Exploitation of Marine Molecules to Manage Alzheimer’s Disease
Abstract
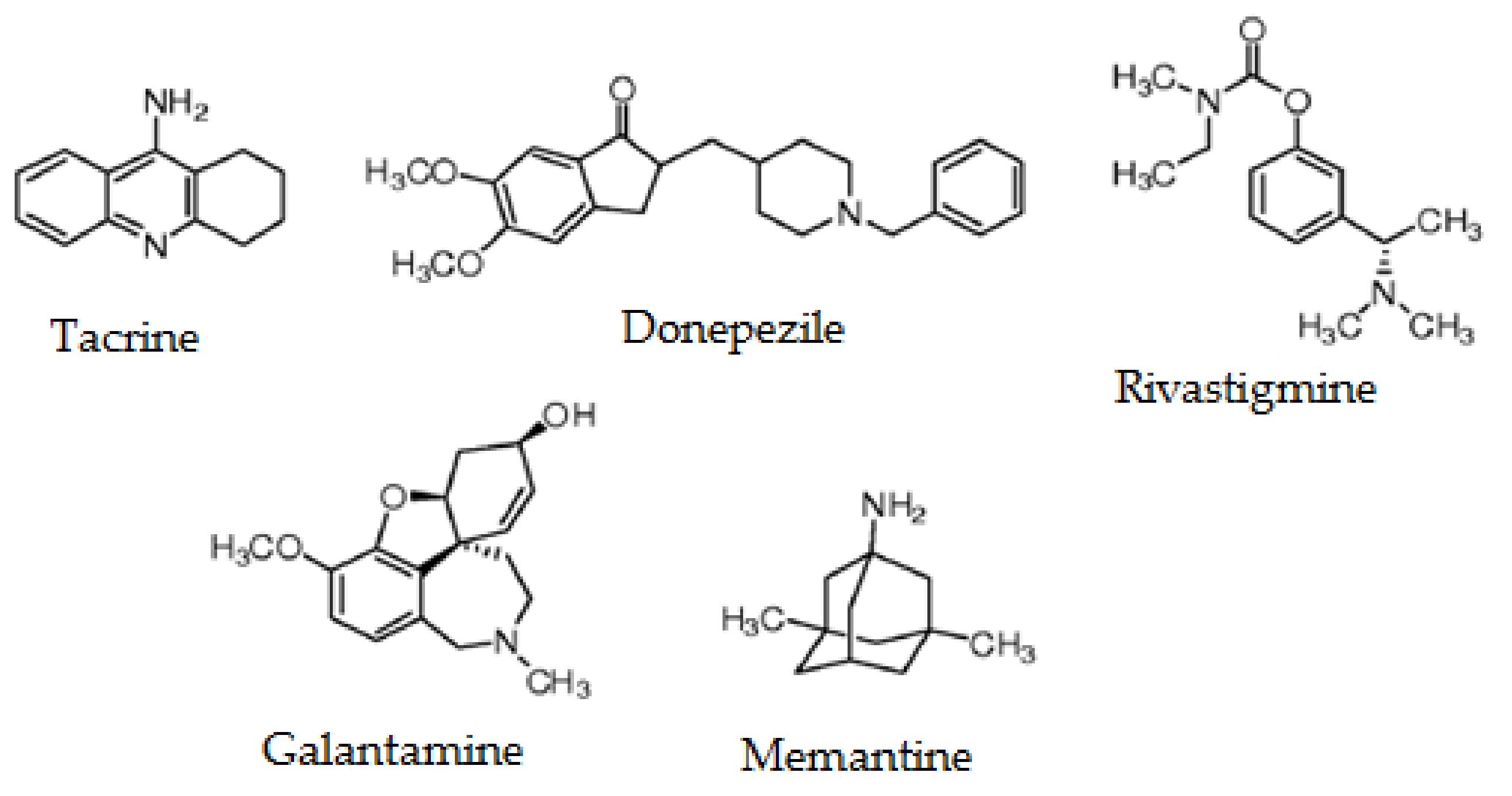
:1. Introduction
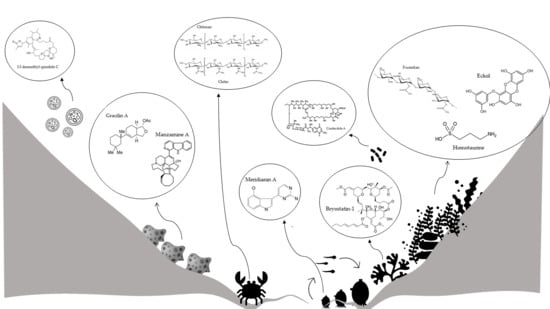
2. Marine Organisms Producing Compounds for Neuroprotection
2.1. Bacterioplankton
2.2. Marine Microalgae
Marine Macroalgae
2.3. Marine Sponges
2.4. Marine Tunicates
2.5. Marine Arthropods
3. Marine Compounds against Alzheimer’s Disease: From Sea to Cells
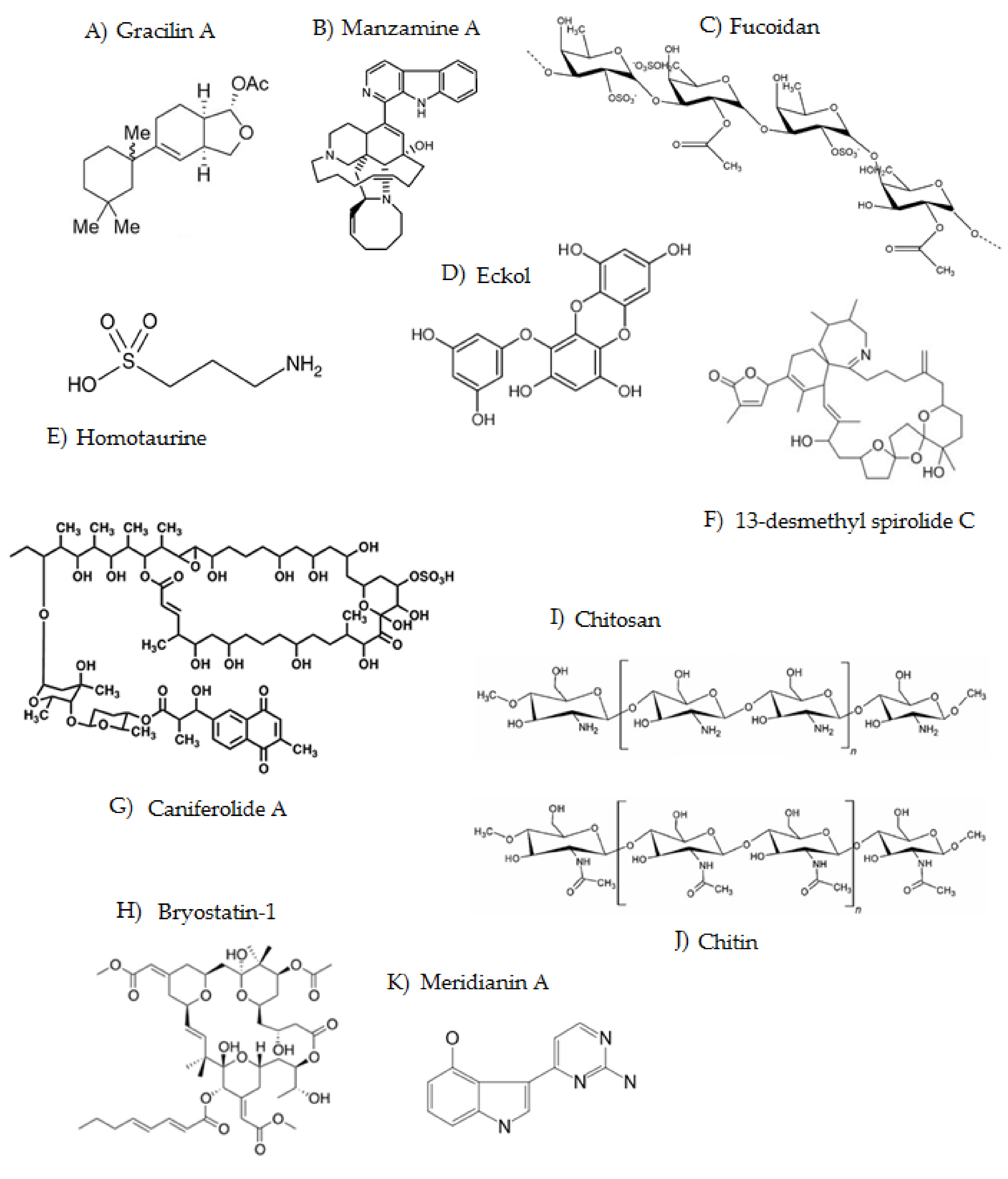
3.1. Gracilins
3.2. Manzamines
3.3. Fucoidan
3.4. Phlorotannins
3.5. Homotaurine
3.6. Spirolides
3.7. Caniferolide A
3.8. Bryostatins
3.9. Chitosan
3.10. Meridianins
4. Isolation of High-Value Molecules with Neuroprotective Effects against Alzheimer’s Disease
4.1. Gracilins
4.2. Manzamines
4.3. Fucoidan
4.4. Phlorotannin
4.5. Homotaurine
4.6. Spirolides
4.7. Caniferolide
4.8. Bryostatins
4.9. Chitosan
4.10. Meridianins
| Compound | Specie | Extraction/Isolation Method | Quantity | Yield (%) | Ref. |
|---|---|---|---|---|---|
| Source: Sponges | |||||
| Gracillin J | Sponginella sp. (Philippines) (5 g) | S-L extraction: water, MeOH/CH2Cl2 (1:1). Clean-up: Sephadex LH-20 column. Purification: reversed-phase HPLC (M.P.: MeCN/H2O, at 1.25 mL/min, 50 min). | 9 mg | 0.18% | [124] |
| Gracillin K | 11 mg | 0.22% | [124] | ||
| Gracillin L | 17 mg | 0.34% | [124] | ||
| Gracillin H and analog | 115 mg | 2.3% | [124] | ||
| Manzamine A | Acanthostrongylophora ingens (1.3 kg) | S-L extraction: MeOH. L-L partitioning: EtOAc and n-BuOH. Clean-up: Diaion HP-20; Sephadex LH-20. Purification: semipreparative reversed-phase HPLC. M.P.: MeOH/H2O/0.1% TFA. | 5 mg | 0.00038% | [153,154] |
| 8-Hydroxymanzamine A | 5 mg | 0.00038% | [153,154] | ||
| Manzamine F | 9 mg | 0.00069% | [153,154] | ||
| manzamine A N-oxide | 1.1 mg | 0.000085% | [153,154] | ||
| 3,4-Dihydromanzamine A N-oxide | 1.1 mg | 0.000085% | [153,154] | ||
| Source: Algae | |||||
| Fucoidan | Sargassum siliquosum | Pretreatment: 95% ethanol, 4 h. S-L extraction: H2O, 100 °C, 1 h. MAE, UAE. EtOH precipitation. Purification: anionic-exchange chromatography, dialysis, | 35 mg/g | 3.5% | [157] |
| Fucoidan | Nizamuddinia zanardinii | 85% EtOH, RT, overnight. UAE: H2O, 55 °C, 20 kHz, 200 W, x2.CaCl2 precipitation, EtOH precipitation. | 35.1 mg/g | 3.51% | [83] |
| Fucoidan | Spirarea japonica | PLE: 0.1% NaOH, 140 °C, 50 bar. CaCl2 precipitation, EtOH precipitation. | 11 mg/g | 1.1% | [158] |
| Eckol, dieckol, dioxinodehydroeckol | Sargassum fusiforme | S-L extraction: ethanol 30% S/L ratio of 1:5) at 25 °C, 30 min. Ethyl acetate partitioning. | 63.61 mg PGE/g | 6.36% | [165] |
| Fucofuropentaphlorethol, pentafuhalol, tetrafucotetraphloretol, and PD | Fucus vesiculosus | S-L extraction: acetone 67% (v/v) at 25 °C. L/S ratio of 70 mL/g. | 2.92 mg PGE/g DS | 0.29% | [166] |
| Dibenzodioxine-1,3,6,8-tetrao, pentafucol, hexafucol, and PD | Fucus vesiculosus | MAE (ethanol 57% (v/v), of 75 °C, 5 min. | 9.8 mg PGE/g DW | 0.98% | [171] |
| Phlorotannins | Fucus vesiculosus | UAE (35 kHz, 30 min, 50% ethanol). | 7.73 mg PGE/g | 0.77% | [172] |
| Hydroxytetrafuhalol, triphlorethol, dihydroxypentafuhalol | Sargassum muticuma | PLE (60 °C and 95% ethanol). | 5.018 mg PGE/g | 0.5% | [167] |
| Eckmaxol | Ecklonia maxima (0.3 kg) | Sephadex LH-20 size-exclusion chromatography. HSCCC using n-hexane/ethyl acetate/methanol/water (2:8:3:7, v/v/v/v). | 5.2 mg | 0.0017% | [168] |
| Homotaurine | Botryocladia leptopoda (1 g) | S-L extraction: 10 mL ethanol 70–80% (v/v). Centrifugation (4000 rpm, 10 min). Collect the supernatant. | 32.3 µg/g | 0.0032% | [173,174] |
| Homotaurine | Gelidium micropterum (1 g) | 32.5 µg/g | 0.0032% | [173,174] | |
| Homotaurine | Caulerpa racemosa (1 g) | 10.56 µg/g | 0.001% | [173,174] | |
| Homotaurine | Cystoseira indica (1 g) | 5.05 µg/g | 0.0005% | [173,174] | |
| Homotaurine | Hypnea boergesenii (1 g) | 702.7 µg/g | 0.0702% | [173,174] | |
| Homotaurine | Gracilaria corticata (1 g) | 474.9 µg/g | 0.0474% | [173,174] | |
| Homotaurine | Gracilaria pygmaea (1 g) | 333.5 µg/g | 0.0333% | [173,174] | |
| Homotaurine | Sargassum tenerrimum (1 g) | S-L extraction: 10 mL ethanol 70% (v/v). | 6.54 µg/g | 0.0006% | [74] |
| Homotaurine | Dyctiota dichotoma (1 g) | 14.62 µg/g | 0.0014% | [74] | |
| Homotaurine | Gracilaria arcuata (1 g) | 6.48 µg/g | 0.0006% | [74] | |
| 13-Desmethyl spirolide C and | Alexandrium ostenfeldii (24 cultures of 20 or 40 L) | S-L extraction: MeOH. L-L extraction: CH2Cl-H2O. Clean up: Sephadex LH-20. Purification: HPLC preparative: Vydac column 201TP C18, F.M. acetonitrile/water (30:70) + 0.1% TFA. | 150 µg | - | [180] |
| 13,19-Didesmethyl spirolide C | 1 mg | - | [180] | ||
| Source: Bacteria | |||||
| Caniferolides A | Streptomyces caniferus CA-271066 (2.3 g appox) | Pretreatment: acetone. L-L extraction: ethyl acetate/water. Clean-up: Sephadex LH-20. Purification: Semipreparative reversed-phase HPLC (Agilent Zorbax RX-C8), M.P.: CH3CN/H2O. Semipreparative reversed-phase HPLC (XBridge C-18) (only for caniferolide D). | 10.0 mg | 0.43% | [25,181] |
| Caniferolides B | 3.6 mg | 0.16% | [25,181] | ||
| Caniferolides C | 4 mg | 0.17% | [25,181] | ||
| Caniferolides D | 1 mg | 0.043% | [25,181] | ||
| Caniferolide C | Streptomyces sp. ISID311 (1 L of culture) crude extract (2.3 g) | Pretreatment: acetone. L-L extraction: ethyl acetate/water. Clean-up: SPE-C18 (55 μm, 20 g). Purification: semipreparative HPLC. | 14.2 mg | 0.62% | [182] |
| Crustaceans | |||||
| Chitosan | Shell power (1 g) | Demineralization: 1 M HCl at 60 °C (30 min). Deproteinization: 3 M NaOH at 80 °C (120 min). | 350 mg | 35% | [185,186] |
| Meridianins A–G | Aplidium falklandicum and Aplidium meridianum | Clean-up: Sephadex LH-20 and silica gel columns. Purification: with TLC using preparative (SiO2) plates and HPLC (reversed-phase semipreparative C18 columns). | 19.11 mg/g DW | 1.91% | [148] |
5. Challenges and Opportunities in the Exploitation of Marine Molecules to Manage Alzheimer’s Disease
6. Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association Report. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G. Treatment Development for Alzheimer’s Disease: How Are We Doing? In GeNeDis; Vlamos, P., Ed.; Springer International Publishing: Cham, Switzerland, 2018; ISBN 9783030326326. [Google Scholar]
- Sanabria-Castro, A.; Alvarado-Echeverría, I.; Monge-Bonilla, C. Molecular pathogenesis of Alzheimer’s disease: An update. Ann. Neurosci. 2017, 24, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Adriaanse, S.M.; Binnewijzend, M.A.A.; Ossenkoppele, R.; Tijms, B.M.; Van Der Flier, W.M.; Koene, T.; Smits, L.L.; Wink, A.M.; Scheltens, P.; Van Berckel, B.N.M.; et al. Widespread disruption of functional brain organization in early-onset Alzheimer’s disease. PLoS ONE 2014, 9, e102995. [Google Scholar] [CrossRef]
- Guerchet, M.; Prina, M.; Prince, M. Policy Brief for Heads of Government: The Global Impact of Dementia 2013–2050; Alzheimer’s Disease International (ADI): London, UK, 2013; pp. 1–8. [Google Scholar]
- Agarwal, M.; Alam, M.R.; Haider, M.K.; Malik, M.Z.; Kim, D.K. Alzheimer’s disease: An overview of major hypotheses and therapeutic options in nanotechnology. Nanomaterials 2021, 11, 59. [Google Scholar] [CrossRef]
- Singh, S.K.; Srivastav, S.; Yadav, A.K.; Srikrishna, S.; Perry, G. Overview of Alzheimer’s Disease and Some Therapeutic Approaches Targeting Aβ by Using Several Synthetic and Herbal Compounds. Oxid. Med. Cell. Longev. 2016, 2016, 7361613. [Google Scholar] [CrossRef] [Green Version]
- Ricciarelli, R.; Fedele, E. The Amyloid Cascade Hypothesis in Alzheimer’s Disease: It’s Time to Change Our Mind. Curr. Neuropharmacol. 2017, 15, 926–935. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The role of NMDA receptors in Alzheimer’s disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s disease mitochondrial cascade hypothesis: Progress and perspectives. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 1219–1231. [Google Scholar] [CrossRef] [Green Version]
- Nizynski, B.; Dzwolak, W.; Nieznanski, K. Amyloidogenesis of Tau protein. Protein Sci. 2017, 26, 2126–2150. [Google Scholar] [CrossRef] [Green Version]
- De Barry, J.; Liégeois, C.M.; Janoshazi, A. Protein kinase C as a peripheral biomarker for Alzheimer’s disease. Exp. Gerontol. 2010, 45, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Musiek, E.S.; Holtzman, D.M. Three dimensions of the amyloid hypothesis: Time, space and “wingmen”. Nat. Neurosci. 2015, 18, 800–806. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Esiri, M.M.; Chance, S.A. Cognitive reserve, cortical plasticity and resistance to Alzheimer’s disease. Alzheimer’s Res. Ther. 2012, 4, 7. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [Green Version]
- Conway, M.E. Alzheimer’s disease: Targeting the glutamatergic system. Biogerontology 2020, 21, 257–274. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Gao, Z.; Zheng, L.; Zhang, C.; Liu, Z.; Yang, Y.; Teng, H.; Hou, L.; Yin, Y.; Zou, X. Protective effects of fucoidan on Aβ25-35 and D-gal-induced neurotoxicity in PC12 cells and D-gal-induced cognitive dysfunction in mice. Mar. Drugs 2017, 15, 77. [Google Scholar] [CrossRef] [Green Version]
- Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Marine biocompounds for neuroprotection—A review. Mar. Drugs 2020, 18, 290. [Google Scholar] [CrossRef]
- Rahman, M.A.; Dash, R.; Sohag, A.A.M.; Alam, M.; Rhim, H.; Ha, H.; Moon, I.S.; Uddin, M.J.; Hannan, M.A. Prospects of Marine Sterols against Pathobiology of Alzheimer’s Disease: Pharmacological Insights and Technological Advances. Mar. Drugs 2021, 19, 167. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-inflammatory activities of marine algae in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef] [Green Version]
- Alvarino, R.; Alonso, E.; Lacret, R.; Oves-Costales, D.; Genilloud, O.; Reyes, F.; Alfonso, A.; Botana, L.M. Caniferolide A, a Macrolide from Streptomyces caniferus, Attenuates Neuroinflammation, Oxidative Stress, Amyloid-Beta, and Tau Pathology in Vitro. Mol. Pharm. 2019, 16, 1456–1466. [Google Scholar] [CrossRef]
- Alonso, E.; Otero, P.; Vale, C.; Alfonso, A.; Antelo, A.; Giménez-llort, L.; Chabaud, L.; Guillou, C.; Botana, L.M. Benefit of 13-desmethyl Spirolide C Treatment in Triple Transgenic Mouse Model of Alzheimer Disease: Beta-Amyloid and Neuronal Markers Improvement. Curr. Alzheimer Res. 2013, 10, 279–289. [Google Scholar] [CrossRef]
- Leirós, M.; Alonso, E.; Rateb, M.E.; Houssen, W.E.; Ebel, R.; Jaspars, M.; Alfonso, A.; Botana, L.M. Gracilins: Spongionella-derived promising compounds for Alzheimer disease. Neuropharmacology 2015, 93, 285–293. [Google Scholar] [CrossRef]
- Ciccone, L.; Shi, C.; di Lorenzo, D.; Van Baelen, A.-C.; Tonali, N. The Positive Side of the Alzheimer’s Disease Amyloid Cross-Interactions: The Case of the Aβ 1-42 Peptide with Tau, TTR, CysC, and ApoA1. Molecules 2020, 25, 2439. [Google Scholar] [CrossRef]
- Kovacs, G.G. Chapter 25—Tauopathies. Handb. Clin. Neurol. 2018, 145, 355–368. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Q.; Jiang, T.; Li, S.; Ye, J.; Zheng, J.; Wang, X.; Liu, Y. A novel small-molecule PROTAC selectively promotes tau clearance to improve cognitive functions in Alzheimer-like models. Theranostics 2021, 11, 5279–5295. [Google Scholar] [CrossRef]
- Barzkar, N.; Jahromi, S.T.; Poorsaheli, H.B.; Vianello, F. Metabolites from Marine Microorganisms, Micro, and Macroalgae: Immense Scope for Pharmacology. Mar. Drugs 2019, 17, 464. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.; Silva, R.; Pinto, M.M.M. Marine Natural Products, Multitarget Therapy and Repurposed Agents in Alzheimer’s Disease. Pharmaceuticals 2020, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, L.; Vandooren, J.; Nencetti, S. Natural Marine and Terrestrial Compounds as Modulators of Matrix Metalloproteinases-2 (MMP-2) and MMP-9 in Alzheimer’s Disease. Pharmaceuticals 2021, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Jo, M. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenical, W. Chemical Studies of Marine Bacteria: Developing a New Resource. Chem. Rev. 1993, 93, 1673–1683. [Google Scholar] [CrossRef]
- Whitehead, R. Natural product chemistry. Annu. Rep. Prog. Chem. Sect. B 1999, 1999, 183–205. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzerseand, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, R.K.; Butman, C.A. Chemical Signaling Processes in the Marine Environment. Biol. Bull. 2000, 198, 168–187. [Google Scholar] [CrossRef]
- Rateb, M.E.; Houssen, W.E.; Schumacher, M.; Harrison, W.T.A.; Diederich, M.; Ebel, R.; Jaspars, M. Bioactive diterpene derivatives from the marine sponge Spongionella sp. J. Nat. Prod. 2009, 72, 1471–1476. [Google Scholar] [CrossRef]
- Kumar, N.; Hovik, A. Extremophiles: Applications and roles in environmental sustainability. Environ. Sustain. 2019, 2, 217–218. [Google Scholar] [CrossRef] [Green Version]
- Zubkov, M.V. Faster growth of the major prokaryotic versus eukaryotic CO2 fixers in the oligotrophic ocean. Nat. Commun. 2014, 5, 3776. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, L.G.E.; Leray, M.; O’Dea, A.; Yuen, B.; Peixoto, R.S.; Pereira, T.J.; Bik, H.M.; Coil, D.A.; Duffy, J.E.; Herre, E.A.; et al. Host-associated microbiomes drive structure and function of marine ecosystems. PLoS Biol. 2019, 17, e3000533. [Google Scholar] [CrossRef] [Green Version]
- Siddharth, S.; Vittal, R. Evaluation of Antimicrobial, Enzyme Inhibitory, Antioxidant and Cytotoxic Activities of Partially Purified Volatile Metabolites of Marine Streptomyces sp.S2A. Microorganisms 2018, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- Stafsnes, M.H.; Josefsen, K.D.; Kildahl-Andersen, G.; Valla, S.; Ellingsen, T.E.; Bruheim, P. Isolation and characterization of marine pigmented bacteria from Norwegian coastal waters and screening for carotenoids with UVA-blue light absorbing properties. J. Microbiol. 2010, 48, 16–23. [Google Scholar] [CrossRef]
- Skovgaard, N. Industrial Microbiology: An Introduction; Wiley-Blackwell: Hoboken, NJ, USA, 2002; Volume 77, ISBN 0632053070. [Google Scholar]
- Raimundo, I.; Silva, S.G.; Costa, R.; Keller-Costa, T. Bioactive secondary metabolites from octocoral-Associated microbes—New chances for blue growth. Mar. Drugs 2018, 16, 485. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.N.; Wang, K.L.; Wu, Z.H.; Tian, R.M.; Liu, G.Z.; Xu, Y. Biological and chemical diversity of bacteria associated with a marine flatworm. Mar. Drugs 2017, 15, 281. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, G.; Ma, W.; Lu, Z.; Sun, C. Marine bacterial polysaccharide EPS11 inhibits cancer cell growth and metastasis via blocking cell adhesion and attenuating filiform structure formation. Mar. Drugs 2019, 17, 50. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, E.; Boyd, K.G.; Pisacane, A.; Peppiatt, C.J.; Burgess, J.G. Marine microbial natural products in antifouling coatings. Biofouling 2000, 16, 215–224. [Google Scholar] [CrossRef]
- Rizzo, C.; Michaud, L.; Hörmann, B.; Gerçe, B.; Syldatk, C.; Hausmann, R.; De Domenico, E.; Lo Giudice, A. Bacteria associated with sabellids (Polychaeta: Annelida) as a novel source of surface active compounds. Mar. Pollut. Bull. 2013, 70, 125–133. [Google Scholar] [CrossRef]
- Qian, P.Y.; Li, Z.; Xu, Y.; Li, Y.; Fusetani, N. Mini-review: Marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling 2015, 31, 101–122. [Google Scholar] [CrossRef]
- Park, S.Y.; Yang, D.; Ha, S.H.; Lee, S.Y. Metabolic Engineering of Microorganisms for the Production of Natural Compounds. Adv. Biosys. 2018, 2, 1700190. [Google Scholar] [CrossRef] [Green Version]
- Hjort, K.; Bergström, M.; Adesina, M.F.; Jansson, J.K.; Smalla, K.; Sjöling, S. Chitinase genes revealed and compared in bacterial isolates, DNA extracts and a metagenomic library from a phytopathogen-suppressive soil. FEMS Microbiol. Ecol. 2010, 71, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Van Elsas, J.D.; Costa, R.; Jansson, J.; Sjöling, S.; Bailey, M.; Nalin, R.; Vogel, T.M.; van Overbeek, L. The metagenomics of disease-suppressive soils—Experiences from the METACONTROL project. Trends Biotechnol. 2008, 26, 591–601. [Google Scholar] [CrossRef]
- Sieg, R.D.; Poulson-Ellestad, K.L.; Kubanek, J. Chemical ecology of the marine plankton. Nat. Prod. Rep. 2011, 28, 388–399. [Google Scholar] [CrossRef]
- Tesson, S.V.M.; Skjøth, A.; Šantl-Temkiv, T. Airborne Microalgae: Insights, Opportunities, and Challenges. Appl. Environ. Microbiol. 2016, 82, 1978–1991. [Google Scholar] [CrossRef] [Green Version]
- Rajvanshi, S.; Sharma, M.P. Micro Algae: A Potential Source of Biodiesel. J. Sustain. Bioenergy Syst. 2012, 2, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive compounds from marine bacteria and fungi: Minireview. Microb. Biotechnol. 2010, 3, 544–563. [Google Scholar] [CrossRef] [Green Version]
- Camacho, F.G.; Rodríguez, J.G.; Mirón, A.S.; García, M.C.C.; Belarbi, E.H.; Chisti, Y.; Grima, E.M. Biotechnological significance of toxic marine dinoflagellates. Biotechnol. Adv. 2007, 25, 176–194. [Google Scholar] [CrossRef]
- Kiuru, P.; Valeria D’Auria, M.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef]
- Abida, H.; Ruchaud, S.; Rios, L.; Humeau, A.; Probert, I.; De Vargas, C.; Bach, S.; Bowler, C. Bioprospecting marine plankton. Mar. Drugs 2013, 11, 4594–4611. [Google Scholar] [CrossRef] [Green Version]
- Otero, P.; Saha, S.K.; Gushin, J.M.; Moane, S.; Barron, J.; Murray, P. Identification of optimum fatty acid extraction methods for two different microalgae Phaeodactylum tricornutum and Haematococcus pluvialis for food and biodiesel applications. Anal. Bioanal. Chem. 2017, 409, 4659–4667. [Google Scholar] [CrossRef]
- Gupta, N.; Pal, M.; Sachdeva, M.; Yadav, M.; Tiwari, A. Thermophilic biohydrogen production for commercial application: The whole picture. Int. J. Energy Res. 2016, 40, 127–145. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Rohit, M.V.; Chiranjeevi, P.; Chandra, R.; Navaneeth, B. Heterotrophic microalgae cultivation to synergize biodiesel production with waste remediation: Progress and perspectives. Bioresour. Technol. 2015, 184, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Scherholz, M.L.; Curtis, W.R. Achieving pH control in microalgal cultures through fed-batch addition of stoichiometrically-balanced growth media. BMC Biotechnol. 2013, 13, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aburai, N.; Sumida, D.; Abe, K. Effect of light level and salinity on the composition and accumulation of free and ester-type carotenoids in the aerial microalga Scenedesmus sp. (Chlorophyceae). Algal Res. 2015, 8, 30–36. [Google Scholar] [CrossRef]
- Ribalet, F.; Wichard, T.; Pohnert, G.; Ianora, A.; Miralto, A.; Casotti, R. Age and nutrient limitation enhance polyunsaturated aldehyde production in marine diatoms. Phytochemistry 2007, 68, 2059–2067. [Google Scholar] [CrossRef]
- Gong, Y.; Guo, X.; Wan, X.; Liang, Z.; Jiang, M. Triacylglycerol accumulation and change in fatty acid content of four marine oleaginous microalgae under nutrient limitation and at different culture ages. J. Basic Microbiol. 2013, 53, 29–36. [Google Scholar] [CrossRef]
- De Morais, M.G.; Vaz, B.D.S.; De Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. Biomed. Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [Green Version]
- Sasso, S.; Pohnert, G.; Lohr, M.; Mittag, M.; Hertweck, C. Microalgae in the postgenomic era: A blooming reservoir for new natural products. FEMS Microbiol. Rev. 2012, 36, 761–785. [Google Scholar] [CrossRef]
- Manoylov, K.M. Taxonomic identification of algae (morphological and molecular): Species concepts, methodologies, and their implications for ecological bioassessment. J. Phycol. 2014, 50, 409–424. [Google Scholar] [CrossRef]
- Osundeko, O.; Ansolia, P.; Gupta, S.K.; Bag, P.; Bajhaiya, A.K. Promises and challenges of growing microalgae in wastewater. In Water Conservation, Recycling and Reuse: Issues and Challenges; Springer: Singapore, 2019; pp. 29–53. [Google Scholar] [CrossRef]
- Motuhi, S.E.; Mehiri, M.; Payri, C.E.; La Barre, S.; Bach, S. Marine Natural Products from New Caledonia-A Review. Mar. Drugs 2016, 14, 58. [Google Scholar] [CrossRef] [Green Version]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenasa, I.; Florou-Paneria, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef]
- Leal, M.C.; Munro, M.H.G.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Rosa, R.; Madeira, C. Biogeography and biodiscovery hotspots of macroalgal marine natural products. Nat. Prod. Rep. 2013, 30, 1380–1390. [Google Scholar] [CrossRef]
- Patra, J.K.; Lee, S.W.; Park, J.G.; Baek, K.H. Antioxidant and Antibacterial Properties of Essential Oil Extracted from an Edible Seaweed Undaria Pinnatifida. J. Food Biochem. 2017, 41, e12278. [Google Scholar] [CrossRef]
- Collins, K.G.; Fitzgerald, G.F.; Stanton, C.; Ross, R.P. Looking beyond the terrestrial: The potential of seaweed derived bioactives to treat non-communicable diseases. Mar. Drugs 2016, 14, 60. [Google Scholar] [CrossRef] [Green Version]
- Duarte, K.; Justino, C.I.L.; Pereira, R.; Freitas, A.C.; Gomes, A.M.; Duarte, A.C.; Rocha-Santos, T.A.P. Green analytical methodologies for the discovery of bioactive compounds from marine sources. Trends Environ. Anal. Chem. 2014, 3, 43–52. [Google Scholar] [CrossRef]
- Sivagnanam, S.P.; Yin, S.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Biological properties of fucoxanthin in oil recovered from two brown seaweeds using supercritical CO2 extraction. Mar. Drugs 2015, 13, 3422–3442. [Google Scholar] [CrossRef]
- Rodrigues, D.; Sousa, S.; Silva, A.; Amorim, M.; Pereira, L.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C.; Freitas, A.C. Impact of enzyme- and ultrasound-assisted extraction methods on biological properties of red, brown, and green seaweeds from the Central West Coast of Portugal. J. Agric. Food Chem. 2015, 63, 3177–3188. [Google Scholar] [CrossRef]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Microwave-hydrothermal extraction and degradation of fucoidan from supercritical carbon dioxide deoiled Undaria pinnatifida. Ind. Eng. Chem. Res. 2013, 52, 7940–7946. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Rittà, M.; Donalisio, M.; Mariatti, F.; You, S.; Lembo, D.; Cravotto, G. Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 124, 131–137. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Bursać Kovačević, D.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef] [Green Version]
- Otero, P.; López-Martínez, M.I.; García-Risco, M.R. Application of pressurized liquid extraction (PLE) to obtain bioactive fatty acids and phenols from Laminaria ochroleuca collected in Galicia (NW Spain). J. Pharm. Biomed. Anal. 2019, 164, 86–92. [Google Scholar] [CrossRef]
- Otero, P.; Quintana, S.E.; Reglero, G.; Fornari, T.; García-Risco, M.R. Pressurized Liquid Extraction (PLE) as an innovative green technology for the effective enrichment of galician algae extracts with high quality fatty acids and antimicrobial and antioxidant properties. Mar. Drugs 2018, 16, 156. [Google Scholar] [CrossRef] [Green Version]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Bell, J.J. The functional roles of marine sponges. Estuar. Coast. Shelf Sci. 2008, 79, 341–353. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Sponges and Their Role in the Marine Environment. 2017. Available online: http://www.fao.org/3/i7775e/i7775e.pdf (accessed on 31 May 2021).
- Balian, E.V.; Lévêque, C.; Segers, H.; Martens, K. (Eds.) Fresh Water Animal Diversity Asses; Springer: Dordrecht, The Netherlands, 2008; ISBN 9781402082580. [Google Scholar]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral Lead Compounds from Marine Sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef] [Green Version]
- Balian, E.V.; Segers, H.; Lévèque, C.; Martens, K. The Freshwater Animal Diversity Assessment: An overview of the results. Hydrobiologia 2008, 595, 627–637. [Google Scholar] [CrossRef]
- Lagos, R.; Tello, M.; Mercado, G.; Garcia, V.; Monasterio, O. Antibacterial and Antitumorigenic Properties of Microcin E492, a Pore- Forming Bacteriocin. Curr. Pharm. Biotechnol. 2009, 10, 74–85. [Google Scholar] [CrossRef]
- Laport, M.; Santos, O.; Muricy, G. Marine Sponges: Potential Sources of New Antimicrobial Drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef]
- Getachew, A.T.; Jacobsen, C.; Holdt, S.L. Emerging technologies for the extraction of marine phenolics: Opportunities and challenges. Mar. Drugs 2020, 18, 389. [Google Scholar] [CrossRef]
- Hotta, K.; Mitsuhara, K.; Takahashi, H.; Inaba, K.; Oka, K.; Gojobori, T.; Ikeo, K. A web-based interactive developmental table for the Ascidian Ciona intestinalis, including 3D real-image embryo reconstructions: I. From fertilized egg to hatching larva. Dev. Dyn. 2007, 236, 1790–1805. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Dong, B. Origins and Bioactivities of Natural Compounds Derived from Marine Ascidians and Their Symbionts. Mar. Drugs 2019, 17, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2001, 18, 1R–49R. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, S. Environmental assessment of a large industrial marine complex based on a community of benthic filter-feeders. Mar. Pollut. Bull. 2002, 44, 605–610. [Google Scholar]
- Mcfall-Ngai, M.; Had, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Lo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, J.M.; Clardy, J.; Crawford, J.M.; Clardy, J. ChemComm Bacterial symbionts and natural products a tripartite aggregate with biological. Chem. Commun. 2011, 47, 7559–7566. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, E.W. The secret to a successful relationship: Lasting chemistry between ascidians and their symbiotic bacteria. Invertebr. Biol. 2015, 134, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Palanisamy, S.K.; Morabito, R.; Remigante, A.; Spanò, N.; La Spada, G.; Giacobbe, S.; Marino, A. Biological activity of extract from Styela plicata and Ascidia mentula (Ascidiacea). J. Biol. Res. 2016, 89. [Google Scholar] [CrossRef] [Green Version]
- Stark, J.L.; Powers, R. Application of NMR and Molecular Docking in Structure-based Drug Discovery. In NMR of Proteins and Small Biomolecules; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–34. [Google Scholar] [CrossRef]
- Plainchont, B.; Nuzillard, J. Structure verification through computer- assisted spectral assignment of NMR spectra. Magn. Reson. Chem. 2013, 51, 54–59. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based plant metabolomics: Where do we stand, where do we go? Trends Biotechnol. 2011, 29, 267–275. [Google Scholar] [CrossRef]
- Ghafor, I.M. Book chapter: Crustaceans. In Crustacea; Diarte-Plata, G., Escamilla-Montes, R., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.A.; De Oliveira, A.R.M. Melanosis in crustaceans: A review. LWT Food Sci. Technol. 2016, 65, 791–799. [Google Scholar] [CrossRef]
- The State of World Fisheries and Aquaculture 2018 (SOFIA): Meeting the Sustainable Development Goals; Food & Agriculture Organization: Rome, Italy, 2018; ISBN 9789251305621.
- Ling, Z.; Bangzhong, Y.I.N.; Qi, L.I.U.; Rong, C.A.O. Purification of Antimicrobial Peptide from Antarctic Krill (Euphausia superba) and its Function Mechanism. J. Ocean Univ. China 2013, 12, 484–490. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Lodhi, G.; Kim, Y.S.; Hwang, J.W.; Kim, S.K.; Jeon, Y.J.; Je, J.Y.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Park, P.J. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef] [Green Version]
- Hamann, M.; Alonso, D.; Martín-Aparicio, E.; Fuertes, A.; Pérez-Puerto, M.J.; Castro, A.; Morales, S.; Navarro, M.L.; del Monte-millán, M.; Medina, M.; et al. Glycogen Synthase Kinase-3 (GSK-3) Inhibitory Activity and Structure–Activity Relationship (SAR) Studies of the Manzamine Alkaloids. Potential for Alzheimer’s Disease. J. Nat. Prod. 2007, 70, 1397–1405. [Google Scholar] [CrossRef]
- Cowan, C.M.; Thai, J.; Krajewski, S.; Reed, J.C.; Nicholson, D.W.; Kaufmann, S.H.; Roskams, A.J. Caspases 3 and 9 send a pro-apoptotic signal from synapse to cell body in olfactory receptor neurons. J. Neurosci. 2001, 21, 7099–7109. [Google Scholar] [CrossRef]
- Hannan, A.; Dash, R.; Haque, N. Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Mar. Drugs 2020, 18, 347. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.H.; Uddin, M.S.; Kim, I.S.; Choi, D.K. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef]
- Alonso, E.; Vale, C.; Vieytes, M.R.; Laferla, F.M.; Giménez-llort, L.; Botana, L.M. Neurochemistry International 13-Desmethyl spirolide-C is neuroprotective and reduces intracellular Aβ and hyperphosphorylated tau in vitro. Neurochem. Int. 2011, 59, 1056–1065. [Google Scholar] [CrossRef]
- Russo, P.; Kisialiou, A.; Lamonaca, P.; Moroni, R.; Prinzi, G.; Fini, M. New Drugs from Marine Organisms in Alzheimer’s Disease. Mar. Drugs 2016, 14, 5. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Alkon, D.L. Bryostatin-1: Pharmacology and Therapeutic Potential as a CNS Drug. CNS Drug Rev. 2006, 12, 1–8. [Google Scholar] [CrossRef]
- Wilkinson, D.G.; Francis, P.T.; Schwam, E.; Payne-Parrish, J. Cholinesterase inhibitors used in the treatment of Alzheimer’s disease: The relationship between pharmacological effects and clinical efficacy. Drugs Aging 2004, 21, 453–478. [Google Scholar] [CrossRef]
- Llorach-Pares, L.; Nonell-Canals, A.; Sanchez-Martinez, M.; Avila, C. Computer-aided drug design applied to marine drug discovery: Meridianins as Alzheimer’s disease therapeutic agents. Mar. Drugs 2017, 15, 366. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.-Y.; Choi, H. Natural products from marine organisms with neuroprotective activity in the experimental models of Alzheimer’s disease, Parkinson’s disease and ischemic brain stroke: Their molecular targets and action mechanisms. Arch. Pharm. Res. 2015, 38, 139–170. [Google Scholar] [CrossRef]
- Mayol, L.; Piccialli, V.; Sica, D. Gracilin A, an unique nor-diterpe metabolite from the marine sponge spongionella gracilis. Tetrahedron Lett. 1985, 26, 1357–1360. [Google Scholar] [CrossRef]
- Abbasov, M.E.; Alvariño, R.; Chaheine, C.M.; Alonso, E.; Jon, A.; Conner, M.L.; Alfonso, A.; Jaspars, M.; Botana, L.M.; Romo, D. Simplified immunosuppressive and neuroprotective agents based on gracilin A. Nat. Chem. 2019, 11, 342–350. [Google Scholar] [CrossRef]
- Leirós, M.; Alonso, E.; Rateb, M.E.; Ebel, R.; Jaspars, M.; Alfonso, A.; Botana, L.M. The Streptomyces metabolite anhydroexfoliamycin ameliorates hallmarks of Alzheimer’s disease in vitro and in vivo. Neuroscience 2015, 305, 26–35. [Google Scholar] [CrossRef]
- Gegunde, S.; Alfonso, A.; Alonso, E.; Alvariño, R.; Botana, L.M. Gracilin-Derivatives as Lead Compounds for Anti-inflammatory Effects. Cell. Mol. Neurobiol. 2020, 40, 603–615. [Google Scholar] [CrossRef]
- Radwan, M.; Hanora, A.; Khalifa, S.; Abou-El-Ela, S.H. Manzamines: A potential for novel cures. Cell Cycle 2012, 11, 1765–1772. [Google Scholar] [CrossRef] [Green Version]
- Althagbi, H.I.; Alarif, W.M.; Al-Footy, K.O.; Abdel-Lateff, A. Marine-Derived Macrocyclic Alkaloids (MDMAs): Chemical and Biological Diversity. Mar. Drugs 2020, 18, 368. [Google Scholar] [CrossRef]
- Karan, D.; Dubey, S.; Pirisi, L.; Nagel, A.; Pina, I.; Choo, Y.; Hamann, M.T. The Marine Natural Product Manzamine A Inhibits Cervical Cancer by Targeting the SIX1 Protein. J. Nat. Prod. 2020, 83, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef] [Green Version]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhang, Q.; Wang, H.; Cui, Y.; Sun, Z.; Yang, J.; Zheng, Y.; Jia, J.; Yu, F.; Wang, X.; et al. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 2009, 617, 33–40. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, N.; Youn, K.; Jo, M.R.; Kim, H.; Lee, D.; Ho, C.; Jun, M. Dieckol Ameliorates Aβ Production via PI3K/Akt/GSK-3β Regulated APP Processing in SweAPP N2a Cell. Mar. Drugs 2021, 19, 152. [Google Scholar] [CrossRef]
- Caltagirone, C.; Ferrannini, L.; Marchionni, N.; Nappi, G.; Scapagnini, G.; Trabucchi, M. The potential protective effect of tramiprosate (homotaurine) against Alzheimer’s disease: A review. Aging Clin. Exp. Res. 2012, 24, 580–587. [Google Scholar] [CrossRef]
- Neuroscience, A.; Martorana, A.; Di Lorenzo, F.; Manenti, G.; Semprini, R.; Koch, G. Homotaurine induces measurable changes of short latency afferent inhibition in a group of mild cognitive impairment individuals. Front. Aging Neurosci. 2014, 6, 254. [Google Scholar] [CrossRef]
- Manzano, S.; Agüera, L.; Aguilar, M.; Olazarán, J. A Review on Tramiprosate (Homotaurine) in Alzheimer’s Disease and Other Neurocognitive Disorders. Front. Neurol. 2020, 11, 614. [Google Scholar] [CrossRef]
- Otero, P.; Alfonso, A.; Vieytes, M.R.; Cabado, A.G.; Vieites, J.M.; Botana, L.M. Effects of environmental regimens on the toxin profile of Alexandrium ostenfeldii. Environ. Toxicol. Chem. 2010, 29, 301–310. [Google Scholar] [CrossRef]
- Otero, P.; Alfonso, A.; Rodríguez, P.; Rubiolo, J.A.; Manuel, J.; Bermúdez, R.; Vieytes, M.R.; Botana, L.M. Pharmacokinetic and toxicological data of spirolides after oral and intraperitoneal administration. FOOD Chem. Toxicol. 2012, 50, 232–237. [Google Scholar] [CrossRef]
- Fonfría, E.S.; Vilariño, N.; Molgó, J.; Aráoz, R.; Otero, P.; Espiña, B.; Louzao, M.C.; Alvarez, M.; Botana, L.M. Detection of 13,19-didesmethyl C spirolide by fluorescence polarization using Torpedo electrocyte membranes. Anal. Biochem. 2010, 403, 102–107. [Google Scholar] [CrossRef]
- Otero, P.; Alfonso, A.; Alfonso, C.; Aráoz, R.; Molgó, J.; Vieytes, M.R.; Botana, L.M. Analytica Chimica Acta First direct fluorescence polarization assay for the detection and quantification of spirolides in mussel samples. Anal. Chim. Acta 2011, 701, 200–208. [Google Scholar] [CrossRef]
- Aldholmi, M.; Marchand, P.; Ourliac-garnier, I.; Le Pape, P.; Ganesan, A. A Decade of Antifungal Leads from Natural Products: 2010–2019. Pharmaceuticals 2019, 12, 182. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Chen, H.; Chang, N.; Xu, Y.; Jiao, J. Unlocking the Drug Potential of the Bryostatin Family: Recent Advances in Product Synthesis and Biomedical Applications. Chem. Eur. J. 2020, 26, 1166–1195. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Tsvetkov, Y.E.; Paulovičová, E.; Paulovičová, L.; Farkaš, P.; Nifantiev, N.E. Synthesis of Biotin-Tagged Chitosan Oligosaccharides and Assessment of Their Immunomodulatory Activity. Front. Chem. 2020, 8, 554732. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Neuroprotective properties of chitosan and its derivatives. Mar. Drugs 2010, 8, 2117–2128. [Google Scholar] [CrossRef]
- Gompel, M.; Leost, M.; Bal De Kier, J.E.; Puricelli, L.; Hernandez Franco, L.; Palermo, J.; Meijer, L. Meridianins, a new family of protein kinase inhibitors isolated from the Ascidian Aplidium meridianum. Bioorg. Med. Chem. Lett. 2004, 14, 1703–1707. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Carbone, M.; Vázquez, J.; Rodríguez, J.; Nieto, R.M.; Varela, M.M.; Gavagnin, M.; Avila, C. Natural products from antarctic colonial ascidians of the genera Aplidium and Synoicum: Variability and defensive role. Mar. Drugs 2012, 10, 1741–1764. [Google Scholar] [CrossRef] [Green Version]
- Zanqui, A.B.; da Silva, C.M.; Ressutte, J.B.; de Morais, D.R.; Santos, J.M.; Eberlin, M.N.; Cardozo-Filho, L.; da Silva, E.A.; Gomes, S.T.M.; Matsushita, M. Extraction and assessment of oil and bioactive compounds from cashew nut (Anacardium occidentale) using pressurized n-propane and ethanol as cosolvent. J. Supercrit. Fluids 2020, 157, 104686. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Dar, A.A.; Sangwan, P.L.; Kumar, A. Chromatography: An important tool for drug discovery. J. Sep. Sci. 2020, 43, 105–119. [Google Scholar] [CrossRef]
- Corey, E.J.; Letavic, M.A. Enantioselective Total Synthesis of Gracilins B and C Using Catalytic Asymmetric Diels-Alder Methodology. J. Am. Chem. Soc. 1995, 117, 9616–9617. [Google Scholar] [CrossRef]
- AlTarabeen, M.; Daletos, G.; Ebrahim, W.; Müller, W.E.G.; Hartmann, R.; Line, W.; Proksch, P. Ircinal E, a new manzamine derivative from the Indonesian marine sponge Acanthostrongylophora ingens. Nat. Prod. Commun. 2015, 10, 1951–1953. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.K.; Riswanto, R.; Won, T.H.; Kim, H.; Elya, B.; Sim, C.J.; Oh, D.C.; Oh, K.B.; Shin, J. Manzamine Alkaloids from an Acanthostrongylophora sp. Sponge. J. Nat. Prod. 2017, 80, 1575–1583. [Google Scholar] [CrossRef]
- Bittkau, K.S.; Neupane, S.; Alban, S. Initial evaluation of six different brown algae species as source for crude bioactive fucoidans. Algal Res. 2020, 45, 101759. [Google Scholar] [CrossRef]
- Jacobsen, C.; Sørensen, A.-D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, Extraction, Characterization, and Applications of Novel Antioxidants from Seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef]
- Wang, S.; Huang, C.; Chen, C.; Chang, C. Isolation and purification of brown algae fucoidan from Sargassum siliquosum and the analysis of anti-lipogenesis activity. Biochem. Eng. J. 2021, 165, 107798. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.; Park, Y.; Woo, H.; Chun, B. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef]
- Liu, X.; Liu, B.; Wei, X.; Shi, Y.; Jia, A.; Wang, C. Structural elucidation of fucoidans from Sargassum pallidum. J. Appl. Phycol. 2020, 33, 523–531. [Google Scholar] [CrossRef]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Liu, D.; Yin, J.Y.; Nie, S.P. Consecutive and progressive purification of food-derived natural polysaccharide: Based on material, extraction process and crude polysaccharide. Trends Food Sci. Technol. 2020, 99, 76–87. [Google Scholar] [CrossRef]
- Cikos, A.M.; Jokic, S.; Subaric, D.; Jerkovic, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M.A. By-Products of Agri-Food Industry as Tannin-Rich Sources: A Review of Tannins’ Biological Activities and Their Potential for Valorization. Foods 2021, 10, 137. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Cassani, L.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Chamorro, F.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Traditional applications of tannin rich extracts supported by scientific data: Chemical composition, bioavailability and bioaccessibility. Foods 2021, 10, 251. [Google Scholar] [CrossRef]
- Setchell, H. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Camargo, P.; Montero, L.; Stiger-Pouvreau, V.; Tanniou, A.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem. 2016, 192, 67–74. [Google Scholar] [CrossRef]
- Chromatography, H.C. Isolation and Purification of a Neuroprotective Phlorotannin from the Marine Algae Ecklonia maxima by Size Exclusion and High-Speed Counter-Current Chromatography. Mar. Drugs 2019, 17, 212. [Google Scholar] [CrossRef] [Green Version]
- Molino, S.; Casanova, N.A.; Rufián Henares, J.Á.; Fernandez Miyakawa, M.E. Natural Tannin Wood Extracts as a Potential Food Ingredient in the Food Industry. J. Agric. Food Chem. 2020, 68, 2836–2848. [Google Scholar] [CrossRef]
- Venkatesan, J.; Keekan, K.K.; Anil, S.; Bhatnagar, I.; Kim, S.-K. Phlorotannins. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 515–527. [Google Scholar] [CrossRef]
- Amarante, S.J.; Catarino, M.D.; Marçal, C.; Silva, A.M.S.; Ferreira, R.; Cardoso, S.M. Microwave-Assisted Extraction of Phlorotannins from Fucus vesiculosus. Mar. Drugs 2020, 18, 559. [Google Scholar] [CrossRef]
- Vázquez-Rodríguez, B.; Gutiérrez-Uribe, J.A.; Antunes-Ricardo, M.; Santos-Zea, L.; Cruz-Suárez, L.E. Ultrasound-assisted extraction of phlorotannins and polysaccharides from Silvetia compressa (Phaeophyceae). J. Appl. Phycol. 2020, 32, 1441–1453. [Google Scholar] [CrossRef]
- Gheytasi, F.; Gholami, M. Extraction and measurement of homotaurine in algae. Res. J. Pharmacogn. 2017, 4, 126. [Google Scholar]
- Mehdinia, A.; Rostami, S.; Dadkhah, S.; Fumani, N.S. Simultaneous screening of homotaurine and taurine in marine macro-algae using liquid chromatography–fluorescence detection. J. Iran. Chem. Soc. 2017, 14, 2135–2142. [Google Scholar] [CrossRef]
- Mehdinia, A.; Fumani, N.S.; Kayyal, T.B.; Ghaderiardakani, F. Homotaurine of marine macroalgae of the Persian Gulf as a potential treatment agent for Alzheimer. J. Persian Gulf 2018, 9, 1–8. [Google Scholar]
- Smith, A.F.; Silvano, E.; Päuker, O.; Guillonneau, R.; Quareshy, M.; Murphy, A.; Mausz, M.A.; Stirrup, R.; Rihtman, B.; Aguilo-Ferretjans, M.; et al. A novel class of sulfur-containing aminolipids widespread in marine roseobacters. ISME J. 2021. [Google Scholar] [CrossRef]
- Otero, P.; Vale, C.; Boente-Juncal, A.; Costas, C.; Carmen Louzao, M.; Botana, L.M. Detection of cyclic imine toxins in dietary supplements of green lipped mussels (Perna canaliculus) and in shellfish mytilus chilensis. Toxins 2020, 12, 613. [Google Scholar] [CrossRef]
- Otero, P.; Miguéns, N.; Rodríguez, I.; Botana, L.M. LC–MS/MS Analysis of the Emerging Toxin Pinnatoxin-G and High Levels of Esterified OA Group Toxins in Galician Commercial Mussels. Toxins 2019, 11, 394. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.; Barreiro, A.; Rodriguez, P.; Otero, P.; Azevedo, J. New Invertebrate Vectors for PST, Spirolides and Okadaic Acid in the North Atlantic. Mar. Drugs 2013, 11, 1936–1960. [Google Scholar] [CrossRef] [Green Version]
- Otero, P.; Alfonso, A.; Alfonso, C.; Vieytes, M.R.; Louzao, M.C.; Botana, A.M.; Botana, L.M. New protocol to obtain spirolides from Alexandrium ostenfeldii cultures with high recovery and purity. Biomed. Chromatogr. 2010, 24, 878–886. [Google Scholar] [CrossRef]
- Pérez-Victoria, I.; Oves-Costales, D.; Lacret, R.; Martín, J.; Sánchez-Hidalgo, M.; Díaz, C.; Cautain, B.; Vicente, F.; Reyes, F. Structure elucidation and biosynthetic gene cluster analysis of caniferolides A–D, new bioactive 36-membered macrolides from the marine-derived Streptomyces caniferus CA-271066. Org. Biomol. Chem. 2019, 17, 2954–2971. [Google Scholar] [CrossRef]
- Ortega, H.E.; Lourenzon, V.B.; Chevrette, M.G.; Ferreira, L.L.G.; Alvarenga, R.F.R.; Melo, W.G.P.; Venâncio, T.; Currie, C.R.; Andricopulo, A.D.; Bugni, T.S.; et al. Antileishmanial macrolides from ant-associated Streptomyces sp. ISID311. Bioorg. Med. Chem. 2021, 32, 116016. [Google Scholar] [CrossRef]
- Catanesi, M.; Caioni, G.; Castelli, V.; Benedetti, E.; Angelo, M.; Cimini, A. Benefits under the Sea: The Role of Marine Compounds in Neurodegenerative Disorders. Mar. Drugs 2021, 19, 24. [Google Scholar] [CrossRef]
- Albadrani, G.M.; Sayed, A.A.; Abdel-Daim, M.M.; Simal-Gandara, J. Anti-Alzheimer’s Molecules Derived from Marine Life: Understanding Molecular Mechanisms and Therapeutic Potential. Mar. Drugs 2021, 19, 251. [Google Scholar] [CrossRef]
- Achur, R.N. Isolation and characterization of chitin from Millipede (Spirobolida). J. Basic Appl. Zool. 2018, 79, 30. [Google Scholar] [CrossRef] [Green Version]
- Mohan, K.; Ravichandran, S.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Rajeevgandhi, C.; Karthick Rajan, D.; Seedevi, P. Extraction and characterization of chitin from sea snail Conus inscriptus (Reeve, 1843). Int. J. Biol. Macromol. 2019, 126, 555–560. [Google Scholar] [CrossRef]
- Ssekatawa, K.; Byarugaba, D.K.; Wampande, E.M.; Moja, T.N.; Nxumalo, E.; Maaza, M.; Sackey, J.; Ejobi, F.; Kirabira, J.B. Isolation and characterization of chitosan from Ugandan edible mushrooms, Nile perch scales and banana weevils for biomedical applications. Sci. Rep. 2021, 11, 4116. [Google Scholar] [CrossRef]
- Netz, N.; Opatz, T. Marine indole alkaloids. Mar. Drugs 2015, 13, 4814–4914. [Google Scholar] [CrossRef] [Green Version]
- Centre, M.B.; Biochemistry, P.; Castellino, V.P.; Tecnolo, P.; Zoologica, S.; Dohrn, A. The marine biodiscovery pipeline and ocean medicines of tomorrow. J. Mar. Biol. Assoc. UK 2016, 96, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Doh, K.-O.; Yeo, Y. Application of polysaccharides for surface modification of nanomedicines. Ther. Deliv. 2012, 3, 1447–1456. [Google Scholar] [CrossRef] [Green Version]
- Lindequist, U. Marine-Derived Pharmaceuticals—Challenges and Opportunities. Biomol. Ther. 2016, 24, 561–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoyo, S.; Plaza, M.; Jaime, L.; Ibañez, E.; Reglero, G.; Señorans, J. Pressurized liquids as an alternative green process to extract antiviral agents from the edible seaweed Himanthalia elongata. J. Appl. Phycol. 2011, 23, 909–917. [Google Scholar] [CrossRef] [Green Version]
- Vá, J.A.; Rodrí, I. Chondroitin Sulfate, Hyaluronic Acid and Chitin/Chitosan Production Using Marine Waste Sources: Characteristics, Applications and Eco-Friendly Processes: A Review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [Green Version]
- Anand, R.; Dip, K.; Ali, A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef]
- Noori, T.; Reza, A.; Sureda, A.; Sobarzo-Sanchez, E.; Shirooie, S. Role of natural products for the treatment of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 898, 173974. [Google Scholar] [CrossRef]
| Compound | Origen | Family | Mechanism of Action | Ref. |
|---|---|---|---|---|
| Sponges | ||||
| Gracilins | Marine sponges (Spongionella gracilis) | Diterpenoid derivatives | Inhibition of the enzyme b-secretase or BACE-1. Anti-inflammatory and antioxidant properties. Reduction in hyperphosphorylation of tau protein. | [39] |
| Manzamines | Marine sponges (Haliclona sp. | Alkaloids with beta-carboline structure | Inhibition of GSK3beta and CDK5. | [114] |
| Macroalgae and microalgae | ||||
| Fucoidans | Brown seaweeds | Sulfated polysaccharides | Block caspase-9 and caspase-3 enzymes. | [115] |
| Phlorotannins | Brown seaweeds (Ecklonia cava, Ecklonia stolonifera) | Polyphenols | Inhibition of the enzymes acetylcholinesterase and butyrylcholinesterase. | [116] |
| Homotaurine | Red seaweeds | Aminosulfonate | Prevention of the formation of a toxic soluble amyloid oligomer. | [117] |
| Spirolides | Alexandrium ostenfeldii/peruvianum dinoflagellates | Cyclic imines | Decrease GSK-3β and ERK in 3xTg mice cortical neurons. Glutamate-induced neurotoxicity inhibition both in control and 3xTg neurons. | [118] |
| Bacteria | ||||
| Caniferoles | Phylum Actinobacteria | Polyol macrolides | Anti-inflammatory and antioxidant action. Blockade of the BACE-1 enzyme. | [25] |
| Marine invertebrates, crustaceans, tunicates | ||||
| Bryostatins | Brown bryozoa (Bugula neritina) | Macrolide lactones | Modulates neuronal synapses under synaptic dysfunctions; improvement of memory, cognition, and spatial learning; decreases amyloid-beta peptide; reappearance of neurotrophic activity. | [119,120] |
| Chitosan | Crustaceans | Polysaccharides | Inhibition of the enzyme acetylcholinesterase. | [121] |
| Meridianins | Tunicates (Aplidium meridianum) | Alkaloid indols | Inhibition of GSK3beta, CK1sigma, DYRK1A, and CLK1. | [122,123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.; Seijas, P.; Otero, P. Exploitation of Marine Molecules to Manage Alzheimer’s Disease. Mar. Drugs 2021, 19, 373. https://doi.org/10.3390/md19070373
Silva M, Seijas P, Otero P. Exploitation of Marine Molecules to Manage Alzheimer’s Disease. Marine Drugs. 2021; 19(7):373. https://doi.org/10.3390/md19070373
Chicago/Turabian StyleSilva, Marisa, Paula Seijas, and Paz Otero. 2021. "Exploitation of Marine Molecules to Manage Alzheimer’s Disease" Marine Drugs 19, no. 7: 373. https://doi.org/10.3390/md19070373
APA StyleSilva, M., Seijas, P., & Otero, P. (2021). Exploitation of Marine Molecules to Manage Alzheimer’s Disease. Marine Drugs, 19(7), 373. https://doi.org/10.3390/md19070373