Optimizing Chitin Depolymerization by Lysozyme to Long-Chain Oligosaccharides
Abstract
1. Introduction
2. Results and Discussion
2.1. Initial Screening of HEWL Activity
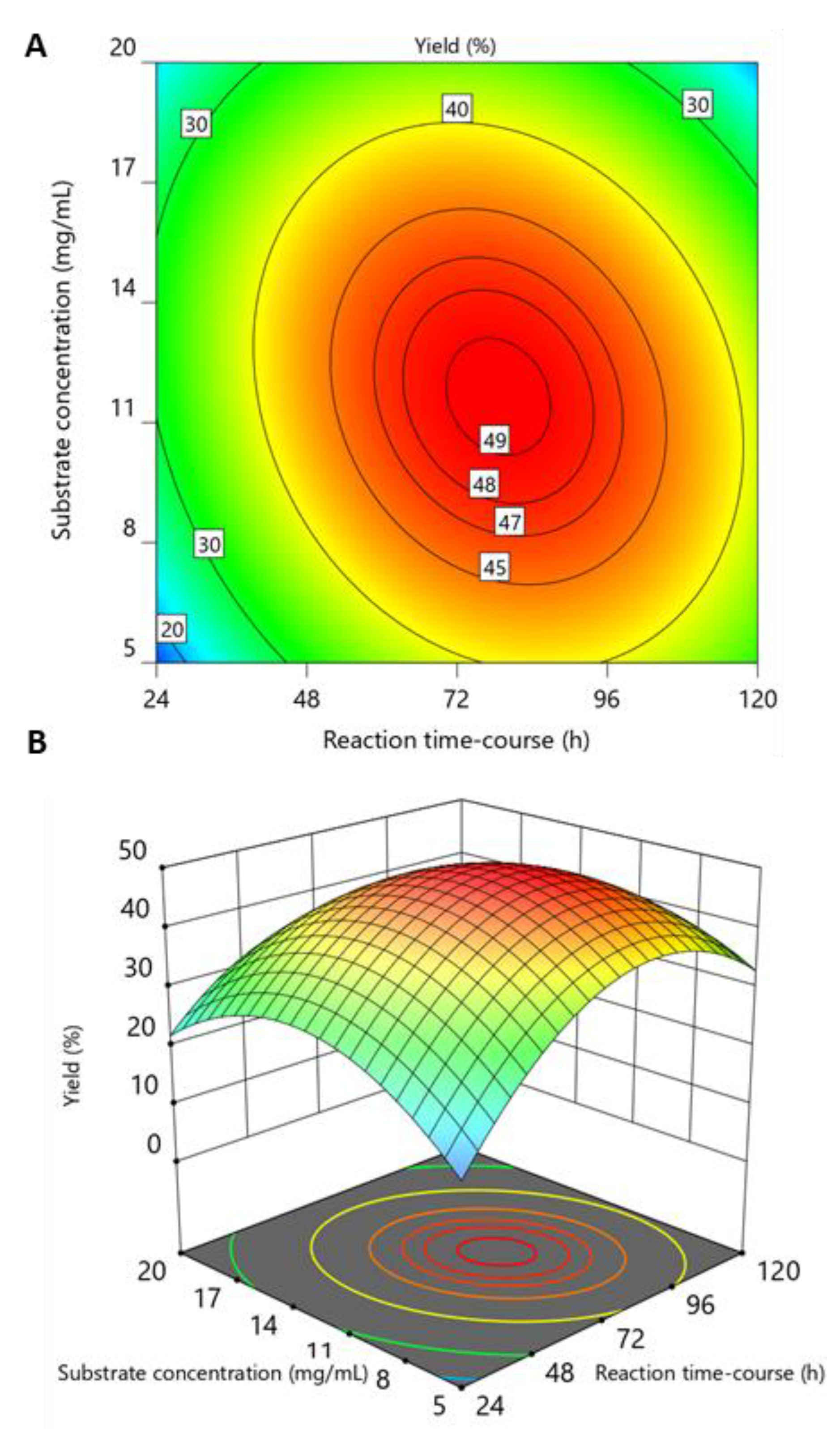
2.2. Optimization of WSC Hydrolysis
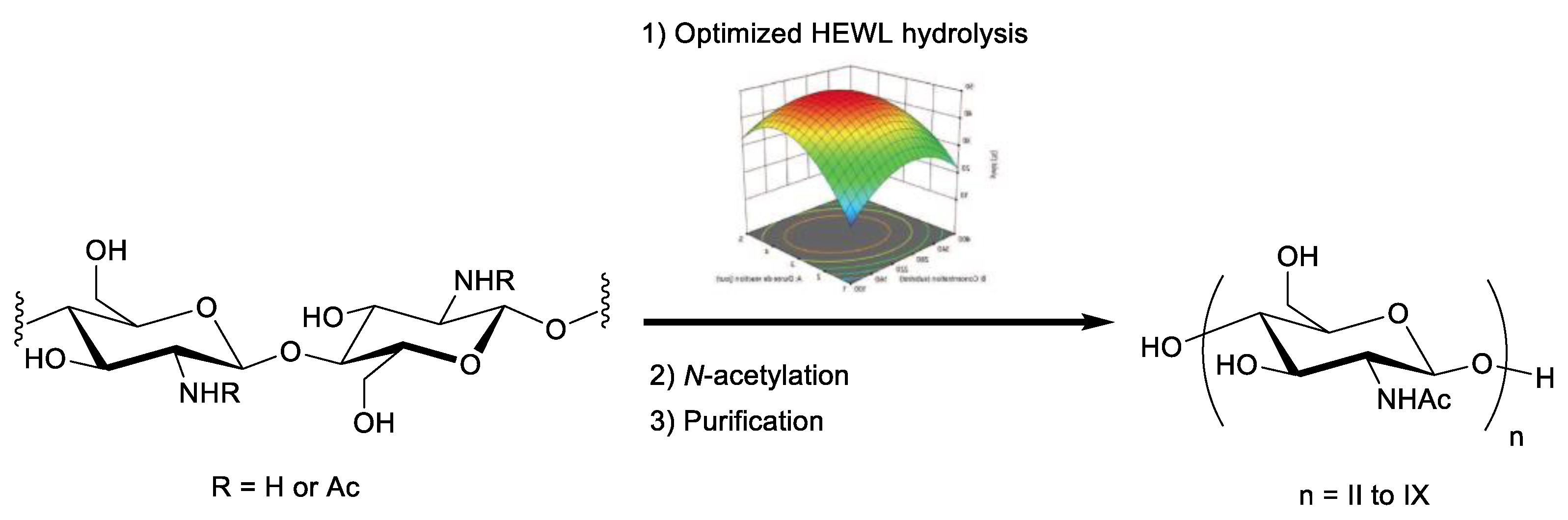
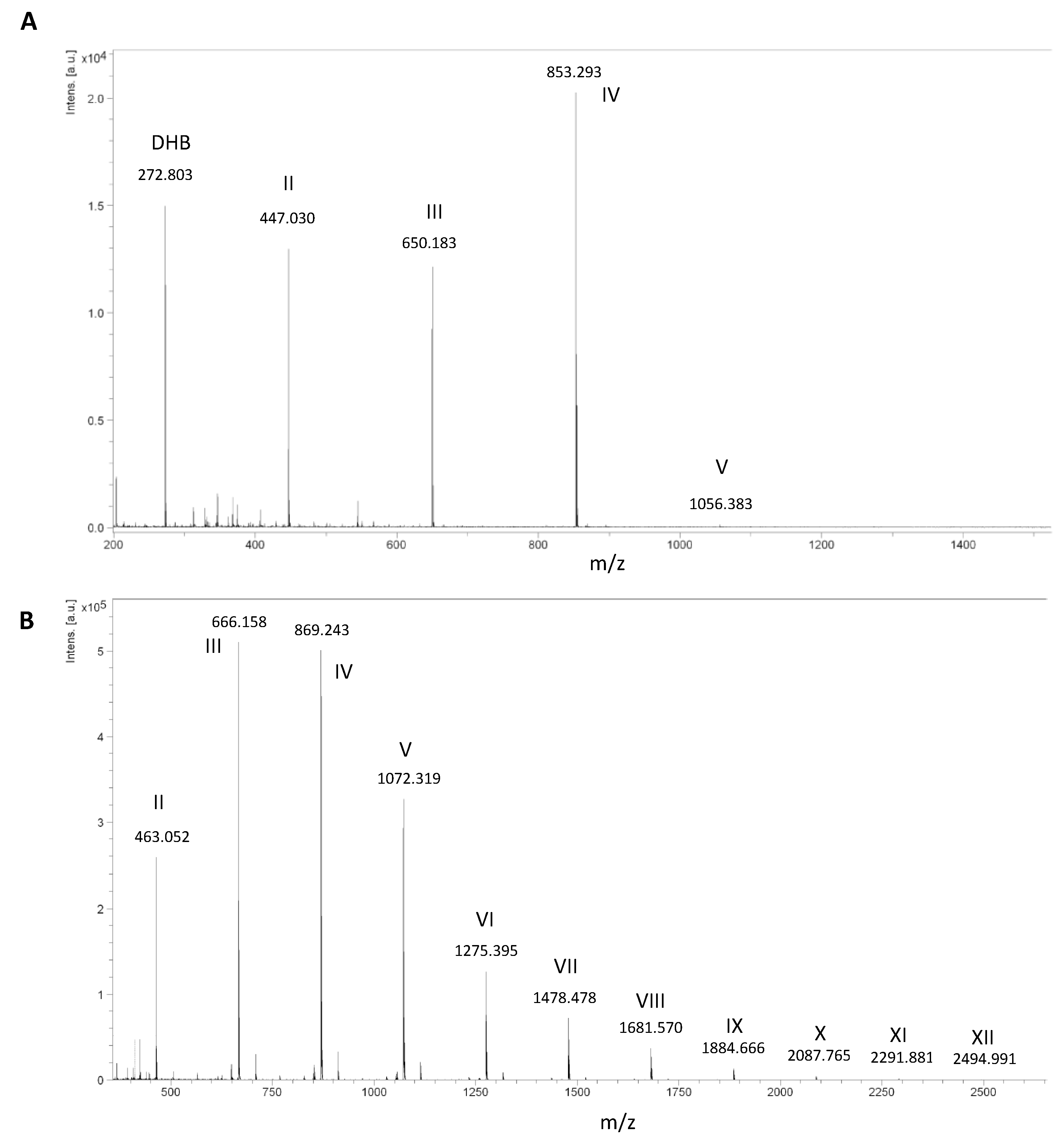
2.3. Validation of the Model and Optimized Synthesis of CO-II to CO-IX
3. Materials and Methods
3.1. Material and Equipment
3.2. Determination of HEWL Activity with 4-Mu-CO-III
3.3. Box-Behnken Design
3.4. Preparation of Water-Soluble Chitin (WSC) DA 0.32/0.42/0.47/0.59
3.5. Experimental Design of WSC Hydrolysis by HEWL
3.6. Optimized Preparation of COs from WSC DA 0.47
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, K.; Xing, R.; Liu, S.; Li, P. Chitin and Chitosan Fragments Responsible for Plant Elicitor and Growth Stimulator. J. Agric. Food Chem. 2020, 68, 12203–12211. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The Undisputed Biomolecule of Great Potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Yan, N.; Chen, X. Sustainability: Don’t Waste Seafood Waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood Waste: A Source for Preparation of Commercially Employable Chitin/Chitosan Materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef]
- Day, R.B.; Okada, M.; Ito, Y.; Tsukada, K.; Zaghouani, H.; Shibuya, N.; Stacey, G. Binding Site for Chitin Oligosaccharides in the Soybean Plasma Membrane. Plant Physiol. 2001, 126, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Halane, M.K.; Gassmann, W.; Stacey, G. The Role of Plant Innate Immunity in the Legume-Rhizobium Symbiosis. Annu. Rev. Plant Biol. 2017, 68, 535–561. [Google Scholar] [CrossRef] [PubMed]
- Bozsoki, Z.; Cheng, J.; Feng, F.; Gysel, K.; Vinther, M.; Andersen, K.R.; Oldroyd, G.; Blaise, M.; Radutoiu, S.; Stougaard, J. Receptor-Mediated Chitin Perception in Legume Roots Is Functionally Separable from Nod Factor Perception. Proc. Natl. Acad. Sci. USA 2017, 114, E8118–E8127. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Rodriguez-Moreno, L.; Mansurkhodzaev, A.; Wang, P.; van den Berg, W.; Gasciolli, V.; Cottaz, S.; Fort, S.; Thomma, B.P.H.J.; Bono, J.J.; et al. A Lysin Motif Effector Subverts Chitin-Triggered Immunity to Facilitate Arbuscular Mycorrhizal Symbiosis. New Phytol. 2020, 225, 448–460. [Google Scholar] [CrossRef]
- Genre, A.; Chabaud, M.; Balzergue, C.; Puech-Pagès, V.; Novero, M.; Rey, T.; Fournier, J.; Rochange, S.; Bécard, G.; Bofante, P.; et al. Short-Chain Chitin Oligomers from Arbuscular Mycorrhizal Fungi Trigger Nuclear Ca2+ Spiking in Medicago Truncatula Roots and Their Production Is Enhanced by Strigolactone. New Phytol. 2013, 198, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, G.; Chabaud, M.; Miyata, K.; Capozzi, M.; Takeda, N.; Kaku, H.; Shibuya, N.; Nakagawa, T.; Barker, D.G.; Genre, A. The Rice LysM Receptor-like Kinase OsCERK1 Is Required for the Perception of Short-Chain Chitin Oligomers in Arbuscular Mycorrhizal Signaling. New Phytol. 2017, 214, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Miller, J.B.; Granqvist, E.; Wiley-Kalil, A.; Gobbato, E.; Maillet, F.; Cottaz, S.; Samain, E.; Venkateshwaran, M.; Fort, S.; et al. Activation of Symbiosis Signaling by Arbuscular Mycorrhizal Fungi in Legumes and Rice. Plant Cell 2015, 27, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Sun, J.; Radhakrishnan, G.V.; Lee, T.; Bozsóki, Z.; Fort, S.; Gavrin, A.; Gysel, K.; Thygesen, M.B.; Andersen, K.R.; et al. A Combination of Chitooligosaccharide and Lipochitooligosaccharide Recognition Promotes Arbuscular Mycorrhizal Associations in Medicago Truncatula. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Z.; Song, C.; Hu, Y.; Han, Z.; She, J.; Fan, G.; Wang, J.; Jin, C.; Chang, J.; et al. Chitin-Induced Dimerization Activates a Plant Immune Receptor. Science 2012, 336, 1160–1164. [Google Scholar] [CrossRef]
- Bozsoki, Z.; Gysel, K.; Hansen, S.B.; Lironi, D.; Krönauer, C.; Feng, F.; de Jong, N.; Vinther, M.; Kamble, M.; Thygesen, M.B.; et al. Ligand-Recognizing Motifs in Plant LysM Receptors Are Major Determinants of Specificity. Science 2020, 369, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sørlie, M.; Vårum, K.M.; Eijsink, V.G.H. Production of Chitooligosaccharides and Their Potential Applications in Medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, B. Recent Advances in the Synthesis of Chitooligosaccharides and Congeners. Tetrahedron 2014, 70, 1023–1046. [Google Scholar] [CrossRef]
- Kuyama, H.; Nakahara, Y.; Nukada, T.; Ito, Y.; Nakahara, Y.; Ogawa, T. Preliminary Communication Stereocontrolled Synthesis of Chitosan Dodecamer. Carbohydr. Res. 1993, 243, 1–7. [Google Scholar] [CrossRef]
- Tyrikos-Ergas, T.; Bordoni, V.; Fittolani, G.; Chaube, M.A.; Grafmüller, A.; Seeberger, P.H.; Delbianco, M. Systematic Structural Characterization of Chitooligosaccharides Enabled by Automated Glycan Assembly. Chem. A Eur. J. 2020, 27, 2321–2325. [Google Scholar] [CrossRef]
- Bredehorst, R.; Pomato, N.; Scheel, O.; Thiem, J. High Yield Preparation of Dimeric to Decameric Chitin Oligomers. Patent Number WO/1996/026965, 6 September 1996. [Google Scholar]
- Hoell, I.A.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Structure and Function of Enzymes Acting on Chitin and Chitosan. Biotechnol. Genet. Eng. Rev. 2010, 27, 331–366. [Google Scholar] [CrossRef]
- Kohri, M.; Kobayashi, A.; Shoda, S. Design and Utilization of Chitinases with Low Hydrolytic Activities. Trends Glycosci. Glycotechnol. 2007, 19, 165–180. [Google Scholar] [CrossRef][Green Version]
- Alsina, C.; Sancho-Vaello, E.; Aranda-Martínez, A.; Faijes, M.; Planas, A. Auxiliary Active Site Mutations Enhance the Glycosynthase Activity of a GH18 Chitinase for Polymerization of Chitooligosaccharides. Carbohydr. Polym. 2021, 252, 117121. [Google Scholar] [CrossRef] [PubMed]
- Alsina, C.; Faijes, M.; Planas, A. Glycosynthase-Type GH18 Mutant Chitinases at the Assisting Catalytic Residue for Polymerization of Chitooligosaccharides. Carbohydr. Res. 2019, 478, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.A.; Boer, H.; Koivula, A.; Samain, E.; Driguez, H.; Armand, S.; Cottaz, S. Engineering Chitinases for the Synthesis of Chitin Oligosaccharides: Catalytic Amino Acid Mutations Convert the GH-18 Family Glycoside Hydrolases into Transglycosylases. J. Mol. Catal. B Enzym. 2012, 74, 89–96. [Google Scholar] [CrossRef]
- Usui, T.; Matsui, H.; Isobe, K. Enzymic Synthesis of Useful Chito-Oligosaccharides Utilizing Transglycosylation by Chitinolytic Enzymes in a Buffer Containing Ammonium Sulfate. Carbohydr. Res. 1990, 203, 65–77. [Google Scholar] [CrossRef]
- Ercan, D.; Demirci, A.; Ercan, D.; Demirci, A. Recent Advances for the Production and Recovery Methods of Lysozyme. Crit. Rev. Biotechnol. 2016, 36, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Chipman, D.M.; Sharon, N. Mechanism of Lysozyme Action. Science 1965, 165, 454–465. [Google Scholar] [CrossRef]
- Pollock, J.J.; Sharon, N. Studies on the Acceptor Specificity of the Lysozyme-Catalyzed Transglycosylation Reaction. Biochemistry 1970, 9, 3913–3925. [Google Scholar] [CrossRef]
- Yang, T.S.; Cunningham, F.E. Stability of Egg White Lysozyme in Combination with Other Antimicrobial Substances. J. Food Prot. 1993, 56, 153–156. [Google Scholar] [CrossRef]
- Berger, L.R.; Weiser, R.S. The β-Glucosaminidase Activity of Egg-White Lysozyme. BBA Biochim. Biophys. Acta 1957, 26, 517–521. [Google Scholar] [CrossRef]
- Amano, K.-I.; Ito, E. The Action of Lysozyme on Partially Deacetylated Chitin. Eur. J. Biochem. 1978, 85, 97–104. [Google Scholar] [CrossRef]
- Stokke, B.T.; Vårum, K.M.; Holme, H.K.; Hjerde, R.J.N.; Smidsrød, O. Sequence Specificities for Lysozyme Depolymerization of Partially N-Acetylated Chitosans. Can. J. Chem. 2006, 73, 1972–1981. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Zhang, J.; Chen, C.; Wu, Q.; Zhang, L.; Zhang, X. Quantitative Determination of Chitinolytic Activity of Lysozyme Using Half-Deacetylated Chitosan as a Substrate. Carbohydr. Polym. 2011, 85, 554–559. [Google Scholar] [CrossRef]
- Pangburn, S.H.; Trescony, P.V.; Heller, J. Lysozyme Degradation of Partially Deacetylated Chitin, Its Films and Hydrogels. Biomaterials 1982, 3, 105–108. [Google Scholar] [CrossRef]
- Lee, K.Y.; Ha, W.S.; Park, W.H. Blood Compatibilitv and Biodegradability of Partially Acylated Chitosan Derivatives. Biomaterials 1995, 16, 1211–1216. [Google Scholar] [CrossRef]
- Aiba, S. Preparation of N-Acetylchitooligosaccharides from Lysozymic Hydrolysates of Partially N-Acetylated Chitosans. Carbohydr. Res. 1994, 261, 297–306. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. J. R. Stat. Soc. Series B Stat. Methodol. 1951, 13, 1–45. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some New Three Level Designs for the Study of Quantitative Variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken Design: An Alternative for the Optimization of Analytical Methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liang, J.; Gao, X.; Liu, G.; Qu, Y. Optimization of an Artificial Cellulase Cocktail for High-Solids Enzymatic Hydrolysis of Cellulosic Materials with Different Pretreatment Methods. Bioresour. Technol. 2020, 295, 122272. [Google Scholar] [CrossRef] [PubMed]
- Angellier, H.; Choisnard, L.; Molina-Boisseau, S.; Ozil, P.; Dufresne, A. Optimization of the Preparation of Aqueous Suspensions of Waxy Maize Starch Nanocrystals Using a Response Surface Methodology. Biomacromolecules 2004, 5, 1545–1551. [Google Scholar] [CrossRef]
- Le Corre, D.; Bras, J.; Choisnard, L.; Dufresne, A. Optimization of the Batch Preparation of Starch Nanocrystals to Reach Daily Time-Scale. Starch/Staerke 2012, 64, 489–496. [Google Scholar] [CrossRef]
- Behera, H.T.; Upadhyay, A.K.; Raina, V.; Ray, L. Optimization of Media Components for the Production of N-Acetylchitooligosaccharide from Chitin by Streptomyces Chilikensis through Taguchi Experimental Design. J. Microbiol. Methods 2019, 159, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.D.; Zeng, H.Y.; Foua, G.B.; Alain, C.; Li, Y.Q. Enzymolysis of Chitosan by Papain and Its Kinetics. Carbohydr. Polym. 2016, 135, 199–206. [Google Scholar] [CrossRef]
- Liu, C.L.; Lan, C.Y.; Fu, C.C.; Juang, R.S. Production of Hexaoligochitin from Colloidal Chitin Using a Chitinase from Aeromonas Schubertii. Int. J. Biol. Macromol. 2014, 69, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hamaguchi, K. Hydrolysis of 4-Methylumbelliferyl N-Acetyl-Chitotrioside Catalyzed by Hen and Turkey Lysozymes: Ph Dependence of the Kinetic Constants. J. Biochem. 1980, 87, 1003–1014. [Google Scholar]
- Ballardie, F.W.; Capon, B.; Cuthbert, M.W.; Dearie, W.M. Some Studies on Catalysis by Lysozyme. Bioorg. Chem. 1977, 6, 483–509. [Google Scholar] [CrossRef]
- Davies, R.C.; Neuberger, A.; Wilson, B.M. The Dependence of Lysozyme Activity on pH and Ionic Strength. BBA Enzymol. 1969, 178, 294–305. [Google Scholar] [CrossRef]
- Ries-Kautt, M.M.; Ducruix, A.F. Relative Effectiveness of Various Ions on the Solubility and Crystal Growth of Lysozyme. J. Biol. Chem. 1989, 264, 745–748. [Google Scholar] [CrossRef]
- Nordtveit, R.J.; Vårum, K.M.; Smidsrød, O. Degradation of Fully Water-Soluble, Partially N-Acetylated Chitosans with Lysozyme. Carbohydr. Polym. 1994, 23, 253–260. [Google Scholar] [CrossRef]
- Lavertu, M.; Darras, V.; Buschmann, M.D. Kinetics and Efficiency of Chitosan Reacetylation. Carbohydr. Polym. 2012, 87, 1192–1198. [Google Scholar] [CrossRef]
- Box, G.E.P.; Hunter, W.G.; Hunter, J.S. Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building; Wiley-Probability and Mathematical Statistics: New York, NY, USA, 1978; ISBN 978-0-471-09315-2. [Google Scholar]
- Yin, Y.; Carter, C.W., Jr. Incomplete Factorial and Response Surface Methods in Experimental Design: Yield Optimization of TRNATrp from In Vitro T7 RNA Polymerase Transcription. Nucleic Acids Res. 1996, 24, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. Information Theory and an Extension of Maximum Likelihood Principle. In Proceedings of the Second International Symposium on Information Theory; Petrov, B.N., Csaki, F., Eds.; Akademiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Burnham, K.P., Anderson, D.R., Eds.; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95364-9. [Google Scholar]
- Nelder, J.A.; Mead, R. A Simplex Method for Function Minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Press, W.H.; Flannery, B.P.; Teukolsky, S.A.; Vetterling, W.T. Numerical Recipes in Pascal: The Art of Scientific Computing; Cambridge University Press: Cambridge, UK, 1989; ISBN 978-0-521-37516-0. [Google Scholar]
- Hattori, T.; Sakabe, Y.; Ogata, M.; Michishita, K.; Dohra, H.; Kawagishi, H.; Totani, K.; Nikaido, M.; Nakamura, T.; Koshino, H.; et al. Enzymatic Synthesis of an α-Chitin-like Substance via Lysozyme-Mediated Transglycosylation. Carbohydr. Res. 2012, 347, 16–22. [Google Scholar] [CrossRef] [PubMed]

| Independent Variables | Symbols | Units | Code Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Acetylation degree | DA | % | 0.32 | 0.42 | 0.59 |
| Substrate concentration | C | mg/mL | 5 | 122.5 | 20 |
| Reaction time-course | t | h | 24 | 72 | 120 |
| Experiment | DA | C | t | Response Y (Yield) |
|---|---|---|---|---|
| 1 | 0 | −1 | 16% | |
| 2 | 0 | −1 | +1 | 34% |
| 3 | 0 | +1 | −1 | 18% |
| 4 | 0 | +1 | +1 | 17% |
| 5 | −1 | 0 | −1 | 15% |
| 6 | −1 | 0 | +1 | 14% |
| 7 | +1 | 0 | −1 | 14% |
| 8 | +1 | 0 | +1 | 26% |
| 9 | −1 | −1 | 0 | 16% |
| 10 | −1 | +1 | 0 | 25% |
| 11 | +1 | −1 | 0 | 25% |
| 12 | +1 | +1 | 0 | 15% |
| 13 | 0 | 0 | 0 | 47% |
| 14 | 0 | 0 | 0 | 49% |
| 15 | 0 | 0 | 0 | 47% |
| 16 | 0 | 0 | 0 | 46% |
| 17 | 0 | 0 | 0 | 49% |
| Source | Sum of Squares | DF | Mean Square | F | p-Value |
|---|---|---|---|---|---|
| Model | 3037.77 | 7 | 433.97 | 20.92 | <0.0001 |
| t | 98.00 | 1 | 98.00 | 4.72 | 0.0578 |
| C | 32.00 | 1 | 32.00 | 1.54 | 0.2456 |
| DA | 12.50 | 1 | 12.50 | 0.6026 | 0.4575 |
| tC | 90.25 | 1 | 90.25 | 4.35 | 0.0666 |
| t2 | 906.76 | 1 | 906.76 | 43.71 | <0.0001 |
| C2 | 573.92 | 1 | 573.92 | 27.67 | 0.005 |
| DA2 | 1040.94 | 1 | 1040.94 | 50.18 | <0.0001 |
| Residual | 186.70 | 9 | 20.74 | / | / |
| Lack of fit | 179.50 | 5 | 35.90 | 19.94 | 0.0063 |
| Pure error | 7.20 | 4 | 1.80 | / | / |
| Cor total | 3224.47 | 16 | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masselin, A.; Rousseau, A.; Pradeau, S.; Fort, L.; Gueret, R.; Buon, L.; Armand, S.; Cottaz, S.; Choisnard, L.; Fort, S. Optimizing Chitin Depolymerization by Lysozyme to Long-Chain Oligosaccharides. Mar. Drugs 2021, 19, 320. https://doi.org/10.3390/md19060320
Masselin A, Rousseau A, Pradeau S, Fort L, Gueret R, Buon L, Armand S, Cottaz S, Choisnard L, Fort S. Optimizing Chitin Depolymerization by Lysozyme to Long-Chain Oligosaccharides. Marine Drugs. 2021; 19(6):320. https://doi.org/10.3390/md19060320
Chicago/Turabian StyleMasselin, Arnaud, Antoine Rousseau, Stéphanie Pradeau, Laure Fort, Rodolphe Gueret, Laurine Buon, Sylvie Armand, Sylvain Cottaz, Luc Choisnard, and Sébastien Fort. 2021. "Optimizing Chitin Depolymerization by Lysozyme to Long-Chain Oligosaccharides" Marine Drugs 19, no. 6: 320. https://doi.org/10.3390/md19060320
APA StyleMasselin, A., Rousseau, A., Pradeau, S., Fort, L., Gueret, R., Buon, L., Armand, S., Cottaz, S., Choisnard, L., & Fort, S. (2021). Optimizing Chitin Depolymerization by Lysozyme to Long-Chain Oligosaccharides. Marine Drugs, 19(6), 320. https://doi.org/10.3390/md19060320







