Abstract
The aim of this study was to evaluate the effects of ingesting fucoidan derived from Okinawa mozuku (Cladosiphon okamuranus) on natural killer (NK) cell activity and to assess its safety in healthy adults via a randomized, double-blind, parallel-group, placebo-controlled pilot study. Subjects were randomly divided into two groups—a placebo group (ingesting citric acid, sucralose, and caramel beverages; n = 20; 45.5 ± 7.8 years (mean ± standard deviation)) and a fucoidan group (3.0 g/day from beverages; n = 20; 47.0 ± 7.6 years); after 12 weeks, blood, biochemical, and immunological tests were performed. Clinically adverse events were not observed in any of the tests during the study period. In addition, adverse events due to the test food were not observed. In the immunological tests, NK cell activity was significantly enhanced at 8 weeks in the fucoidan group, compared to before ingestion (0 weeks). In addition, a significantly enhanced NK cell activity was observed in male subjects at 8 weeks, compared with the placebo group. These results confirm that Okinawa mozuku-derived fucoidan enhances NK cell activity and suggest that it is a safe food material.
1. Introduction
Fucoidan is a general term for the sulfated polysaccharides contained in brown algae, the chemical structure of which differs depending on the seaweed species [1]. Edible seaweeds such as Kombu (Kjellmaniella crassifolia), Wakame (Undaria pinnatifida), and Okinawa mozuku (Cladosiphon okamuranus) are the main raw materials for fucoidan in Japan. In this study, fucoidan was extracted from Okinawa mozuku, which is an endemic species that grows only in the Okinawa Islands of Japan. Previous studies of Okinawa mozuku-derived fucoidan have reported its anticoagulant [2], anti-inflammatory [2], antiviral [3,4,5], anti-HTLV-1 [6,7], antitumor [8,9], antihepatitis [10], and antiulcer [11] effects. Okinawa mozuku-derived fucoidan has a chemical structure including a sulfate group and uronic acid bonded to the main fucose chain [12]. Although Okinawa mozuku-derived fucoidan has a high molecular weight, it is absorbed and has been detected in human blood and urine after ingestion [13,14,15]. In a previous study [16], we reported that the frequency of mozuku intake affects the absorption of fucoidan and the negativity of Helicobacter pylori antibody titers. Previous studies have also reported the immune effects of fucoidan. For example, we reported that Okinawa mozuku-derived fucoidan has an immune cell proliferative effect in mice, enhances macrophage phagocytosis, increases IgM, IgG, and IgA production, and suppresses IgE production [17]. Nagamine et al. [18] also reported that fucoidan activates natural killer (NK) cells in male cancer survivors.
In the present pilot study, we conducted a clinical trial using a placebo-controlled, randomized, double-blind, parallel-group comparison method. The primary outcome was to evaluate the effects of ingesting Okinawa mozuku-derived fucoidan on NK cells derived from healthy humans. The secondary outcome was to evaluate the safety of fucoidan consumption in healthy subjects under the observation of a medical doctor. In summary, adverse events due to the test food were not observed and Okinawa mozuku-derived fucoidan enhanced NK cell activity, especially in male subjects.
2. Results
2.1. Subject Background and Test Food Ingestion Rate
The background of the 39 subjects that completed the study is shown in Table 1. In the fucoidan group, the mean age (± standard deviation) of male and female subjects was 44.5 ± 8.0 and 46.5 ± 7.0 years, respectively; in the placebo group, these respective mean ages were 46.7 ± 6.5 years and 45.7 ± 7.1 years. The ingestion rate of the test food was 100.0%, 98.8%, 96.4%, 95.8%, and 86.9% in 33, 2, 2, 1, and 1 subject(s), respectively. The study started with 20 subjects in the fucoidan and placebo groups; however, one subject in the fucoidan group did not appear on the examination day after 8 weeks and could not be contacted. Therefore, in the fucoidan group, statistical analysis was performed with 20 subjects up to 4 weeks and 19 subjects after 8 weeks (Figure 1).

Table 1.
Background of subjects in the placebo and fucoidan groups in the present study.
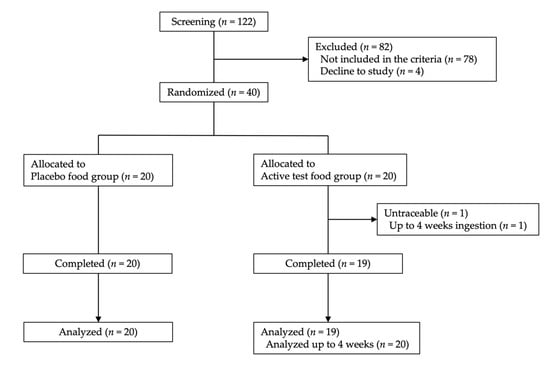
Figure 1.
Flow diagram of the clinical study procedure. Values in parentheses show the number of participants (n).
2.2. Immnologycal Assessment
2.2.1. NK Cell Activity
In the fucoidan group, a significant increase in NK cell activity was observed at 8 weeks after ingestion when compared with NK cell activity at week 0 (Figure 2a). In male subjects in the fucoidan group, a significant increase in NK cell activity was observed at 8 weeks, relative to activity at week 0 in the placebo group, after fucoidan ingestion (Figure 2b). On the other hand, there was no significant effect of fucoidan on NK cell activity in female subjects (Figure 2c). In addition, NK cell activity results were described in Supplementary Materials (Tables S1 and S2).
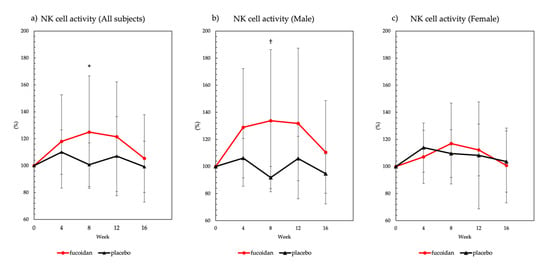
Figure 2.
Natural killer (NK) cell activity. NK cell activity was expressed as relative to basal value (100%). All data were presented as mean ± standard deviation (n = 20 subjects per group). NK cell activity in (a) all subjects, (b) males, and (c) females. * Significant difference compared with week 0 within the fucoidan group (p < 0.05, Dunnett’s multiple test). † Significant difference between the placebo and fucoidan groups within the same week (p < 0.05, t-test).
2.2.2. Interferon-γ (IFN-γ) and Interleukin-2 (IL-2) Concentrations in the Blood
In tests of plasma IFN-γ concentrations, the number of fucoidan group subjects in which IFN-γ was detected (0, 4, and 8 weeks: 4 cases per week; 12 weeks: 6 cases) during the ingestion period tended to increase, whereas the number of placebo group subjects in which IFN-γ was detected (0 weeks: 5 cases; 4 weeks: 6 cases; 8 weeks: 3 cases; and 12 weeks: 4 cases) tended to decrease (Table 2). On the other hand, plasma IL-2 concentrations were below the detection limit in all subjects during the test period (data not shown). In addition, blood test results were graphed in Supplementary Materials (Figure S1).

Table 2.
Number of detection in IFN-γ.
In addition, the number of detections in IFN-γ results were graphed in Supplementary Materials (Figure S2).
2.3. Safty Assessment
2.3.1. Blood Tests
There were no significant differences in white blood cell count, hemoglobin, platelet count, or hematocrit between the placebo and fucoidan groups during the study period (Table 3). In addition, blood test results were graphed in Supplementary Materials (Figures S2 and S3).

Table 3.
Blood test results.
2.3.2. Biochemical Tests
The results of the biochemical tests showed no abnormalities (Table 4). In the fucoidan and placebo group, compared with measurements at the same week, significant changes were observed at 12 and 16 weeks for magnesium (Mg), 8 weeks for iron (Fe). In addition, blood test results were graphed in Supplementary Materials (Figures S4–S12).

Table 4.
Biochemical test results.
2.3.3. Evaluation of Adverse Events
To evaluate the safety of the test food, adverse events during the ingestion period were recorded. Across both groups, 28 adverse events were observed during the ingestion period. However, following interviews and medical examinations by a doctor, it was concluded that serious adverse events had not occurred, and causal relationships with the test food did not exist. In addition, subjects recovered from all adverse events without further problems (Table 5).

Table 5.
List of adverse events during the ingestion period.
3. Discussion
3.1. NK Cell Activity
The raw materials for fucoidan, i.e., edible seaweeds such as kombu, wakame, and mozuku, have a long history of being consumed as food in Japan. In the present study, fucoidan derived from mozuku (C. okamuranus) was used as a test sample. In previous studies, fucoidan has been reported to have effects on immunity in animals [17,19] and humans [18,20]. In the present study, healthy subjects were randomly allocated to fucoidan and placebo groups, and peripheral-blood-derived NK cell activity was evaluated after daily oral ingestion of fucoidan (3 g) for 12 weeks. Results showed that NK cell activity was significantly enhanced in the fucoidan group after ingestion of fucoidan; specifically, this activity was significantly enhanced in male subjects but not in female subjects. Normal cells have MHC class I receptors on their surface that suppress the activity of NK cells. However, cancer cells and virus-infected cells have decreased or deficient MHC class I expression, in which case the activation signal for NK cells is increased; this is a characteristic of attacking and removing cancer cells and virus-infected cells. In the present study, however, healthy adults were tested; thus, conditions related to cancer and virus-infected cells are not relevant.
In general, the activation of NK cells requires the secretion of cytokines, such as IFN-γ, IL-2, and IL-12, by macrophages and helper T cells. In the present study, IFN-γ in the blood tended to increase over time in fucoidan group subjects, whereas IL-2 concentrations did not change significantly in any subject in either group during the ingestion and washout periods. Previously, Takahashi et al. [21] conducted an oral ingestion study of fucoidan in subjects with advanced cancer and reported that the production of IL-1β, IL-6, and TNF-α was significantly reduced 2 weeks after ingestion. In addition, Ohnogi et al. [22] reported that healthy subjects (one male and 14 female) who ingested fucoidan derived from Gagome kombu (Kjellmaniella crassifolia) for 4 weeks showed a significant suppression in the decrease of IFN-γ and IL-2 production. In a previous study in which fucoidan was administered to mice, NK cell activation significantly enhanced the production of IFN-γ [23]. Similarly, Murayama et al. [24] reported increased IFN-γ production in mice administered fucoidan, suggesting that the mechanism of action was via helper Th1 cell enhancement.
Previous studies have reported that macrophages are involved in the activation of NK cells by fucoidan. For example, we previously revealed that fucoidan significantly enhances IL-2 and IFN-γ levels in mice as well as macrophage phagocytosis [17]. Additional flows, other than those of cytokines, are involved in the activation of macrophages, e.g., radical scavenger receptors are involved. These receptors widely recognize negatively charged macromolecules such as low-density lipoproteins, lipopolysaccharides, and lipoteichoic acid [25,26]. Since fucoidan is a negatively charged polymer to which a sulfate group is bound, it is considered that fucoidan activity is enhanced via the radical scavenger receptor of macrophages. In addition, Miyazaki et al. [27] reported that fucoidan binds to the plasma membrane of macrophages to increase the production of nitric oxide and TNF-α. This suggests that the reaction is mediated by several pattern recognition receptors, such as Dectin-1, on the cell surface rather than through the phagocytosis of macrophages. Thus, it can be inferred that cytokines such as IFN-γ, which are produced by macrophages, are involved in the activation of NK cells by fucoidan. Overall, no significant change in IFN-γ concentration was observed in the present study; however, the cause of the seemingly fucoidan-mediated time-dependent increase in subjects with IFN-γ in the blood requires further investigation.
Innate and adaptive immunity tends to be higher in women than in men, but the number of NK cells in men is higher than that in women [28]. Specifically, the normal range of NK cell activity is higher in adult males (post-puberty/adulthood) but becomes higher in females at old age [29]. In addition, hormones, genes, environment, age, etc. are also related to gender-specific differences in immune responses [30]. The mean age of the subjects in the fucoidan ingestion group in the present study was 47.0 ± 7.6 years, which is the period in which male NK cell activity is high. In addition, Nagamine et al. [18] conducted a fucoidan administration study in cancer survivors and reported that the NK cell activity of older male subjects (mean age: 73.9 ± 4.9; n = 11) was significantly higher than that of female subjects (mean age: 59.0 ± 7.7; n = 4). In a previous ex vivo study using ovariectomized rats [31], it was reported that fucoidan-treated NK cells had enhanced tumoricidal activity, but fucoidan-free standard diet-treated NK cells did not. This result suggests that fucoidan supplementation induces NK cell activity, regulating immunity caused by estrogen deficiency. In other words, female immune regulation is affected by organs such as the ovaries, and fucoidan may act on postmenopausal immunoregulation. According to these results, mozuku-derived fucoidan seems to enhance the activity of NK cells in males. The underlying mechanism of this effect, however, has yet to be evidenced and requires further investigation. In the future, we would like to increase the number of subjects and investigate the immunomodulatory effect of mozuku derived from fucoidan.
3.2. Safety Assessment
In the present study, fucoidan did not cause problematic adverse events when ingested at 3 g per day for 12 weeks. In addition, abnormalities were not detected in blood and biochemical tests. Although adverse events were occasionally observed during the study period, all were judged by the medical doctor to be unrelated to fucoidan ingestion, and patients recovered after the study. These results are consistent with mozuku, the raw material of fucoidan, being a type of seaweed that is often eaten in Japan and considered highly safe as a food item. Similar to the present results, Abe et al. [32] reported blood and urinalysis test results showing that ingestion of 4 g of mozuku-derived fucoidan daily for 2 weeks was safe. In addition, we previously reported no abnormalities in blood and biochemical tests performed on healthy Japanese adults following ingestion of 2 g of mozuku-derived fucoidan daily for 4 weeks [33]. High intake of fucoidan has been reported to cause diarrhea [7]; however, fucoidan intake in suitable amounts can improve defecation [34]. Diarrhea was not reported by the participants of the present study.
In conclusion, we have shown that Okinawa mozuku-derived fucoidan is safe as a food item and that it enhances NK cell activity, especially in males. In future research, we intend to investigate the effects of fucoidan on dendritic cells, macrophages, and cytokines (as examples) to elucidate the immunomodulatory action of these sulfated polysaccharides.
4. Materials and Methods
4.1. Materials
A beverage containing 1.5 g/50 mL of fucoidan derived from Okinawa mozuku (South Product, Uruma, Japan) was used as a sample for the fucoidan treatment group; citric acid and sucralose were added to the raw materials (which included 51.3% l-fucose, 18.8% sulfate ions, 14.4% uronic acid; mean molecular weight: 73.4 kDa). For the placebo group, citric acid and sucralose were blended in a beverage with caramel, which was used to ensure that the appearance of the placebo beverage did not differ from that of the fucoidan beverage.
4.2. Subjects
The study was conducted according to the Declaration of Helsinki. The study implementation plan, subject diary, and consent form were approved by the ethics review committee of Nihonbashi Cardiology Clinic, Tokyo, Japan (UMIN000043804), who also gave final approval to conduct the study. Moreover, the study was conducted under the guidance of a doctor at the Shinagawa Season Terrace Healthcare Clinic, Tokyo, Japan. The subjects were healthy men and women between the ages of 20 and 65 years old; each subject gave written consent after being provided with a sufficient explanation of the study. The target number of participants was set as the number required for statistical analysis. Subjects were recruited by KSO Corporation (Tokyo, Japan) and were assigned by block randomization to ensure that independent variables, e.g., age, gender, etc., did not differ significantly.
4.3. Study Design
The controller assigned subjects to two groups—the fucoidan and placebo groups—in a randomized, double-blind, parallel-group, placebo-controlled study (Figure 1). Both groups ingested two bottles containing samples per day. In a previous study [13], the amount of fucoidan detected in human blood and urine was 1 g/day. In addition, Abe et al. [32] administered fucoidan at a dose of 4.05 g/day, which was determined to be safe for human consumption; thus, the dose in this study was set to 3 g/day. The study was conducted from 17 July to 26 December 2016 (Figure 3).
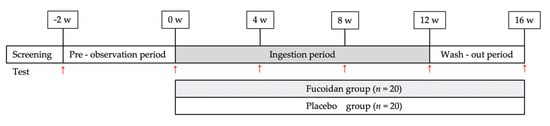
Figure 3.
Overall study design. w: week; red arrows: testing of subjects; n: number of subjects.
4.4. Examination
All specimen measurements were performed by LSI Medience Corporation (Tokyo, Japan).
4.4.1. NK Cell Activity
NK cell activity was measured in the K562 cell line (Dainippon Pharmaceutical, Japan) labeled with 51Cr using a cytotoxicity test. The NK cell activity test used subject blood on the day of first ingestion (week 0) and after 4, 8, 12, and 16 weeks. Blood samples were collected into heparinized tubes. After centrifugation of the blood samples with a lymphocyte separation medium, interface mononuclear cells were collected and suspended in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS). The samples were centrifuged again, and mononuclear cells were collected and mixed with 51Cr-labeled target cells (K562) at a ratio of 50:1. The cell mixture was cultured at 37 °C and 5% CO2 for 4 h. The 51Cr released from the target cells by NK cell cytotoxicity was determined using a gamma counter (ARC370, Hitachi Aloka Medical, Mitaka, Japan). The percentage of cytotoxicity was calculated as follows: cytotoxicity (%) = (experimental 51Cr release − spontaneous 51Cr release)/(maximal 51Cr release − spontaneous 51Cr release) × 100.
4.4.2. INF-γ and IL-2 Concentrations in the Blood
IFN-γ (Human IFN-gamma Quantikine ELISA Kit, R&D Systems, Minneapolis, MN, USA) and IL-2 (Human IL-2 Quantikine ELISA Kit, R&D Systems, Minneapolis, MN, USA) concentrations in the plasma of subjects were measured using sandwich ELISA on the day of first ingestion (week 0) and after 4, 8, 12, and 16 weeks.
4.4.3. Blood Tests, Biochemical Tests, and Safety Assessment
Blood tests (white blood cells, red blood cells, hemoglobin, hematocrit, and platelets) and biochemical tests (aspartate aminotransferase, alanine aminotransferase, LDH, T-bil, alkaline phosphatase, γ-glutamyl transpeptidase, creatine kinase, fasting blood sugar, HbA1c, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, total protein, albumin, urea nitrogen, CRE, uric acid, sodium, chlorine, potassium, calcium, inorganic phosphorus, magnesium, and iron) were performed on the day of first ingestion (week 0) and 4, 8, 12, and 16 weeks later. In addition, during the same period, a doctor interviewed and examined the subjects as a safety evaluation.
4.5. Statistical Analysis
Data are presented as means ± standard deviation. Data were analyzed using Dunnett’s multiple-test within groups and Welch’s t-test between groups. p < 0.05 was considered to indicate statistical significance. Statcel version 4 software (OMS Publishing, Japan) was used to conduct all statistical analyses.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/md19060340/s1. Table S1: NK cell activity in fucoidan group; Table S2: NK cell activity in placebo group; Figure S1: Detection number of IFN-γ; Figure S2: Blood test result (WBC, RBC and Hb); Figure S3: Blood test result (Ht and Plt); Figure S4: Biochemical test result (AST, ALT and LF); Figure S5: Biochemical test result (T-bill, ALP and γ-GT); Figure S6: Biochemical test result (CK, FBS and HbA1c); Figure S7: Biochemical test result (TC, LDL-C and HDL-C); Figure S8: Biochemical test result (TG, TP and Alb); Figure S9: Biochemical test result (UN, CRE and UA); Figure S10: Biochemical test result (Na, Cl and K); Figure S11: Biochemical test result (Ca, IP and Mg); Figure S12: Biochemical test result (Fe).
Author Contributions
M.T., T.N., and M.I. designed the study; M.T. wrote the manuscript; T.N., T.M., and M.I. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by South Product.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee Nihonbashi Cardiology Clinic (Protocol code: OSP-001-05, date of approval: 4 July 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are not publicly available due to privacy.
Acknowledgments
We would like to thank Eiji Yoshiokawa and Nobuhiko Shioya (KSO Corporation) for support with all experiments and statistical analysis.
Conflicts of Interest
M.T. and M.I. received compensation from South Product. Test material (Okinawa mozuku fucoidan) was provided by South Product.
References
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D′Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Prokofjeva, M.M.; Imbs, T.I.; Shevchenko, N.M.; Spirin, P.V.; Horn, S.; Fehse, B.; Zvyagintseva, T.N.; Prassolov, V.S. Fucoidans as potential inhibitors of HIV-1. Mar. Drugs 2013, 11, 3000–3014. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.T.; Ly, B.M.; Van, T.T.T.; Van Quang, N.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohyd. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Hidari, K.I.; Takahashi, N.; Arihara, M.; Nagaoka, M.; Morita, K.; Suzuki, T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem. Bioph. Res. Commun. 2008, 376, 91–95. [Google Scholar] [CrossRef]
- Haneji, K.; Matsuda, T.; Tomita, M.; Kawakami, H.; Ohshiro, K.; Uchihara, J.N.; Masuda, M.; Takasu, N.; Tanaka, Y.; Ohta, T.; et al. Fucoidan extracted from Cladosiphon okamuranus Tokida induces apoptosis of human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. Nutr. Cancer 2005, 52, 189–201. [Google Scholar] [CrossRef]
- Araya, N.; Takahashi, K.; Sato, T.; Nakamura, T.; Sawa, C.; Hasegawa, D.; Ando, H.; Aratani, S.; Yagishita, N.; Fujii, R.; et al. Fucoidan therapy decreases the proviral load in patients with human T-lymphotropic virus type-1-assoiciated neurological disease. Antivir. Ther. 2011, 16, 89–98. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The anti-cancer effects of fucoidan: A review of both in vivo and in vitro investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef]
- Mori, N.; Nakasone, K.; Tomimori, K.; Ishikawa, C. Beneficial effects of fucoidan in patients with chronic hepatitis C virus infection. World J. Gastroenterol. 2012, 18, 2225–2230. [Google Scholar] [CrossRef]
- Choi, J.I.; Raghavendran, H.R.B.; Sung, N.Y.; Kim, J.H.; Chun, B.S.; Ahn, D.H.; Choi, H.S.; Kang, K.W.; Lee, J.W. Effect of fucoidan on aspirin-induced stomach ulceration in rats. Chem. Biol. Interact. 2010, 183, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Shibata, H.; Kimura-Takagi, I.; Hashimoto, S.; Kimura, K.; Makino, T.; Aiyama, R.; Ueyama, S.; Yokokura, T. Structural study of fucoidan from Cladosiphon okamuranus Tokida. Glycoconj. J. 1999, 16, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Tokita, Y.; Nakajima, K.; Mochida, H.; Iha, M.; Nagamine, T. Development of a fucoidan-specific antibody and measurement of fucoidan in serum and urine by sandwich ELISA. Biosci. Biotech. Biochem. 2010, 74, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, T.; Nakazato, K.; Tomioka, S.; Iha, M.; Nakajima, K. Intestinal absorption of fucoidan extracted from the brown seaweed, Cladosiphon okamuranus. Mar. Drugs 2015, 13, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Kadena, K.; Tomori, M.; Iha, M.; Nagamine, T. Absorption study of mozuku fucoidan in Japanese volunteers. Mar. Drugs 2018, 16, 254. [Google Scholar] [CrossRef]
- Tomori, M.; Nagamine, T.; Iha, M. Are Helicobacter pylori infection and fucoidan consumption associated with fucoidan absorption? Mar. Drugs 2020, 18, 235. [Google Scholar] [CrossRef]
- Tomori, M.; Nagamine, T.; Miyamoto, T.; Iha, M. Evaluation of the immunomodulatory effects of fucoidan derived from Cladosiphon okamuranus Tokida in mice. Mar. Drugs 2019, 17, 547. [Google Scholar] [CrossRef]
- Nagamine, T.; Kadena, K.; Tomori, M.; Nakajima, K.; Iha, M. Activation of NK cells in male cancer survivors by fucoidan extracted from Cladosiphon okamuranus. Mol. Clin. Oncol. 2020, 12, 81–88. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Hashimoto, M.; Nakano, T. Suppression of Th2 immune responses by mekabu fucoidan from Undaria pinnatifida sporophylls. Int. Arch. Allergy Immunol. 2005, 137, 289–294. [Google Scholar] [CrossRef]
- Negishi, H.; Mori, M.; Mori, H.; Yamori, Y. Supplementation of elderly Japanese men and women with fucoidan from seaweed increases immune responses to seasonal influenza vaccination. J. Nutr. 2013, 143, 1794–1798. [Google Scholar] [CrossRef]
- Takahashi, H.; Kawaguchi, M.; Kitamura, K.; Narumiya, S.; Kawamura, M.; Tengan, I.; Nishimoto, S.; Hanamure, Y.; Majima, Y.; Tsubura, S.; et al. An exploratory study on the anti-inflammatory effects of fucoidan in relation to quality of life in advanced cancer patients. Integr. Cancer Ther. 2018, 17, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Ohnogi, H.; Naito, Y.; Higashimura, Y.; Uno, K.; Yoshikawa, T. Immune efficacy and safety of fucoidan extracted from Gagome kombu (Kjellmaniella crassifolia) in healthy Japanese subjects. Jpn. J. Complement. Altern. Med. 2015, 12, 87–93. [Google Scholar]
- Zhang, W.; Okimura, T.; Oda, T.; Jin, O. Ascophyllan induces activation of natural killer cells in mice in vivo and in vitro. Mar. Drugs 2019, 17, 197. [Google Scholar] [CrossRef] [PubMed]
- Murayama, H.; Tamauchi, H.; Iizuka, M.; Nakano, T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Med. 2006, 72, 1415–1417. [Google Scholar] [CrossRef]
- Doi, T.; Higashino, K.I.; Kurihara, Y.; Wada, Y.; Miyazaki, T.; Nakamura, H.; Uesugi, S.; Imanishi, T.; Kawabe, Y.; Itakura, H. Charged collagen structure mediate the recognition of negatively charged macromolecules by macrophage scavenger receptors. J. Biol. Chem. 1993, 268, 2126–2133. [Google Scholar] [CrossRef]
- Lin, Z.; Tan, X.; Zhang, Y.; Li, F.; Luo, P.; Liu, H. Molecular targets and related biologic activities of fucoidan: A review. Mar. Drugs 2020, 18, 376. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Iwaihara, Y.; Bak, J.; Nakano, H.; Takeuchi, S.; Takeuchi, H.; Matsui, T.; Tachikawa, D. The cooperative induction of macrophage activation by fucoidan derived Cladosiphon okamuranus and β-glucan derived from Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2019, 516, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, P.; Deane, J.A.; Difilippantonio, M.J.; Tarasenko, T.; Satterthwaite, A.B.; Bolland, S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 2006, 312, 1669–1672. [Google Scholar] [CrossRef]
- Ngo, S.T.; Steyn, F.J.; McCombe, P.A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 2014, 35, 347–369. [Google Scholar] [PubMed]
- Namkoong, S.; Kim, J.Y.; Kim, T.; Sohn, H.E. Immunomodulatory effects of fucoidan on NK cells in ovariectomized rats. Korean J. Plant Res. 2012, 25, 317–322. [Google Scholar] [CrossRef]
- Abe, S.; Hiramatsu, K.; Ichikawa, O.; Kawamoto, H.; Kasagi, T.; Miki, Y.; Kimura, T.; Ikeda, T. Safety evaluation of excessive ingestion of mozuku fucoidan in human. J. Food Sci. 2013, 78, T648–T651. [Google Scholar] [CrossRef] [PubMed]
- Tomori, M.; Hirata, T.; Nagamine, T.; Iha, M. Safety evaluation of long-term and excessive ingestion of Cladosiphon okamuranus fucoidan in healthy subjects. A randomized, double-blind, placebo-controlled, parallel study. Jpn. Pharmacol. Ther. 2017, 45, 447–461. (In Japanese) [Google Scholar]
- Tomori, M.; Matsuda, K.; Nakamura, Y.; Kadena, K.; Shimoji, S.; Nagamine, T.; Iha, M. Effect of Cladosiphon okamuranus fucoidan containing drink on improvement of defecation in healthy subjects with mild constipation—A randomized, doble-blind, placebo-controlled, crossover study. Jpn. Pharmacol. Ther. 2016, 44, 1621–1626. (In Japanese) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).