The Use of Invasive Algae Species as a Source of Secondary Metabolites and Biological Activities: Spain as Case-Study
Abstract
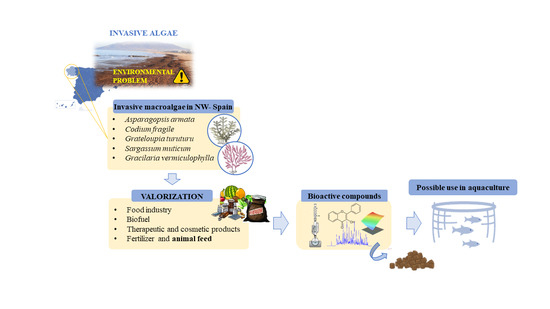
1. Introduction
2. Possible Exploitation of the Invasive Species
2.1. Food Industry
2.2. Biofuel
2.3. Therapeutic and Cosmetic Products
2.4. Fertilizer and Animal Feed
3. Main Invasive Species of Northwest Spain and Their Bioactive Compounds
3.1. Polysaccharides
3.2. Lipids
3.3. Proteins
3.4. Pigments
3.5. Vitamins
3.6. Phenolic Compounds
3.7. Other Minor Compounds
4. Current Strategies to Obtain Bioactive Compounds from Algae
4.1. Conventional Extraction Techniques
4.2. Novel Extraction Techniques
5. Algae as Supplement of Diets in Aquaculture
6. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Máximo, P.; Ferreira, L.M.; Branco, P.; Lima, P.; Lourenço, A. Secondary metabolites and biological activity of invasive macroalgae of southern Europe. Mar. Drugs 2018, 16, 265. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Shackleton, C.M.; Kull, C.A. The role of invasive alien species in shaping local livelihoods and human well-being: A review. J. Environ. Manag. 2019, 229, 145–157. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Cebrian, E.; Francour, P.; Galil, B.; Savini, D. Monitoring Marine Marine Protected in Mediterranean Invasive Species Areas (MPAs)—A Strategy and Practical Guide for Managers; IUCN: Malaga, Spain, 2013. [Google Scholar]
- Commision European. Invasive Alien Species of Union Concern; Commision European: Luxembourg, 2020. [Google Scholar]
- Milledge, J.J.; Nielsen, B.V.; Bailey, D. High-value products from macroalgae: The potential uses of the invasive brown seaweed, Sargassum muticum. Rev. Environ. Sci. Biotechnol. 2015, 15, 67–88. [Google Scholar] [CrossRef]
- Davoult, D.; Surget, G.; Stiger-Pouvreau, V.; Noisette, F.; Riera, P.; Stagnol, D.; Androuin, T.; Poupart, N. Multiple effects of a Gracilaria vermiculophylla invasion on estuarine mudflat functioning and diversity. Mar. Environ. Res. 2017, 131, 227–235. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Neugebauer, A.; Silva, J.; Thomas, O.P.; Botana, L.M.; Gaspar, H.; Pedrosa, R. Marine invasive macroalgae: Turning a real threat into a major opportunity—the biotechnological potential of Sargassum muticum and Asparagopsis armata. Algal Res. 2018, 34, 217–234. [Google Scholar] [CrossRef]
- Machmudah, S.; Diono, W.; Kanda, H.; Goto, M. Supercritical fluids extraction of valuable compounds from algae: Future perspectives and challenges. Eng. J. 2018, 22, 13–30. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Fernández-Segovia, I.; Lerma-García, M.J.; Fuentes, A.; Barat, J.M. Characterization of Spanish powdered seaweeds: Composition, antioxidant capacity and technological properties. Food Res. Int. 2018, 111, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zavaglia, A.; Prieto Lage, M.A.; Jiménez-López, C.; Mejuto, J.C.; Simal-Gándara, J. The Potential of Seaweeds as a Source of Functional Ingredients of Prebiotic and Antioxidant Value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef]
- Gomez, L.P.; Alvarez, C.; Zhao, M.; Tiwari, U.; Curtin, J.; Garcia-Vaquero, M.; Tiwari, B.K. Innovative processing strategies and technologies to obtain hydrocolloids from macroalgae for food applications. Carbohydr. Polym. 2020, 248, 116784. [Google Scholar] [CrossRef]
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef]
- Matos, Â.P. The Impact of Microalgae in Food Science and Technology. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Soleymani, M.; Rosentrater, K.A. Techno-economic analysis of biofuel production from macroalgae (Seaweed). Bioengineering 2017, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Culaba, A.B.; Ubando, A.T.; Ching, P.M.L.; Chen, W.H.; Chang, J.S. Biofuel from microalgae: Sustainable pathways. Sustainability 2020, 12, 9. [Google Scholar] [CrossRef]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Chong, W.T.; Lam, M.K.; Loh, P.K.; Vellayan, V. Microalgae biofuels as an alternative to fossil fuel for power generation. Renew. Sustain. Energy Rev. 2016, 58, 180–197. [Google Scholar] [CrossRef]
- Shuba, E.S.; Kifle, D. Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a source of valuable antimicrobial compounds: Extraction and applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef]
- Gopeechund, A.; Bhagooli, R.; Neergheen, V.S.; Bolton, J.J.; Bahorun, T. Anticancer activities of marine macroalgae: Status and future perspectives. In Biodiversity and Biomedicine; Ozturk, M., Egamberdieva, D., Pešić, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 257–275. ISBN 9780128195413. [Google Scholar]
- Cikoš, A.-M.; Jerković, I.; Molnar, M.; Šubarić, D.; Jokić, S. New trends for macroalgal natural products applications. Nat. Prod. Res. 2019, 1–12. [Google Scholar] [CrossRef]
- Kim, S.K. Marine Cosmeceuticals: Trends and Prospects, 1st ed.; Kim, S.K., Ed.; Tayor & Francis Group: Boca Ratón, FL, USA, 2012; ISBN 9781439860281. [Google Scholar]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive components from seaweeds: Cosmetic applications and future development. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Verdy, C.; Branka, J.-E.; Mekideche, N. Quantitative assessment of lactate and progerin production in normal human cutaneous cells during normal ageing: Effect of an Alaria esculenta extract. Int. J. Cosmet. Sci. 2011, 33, 462–466. [Google Scholar] [CrossRef]
- Nizard, C.; Friguet, B.; Moreau, M.; Bulteau, A.-L.; Saunois , A. Use of Phaeodactylum Algae Extract as Cosmetic Agent Promoting the Proteasome Activity of Skin Cells and Cosmetic Composition Comprising Same. U.S. Patent 7,220,417, 22 May 2007. [Google Scholar]
- Hwang, E.; Park, S.Y.; Sun, Z.; Shin, H.S.; Lee, D.G.; Yi, T.H. The Protective Effects of Fucosterol Against Skin Damage in UVB-Irradiated Human Dermal Fibroblasts. Mar. Biotechnol. 2014, 16, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Joe, M.J.; Kim, S.N.; Choi, H.Y.; Shin, W.S.; Park, G.M.; Kang, D.W.; Yong, K.K. The inhibitory effects of eckol and dieckol from Ecklonia stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Le Lann, K.; Surget, G.; Couteau, C.; Coiffard, L.; Cérantola, S.; Gaillard, F.; Larnicol, M.; Zubia, M.; Guérard, F.; Poupart, N.; et al. Sunscreen, antioxidant, and bactericide capacities of phlorotannins from the brown macroalga Halidrys siliquosa. J. Appl. Phycol. 2016, 28, 3547–3559. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kim, E.A.; Son, K.T.; Jeon, Y.J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.M.; Qian, Z.J.; Kim, M.M.; Nam, K.W.; Kim, S.K. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem. 2009, 78, 98–105. [Google Scholar] [CrossRef]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B Biol. 2009, 95, 101–107. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Ahn, G. Fucoidan refined by Sargassum confusum indicate protective effects suppressing photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes. Int. J. Biol. Macromol. 2020, 164, 149–161. [Google Scholar] [CrossRef]
- Guinea, M.; Franco, V.; Araujo-Bazán, L.; Rodríguez-Martín, I.; González, S. In vivo UVB-photoprotective activity of extracts from commercial marine macroalgae. Food Chem. Toxicol. 2012, 50, 1109–1117. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Choi, J.S.; Moon, W.S.; Choi, J.N.; Do, K.H.; Moon, S.H.; Cho, K.K.; Han, C.J.; Choi, I.S. Effects of seaweed Laminaria japonica extracts on skin moisturizing activity in vivo. J. Cosmet Sci. 2013, 64, 193–205. [Google Scholar] [PubMed]
- Leelapornpisid, P.; Mungmai, L.; Sirithunyalug, B.; Jiranusornkul, S.; Peerapornpisal, Y. A novel moisturizer extracted from freshwater macroalga [Rhizoclonium hieroglyphicum (C. Agardh) Kützing] for skin care cosmetic. Chiang Mai J. Sci 2014, 41, 1195–1207. [Google Scholar]
- Kim, S.-K.; Babitha, S.; Kim, E.-K. Effect of Marine Cosmeceuticals on the Pigmentation of Skin. In Marine Cosmeceuticals; CRC Press: Boca Ratón, FL, USA, 2011; pp. 63–65. [Google Scholar]
- Wang, H.D.; Chen, C.; Huynh, P.; Chang, J. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.C. The potential of Arthrospira platensis extract as a tyrosinase inhibitor for pharmaceutical or cosmetic applications. S. Afr. J. Bot. 2018, 119, 236–243. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Bak, S.S.; Ahn, B.N.; Kim, J.A.; Shin, S.H.; Kim, J.C.; Kim, M.K.; Sung, Y.K.; Kim, S.K. Ecklonia cava promotes hair growth. Clin. Exp. Dermatol. 2013, 38, 904–910. [Google Scholar] [CrossRef]
- Atzori, G.; Nissim, W.G.; Rodolfi, L.; Niccolai, A.; Biondi, N.; Mancuso, S.; Tredici, M.R. Algae and Bioguano as promising source of organic fertilizers. J. Appl. Phycol. 2020, 32, 3971–3981. [Google Scholar] [CrossRef]
- Akila, V.; Manikandan, A.; Sahaya Sukeetha, D.; Balakrishnan, S.; Ayyasamy, P.M.; Rajakumar, S. Biogas and biofertilizer production of marine macroalgae: An effective anaerobic digestion of Ulva sp. Biocatal. Agric. Biotechnol. 2019, 18, 101035. [Google Scholar] [CrossRef]
- Hashem, H.A.; Mansour, H.A.; El-Khawas, S.A.; Hassanein, R.A. The potentiality of marine macro-algae as bio-fertilizers to improve the productivity and salt stress tolerance of canola (Brassica napus L.) plants. Agronomy 2019, 9, 146. [Google Scholar] [CrossRef]
- Stirk, W.A.; Rengasamy, K.R.R.; Kulkarni, M.G.; Staden, J. Plant Biostimulants from Seaweed. In The Chemical Biology of Plant Biostimulants; Geelen, D., Lin, X., Eds.; Wiley: Chichester, UK, 2020; pp. 31–55. [Google Scholar]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.R.G.; Fonseca, A.J.M.; Cortez, P.P.; Cabrita, A.R.J. In vitro evaluation of macroalgae as unconventional ingredients in ruminant animal feeds. Algal Res. 2019, 40, 101481. [Google Scholar] [CrossRef]
- Bansemer, M.S.; Qin, J.G.; Harris, J.O.; Duong, D.N.; Hoang, T.H.; Howarth, G.S.; Stone, D.A.J. Growth and feed utilisation of greenlip abalone (Haliotis laevigata) fed nutrient enriched macroalgae. Aquaculture 2016, 452, 62–68. [Google Scholar] [CrossRef]
- Sáez, M.I.; Vizcaíno, A.; Galafat, A.; Anguís, V.; Fernández-Díaz, C.; Balebona, M.C.; Alarcón, F.J.; Martínez, T.F. Assessment of long-term effects of the macroalgae Ulva ohnoi included in diets on Senegalese sole (Solea senegalensis) fillet quality. Algal Res. 2020, 47, 101885. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Gouveia, A.; Rema, P.; Matos, J.; Gomes, E.F.; Pinto, I.S. Evaluation of three seaweeds Gracilaria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2006, 252, 85–91. [Google Scholar] [CrossRef]
- Passos, R.; Correia, A.P.; Ferreira, I.; Pires, P.; Pires, D.; Gomes, E.; do Carmo, B.; Santos, P.; Simões, M.; Afonso, C.; et al. Effect on health status and pathogen resistance of gilthead seabream (Sparus aurata) fed with diets supplemented with Gracilaria gracilis. Aquaculture 2021, 531, 735888. [Google Scholar] [CrossRef]
- Boletín Oficial del Estado. Real Decreto 630/2013, de 2 de Agosto, por el que se Regula el Catálogo Español de Especies Exóticas Invasoras; Boletín Oficial del Estado: Madrid, Spain, 2013.
- Mulas, M.; Bertocci, I. Devil’s tongue weed (Grateloupia turuturu Yamada) in northern Portugal: Passenger or driver of change in native biodiversity? Mar. Environ. Res. 2016, 118, 1–9. [Google Scholar] [CrossRef]
- Altamirano, M.; Muñoz, A.R.; de la Rosa, J.; Barrajón-Mínguez, A.; Barrajón-Domenech, A.; Moreno-Robledo, C.; del Arroyo, M.C. The invasive species Asparagopsis taxiformis (Bonnemaisoniales, Rhodophyta) on andalusian coasts (Southern Spain): Reproductive stages, new records and invaded communities. Acta Botánica Malacit. 2008, 23, 5–15. [Google Scholar] [CrossRef]
- Bellissimo, G.; Galfo, F.; Nicastro, A.; Costantini, R.; Castriota, L. First record of the invasive green alga Codium fragile ssp. fragile (Chlorophyta, Bryopsidales) in Abruzzi waters, central Adriatic sea. Acta Adriat. 2018, 59, 207–212. [Google Scholar] [CrossRef]
- Gennaro, P.; Piazzi, L. The indirect role of nutrients in enhancing the invasion of Caulerpa racemosa var cylindracea. Biol. Invasions 2014, 16, 1709–1717. [Google Scholar] [CrossRef]
- Ornano, L.; Sanna, C.; Serafini, M.; Bianco, A.; Donno, Y.; Ballero, M. Phytochemical study of Caulerpa racemosa (Forsk.) J. Agarth, an invading alga in the habitat of La Maddalena Archipelago. Nat. Prod. Res. 2014, 28, 1795–1799. [Google Scholar] [CrossRef]
- Capdevila-Argüelles, L.; Zilletti, B.; Suárez Álvarez, V.Á. Plan. Extratéxico galego de Xestión das Especies Exóticas Invasoras e Para o Desenvolvemento Dun Sistema Esandarizado de Análise de Riscos Para as Especies Exóticas en Galicia; Xunta de Galicia: Santiago, Chile; Galicia, Spain, 2012. [Google Scholar]
- Haslin, C.; Lahaye, M.; Pellegrini, M.; Chermann, J.C. In Vitro Anti-HIV Activity of Sulfated Cell-Wall Polysaccharides from Gametic, Carposporic and Tetrasporic Stages of the Mediterraean Red Alga Asparagopsis armata. Planta Med. 2001, 67, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Bouhlal, R.; Riadi, H.; Bourgougnon, N. Antiviral activity of the extracts of Rhodophyceae from Morocco. Afr. J. Biotechnol. 2010, 9, 7968–7975. [Google Scholar] [CrossRef]
- Andrade, P.B.; Barbosa, M.; Pedro, R.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Fakhfakh, J.; Sassi, S.; Elleuch, M.; Gargouri, L. Physico-chemical characterization and beneficial effects of seaweed sulfated polysaccharide against oxydatif and cellular damages caused by alloxan in diabetic rats. Int. J. Biol. Macromol. 2018, 117, 407–417. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Jardak, N.; Hajkacem, F.; Chaaben, R.; Jribi, I.; El Feki, A.; Rebai, T.; Jamoussi, K.; Fki, L.; Belghith, H.; et al. Anti-obesity effect and protection of liver-kidney functions by Codium fragile sulphated polysaccharide on high fat diet induced obese rats. Int. J. Biol. Macromol. 2017, 102, 119–129. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Je, J.G.; Jayawardena, T.U.; Kim, Y.S.; Ko, J.Y.; Fu, X.; Jeon, Y.J. Protective effects of sulfated polysaccharides isolated from the enzymatic digest of Codium fragile against hydrogen peroxide-induced oxidative stress in in vitro and in vivo models. Algal Res. 2020, 48, 101891. [Google Scholar] [CrossRef]
- Athukorala, Y.; Lee, K.W.; Kim, S.K.; Jeon, Y.J. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour. Technol. 2007, 98, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Ciancia, M.; Quintana, I.; Vizcargüénaga, M.I.; Kasulin, L.; de Dios, A.; Estevez, J.M.; Cerezo, A.S. Polysaccharides from the green seaweeds Codium fragile and C. vermilara with controversial effects on hemostasis. Int. J. Biol. Macromol. 2007, 41, 641–649. [Google Scholar] [CrossRef]
- Surayot, U.; You, S.G. Structural effects of sulfated polysaccharides from Codium fragile on NK cell activation and cytotoxicity. Int. J. Biol. Macromol. 2017, 98, 117–124. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.L.; Jeong, S.; Kim, B.R.; Na, Y.J.; Jo, M.J.; Yun, H.K.; Jeong, Y.A.; Kim, D.Y.; Kim, B.G.; et al. Codium fragile F2 sensitize colorectal cancer cells to TRAIL-induced apoptosis via c-FLIP ubiquitination. Biochem. Biophys. Res. Commun. 2019, 508, 1–8. [Google Scholar] [CrossRef]
- Marshall, R.A.; Hamilton, J.T.G.; Dring, M.J.; Harper, D.B. Do vesicle cells of the red alga Asparagopsis (Falkenbergia stage) play a role in bromocarbon production? Chemosphere 2003, 52, 471–475. [Google Scholar] [CrossRef]
- McConnell, O.; Fenical, W. Halogen chemistry of the red alga asparagopsis. Phytochemistry 1977, 16, 367–374. [Google Scholar] [CrossRef]
- Woolard, F.X.; Moore, R.E.; Roller, P.P. Halogenated acetic and acrylic acids from the red alga asparagopsis taxiformis. Phytochemistry 1979, 18, 617–620. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Salah, H.B.; Hamza, A.; El feki, A.; Allouche, N.; El feki, L.; Belguith, K. Characterization and evaluating of antioxidant and antihypertensive properties of green alga (Codium fragile) from the coast of Sfax. J. Pharmacogn. Phytochem. 2017, 6, 186–191. [Google Scholar]
- Ortiz, J.; Uquiche, E.; Robert, P.; Romero, N.; Quitral, V.; Llantén, C. Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. Eur. J. Lipid Sci. Technol. 2009, 320–327. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Abreu, M.H.; Rocha, S.M.; Silvestre, A.J.D. Chlorophyta and Rhodophyta macroalgae: A source of health promoting phytochemicals. Food Chem. 2015, 183, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.P.; Rodríguez-Hermida, V.; Pérez-Larrán, P.; Conde, E.; Liveri, M.T.; Ribeiro, D.; Fernandes, E.; Domínguez, H. In vitro bioactive properties of phlorotannins recovered from hydrothermal treatment of Sargassum muticum. Sep. Purif. Technol. 2016, 167, 117–126. [Google Scholar] [CrossRef]
- Kendel, M.; Barnathan, G.; Fleurence, J.; Rabesaotra, V.; Wielgosz-Collin, G. Non-methylene interrupted and hydroxy fatty acids in polar lipids of the alga Grateloupia turuturu over the four seasons. Lipids 2013, 48, 535–545. [Google Scholar] [CrossRef]
- Kendel, M.; Couzinet-Mossion, A.; Viau, M.; Fleurence, J.; Barnathan, G.; Wielgosz-Collin, G. Seasonal composition of lipids, fatty acids, and sterols in the edible red alga Grateloupia turuturu. J. Appl. Phycol. 2013, 25, 425–432. [Google Scholar] [CrossRef]
- Lee, J.B.; Ohta, Y.; Hayashi, K.; Hayashi, T. Immunostimulating effects of a sulfated galactan from Codium fragile. Carbohydr. Res. 2010, 345, 1452–1454. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fernández, P.V.; Arata, P.X.; Ciancia, M. Polysaccharides from Codium Species; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, ISBN 9780124080621. [Google Scholar]
- Yang, Y.; Park, J.; You, S.G.; Hong, S. Immuno-stimulatory effects of sulfated polysaccharides isolated from Codium fragile in olive flounder, Paralichthys olivaceus. In Fish Shellfish Immunology; Elsevier Inc.: Alpharetta, GA, USA, 2019; Volume 87, pp. 609–614. [Google Scholar] [CrossRef]
- Zhang, W.; Hwang, J.; Park, H.; Lim, S.; Go, S. Human Peripheral Blood Dendritic Cell and T Cell Activation by Codium fragile Polysaccharide. Mar. Drugs 2020, 18, 535. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, P.; Montero, L.; Stiger-pouvreau, V.; Tanniou, A.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem. 2016, 192, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. A green approach for alginate extraction from Sargassum muticum brown seaweed using ultrasound-assisted technique. Int. J. Biol. Macromol. 2019, 124, 451–459. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Holmer, M. Potential effects of the invasive species Gracilaria vermiculophylla on Zostera marina metabolism and survival. Mar. Environ. Res. 2010, 69, 345–349. [Google Scholar] [CrossRef]
- Pereira, L. Edible Seaweeds of the World; CRC Press: Boca Ratón, FL, USA, 2016. [Google Scholar]
- Cardoso, I.; Cotas, J.; Rodrigues, A.; Ferreira, D.; Osório, N.; Pereira, L. Extraction and analysis of compounds with antibacterial potential from the red alga Grateloupia turuturu. J. Mar. Sci. Eng. 2019, 7, 220. [Google Scholar] [CrossRef]
- Sheu, J.; Huang, S.; Duh, C. Cytotoxic Oxygenated Desmosterols of the Red Alga Galaxaura marginata. J. Nat. Prod. 1996, 59, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Silva, J.; Pedrosa, R. The marine invasive seaweeds Asparagopsis armata and Sargassum muticum as targets for greener antifouling solutions. Sci. Total Environ. 2021, 750, 141372. [Google Scholar] [CrossRef] [PubMed]
- Pinteus, S.; Lemos, M.F.L.; Simões, M.; Alves, C.; Silva, J.; Gaspar, H.; Martins, A.; Rodrigues, A.; Pedrosa, R. Marine invasive species for high-value products’ exploration—Unveiling the antimicrobial potential of Asparagopsis armata against human pathogens. Algal Res. 2020, 52, 102091. [Google Scholar] [CrossRef]
- Lee, C.; Park, G.H.; Ahn, E.M.; Kim, B.A.; Park, C.I.; Jang, J.H. Protective effect of Codium fragile against UVB-induced pro-inflammatory and oxidative damages in HaCaT cells and BALB/c mice. Fitoterapia 2013, 86, 54–63. [Google Scholar] [CrossRef]
- Kim, A.D.; Lee, Y.; Kang, S.H.; Kim, G.Y.; Kim, H.S.; Hyun, J.W. Cytotoxic effect of clerosterol isolated from Codium fragile on A2058 human melanoma cells. Mar. Drugs 2013, 11, 418–430. [Google Scholar] [CrossRef]
- Ganesan, P.; Noda, K.; Manabe, Y.; Ohkubo, T.; Tanaka, Y.; Maoka, T.; Sugawara, T.; Hirata, T. Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 497–503. [Google Scholar] [CrossRef]
- Silva, J.; Martins, A.; Alves, C.; Pinteus, S.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Natural Approaches for Neurological Disorders-The Neuroprotective Potential of Codium tomentosum. Molecules 2020, 25, 5478. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, Y.; Liu, K.; Hua, H.; Zhu, H.; Pei, Y. Gracilarioside and gracilamides from the Red alga Gracilaria asiatica. J. Nat. Prod. 2006, 69, 1488–1491. [Google Scholar] [CrossRef]
- Barceló-villalobos, M.; Figueroa, F.L.; Korbee, N. Production of Mycosporine-Like Amino Acids from Gracilaria vermiculophylla (Rhodophyta) Cultured Through One Year in an Integrated Multi-trophic Aquaculture (IMTA) System. Mar. Biotechnol 2017, 19, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Matsubara, K.; Ohkubo, T.; Tanaka, Y.; Noda, K.; Sugawara, T.; Hirata, T. Anti-angiogenic effect of siphonaxanthin from green alga, Codium fragile. Phytomedicine 2010, 17, 1140–1144. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Ahn, G.N.; Kang, S.M.; Kang, D.H.; Affan, A.; Oh, C.; Jung, W.K.; Jeon, Y.J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.-J.; Moon, J.-Y.; Kim, S.S.; Yang, K.-W.; Lee, W.J.; Lee, N.H.; Hyun, C.-G. Jeju seaweeds suppress lipopolysaccharide-stimulated proinflammatory response in RAW 264. 7 murine macrophages. Asian Pac. J. Trop. Biomed. 2014, 4, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Le Guillard, C.; Dumay, J.; Donnay-Moreno, C.; Bruzac, S.; Ragon, J.Y.; Fleurence, J.; Bergé, J.P. Ultrasound-assisted extraction of R-phycoerythrin from Grateloupia turuturu with and without enzyme addition. Algal Res. 2015, 12, 522–528. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Zubia, M.; Fabre, M.; Deslandes, E.; Shannon, C. Antioxidant and cytotoxic activities of some red algae (Rhodophyta) from Brittany coasts (France). Botenica Mar. 2009, 52, 268–277. [Google Scholar] [CrossRef]
- Klejdus, B.; Plaza, M.; Snóblová, M.; Lojková, L. Development of new efficient method for isolation of phenolics from sea algae prior to their rapid resolution liquid chromatographic—tandem mass spectrometric determination. J. Pharm. Biomed. Anal. 2017, 135, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Luisa, M.; Nogueira, D.R.; González-lópez, N.; Conde, E.; Moure, A.; Pilar, M. Potential of antioxidant extracts produced by aqueous processing of renewable resources for the formulation of cosmetics. Ind. Crop. Prod. 2014, 58, 104–110. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic compounds from three brown seaweed species using LC-DAD—ESI-MS/MS. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.P.; Conde, E.; Domínguez, H.; Moure, A. Ecofriendly extraction of bioactive fractions from Sargassum muticum. Process Biochem. 2019, 79, 166–173. [Google Scholar] [CrossRef]
- Pérez-Larrán, P.; Torres, M.D.; Flórez-Fernández, N.; Balboa, E.M.; Moure, A.; Domínguez, H. Green technologies for cascade extraction of Sargassum muticum bioactives. J. Appl. Phycol. 2019, 31, 2481–2495. [Google Scholar] [CrossRef]
- Mata, L.; Silva, J.; Schuenhoff, A.; Santos, R. The effects of light and temperature on the photosynthesis of the Asparagopsis armata tetrasporophyte (Falkenbergia rufolanosa), cultivated in tanks. Aquaculture 2006, 252, 12–19. [Google Scholar] [CrossRef]
- Jacinto, M.S.C.; Monteiro, H.R.; Lemos, M.F.L. Impact of the invasive macroalgae Asparagopsis armata on coastal environments: An ecotoxicological assessment. Curr. Opin. Biotechnol. 2013, 24S, S75. [Google Scholar] [CrossRef]
- De Nys, R.; Steinberg, P.D.; Willemsen, P.; Dworjanyn, S.A.; Gabelish, C.L.; King, R.J. Broad spectrum effects of secondary metabolites from the red alga delisea pulchra in antifouling assays. Biofouling 1995, 8, 259–271. [Google Scholar] [CrossRef]
- Schuenhoff, A.; Mata, L.; Santos, R. The tetrasporophyte of Asparagopsis armata as a novel seaweed biofilter. Aquaculture 2006, 252, 3–11. [Google Scholar] [CrossRef]
- Paul, N.; Nys, R. De Chemical defence against bacteria in the red alga Asparagopsis armata: Linking structure with function. Mar. Ecol. Prog. Ser. 2006, 306, 87–101. [Google Scholar] [CrossRef]
- Choi, J.H.; Sapkota, K.; Park, S.E.; Kim, S.; Kim, S.J. Thrombolytic, anticoagulant and antiplatelet activities of codiase, a bi-functional fibrinolytic enzyme from Codium fragile. Biochimie 2013, 95, 1266–1277. [Google Scholar] [CrossRef]
- Alhazzaa, R.; Nichols, P.D.; Carter, C.G. Sustainable alternatives to dietary fish oil in tropical fish aquaculture. Rev. Aquac. 2019, 11, 1195–1218. [Google Scholar] [CrossRef]
- Ummat, V.; Sivagnanam, S.P.; Rajauria, G.; O’Donnell, C.; Tiwari, B.K. Advances in pre-treatment techniques and green extraction technologies for bioactives from seaweeds. Trends Food Sci. Technol. 2021, 110, 90–106. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparativeperspective. Curr. Opin. Food Sci. 2021. [Google Scholar] [CrossRef]
- Mendes, M.; Pereira, R.; Sousa Pinto, I.; Carvalho, A.P.; Gomes, A.M. Antimicrobial activity and lipid profile of seaweed extracts from the North Portuguese Coast. Int. Food Res. J. 2013, 20, 3337–3345. [Google Scholar]
- Kamarudin, A.A.; Mohd, E.N.; Saad, N.; Sayuti, N.H.; Nor, N.A. Heat assisted extraction of phenolic compounds from Eleutherine bulbosa (Mill.) bulb and its bioactive profiles using response surface methodology. Ind. Crops Prod. 2020, 144, 112064. [Google Scholar] [CrossRef]
- Genovese, G.; Tedone, L.; Hamann, M.T.; Morabito, M. The Mediterranean Red Alga Asparagopsis: A Source of Compounds against Leishmania. Mar. Drugs 2009, 7, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Silvestre, L.; Rocha, M.I.; Rodrigues, M.J.; Vizetto-duarte, C.; Pereira, H.; Barreira, L.; Varela, J.; Custódio, L.; Silvestre, L.; et al. Methanol extracts from Cystoseira tamariscifolia and Cystoseira nodicaulis are able to inhibit cholinesterases and protect a human dopaminergic cell line from hydrogen peroxide- induced cytotoxicity from hydrogen peroxide-induced cytotoxicity. Pharm. Biol. 2016, 54, 1687–1696. [Google Scholar] [CrossRef]
- Kang, C.-H.; Choi, Y.H.; Park, S.-Y.; Kim, G.-Y. Anti-Inflammatory Effects of Methanol Extract of Codium fragile in Lipopolysaccharide-Stimulated RAW 264.7 Cells. J. Med. Food 2011, 15, 44–50. [Google Scholar] [CrossRef]
- Dilshara, M.G.; Jayasooriya, R.G.P.T.; Kang, C.H.; Choi, Y.H.; Kim, G.Y. Methanol extract of Codium fragile inhibits tumor necrosis factor-α-induced matrix metalloproteinase-9 and invasiveness of MDA-MB-231 cells by suppressing nuclear factor-κB activation. Asian Pac. J. Trop. Med. 2016, 9, 535–541. [Google Scholar] [CrossRef]
- Lee, S.A.; Moon, S.M.; Choi, Y.H.; Han, S.H.; Park, B.R.; Choi, M.S.; Kim, J.S.; Kim, Y.H.; Kim, D.K.; Kim, C.S. Aqueous extract of Codium fragile suppressed inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells and carrageenan-induced rats. Biomed. Pharmacother. 2017, 93, 1055–1064. [Google Scholar] [CrossRef]
- Yoon, H.-D.; Jeong, E.-J.; Choi, J.-W.; Lee, M.-S.; Park, M.-A.; Yoon, N.-Y.; Kim, Y.-K.; Cho, D.-M.; Kim, J.-I.; Kim, H.-R. Anti-inflammatory Effects of Ethanolic Extracts from Codium fragile on LPS-Stimulated RAW 264.7 Macrophages via Nuclear Factor kappaB Inactivation. Fish. Aquat. Sci. 2012, 14, 267–274. [Google Scholar] [CrossRef]
- Moon, S.M.; Lee, S.A.; Han, S.H.; Park, B.R.; Choi, M.S.; Kim, J.S.; Kim, S.G.; Kim, H.J.; Chun, H.S.; Kim, D.K.; et al. Aqueous extract of Codium fragile alleviates osteoarthritis through the MAPK/NF-κB pathways in IL-1β-induced rat primary chondrocytes and a rat osteoarthritis model. Biomed. Pharmacother. 2018, 97, 264–270. [Google Scholar] [CrossRef]
- García-Bueno, N.; Decottignies, P.; Turpin, V.; Dumay, J.; Paillard, C.; Stiger-Pouvreau, V.; Kervarec, N.; Pouchus, Y.-F.; Marín-Atucha, A.A.; Fleurence, J. Seasonal antibacterial activity of two red seaweeds, Palmaria palmata and Grateloupia turuturu, on European abalone pathogen Vibrio harveyi. Aquat. Living Resour. 2014, 27, 83–89. [Google Scholar] [CrossRef]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Fino, N.; Rodrigues, A.I.; Mendes, S.; Pedrosa, R. Cytoprotective effect of seaweeds with high antioxidant activity from the Peniche coast (Portugal). Food Chem. 2017, 218, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Li, Y.; Ahn, B.; Eom, S.; Domínguez, H.; Jiménez, C.; Rodríguez, J. Photodamage attenuation effect by a tetraprenyltoluquinol chromane meroterpenoid isolated from Sargassum muticum. J. Photochem. Photobiol. B Biol. 2015, 148, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, E.; Mendiola, J.A.; Castro-Puyana, M. Supercritical Fluid Extraction; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780123849472. [Google Scholar]
- Montero, L.; Sánchez-Camargo, A.P.; García-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Rai, D.K.; Hossain, M. Analytical techniques for bioactives from seaweed. In Seaweed Sustainability: Food and Non-Food Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 271–287. ISBN 9780124199583. [Google Scholar]
- Fraga-Corral, M.; Carpena, M.; Garcia-Oliveira, P.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Analytical Metabolomics and Applications in Health, Environmental and Food Science. Crit. Rev. Anal. Chem. 2020, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, E.; Lozano, S.; Guillén, J. Efficiency data analysis in EU aquaculture production. Aquaculture 2020, 520, 734962. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; Volume 35, ISBN 9789251060292. [Google Scholar]
- Jones, S.W.; Karpol, A.; Friedman, S.; Maru, B.T.; Tracy, B.P. Recent advances in single cell protein use as a feed ingredient in aquaculture. Curr. Opin. Biotechnol. 2020, 61, 189–197. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Hasan, M.R.; Metian, M. Demand and Supply of Feed Ingredients for Farmed Fish and Crustaceans: Trends and Prospects; FAO: Rome, Italy, 2011; Volume 564, ISBN 9789251069332. [Google Scholar]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Soler-vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquacultre 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Castanho, S.; Califano, G.; Soares, F.; Costa, R.; Mata, L.; Pousão-Ferreira, P.; Ribeiro, L. The effect of live feeds bathed with the red seaweed Asparagopsis armata on the survival, growth and physiology status of Sparus aurata larvae. Fish. Physiol. Biochem. 2017, 43, 1043–1054. [Google Scholar] [CrossRef]
- Félix, R.; Félix, C.; Januário, A.P.; Carmona, A.M.; Baptista, T.; Gonçalves, R.A.; Sendão, J.; Novais, S.C.; Lemos, M.F.L. Tailoring shrimp aquafeed to tackle Acute Hepatopancreatic Necrosis Disease by inclusion of industry-friendly seaweed extracts. Aquaculture 2020, 529, 735661. [Google Scholar] [CrossRef]
- Pereira, R.; Valente, L.M.P.; Sousa-Pinto, I.; Rema, P. Apparent nutrient digestibility of seaweeds by rainbow trout (Oncorhynchus mykiss) and Nile tilapia (Oreochromis niloticus). Algal Res. 2012, 1, 77–82. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Rema, P.; Ferraro, V.; Pintado, M.; Sousa-Pinto, I.; Cunha, L.M.; Oliveira, M.B.; Araújo, M. Iodine enrichment of rainbow trout flesh by dietary supplementation with the red seaweed Gracilaria vermiculophylla. Aquaculture 2015, 446, 132–139. [Google Scholar] [CrossRef]
- Araújo, M.; Rema, P.; Sousa-Pinto, I.; Cunha, L.M.; Peixoto, M.J.; Pires, M.A.; Seixas, F.; Brotas, V.; Beltrán, C.; Valente, L.M.P. Dietary inclusion of IMTA-cultivated Gracilaria vermiculophylla in rainbow trout (Oncorhynchus mykiss) diets: Effects on growth, intestinal morphology, tissue pigmentation, and immunological response. J. Appl. Phycol. 2016, 28, 679–689. [Google Scholar] [CrossRef]
- Magnoni, L.J.; Martos-Sitcha, J.A.; Queiroz, A.; Calduch-Giner, J.A.; Gonçalves, J.F.M.; Rocha, C.M.R.; Abreu, H.T.; Schrama, J.W.; Ozorio, R.O.A.; Perez-Sanchez, J. Dietary supplementation of heat-treated Gracilaria and Ulva seaweeds enhanced acute hypoxia tolerance in gilthead sea bream (Sparus aurata). Biol. Open 2017, 6, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Rosas, R.E.; Rivas-Vega, M.E.; Miranda-Baeza, A.; Piña-Valdez, P.; Nieves-Soto, M. Effects of a co-culture of marine algae and shrimp (Litopenaeus vannamei) on the growth, survival and immune response of shrimp infected with Vibrio parahaemolyticus and white spot virus (WSSV). Fish. Shellfish Immunol. 2019, 87, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Qadir, A.-M.; Mohammed, A.-P.; Adamu, K.M.; Abdulraheem, A.-A. Inclusion of Sargassum muticum and Parkia biglobosa in diets for African Catfish (Clarias gariepinus) elevates feed utilization, growth and immune parameters. Afr. J. Agric. Res. 2020, 15, 134–139. [Google Scholar] [CrossRef][Green Version]
- Yeganeh, S.; Adel, M. Effects of dietary algae (Sargassum ilicifolium) as immunomodulator and growth promoter of juvenile great sturgeon (Huso huso Linnaeus, 1758). J. Appl. Phycol. 2019, 31, 2093–2102. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, H.; Zhang, H. The effect of Sargassum fusiforme polysaccharide extracts on vibriosis resistance and immune activity of the shrimp, Fenneropenaeus chinensis. Fish. Shellfish Immunol. 2006, 20, 750–757. [Google Scholar] [CrossRef]
- Mulvaney, W.J.; Winberg, P.C.; Adams, L. Comparison of macroalgal (Ulva and Grateloupia spp.) and formulated terrestrial feed on the growth and condition of juvenile abalone. J. Appl. Phycol. 2013, 25, 815–824. [Google Scholar] [CrossRef]
- García-Bueno, N.; Turpin, V.; Cognie, B.; Dumay, J.; Morançais, M.; Amat, M.; Pédron, J.-M.; Atucha, A.M.; Fleurence, J.; Decottignies, P. Can the European abalone Haliotis tuberculata survive on an invasive algae? A comparison of the nutritional value of the introduced Grateloupia turuturu and the native Palmaria palmata, for the commercial European abalone industry. J. Appl. Phycol. 2016, 28, 2427–2433. [Google Scholar] [CrossRef]
| Treatment | Specie | Compound | Result | Ref. |
|---|---|---|---|---|
| Skin aging | Alaria esculenta | Extract | Decline the amount of progerin in aged fibroblasts at the lowest tested concentration (not for younger cells) | [27] |
| Phaeodactylum tricornutum | Ethanol extract | Protecting the skin from the adverse effects of UV exposure; preventing and/or delaying the appearance of skin aging effects | [28] | |
| Hizikia fusiformis | Fucosterol | Inhibit metalloproteinase-1 expression | [29] | |
| Ecklonia stolonifera | Phlorotannins | Inhibit metalloproteinase-1 expression | [30] | |
| Sunscreen | Halidrys siliquosa | Phlorotannins | UV-filter activity | [31] |
| Brown seaweeds | Phlorotannins | Protective effect against photo-oxidative stress | [32] | |
| Corallina pilulifera | Phenolic compounds | Anti-photoaging activity and inhibition of matrix metalloproteinase | [33] | |
| Sargassum spp. | Fucoxanthin | Protective effect on UV-B induced cell damage | [34] | |
| Sargassum confusum | Fucoidan | Suppress photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes | [35] | |
| Macrocystis pyrifera, Porphyra columbina | Acetone extracts | In vivo UVB-photoprotective activity | [36] | |
| Moisturizer | Fucus vesiculosus | Fucoidan | Inhibition of hyaluronidase enzyme | [37] |
| Laminaria japonica | 5% water:propylene glycol (50:50) extracts | Hydration with the alga extract increased by 14.44% compared with a placebo | [38] | |
| Rhizoclonium hieroglyphicum | Polysaccharides and amino acids | Similar moisturizing effects to hyaluronic acid and glycerin | [39] | |
| Whitening | Nannochloropsis oculata | Zeaxanthin | Antityrosinase activity | [40] |
| Laminaria japonica | Fucoxanthin | Antityrosinase activity | [41] | |
| Arthrospira platensis | Ethanol extract | Antityrosinase activity | [42] | |
| Hair care | Chlorella spp. | Intact microalga cells | Soften and make flexible both skin and hair | [43] |
| Ecklonia cava | Dioxinodehydroeckol | Promote hair growth | [44] |
| Specie | Taxonomy | Native Distribution | Distribution in Spain | Other Regions in Which They are Invasive | Principal Uses |
|---|---|---|---|---|---|
| Red species | |||||
| Acrothamnion preissii | Phylum: Rhodophyta Class: Florideophyceae Orden: Ceramiales Family: Ceramiaceae | Western Australia | All Spain | Temperate coastlines on the Pacific coast of North America and western coasts of Europe | - Unknown |
| Asparagopsis armata | Phylum: Rhodophyta Class: Florideophyceae Orden: Bonnemaisoniales Family: Bonnemaisoniaceae | Indo-Pacific Ocean | All Spain | Mediterranean, Portugal, and Ireland | - Pharmaceutical potential as antibiotic |
| Asparagopsis taxiformis | Phylum: Rodophyta Class: Rhodoplayceae Orden: Nemaliales Family: Bonnemaisoniaceae | Australia and New Zealand | Except Canarias | Portugal | - Human consumption - Antifungal |
| Grateloupia turuturu | Phylum: Rhodophyta Class: Florideophyaceae Orden: Halymeniales Family: Halymeniaceae | Pacific Ocean | All Spain | North America, Europe, and Oceania | - Human consumption - Fertilizer |
| Lophocladia lallemandii | Phylum: Rhodophyta Class: Florideophyceae Order: Ceramiales Family: Rhodomelaceae | Indo-Pacific Ocean | All Spain | Mediterranean | - Unknown |
| Womersleyella setacea | Phylum: Rhodophyta Class: Rhodophyceae Order: Ceramiales Family: Rhodomelaceae | Indo-Pacific Ocean | All Spain | Mediterranean | - Unknown |
| Brown species | |||||
| Gracilaria vermiculophylla | Phylum: Rhodophyta Class: Florideophyceae Orden: Gracilariales Family: Gracilariaceae | North-east Pacific | All Spain | Europe and North America | - Animal feed - Biofuels - Fertilizer - Human consumption |
| Sargassum muticum | Phylum: Ochrophyta Class: Phaeophyceae Order: Fucales Family: Sargassaceae | Indo-Pacific Ocean | All Spain | Pacific Coast of North America, North Sea, Portugal, and the Mediterranean | - Animal feed - Food additive - Pesticide |
| Stypopodium schimperi | Phylum: Ochrophyta Class: Phaeophyceae Order: Dictyotales Family: Dictyotaceae | Indo-Pacific Ocean and Red Sea | All Spain | Africa and Southwest Asia | - Unknown |
| Undaria pinnatifida | Phylum: Heterokontophyta Class: Phaeophyceae Order: Laminariales Family: Alariaceae | Asia | All Spain | Europe | - Human consumption - Animal feed |
| Green species | |||||
| Caulerpa taxifolia | Phylum: Chlorophyta Class: Bryopsidophyceae Orden: Bryopsidales Family: Caulerpaceae | Tropical area | All Spain | Mediterranean, California, and southern Australia | - Laboratory use |
| Codium fragile | Phylum: Chlorophyta Class: Chlorophyceae Orden: Codiales Family: Codiaceae | North of the Pacific Ocean and coast of Japan | All Spain | Widespread in the Mediterranean | - Human consumption |
| Caulerpa racemosa | Phylum: Chlorophyta Class: Bryopsidophyceae Orden: Bryopsidales Family: Caulerpaceae | Tropical areas | Except Canarias | Mediterranean: from Spain to Turkey | - Human consumption |
| Diatoms | |||||
| Didymosphenia geminata | Phylum: Ochrophyta Class: Bacillariophyceae Orden: Cymbellales Family: Gomphonemataceae | Boreal and alpine regions of North America and Northern Europe | All Spain | New Zealand and Patagonia, South America | - Ornamental |
| Bioactive compounds | Invasive Macroalgae | ||||
|---|---|---|---|---|---|
 |  |  |  |  | |
| Asparagopsis armata | Codium fragile | Gracilaria vermiculophylla | Sargassum muticum | Grateloupia turuturu | |
| Polysaccharides | Sulphated galactan derivatives, Mannitol | Sulphated polysaccharides | Fucoidans, Alginate, Glucuronic acid, Mannuronic acid, Laminarin | ||
| Lipids | Cholestanol, Cholesta-5,25-diene-3,24 -diol, Palmitic acid, Stearic acid | Clerosterol | Cholesterol, 1-tetradecanol, 1-hexadecanol, 1-octadecanol, 1-eicosanol, 1-docosanol, Sterols, Monoacylglycerol | α -Linolenic acid | Phospholipids, Glycolipids, Eicosapentaenoic acid |
| Proteins | Mycrosporine-like aminoacids* | ||||
| Pigments | β-carotene, Siphonaxanthin | Fucoxanthin | R-phycoerythrin | ||
| Vitamins | α, β, γ, δ-tocopherol, γ-tocotrienol | α-tocopherol | α, γ-tocopherol | α-tocopherol, Phytonadione (vitamin K1) | |
| Phenolic compounds | Not specified | Flavonoids, tannins | Gallic acid, Protocatechuic acid, Gentisic acid, Hydroxybenzoic acid, vVnillic acid, Syringic acid | Hydroxybenzoic acid, Gallic acid, Vanillic acid, Protocatechuic acid, Caffeic acid, Syringic acid, Chlorogenic acid, Coumaric acid, Phlorotannins, Fuhalols, Phlorethols, Hydroxyfuhalols, Monofuhalol A, | |
| Other compounds | Halogenated compounds, Halogenated ketones, 1,1-dibromo-3-iodo-2- propanone, 1,3-dibromo-2- propanone, 1,3-dibromo-1-chloro-2- propanone (±) form, Halogenated carboxylic acids, Dibromoacetic acid, Bromochloroacetic acid, Dibromoacrylic acid, Halogenated alkanes, Bromoform, Dibromochloromethane | Serine protease | Long chain aliphatic alcohols | Tetrapernyltaluquinol meroterpenoid with a chrome moiety | Squalene |
| Reference | [72,73,74] | [75,76] | [77] | [78] | [79,80] |
| Method | Conditions | Compounds | Activities | Model/Assay | Ref. |
|---|---|---|---|---|---|
| Asparagopsis armata | |||||
| Soxhlet | Chloroform-methanol (3:2), dichloromethane (100%), methanol (100%), and water (100%), 8 h | - | Anti-Herpes Simplex Virus and cytotoxicity | Neutral red dye method on Vero cells. | [63] |
| Mac | Hexane, dichloromethane, and ethanol | Halogenated compounds | Antiprotozoal | Leishmania donovani promastigotes cultures | [123] |
| Mac | 0.025 g/mL; methanol, 16 h, 20 °C | Phenolic compounds | Antioxidant and neuroprotective | DPPH, CCA, ICA. AChE, BuChE, TYRO inhibition. In vivo MTT assay on SH-SY5Y cells on H2O2 induced cytotoxicity. | [124] |
| HAE | 0.04 g/mL; distilled water, 5 h, 96 °C | Polysaccharides | Anti-HIV | Human immunodeficiency virus (HIV) induced syncytium formation on MT4 cells. | [62] |
| PLE | Dichloromethane methanol (1:1; v:v); 75 °C, 1500 psi, 7 min (×2) | Phenolic compounds | Antioxidant and cytotoxicity | Radical-scavenging activity (DPPH). Reducing activity. Daudi, Jurkat and K562 cell lines. | [106] |
| Codium fragile | |||||
| Mac | 80% methanol (×3). Butanol and ethyl-acetate fractions. | Clerosterol | Antioxidant and anti-inflammatory | In vivo MTT assay on human keratinocyte HaCaT cells irradiated with UVB and BALB/c mice models. Expression of pro-inflammatory proteins and mediators | [94] |
| Mac | Hexane, ethyl, and methanol (×3) | - | Antioxidant and anti-hypertensive | DPPH and ABTS inhibition In vitro ACE inhibitory assay | [75] |
| Mac | 80% methanol | - | Anti-inflammatory | Lipopolysaccharide-stimulated RAW 264.7 | [125] |
| Mac | 80% methanol | - | Anti-cancer | Human breast cancer cell line MDA-MB-231 | [126] |
| HAE | 0.02 g/mL; water, 12 h, 60 °C | Polysaccharides | Anticoagulant | APTT assay on human blood | [68] |
| HAE | 10 vol, distilled water, 1 h, 95 °C | - | Anti-inflammatory and anti-edema | LPS-stimulated RAW 264.7 and carrageenan-induced paw edema in male Sprague-Dawley rats. | [127] |
| HAE | Ethanol 96% (v/v), 3 h, 70 °C (×3) | - | Anti-inflammatory | LPS-stimulated RAW 264.7. | [128] |
| HAE | Distilled water, 4 h, 90 °C. | - | Anti-inflammatory, alleviation of cartilage destruction | Primary chondrocytes cells, osteoarthritis rat model. | [129] |
| Gracilaria vermiculophylla | |||||
| Mac | 0.1 g/mL; water or ethanol, 96%, 12 h, room temperature. | Phenolic compounds | Antioxidant | In vitro assays (DPPH, FRAP, ferrous ion-chelating) and liposome model system. | [105] |
| Soxhlet | 0.3 g/mL; ethyl acetate; 72 h. | - | Antimicrobial | Strains of S. enteritidis, P. Aeruginosa and L. innocua | [121] |
| Grateloupia turututu | |||||
| S/L | 1/20 ratio (w/v), water, 20 min, phosphate buffer (20 mM, pH 7.1) | - | Antibacterial | European abalone pathogen Vibrio harveyi | [130] |
| Sargassum muticum | |||||
| Mac | 0.01 g/mL; 80% methanol, 24 h, RT. | Fucoxanthin | Anti-inflammatory | LPS-stimulated RAW 264.7 macrophages | [103] |
| Mac | 0.1 g/mL; Water or ethanol, 96%, 12 h, RT. | Phenolic compounds | Antioxidant | In vitro assays (DPPH, FRAP, ferrous ion-chelating) and liposome model system | [105] |
| Mac | Dichloromethane or methanol, 1:4 (w/v), 12 h. | Phenolic compounds | Antioxidant and cytoprotective effect | In vitro assays (DPPH and ORAC) Protective effect on MCF-7 cells | [131] |
| HAE | Methanol:water (1:10), 3 h, 65 °C (×3) | Chromane meroterpenoid | Photodamage attenuation | Human dermal fibroblasts | [132] |
| SFE | CO2, 10% ethanol, 15.2 MPa, 60 °C, 90 min (static) | - | Antioxidant | Not reported | [133] |
| PLE | Ethanol:water (95:5); 160 °C, 10.3 MPa, 20 min (×2) | Phlorotannins | Antiproliferative | HT-29 adenocarcinoma colon cancer cells | [134] |
| UAE | Water at S/L ratio of 1:20; 5–30 min, RT (25 °C), 5 A, 150 W and 40 Hz. | Alginate | Cytotoxic effect | A549, HCT- 116, PSN1, and T98G cells | [87] |
| Autohydrolisis | 96% ethanol | - | Antioxidant, anti-inflammatory and anti-irritant | In vitro assays (FRAP, DPPH and ABTS). Reconstructed human epidermis test method. Irritability assays with the Episkin test. | [108] |
| Autohydrolisis | RT, formaldehyde 1% (15 h), sulfuric acid 0.2 N (4 h), and sodium carbonate 1% (15 h). | Phlorotannins | Anti-tumor and anti-inflammatory | A549, HCT-116, PSN1, and T98G cells. Neutrophils’ oxidative burst oxidation of luminol. | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.G.; Fraga-Corral, M.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. The Use of Invasive Algae Species as a Source of Secondary Metabolites and Biological Activities: Spain as Case-Study. Mar. Drugs 2021, 19, 178. https://doi.org/10.3390/md19040178
Pereira AG, Fraga-Corral M, Garcia-Oliveira P, Lourenço-Lopes C, Carpena M, Prieto MA, Simal-Gandara J. The Use of Invasive Algae Species as a Source of Secondary Metabolites and Biological Activities: Spain as Case-Study. Marine Drugs. 2021; 19(4):178. https://doi.org/10.3390/md19040178
Chicago/Turabian StylePereira, Antia G., Maria Fraga-Corral, Paula Garcia-Oliveira, Catarina Lourenço-Lopes, Maria Carpena, Miguel A. Prieto, and Jesus Simal-Gandara. 2021. "The Use of Invasive Algae Species as a Source of Secondary Metabolites and Biological Activities: Spain as Case-Study" Marine Drugs 19, no. 4: 178. https://doi.org/10.3390/md19040178
APA StylePereira, A. G., Fraga-Corral, M., Garcia-Oliveira, P., Lourenço-Lopes, C., Carpena, M., Prieto, M. A., & Simal-Gandara, J. (2021). The Use of Invasive Algae Species as a Source of Secondary Metabolites and Biological Activities: Spain as Case-Study. Marine Drugs, 19(4), 178. https://doi.org/10.3390/md19040178