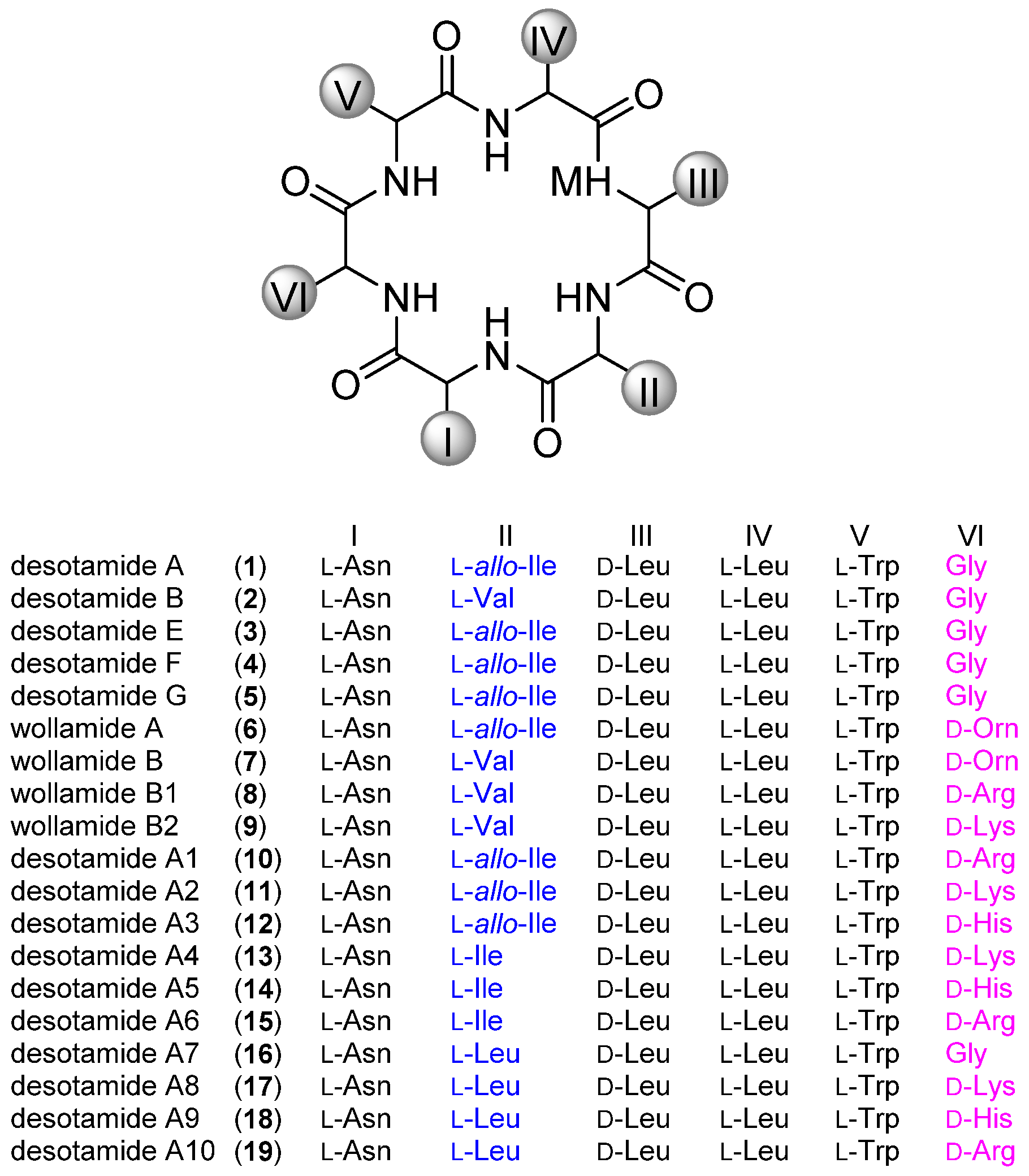
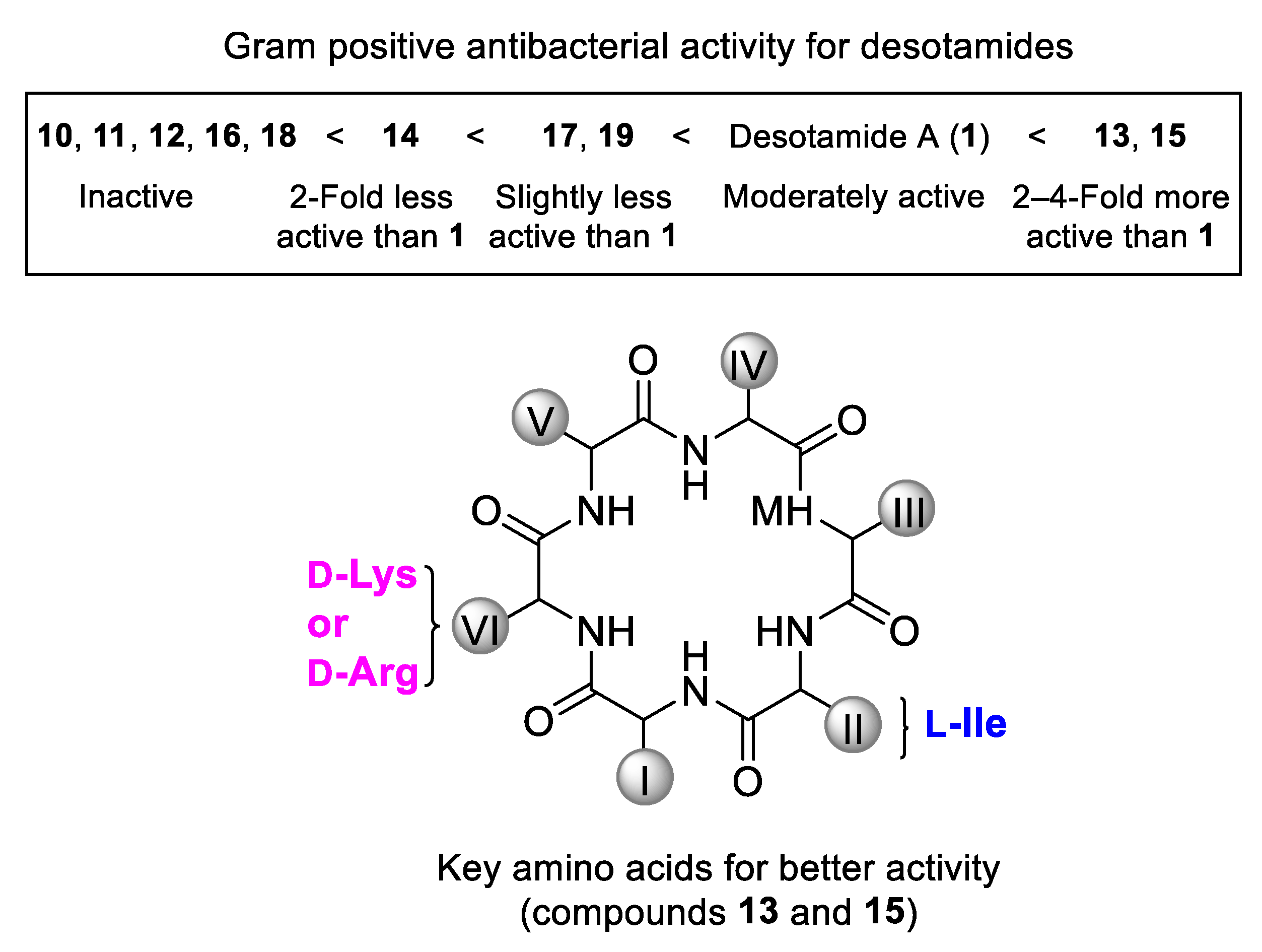
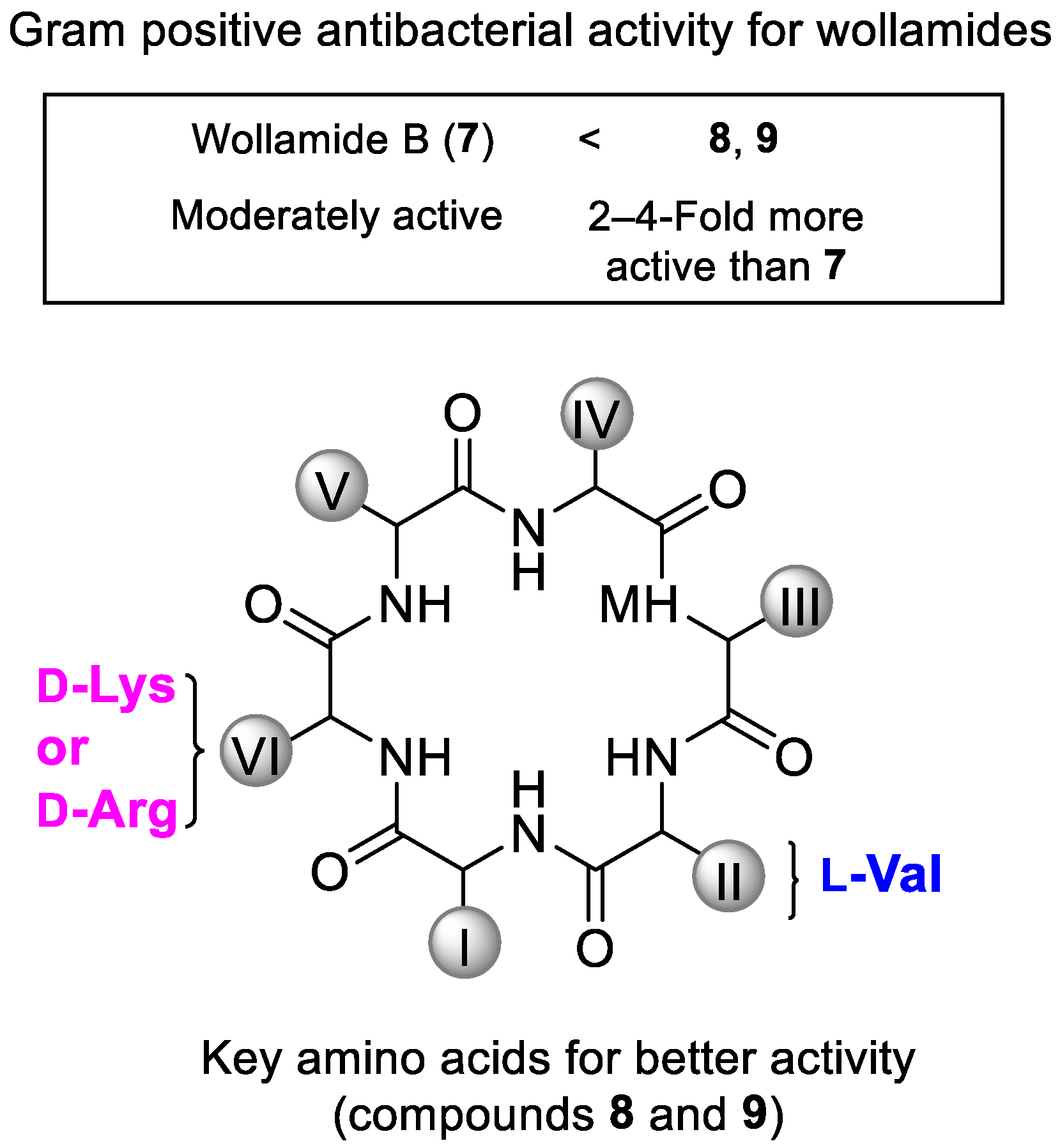
3.2. General Procedure for the Solid-Phase Synthesis of Cyclic Hexapeptides
The first Fmoc-protected amino acid (1.5 equiv.), HBTU (3 equiv.) and DIEA (3 equiv.) were added into a solution of 2-chlorotrityl chloride (2-CTC) in DMF at room temperature. After reaction for 1.5 h, the mixture was capped with methanol for 0.5 h. The reaction was washed three times with DCM and DMF (40 mL, 1 min each), then concentrated under vacuum, and redissolved with 20% piperidine in DMF for the removal of the first Fmoc group. Subsequently, elongation of the peptides was achieved with another Fmoc-amino acid using a mixture of HBTU and DIEA in DMF. After 0.5 h, the mixture was washed three times with DMF and then DCM. The procedure of deprotection, washing, coupling and washing was repeated for the synthesis of the linear hexapeptide. The cleavage of linear precursor from the resin and final global deprotection were processed with a solution of TFA/thioanisole/phenol/ddH2O (33/2/2/1/2, v/v). After stirring in dark for 2 h, the reaction mixture was filtrated and subsequently washed with TFA. The addition of pre-chilled diethyl ether and centrifugation afforded the crude precipitated product, which was then washed three times with pre-chilled diethyl ether. The crude linear hexapeptide was purified with RP-HPLC (0.1% TFA of CH3CN/H2O system) at a flow rate of 23 mL/min. For macrocyclization, the purified linear hexapeptides were first dissolved in DMF to give a 10−3–10−4 M solution and HBTU was added to this solution under stirring. DIEA was then added to this dilute solution to adjust the pH value to 8.0–9.0. The reaction mixture was stirred at room temperature for 2 d. Following these, the reaction mixture was concentrated under vacuum before purification by RP-HPLC.
Wollamide B (7): cyclo(Asn-Val-d-Leu-Leu-Trp-d-Orn). 1H NMR (DMSO-d6, 400 MHz) δ 10.85 (br d, 1H, J = 1.6 Hz), 8.38 (br d, 1H, J = 5.6 Hz), 8.35 (br d, 1H, J = 7.9 Hz), 8.29 (br d, 1H, J = 7.9 Hz), 7.69 (br d, 1H, J = 7.5 Hz), 7.6–7.7 (m, 2H), 7.56 (br s, 1H), 7.53 (d, 1H, J = 7.8 Hz), 7.49 (br d, 1H, J = 8.2 Hz), 7.39 (br d, 1H, J = 8.2 Hz), 7.33 (d, 1H, J = 8.0 Hz), 7.14 (d, 1H, J = 2.1 Hz), 7.0–7.1 (m, 1H), 7.0–7.0 (m, 1H), 7.0–7.0 (m, 1H), 4.6–4.6 (m, 1H), 4.44 (q, 1H, J = 7.1 Hz), 4.3–4.4 (m, 2H), 4.1–4.2 (m, 1H), 4.02 (dd, 1H, J = 4.3, 7.6 Hz), 3.19 (br dd, 1H, J = 4.1, 14.6 Hz), 2.96 (br dd, 1H, J = 10.3, 14.6 Hz), 2.6–2.7 (m, 4H), 2.2–2.3 (m, 1H), 1.4–1.6 (m, 8H), 1.2–1.4 (m, 2H), 0.8–0.9 (m, 18H). 13C NMR (DMSO-d6, 101 MHz) δ 173.5, 171.8, 171.6, 171.2, 170.7, 170.6, 170.6, 136.1, 127.0, 123.6, 120.9, 118.4, 118.1, 111.4, 110.0, 58.6, 55.2, 51.9, 51.8, 50.4, 49.5, 41.9, 40.4, 38.3, 37.1, 28.8, 27.2, 26.6, 24.4, 24.1, 23.2, 22.6, 22.5, 22.4, 22.1, 19.1, 17.0. (+)-HRESIMS m/z 740.4445 [M + H]+ (calcd for C37H58N9O7, 740.4454).
Wollamide B1 (8): cyclo(Asn-Val-d-Leu-Leu-Trp-d-Arg). 1H NMR (DMSO-d6, 400 MHz) δ 10.80 (s, 1H), 8.38 (br d, 1H, J = 5.4 Hz), 8.35 (br d, 1H, J = 7.7 Hz), 8.30 (br d, 1H, J = 8.0 Hz), 7.72 (br d, 1H, J = 7.2 Hz), 7.54 (br s, 1H), 7.52 (br d, 1H, J = 8.0 Hz), 7.4–7.5 (m, 4H), 7.33 (d, 1H, J = 8.0 Hz), 7.14 (br d, 1H, J = 1.8 Hz), 7.06 (br t, 2H, J = 7.3 Hz), 7.0–7.0 (m, 3H), 4.5–4.7 (m, 1H), 4.45 (q, 1H, J = 7.2 Hz), 4.3–4.3 (m, 2H), 4.12 (q, 1H, J = 7.2 Hz), 4.00 (dd, 1H, J = 4.3, 7.5 Hz), 3.21 (br dd, 1H, J = 3.8, 14.6 Hz), 3.02 (dt, 1H, J = 4.0, 6.5 Hz), 2.9–3.0 (m, 4H), 2.2–2.3 (m, 1H), 1.4–1.6 (m, 9H), 1.0–1.2 (m, 1H), 0.8–0.9 (m, 18H).13C NMR (DMSO-d6, 101 MHz) δ 173.5, 171.7, 171.6, 171.3, 170.7, 170.6, 170.6, 156.7, 136.1, 127.0, 123.7, 120.9, 118.4, 118.1, 111.3, 110.0, 58.6, 55.1, 52.3, 51.9, 50.5, 49.5, 42.0, 40.4, 40.2, 37.2, 28.7, 27.2, 26.9, 24.5, 24.4, 24.1, 22.6, 22.5, 22.4, 22.1, 19.1, 17.0. (+)-HRESIMS m/z 782.4674 [M + H]+ (calcd for C38H60N11O7, 782.4672).
Wollamide B2 (9): cyclo(Asn-Val-d-Leu-Leu-Trp-d-Lys). 1H NMR (DMSO-d6, 400 MHz) δ 10.84 (d, 1H, J = 1.8 Hz), 8.3–8.4 (m, 2H), 8.28 (br d, 1H, J = 8.0 Hz), 7.67 (br s, 2H), 7.65 (br s, 1H), 7.53 (br d, 2H, J = 7.2 Hz), 7.45 (br d, 1H, J = 8.0 Hz), 7.4–7.4 (m, 1H), 7.33 (d, 1H, J = 8.0 Hz), 7.15 (d, 1H, J = 2.0 Hz), 7.06 (t, 1H, J = 7.7 Hz), 7.00 (s, 1H), 6.98 (t, 1H, J = 7.7 Hz), 4.6–4.6 (m, 1H), 4.45 (q, 1H, J = 7.1 Hz), 4.2–4.4 (m, 2H), 4.09 (q, 1H, J = 7.2 Hz), 3.99 (dd, 1H, J = 4.4, 7.5 Hz), 3.22 (br dd, 1H, J = 3.8, 14.5 Hz), 2.93 (br dd, 1H, J = 10.7, 14.4 Hz), 2.5–2.7 (m, 4H), 2.2–2.3 (m, 1H), 1.3–1.7 (m, 11H), 1.0–1.1 (m, 1H), 0.8–0.9 (m, 18H). 13C NMR (DMSO-d6, 101 MHz) δ 173.5, 171.7, 171.6, 171.4, 170.8, 170.6, 170.5, 136.1, 127.0, 123.7, 120.9, 118.3, 118.1, 111.3, 110.1, 58.6, 55.1, 52.3, 51.9, 50.5, 49.5, 42.0, 38.6, 38.6, 37.2, 29.2, 28.8, 27.1, 26.4, 24.5, 24.1, 22.6, 22.5, 22.5, 22.1, 21.7, 19.1, 17.0. (+)-HRESIMS m/z 754.4609 [M + H]+ (calcd for C38H60N9O7, 754.4610).
Desotamide A1 (10): cyclo(Asn-allo-Ile-d-Leu-Leu-Trp-d-Arg). 1H NMR (DMSO-d6, 400 MHz) δ 10.8–10.8 (m, 1H), 8.29 (br d, 1H, J = 6.9 Hz), 8.20 (br d, 1H, J = 8.3 Hz), 7.98 (br d, 1H, J = 7.4 Hz), 7.88 (br d, 1H, J = 7.4 Hz), 7.57 (d, 1H, J = 7.8 Hz), 7.52 (br d, 1H, J = 7.3 Hz), 7.4–7.5 (m, 4H), 7.33 (d, 1H, J = 8.0 Hz), 7.13 (d, 1H, J = 2.1 Hz), 7.0–7.1 (m, 2H), 6.9–7.0 (m, 3H), 4.48 (q, 1H, J = 6.8 Hz), 4.3–4.4 (m, 1H), 4.3–4.3 (m, 1H), 4.19 (dd, 1H, J = 5.6, 7.9 Hz), 4.0–4.1 (m, 2H), 3.16 (br dd, 1H, J = 5.0, 14.5 Hz), 3.0–3.1 (m, 1H), 2.95 (br d, 2H, J = 5.9 Hz), 1.86 (td, 1H, J = 6.4, 13.1 Hz), 1.2–1.7 (m, 12H), 1.1–1.2 (m, 1H), 1.0–1.1 (m, 1H), 0.89 (br t, 6H, J = 6.7 Hz), 0.8–0.9 (m, 5H), 0.8–0.8 (m, 4H), 0.77 (d, 3H, J = 6.9 Hz). 13C NMR (DMSO-d6, 101 MHz) δ 172.1, 171.9, 171.4, 171.0, 170.9, 170.6, 170.5, 156.6, 136.1, 127.1, 123.6, 120.9, 118.3, 118.3, 111.3, 110.2, 55.7, 55.0, 52.8, 52.5, 51.4, 49.7, 40.9, 40.4, 40.4, 36.5, 36.3, 27.7, 27.4, 25.6, 24.6, 24.3, 24.2, 22.9, 22.6, 22.5, 21.0, 14.8, 11.5. (+)-HRESIMS m/z 796.4832 [M + H]+ (calcd for C39H62N11O7, 796.4828).
Desotamide A2 (11): cyclo(Asn-allo-Ile-d-Leu-Leu-Trp-d-Lys). 1H NMR (DMSO-d6, 400 MHz) δ 10.8–10.9 (m, 1H), 8.2–8.3 (m, 1H), 8.19 (br d, 1H, J = 8.3 Hz), 8.00 (br d, 1H, J = 7.3 Hz), 7.83 (br d, 1H, J = 7.4 Hz), 7.67 (br s, 2H), 7.58 (d, 1H, J = 7.8 Hz), 7.51 (br d, 1H, J = 7.3 Hz), 7.4–7.5 (m, 1H), 7.37 (br d, 1H, J = 8.2 Hz), 7.3–7.3 (m, 1H), 7.14 (d, 1H, J = 2.0 Hz), 7.0–7.1 (m, 1H), 7.0–7.0 (m, 1H), 6.9–7.0 (m, 1H), 4.47 (q, 1H, J = 6.9 Hz), 4.3–4.4 (m, 1H), 4.3–4.3 (m, 1H), 4.18 (dd, 1H, J = 5.8, 8.0 Hz), 4.1–4.1 (m, 1H), 4.0–4.1 (m, 1H), 3.16 (br dd, 1H, J = 4.9, 14.6 Hz), 2.99 (br dd, 1H, J = 9.9, 14.5 Hz), 2.4–2.7 (m, 4H), 1.85 (td, 1H, J = 6.4, 13.1 Hz), 1.6–1.7 (m, 1H), 1.4–1.6 (m, 6H), 1.2–1.4 (m, 5H), 1.0–1.1 (m, 2H), 0.9–0.9 (m, 3H), 0.88 (br s, 1H), 0.8–0.9 (m, 3H), 0.8–0.8 (m, 1H), 0.8–0.8 (m, 3H), 0.77 (d, 3H, J = 6.8 Hz).13C NMR (DMSO-d6, 101 MHz) δ 172.1, 171.8, 171.4, 171.0, 170.8, 170.7, 170.4, 136.1, 127.2, 123.6, 120.8, 118.3, 118.2, 111.3, 110.2, 55.7, 55.1, 52.8, 52.6, 51.4, 49.7, 40.8, 40.4, 38.5, 36.5, 36.3, 30.0, 27.3, 26.5, 25.6, 24.2, 24.2, 22.9, 22.6, 22.5, 21.8, 21.0, 14.7, 11.5. (+)-HRESIMS m/z 768.4763 [M + H]+ (calcd for C39H62N9O7, 768.4767).
Desotamide A3 (12): cyclo(Asn-allo-Ile-d-Leu-Leu-Trp-d-His). 1H NMR (DMSO-d6, 400 MHz) δ 14.14 (br s, 2H), 10.84 (s, 1H), 8.9–8.9 (m, 1H), 8.26 (br d, 1H, J = 6.9 Hz), 8.19 (br d, 1H, J = 8.2 Hz), 8.07 (br dd, 2H, J = 3.5, 7.5 Hz), 7.63 (br d, 1H, J = 7.0 Hz), 7.5–7.6 (m, 3H), 7.33 (d, 1H, J = 8.2 Hz), 7.13 (s, 1H), 7.1–7.1 (m, 1H), 7.0–7.1 (m, 1H), 6.9–7.0 (m, 2H), 4.4–4.5 (m, 2H), 4.3–4.4 (m, 1H), 4.25 (q, 1H, J = 6.9 Hz), 4.1–4.2 (m, 1H), 4.1–4.1 (m, 1H), 3.1–3.2 (m, 1H), 2.9–3.1 (m, 2H), 2.76 (br dd, 1H, J = 9.0, 15.2 Hz), 1.85 (td, 1H, J = 6.5, 12.9 Hz), 1.6–1.7 (m, 1H), 1.4–1.6 (m, 6H), 1.3–1.4 (m, 1H), 1.0–1.2 (m, 2H), 0.89 (br d, 3H, J = 6.8 Hz), 0.87 (br d, 3H, J = 6.3 Hz), 0.8–0.9 (m, 9H), 0.78 (d, 3H, J = 6.8 Hz). 13C NMR (DMSO-d6, 101 MHz) δ 172.3, 171.9, 171.6, 171.0, 170.7, 170.6, 169.2, 136.1, 133.6, 129.3, 127.1, 123.5, 120.9, 118.3, 116.6, 111.3, 109.9, 56.0, 55.2, 52.6, 51.6, 51.5, 49.6, 40.7, 40.4, 40.4, 36.4, 27.5, 25.8, 25.5, 24.2, 22.9, 22.5, 22.4, 21.0, 14.9, 11.5. (+)-HRESIMS m/z 777.4403 [M + H]+ (calcd for C39H57N10O7, 777.4406).
Desotamide A4 (13): cyclo(Asn-Ile-d-Leu-Leu-Trp-d-Lys). 1H NMR (DMSO-d6, 400 MHz) δ 10.84 (s, 1H), 8.3–8.4 (m, 2H), 8.25 (br d, 1H, J = 7.9 Hz), 7.66 (br s, 2H), 7.63 (br d, 1H, J = 7.3 Hz), 7.55 (br s, 1H), 7.5–7.5 (m, 1H), 7.4–7.5 (m, 1H), 7.4–7.4 (m, 1H), 7.33 (d, 1H, J = 8.2 Hz), 7.15 (d, 1H, J = 2.0 Hz), 7.0–7.1 (m, 1H), 7.0–7.0 (m, 1H), 6.9–7.0 (m, 1H), 4.5–4.6 (m, 1H), 4.43 (q, 1H, J = 7.1 Hz), 4.2–4.3 (m, 2H), 4.0–4.1 (m, 1H), 4.01 (dd, 1H, J = 4.5, 7.2 Hz), 3.20 (br dd, 1H, J = 3.9, 14.6 Hz), 2.93 (br dd, 1H, J = 10.6, 14.5 Hz), 2.6–2.7 (m, 4H), 1.9–2.0 (m, 1H), 1.2–1.6 (m, 13H), 1.0–1.1 (m, 1H), 0.8–0.9 (m, 18H);13C NMR (DMSO-d6, 101 MHz) δ 173.5, 171.7, 171.6, 171.4, 170.8, 170.5, 170.5, 136.1, 127.0, 123.7, 120.9, 118.3, 118.1, 111.3, 110.1, 58.3, 55.2, 52.3, 51.8, 50.5, 49.5, 41.8, 40.4, 38.6, 37.0, 35.5, 29.1, 27.1, 26.4, 24.5, 24.1, 23.9, 22.5, 22.5, 22.0, 21.7, 15.6, 11.8. (+)-HRESIMS m/z 768.4774 [M + H]+ (calcd for C39H62N9O7, 768.4767).
Desotamide A5 (14): cyclo(Asn-Ile-d-Leu-Leu-Trp-d-His). 1H NMR (DMSO-d6, 400 MHz) δ 14.0–14.2 (m, 1H), 10.8–10.9 (m, 1H), 8.9–8.9 (m, 1H), 8.4–8.5 (m, 1H), 8.44 (br s, 1H), 8.15 (br d, 1H, J = 8.2 Hz), 7.71 (br s, 1H), 7.59 (br dd, 2H, J = 3.0, 8.3 Hz), 7.52 (s, 1H), 7.5–7.5 (m, 1H), 7.33 (d, 1H, J = 8.2 Hz), 7.17 (s, 1H), 7.1–7.2 (m, 1H), 7.10 (d, 1H, J = 2.1 Hz), 7.0–7.1 (m, 1H), 6.9–7.0 (m, 1H), 4.5–4.6 (m, 2H), 4.43 (q, 1H, J = 7.4 Hz), 4.36 (dt, 1H, J = 5.0, 9.0 Hz), 4.3–4.3 (m, 1H), 4.06 (br dd, 1H, J = 4.3, 6.8 Hz), 3.1–3.2 (m, 1H), 3.0–3.1 (m, 1H), 3.0–3.0 (m, 1H), 2.8–2.9 (m, 2H), 2.63 (dd, 1H, J = 5.0, 16.0 Hz), 1.9–2.0 (m, 1H), 1.2–1.6 (m, 8H), 0.8–0.9 (m, 18H). 13C NMR (DMSO-d6, 101 MHz) δ 174.1, 172.0, 171.8, 171.1, 170.9, 170.8, 169.7, 136.1, 133.5, 129.2, 126.9, 123.5, 121.0, 118.4, 118.1, 116.9, 111.4, 109.8, 58.7, 55.7, 52.1, 51.1, 50.4, 49.6, 41.8, 36.4, 35.4, 27.2, 25.0, 24.5, 24.1, 23.9, 22.6, 22.4, 22.4, 22.1, 15.6, 11.8. (+)-HRESIMS m/z 777.4404 [M + H]+ (calcd for C39H57N10O7, 777.4406).
Desotamide A6 (15): cyclo(Asn-Ile-d-Leu-Leu-Trp-d-Arg). 1H NMR (DMSO-d6, 400 MHz) δ 10.8–10.8 (m, 1H), 8.34 (br s, 1H), 8.33 (br s, 1H), 8.26 (br d, 1H, J = 7.9 Hz), 7.68 (br d, 1H, J = 7.2 Hz), 7.56 (br s, 1H), 7.51 (d, 1H, J = 7.8 Hz), 7.4–7.5 (m, 4H), 7.33 (d, 1H, J = 8.0 Hz), 7.14 (d, 1H, J = 2.1 Hz), 7.0–7.1 (m, 2H), 6.9–7.0 (m, 3H), 4.5–4.6 (m, 1H), 4.43 (q, 1H, J = 7.2 Hz), 4.3–4.4 (m, 2H), 4.12 (q, 1H, J = 7.2 Hz), 4.02 (dd, 1H, J = 4.4, 7.3 Hz), 3.20 (br dd, 1H, J = 4.0, 14.6 Hz), 2.9–3.0 (m, 3H), 2.6–2.7 (m, 2H), 1.98 (dt, 1H, J = 4.6, 10.3 Hz), 1.2–1.6 (m, 13H), 1.0–1.2 (m, 1H), 0.91 (d, 7H, J = 6.3 Hz), 0.8–0.9 (m, 13H).13C NMR (DMSO-d6, 101 MHz) δ 173.5, 171.7, 171.6, 171.3, 170.8, 170.6, 170.5, 156.7, 136.1, 126.9, 123.7, 120.9, 118.4, 118.1, 111.3, 110.0, 58.3, 55.2, 52.2, 51.8, 50.5, 49.5, 41.8, 40.4, 40.2, 37.0, 35.5, 27.1, 26.9, 24.6, 24.5, 24.1, 23.9, 22.5, 22.5, 22.5, 22.0, 15.7, 11.8. (+)-HRESIMS m/z 796.4823 [M + H]+ (calcd for C39H62N11O7, 796.4828).
Desotamide A7 (16): cyclo(Asn-Ile-d-Leu-Leu-Trp-Gly). 1H NMR (DMSO-d6, 400 MHz) δ 10.83 (s, 1H), 8.62 (br d, 1H, J = 7.0 Hz), 8.34 (br d, 1H, J = 5.9 Hz), 8.2–8.3 (m, 1H), 7.88 (br t, 1H, J = 5.4 Hz), 7.69 (br d, 1H, J = 8.0 Hz), 7.67 (br s, 1H), 7.63 (br d, 1H, J = 8.4 Hz), 7.51 (d, 1H, J = 7.8 Hz), 7.32 (d, 1H, J = 8.0 Hz), 7.1–7.2 (m, 1H), 7.1–7.1 (m, 1H), 7.06 (t, 1H, J = 7.3 Hz), 6.98 (t, 1H, J = 7.3 Hz), 4.52 (td, 1H, J = 5.3, 8.3 Hz), 4.3–4.4 (m, 2H), 4.19 (q, 1H, J = 7.2 Hz), 4.07 (ddd, 1H, J = 4.1, 6.7, 10.5 Hz), 3.87 (br dd, 1H, J = 6.3, 16.1 Hz), 3.31 (br dd, 1H, J = 4.6, 16.0 Hz), 3.1–3.2 (m, 1H), 2.98 (br dd, 1H, J = 10.0, 14.6 Hz), 2.84 (br dd, 1H, J = 5.3, 16.0 Hz), 2.63 (br dd, 1H, J = 5.4, 15.9 Hz), 1.4–1.7 (m, 9H), 0.8–0.9 (m, 18H). 13C NMR (DMSO-d6, 101 MHz) δ 172.8, 172.1, 171.9, 171.5, 170.9, 170.9, 168.9, 136.1, 127.0, 123.5, 120.9, 118.3, 118.1, 111.3, 110.0, 55.3, 51.9, 51.9, 50.8, 49.2, 43.3, 41.5, 40.4, 40.4, 36.7, 27.4, 24.5, 24.2, 24.2, 23.1, 22.6, 22.5, 22.4, 22.1, 20.8. (+)-HRESIMS m/z 697.4025 [M + H]+ (calcd for C35H53N8O7, 697.4032).
Desotamide A8 (17): cyclo(Asn-Ile-d-Leu-Leu-Trp-d-Lys). 1H NMR (DMSO-d6, 400 MHz) δ 10.8–10.9 (m, 1H), 8.70 (br d, 1H, J = 7.0 Hz), 8.42 (br d, 1H, J = 5.4 Hz), 8.25 (br d, 1H, J = 8.3 Hz), 7.67 (br s, 2H), 7.59 (br d, 1H, J = 7.0 Hz), 7.55 (br s, 1H), 7.53 (d, 1H, J = 7.8 Hz), 7.46 (br d, 2H, J = 8.2 Hz), 7.33 (d, 1H, J = 8.2 Hz), 7.14 (d, 1H, J = 2.1 Hz), 7.0–7.1 (m, 1H), 7.02 (br s, 1H), 7.0–7.0 (m, 1H), 4.5–4.6 (m, 1H), 4.4–4.5 (m, 1H), 4.30 (ddd, 1H, J = 4.3, 8.2, 10.4 Hz), 4.1–4.2 (m, 1H), 4.0–4.1 (m, 1H), 4.0–4.0 (m, 1H), 3.21 (br dd, 1H, J = 3.9, 14.4 Hz), 2.93 (br dd, 1H, J = 10.6, 14.5 Hz), 2.5–2.7 (m, 4H), 1.3–1.7 (m, 14H), 1.0–1.2 (m, 1H), 0.9–0.9 (m, 12H), 0.84 (d, 3H, J = 6.5 Hz), 0.80 (d, 3H, J = 6.3 Hz);13C NMR (DMSO-d6, 101 MHz) δ 173.0, 171.8, 171.8, 171.5, 171.2, 170.8, 170.5, 136.1, 127.0, 123.7, 120.9, 118.3, 118.1, 111.3, 110.1, 55.1, 52.3, 52.1, 51.9, 50.5, 49.4, 42.0, 40.4, 38.6, 38.6, 37.2, 29.1, 27.2, 26.4, 24.5, 24.2, 24.1, 23.1, 22.7, 22.5, 22.4, 22.0, 21.7, 20.6. (+)-HRESIMS m/z 768.4768 [M + H]+ (calcd for C39H62N9O7, 768.4767).
Desotamide A9 (18): cyclo(Asn-Ile-d-Leu-Leu-Trp-d-His). 1H NMR (DMSO-d6, 400 MHz) δ 14.11 (br s, 2H), 10.86 (s, 1H), 8.91 (d, 1H, J = 1.0 Hz), 8.83 (br d, 1H, J = 6.5 Hz), 8.54 (br d, 1H, J = 4.8 Hz), 8.13 (br d, 1H, J = 8.2 Hz), 7.75 (s, 1H), 7.62 (br d, 1H, J = 8.5 Hz), 7.55 (br dd, 2H, J = 3.8, 8.2 Hz), 7.51 (d, 1H, J = 7.8 Hz), 7.34 (d, 1H, J = 8.0 Hz), 7.18 (s, 1H), 7.16 (br s, 1H), 7.09 (s, 1H), 7.0–7.1 (m, 1H), 7.0–7.0 (m, 1H), 4.5–4.5 (m, 2H), 4.4–4.5 (m, 1H), 4.37 (dt, 1H, J = 5.0, 9.1 Hz), 4.1–4.2 (m, 1H), 4.0–4.0 (m, 1H), 3.1–3.2 (m, 2H), 3.0–3.0 (m, 1H), 2.8–2.9 (m, 2H), 2.63 (br dd, 1H, J = 4.9, 15.9 Hz), 1.4–1.6 (m, 9H), 0.8–0.9 (m, 18H); 13C NMR (DMSO-d6, 101 MHz) δ 173.6, 172.1, 172.0, 171.9, 171.1, 170.8, 169.6, 136.1, 133.5, 129.3, 126.9, 123.4, 120.9, 118.4, 118.1, 116.8, 111.3, 109.8, 55.8, 52.4, 52.3, 51.1, 50.4, 49.5, 41.8, 40.4, 36.4, 25.9, 25.8, 24.6, 24.2, 24.1, 23.1, 22.6, 22.4, 22.3, 22.1, 20.6. (+)-HRESIMS m/z 768.4768 [M + H]+ (calcd for C39H62N9O7, 768.4767).
Desotamide A0 (19): cyclo(Asn-Ile-d-Leu-Leu-Trp-d-Arg). 1H NMR (400 MHz, DMSO-d6) δ ppm 0.79–0.93 (m, 18 H) 1.32–1.68 (m, 13 H) 2.65 (qd, J = 15.64, 6.02 Hz, 4 H) 2.96 (br dd, J = 14.56, 10.29 Hz, 1 H) 3.18 (br dd, J = 14.62, 4.08 Hz, 1 H) 4.02 (ddd, J = 10.63, 6.81, 4.14 Hz, 1 H) 4.11–4.19 (m, 2 H) 4.27–4.35 (m, 1 H) 4.44 (q, J = 7.11 Hz, 1 H) 4.54–4.60 (m, 1 H) 6.96–7.01 (m, 1 H) 7.01–7.04 (m, 1 H) 7.04–7.09 (m, 1 H) 7.13 (d, J = 1.88 Hz, 1 H) 7.33 (d, J = 8.03 Hz, 1 H) 7.44 (br d, J = 8.16 Hz, 1 H) 7.51 (br d, J = 5.40 Hz, 1 H) 7.53 (br d, J = 5.14 Hz, 1 H) 7.59 (br s, 1 H) 7.61 (br s, 1 H) 7.65 (br s, 2 H) 8.21–8.28 (m, 1 H) 8.41 (br d, J = 5.27 Hz, 1 H) 8.70 (br d, J = 7.03 Hz, 1 H) 10.84 (d, J = 1.25 Hz, 1 H);13C NMR (DMSO-d6, 101 MHz) δ 173.0, 171.9, 171.8, 171.6, 171.0, 170.8, 170.6, 136.1, 127.0, 123.6, 120.9, 118.4, 118.1, 111.3, 110.0, 55.3, 52.2, 51.9, 51.8, 50.5, 49.4, 41.9, 40.4, 38.2, 37.1, 27.2, 26.5, 25.9, 25.8, 24.5, 24.2, 24.1, 23.2, 23.2, 22.6, 22.5, 22.4, 22.1, 20.6. (+)-HRESIMS m/z 777.4407 [M + H]+ (calcd for C39H57N10O7, 777.4406).