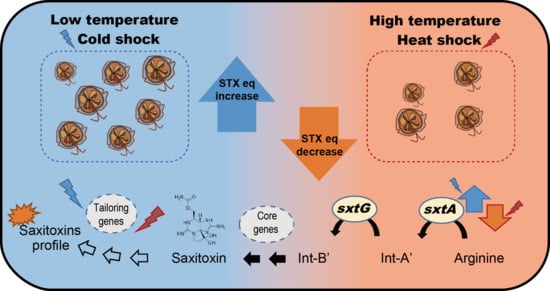
Low Temperature and Cold Stress Significantly Increase Saxitoxins (STXs) and Expression of STX Biosynthesis Genes sxtA4 and sxtG in the Dinoflagellate Alexandrium catenella
Abstract
:1. Introduction
2. Results
2.1. Effects of Temperature on Cell Growth and Size
2.2. Phylogenetic Relationships of sxtA4 and sxtG and Characterization
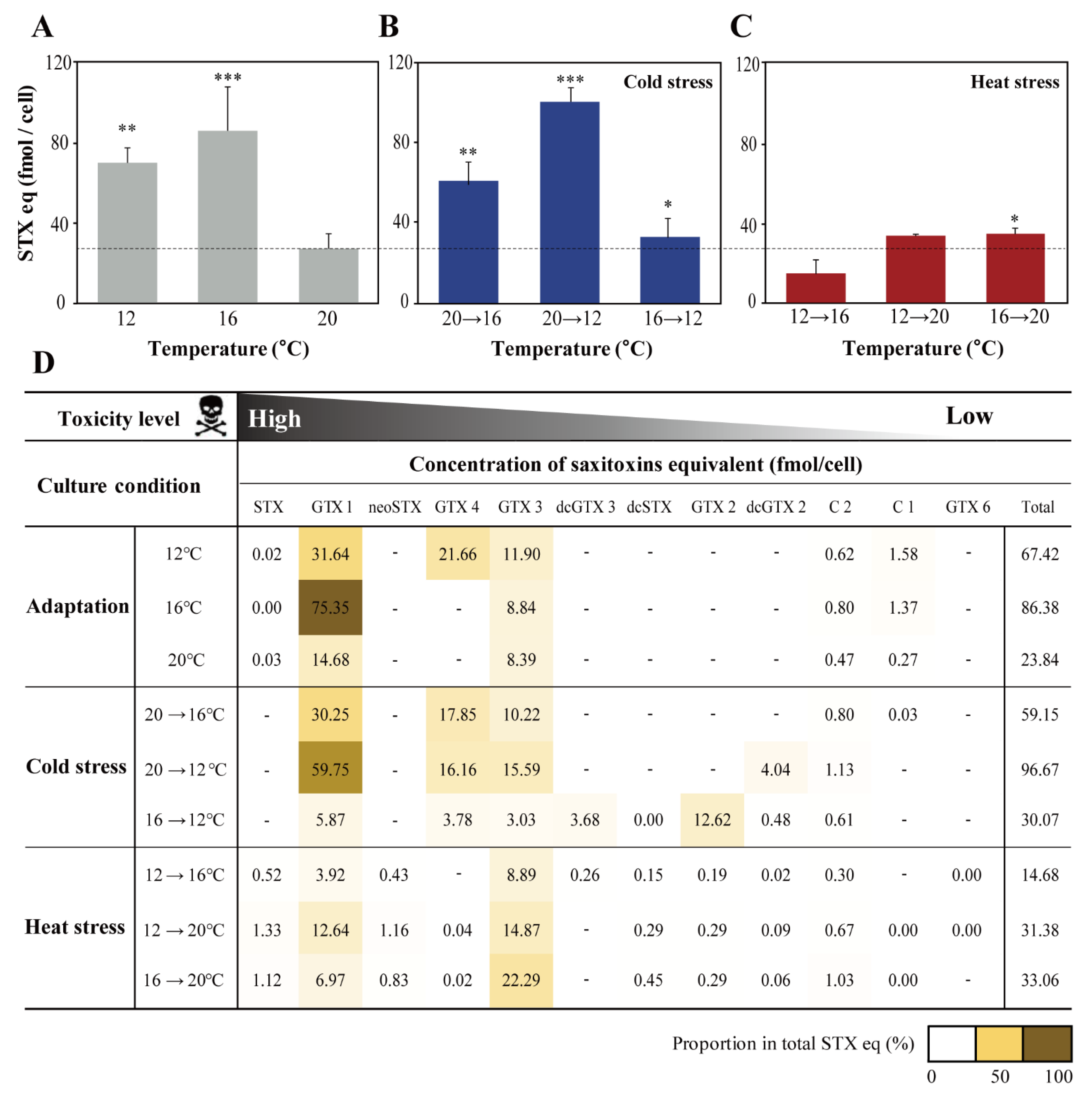
2.3. Effect of Water Temperature on STXs
2.4. Effects of Different Temperatures on sxtA4 and sxtG Transcription
2.5. Correlation of Temperature, STXs eq and Sxt Genes Expression
3. Discussion
4. Materials and Methods
4.1. Cell culture and Adaptation
4.2. Design for Temperature Experiments
4.3. Cell Density and Size Measurements
4.4. DNA and RNA Extraction and cDNA Synthesis
4.5. Cloning of sxtA4, sxtG and 28S rRNA
4.6. Gene Characterization and Phylogenetic Analysis
4.7. Quantitative Real-Time PCR
4.8. High-Pressure Liquid Chromatography-Fluorescence Detection (HPLC-FLD)
4.9. Statistical Analysis and Principal Component Analysis (PCA)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, F.J.R. Ecology of dinoflagellates. In The Biology of Dinoflagellates; Blackwell Scientific Publications: Oxford, UK, 1987. [Google Scholar]
- Taylor, F.J.R.; Hoppenrath, M.; Saldarriaga, J.F. Dinoflagellate diversity and distribution. Biodiver. Conserv. 2008, 17, 407–418. [Google Scholar] [CrossRef]
- Murray, S.A.; Wiese, M.; Stüken, A.; Brett, S.; Kellmann, R.; Hallegraeff, G.; Neilan, B.A. sxtA-based quantitative molecular assay to identify saxitoxin-producing harmful algal blooms in marine waters. Appl. Environ. Microbiol. 2011, 77. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.A.; Wiese, M.; Neilan, B.A.; Orr, R.J.; de Salas, M.; Brett, S.; Hallegraeff, G. A reinvestigation of saxitoxin production and sxtA in the ‘non-toxic’ Alexandrium tamarense Group V clade. Harmful Algae 2012, 18, 96–104. [Google Scholar] [CrossRef]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.Z. Neurotoxins from marine dinoflagellates: A brief review. Mar. Drugs 2008, 6, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.; Lazensky, R.; Faris, J.; Fleming, L.E.; Kirkpatrick, B.; Watkins, S.; Ullmannd, S.; Kohlere, K.; Hoagland, P. Assessing the impact of shellfish harvesting area closures on neurotoxic shellfish poisoning (NSP) incidence during red tide (Karenia brevis) blooms. Harmful Algae 2015, 43, 13–19. [Google Scholar] [CrossRef]
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazachariou, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First detection of tetrodotoxin in Greek shellfish by UPLC-MS/MS potentially linked to the presence of the dinoflagellate Prorocentrum minimum. Toxins 2015, 7, 1779–1807. [Google Scholar] [CrossRef] [Green Version]
- Glibert, P.M.; Pitcher, G. Global Ecology and Oceanography of Harmful Algal Blooms, Science Plan; SCOR and IOC: Baltimore, MD, USA; Paris, France, 2001; p. 87. [Google Scholar]
- De Carvalho, M.; Jacinto, J.; Ramos, N.; de Oliveira, V.; e Melo, T.P.; de Sá, J. Paralytic shellfish poisoning: Clinical and electrophysiological observations. J. Neurol. 1998, 245, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu. Rev. Pharmacol. 1980, 20, 15–43. [Google Scholar] [CrossRef]
- Cestèle, S.; Catterall, W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie 2000, 82, 883–892. [Google Scholar] [CrossRef]
- Wang, J.; Salata, J.J.; Bennett, P.B. Saxitoxin is a gating modifier of HERG K+ channels. J. Gen. Physiol. 2003, 121, 583–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schantz, E.J.; Mold, J.; Stanger, D.; Shavel, J.; Riel, F.; Bowden, J.; Lynch, J.; Wyler, R.; Riegel, B.; Sommer, H. Paralytic shellfish poison VI. A procedure for the isolation and purification of the poison from toxic clams and mussel tissues. J. Am. Chem. Soc. 1957, 79, 5230–5235. [Google Scholar] [CrossRef]
- Shumway, S.E. Phycotoxin-related shellfish poisoning: Bivalve molluscs are not the only vectors. Rev. Fish. Sci. 1995, 3, 1–31. [Google Scholar] [CrossRef]
- Usup, G.; Pin, L.C.; Ahmad, A.; Teen, L.P. Alexandrium (Dinophyceae) species in Malaysian waters. Harmful Algae 2002, 1, 265–275. [Google Scholar] [CrossRef]
- Lim, P.T.; Leaw, C.P.; Usup, G.; Kobiyama, A.; Koike, K.; Ogata, T. Effects of light and temperature on growth, nitrate uptake, and toxin production of two tropical dinoflagellates: Alexandrium tamiyavanichii and Alexandrium minutum (Dinophyceae). J. Phycol. 2006, 42, 786–799. [Google Scholar] [CrossRef]
- Hattenrath-Lehmann, T.K.; Smith, J.L.; Wallace, R.B.; Merlo, L.R.; Koch, F.; Mittelsdorf, H.; Goleski, J.A.; Anderson, D.M.; Gobler, C.J. The effects of elevated CO2 on the growth and toxicity of field populations and cultures of the saxitoxin-producing dinoflagellate, Alexandrium fundyense. Limnol. Oceanogr. 2015, 60, 198–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirés, S.; Delgado, A.; González-Pleiter, M.; Quesada, A. Temperature influences the production and transport of saxitoxin and the expression of sxt genes in the cyanobacterium Aphanizomenon gracile. Toxins 2017, 9, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, D.F.; Lu, Y.H. Influence of environmental and nutritional factors on growth, toxicity, and toxin profile of dinoflagellate Alexandrium minutum. Toxicon 2000, 38, 1491–1503. [Google Scholar] [CrossRef]
- Vargas, S.R.; dos Santos, P.V.; Bottino, F.; do Carmo Calijuri, M. Effect of nutrient concentration on growth and saxitoxin production of Raphidiopsis raciborskii (Cyanophyta) interacting with Monoraphidium contortum (Chlorophyceae). J. Appl. Phycol. 2020, 32, 421–430. [Google Scholar] [CrossRef]
- Lilly, E.L.; Kulis, D.M.; Gentien, P.; Anderson, D.M. Paralytic shellfish poisoning toxins in France linked to a human-introduced strain of Alexandrium catenella from western Pacific: Evidence from DNA and toxin analysis. J. Plankton. Res. 2002, 24, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Vila, M.; Giacobbe, M.G.; Masó, M.; Gangemi, E.; Penna, A.; Sampedro, N.; Azzaro, F.; Camp, J.; Galluzzi, L.; Galluzzi, L. A comparative study on recurrent blooms of Alexandrium minutum in two Mediterranean coastal areas. Harmful Algae 2005, 4, 673–695. [Google Scholar] [CrossRef]
- Penna, A.; Garcés, E.; Vila, M.; Giacobbe, M.G.; Fraga, S.; Lugliè, A.; Bravo, I.; Bertozzini, E.; Vernesi, C. Alexandrium catenella (Dinophyceae), a toxic ribotype expanding in the NW Mediterranean Sea. Mar. Biol. 2005, 148, 13–23. [Google Scholar] [CrossRef]
- Genovesi, B.; Shin-Grzebyk, M.S.; Grzebyk, D.; Laabir, M.; Gagnaire, P.A.; Vaquer, A.; Pastoureaud, A.; Lasserre, B.; Collos, Y.; Berrebi, P.; et al. Assessment of cryptic species diversity within blooms and cyst bank of the Alexandrium tamarense complex (Dinophyceae) in a Mediterranean lagoon facilitated by semi-multiplex PCR. J. Plankton. Res. 2011, 33, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Vandersea, M.W.; Kibler, S.R.; Tester, P.A.; Holderied, K.; Hondolero, D.E.; Powell, K.; Baird, S.; Doroff, A.; Dugan, D.; Litaker, R.W. Environmental factors influencing the distribution and abundance of Alexandrium catenella in Kachemak bay and lower cook inlet, Alaska. Harmful Algae 2018, 77, 81–92. [Google Scholar] [CrossRef]
- Lim, P.T.; Ogata, T. Salinity effect on growth and toxin production of four tropical Alexandrium species (Dinophyceae). Toxicon 2005, 45, 699–710. [Google Scholar] [CrossRef]
- Perini, F.; Galluzzi, L.; Dell’Aversano, C.; Iacovo, E.D.; Tartaglione, L.; Ricci, F.; Forino, M.; Ciminiello, P.; Penna, A. SxtA and sxtG gene expression and toxin production in the Mediterranean Alexandrium minutum (Dinophyceae). Mar. Drugs 2014, 12, 5258–5276. [Google Scholar] [CrossRef] [PubMed]
- Eckford-Soper, L.K.; Bresnan, E.; Lacaze, J.P.; Green, D.H.; Davidson, K. The competitive dynamics of toxic Alexandrium fundyense and non-toxic Alexandrium tamarense: The role of temperature. Harmful Algae 2016, 53, 135–144. [Google Scholar] [CrossRef]
- Wang, D.Z.; Hsieh, D.P. Effects of nitrate and phosphate on growth and C2 toxin productivity of Alexandrium tamarense CI01 in culture. Mar. Pollut. Bull. 2002, 45, 286–289. [Google Scholar] [CrossRef]
- Kellmann, R.; Mihali, T.K.; Jeon, Y.J.; Pickford, R.; Pomati, F.; Neilan, B.A. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Appl. Environ. Microbiol. 2008, 74, 4044–4053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihali, T.K.; Kellmann, R.; Neilan, B.A. Characterisation of the paralytic shellfish toxin biosynthesis gene clusters in Anabaena circinalis AWQC131C and Aphanizomenon sp. NH-5. BMC Biochem. 2009, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agostino, P.M.; Al-Sinawi, B.; Mazmouz, R.; Muenchhoff, J.; Neilan, B.A.; Moffitt, M.C. Identification of promoter elements in the Dolichospermum circinale AWQC131C saxitoxin gene cluster and the experimental analysis of their use for heterologous expression. BMC Microbiol. 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.; Tsuchiya, S.; Omura, T.; Koike, K.; Oikawa, H.; Konoki, K.; Oshima, Y.; Yotsu-Yamashita, M. Metabolomic study of saxitoxin analogues and biosynthetic intermediates in dinoflagellates using 15 N-labelled sodium nitrate as a nitrogen source. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Akbar, M.A.; Mohd Yusof, N.Y.; Tahir, N.I.; Ahmad, A.; Usup, G.; Sahrani, F.K.; Bunawan, H. Biosynthesis of saxitoxin in marine dinoflagellates: An omics perspective. Mar. Drugs. 2020, 18, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisecaver, J.H.; Brosnahan, M.L.; Hackett, J.D. Horizontal gene transfer is a significant driver of gene innovation in dinoflagellates. Genome Biol. Evol. 2013, 5, 2368–2381. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Lin, S.; Huang, L.; Lu, W.; Li, M.; Liu, S. Suppression subtraction hybridization analysis revealed regulation of some cell cycle and toxin genes in Alexandrium catenella by phosphate limitation. Harmful Algae 2014, 39, 26–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.F.; Lin, L.; Wang, D.Z. Whole transcriptomic analysis provides insights into molecular mechanisms for toxin biosynthesis in a toxic dinoflagellate Alexandrium catenella (ACHK-T). Toxins 2017, 9, 213. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Wang, H.; Suh, Y.S.; Ki, J.S. Transcriptomic profiles reveal the genome-wide responses of the harmful dinoflagellate Cochlodinium polykrikoides when exposed to the algicide copper sulfate. BMC Genom. 2016, 17, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.; Barua, A.; Ruvindy, R.; Savela, H.; Ajani, P.A.; Murray, S.A. The genetic basis of toxin biosynthesis in dinoflagellates. Microorganisms 2019, 7, 222. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Guo, R.; Lim, W.A.; Allen, A.E.; Ki, J.S. Comparative transcriptomics of toxin synthesis genes between the non-toxin producing dinoflagellate Cochlodinium polykrikoides and toxigenic Alexandrium pacificum. Harmful Algae 2020, 93. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kim, H.; Ki, J.S. Transcriptome survey and toxin measurements reveal evolutionary modification and loss of saxitoxin biosynthesis genes in the dinoflagellates Amphidinium carterae and Prorocentrum micans. Ecotoxicol. Environ. Saf. 2020, 195. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kim, H.; Ki, J.S. Transcriptomic identification and expression analysis of cold shock domain protein (CSP) genes in the marine dinoflagellate Prorocentrum minimum. J. Appl. Phycol. 2021, 33, 843–845. [Google Scholar] [CrossRef]
- Lukowski, A.L.; Mallik, L.; Hinze, M.E.; Carlson, B.M.; Ellinwood, D.C.; Pyser, J.B.; Koutmos, M.; Narayan, A.R. Substrate promiscuity of a paralytic shellfish toxin amidinotransferase. ACS Chem. Biol. 2020, 15, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Stüken, A.; Orr, R.J.; Kellmann, R.; Murray, S.A.; Neilan, B.A.; Jakobsen, K.S. Discovery of nuclear-encoded genes for the neurotoxin saxitoxin in dinoflagellates. PLoS ONE 2011, 6, e20096. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.A.; Ruvindy, R.; Kohli, G.S.; Anderson, D.M.; Brosnahan, M.L. Evaluation of sxtA and rDNA qPCR assays through monitoring of an inshore bloom of Alexandrium catenella Group 1. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Orr, R.J.; Stüken, A.; Murray, S.A.; Jakobsen, K.S. Evolution and distribution of saxitoxin biosynthesis in dinoflagellates. Mar. Drugs 2013, 11, 2814–2828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza-Flores, A.; Leyva-Valencia, I.; Band-Schmidt, C.J.; Galindo-Sánchez, C.E.; Bustillos-Guzmán, J.J. Identification of the gene sxtA (domains sxtA1 and sxtA4) in Mexican strains of Gymnodinium catenatum (Dinophyceae) and their evolution. Front. Mar. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Oh, S.J.; Park, J.A.; Kwon, H.K.; Yang, H.S.; Lim, W. Ecophysiological studies on the population dynamics of two toxic dinoflagellates Alexandrium tamarense and Alexandrium catenella isolated from the southern coast of Korea-I. Effects of temperature and salinity on the growth. J. Korean Soc. Mar. Environ. Energy 2012, 15, 133–141. [Google Scholar] [CrossRef]
- Shin, H.H.; Li, Z.; Kim, E.S.; Park, J.W.; Lim, W.A. Which species, Alexandrium catenella (Group I) or A. pacificum (Group IV), is really responsible for past paralytic shellfish poisoning outbreaks in Jinhae-Masan Bay, Korea? Harmful Algae 2017, 68, 31–39. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, P.M.; Song, X.; Neilan, B.A.; Moffitt, M.C. Comparative proteomics reveals that a saxitoxin-producing and a nontoxic strain of Anabaena circinalis are two different ecotypes. J. Proteome Res. 2014, 13, 1474–1484. [Google Scholar] [CrossRef]
- D’Agostino, P.M.; Song, X.; Neilan, B.A.; Moffitt, M.C. Proteogenomics of a saxitoxin-producing and non-toxic strain of Anabaena circinalis (cyanobacteria) in response to extracellular NaCl and phosphate depletion. Environ. Microbiol. 2016, 18, 461–476. [Google Scholar] [CrossRef]
- John, U.; Litaker, R.W.; Montresor, M.; Murray, S.; Brosnahan, M.L.; Anderson, D.M. Formal revision of the Alexandrium tamarense species complex (Dinophyceae) taxonomy: The introduction of five species with emphasis on molecular-based (rDNA) classification. Protist 2014, 165, 779–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lilly, E.L.; Halanych, K.M.; Anderson, D.M. Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae). J. Phycol. 2007, 43, 1329–1338. [Google Scholar] [CrossRef]
- Mertens, K.N.; Adachi, M.; Anderson, D.M.; Band-Schmidt, C.J.; Bravo, I.; Brosnahan, M.L.; Bolch, C.J.S.; Calado, A.J.; Carbonell-Moore, M.C.; Chomérat, N. Morphological and phylogenetic data do not support the split of Alexandrium into four genera. Harmful Algae 2020, 98. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.M.; Munoz, M.G.; Contreras, A.M. Temperature as a factor regulating growth and toxin content in the dinoflagellate Alexandrium catenella. Harmful Algae 2006, 5, 762–769. [Google Scholar] [CrossRef]
- Laabir, M.; Jauzein, C.; Genovesi, B.; Masseret, E.; Grzebyk, D.; Cecchi, P.; Vaquer, A.; Perrin, Y.; Collos, Y. Influence of temperature, salinity and irradiance on the growth and cell yield of the harmful red tide dinoflagellate Alexandrium catenella colonizing Mediterranean waters. J. Plankton Res. 2011, 33, 1550–1563. [Google Scholar] [CrossRef]
- Laabir, M.; Collos, Y.; Masseret, E.; Grzebyk, D.; Abadie, E.; Savar, V.; Sibat, M.; Amzil, Z. Influence of environmental factors on the paralytic shellfish toxin content and profile of Alexandrium catenella (Dinophyceae) isolated from the Mediterranean Sea. Mar. Drugs 2013, 11, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization (FAO)/World Health Organization (WHO). Technical Paper on Toxicity Equivalency Factors for Marine Biotoxins Associated with Bivalve Molluscs; FAO: Rome, Italy, 2016; p. 108. [Google Scholar]
- Abdulhussain, A.H.; Cook, K.B.; Turner, A.D.; Lewis, A.M.; Elsafi, M.A.; Mayor, D.J. The influence of the toxin producing Dinoflagellate, Alexandrium catenella (1119/27), on the feeding and survival of the marine Copepod, Acartia tonsa. Harmful Algae 2020, 98. [Google Scholar] [CrossRef]
- Etheridge, S.M.; Roesler, C.S. Effects of temperature, irradiance, and salinity on photosynthesis, growth rates, total toxicity, and toxin composition for Alexandrium fundyense isolates from the Gulf of Maine and Bay of Fundy. Deep Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 2491–2500. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Ogata, T.; Kaga, S.; Yoshida, M.; Fukuyo, Y.; Kodama, M. Accumulation of paralytic shellfish toxins in the scallop Patinopecten yessoensis caused by the dinoflagellate Alexandrium catenella in Otsuchi Bay, Iwate Prefecture, northern Pacific coast of Japan. Fish. Sci. 2001, 67, 1157–1162. [Google Scholar] [CrossRef]
- Tillmann, U.; John, U. Toxic effects of Alexandrium spp. on heterotrophic dinoflagellates: An allelochemical defence mechanism independent of PSP-toxin content. Mar. Ecol. Prog. Ser. 2002, 230, 47–58. [Google Scholar] [CrossRef]
- Hamasaki, K.; Horie, M.; Tokimitsu, S.; Toda, T.; Taguchi, S. Variability in toxicity of the dinoflagellate Alexandrium tamarense isolated from Hiroshima Bay, western Japan, as a reflection of changing environmental conditions. J. Plankton Res. 2001, 23, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Band-Schmidt, C.J.; Lilly, E.L.; Anderson, D.M. Identification of Alexandrium affine and A. margalefii (Dinophyceae) using DNA sequencing and LSU rDNA-based RFLP-PCR assays. Phycologia 2003, 42, 261–268. [Google Scholar] [CrossRef]
- Anderson, D.M.; Kulis, D.M.; Sullivan, J.J.; Hall, S.; Lee, C. Dynamics and physiology of saxitoxin production by the dinoflagellates Alexandrium spp. Mar. Biol. 1990, 104, 511–524. [Google Scholar] [CrossRef]
- Lim, P.T.; Leaw, C.P.; Kobiyama, A.; Ogata, T. Growth and toxin production of tropical Alexandrium minutum halim (Dinophyceae) under various nitrogen to phosphorus ratios. J. Appl. Phycol. 2010, 22, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.Ø.; Moestrup, Ø. Autecology of the toxic dinoflagellate Alexandrium ostenfeldii: Life history and growth at different temperatures and salinities. Eur. J. Phycol. 1997, 32, 9–18. [Google Scholar] [CrossRef]
- Cho, Y.; Ogawa, M.; Yotsu-Yamashita, M.; Oshima, Y. Effect of 5-fluoro-2′-deoxyuridine on toxin production and cell cycle regulation in marine dinoflagellate, Alexandrium tamarense. Harmful Algae 2014, 32, 64–72. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Cho, Y.; Konoki, K.; Nagasawa, K.; Oshima, Y.; Yotsu-Yamashita, M. Biosynthetic route towards saxitoxin and shunt pathway. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ongley, S.E.; Pengelly, J.J.; Neilan, B.A. Elevated Na+ and pH influence the production and transport of saxitoxin in the cyanobacteria Anabaena circinalis AWQC131C and Cylindrospermopsis raciborskii T3. Environ. Microbiol. 2016, 18, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Geffroy, S.; Lechat, M.M.; Le Gac, M.; Rovillon, G.A.; Marie, D.; Bigeard, E.; Malo, F.; Amzil, Z.; Guillou, L.; Caruana, A. From the sxtA4 gene to saxitoxin production: What controls the variability among Alexandrium minutum and Alexandrium pacificum strains? Front. Microbiol. 2021, 12, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Hii, K.S.; Lim, P.T.; Kon, N.F.; Takata, Y.; Usup, G.; Leaw, C.P. Physiological and transcriptional responses to inorganic nutrition in a tropical Pacific strain of Alexandrium minutum: Implications for the saxitoxin genes and toxin production. Harmful Algae 2016, 56, 9–21. [Google Scholar] [CrossRef]
- Wiese, M.; Murray, S.A.; Alvin, A.; Neilan, B.A. Gene expression and molecular evolution of sxtA4 in a saxitoxin producing dinoflagellate Alexandrium catenella. Toxicon 2014, 92, 102–112. [Google Scholar] [CrossRef]
- Jansen, R.; Greenbaum, D.; Gerstein, M. Relating whole-genome expression data with protein-protein interactions. Genome Res. 2002, 12, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Greenbaum, D.; Colangelo, C.; Williams, K.; Gerstein, M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; Hernández-Sandoval, F.E.; Núñez-Vázquez, E.J.; López-Cortés, D.J. Effect of temperature on growth and paralytic toxin profiles in isolates of Gymnodinium catenatum (Dinophyceae) from the Pacific coast of Mexico. Toxicon 2014, 90, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Sako, Y.; Yoshida, T.; Uchida, A.; Arakawa, O.; Noguchi, T.; Ishida, Y. Purification and characterization of a sulfotransferase specific to N-21 of saxitoxin and gonyautoxin 2+3 from the toxic dinoflagellate Gymnodinium catenatum (Dinophyceae). J. Phycol. 2001, 37, 1044–1051. [Google Scholar] [CrossRef]
- Llewellyn, L.E.; Negri, A.P.; Doyle, J.; Baker, P.D.; Beltran, E.C.; Neilan, B.A. Radioreceptor assays for sensitive detection and quantitation of saxitoxin and its analogues from strains of the freshwater cyanobacterium, Anabaena circinalis. Environ. Sci. Technol. 2001, 35, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Soto-Liebe, K.; Murillo, A.A.; Krock, B.; Stucken, K.; Fuentes-Valdés, J.J.; Trefault, N.; Cembella, A.; Vásquez, M. Reassessment of the toxin profile of Cylindrospermopsis raciborskii T3 and function of putative sulfotransferases in synthesis of sulfated and sulfonated PSP toxins. Toxicon 2010, 56, 1350–1361. [Google Scholar] [CrossRef] [Green Version]
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant. Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals, 1st ed.; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Richards, E.; Reichardt, M.; Rogers, S. Preparation of genomic DNA from plant tissue. In Current Protocols in Molecular Biology; Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., Struhl, K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1994; pp. 2–3. [Google Scholar]
- Ruvindy, R.; Bolch, C.J.; MacKenzie, L.; Smith, K.F.; Murray, S.A. qPCR assays for the detection and quantification of multiple paralytic shellfish toxin-producing species of Alexandrium. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Thangaraj, P.; Park, T.G.; Ki, J.S. Molecular cloning reveals co-occurring species behind red tide blooms of the harmful dinoflagellate Cochlodinium polykrikoides. Biochem. Syst. Ecol. 2017, 70, 29–34. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Rey, V.; Botana, A.M.; Antelo, A.; Alvarez, M.; Botana, L.M. Rapid analysis of paralytic shellfish toxins and tetrodotoxins by liquid chromatography-tandem mass spectrometry using a porous graphitic carbon column. Food chem. 2018, 269, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Mok, J.S.; Song, K.C.; Lee, K.J.; Kim, J.H. Variation and profile of paralytic shellfish poisoning toxins in Jinhae bay, Korea. Fish. Aquat. Sci. 2013, 16, 137–142. [Google Scholar] [CrossRef] [Green Version]


| Species | Strain | Temperature | Toxins | STXs eq (fmol/cell) | Reference |
|---|---|---|---|---|---|
| Alexandrium catenella | ACC02 | 10–16 °C | electrophysiological test | 3.427.7 | [56] |
| CCAP1119/27 | 15 °C | STX, neoSTX, dxSTX, GTX1-6, C1–2, C4 | 2732.5 fg STXs eq/cell | [60] | |
| ATTL01 ATTL02 | 15 °C | GTX 1,4,5, C1–2 | 5.3–44.3 fg STXs eq/cell | [22] | |
| BAH91 | 15 °C | STX, B1-2, C1–2 | 9.9 | [61] | |
| Otsuchi Bay isolated | 15 °C | STX, neoSTX, GTX1, C1–2 | 34.5 | [62] | |
| ACT03 | 10–30 °C | GTX3-5, C1–4 | 2.9–50.3 | [58] | |
| Alexandrium fundyense | BOF | 5–20 °C | GTX1–4, STX, neoSTX | 211–544 | [61] |
| MI | 5–20 °C | GTX1–4, STX, neoSTX | 100–532 | [61] | |
| Alexandrium tamrense | BAH181 | 15 °C | GTX1–4, neoSTX, STX, B1–2, C1–2 | 42.3 | [63] |
| GTPP01 | 15 °C | GTX1–4, neoSTX, STX, B1–2, C1–2 | 33.4 | [63] | |
| ATHS-95 | 17 °C | GTX1–4, C1–4 | 1.35–2.7 | [64] | |
| Alexandrium minutum | AmSp01 | 25 °C | GTX1, 3, 4, neoSTX | 11.2–12.8 | [17] |
| AmSp03 | 25 °C | GTX1, 4, neoSTX | 9.1–11.8 | [17] | |
| AmSp04 | 25 °C | GTX1, 3, 4, neoSTX | 5.1–11.2 | [17] | |
| AmSp05 | 25 °C | GTX1–4, neoSTX, dcSTX, | 3.0–9.5 | [17] | |
| AmSp17 | 25 °C | GTX1, 3, 4, dcSTX, neoSTX | 5.6–6.3 | [17] | |
| AL3T | 15 °C | GTX1–4 | 3 | [63] | |
| Alexandrium lusitanicum | BAH91 | 15 °C | GTX1–4 | 16 | [63] |
| Alexandrium affine | AABCV-1 | 15–34 °C | non-toxic | non-toxic | [65] |
| CCMP112 | 16–20 °C | non-toxic | non-toxic | [3] | |
| CS 312/02 | 16–20 °C | non-toxic | non-toxic | [3] | |
| Alexandrium andersonii | CCMP1597 | 16–20 °C | non-toxic | non-toxic | [3] |
| CCMP2222 | 16–20 °C | non-toxic | non-toxic | [3] |
| Gene | Primer | Nucleotide Sequence (5’→3’) | Remark | Source |
|---|---|---|---|---|
| sxtA4 | Sxt007F | ATGCTCAACATGGGAGTCATCC | ORF | [45] |
| Sxt008R | GGGTCCAGTAGATGTTGACGATG | ORF | [45] | |
| sxtA4qF | GAGCAACCCTTCGGGTATGGT | qRT-PCR | This study | |
| sxtA4qR | TCAGAATGCCGAACTTCTCGTCG | qRT-PCR | This study | |
| sxtG | sxtG001F | GCCGATGTATGACTTCTACAAGAG | ORF | This study |
| sxtG002F | CATCCCAGACTGGTACATGC | ORF | This study | |
| sxtG001R | CCGTATGGATGTACCTGTGC | ORF | This study | |
| sxtG002R | AGAGCGTGTTCAAGTGGTAGC | ORF | This study | |
| sxtGqF | GGACATGGACGAGAATAGCTG | qRT-PCR | This study | |
| sxtGqR | GATGGCGAGCACGTTTATGC | qRT-PCR | This study | |
| α-tubulin | TUA qF | CTTCCAGGGCTTCATGGTG | qRT-PCR | This study |
| TUA qR | AGACACGTTTGGCTCCTG | qRT-PCR | This study | |
| Actin | ACT-US-408-F | ACTTGATTTGCTTGGTGGGAG | qRT-PCR | [84] |
| ACT-US-645-R | AAGTCCAAGGAAGGAAGCATC | qRT-PCR | [84] | |
| 28S rRNA | 28F01 | CCGCTGAATTTAAGCATATAAGTAAGC | rRNA | [85] |
| 28R691 | CTTGGTCCGTGTTTCAAGAC | rRNA | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Park, H.; Wang, H.; Yoo, H.Y.; Park, J.; Ki, J.-S. Low Temperature and Cold Stress Significantly Increase Saxitoxins (STXs) and Expression of STX Biosynthesis Genes sxtA4 and sxtG in the Dinoflagellate Alexandrium catenella. Mar. Drugs 2021, 19, 291. https://doi.org/10.3390/md19060291
Kim H, Park H, Wang H, Yoo HY, Park J, Ki J-S. Low Temperature and Cold Stress Significantly Increase Saxitoxins (STXs) and Expression of STX Biosynthesis Genes sxtA4 and sxtG in the Dinoflagellate Alexandrium catenella. Marine Drugs. 2021; 19(6):291. https://doi.org/10.3390/md19060291
Chicago/Turabian StyleKim, Hansol, Hyunjun Park, Hui Wang, Hah Young Yoo, Jaeyeon Park, and Jang-Seu Ki. 2021. "Low Temperature and Cold Stress Significantly Increase Saxitoxins (STXs) and Expression of STX Biosynthesis Genes sxtA4 and sxtG in the Dinoflagellate Alexandrium catenella" Marine Drugs 19, no. 6: 291. https://doi.org/10.3390/md19060291
APA StyleKim, H., Park, H., Wang, H., Yoo, H. Y., Park, J., & Ki, J.-S. (2021). Low Temperature and Cold Stress Significantly Increase Saxitoxins (STXs) and Expression of STX Biosynthesis Genes sxtA4 and sxtG in the Dinoflagellate Alexandrium catenella. Marine Drugs, 19(6), 291. https://doi.org/10.3390/md19060291