The Microbial Community Associated with Rhizostoma pulmo: Ecological Significance and Potential Consequences for Marine Organisms and Human Health
Abstract
1. Introduction
2. Results
2.1. Bacterial Enumeration: Comparative Analysis
2.2. Microbial Profiles Related to Potential Carbon Sources Utilization: Comparative Analysis
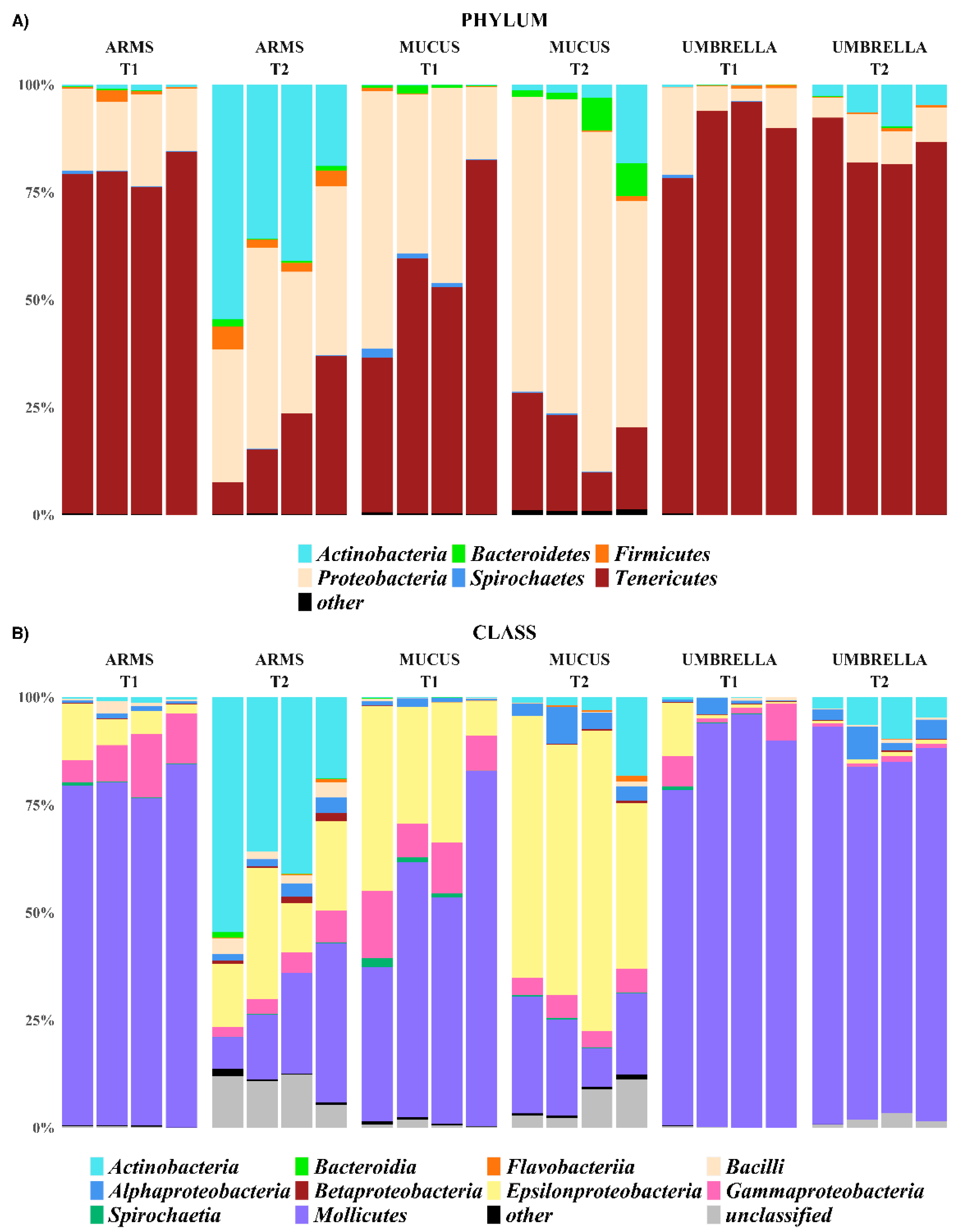
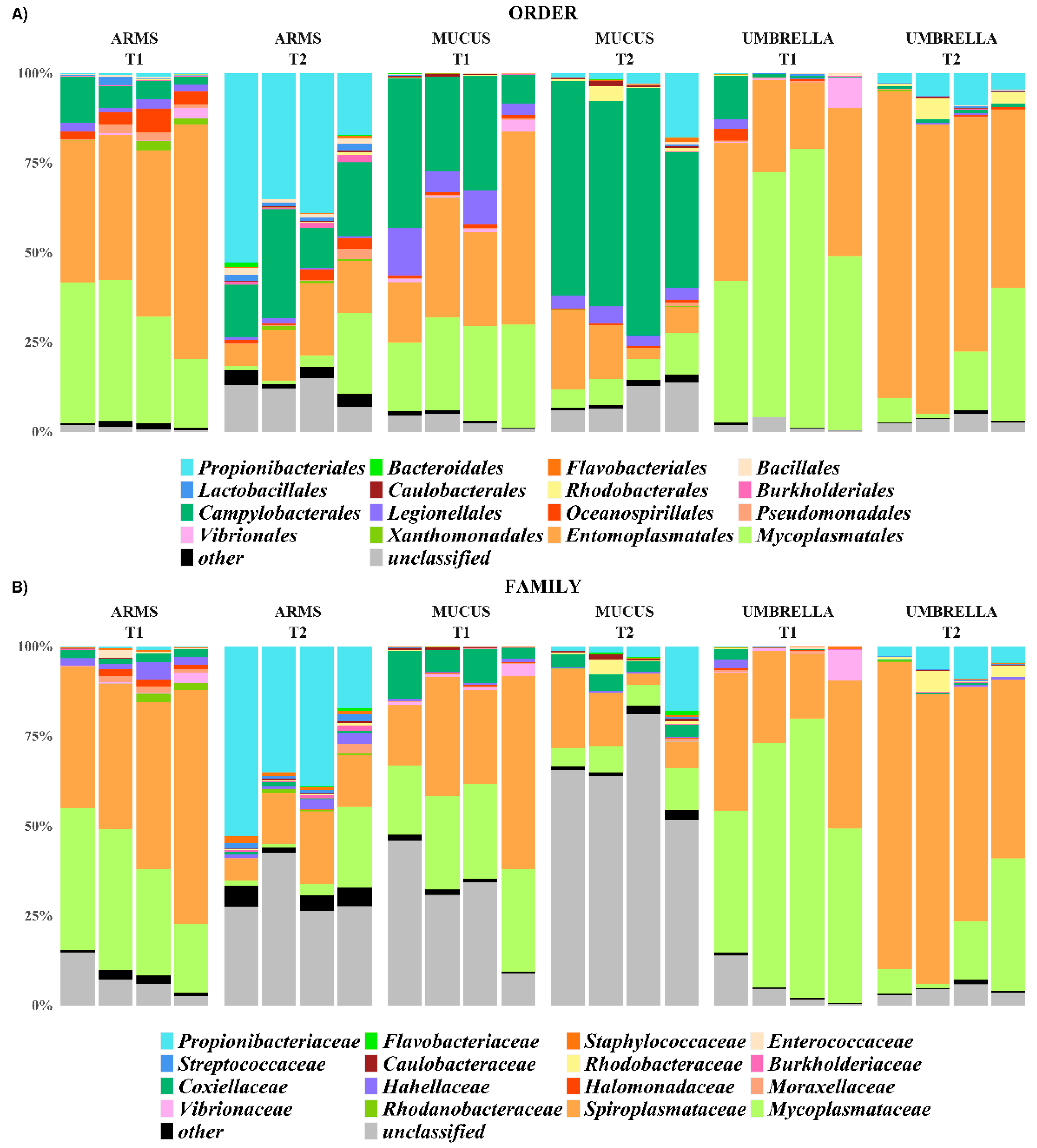
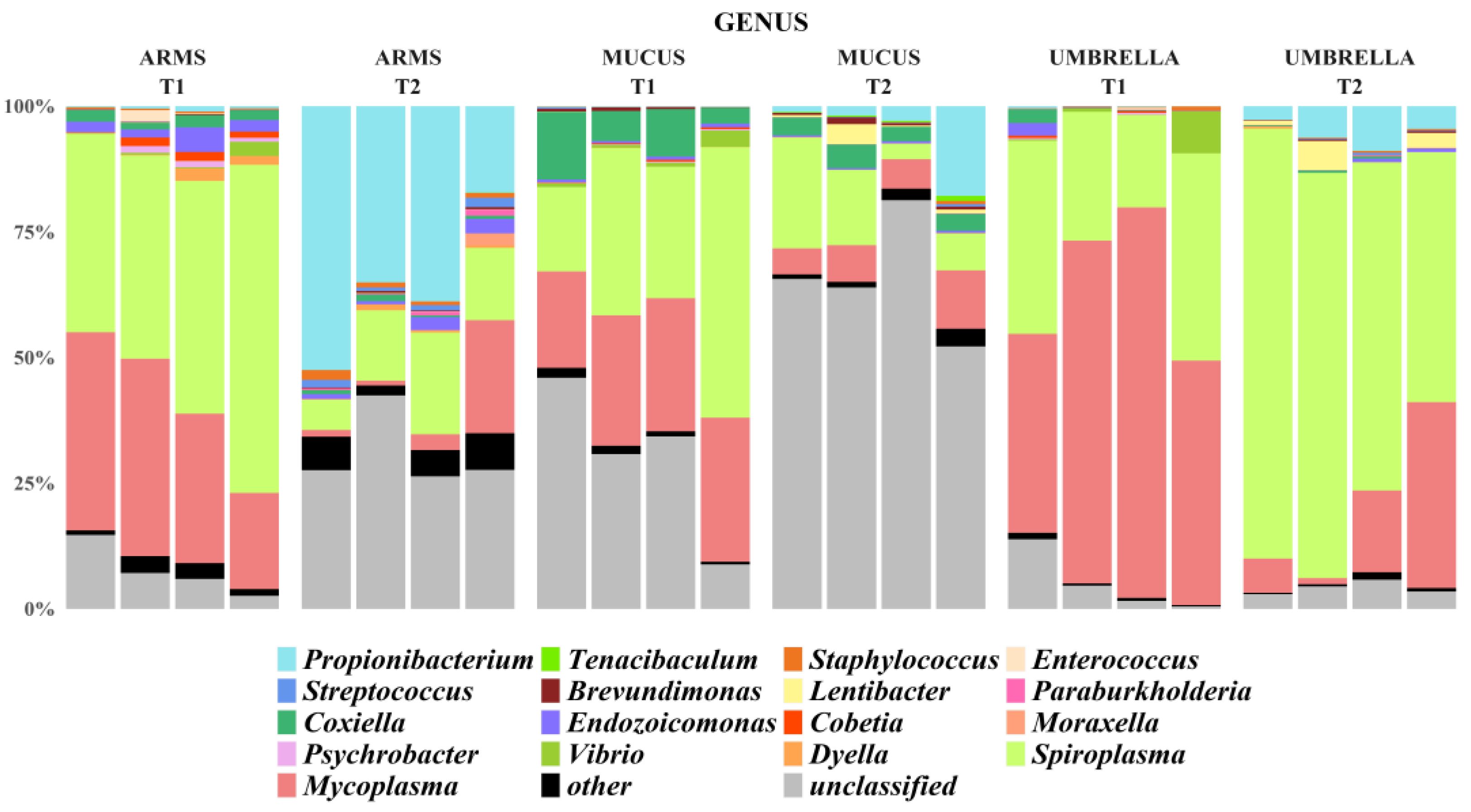
2.3. Microbial Diversity: Comparative Analysis
3. Discussion
4. Materials and Methods
4.1. Animals, Collection, and Sample Preparation
4.2. Microbiological Analyses
4.3. BIOLOG EcoPlate Inoculation and Incubation
4.4. DNA Extraction
4.5. 16S rDNA Library Preparation and Sequencing
4.6. Taxonomic and Phylogenetic Analyses
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boero, F.; Brotz, L.; Gibbons, M.J.; Piraino, S.; Zampardi, S. Impacts and Effects of Ocean Warming on Jellyfish. In Explaining Ocean Warming: Causes, Scale, Effects and Consequences; IUCN: Gland, Switzerland, 2016; pp. 213–237. [Google Scholar] [CrossRef]
- Chi, X.; Mueller-Navarra, D.C.; Hylander, S.; Sommer, U.; Javidpour, J. Food quality matters: Interplay among food quality, food quantity and temperature affecting life history traits of Aurelia aurita (Cnidaria: Scyphozoa) polyps. Sci. Total Environ. 2019, 656, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Purcell, J.E. Climate effects on formation of jellyfish and ctenophore blooms: A review. J. Mar. Biol. Assoc. UK 2005, 85, 461–476. [Google Scholar] [CrossRef]
- Purcell, J.E. Environmental effects on asexual reproduction rates of the scyphozoan Aurelia labiata. Mar. Ecol. Prog. Ser. 2007, 348, 183–196. [Google Scholar] [CrossRef]
- Purcell, J.E. Jellyfish and Ctenophore Blooms Coincide with Human Proliferations and Environmental Perturbations. Ann. Rev. Mar. Sci. 2012, 4, 209–235. [Google Scholar] [CrossRef]
- Licandro, P.; Conway, D.V.P.; Daly Yahia, M.N.; Fernandez de Puelles, M.L.; Gasparini, S.; Hecq, J.H.; Tranter, P.; Kirby, R.R. A blooming jellyfish in the northeast Atlantic and Mediterranean. Biol. Lett. 2010, 6, 688–691. [Google Scholar] [CrossRef]
- Condon, R.H.; Duarte, C.M.; Pitt, K.A.; Robinson, K.L.; Lucas, C.H.; Sutherland, K.R.; Mianzan, H.W.; Bogeberg, M.; Purcell, J.E.; Decker, M.B.; et al. Recurrent jellyfish blooms are a consequence of global oscillations. Proc. Natl. Acad. Sci. USA 2013, 110, 1000–1005. [Google Scholar] [CrossRef]
- Gibbons, M.J.; Richardson, A.J. Beyond the jellyfish joyride and global oscillations: Advancing jellyfish research. J. Plankton Res. 2013, 35, 929–938. [Google Scholar] [CrossRef]
- Duarte, C.M.; Pitt, K.A.; Lucas, C.H.; Purcell, J.E.; Uye, S.-I.; Robinson, K.; Malej, A.; Graham, W.M.; Madin, L.; Atienza, D.; et al. Is global ocean sprawl a cause of jellyfish blooms? Front. Ecol. Environ. 2013, 11, 246. [Google Scholar] [CrossRef]
- Pitt, K.A.; Lucas, C.H.; Condon, R.H.; Duarte, C.M.; Stewart-Koster, B. Claims That Anthropogenic Stressors Facilitate Jellyfish Blooms Have Been Amplified Beyond the Available Evidence: A Systematic Review. Front. Mar. Sci. 2018, 5, 451. [Google Scholar] [CrossRef]
- Kogovšek, T.; Bogunović, B.; Malej, A. Recurrence of bloom-forming scyphomedusae: Wavelet analysis of a 200-year time series. Hydrobiologia 2010, 645, 81–96. [Google Scholar] [CrossRef]
- Brotz, L.; Pauly, D. Jellyfish populations in the Mediterranean Sea. Acta Adriat. 2012, 53, 213–231. [Google Scholar]
- Mariottini, G.L.; Pane, L. Mediterranean jellyfish venoms: A review on scyphomedusae. Mar. Drugs 2010, 8, 1122–1152. [Google Scholar] [CrossRef] [PubMed]
- De Donno, A.; Idolo, A.; Bagordo, F.; Grassi, T.; Leomanni, A.; Serio, F.; Guido, M.; Canitano, M.; Zampardi, S.; Boero, F.; et al. Impact of stinging jellyfish proliferations along south Italian coasts: Human health hazards, treatment and social costs. Int. J. Environ. Res. Public Health 2014, 11, 2488–2503. [Google Scholar] [CrossRef] [PubMed]
- Purcell, J.E.; Baxter, E.J.; Fuentes, V.L. Jellyfish as products and problems of aquaculture. In Advances in Aquaculture Hatchery Technology; Woodhead Publishing: Cambridge, UK, 2013; pp. 404–430. [Google Scholar]
- Milisenda, G.; Rosa, S.; Fuentes, V.L.; Boero, F.; Guglielmo, L.; Purcell, J.E.; Piraino, S. Jellyfish as prey: Frequency of predation and selective foraging of Boops boops (Vertebrata, Actinopterygii) on the mauve stinger Pelagia noctiluca (Cnidaria, Scyphozoa). PLoS ONE 2014, 9, e94600. [Google Scholar] [CrossRef]
- Milisenda, G.; Rossi, S.; Vizzini, S.; Fuentes, V.L.; Purcell, J.E.; Tilves, U.; Piraino, S. Seasonal variability of diet and trophic level of the gelatinous predator Pelagia noctiluca (Scyphozoa). Sci. Rep. 2018, 8, 12140. [Google Scholar] [CrossRef]
- Canepa, A.; Fuentes, V.; Sabatés, A.; Piraino, S.; Boero, F.; Gili, J.-M. Pelagia noctiluca in the Mediterranean Sea. In Jellyfish Bloom; Springer: Dordrecht, The Netherlands, 2014; pp. 237–266. [Google Scholar]
- Sommer, U.; Lengfellner, K. Climate change and the timing, magnitude, and composition of the phytoplankton spring bloom. Glob. Chang. Biol. 2008, 14, 1199–1208. [Google Scholar] [CrossRef]
- West, E.J.; Pitt, K.A.; Welsh, D.T.; Koop, K.; Rissik, D. Top-down and bottom-up influences of jellyfish on primary productivity and planktonic assemblages. Limnol. Oceanogr. 2009, 54, 2058–2071. [Google Scholar] [CrossRef]
- Hosia, A.; Augustin, C.B.; Dinasquet, J.; Granhag, L.; Paulsen, M.L.; Riemann, L.; Rintala, J.-M.; Setälä, O.; Talvitie, J.; Titelman, J. Autumnal bottom-up and top-down impacts of Cyanea capillata: A mesocosm study. J. Plankton Res. 2015, 37, 1042–1055. [Google Scholar] [CrossRef]
- Manzari, C.; Fosso, B.; Marzano, M.; Annese, A.; Caprioli, R.; D’Erchia, A.M.; Gissi, C.; Intranuovo, M.; Picardi, E.; Santamaria, M.; et al. The influence of invasive jellyfish blooms on the aquatic microbiome in a coastal lagoon (Varano, SE Italy) detected by an Illumina-based deep sequencing strategy. Biol. Invasions 2015, 17, 923–940. [Google Scholar] [CrossRef]
- Tinta, T.; Kogovšek, T.; Malej, A.; Turk, V. Jellyfish modulate bacterial dynamic and community structure. PLoS ONE 2012, 7, e39274. [Google Scholar] [CrossRef]
- Sweetman, A.K.; Chapman, A. First assessment of flux rates of jellyfish carcasses (jelly-falls) to the benthos reveals the importance of gelatinous material for biological C-cycling in jellyfish-dominated ecosystems. Front. Mar. Sci. 2015, 2, 47. [Google Scholar] [CrossRef]
- Bosch-Belmar, M.; Giomi, F.; Rinaldi, A.; Mandich, A.; Fuentes, V.; Mirto, S.; Sarà, G.; Piraino, S. Concurrent environmental stressors and jellyfish stings impair caged European sea bass (Dicentrarchus labrax) physiological performances. Sci. Rep. 2016, 6, 27929. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Belmar, M.; Azzurro, E.; Pulis, K.; Milisenda, G.; Fuentes, V.; Kéfi-Daly Yahia, O.; Micallef, A.; Deidun, A.; Piraino, S. Jellyfish blooms perception in Mediterranean finfish aquaculture. Mar. Policy 2017, 76, 1–7. [Google Scholar] [CrossRef]
- Palmieri, M.G.; Barausse, A.; Luisetti, T.; Turner, K. Jellyfish blooms in the Northern Adriatic Sea: Fishermen’s perceptions and economic impacts on fisheries. Fish. Res. 2014, 155, 51–58. [Google Scholar] [CrossRef]
- Chelsky, A.; Pitt, K.A.; Ferguson, A.J.P.; Bennett, W.W.; Teasdale, P.R.; Welsh, D.T. Decomposition of jellyfish carrion in situ: Short-term impacts on infauna, benthic nutrient fluxes and sediment redox conditions. Sci. Total Environ. 2016, 566–567, 929–937. [Google Scholar] [CrossRef]
- Delannoy, C.M.J.; Houghton, J.D.R.; Fleming, N.E.C.; Ferguson, H.W. Mauve Stingers (Pelagia noctiluca) as carriers of the bacterial fish pathogen Tenacibaculum maritimum. Aquaculture 2011, 311, 255–257. [Google Scholar] [CrossRef]
- Bosch-Belmar, M.; Kéfi-Daly Yahia, O.; M’Rabet, C.; Dhaouadi, R.; Fuentes, V.; Chalghaf, M.; Piraino, S. Effects of Pelagia noctiluca jellyfish swarms on caged gilthead sea bream. In Proceedings of the International Council for the Exploration of the Sea Annual Science Conference (ICES ASC 2014), A Coruna, Spain, 15–19 September 2014; p. 3728. [Google Scholar]
- Doyle, T.K.; De Haas, H.; Cotton, D.; Dorschel, B.; Cummins, V.; Houghton, J.D.R.; Davenport, J.; Hays, G.C. Widespread occurrence of the jellyfish Pelagia noctiluca in Irish coastal and shelf waters. J. Plankton Res. 2008, 30, 963–968. [Google Scholar] [CrossRef]
- Fringuelli, E.; Savage, P.D.; Gordon, A.; Baxter, E.J.; Rodger, H.D.; Graham, D.A. Development of a quantitative real-time PCR for the detection of Tenacibaculum maritimum and its application to field samples. J. Fish. Dis. 2012, 35, 579–590. [Google Scholar] [CrossRef]
- Ferguson, H.W.; Christian, M.J.D.; Hay, S.; Nicolson, J.; Sutherland, D.; Crumlish, M. Jellyfish as Vectors of Bacterial Disease for Farmed Salmon (Salmo salar). J. Vet. Diagn. Investig. 2010, 22, 376–382. [Google Scholar] [CrossRef]
- Stabili, L.; Parisi, M.G.; Parrinello, D.; Cammarata, M. Cnidarian Interaction with Microbial Communities: From Aid to Animal’s Health to Rejection Responses. Mar. Drugs 2018, 16, 296. [Google Scholar] [CrossRef]
- Tinta, T.; Kogovšek, T.; Klun, K.; Malej, A.; Herndl, G.J.; Turk, V. Jellyfish-Associated Microbiome in the Marine Environment: Exploring Its Biotechnological Potential. Mar. Drugs 2019, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Bruno, D.W.; Ellis, A.E. Multiple hepatic cysts in farmed Atlantic salmon, Salmo salar L. J. Fish. Dis. 1986, 9, 79–81. [Google Scholar] [CrossRef]
- Margulis, L.; Thorington, G.; Berger, B.; Stolz, J. Endosymbiotic bacteria associated with the intracellular green algae of Hydra viridis. Curr. Microbiol. 1978, 1, 227–232. [Google Scholar] [CrossRef]
- Kos Kramar, M.; Tinta, T.; Lučić, D.; Malej, A.; Turk, V. Bacteria associated with moon jellyfish during bloom and post-bloom periods in the Gulf of Trieste (northern Adriatic). PLoS ONE 2019, 14, e0198056. [Google Scholar] [CrossRef]
- Basso, L.; Rizzo, L.; Marzano, M.; Intranuovo, M.; Fosso, B.; Pesole, G.; Piraino, S.; Stabili, L. Jellyfish summer outbreaks as bacterial vectors and potential hazards for marine animals and humans health? The case of Rhizostoma pulmo (Scyphozoa, Cnidaria). Sci. Total Environ. 2019, 692, 305–318. [Google Scholar] [CrossRef]
- Kushmaro, A.; Kramarsky-Winter, E. Bacteria as a Source of Coral Nutrition. Coral Heal. Dis. 2004, 231–241. [Google Scholar]
- Davy, S.K.; Allemand, D.; Weis, V.M. Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol. Mol. Biol. Rev. 2012, 76, 229–261. [Google Scholar] [CrossRef]
- Thompson, J.R.; Rivera, H.E.; Closek, C.J.; Medina, M. Microbes in the coral holobiont: Partners through evolution, development, and ecological interactions. Front. Cell. Infect. Microbiol. 2015, 4, 176. [Google Scholar] [CrossRef]
- Lesser, M.P. Discovery of Symbiotic Nitrogen-Fixing Cyanobacteria in Corals. Science 2004, 305, 997–1000. [Google Scholar] [CrossRef]
- Kelman, D.; Kashman, Y.; Rosenberg, E.; Kushmaro, A.; Loya, Y. Antimicrobial activity of Red Sea corals. Mar. Biol. 2006, 149, 357–363. [Google Scholar] [CrossRef]
- Ritchie, K.B.; Smith, G.W. Microbial Communities of Coral Surface Mucopolysaccharide Layers. In Coral Health and Disease; Springer: Berlin/Heidelberg, Germany, 2004; pp. 259–264. [Google Scholar]
- Disalvo, L.H. Isolation of Bacteria from the Corallum of Porites lobate (Vaughn) and Its Possible Significance. Am. Zool. 1969, 9, 735–740. [Google Scholar] [CrossRef]
- Ritchie, K.B. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar. Ecol. Prog. Ser. 2006, 322, 1–14. [Google Scholar] [CrossRef]
- Murillo-Rincon, A.P.; Klimovich, A.; Pemöller, E.; Taubenheim, J.; Mortzfeld, B.; Augustin, R.; Bosch, T.C.G. Spontaneous body contractions are modulated by the microbiome of Hydra. Sci. Rep. 2017, 7, 15937. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.D.; Kling, J.D.; Araya, R.; Ceh, J. Jellyfish Life Stages Shape Associated Microbial Communities, While a Core Microbiome Is Maintained Across All. Front. Microbiol. 2018, 9, 1534. [Google Scholar] [CrossRef]
- Viver, T.; Orellana, L.H.; Hatt, J.K.; Urdiain, M.; Díaz, S.; Richter, M.; Antón, J.; Avian, M.; Amann, R.; Konstantinidis, K.T.; et al. The low diverse gastric microbiome of the jellyfish Cotylorhiza tuberculata is dominated by four novel taxa. Environ. Microbiol. 2017, 19, 3039–3058. [Google Scholar] [CrossRef]
- Cortés-Lara, S.; Urdiain, M.; Mora-Ruiz, M.; Prieto, L.; Rosselló-Móra, R. Prokaryotic microbiota in the digestive cavity of the jellyfish Cotylorhiza tuberculata. Syst. Appl. Microbiol. 2015, 38, 494–500. [Google Scholar] [CrossRef]
- Weiland-Bräuer, N.; Neulinger, S.C.; Pinnow, N.; Künzel, S.; Baines, J.F.; Schmitz, R.A. Composition of Bacterial Communities Associated with Aurelia aurita Changes with Compartment, Life Stage, and Population. Appl. Environ. Microbiol. 2015, 81, 6038–6052. [Google Scholar] [CrossRef]
- Blanchet, M.; Pringault, O.; Bouvy, M.; Catala, P.; Oriol, L.; Caparros, J.; Ortega-Retuerta, E.; Intertaglia, L.; West, N.; Agis, M.; et al. Changes in bacterial community metabolism and composition during the degradation of dissolved organic matter from the jellyfish Aurelia aurita in a Mediterranean coastal lagoon. Environ. Sci. Pollut. Res. 2014, 22, 13638–13653. [Google Scholar] [CrossRef]
- Cleary, D.F.R.; Becking, L.E.; Polónia, A.R.M.; Freitas, R.M.; Gomes, N.C.M. Jellyfish-associated bacterial communities and bacterioplankton in Indonesian Marine lakes. Fems Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef]
- Daley, M.C.; Urban-Rich, J.; Moisander, P.H. Bacterial associations with the hydromedusa Nemopsis bachei and scyphomedusa Aurelia aurita from the North Atlantic Ocean. Mar. Biol. Res. 2016, 12, 1088–1100. [Google Scholar] [CrossRef]
- Bleve, G.; Ramires, F.A.; Gallo, A.; Leone, A. Identification of safety and quality parameters for preparation of jellyfish based novel food products. Foods Switz. 2019, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Lilley, M.K.S.; Houghton, J.D.R.; Hays, G.C. Distribution, extent of inter-annual variability and diet of the bloom-forming jellyfish Rhizostoma in European waters. J. Mar. Biol. Assoc. UK 2008, 89, 39–48. [Google Scholar] [CrossRef]
- Fuentes, V.; Straehler-Pohl, I.; Atienza, D.; Franco, I.; Tilves, U.; Gentile, M.; Acevedo, M.; Olariaga, A.; Gili, J.-M. Life cycle of the jellyfish Rhizostoma pulmo (Scyphozoa: Rhizostomeae) and its distribution, seasonality and inter-annual variability along the Catalan coast and the Mar Menor (Spain, NW Mediterranean). Mar. Biol. 2011, 158, 2247–2266. [Google Scholar] [CrossRef]
- Omori, M.; Nakano, E. Jellyfish fisheries in southeast Asia. Hydrobiologia 2001, 451, 19–26. [Google Scholar] [CrossRef]
- Muhammed, F.; Sultana, R. New record of edible jellyfish, Rhizostoma pulmo (Cnidaria: Scyphozoa: Rhizostomitidae) from Pakistani waters. Mar. Biodivers. Rec. 2008, 1. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.M.; Durante, M.; Meli, F.; Piraino, S. The Bright Side of Gelatinous Blooms: Nutraceutical Value and Antioxidant Properties of Three Mediterranean Jellyfish (Scyphozoa). Mar. Drugs 2015, 13, 4654–4681. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Lecci, R.M.; Durante, M.; Piraino, S. Extract from the zooxanthellate jellyfish Cotylorhiza tuberculata modulates gap junction intercellular communication in human cell cultures. Mar. Drugs 2013, 11, 1728–1762. [Google Scholar] [CrossRef] [PubMed]
- Angilè, F.; Del Coco, L.; Girelli, C.R.; Basso, L.; Rizzo, L.; Piraino, S.; Stabili, L.; Fanizzi, F.P. (1)H NMR Metabolic Profile of Scyphomedusa Rhizostoma pulmo (Scyphozoa, Cnidaria) in Female Gonads and Somatic Tissues: Preliminary Results. Molecules 2020, 25, 806. [Google Scholar] [CrossRef]
- De Domenico, S.; De Rinaldis, G.; Paulmery, M.; Piraino, S.; Leone, A. Barrel Jellyfish (Rhizostoma pulmo) as Source of Antioxidant Peptides. Mar. Drugs 2019, 17, 134. [Google Scholar] [CrossRef]
- Stabili, L.; Rizzo, L.; Fanizzi, F.P.; Angilè, F.; Del Coco, L.; Girelli, C.R.; Lomartire, S.; Piraino, S.; Basso, L. The Jellyfish Rhizostoma pulmo (Cnidaria): Biochemical Composition of Ovaries and Antibacterial Lysozyme-like Activity of the Oocyte Lysate. Mar. Drugs 2019, 17, 17. [Google Scholar] [CrossRef]
- Prieto, L.; Enrique-Navarro, A.; Li Volsi, R.; Ortega, M.J. The Large Jellyfish Rhizostoma luteum as Sustainable a Resource for Antioxidant Properties, Nutraceutical Value and Biomedical Applications. Mar. Drugs 2018, 16, 396. [Google Scholar] [CrossRef] [PubMed]
- Pastor, F.; Valiente, J.A.; Palau, J.L. Sea Surface Temperature in the Mediterranean: Trends and Spatial Patterns (1982–2016). Pure Appl. Geophys. 2018, 175, 4017–4029. [Google Scholar] [CrossRef]
- Basso, L.; Rizzo, L.; Piraino, S.; Stabili, L. Metabolic diversity of microbial community associated with Rhizostoma pulmo (Scyphozoa: Rhizostomeae). J. Mar. Microbiol. 2017, 1, 5–8. [Google Scholar]
- Torri, L.; Tuccillo, F.; Bonelli, S.; Piraino, S.; Leone, A. The attitudes of Italian consumers towards jellyfish as novel food. Food Qual. Prefer. 2020, 79, 1–10. [Google Scholar] [CrossRef]
- Niggl, W.; Naumann, M.S.; Struck, U.; Manasrah, R.; Wild, C. Organic matter release by the benthic upside-down jellyfish Cassiopea sp. fuels pelagic food webs in coral reefs. J. Exp. Mar. Bio. Ecol. 2010, 384, 99–106. [Google Scholar] [CrossRef]
- Turk, V.; Lučić, D.; Flander-Putrle, V.; Malej, A. Feeding of Aurelia sp. (Scyphozoa) and links to the microbial food web. Mar. Ecol. 2008, 29, 495–505. [Google Scholar] [CrossRef]
- Titelman, J.; Riemann, L.; Sørnes, T.A.; Nilsen, T.; Griekspoor, P.; Båmstedt, U. Turnover of dead jellyfish: Stimulation and retardation of microbial activity. Mar. Ecol. Prog. Ser. 2006, 325, 43–58. [Google Scholar] [CrossRef]
- Condon, R.H.; Steinberg, D.K.; del Giorgio, P.A.; Bouvier, T.C.; Bronk, D.A.; Graham, W.M.; Ducklow, H.W. Jellyfish blooms result in a major microbial respiratory sink of carbon in marine systems. Proc. Natl. Acad. Sci. USA 2011, 108, 10225–10230. [Google Scholar] [CrossRef]
- Bythell, J.C.; Wild, C. Biology and ecology of coral mucus release. J. Exp. Mar. Bio. Ecol. 2011, 408, 88–93. [Google Scholar] [CrossRef]
- Martin, R.; Walther, P. Protective mechanisms against the action of nematocysts in the epidermis of Cratena peregrina and Flabellina affinis (Gastropoda, Nudibranchia). Zoomorphology 2003, 122, 25–32. [Google Scholar] [CrossRef]
- Ames, C.L.; Klompen, A.M.L.; Badhiwala, K.; Muffett, K.; Reft, A.J.; Kumar, M.; Janssen, J.D.; Schultzhaus, J.N.; Field, L.D.; Muroski, M.E.; et al. Cassiosomes are stinging-cell structures in the mucus of the upside-down jellyfish Cassiopea xamachana. Commun. Biol. 2020, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Ducklow, H.W.; Mitchell, R. Composition of mucus released by coral reef coelenterates1. Limnol. Oceanogr. 1979, 24, 706–714. [Google Scholar] [CrossRef]
- Bakshani, C.R.; Morales-Garcia, A.L.; Althaus, M.; Wilcox, M.D.; Pearson, J.P.; Bythell, J.C.; Burgess, J.G. Evolutionary conservation of the antimicrobial function of mucus: A first defence against infection. Npj Biofilms Microbiomes 2018, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.B. Experiments on homing in the limpet Siphonaria normalis. Anim. Behav. 1969, 17, 679–682. [Google Scholar] [CrossRef]
- McFaruume, I.D. Trail-following and trail-searching behaviour in homing of the intertidal gastropod mollusc, Onchidium verruculatum. Mar. Behav. Physiol. 1980, 7, 95–108. [Google Scholar] [CrossRef]
- Condon, R.H.; Steinberg, D.K.; Bronk, D.A. Production of dissolved organic matter and inorganic nutrients by gelatinous zooplankton in the York River estuary, Chesapeake Bay. J. Plankton Res. 2010, 32, 153–170. [Google Scholar] [CrossRef]
- Patwa, A.; Thiéry, A.; Lombard, F.; Lilley, M.K.S.; Boisset, C.; Bramard, J.-F.; Bottero, J.-Y.; Barthélémy, P. Accumulation of nanoparticles in “jellyfish” mucus: A bio-inspired route to decontamination of nano-waste. Sci. Rep. 2015, 5, 11387. [Google Scholar] [CrossRef]
- Azam, F. Oceanography: Microbial Control of Oceanic Carbon Flux: The Plot Thickens. Science 1998, 280, 694–696. [Google Scholar] [CrossRef]
- Simu, K.; Holmfeldt, K.; Zweifel, U.L.; Hagström, A. Culturability and coexistence of colony-forming and single-cell marine bacterioplankton. Appl. Environ. Microbiol. 2005, 71, 4793–4800. [Google Scholar] [CrossRef]
- Stabili, L.; Gravili, C.; Piraino, S.; Boero, F.; Alifano, P. Vibrio harveyi Associated with Aglaophenia octodonta (Hydrozoa, Cnidaria). Microb. Ecol. 2006, 52, 603–608. [Google Scholar] [CrossRef]
- Hagström, A.; Pinhassi, J.; Zweifel, U.L. Biogeographical diversity among marine bacterioplankton. Aquat. Microb. Ecol. 2000, 21, 231–244. [Google Scholar] [CrossRef]
- Calow, P. Why Some Metazoan Mucus Secretions are More Susceptible to Microbial Attack than Others. Am. Nat. 1979, 114, 149–152. [Google Scholar] [CrossRef]
- Stabili, L.; Schirosi, R.; Licciano, M.; Giangrande, A. Role of Myxicola infundibulum (Polychaeta, Annelida) mucus: From bacterial control to nutritional home site. J. Exp. Mar. Bio. Ecol. 2014, 461, 344–349. [Google Scholar] [CrossRef]
- Nguyen-Kim, H.; Bouvier, T.; Bouvier, C.; Doan-Nhu, H.; Nguyen-Ngoc, L.; Rochelle-Newall, E.; Baudoux, A.-C.; Desnues, C.; Reynaud, S.; Ferrier-Pages, C.; et al. High occurrence of viruses in the mucus layer of scleractinian corals. Environ. Microbiol. Rep. 2014, 6, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Gil-Agudelo, D.L.; Myers, C.; Smith, G.W.; Kim, K. Changes in the microbial communities associated with Gorgonia ventalina during aspergillosis infection. Dis. Aquat. Organ. 2006, 69, 89–94. [Google Scholar] [CrossRef]
- Razin, S.; Yogev, D.; Naot, Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 1998, 62, 1094–1156. [Google Scholar] [CrossRef]
- Altamiranda, M.J.; Salazar, V.M.; Briñez, R.B. Presencia de Spiroplasma penaei en plancton, bentos y fauna acompañante en fincas camaroneras de Colombia. Rev. Mvz Córdoba 2011, 2576–2583. [Google Scholar] [CrossRef][Green Version]
- Davis, J.; Fricke, W.F.; Hamann, M.T.; Esquenazi, E.; Dorrestein, P.C.; Hill, R.T. Characterization of the bacterial community of the chemically defended Hawaiian sacoglossan Elysia rufescens. Appl. Environ. Microbiol. 2013, 79, 7073–7081. [Google Scholar] [CrossRef]
- Fernandez-Piquer, J.; Bowman, J.P.; Ross, T.; Tamplin, M.L. Molecular analysis of the bacterial communities in the live Pacific oyster (Crassostrea gigas) and the influence of postharvest temperature on its structure. J. Appl. Microbiol. 2012, 112, 1134–1143. [Google Scholar] [CrossRef]
- Zimmer, R.L.; Woollacott, R.M. Mycoplasma-Like Organisms: Occurrence with the Larvae and Adults of a Marine Bryozoan. Science 1983, 220, 208–210. [Google Scholar] [CrossRef]
- Liang, T.; Li, X.; Du, J.; Yao, W.; Sun, G.; Dong, X.; Liu, Z.; Ou, J.; Meng, Q.; Gu, W.; et al. Identification and isolation of a spiroplasma pathogen from diseased freshwater prawns, Macrobrachium rosenbergii, in China: A new freshwater crustacean host. Aquaculture 2011, 318, 1–6. [Google Scholar] [CrossRef]
- Hao, W.; Gerdts, G.; Peplies, J.; Wichels, A. Bacterial communities associated with four ctenophore genera from the German Bight (North Sea). Fems Microbiol. Ecol. 2014, 91, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-B.; Guo, F.; Zhao, J.; Li, W.-D.; Ke, C.-H. Molecular analysis of the intestinal bacterial flora in cage-cultured adult small abalone, Haliotis diversicolor. Aquac. Res. 2010, 41, e760–e769. [Google Scholar] [CrossRef]
- Kellogg, C.A.; Lisle, J.T.; Galkiewicz, J.P. Culture-independent characterization of bacterial communities associated with the cold-water coral Lophelia pertusa in the northeastern Gulf of Mexico. Appl. Environ. Microbiol. 2009, 75, 2294–2303. [Google Scholar] [CrossRef]
- Romalde, J.L.; Barja, J.L. Bacteria in molluscs: Good and bad guys. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010; pp. 136–147. [Google Scholar]
- Wang, W. Bacterial diseases of crabs: A review. J. Invertebr. Pathol. 2011, 106, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.M.; Johnson, M.D. Mycoplasmal panencephalitis: A neuropathologic documentation. Acta Neuropathol. 2012, 124, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Neulinger, S.C.; Gärtner, A.; Järnegren, J.; Ludvigsen, M.; Lochte, K.; Dullo, W.C. Tissue-associated “Candidatus Mycoplasma corallicola” and filamentous bacteria on the cold-water coral Lophelia pertusa (Scleractinia). Appl. Environ. Microbiol. 2009, 75, 1437–1444. [Google Scholar] [CrossRef]
- Vega-Orellana, O.M. Estudio de Microorganismos de la Clase Mollicutes en Organismos Marinos. Ph.D. Thesis, University of Las Palmas de Gran Canaria, Las Palmas, Spain, 2014. [Google Scholar]
- Al Masalma, M.; Armougom, F.; Scheld, W.M.; Dufour, H.; Roche, P.; Drancourt, M.; Raoult, D. The Expansion of the Microbiological Spectrum of Brain Abscesses with Use of Multiple 16S Ribosomal DNA Sequencing. Clin. Infect. Dis. 2009, 48, 1169–1178. [Google Scholar] [CrossRef]
- Haulena, M.; Gulland, F.M.D.; Lawrence, J.A.; Fauquier, D.A.; Jang, S.; Aldridge, B.; Spraker, T.; Thomas, L.C.; Brown, D.R.; Wendland, L.; et al. Lesions associated with a novel Mycoplasma sp. in california sea lions (Zalophus californianus) undergoing rehabilitation. J. Wildl. Dis. 2006, 42, 40–45. [Google Scholar] [CrossRef]
- Wallace, J.C.; Youngblood, J.E.; Port, J.A.; Cullen, A.C.; Smith, M.N.; Workman, T.; Faustman, E.M. Variability in metagenomic samples from the Puget Sound: Relationship to temporal and anthropogenic impacts. PLoS ONE 2018, 13, e0192412. [Google Scholar] [CrossRef]
- Li, Z.; Qu, Z.; Zhang, X.; Zhang, X.-H. Lentibacter algarum gen. nov., sp. nov., isolated from coastal water during a massive green algae bloom. Int. J. Syst. Evol. Microbiol. 2012, 62, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Hahnke, S.; Brock, N.L.; Zell, C.; Simon, M.; Dickschat, J.S.; Brinkhoff, T. Physiological diversity of Roseobacter clade bacteria co-occurring during a phytoplankton bloom in the North Sea. Syst. Appl. Microbiol. 2013, 36, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.; Layton, R.; Owens, L.; Ketheesan, N.; Govan, B. Evidence for the classification of a crayfish pathogen as a member of the genus Coxiella. Lett. Appl. Microbiol. 2007, 45, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.; Owens, L. Infectivity, transmission and 16S rRNA sequencing of a rickettsia, Coxiella cheraxi sp. nov., from the freshwater crayfish Cherax quadricarinatus. Dis. Aquat. Organ. 2000, 41, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Hyun, D.-W.; Shin, N.-R.; Kim, M.-S.; Oh, S.J.; Kim, P.S.; Whon, T.W.; Bae, J.-W. Endozoicomonas atrinae sp. nov., isolated from the intestine of a comb pen shell Atrina pectinata. Int. J. Syst. Evol. Microbiol. 2014, 64, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.L.; Paul, V.J.; Teplitski, M. Community shifts in the surface microbiomes of the coral Porites astreoides with unusual lesions. PLoS ONE 2014, 9, e100316. [Google Scholar] [CrossRef]
- Vezzulli, L.; Pezzati, E.; Huete-Stauffer, C.; Pruzzo, C.; Cerrano, C. 16SrDNA Pyrosequencing of the Mediterranean Gorgonian Paramuricea clavata Reveals a Link among Alterations in Bacterial Holobiont Members, Anthropogenic Influence and Disease Outbreaks. PLoS ONE 2013, 8, e67745. [Google Scholar] [CrossRef]
- Nishijima, M.; Adachi, K.; Katsuta, A.; Shizuri, Y.; Yamasato, K. Endozoicomonas numazuensis sp. nov., a gammaproteobacterium isolated from marine sponges, and emended description of the genus Endozoicomonas Kurahashi and Yokota 2007. Int. J. Syst. Evol. Microbiol. 2013, 63, 709–714. [Google Scholar] [CrossRef]
- Pike, R.E.; Haltli, B.; Kerr, R.G. Description of Endozoicomonas euniceicola sp. nov. and Endozoicomonas gorgoniicola sp. nov., bacteria isolated from the octocorals Eunicea fusca and Plexaura sp., and an emended description of the genus Endozoicomonas. Int. J. Syst. Evol. Microbiol. 2013, 63, 4294–4302. [Google Scholar] [CrossRef]
- Yang, C.S.; Chen, M.H.; Arun, A.B.; Chen, C.A.; Wang, J.T.; Chen, W.M. Endozoicomonas montiporae sp. nov., isolated from the encrusting pore coral Montipora aequituberculata. Int. J. Syst. Evol. Microbiol. 2009, 60, 1158–1162. [Google Scholar] [CrossRef]
- Neave, M.J.; Michell, C.T.; Apprill, A.; Voolstra, C.R. Endozoicomonas genomes reveal functional adaptation and plasticity in bacterial strains symbiotically associated with diverse marine hosts. Sci. Rep. 2017, 7, 40579. [Google Scholar] [CrossRef]
- Stabili, L.; Cardone, F.; Alifano, P.; Tredici, S.M.; Piraino, S.; Corriero, G.; Gaino, E. Epidemic Mortality of the Sponge Ircinia variabilis (Schmidt, 1862) Associated to Proliferation of a Vibrio Bacterium. Microb. Ecol. 2012, 64, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Gravili, C.; Tredici, S.M.; Boero, F.; Alifano, P. Association of a luminous Vibrio sp., taxonomically related to Vibrio harveyi, with Clytia linearis (Thornely, 1900) (Hydrozoa, Cnidaria). J. Exp. Mar. Bio. Ecol. 2011, 396, 77–82. [Google Scholar] [CrossRef]
- Stabili, L.; Gravili, C.; Tredici, S.M.; Piraino, S.; Talà, A.; Boero, F.; Alifano, P. Epibiotic Vibrio Luminous Bacteria Isolated from Some Hydrozoa and Bryozoa Species. Microb. Ecol. 2008, 56, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Frans, I.; Michiels, C.W.; Bossier, P.; Willems, K.A.; Lievens, B.; Rediers, H. Vibrio anguillarum as a fish pathogen: Virulence factors, diagnosis and prevention. J. Fish. Dis. 2011, 34, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gil, B.; Thompson, C.C.; Matsumura, Y.; Sawabe, T.; Iida, T.; Christen, R.; Sawabe, T. The Family Vibrionaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 659–747. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Okoh, A.I. Emerging Vibrio species: An unending threat to public health in developing countries. Res. Microbiol. 2008, 159, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Chen, X.; Hu, J.; Shen, C.; Ding, L. Exploring the potential environmental functions of viable but non-culturable bacteria. World J. Microbiol. Biotechnol. 2013, 29, 2213–2218. [Google Scholar] [CrossRef]
- Al-Saari, N.; Mohamad, A.; Fathin-Amirah, M.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A Review on Disease Development and Prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef]
- Roux, F.L.; Wegner, K.M.; Baker-Austin, C.; Vezzulli, L.; Osorio, C.R.; Amaro, C.; Ritchie, J.M.; Defoirdt, T.; Destoumieux-Garzón, D.; Blokesch, M.; et al. The emergence of Vibrio pathogens in Europe: Ecology, evolution, and pathogenesis (Paris, 11–12th March 2015). Front. Microbiol. 2015, 6, 830. [Google Scholar] [CrossRef]
- Harvell, C.D. Climate Warming and Disease Risks for Terrestrial and Marine Biota. Sci. 80 2002, 296, 2158–2162. [Google Scholar] [CrossRef]
- Tout, J.; Siboni, N.; Messer, L.F.; Garren, M.; Stocker, R.; Webster, N.S.; Ralph, P.J.; Seymor, J.R. Increased seawater temperature increases the abundance and alters the structure of natural Vibrio populations associated with the coral Pocillopora damicornis. Front. Microbiol. 2015, 6, 432. [Google Scholar] [CrossRef] [PubMed]
- Sinatra, J.A.; Colby, K. Notes from the Field: Fatal Vibrio anguillarum Infection in an Immunocompromised Patient—Maine, 2017. Mmwr Morb. Mortal. Wkly. Rep. 2018, 67, 962–963. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, L.; Colwell, R.R.; Pruzzo, C. Ocean Warming and Spread of Pathogenic Vibrios in the Aquatic Environment. Microb. Ecol. 2013, 65, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, L.; Grande, C.; Reid, P.C.; Hélaouët, P.; Edwards, M.; Höfle, M.G.; Brettar, I.; Colwell, R.R.; Pruzzo, C. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc. Natl. Acad. Sci. USA 2016, 113, E5062–E5071. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Herbst, S.; Rechenburg, A.; Suk, J.E.; Höser, C.; Schreiber, C.; Kistemann, T. Climate Change Impact Assessment of Food- and Waterborne Diseases. Crit. Rev. Environ. Sci. Technol. 2012, 42, 857–890. [Google Scholar] [CrossRef] [PubMed]
- Baedke, J.; Fábregas-Tejeda, A.; Nieves Delgado, A. The holobiont concept before Margulis. J. Exp. Zool. Part. B Mol. Dev. Evol. 2020, 334, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Prasse, D.; Weiland-Bräuer, N.; Jaspers, C.; Reusch, T.B.H.; Schmitz, R.A. Evaluating the quorum quenching potential of bacteria associated to Aurelia aurita and Mnemiopsis leidyi. bioRxiv 2019. [Google Scholar] [CrossRef]
- Van der Ploeg, J.R. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J. Bacteriol. 2005, 187, 3980–3989. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, L.; Boutry, C.; Guédon, E.; Guillot, A.; Ibrahim, M.; Grossiord, B.; Hols, P. Quorum-sensing regulation of the production of Blp bacteriocins in Streptococcus thermophilus. J. Bacteriol. 2007, 189, 7195–7205. [Google Scholar] [CrossRef]
- Campbell, B.J.; Engel, A.S.; Porter, M.L.; Takai, K. The versatile ε-proteobacteria: Key players in sulphidic habitats. Nat. Rev. Microbiol. 2006, 4, 458–468. [Google Scholar] [CrossRef]
- Kern, M.; Simon, J. Electron transport chains and bioenergetics of respiratory nitrogen metabolism in Wolinella succinogenes and other Epsilonproteobacteria. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sasaki, T.; Suzuki, M.; Nogi, Y.; Miwa, T.; Takai, K.; Nealson, K.H.; Horikoshi, K. Novel chemoautotrophic endosymbiosis between a member of the Epsilonproteobacteria and the hydrothermal-vent gastropod Alviniconcha aff. hessleri (Gastropoda: Provannidae) from the Indian Ocean. Appl. Environ. Microbiol. 2005, 71, 5440–5450. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sasaki, T.; Suzuki, M.; Tsuchida, S.; Nealson, K.H.; Horikoshi, K. Molecular phylogenetic and isotopic evidence of two lineages of chemoautotrophic endosymbionts distinct at the subdivision level harbored in one host-animal type: The genus Alviniconcha (Gastropoda: Provannidae). Fems Microbiol. Lett. 2005, 249, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Fraschetti, S.; Alifano, P.; Tredici, M.S.; Stabili, L. Association of Vibrio community with the Atlantic Mediterranean invasive alga Caulerpa cylindracea. J. Exp. Mar. Bio. Ecol. 2016, 475, 129–136. [Google Scholar] [CrossRef]
- Rizzo, L.; Fraschetti, S.; Alifano, P.; Pizzolante, G.; Stabili, L. The alien species Caulerpa cylindracea and its associated bacteria in the Mediterranean Sea. Mar. Biol. 2016, 163, 4. [Google Scholar] [CrossRef]
- Rizzo, L.; Pusceddu, A.; Stabili, L.; Alifano, P.; Fraschetti, S. Potential effects of an invasive seaweed (Caulerpa cylindracea, Sonder) on sedimentary organic matter and microbial metabolic activities. Sci. Rep. 2017, 7, 12113. [Google Scholar] [CrossRef]
- Stabili, L.; Rizzo, L.; Pizzolante, G.; Alifano, P.; Fraschetti, S. Spatial distribution of the culturable bacterial community associated with the invasive alga Caulerpa cylindracea in the Mediterranean Sea. Mar. Environ. Res. 2017, 125, 90–98. [Google Scholar] [CrossRef]
- Gryta, A.; Frąc, M.; Oszust, K. The application of the Biolog EcoPlate approach in ecotoxicological evaluation of dairy sewage sludge. Appl. Biochem. Biotechnol. 2014, 174, 1434–1443. [Google Scholar] [CrossRef]
- Truu, M.; Juhanson, J.; Truu, J. Microbial biomass, activity and community composition in constructed wetlands. Sci. Total Environ. 2009, 407, 3958–3971. [Google Scholar] [CrossRef]
- Rüger, H.-J.; Krambeck, H.-J. Evaluation of the BIOLOG Substrate Metabolism System for Classification of Marine Bacteria. Syst. Appl. Microbiol. 1994, 17, 281–288. [Google Scholar] [CrossRef]
- Gamo, M.; Shoji, T. A Method of Profiling Microbial Communities Based on a Most-Probable-Number Assay That Uses BIOLOG Plates and Multiple Sole Carbon Sources. Appl. Environ. Microbiol. 1999, 65, 4419–4424. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.P.; Grove, J.A.; Gehder, M.; Anderson, W.A.; Legge, R.L. Data transformations in the analysis of community-level substrate utilization data from microplates. J. Microbiol. Methods 2007, 69, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Frąc, M.; Oszust, K.; Lipiec, J. Community level physiological profiles (CLPP), characterization and microbial activity of soil amended with dairy sewage sludge. Sensors 2012, 12, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, D141–D145. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 2010, 26, 1899–1900. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Pielou, E.C. Shannon’s formula as a measure of specific diversity: Its use and misuse. Am. Nat. 1966, 100, 463–465. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 125–349. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.B.; Simpson, G.L.; Friendly, M.; Solymos, P.; Stevens, M.H.M.; Wagner, H.; et al. Vegan: Community Ecology Package. R Package Version 1.17-4. 2010. Available online: http://CRAN.R-project.org/package=vegan (accessed on 17 December 2017).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Anderson, M.J.; Willis, T.J. Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Permanova+ for Primer: Guide to Software and Statistical Methods; Primer-E: Plymouth, UK, 2006. [Google Scholar]
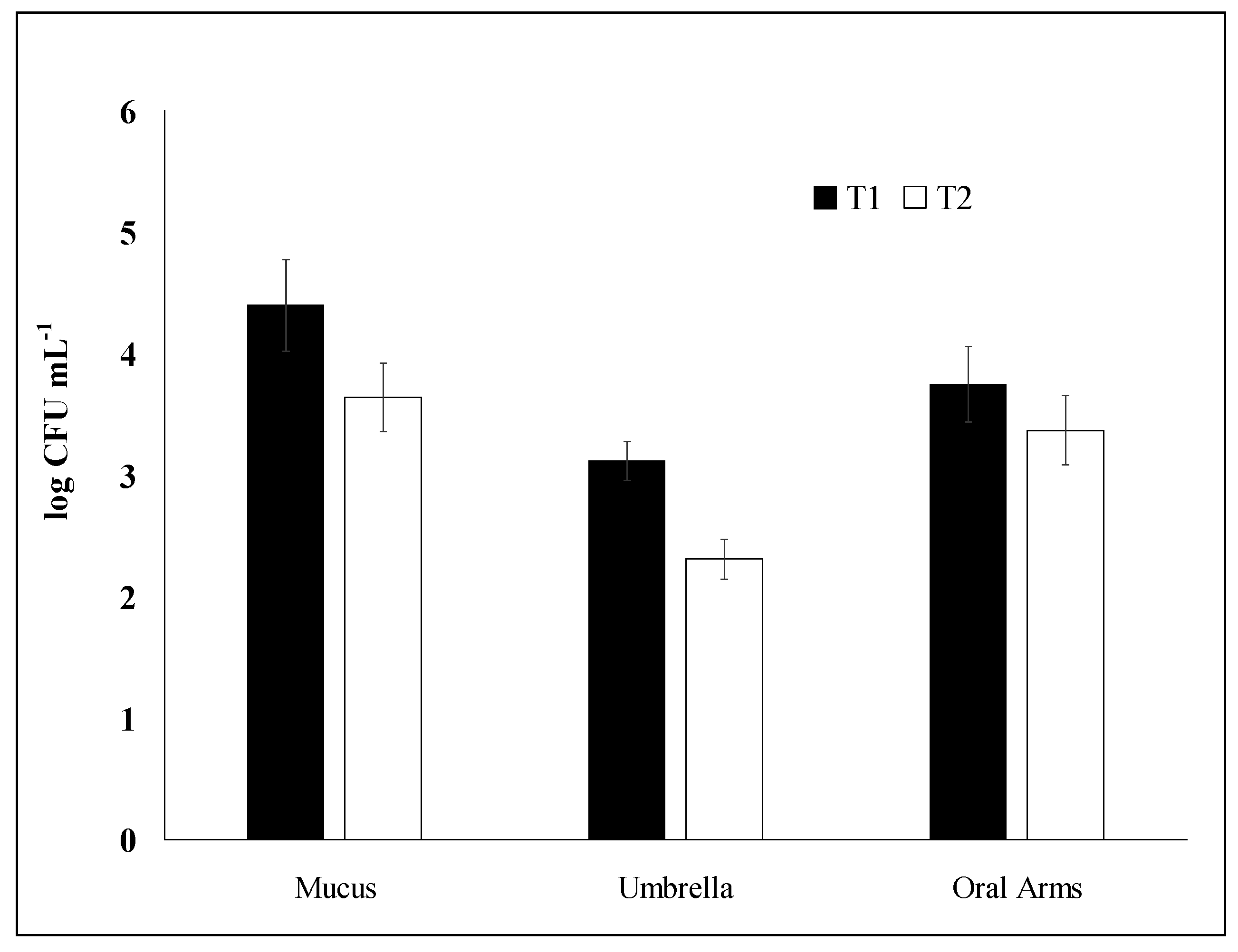


| Source | df | MS | Pseudo-F | P(perm) | MS | Pseudo-F | P(perm) |
|---|---|---|---|---|---|---|---|
| Heterotrophic Abundance | Optical Density | ||||||
| C | 2 | 4.26 × 108 | 1.86 | 22.41 | 1.14 | ||
| T | 1 | 4.17 × 108 | 13.72 | 10.46 | 9.18 | ||
| CxT | 2 | 2.29 × 108 | 7.55 | *** | 19.71 | 17.29 | *** |
| Res | 12 | 3.04 × 107 | 1.14 | ||||
| Tot | 17 | ||||||
| t | P(MC) | t | P(MC) | t | P(MC) | t | P(MC) | |
|---|---|---|---|---|---|---|---|---|
| Heterotrophic Abundance | Optical Density | |||||||
| T1 | T2 | T1 | T2 | |||||
| M vs U | 3.61 | * | 5.97 | ** | 4.56 | *** | 3.72 | * |
| M vs A | 2.1 | * | 2.02 | ns | 5.67 | *** | 3.61 | * |
| U vs A | 3.51 | ** | 2.91 | * | 3.60 | ** | 3.84 | * |
| Oral Arms | Mucus | Umbrella | ||||
|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | |
| γ−Hydroxybutyric Acid | + | + | − | + | − | − |
| d−Glucosaminic Acid | + | + | − | + | − | − |
| d−Galacturonic Acid | + | + | + | + | − | − |
| l−Phenylalanine | + | + | + | − | − | − |
| d−Xylose | + | − | − | + | − | − |
| Pyruvic Acid Methyl Ester | + | − | − | − | − | − |
| l−Threonine | − | + | + | − | − | − |
| Glucose−1−Phosphate | − | + | + | + | − | + |
| Glycyl−l−Glutamic Acid | − | + | + | + | − | − |
| d.l−α−Glycerol Phosphate | − | + | − | − | − | − |
| l−Arginine | − | + | + | + | − | − |
| Tween 40 | − | + | + | + | + | − |
| N−Acetyl−d−Glucosamine | − | + | − | − | − | − |
| d−Mannitol | − | + | − | − | − | − |
| Glycogen | − | + | + | − | − | − |
| β−Methyl−d−Glucoside | − | − | + | + | − | − |
| d−Galactonic Acid γ−Lactone | − | − | − | − | − | − |
| l−Asparagine | − | − | + | − | − | − |
| i−Erythritol | − | − | + | − | − | − |
| 2−Hydroxy Benzoic Acid | − | − | − | + | − | − |
| Tween 80 | − | − | − | − | + | − |
| 4−Hydroxy Benzoic Acid | − | − | − | − | − | − |
| l−Serine | − | − | + | + | − | − |
| α−Cyclodextrin | − | − | + | + | − | − |
| Itaconic Acid | − | − | − | + | − | − |
| d−Cellobiose | − | − | + | + | − | − |
| α−Ketobutyric Acid | − | − | + | − | − | − |
| Phenylethyl−amine | − | − | − | − | − | − |
| α−d−Lactose | − | − | + | + | − | − |
| d−Malic Acid | − | − | − | − | − | − |
| Putrescine | − | − | − | + | + | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabili, L.; Rizzo, L.; Basso, L.; Marzano, M.; Fosso, B.; Pesole, G.; Piraino, S. The Microbial Community Associated with Rhizostoma pulmo: Ecological Significance and Potential Consequences for Marine Organisms and Human Health. Mar. Drugs 2020, 18, 437. https://doi.org/10.3390/md18090437
Stabili L, Rizzo L, Basso L, Marzano M, Fosso B, Pesole G, Piraino S. The Microbial Community Associated with Rhizostoma pulmo: Ecological Significance and Potential Consequences for Marine Organisms and Human Health. Marine Drugs. 2020; 18(9):437. https://doi.org/10.3390/md18090437
Chicago/Turabian StyleStabili, Loredana, Lucia Rizzo, Lorena Basso, Marinella Marzano, Bruno Fosso, Graziano Pesole, and Stefano Piraino. 2020. "The Microbial Community Associated with Rhizostoma pulmo: Ecological Significance and Potential Consequences for Marine Organisms and Human Health" Marine Drugs 18, no. 9: 437. https://doi.org/10.3390/md18090437
APA StyleStabili, L., Rizzo, L., Basso, L., Marzano, M., Fosso, B., Pesole, G., & Piraino, S. (2020). The Microbial Community Associated with Rhizostoma pulmo: Ecological Significance and Potential Consequences for Marine Organisms and Human Health. Marine Drugs, 18(9), 437. https://doi.org/10.3390/md18090437










