Biochemical Characterization of Cassiopea andromeda (Forsskål, 1775), Another Red Sea Jellyfish in the Western Mediterranean Sea
Abstract
1. Introduction
2. Results and Discussion
2.1. Jellyfish Biometric Data
2.2. Jellyfish Biomass Composition
2.2.1. Amino Acid Composition of Whole C. andromeda Jellyfish
2.2.2. Jellyfish Soluble Compounds
2.2.3. Jellyfish Lipid Composition
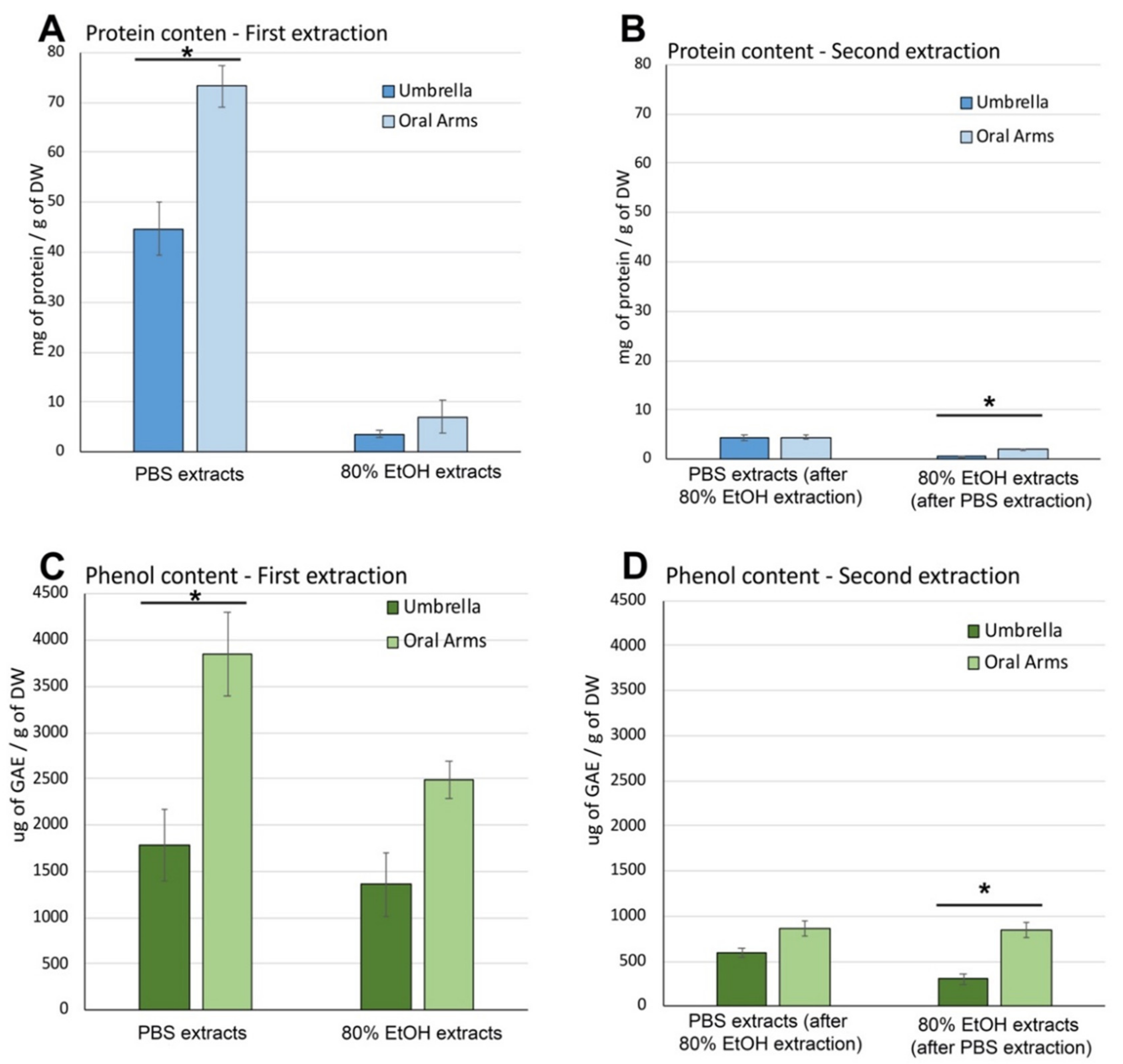
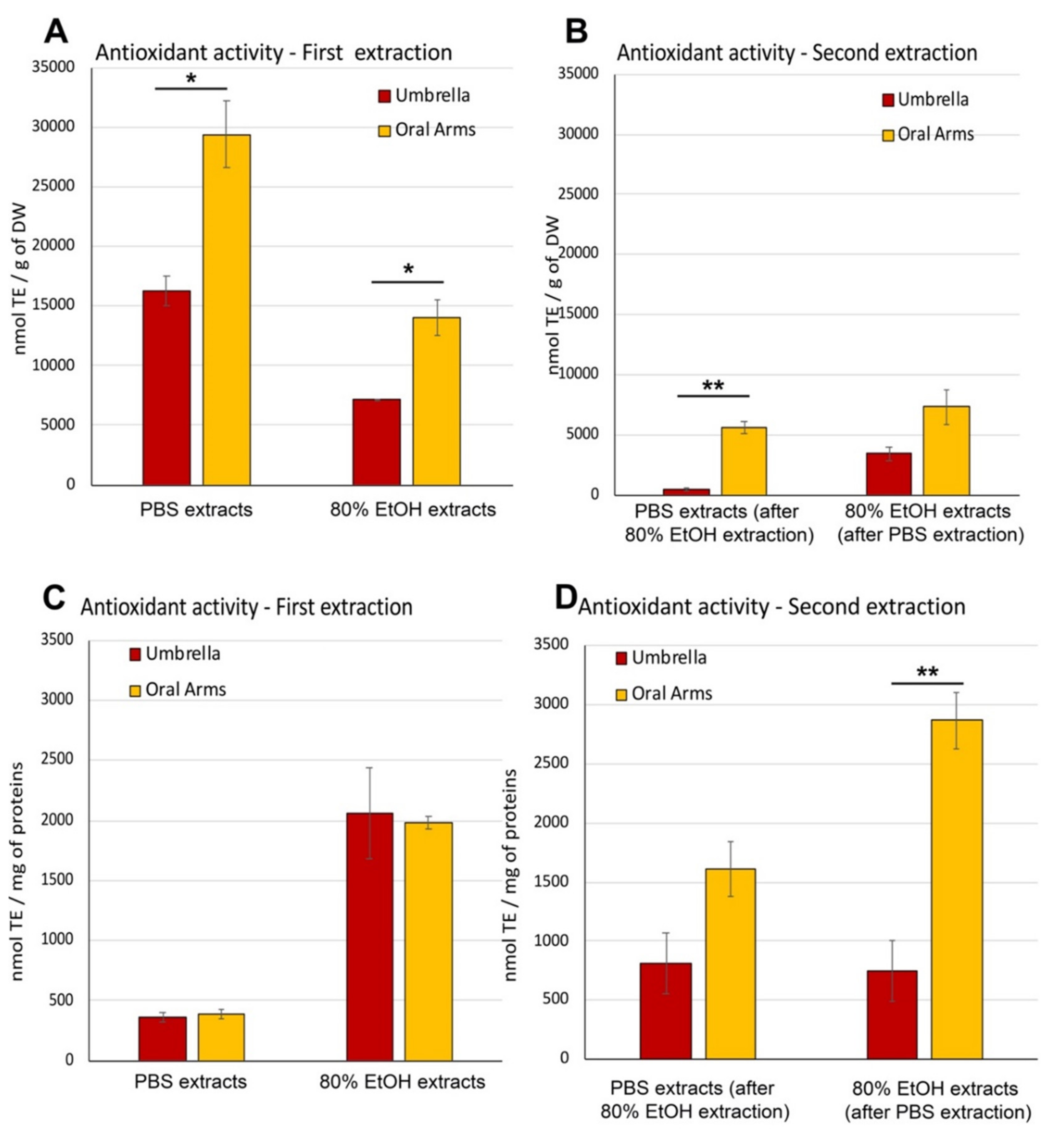
2.2.4. Soluble Biomass Distribution in Umbrella and Oral Arms
2.2.5. Partial Characterization of Soluble Compounds in Umbrella and Oral Arms of Cassiopea andromeda
Soluble Protein and Phenolic Compound Content in Aqueous and Hydroalcoholic Extracts
Antioxidant Activity in Aqueous and Hydroalcoholic Extracts
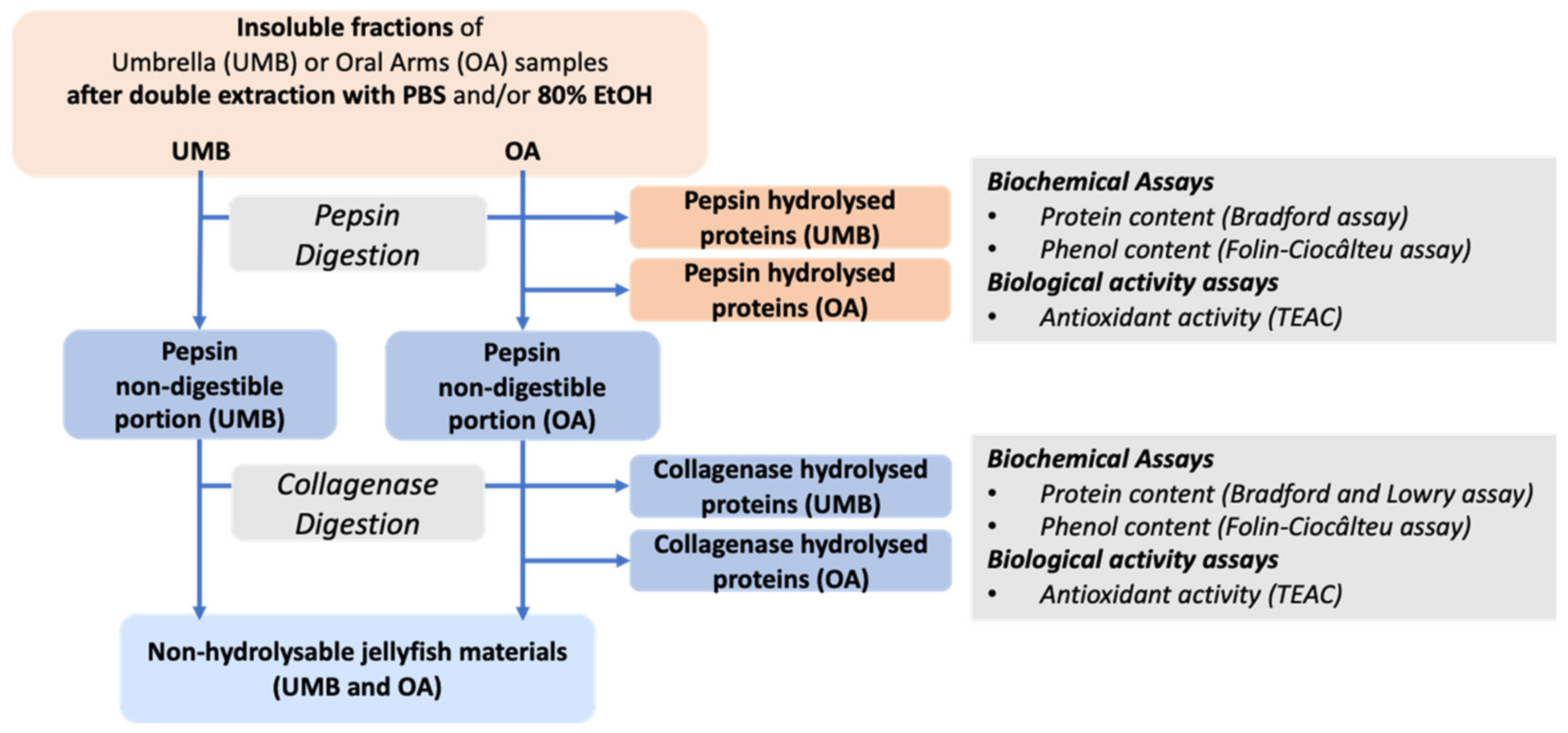
2.2.6. Umbrella and Oral Arms Insoluble Biomass Characterization
Protein Content in Pepsin- and Collagenase-Hydrolyzed Jellyfish Fractions
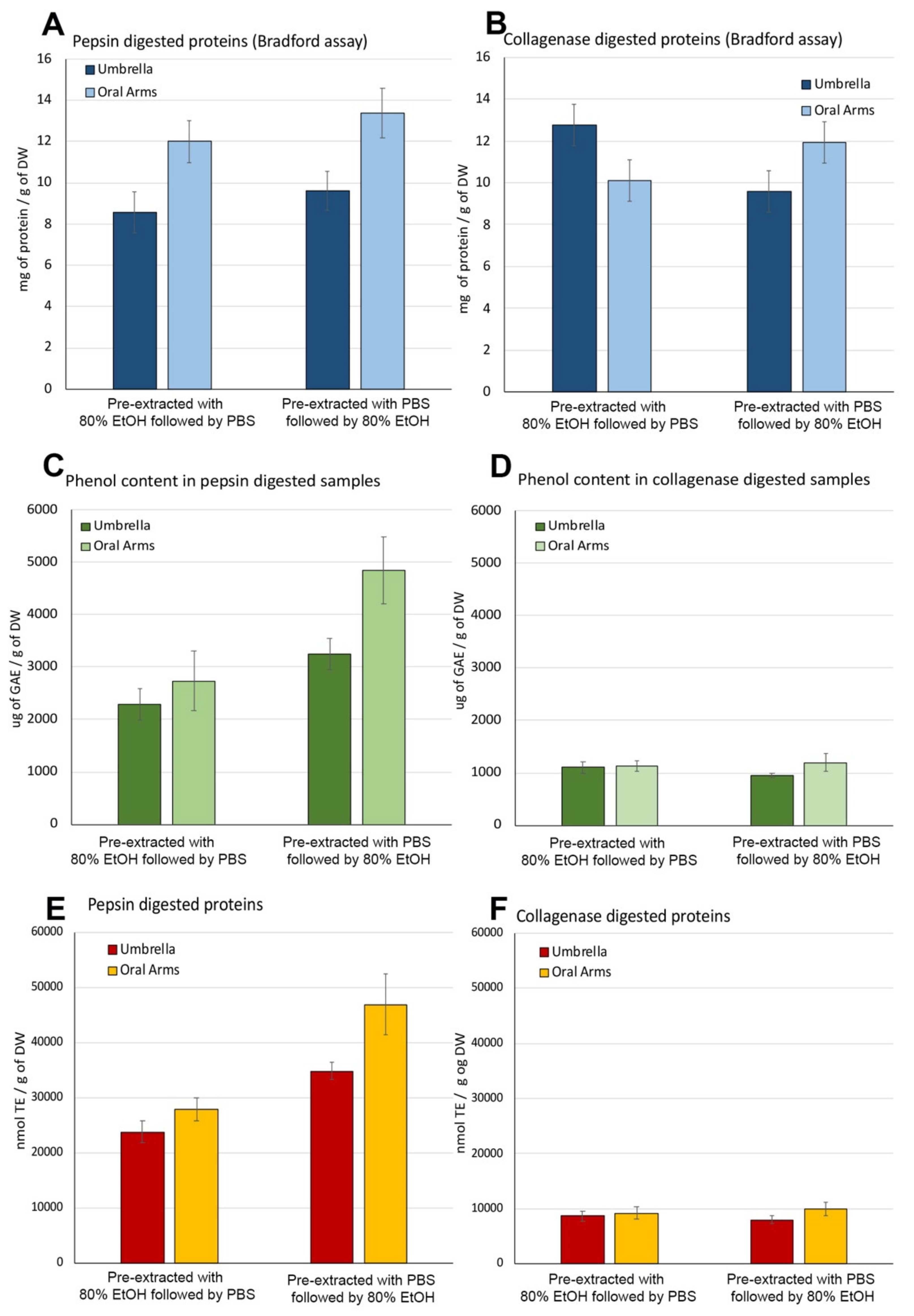
Total Content of Phenolic Compounds in Pepsin- and Collagenase-Hydrolyzed Fractions
Antioxidant Activity in Pepsin- and Collagenase-Hydrolyzed Fractions
3. Materials and Methods
3.1. Chemicals, Materials, and Equipment
3.2. Jellyfish Samples
3.3. Amino Acid Analysis
3.4. Aqueous and Hydroalcoholic Extractions of Soluble Compounds
3.5. Sequential Hydrolysis of Insoluble Compounds
3.6. Protein Quantification
3.7. Quantification of Phenolic Compounds
3.8. In Vitro Antioxidant Activity Assay
3.9. Total Lipid Extraction
3.10. Fatty Acid Profiles Analysis
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Galil, B.S.; Gershwin, L.A.; Zorea, M.; Rahav, A.; Rothman, S.B.; Fine, M.; Lubinevsky, H.; Douek, J.; Paz, G.; Rinkevich, B. Cotylorhiza erythraea Stiasny, 1920 (Scyphozoa: Rhizostomeae: Cepheidae), yet another erythraean jellyfish from the Mediterranean coast of Israel. Mar. Biodivers. 2017, 47, 229–235. [Google Scholar] [CrossRef]
- Galil, B.S.; Spanier, E.; Ferguson, W.W. The Scyphomedusae of the Mediterranean coast of Israel, including two Lessepsian migrants new to the Mediterranean. Zool. Meded. 1990, 64, 95–105. [Google Scholar]
- Deidun, A.; Gauci, A.; Sciberras, A.; Piraino, S. Back with a bang—An unexpected massive bloom of Cassiopea andromeda (Forskaal, 1775) in the Maltese Islands, nine years after its first appearance. BioInvasions Rec. 2018, 7, 399–404. [Google Scholar] [CrossRef]
- Schembri, P.J.; Deidun, A.; Vella, P.J. First record of Cassiopea andromeda (Scyphozoa: Rhizostomeae: Cassiopeidae) from the central Mediterranean Sea. Mar. Biodivers. Rec. 2010, 3, e6. [Google Scholar] [CrossRef]
- Amor, K.O.B.; Rifi, M.; Ghanem, R.; Draeif, I.; Zaouali, J.; Ben Souissi, J. Update of alien fauna and new records from Tunisian marine waters. Mediterr. Mar. Sci. 2015, 17, 124–143. [Google Scholar] [CrossRef]
- Piraino, S.; Deidun, A.; Fuentes, V.; Daly Yahia, N.; Kefi Daly Yahia, O.; Marambio, M.; Gueroun, S. Guidelines for the Identification of Mediterranean Jellyfish and Other Gelatinous Organisms with a First Aid Protocol for Possible Sting Treatment. 2014. Available online: http://www.enpicbcmed.eu/sites/default/files/med-jellyrisk_guidelines.pdf (accessed on 22 April 2020).
- Cillari, T.; Andaloro, F.; Castriota, L. First documented record of Cassiopea cf andromeda (Cnidaria: Scyphozoa) in Italian waters. Cah. Biol. Mar. 2018, 59, 193–195. [Google Scholar]
- Rubio, M. Una Medusa Tropical, Nueva Amenaza Para la Biodiversidad Del Mar Menor. Elclickverde. 2017. Available online: https://elclickverde.com/reportajes/una-medusa-tropical-nueva-amenaza-para-la-biodiversidad-del-mar-menor (accessed on 22 April 2020).
- Maggio, T.; Allegra, A.; Bosch-Belmar, M.; Cillari, T.; Cuttitta, A.; Falautano, M.; Milisenda, G.; Nicosia, A.; Perzia, P.; Sinopoli, M.; et al. Molecular identity of the non-indigenous Cassiopea sp. from Palermo Harbour (central Mediterranean Sea). J. Mar. Biol. Assoc. UK 2019, 99, 1765–1773. [Google Scholar] [CrossRef]
- Arai, Y.; Gotoh, R.O.; Yokoyama, J.; Sato, C.; Okuizumi, K.; Hanzawa, N. Phylogenetic relationships and morphological variations of upside-down jellyfishes, Cassiopea spp. inhabiting Palau Islands. Biogeography 2017, 19, 133–141. [Google Scholar]
- Morandini, A.C.; Stampar, S.N.; Maronna, M.M.; Da Silveira, F.L. All non-indigenous species were introduced recently? The case study of Cassiopea (Cnidaria: Scyphozoa) in Brazilian waters. J. Mar. Biol. Assoc. UK 2016, 97, 321–328. [Google Scholar] [CrossRef]
- Holland, B.S.; Dawson, M.N.; Crow, G.L.; Hofmann, D.K. Global phylogeography of Cassiopea (Scyphozoa: Rhizostomeae): Molecular evidence for cryptic species and multiple invasions of the Hawaiian Islands. Mar. Biol. 2004, 145, 1119–1128. [Google Scholar] [CrossRef]
- Lampert, K.P.; Bürger, P.; Striewski, S.; Tollrian, R. Lack of association between color morphs of the Jellyfish Cassiopea andromeda and zooxanthella clade. Mar. Ecol. 2012, 33, 364–369. [Google Scholar] [CrossRef]
- Banha, T.N.S.; Mies, M.; Güth, A.Z.; Pomory, C.M.; Sumida, P.Y.G. Juvenile Cassiopea andromeda medusae are resistant to multiple thermal stress events. Mar. Biol. 2020, 167, 1–13. [Google Scholar] [CrossRef]
- LaJeunesse, T.C.; Loh, W.; Trench, R.K. Do introduced endosymbiotic dinoflagellates ‘take’ to new hosts? Biol. Invasions. 2009, 11, 995–1003. [Google Scholar] [CrossRef]
- Djeghri, N.; Pondaven, P.; Stibor, H.; Dawson, M.N. Review of the diversity, traits, and ecology of zoox- anthellate jellyfishes. Mar. Biol. 2019, 166, 1–19. [Google Scholar] [CrossRef]
- Brotz, L.; Pauly, D. Studying jellyfish fisheries: Toward accurate national catch reports and appropriate methods. In Jellyfish: Ecology, Distribution Patterns and Human Interactions; Mariottini, G.L., Ed.; Nova Publishers: Hauppauge, NY, USA, 2017; pp. 313–329. Available online: https://www.researchgate.net/publication/312492290_Studying_jellyfish_fisheries_toward_accurate_national_catch_reports_and_appropriate_methods_for_stock_assessments (accessed on 10 May 2020).
- Leone, A.; Lecci, R.M.; Milisenda, G.; Piraino, S. Mediterranean jellyfish as novel food: Effects of thermal processing on antioxidant, phenolic, and protein contents. Eur. Food Res. Technol. 2019, 245, 1611–1627. [Google Scholar] [CrossRef]
- Bleve, G.; Ramires, F.A.; Gallo, A.; Leone, A. Identification of safety and quality parameters for preparation of jellyfish based novel food products. Foods 2019, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Bleve, G.; Ramires, F.A.; De Domenico, S.; Gallo, A.; Leone, A. An alum-free jellyfish treatment for food applications. Front. Nutr. 2021, 8, 718798. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.; Durante, M.; Meli, F.; Piraino, S. The bright side of gelatinous blooms: Nutraceutical value and antioxidant properties of three mediterranean jellyfish (Scyphozoa). Mar. Drugs 2015, 13, 4654–4681. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Lecci, R.M.; Durante, M.; Piraino, S. Extract from the zooxanthellatae jellyfish Cotylorhiza tuberculata modulates gap junction intercellular communication in human cell cultures. Mar. Drugs 2013, 11, 1728–1762. [Google Scholar] [CrossRef]
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.M.; Calado, R. Trends in the Discovery of New Marine Natural Products from Invertebrates over the Last Two Decades—Where and What Are We Bioprospecting? PLoS ONE 2012, 7, e30580. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.E.F.; Moustafa, S.; El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine natural products: A source of novel anticancer drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Peixe, L.; Gomes, N.C.; Calado, R. Cnidarians as a source of new marine bioactive com-pounds—An overview of the last decade and future steps for bioprospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef]
- Stabili, L.; Schirosi, R.; Parisi, M.G.; Piraino, S.; Cammarata, M. The Mucus of Actinia equina (Anthozoa, Cnidaria): An Unexplored Resource for Potential Applicative Purposes. Mar. Drugs 2015, 13, 5276–5296. [Google Scholar] [CrossRef] [PubMed]
- Schmich, J.; Kraus, Y.; De Vito, D.; Graziussi, D.; Boero, F.; Piraino, S. Induction of reverse development in two marine Hydrozoans. Int. J. Dev. Biol. 2007, 51, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Brotz, L.; Cheung, W.W.L.; Kleisner, K.; Pakhomov, E.; Pauly, D. Increasing jellyfish populations: Trends in Large Marine Ecosystems. Hydrobiologia 2012, 690, 3–20. [Google Scholar] [CrossRef]
- D’Amico, P.; Leone, A.; Giusti, A.; Armani, A. Jellyfish and humans: Not just negative interactions. In Jellyfish: Ecology, Distribution Patterns and Human Interactions; Mariottini, G.L., Ed.; Nova Publishers: Hauppauge, NY, USA, 2017; pp. 331–352. Available online: http://hdl.handle.net/11568/795143 (accessed on 22 April 2020).
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.M.; Wang, J.F.; Zha, X.Q.; Pan, L.H.; Zhang, H.L.; Luo, J.P. Structural characterization, and immunomodulatory activity of a new polysaccharide from jellyfish. Carbohydr. Polym. 2017, 159, 188–194. [Google Scholar] [CrossRef]
- Lucas, C.H.; Pitt, K.A.; Purcell, J.E.; Lebrato, M.; Condon, R.H. What’s in a jellyfish? Proximate and elemental composition and biometric relationships for use in biogeochemical studies. Ecology 2011, 92, 1704. [Google Scholar] [CrossRef]
- Merquiol, L.; Romano, G.; Ianora, A.; D’Ambra, I. Biotechnological Applications of Scyphomedusae. Mar. Drugs 2019, 17, 604. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z. In vitro Evaluation of Natural Marine Sponge Collagen as a Scaffold for Bone Tissue Engineering. Int. J. Biol. Sci. 2011, 7, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.M.; Sasaki, S.; McClements, D.J.; Decker, E.A. Mechanisms of the antioxidant activity of a high molecular weight fraction of whey. J. Agric. Food Chem. 2000, 48, 1473–1478. [Google Scholar] [CrossRef]
- Khong, N.M.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Nishikawa, J. Nutritional composition, and total collagen content of three commercially important edible jellyfish. Food Chem. 2016, 196, 953–960. [Google Scholar] [CrossRef]
- Addad, S.; Esposito, J.Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, characterization, and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef]
- Barzideh, Z.; Latiff, A.A.; Gan, C.Y.; Benjakul, S.; Karim, A.A. Isolation and characterisation of collagen from the ribbon jellyfish (Chrysaora sp.). Int. J. Food Sci. Tech. 2014, 49, 1490–1499. [Google Scholar] [CrossRef]
- Jankangram, W.; Chooluck, S.; Pomthong, B. Comparison of the properties of collagen extracted from dried jellyfish and dried squid. Afr. J. Biotechnol. 2016, 15, 642–648. [Google Scholar] [CrossRef]
- De Domenico, S.; De Rinaldis, G.; Paulmery, M.; Piraino, S.; Leone, A. Barrel Jellyfish (Rhizostoma pulmo) as Source of Antioxidant Peptides. Mar. Drugs 2019, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Yu, R.H.; Li, M.Z.; Li, C.J.; Chen, H.Q.; Jiang, Y.; Tang, T.; Qiab, W.Y.; Xu, H.M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. 2019, 22, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Ueno, M.; Goto, Y.; Shiraishi, R.; Doi, M.; Akiyama, K.; Yamauchi, S. Immunostimulation Effect of Jellyfish Collagen. Biosci. Biotechnol. Biochem. 2006, 70, 2131–2137. [Google Scholar] [CrossRef]
- Lucas, C.H. Biochemical composition of the mesopelagic coronate jellyfish Periphylla periphylla from the Gulf of Mexico. J. Mar. Biol. Assoc. UK 2009, 89, 77–81. [Google Scholar] [CrossRef]
- De Souza, L.M.; Iacomini, M.; Gorin, P.A.J.; Sari, R.S.; Haddad, M.A.; Sassaki, G.L. Glyco- and sphingophosphonolipids from the medusa Phyllorhiza punctata: NMR and ESI-MS/MS fingerprints. Chem. Phys. Lipids 2007, 145, 85–96. [Google Scholar] [CrossRef]
- Thè, J.; de Sousa Barroso, H.; Mammone, M.; Viana, M.; Servulo, C.; Melo, B.; Mies, M.; Banha, T.N.S.; Morandini, A.C.; Rossi, S.; et al. Aquaculture facilities promote populational stability throughout seasons and increase medusae size for the invasive jellyfish Cassiopea andromeda. Mar. Environ. Res. 2020, 162, 105161. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, Characterization, and evaluation of collagen from jellyfish Rhopilema esculentum Kishinouye for use in haemostatic applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Lanier, T.C.; Hultin, H.O. Characteristics of edible muscle tissues. Food Chem. 1996, 3, 879–942. [Google Scholar]
- Holmgren, S.K.; Taylor, K.M.; Bretscher, L.E.; Raines, R.T. Code for collagen’s stability deciphered. Nature 1998, 392, 666–667. [Google Scholar] [CrossRef]
- Whelan, J. Dietary stearidonic acid is a long chain (n-3) polyunsaturated fatty acid with potential health benefits. J. Nutr. 2009, 139, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Surette, M.E. Dietary omega-3 PUFA and health: Stearidonic acid-containing seed oils as effective and sustainable alternatives to traditional marine oils. Mol. Nutr. Food Res. 2013, 57, 748–759. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Aung, T.; Halsey, J.; Kromhout, D.; Hertzel, C.G.; Marchioli, R.; Tavazzi, L.; Geleijnse, J.M.; Rauch, B.; Ness, A.; Galan, P.; et al. For the Omega-3 Treatment Trialists’ Collaboration. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks meta-analysis of 10 trials involving 77917 Individuals. JAMA Cardiol. 2018, 3, 225–233. [Google Scholar] [CrossRef]
- Maillard, V.; Bougnoux, P.; Ferrari, P.; Jourdan, M.L.; Pinault, M.; Lavillonnière, F.; Body, G.; Le Floch, O.; Chajès, V. N-3 and N-6 fatty acids in breast adipose tissue and relative risk of breast cancer in a case-control study in Tours, France. Int. J. Cancer 2002, 98, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Park, S.G.; Kim, B.; Lee, J.; Huang, W.X.; Sung, H.J. Dynamics of prolate jellyfish with a jet-based locomotion. J. Fluids Struct. 2015, 57, 331–343. [Google Scholar] [CrossRef]
- Costello, J.H.; Colin, S.P.; Dabiri, J.O. Medusan morphospace: Phylogenetic constraints, biomechanical solutions, and ecological consequences. Invertebr. Biol. 2008, 127, 265–290. [Google Scholar] [CrossRef]
- McHenry, M.J.; Jed, J. The ontogenetic scaling of hydrodynamics and swimming performance in jellyfish (Aurelia aurita). J. Exp. Biol. 2003, 206, 4125–4137. [Google Scholar] [CrossRef]
- Colin, S.P.; Costello, J.H. Morphology, swimming performance and propulsive mode of six co-occurring hydromedusae. J. Exp. Biol. 2002, 205, 427–437. [Google Scholar] [CrossRef]
- Purcell, J.E. Jellyfish and Ctenophore Blooms Coincide with Human Proliferations and Environmental Perturbations. Annu. Rev. Mar. 2012, 4, 209–235. [Google Scholar] [CrossRef]
- Pitt, K.A.; Clement, A.L.; Connolly, R.M.; Thibault-Botha, D. Predation by jellyfish on large and emergent zooplankton: Implications for benthic–pelagic coupling. Estuar. Coast. Mar. Sci. 2008, 76, 827–833. [Google Scholar] [CrossRef]
- Hamlet, C.; Miller, L.A. Feeding Currents of the Upside-Down Jellyfish in the Presence of Background Flow. Bull. Math. Biol. 2012, 74, 2547–2569. [Google Scholar] [CrossRef] [PubMed]
- Hamlet, C.; Santhanakrishnan, A.; Miller, L.A. A numerical study of the effects of bell pulsation dynamics and oral arms on the exchange currents generated by the upside-down jellyfish Cassiopea xamachana. J. Exp. Biol. 2011, 214, 1911–1921. [Google Scholar] [CrossRef]
- Latyshev, N.A.; Naumenko, N.V.; Svetashev, V.I.; Latypov, Y.Y. Fatty acids of reef-building corals. Mar. Ecol. Progr. Ser. 1991, 76, 295–301. [Google Scholar] [CrossRef]
- Dos Santos, R.; Carvalho, A.L.; Roque, A.C.A. Renaissance of protein crystallization and precipitation in biopharmaceuticals purification. Biotechnol. Adv. 2017, 35, 41–50. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Hirano, A.; Arakawa, T.; Shiraki, K. Mechanistic insights into protein precipitation by alcohol. Int. J. Biol. Macromol. 2012, 50, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Hydrolysates of fish skin collagen: An opportunity for valorising fish industry by-products. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Isolation, and characterization of collagen from bigeye snapper (Priacanthus macracanthus) skin. J. Sci. Food Agric. 2005, 85, 1203–1210. [Google Scholar] [CrossRef]
- Grienke, U.; Silke, J.; Tasdemir, D. Bioactive compounds from marine mussels and their effects on human health. Food Chem. 2014, 142, 48–60. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lopez, J.M.; Imperial, S.; Valderrama, R.; Navarro, S. An improved Bradford protein for collagen proteins. Clin. Chim. Acta 1993, 220, 91–100. [Google Scholar] [CrossRef]
- Komsa-Penkova, R.; Spirova, R.; Bechev, B. Modification of Lowry’s method for collagen concentration measurement. J. Biochem. Bioph. Meth. 1996, 32, 33–43. [Google Scholar] [CrossRef]
- Kiew, P.L.; Mashitah, M.D. Modified Lowry’s Method for Acid and Pepsin Soluble Collagen Measurement from Clarias Species Muscles. Sci. Rep. 2013, 2, 1–5. [Google Scholar]
- Barzideh, Z.; Latiff, A.A.; Gan, C.; Alias, A.K. ACE inhibitory and antioxidant activities of collagen hydrolysates from the ribbon Jellyfish (Chrysaora sp.). Food Technol. Biotecnol. 2014, 52, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, M.; Seok, J.H.; Kim, S.; Lee, D.B.; Bae, G.; Bae, H.I.; Bae, S.Y.; Hong, Y.M.; Kwon, S.O.; et al. Antiviral Effects of Black Raspberry (Rubus coreanus) Seed and Its Gallic Acid against Influenza Virus Infection. Viruses 2016, 8, 157. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ohnaka, K.; Morita, M.; Toyomura, K.; Kono, S.; Ueki, T.; Tanaka, M.; Kakeji, Y.; Maehara, Y.; Okamura, T.; et al. Dietary polyphenols and colorectal cancer risk: The Fukuoka colorectal cancer study. World. J. Gastroenterol. 2013, 19, 2683–2690. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. AJCN 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. AJCN 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Hsieh, Y.P.; Leong, F.M.; Rudloe, J. Jellyfish as food. Hydrobiologia 2001, 451, 11–17. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, C.; Zhao, J.; Shi, X.; Sun, M.; Liu, J.; Fu, Y.; Jin, W.; Zhu, B. Separation and characterization of antioxidative and angiotensin converting enzyme inhibitory peptide from jellyfish gonad hydrolysate. Molecules 2018, 23, 94. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, Y.; Shi, R.; Qiao, W.; Tang, Z. Sun Production of the angiotensin I converting enzyme inhibitory peptides and isolation of four novel peptides from jellyfish (Rhopilema esculentum) protein hydrolysate. J. Sci. Food Agric. 2016, 96, 3240–3248. [Google Scholar] [CrossRef]
- Fan, J.; Zhuang, Y.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients 2013, 5, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Sun, L.; Zhang, Y.; Liu, G. Antihypertensive effect of long-term oral administration of jellyfish (Rhopilema esculentum) collagen peptides on renovascular hypertension. Mar. Drugs 2012, 10, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Hou, H.; Zhao, X.; Zhang, Z.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV Irradiation. J. Food Sci. 2009, 74, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.; Li-Chen, C.; Eunice, C.H.; Jao, L.C. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem. 2011, 126, 617–622. [Google Scholar] [CrossRef]
- Etemadian, Y.; Ghaemi, V.; Shaviklo, A.R.; Pourashouri, P.; Mahoonak, A.R.S.; Rafipour, F. Development of animal/plant-based protein hydrolysate and its application in food, feed and nutraceutical industries: State of the art. J. Clean. Prod. 2021, 278, 123219. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Munekata, P.E.S.; Gomez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- De Domenico, S.; Giudetti, A. Nutraceutical intervention in ageing brain. J. Clin. Gerontol. Geriatr. 2017, 65, 79–92. [Google Scholar]
- Adebiyi, A.P.; Adebiyi, A.O.; Yamashita, J.; Ogawa, T.; Muramoto, K. Purification and characterization of antioxidative peptides derived from rice bran protein hydrolysates. Eur. Food Res. Technol. 2009, 228, 553–563. [Google Scholar] [CrossRef]
- Gulcin, I.; Şat, İ.G.; Beydemir, Ş.; Elmastaş, M.; Küfrevioǧlu, Ö.İ. Comparison of antioxidant activity of clove (Eugenia caryophylata) buds and lavender (Lavandula stoechas). Food Chem. 2004, 87, 393–400. [Google Scholar] [CrossRef]
- Gajanan, P.G.; Elavarasan, K.; Shamasundar, B.A. Bioactive and functional properties of protein hydrolysates from fish frame processing waste using plant proteases. ESPR 2016, 23, 24901–24911. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Gorguc, A.; Gencdag, E.; Yilmaz, F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments—A review. Food Res. Int. 2020, 136, 109504. [Google Scholar] [CrossRef]
- Karoud, W.; Sila, A.; Krichen, F.; Martinez-Alvarez, O.; Bougatef, A. Characterization, Surface Properties and Biological Activities of Protein Hydrolysates Obtained from Hake (Merluccius merluccius) Heads. Waste Biomass Valorization 2019, 10, 287–297. [Google Scholar] [CrossRef]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from Fish By-product Protein Hydrolysates and Its Functional Properties: An Overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef]
- Colgrave, M.L.; Allingham, P.G.; Jones, A. Hydroxyproline quantification for the estimation of collagen in tissue using multiple reaction monitoring mass spectrometry. J. Chromatogr. A 2008, 1212, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, L.M.; Fernando, S.; Marcela, S.; Salette, R.; Lima José, L.F.C. Rapid microplate high-throughput methodology for assessment of Folin–Ciocalteu reducing capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]


| Specimens | Umbrella Diameter (cm) | Fresh Weight (g) | Dry Weight (g) | Yield (% FW) |
|---|---|---|---|---|
| A1 | 15.5 | 245.2 | 18.6 | 7.6 |
| A2 | 14.0 | 233.5 | 16.3 | 7.0 |
| A3 | 17.5 | 296.5 | 22.4 | 7.6 |
| A4 | 16.5 | 288.4 | 19.8 | 6.9 |
| A5 | 14.0 | 202.5 | 15.7 | 7.8 |
| A6 | 14.5 | 264.9 | 19.8 | 7.5 |
| A7 | 15.5 | 210.1 | 17.0 | 8.1 |
| A8 | 13.5 | 152.4 | 11.6 | 7.6 |
| A9 | 14.0 | 204.5 | 15.8 | 7.7 |
| Mean | 15.0 | 233.1 | 17.4 | 7.5 |
| SD | ±1.3 | ±46.1 | ±3.1 | ±0.4 |
| Cassiopea andromeda Whole Jellyfish | |||
|---|---|---|---|
| Amino Acids | Percentage per Dry Weight (% of DW) | Percentage of the Total AA (%) | |
| Mean | ±SD | ||
| Alanine (Ala) | 0.96 | ±0.02 | 5.98 |
| Arginine (Arg) | 1.02 | ±0.02 | 6.34 |
| Aspartic acid + Asparagine (Asx) | 1.29 | ±0.07 | 8.07 |
| Cystine (Cys-Cys) | 0.28 | ±0.07 | 1.76 |
| Glutamic acid + Glutamine (Glx) | 1.80 | ±0.03 | 11.26 |
| Glycine (Gly) | 1.72 | ±0.12 | 10.73 |
| Histidine (His) e | 0.34 | ±0.00 | 2.13 |
| Hydroxylysine | Nd | Nd | 0 |
| Hydroxyproline | 0.26 | ±0.02 | 1.64 |
| Isoleucine (Ile) e | 0.56 | ±0.00 | 3.52 |
| Leucine (Leu) e | 0.94 | ±0.02 | 5.84 |
| Lysine (Lys) e | 1.06 | ±0.01 | 6.62 |
| Methionine (Met) e | 0.22 | ±0.00 | 1.38 |
| Methionine sulfoxide | Nd | Nd | 0 |
| Phenylalanine (Phe) e | 0.73 | ±0.02 | 4.58 |
| Proline (Pro) | 0.97 | ±0.02 | 6.04 |
| Serine (Ser) | 0.81 | ±0.00 | 5.07 |
| Taurine | 0.96 | ±0.02 | 6.01 |
| Threonine (Thr) e | 0.53 | ±0.10 | 3.32 |
| Tryptophan (Trp) e | Nd | Nd | 0 |
| Tyrosine (Tyr) | 0.44 | ±0.04 | 2.74 |
| Valine (Val) e | 0.79 | ±0.00 | 4.91 |
| Ammonia | 0.33 | ±0.01 | 2.06 |
| Total (AA only) | 15.68 | ±0.09 | |
| Total (AA + ammonia) | 16.01 | ±0.10 | 100 |
| Whole Jellyfish (WJ) | ||||
|---|---|---|---|---|
| Extraction Solvents | WJ DW (g) | Extract DW (g) | Yield (%DW) | Yield * (% FW) |
| Mean ± SD | ||||
| PBS | 1.629 ± 0.015 | 1.553 ± 0.011 | 83.4 ± 15.8 | 7.1 * |
| 80% EtOH | 1.517 ± 0.051 | 0.650 ± 0.009 | 42.5 ± 0.9 | 3.2 * |
| Total Lipids in Cassiopea andromeda | ||
|---|---|---|
| Total Lipids | Whole Jellyfish (WJ) | Hydroalcoholic Extract (80% EtOH Extract) |
| Total lipids by DW (mg/g of lyophylized whole JF) | 9.4 ± 0.4 | 6.2 ± 0.5 |
| Percentage of DW (%) | 0.94% | 0.62% |
| Lipids in hydroalcoholic extract (mg/g of 80% EtOH) | - | 15.5 ± 0.5 |
| Total lipids by FW (mg/g of FW) * | 0.67 ± 0.07 | 0.46 ± 0.09 |
| Percentage of FW * (%) | 0.07% | 0.05% |
| Cassiopea andromeda Fatty Acid Composition | ||
|---|---|---|
| Fatty Acid (FA) | Whole Jellyfish (WJ) | HydroalcoholicExtract (80% EtOH Extract) |
| % | % | |
| Saturated FA (SFA) % | ||
| Lauric acid C12:0 | 9.3 ± 0.9 | 1.5 ± 0.2 |
| Myristic acid C14:0 | 4.2 ± 0.4 | 5.2 ± 0.5 |
| Palmitic acid C16:0 | 21.9 ± 2.2 | 13.9 ± 1.4 |
| Stearic acid C18:0 | 12.5 ± 1.2 | 9.9 ± 0.9 |
| Arachidic acid C20:0 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| Total SFA | 48.5 ± 4.8 | 31.1 ± 3.1 |
| Monounsaturated FA (MUFA) % | ||
| Palmitoleic acid C16:1 (ω7) | 4.3 ± 0.4 | 3,3 ± 0.3 |
| Oleic acid C18:1 cis-9 (ω9) | 2.8 ± 0.3 | 2.3 ± 0.3 |
| Isoleic acid C18:1 trans-10 | 0.5 ± 0.1 | 0.6 ± 0.1 |
| Total MUFA | 7.5 ± 0.8 | 6.1 ± 0.6 |
| Polyunsaturated FA (PUFA) % | ||
| Linoleic acid C18:2 cis-9,12 (ω6) | 0.8 ± 0.1 | 1.9 ± 0.2 |
| Isolinoleic acid C18:2 cis-6,9 (ω9) | 0.5 ± 0.1 | -- |
| Linolenic acid C18:3 cis-9,12,15 (ω3) | 2.6 ± 0.3 | 3.2 ± 0.3 |
| Stearidonic acid C18:4 (ω3) | 7.4 ± 0.7 | 7.9 ± 0.8 |
| Arachidonic acid C20:4 (ω6) | 14.2 ± 1.4 | 19.2 ± 1.9 |
| Eicosapentaenoic acid C20:5 (ω3) | 2.1 ± 0.2 | 3.5 ± 0.3 |
| Docosatetraenoic acid C22:4 (ω6) | 2.9 ± 0.3 | 4.2 ± 0.4 |
| Docosapentaenoic acid C22:5 (ω3) | 2.5 ± 0.2 | 5.1 ± 0.5 |
| Docosahexaenoic acid C22:6 (ω3) | 11.0 ± 1.1 | 17.8 ± 1.8 |
| Total PUFA | 44.0 ± 4.4 | 62.8 ± 6.3 |
| Total fatty acids (%) | 100.0 | 100.0 |
| Fatty acids Σω6 | 17.9 | 25.3 |
| Fatty acids Σω3 | 25.6 | 37.5 |
| Ratio ω6/ω3 | 0.7 | 0.7 |
| Umbrella (UMB) | Oral Arms (OA) | |||||
|---|---|---|---|---|---|---|
| Extraction Solutions | UMB DW (g) | Extract DW (g) | Yield (%DW) | OA DW (g) | Extract DW (g) | Yield (%DW) |
| Mean ±SD | Mean ±SD | |||||
| PBS | 0.652 ± 0.155 | 0.616 ± 0.10 | 94.5 ± 0.92 | 0.978 ± 0.15 | 0.937 ± 0.01 | 95.8 ± 0.81 |
| 80% EtOH | 0.515 ± 0.172 | 0.214 ± 0.07 | 41.6 ± 0.09 | 1.003 ± 0.08 | 0.436 ± 0.04 | 43.5 ± 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rinaldis, G.; Leone, A.; De Domenico, S.; Bosch-Belmar, M.; Slizyte, R.; Milisenda, G.; Santucci, A.; Albano, C.; Piraino, S. Biochemical Characterization of Cassiopea andromeda (Forsskål, 1775), Another Red Sea Jellyfish in the Western Mediterranean Sea. Mar. Drugs 2021, 19, 498. https://doi.org/10.3390/md19090498
De Rinaldis G, Leone A, De Domenico S, Bosch-Belmar M, Slizyte R, Milisenda G, Santucci A, Albano C, Piraino S. Biochemical Characterization of Cassiopea andromeda (Forsskål, 1775), Another Red Sea Jellyfish in the Western Mediterranean Sea. Marine Drugs. 2021; 19(9):498. https://doi.org/10.3390/md19090498
Chicago/Turabian StyleDe Rinaldis, Gianluca, Antonella Leone, Stefania De Domenico, Mar Bosch-Belmar, Rasa Slizyte, Giacomo Milisenda, Annalisa Santucci, Clara Albano, and Stefano Piraino. 2021. "Biochemical Characterization of Cassiopea andromeda (Forsskål, 1775), Another Red Sea Jellyfish in the Western Mediterranean Sea" Marine Drugs 19, no. 9: 498. https://doi.org/10.3390/md19090498
APA StyleDe Rinaldis, G., Leone, A., De Domenico, S., Bosch-Belmar, M., Slizyte, R., Milisenda, G., Santucci, A., Albano, C., & Piraino, S. (2021). Biochemical Characterization of Cassiopea andromeda (Forsskål, 1775), Another Red Sea Jellyfish in the Western Mediterranean Sea. Marine Drugs, 19(9), 498. https://doi.org/10.3390/md19090498










