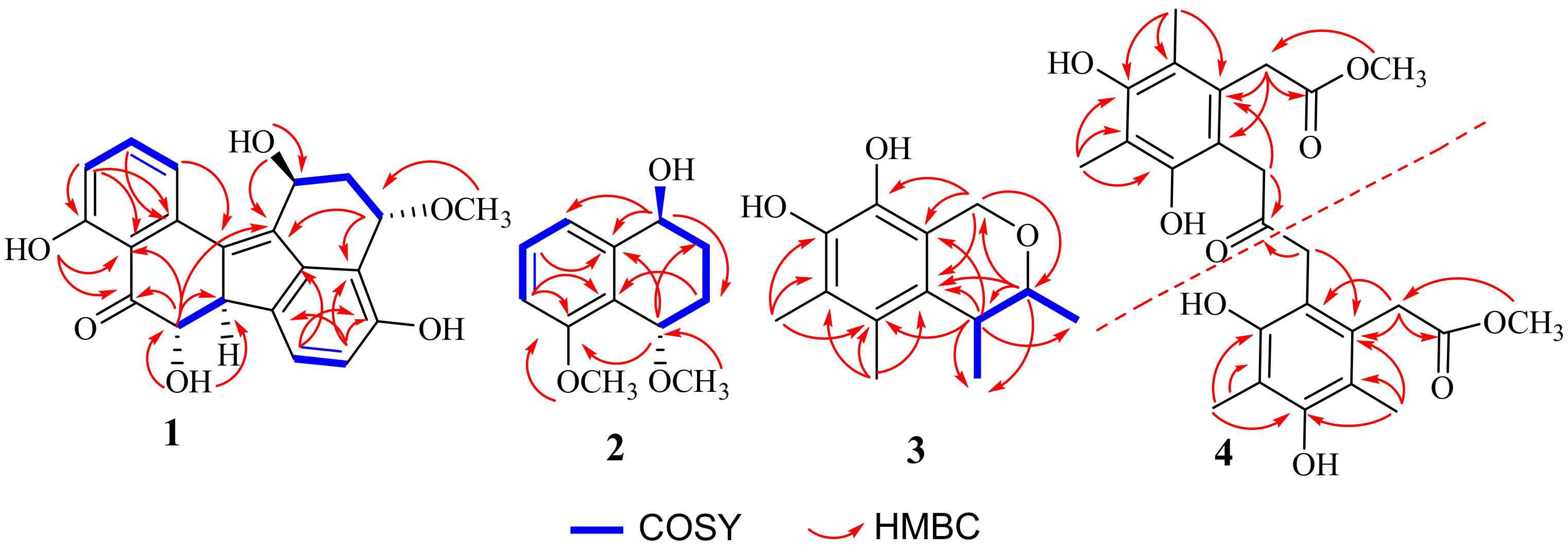

| Position | δC, Type | δH, (Mult., J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1 | 62.5, CH | 5.22, m | H-2 | |
| 2α 2β | 39.7, CH2 | 2.50, m 1.68, m | H-1, 3 | |
| 3 | 70.4, CH | 4.74, t (3.0) | H-2 | C-3a, 12c |
| 4 | 154.4, C | |||
| 5 | 112.9, CH | 6.71, d (8.0) | H-6 | C-3a, 4, 6a |
| 6 | 125.5, CH | 7.38, d (8.0) | H-5 | C-4, 12d |
| 6a | 134.4, C | |||
| 6b | 56.1, CH | 3.94, dd (12.4, 3.1) | H-7 | |
| 7 | 76.4, CH | 3.78, dd (12.3, 5.6) | H-6b | C-6b, 8, 8a, 12c |
| 8 | 206.5, C | |||
| 8a | 114.0, C | |||
| 9 | 161.4, C | |||
| 10 | 115.6, CH | 6.84, d (8.2) | H-11 | C-8a, 9, 12a |
| 11 | 136.1, CH | 7.55, d (8.0) | H-10, 12 | C-12a |
| 12 | 121.5, CH | 7.43, d (7.7) | H-11 | C-12b |
| 12a | 138.2, C | |||
| 12b | 134.2, C | |||
| 12c | 140.0, C | |||
| 12d | 144.9, C | |||
| 1-OH | 5.06, d (7.8) | C-1, 12c | ||
| 4-OH | 9.54, s | |||
| 7-OH | 6.17, d (5.9) | C-6b, 7 | ||
| 9-OH | 12.32, s | C-8, 8a | ||
| 3-OCH3 | 55.9, CH3 | 3.29, s | C-3 |
| Position | δC, Type | δH (Mult., J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1 | 67.8, CH | 4.41, m | H-2 | C-3, 8, 8a |
| 2 | 27.2, CH2 | 1.80, m | H-1, 3 | |
| 3α 3β | 24.7, CH2 | 2.09, m 1.51, m | H-2, 4 | C-4a |
| 4 | 69.8, CH | 4.35, t (2.8) | H-3 | C-2, 5, 8a |
| 4a | 124.8, C | |||
| 5 | 157.5, C | |||
| 6 | 128.5, CH | 7.24, d (7.9) | H-7 | C-4a, 5 |
| 7 | 108.8, CH | 6.83, d (8.1) | H-6, 8 | C-8a |
| 8 | 118.7, CH | 7.14, d (7.7) | H-7 | |
| 8a | 143.1, C | |||
| 1-OH | 5.28, s | |||
| 4-OCH3 | 56.7, CH3 | 3.31, s | C-4 | |
| 5-OCH3 | 55.7, CH3 | 3.75, s | C-5 |
| Position | δC, Type | δH (Mult., J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 2 | 76.0, CH | 3.86, qd (6.6, 2.6) | H-3, 11 | C-3, 3a, 8, 12 |
| 3 | 36.4, CH | 2.63, qd (6.8, 2.6) | H-2, 12 | C-3a, 4, 7a, 11, 12 |
| 3a | 134,8, C | |||
| 4 | 115.9, C | |||
| 5 | 111.3, C | |||
| 6 | 153.3, C | |||
| 7 | 149.6, C | |||
| 7a | 114.4, C | |||
| 8 | 60.8, CH2 | 4.65, d (15.2) 4.58, d (15.2) | C-2, 3a, 7, 7a | |
| 9 | 11.1, CH3 | 2.10, s | C-3a, 4, 5 | |
| 10 | 9.1, CH3 | 2.10, s | C-4, 5, 6 | |
| 11 | 21.0, CH3 | 1.18, d (6.8) | ||
| 12 | 18.2, CH3 | 1.19, d (6.6) |
| Position | δC, Type | δH (Mult., J in Hz) | HMBC |
|---|---|---|---|
| 1 (1′) | 118.7, C | ||
| 2 (2′) | 130.6, C | ||
| 3 (3′) | 157.5, C | ||
| 4 (4′) | 112.5, C | ||
| 5 (5′) | 155.3, C | ||
| 6 (6′) | 123.6, C | ||
| 7 (7′) | 36.5, CH2 | 3.73, s | C-1 (1′), 6 (6′), 8 (8′) |
| 8 (8′) | 173.8, C | ||
| 9 (9′) | 9.0, CH3 | 2.10, s | C-1 (1′), 2 (2′), 3 (3′) |
| 10 (10′) | 12.1, CH3 | 2.07, s | C-3 (3′), 4 (4′), 5 (5′) |
| 11 (11′) | 32.5, CH2 | 2.50, s | C-1 (1′), 12 |
| 12 | 207.9, C | ||
| 8-OCH3 | 52.5, CH3 | 3.67, s | C-8 (8′) |
| Compounds | IC50 (μM) |
|---|---|
| 1 | 14.42 ± 0.11 |
| 2 | 32.48 ± 0.19 |
| 3 | 18.03 ± 0.14 |
| 4 | 16.66 ± 0.21 |
| 5 | >80 |
| 6 | 21.05 ± 0.13 |
| 7 | >80 |
| 8 | >80 |
| 9 | >80 |
| 10 | >80 |
| 11 | >80 |
| Dexamethasoneb | 16.12 ± 1.41 μM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Huang, J.; Zhou, D.; Zhang, W.; Zhang, Y.; Li, J.; Yang, R.; Huang, X. Polyketide Derivatives, Guhypoxylonols A–D from a Mangrove Endophytic Fungus Aspergillus sp. GXNU-Y45 That Inhibit Nitric Oxide Production. Mar. Drugs 2022, 20, 5. https://doi.org/10.3390/md20010005
Qin X, Huang J, Zhou D, Zhang W, Zhang Y, Li J, Yang R, Huang X. Polyketide Derivatives, Guhypoxylonols A–D from a Mangrove Endophytic Fungus Aspergillus sp. GXNU-Y45 That Inhibit Nitric Oxide Production. Marine Drugs. 2022; 20(1):5. https://doi.org/10.3390/md20010005
Chicago/Turabian StyleQin, Xiaoya, Jiguo Huang, Dexiong Zhou, Wenxiu Zhang, Yanjun Zhang, Jun Li, Ruiyun Yang, and Xishan Huang. 2022. "Polyketide Derivatives, Guhypoxylonols A–D from a Mangrove Endophytic Fungus Aspergillus sp. GXNU-Y45 That Inhibit Nitric Oxide Production" Marine Drugs 20, no. 1: 5. https://doi.org/10.3390/md20010005
APA StyleQin, X., Huang, J., Zhou, D., Zhang, W., Zhang, Y., Li, J., Yang, R., & Huang, X. (2022). Polyketide Derivatives, Guhypoxylonols A–D from a Mangrove Endophytic Fungus Aspergillus sp. GXNU-Y45 That Inhibit Nitric Oxide Production. Marine Drugs, 20(1), 5. https://doi.org/10.3390/md20010005





