A Method of Solubilizing and Concentrating Astaxanthin and Other Carotenoids
Abstract
:1. Introduction
2. Results and Discussion
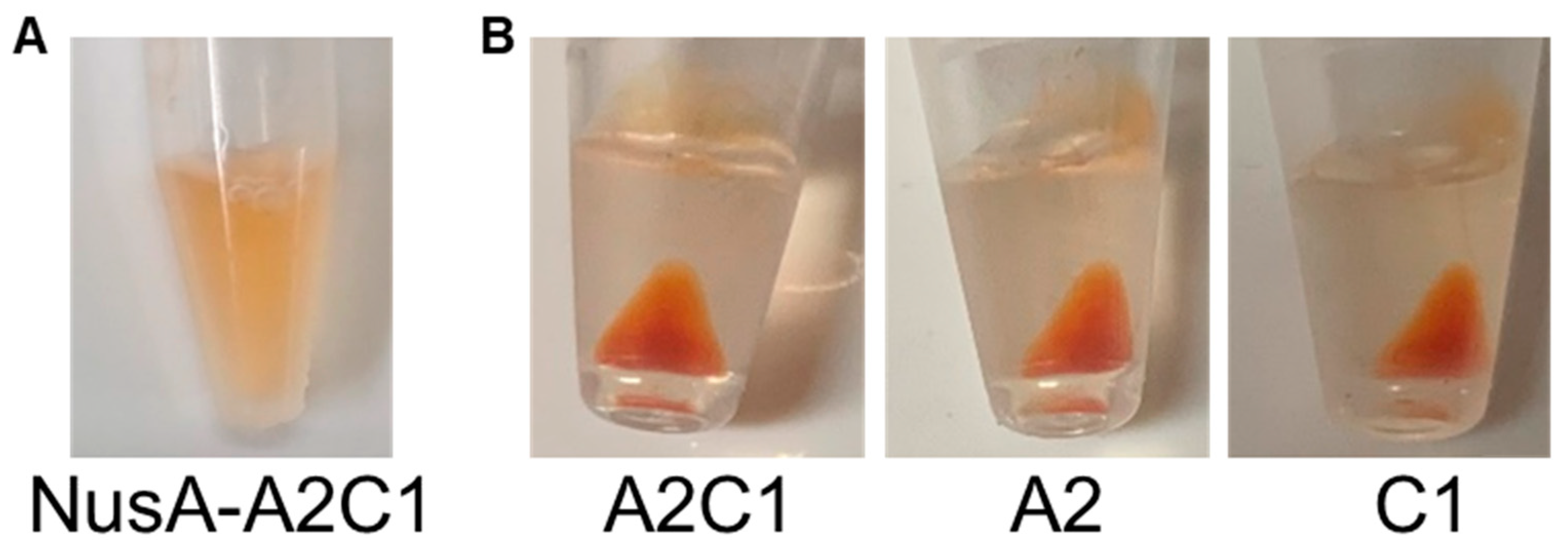
2.1. Optimization of Crustacyanin Preparation
2.2. Carotenoid Recovery by Insoluble Crustacyanin
3. Materials and Methods
3.1. Strains, Plasmids and Transformation
3.2. Preparation of Soluble NusA-Crustacyanin
3.3. Preparation of NusA-free Crustacyanin
3.4. Concentrating Carotenoids Using NusA-free Crustacyanin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Vachali, P.; Bhosale, P.; Bernstein, P.S. Microbial carotenoids. Methods Mol. Biol. 2012, 898, 41–59. [Google Scholar]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 12. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Hara, K.Y.; Morita, T.; Nishimura, A.; Sasaki, D.; Ishii, J.; Ogino, C.; Kizaki, N.; Kondo, A. Enhancement of astaxanthin production in Xanthophyllomyces dendrorhous by efficient method for the complete deletion of genes. Microb. Cell Fact. 2016, 15, 155. [Google Scholar] [CrossRef] [Green Version]
- Gervasi, T.; Santini, A.; Daliu, P.; Salem, A.Z.; Gervasi, C.; Pellizzeri, A.V.; Salem, Z.M.; Gervasi, C.; Pellizzeri, V.; Barrega, L.; et al. Astaxanthin production by Xanthophyllomyces dendrorhous growing on a low cost substrate. Agroforest Syst. 2020, 94, 1229–1234. [Google Scholar] [CrossRef]
- Villegas-Méndez, M.Á.; Aguilar-Machado, D.E.; Balagurusamy, N.; Montañez, J.; Morales-Oyervides, L. Agro-industrial wastes for the synthesis of carotenoids by Xanthophyllomyces dendrorhous: Mesquite pods-based medium design and optimization. Biochem. Eng. J. 2019, 150, 107260. [Google Scholar] [CrossRef]
- Korumilli, T.; Mishra, S.; Korukonda, J.R. Production of astaxanthin by Xanthophyllomyces dendrorhous on fruit waste extract and optimization of key parameters using Taguchi method. J. Biochem. Technol. 2020, 11, 25. [Google Scholar]
- Satoh, A.; Tsuji, S.; Okada, Y.; Murakami, N.; Urami, M.; Nakagawa, K.; Ishikura, M.; Katagiri, M.; Koga, Y.; Shirasawa, T. Preliminary clinical evaluation of toxicity and efficacy of a new astaxanthin-rich Haematococcus pluvialis extract. J. Clin. Biochem. Nutr. 2009, 44, 280–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, D.N.; Jena, G.B. Intervention of astaxanthin against cyclophosphamide-induced oxidative stress and DNA damage: A study in mice. Chem.-Biol. Interact. 2009, 180, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.Y.; Morita, T.; Endo, Y.; Mochizuki, M.; Araki, M.; Kondo, A. Evaluation and screening of efficient promoters to improve astaxanthin production in Xanthophyllomyces dendrorhous. Appl. Microbiol. Biotechnol. 2014, 98, 6787–6793. [Google Scholar] [CrossRef] [PubMed]
- Panis, G.; Rosales, C.J. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal. Res. 2016, 18, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Brunner, G.J. Supercritical fluids: Technology and application to food processing. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Valderrama, J.O.; Perrut, M.; Majewski, W. Extraction of astaxantine and phycocyanine from microalgae with supercritical carbon dioxide. J. Chem. Eng. Data 2003, 48, 827–830. [Google Scholar] [CrossRef]
- Nobre, B.; Marcelo, F.; Passos, R.; Beiro, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Perrut, M. Supercritical fluid applications: Industrial developments and economic issues. Ind. Eng. Chem. Res. 2000, 39, 4531–4535. [Google Scholar] [CrossRef]
- Pan, J.L.; Wang, H.M.; Chen, C.Y.; Chang, J.S. Extraction of astaxanthin from Haematococcus pluvialis by supercritical carbon dioxide fluid with ethanol modifier. Eng. Life Sci. 2012, 12, 638–647. [Google Scholar] [CrossRef]
- Todd, R.; Baroutian, S.J. A techno-economic comparison of subcritical water, supercritical CO2 and organic solvent extraction of bioactives from grape marc. J. Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef]
- Park, J.Y.; Oh, Y.K.; Choi, S.A.; Kim, M.C. Recovery of astaxanthin-containing oil from Haematococcus pluvialis by nano-dispersion and oil partitioning. Appl. Biochem. Biotechnol. 2020, 190, 1304–1318. [Google Scholar] [CrossRef] [PubMed]
- Wald, G.; Nathanson, N. Crustacyanin, the blue carotenoid-protein of the lobster shell. Biol. Bull. 1948, 95, 249. [Google Scholar]
- Chayen, N.E.; Cianci, M.; Grossmann, J.G.; Habash, J.; Helliwell, J.R.; Nneji, G.A.; Raftery, J.; Rizkallah, P.J.; Zagalsky, P.F. Unravelling the structural chemistry of the colouration mechanism in lobster shell. Acta Crystallogr. 2003, 59, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Folli, C.; Pincolini, E.; McClintock, T.S.; Rössle, M.; Berni, R.; Cianci, M. Structural characterization of recombinant crustacyanin subunits from the lobster Homarus americanus. Acta Crystallogr. 2012, 68, 846–853. [Google Scholar]
- Davis, G.D.; Elisee, C.; Newham, D.M.; Harrison, R.G. New fusion protein systems designed to give soluble expression in Escherichia coli. Biotechnol. Bioeng. 1999, 65, 382–388. [Google Scholar] [CrossRef]
- Hara, K.Y.; Kageyama, Y.; Tanzawa, N.; Hirono-Hara, Y.; Kikukawa, H.; Wakabayashi, K. Development of astaxanthin production from citrus peel extract using Xanthophyllomyces dendrorhous. Environ. Sci. Pollut. Res. 2021, 28, 12640–12647. [Google Scholar] [CrossRef] [PubMed]
- Kikukawa, H.; Okaya, T.; Maoka, T.; Miyazaki, M.; Murofushi, K.; Kato, T.; Hirono-Hara, Y.; Katsumata, M.; Miyahara, S.; Hara, K.Y. Carotenoid Nostoxanthin production by Sphingomonas sp. SG73 isolated from deep sea sediment. Mar. Drugs 2021, 19, 274. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, K.Y.; Yagi, S.; Hirono-Hara, Y.; Kikukawa, H. A Method of Solubilizing and Concentrating Astaxanthin and Other Carotenoids. Mar. Drugs 2021, 19, 462. https://doi.org/10.3390/md19080462
Hara KY, Yagi S, Hirono-Hara Y, Kikukawa H. A Method of Solubilizing and Concentrating Astaxanthin and Other Carotenoids. Marine Drugs. 2021; 19(8):462. https://doi.org/10.3390/md19080462
Chicago/Turabian StyleHara, Kiyotaka Y., Shuwa Yagi, Yoko Hirono-Hara, and Hiroshi Kikukawa. 2021. "A Method of Solubilizing and Concentrating Astaxanthin and Other Carotenoids" Marine Drugs 19, no. 8: 462. https://doi.org/10.3390/md19080462
APA StyleHara, K. Y., Yagi, S., Hirono-Hara, Y., & Kikukawa, H. (2021). A Method of Solubilizing and Concentrating Astaxanthin and Other Carotenoids. Marine Drugs, 19(8), 462. https://doi.org/10.3390/md19080462






