Culturable Microorganisms Associated with Sea Cucumbers and Microbial Natural Products
Abstract
1. Introduction
2. Microorganisms Associated with Sea Cucumbers
2.1. Geographical Distribution of Microorganisms Associated with Sea Cucumbers
2.2. Culturable Microorganisms Associated with Sea Cucumbers
2.2.1. Bacteria
2.2.2. Fungi
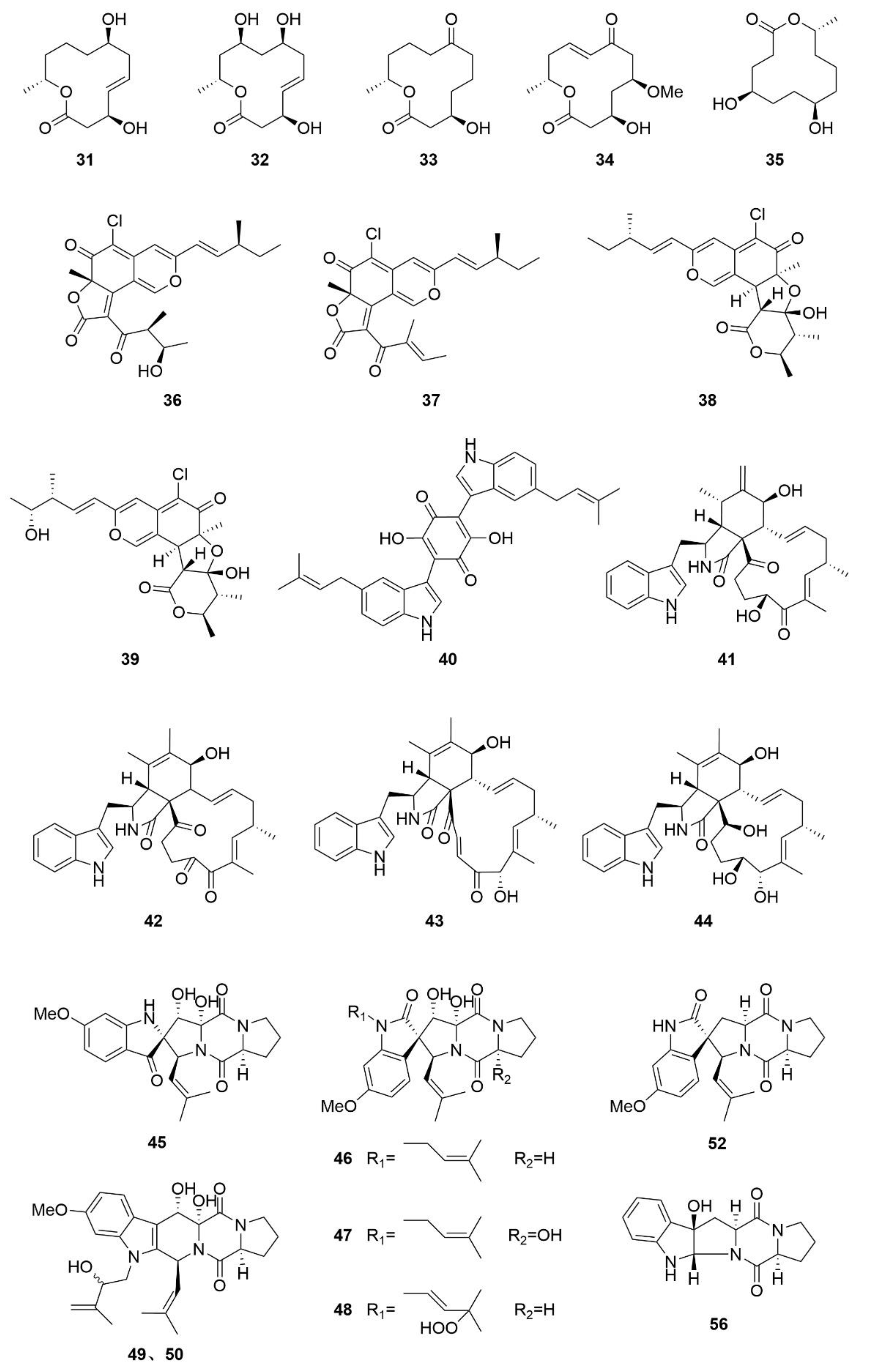
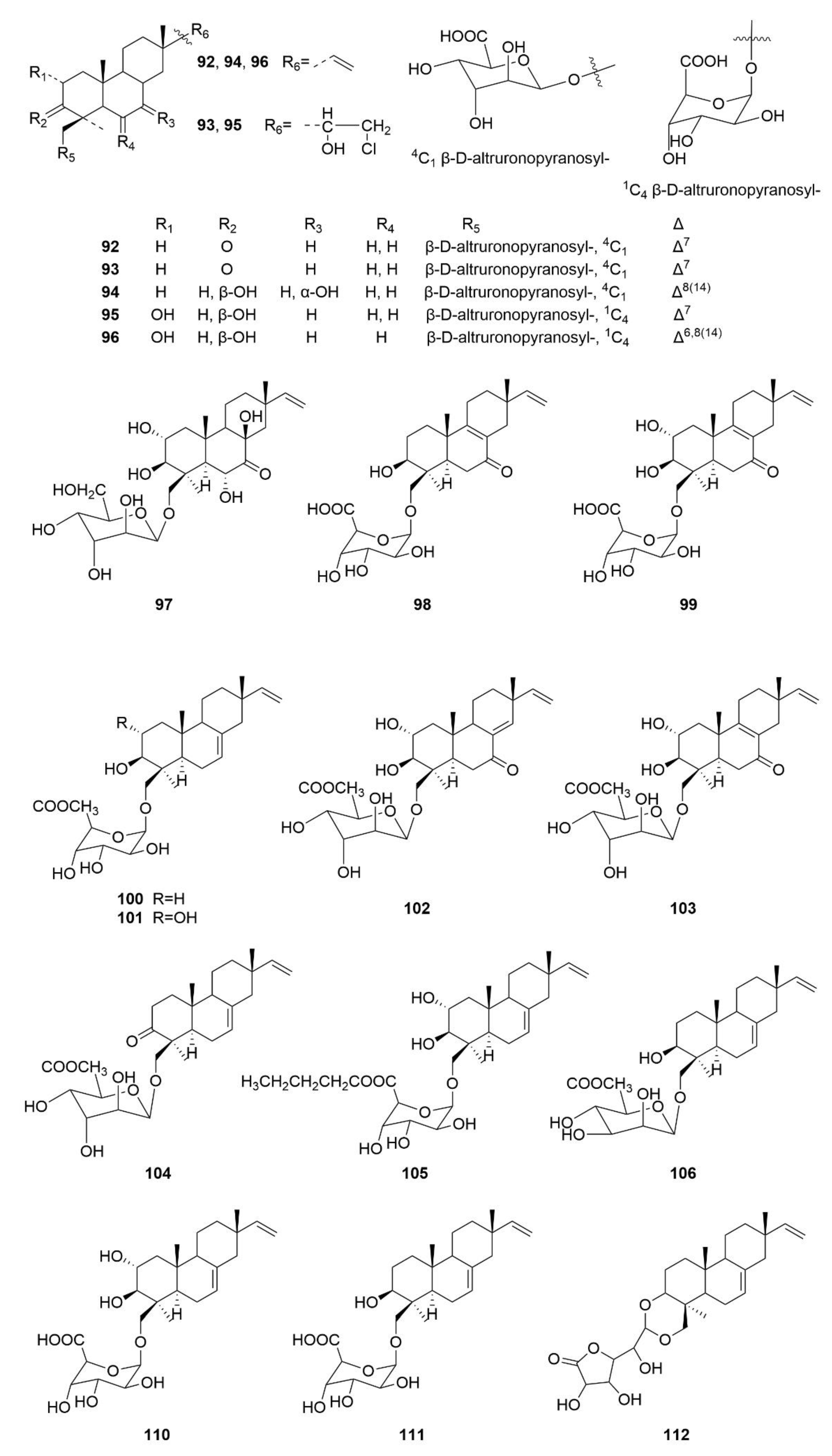
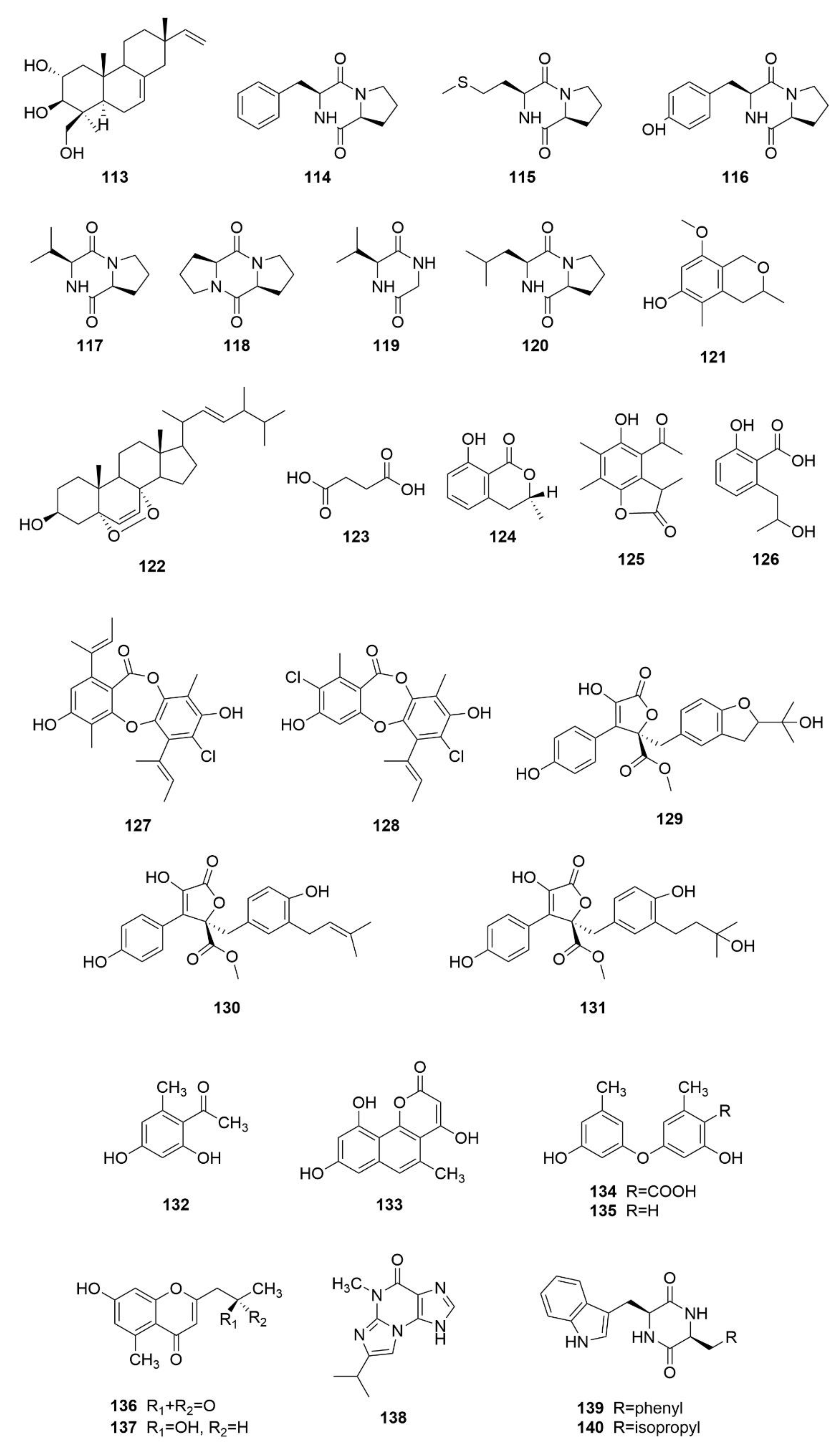
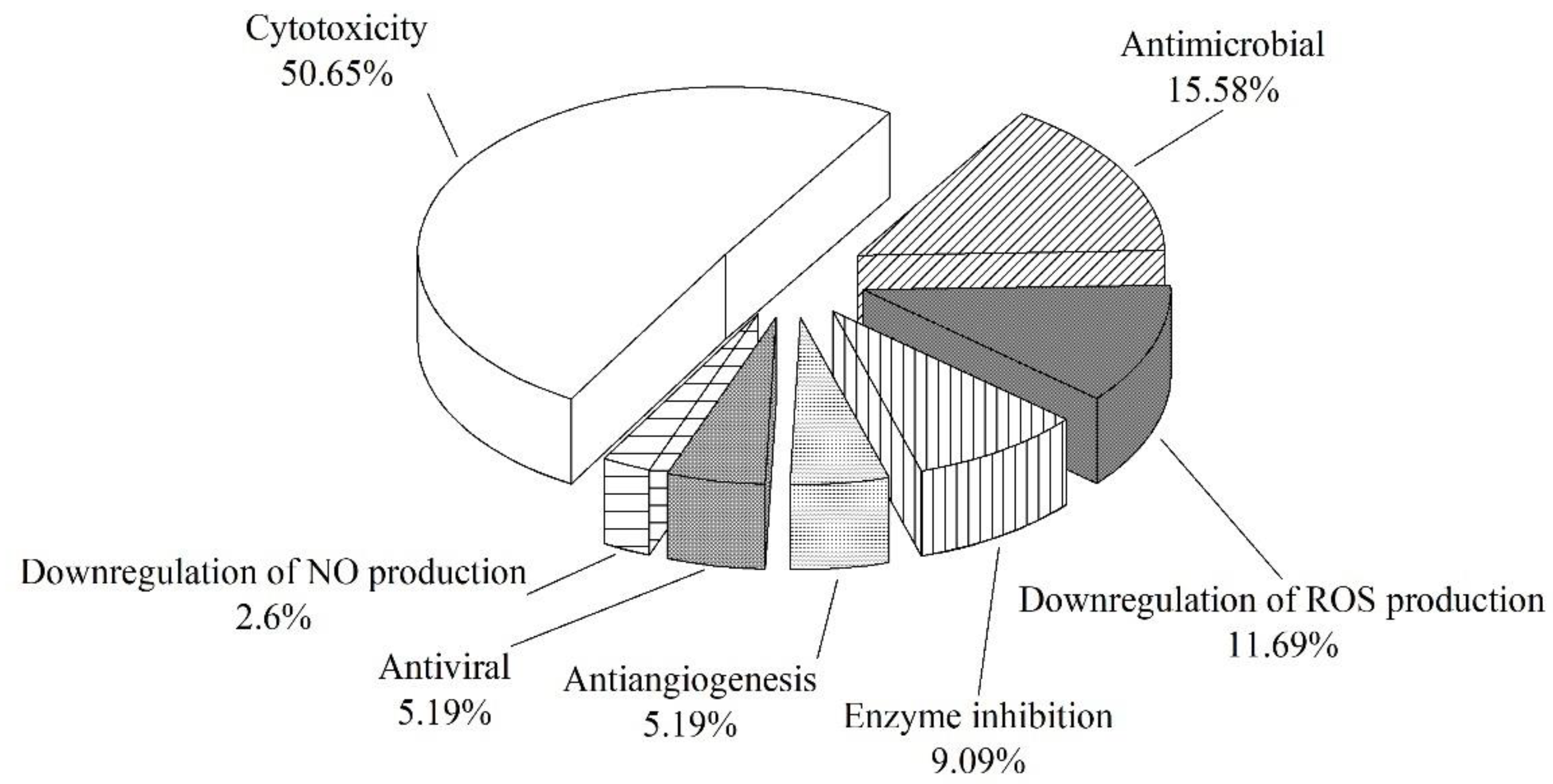
3. Structures and Bioactivities of Natural Products
3.1. Polyketides
3.2. Alkaloids
3.3. Terpenoids
3.4. Other Types of Compounds Isolated from Sea-Cucumber-Associated Microorganisms
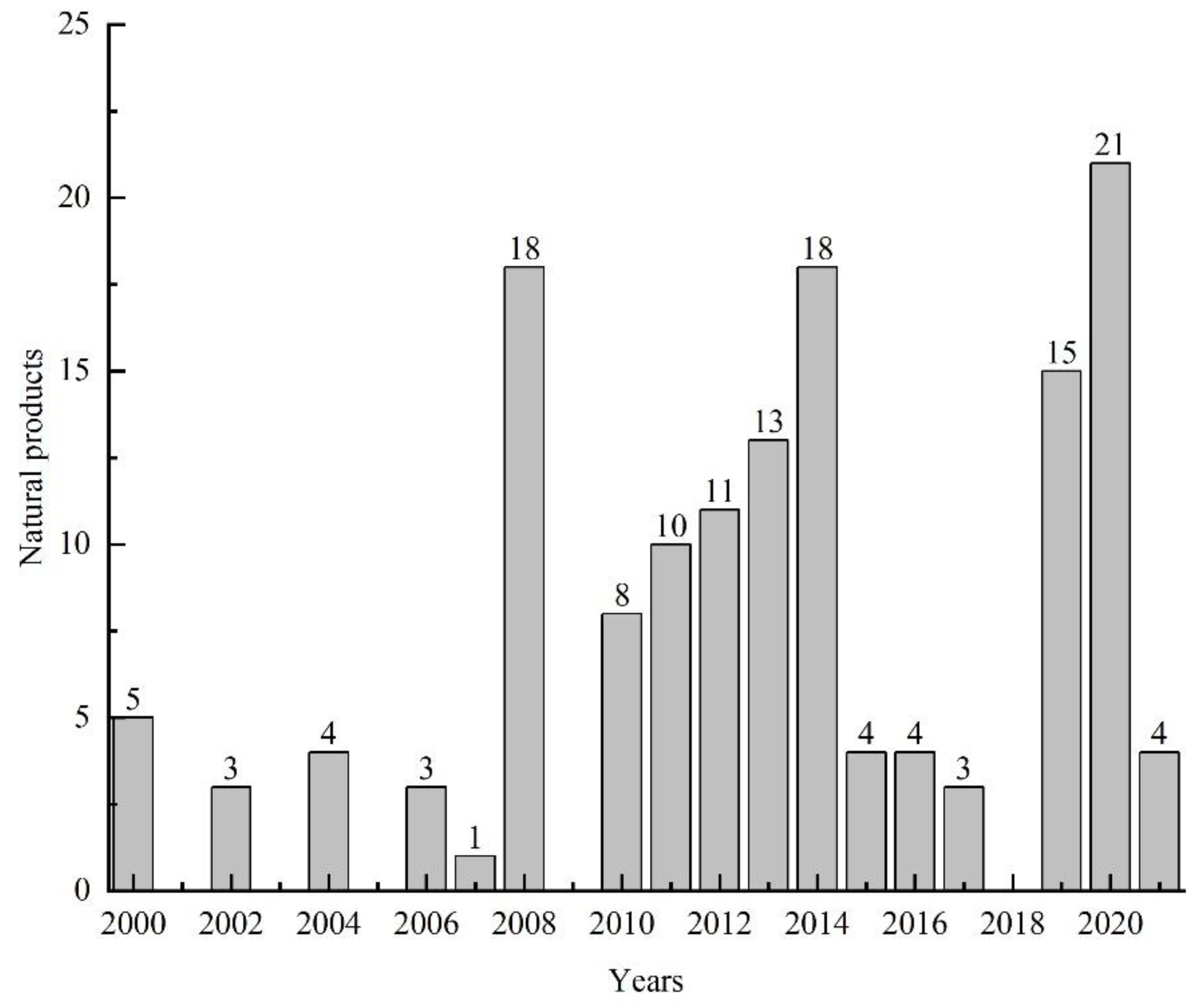
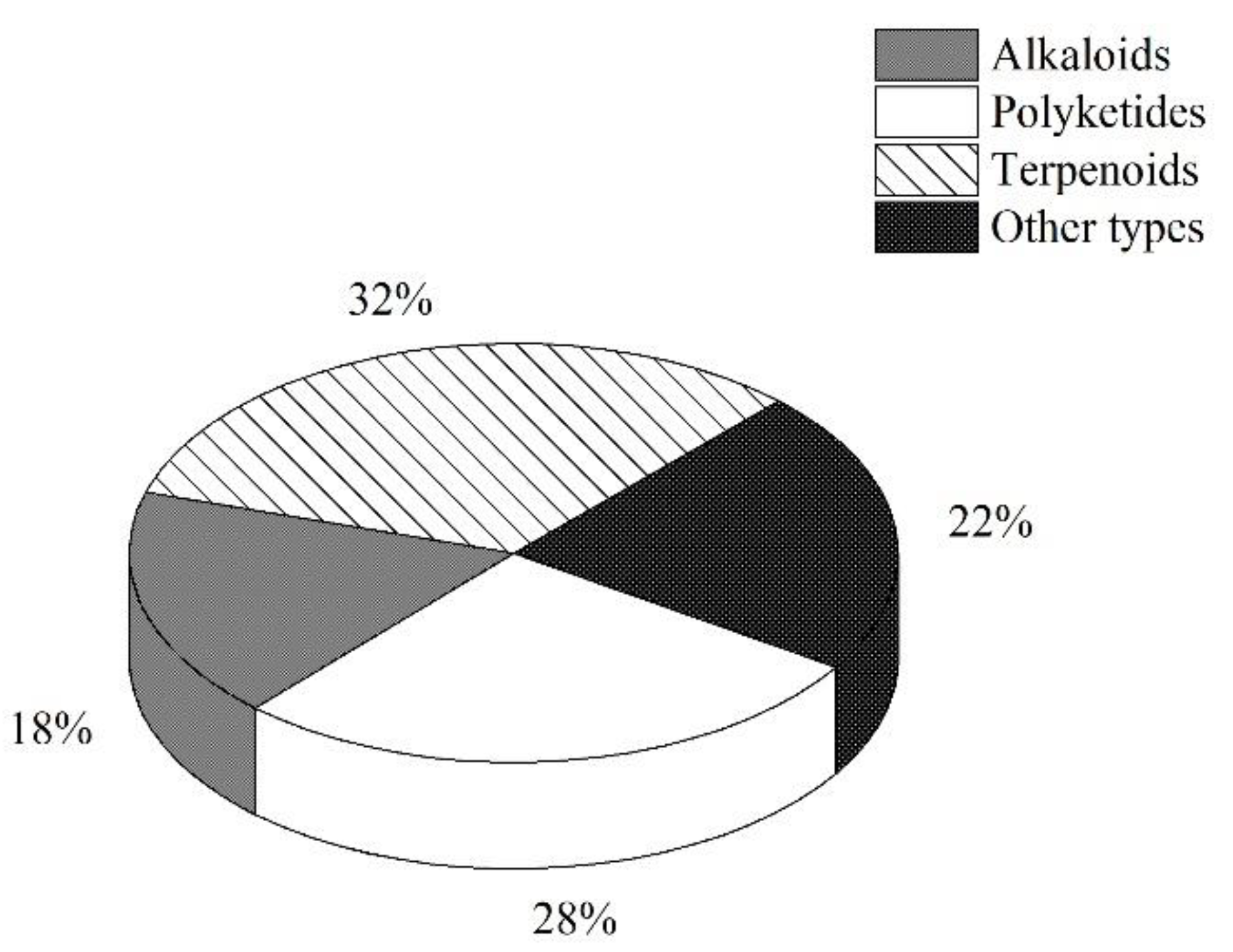
3.5. Summary of the Natural Products Isolated from Microorganisms Associated with Sea Cucumbers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kiew, P.L.; Don, M.M. Jewel of the seabed: Sea cucumbers as nutritional and drug candidates. Int. J. Food Sci. Nutr. 2012, 63, 616–636. [Google Scholar] [CrossRef]
- Mondol, M.A.M.; Shin, H.J.; Rahman, M.A.; Islam, M.T. Sea cucumber glycosides: Chemical structures, producing species and important biological properties. Mar. Drugs 2017, 15, 317. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Nakahara, T.; Miyazaki, M.; Nogi, Y.; Taniyama, S.; Arakawa, O.; Inoue, T.; Kudo, T. Diversity and function of aerobic culturable bacteria in the intestine of the sea cucumber Holothuria leucospilota. J. Gen. Appl. Microbiol. 2012, 58, 447–456. [Google Scholar] [CrossRef]
- Gao, F.; Li, F.; Tan, J.; Yan, J.; Sun, H. Bacterial community composition in the gut content and ambient sediment of sea cucumber Apostichopus japonicus revealed by 16S rRNA gene pyrosequencing. PLoS ONE 2014, 9, e100092. [Google Scholar] [CrossRef]
- Chen, L.; Du, S.; Qu, W.Y.; Guo, F.R.; Wang, G.Y. Biosynthetic potential of culturable bacteria associated with Apostichopus japonicus. J. Appl. Microbiol. 2019, 127, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Seo, E.Y.; Epstein, S.S.; Joung, Y.; Han, J.; Parfenova, V.V.; Belykh, O.I.; Gladkikh, A.S.; Ahn, T.S. Application of a new cultivation technology, I-tip, for studying microbial diversity in freshwater sponges of Lake Baikal, Russia. FEMS Microbiol. Ecol. 2014, 90, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Wargasetia, T.L. Mechanisms of cancer cell killing by sea cucumber-derived compounds. Investig. New Drugs 2017, 35, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Taiyeb-Ali, T.B.; Zainuddin, S.L.; Swaminathan, D.; Yaacob, H. Efficacy of ‘Gamadent’ toothpaste on the healing of gingival tissues: A preliminary report. J. Oral Sci. 2003, 45, 153–159. [Google Scholar] [CrossRef]
- Shi, S.; Feng, W.; Hu, S.; Liang, S.; An, N.; Mao, Y. Bioactive compounds of sea cucumbers and their therapeutic effects. Chin. J. Oceanol. Limnol. 2016, 34, 549–558. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Northern sea cucumber (Cucumaria frondosa): A potential candidate for functional food, nutraceutical, and pharmaceutical sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef]
- Chari, A.; Mazumder, A.; Lau, K.; Catamero, D.; Galitzeck, Z.; Jagannath, S. A phase II trial of TBL-12 sea cucumber extract in patients with untreated asymptomatic myeloma. Br. J. Haematol. 2018, 180, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.W. The secret to a successful relationship: Lasting chemistry between ascidians and their symbiotic bacteria. Invertebr. Biol. 2015, 134, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Bewley, C.A.; Holland, N.D.; Faulkner, D.J. Two classes of metabolites from Theonella swinhoei are localized in distinct populations of bacterial symbionts. Experientia 1996, 52, 716–722. [Google Scholar] [CrossRef]
- Bewley, C.A.; Faulkner, D.J. Lithistid sponges: Star performers or hosts to the stars. Angew. Chem. Int. Ed. 1998, 37, 2162–2178. [Google Scholar] [CrossRef]
- Piel, J. Metabolites from symbiotic bacteria. Nat. Prod. Rep. 2004, 21, 519–538. [Google Scholar] [CrossRef]
- Zhang, X.; Nakahara, T.; Murase, S.; Nakata, H.; Inoue, T.; Kudo, T. Physiological characterization of aerobic culturable bacteria in the intestine of the sea cucumber Apostichopus japonicus. J. Gen. Appl. Microbiol. 2013, 59, 1–10. [Google Scholar] [CrossRef][Green Version]
- Kurahashi, M.; Fukunaga, Y.; Sakiyama, Y.; Harayama, S.; Yokota, A. Iamia majanohamensis gen. nov., sp. nov., an actinobacterium isolated from sea cucumber Holothuria edulis, and proposal of Iamiaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 869–873. [Google Scholar] [CrossRef]
- Gozari, M.; Bahador, N.; Jassbi, A.R.; Mortazavi, M.S.; Eftekhar, E. Antioxidant and cytotoxic activities of metabolites produced by a new marine Streptomyces sp. isolated from the sea cucumber Holothuria leucospilota. Iran. J. Fish. Sci. 2018, 17, 413–426. [Google Scholar] [CrossRef]
- Pivkin, M.V. Filamentous fungi associated with holothurians from the Sea of Japan, off the primorye coast of Russia. Biol. Bull. 2000, 198, 101–109. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Kuznetsova, T.A.; Isakov, V.V.; Pivkin, M.V.; Prokof’eva, N.G.; Elyakov, G.B. New diterpenic altrosides of the fungus Acremonium striatisporum isolated from a sea cucumber. J. Nat. Prod. 2000, 63, 848–850. [Google Scholar] [CrossRef]
- Marchese, P.; Garzoli, L.; Gnavi, G.; O’Connell, E.; Bouraoui, A.; Mehiri, M.; Murphy, J.M.; Varese, G.C. Diversity and bioactivity of fungi associated with the marine sea cucumber Holothuria poli: Disclosing the strains potential for biomedical applications. J. Appl. Microbiol. 2020, 129, 612–625. [Google Scholar] [CrossRef]
- Gong, J.; Tang, H.; Geng, W.L.; Liu, B.S.; Sun, P.; Li, L.; Li, Z.Y.; Zhang, W. Cyclic dipeptides in actinomycete Brevibacterium sp. associated with sea cucumber Apostichopus japonicus Selenka: Isolation and identification. Acad. J. Second Mil. Med. Univ. 2012, 33, 1284–1287. (In Chinese) [Google Scholar] [CrossRef]
- Enomoto, M.; Nakagawa, S.; Sawabe, T. Microbial communities associated with Holothurians: Presence of unique bacteria in the coelomic fluid. Microbes Environ. 2012, 27, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, J.T.; Kellermann, M.Y.; Versluis, D.; Putra, M.Y.; Murniasih, T.; Mohr, K.I.; Wink, J.; Engelmann, M.; Praditya, D.F.; Steinmann, E.; et al. Biotechnological potential of bacteria isolated from the sea cucumber Holothuria leucospilota and Stichopus vastus from Lampung, Indonesia. Mar. Drugs 2019, 17, 635. [Google Scholar] [CrossRef]
- Alipiah, N.M.; Ramli, N.H.S.; Low, C.F.; Shamsudin, M.N.; Yusoff, F.M. Protective effects of sea cucumber surface-associated bacteria against Vibrio harveyi in brown-marbled grouper fingerlings. Aquacult. Environ. Interact. 2016, 8, 147–155. [Google Scholar] [CrossRef]
- Xia, X.; Qi, J.; Wei, F.; Jia, A.; Yuan, W.; Meng, X.; Zhang, M.; Liu, C.; Wang, C. Isolation and characterization of a new Benzofuran from the fungus Alternaria sp. (HS-3) associated with a sea cucumber. Nat. Prod. Commun. 2011, 6, 1913–1914. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Xia, X.K.; Qi, J.; Zhang, Y.G.; Yuan, W.P.; Meng, X.M.; Jia, A.R.; Sun, Y.J.; Hu, W. The secondary metabolites of the HS-3 Alternaria sp. fungus associated with holothurians. J. Chin. Med. Mater. 2010, 33, 1875–1877. (In Chinese) [Google Scholar] [CrossRef]
- Qi, J.; Jiang, L.; Zhao, P.; Chen, H.; Jia, X.; Zhao, L.; Dai, H.; Hu, J.; Liu, C.; Shim, S.H.; et al. Chaetoglobosins and azaphilones from Chaetomium globosum associated with Apostichopus japonicus. Appl. Microbiol. Biotechnol. 2020, 104, 1545–1553. [Google Scholar] [CrossRef]
- Farouk, A.E.; Ghouse, F.A.H.; Ridzwan, B.H. New bacterial species isolated from malaysia sea cucumbers with optimized secreted antibacterial activity. Am. J. Biochem. Biotechnol. 2007, 3, 60–65. [Google Scholar]
- Kamarudin, K.R. Microbial population in the coelomic fluid of Stichopus chloronotus and Holothuria (Mertensiothuria) leucospilota collected from Malaysian waters. Sains Malays. 2014, 43, 1013–1021. [Google Scholar]
- Li, Z.; Huang, X.S.; Zheng, B.D.; Deng, K.B. Biodiversity analysis and pathogen inhibition mechanism of the endogenous bacteria in Fujian Apostichopus japonicus. Sci. Technol. Food Ind. 2018, 39, 137–141, 170. (In Chinese) [Google Scholar] [CrossRef]
- Bogatyrenko, E.A.; Buzoleva, L.S. Characterization of the gut bacterial community of the Japanese sea cucumber Apostichopus japonicus. Microbiology 2016, 85, 116–123. [Google Scholar] [CrossRef]
- Jo, J.; Choi, H.; Lee, S.-G.; Oh, J.; Lee, H.-G.; Park, C. Draft genome sequences of Pseudoalteromonas tetraodonis CSB01KR and Pseudoalteromonas lipolytica CSB02KR, isolated from the gut of the sea cucumber Apostichopus japonicus. Genome Announc. 2017, 5, e00627-17. [Google Scholar] [CrossRef]
- Tan, J.J.; Liu, X.Y.; Yang, Y.; Li, F.H.; Tan, C.H.; Li, Y.M. Aspergillolide, a new 12-membered macrolide from sea cucumber-derived fungus Aspergillus sp. S-3-75. Nat. Prod. Res. 2020, 34, 1131–1137. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, M.; Zhao, J.; Liao, Z.; Qi, J.; Wang, X.; Jiang, W.; Xia, X. A Meroterpenoid isolated from the fungus Aspergillus sp. Nat. Prod. Commun. 2019, 14, 1–3. [Google Scholar] [CrossRef]
- Sun, P.; Xu, D.X.; Mandi, A.; Kurtan, T.; Li, T.J.; Schulz, B.; Zhang, W. Structure, absolute configuration, and conformational study of 12-membered macrolides from the fungus Dendrodochium sp. associated with the sea cucumber Holothuria nobilis Selenka. J. Org. Chem. 2013, 78, 7030–7047. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Sun, P.; Xu, D.X.; Tang, H.; Liu, B.S.; Zhang, W. Identification of secondary metabolites of the fungus Phialemonium sp. associated with the South China sea cucumber Holothuria nobilis Selenka. Acad. J. Second Mil. Med. Univ. 2014, 35, 988–991. (In Chinese) [Google Scholar] [CrossRef]
- Xia, X.K.; Qi, J.; Liu, C.H.; Zhang, Y.G.; Jia, A.R.; Yuan, W.P.; Liu, X.; Zhang, M.S. Polyketones from Aspergillus terreus associated with Apostichopus japonicus. Mod. Food Sci. Technol. 2014, 30, 10–14, 62. (In Chinese) [Google Scholar] [CrossRef]
- Xia, X.; Zhang, J.; Zhang, Y.; Wei, F.; Liu, X.; Jia, A.; Liu, C.; Li, W.; She, Z.; Lin, Y. Pimarane diterpenes from the fungus Epicoccum sp. HS-1 associated with Apostichopus japonicus. Bioorg. Med. Chem. Lett. 2012, 22, 3017–3019. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fang, Y.; Zhu, T.; Zhang, M.; Lin, A.; Gu, Q.; Zhu, W. Seven new prenylated indole diketopiperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus. Tetrahedron 2008, 64, 7986–7991. [Google Scholar] [CrossRef]
- Wang, F.-Z.; Zhang, M.; Sun, W.; Gu, Q.-Q.; Zhu, W.-M. 5a-Hydroxy-9-methoxy-11-(3-methyl-2-butenyl)-12-(2-methyl-1-propenyl)-2,3,11,12-tetrahydro-1H,5H-pyrrolo[1′′,2′′:4′,5′]pyrazino[1′,2′:1,6]pyrido[3,4-b]indole-5,6,14(5aH,14aH)-trione. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, o1859–o1860. [Google Scholar] [CrossRef]
- Xia, X.K.; Liu, X.; Zhang, Y.G.; Yuan, W.P.; Zhang, M.S.; Wan, X.J.; Meng, X.M.; Liu, C.H. Study on the second metabolisms from fungus HS-1 Epicoccum spp. from the sea cucumber in Yellow Sea. J. Chin. Med. Mater. 2010, 33, 1577–1579. (In Chinese) [Google Scholar] [CrossRef]
- Qi, J.; Zhao, P.; Zhao, L.; Jia, A.; Liu, C.; Zhang, L.; Xia, X. Anthraquinone derivatives from a sea cucumber-derived Trichoderma sp. fungus with antibacterial activities. Chem. Nat. Compd. 2020, 56, 112–114. [Google Scholar] [CrossRef]
- Xia, X.; Liu, X.; Koo, D.C.; Sun, Z.; Shim, S. Chemical constituents of Fusarium sp. fungus associated with sea cucumbers. Chem. Nat. Compd. 2014, 50, 1103–1105. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Kalinovsky, A.I.; Antonov, A.S.; Zhuravleva, O.I.; Khudyakova, Y.V.; Aminin, D.L.; Yurchenko, A.N.; Pivkin, M.V. Isolation and structures of virescenosides from the marine-derived fungus Acremonium striatisporum. Phytochem. Lett. 2016, 15, 66–71. [Google Scholar] [CrossRef]
- Qi, J.; Zhao, B.; Zhao, P.; Jia, A.; Zhang, Y.; Liu, X.; Liu, C.; Zhang, L.; Xia, X. Isolation and characterization of antiangiogenesis compounds from the fungus Aspergillus terreus associated with Apostichopus japonicus using zebrafish assay. Nat. Prod. Commun. 2017, 12, 261–262. [Google Scholar] [CrossRef]
- Tae, H.; Sohng, J.K.; Park, K. MapsiDB: An integrated web database for type I polyketide synthases. Bioprocess. Biosyst. Eng. 2009, 32, 723–727. [Google Scholar] [CrossRef]
- Risdian, C.; Mozef, T.; Wink, J. Biosynthesis of polyketides in Streptomyces. Microorganisms 2019, 7, 124. [Google Scholar] [CrossRef]
- Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 2009, 48, 4688–4716. [Google Scholar] [CrossRef]
- Hussain, H.; Al-Sadi, A.M.; Schulz, B.; Steinert, M.; Khan, A.; Green, I.R.; Ahmed, I. A fruitful decade for fungal polyketides from 2007 to 2016: Antimicrobial activity, chemotaxonomy and chemodiversity. Future Med. Chem. 2017, 9, 1631–1648. [Google Scholar] [CrossRef]
- Staunton, J.; Weissman, K.J. Polyketide biosynthesis: A millennium review. Nat. Prod. Rep. 2001, 18, 380–416. [Google Scholar] [CrossRef]
- Xu, D.X.; Sun, P.; Kurtan, T.; Mandi, A.; Tang, H.; Liu, B.; Gerwick, W.H.; Wang, Z.W.; Zhang, W. Polyhydroxy cyclohexanols from a Dendrodochium sp. fungus associated with the sea cucumber Holothuria nobilis Selenka. J. Nat. Prod. 2014, 77, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Pavesi, C.; Flon, V.; Mann, S.; Leleu, S.; Prado, S.; Franck, X. Biosynthesis of azaphilones: A review. Nat. Prod. Rep. 2021, 38, 1058–1071. [Google Scholar] [CrossRef] [PubMed]
- Schlager, S.; Drager, B. Exploiting plant alkaloids. Curr. Opin. Biotechnol. 2016, 37, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Desgagné-Penix, I. Distribution of alkaloids in woody plants. Plant Sci. Today 2017, 4, 137–142. [Google Scholar] [CrossRef]
- Zotchev, S.B. Alkaloids from marine bacteria. Adv. Bot. Res. 2013, 68, 301–333. [Google Scholar] [CrossRef]
- Souza, C.R.M.; Bezerra, W.P.; Souto, J.T. Marine alkaloids with anti-inflammatory activity: Current knowledge and future perspectives. Mar. Drugs 2020, 18, 147. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, W.; Guo, Q.; Yu, W.; Zhang, Y.; He, B. Bioactivities and future perspectives of Chaetoglobosins. J. Evid. Based Complement. Altern. Med. 2020, 2020, 8574084. [Google Scholar] [CrossRef]
- Wang, F.-Z.; Li, D.-H.; Zhu, T.-J.; Zhang, M.; Gu, Q.-Q. Pseurotin A1 and A2, two new 1-oxa-7-azaspiro[4.4]non-2-ene-4,6-diones from the holothurian-derived fungus Aspergillus fumigatus WFZ-25. Can. J. Chem. 2011, 89, 72–76. [Google Scholar] [CrossRef]
- Kim, S.K.; Li, Y.X. Biological activities and health effects of terpenoids from marine fungi. Adv. Food Nutr. Res. 2012, 65, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Gozari, M.; Alborz, M.; El-Seedi, H.R.; Jassbi, A.R. Chemistry, biosynthesis and biological activity of terpenoids and meroterpenoids in bacteria and fungi isolated from different marine habitats. Eur. J. Med. Chem. 2021, 210, 112957. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Xia, X.K.; Jia, A.R.; Liu, X.; Zhang, M.S.; Liu, C.H. Separation and preparation of chemical components from sea cucmber-derived fungus Epicoccum sp. by two-dimensional high-throughput chromatography. Shandong Sci. 2015, 28, 14–18, 24. (In Chinese) [Google Scholar] [CrossRef]
- Xia, X.; Qi, J.; Liu, Y.; Jia, A.; Zhang, Y.; Liu, C.; Gao, C.; She, Z. Bioactive isopimarane diterpenes from the fungus, Epicoccum sp. HS-1, associated with Apostichopus japonicus. Mar. Drugs 2015, 13, 1124–1132. [Google Scholar] [CrossRef]
- Berrue, F.; McCulloch, M.W.; Kerr, R.G. Marine diterpene glycosides. Bioorg. Med. Chem. 2011, 19, 6702–6719. [Google Scholar] [CrossRef] [PubMed]
- Afiyatullov, S.S.; Kalinovsky, A.I.; Kuznetsova, T.A.; Isakov, V.V.; Pivkin, M.V.; Dmitrenok, P.S.; Elyakov, G.B. New diterpene glycosides of the fungus Acremonium striatisporum isolated from a sea cucumber. J. Nat. Prod. 2002, 65, 641–644. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Kalinovsky, A.I.; Kuznetsova, T.A.; Pivkin, M.V.; Prokof’eva, N.G.; Dmitrenok, P.S.; Elyakov, G.B. New glycosides of the fungus Acremonium striatisporum isolated from a sea cucumber. J. Nat. Prod. 2004, 67, 1047–1051. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Kalinovsky, A.I.; Pivkin, M.V.; Dmitrenok, P.S.; Kuznetsova, T.A. New diterpene glycosides of the fungus Acremonium striatisporum isolated from a sea cucumber. Nat. Prod. Res. 2006, 20, 902–908. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Kalinovsky, A.I.; Antonov, A.S. New Virescenosides from the marine-derived fungus Acremonium striatisporum. Nat. Prod. Commun. 2011, 6, 1063–1068. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Antonov, A.S.; Oleinikova, G.K.; Khudyakova, Y.V.; Popov, R.S.; Denisenko, V.A.; Pislyagin, E.A.; Chingizova, E.A.; Afiyatullov, S.S. Virescenosides from the Holothurian-associated fungus Acremonium striatisporum Kmm 4401. Mar. Drugs 2019, 17, 616. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, J.T.; Kellermann, M.Y.; Kock, M.; Putra, M.Y.; Murniasih, T.; Mohr, K.I.; Wink, J.; Praditya, D.F.; Steinmann, E.; Schupp, P.J. Anti-Infective and antiviral activity of valinomycin and its analogues from a sea cucumber-associated bacterium, Streptomyces sp. SV 21. Mar. Drugs 2021, 19, 81. [Google Scholar] [CrossRef] [PubMed]






| Sea Cucumbers | Microorganism Genera | References | |||
|---|---|---|---|---|---|
| Family | Genus | Species | Bacteria | Fungi | |
| Cucumariidae | Cucumaria | japonica | 0 | 2 | [20,36] |
| Holothuriidae | Holothuria | atra | 2 | 0 | [30] |
| edulis | 1 | 0 | [18] | ||
| leucospilota | 36 | 0 | [4,19,25,31] | ||
| nobilis | 0 | 3 | [35,37,38] | ||
| poli | 0 | 16 | [22] | ||
| Sclerodactylidae | Eupentacta | fraudatrix | 0 | 13 | [20,21] |
| Stichopodidae | Apostichopus | japonicus | 54 | 12 | [6,17,20,23,24,29,32,33,34,39,40] |
| Stichopus | badionotus | 6 | 0 | [26] | |
| chloronotus | 3 | 0 | [31] | ||
| japonicus | 0 | 1 | [41,42] | ||
| vastus | 15 | 0 | [25] | ||
| Kingdom | Phylum | Class | Family | Genus | References |
|---|---|---|---|---|---|
| Bacteria | Actinobacteria | Acidimicrobiia | Iamiaceae | Iamia | [18] |
| Actinomycetia | Brevibacteriaceae | Brevibacterium | [23,25] | ||
| Corynebacteriaceae | Corynebacterium | [25] | |||
| Dermabacteraceae | Brachybacterium | [6] | |||
| Dermacoccaceae | Dermacoccus | [25] | |||
| Dietziaceae | Dietzia | [25] | |||
| Gordoniaceae | Williamsia | [24] | |||
| Intrasporangiaceae | Janibacter | [25] | |||
| Kytococcaceae | Kytococcus | [25,31] | |||
| Microbacteriaceae | Microbacterium | [6,32] | |||
| Micrococcaceae | Glutamicibacter | [6,25] | |||
| Kocuria | [25] | ||||
| Micrococcus | [4,6,24,25,31,33] | ||||
| Rothia | [24,25,31] | ||||
| Nocardioidaceae | Nocardioides | [25] | |||
| Nocardiopsaceae | Nocardiopsis | [4,6,17] | |||
| Oerskoviaceae | Paraoerskovia | [4] | |||
| Ornithinimicrobiaceae | Ornithinimicrobium | [25] | |||
| Serinicoccus | [25] | ||||
| Promicromonosporaceae | Cellulosimicrobium | [6,25] | |||
| Isoptericola | [25] | ||||
| Propionibacteriaceae | Pseudopropionibacterium | [25] | |||
| Streptomycetaceae | Streptomyces | [6,17,19,25] | |||
| Bacteroidetes | Cytophagia | Cytophagaceae | Cytophaga | [24] | |
| Flavobacteriia | Flavobacteriaceae | Flavobacterium | [33] | ||
| Lacinutrix | [24] | ||||
| Maribacter | [24] | ||||
| Psychroserpens | [24] | ||||
| Ulvibacter | [24] | ||||
| Winogradskyella | [24] | ||||
| Zobellia | [24] | ||||
| Firmicutes | Bacilli | Bacillaceae | Bacillus | [4,6,17,24,25,30,31,32,33] | |
| Geomicrobium | [4,17] | ||||
| Gracilibacillus | [4,17] | ||||
| Halobacillus | [4,6,17] | ||||
| Halolactibacillus | [17] | ||||
| Oceanobacillus | [4,17] | ||||
| Salsuginibacillus | [17] | ||||
| Virgibacillus | [4,6,17] | ||||
| Planococcaceae | Lysinibacillus | [17] | |||
| Planococcus | [26] | ||||
| Sporosarcina | [4,17] | ||||
| Staphylococcaceae | Staphylococcus | [4,25] | |||
| Unidentified | Exiguobacterium | [26,31] | |||
| Proteobacteria | Alphaproteobacteria | Ahrensiaceae | Ahrensia | [24] | |
| Erythrobacteraceae | Erythrobacter | [25] | |||
| Rhizobiaceae | Agrobacterium | [24] | |||
| Rhodobacteraceae | Epibacterium | [25] | |||
| Marinosulfonomonas | [24] | ||||
| Octadecabacter | [24] | ||||
| Paracoccus | [25] | ||||
| Roseobacter | [24] | ||||
| Ruegeria | [4] | ||||
| Sphingomonadaceae | Sphingomonas | [24,26] | |||
| Stappiaceae | Pseudovibrio | [17] | |||
| Betaproteobacteria | Comamonadaceae | Acidovorax | [24] | ||
| Gammaproteobacteria | Aeromonadaceae | Aeromonas | [33] | ||
| Oceanisphaera | [32] | ||||
| Alteromonadaceae | Alteromonas | [24] | |||
| Colwelliaceae | Colwellia | [24] | |||
| Enterobacteriaceae | Enterobacter | [33] | |||
| Klebsiella | [30] | ||||
| Erwiniaceae | Pantoea | [25] | |||
| Ferrimonadaceae | Ferrimonas | [17] | |||
| Halomonadaceae | Halomonas | [4,33] | |||
| Idiomarinaceae | Pseudidiomarina | [32] | |||
| Lysobacteraceae | Stenotrophomonas | [31] | |||
| Moraxellaceae | Acinetobacter | [25,32] | |||
| Psychrobacter | [24,25,26] | ||||
| Oceanospirillaceae | Marinobacterium | [32] | |||
| Marinomonas | [24,32] | ||||
| Pseudoalteromonadaceae | Pseudoalteromonas | [4,17,24,26,32,33,34] | |||
| Pseudomonadaceae | Pseudomonas | [6,17,24,25,31,32,33] | |||
| Psychromonadaceae | Psychromonas | [24] | |||
| Shewanellaceae | Shewanella | [4,6,24,32] | |||
| Vibrionaceae | Aliivibrio | [24] | |||
| Photobacterium | [4] | ||||
| Vibrio | [4,6,24,25,26,31,32,33] | ||||
| Fungi | Ascomycota | Dothideomycetes | Cladosporiaceae | Cladosporium | [20,22] |
| Didymellaceae | Epicoccum | [20,40,43] | |||
| Pleosporaceae | Alternaria | [20,22,27,28] | |||
| Ulocladium | [20] | ||||
| Saccotheciaceae | Aureobasidium | [22] | |||
| Torulaceae | Dendryphiella | [20] | |||
| Eurotiomycetes | Aspergillaceae | Aspergillus | [20,22,35,36,39,41,42] | ||
| Emericella | [22] | ||||
| Paecilomyces | [22] | ||||
| Penicillium | [20,22] | ||||
| Onygenaceae | Auxarthron | [22] | |||
| Leotiomycetes | Myxotrichaceae | Oidiodendron | [20] | ||
| Ploettnerulaceae | Cadophora | [22] | |||
| Sclerotiniaceae | Botryophialophora | [20] | |||
| Sordariomycetes | Bionectriaceae | Dendrodochium | [37] | ||
| Cephalothecaceae | Phialemonium | [38] | |||
| Chaetomiaceae | Chaetomium | [20,22,29] | |||
| Cordycipitaceae | Beauveria | [20] | |||
| Hypocreaceae | Acrostalagmus | [22] | |||
| Trichoderma | [20,22,44] | ||||
| Nectriaceae | Fusarium | [45] | |||
| Plectosphaerellaceae | Verticillium | [20] | |||
| Stachybotryaceae | Stachybotrys | [22] | |||
| Tilachlidiaceae | Tilachlidium | [20] | |||
| Unidentified | Acremonium | [20,21,22] | |||
| Unidentified | Myrothecium | [22] | |||
| Unidentified | Stilbella | [20] | |||
| Unidentified | Unidentified | Myriodontium | [22] | ||
| Unidentified | Unidentified | Phialophorophoma | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wang, X.-Y.; Liu, R.-Z.; Wang, G.-Y. Culturable Microorganisms Associated with Sea Cucumbers and Microbial Natural Products. Mar. Drugs 2021, 19, 461. https://doi.org/10.3390/md19080461
Chen L, Wang X-Y, Liu R-Z, Wang G-Y. Culturable Microorganisms Associated with Sea Cucumbers and Microbial Natural Products. Marine Drugs. 2021; 19(8):461. https://doi.org/10.3390/md19080461
Chicago/Turabian StyleChen, Lei, Xiao-Yu Wang, Run-Ze Liu, and Guang-Yu Wang. 2021. "Culturable Microorganisms Associated with Sea Cucumbers and Microbial Natural Products" Marine Drugs 19, no. 8: 461. https://doi.org/10.3390/md19080461
APA StyleChen, L., Wang, X.-Y., Liu, R.-Z., & Wang, G.-Y. (2021). Culturable Microorganisms Associated with Sea Cucumbers and Microbial Natural Products. Marine Drugs, 19(8), 461. https://doi.org/10.3390/md19080461






