Implementation of kLa-Based Strategy for Scaling Up Porphyridium purpureum (Red Marine Microalga) to Produce High-Value Phycoerythrin, Fatty Acids, and Proteins
Abstract
1. Introduction
2. Result and Discussion
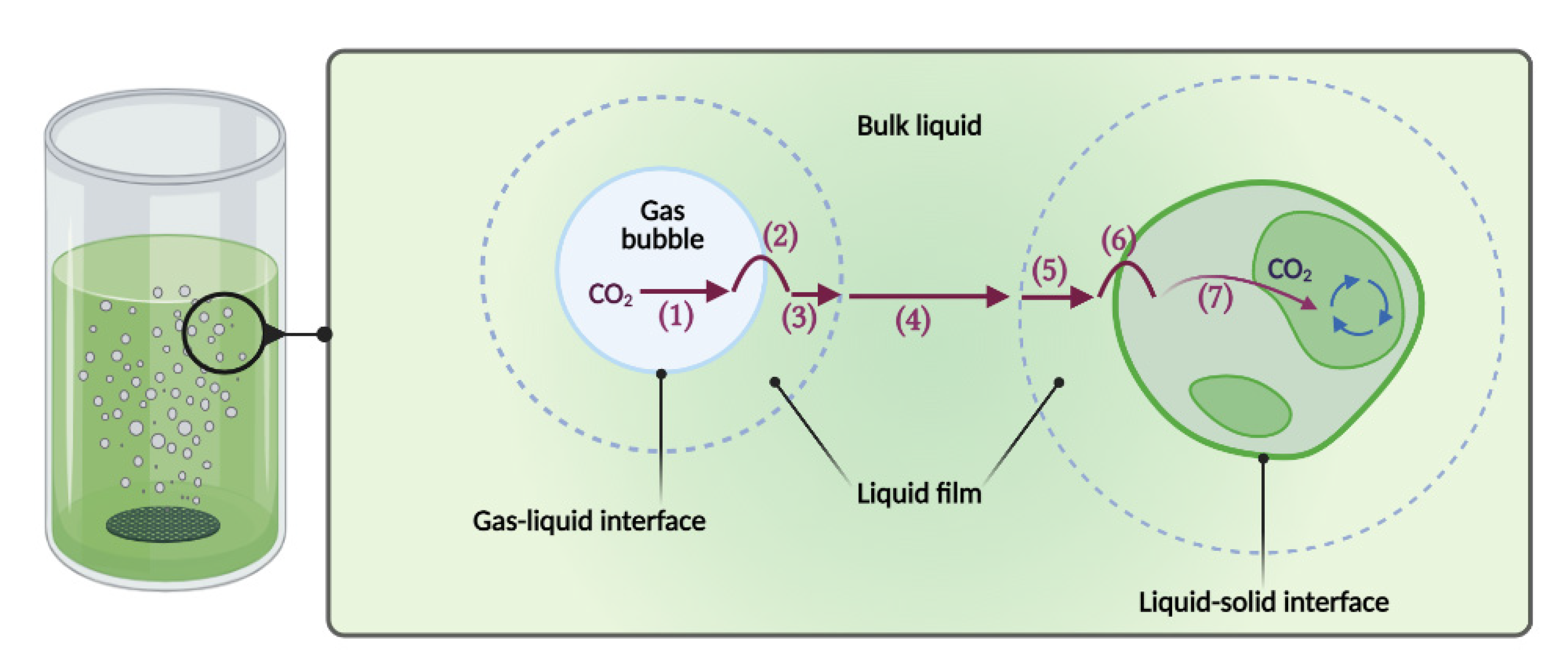
2.1. Volumetric Mass Transfer Coefficients
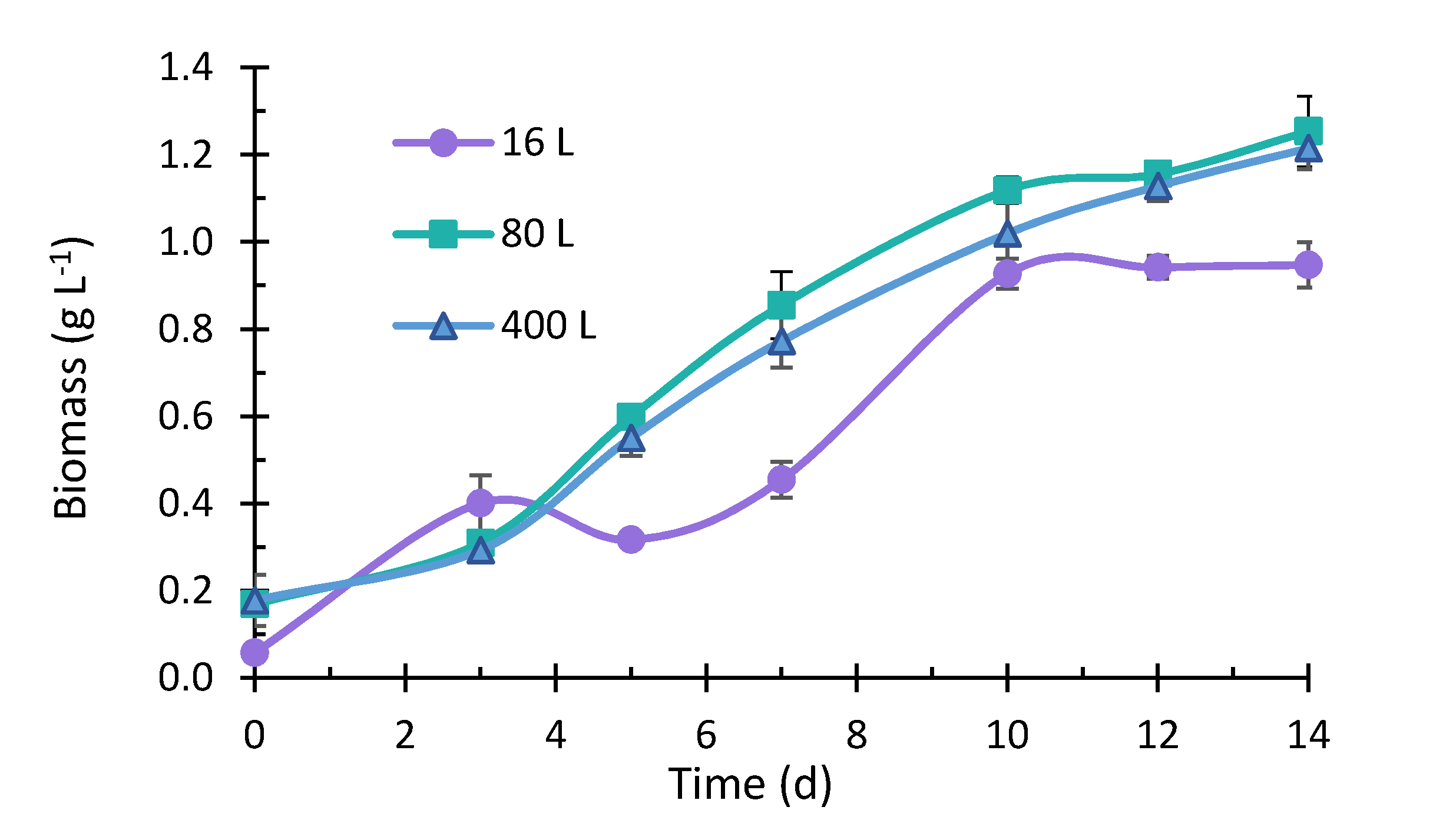
2.2. Microalgae Growth
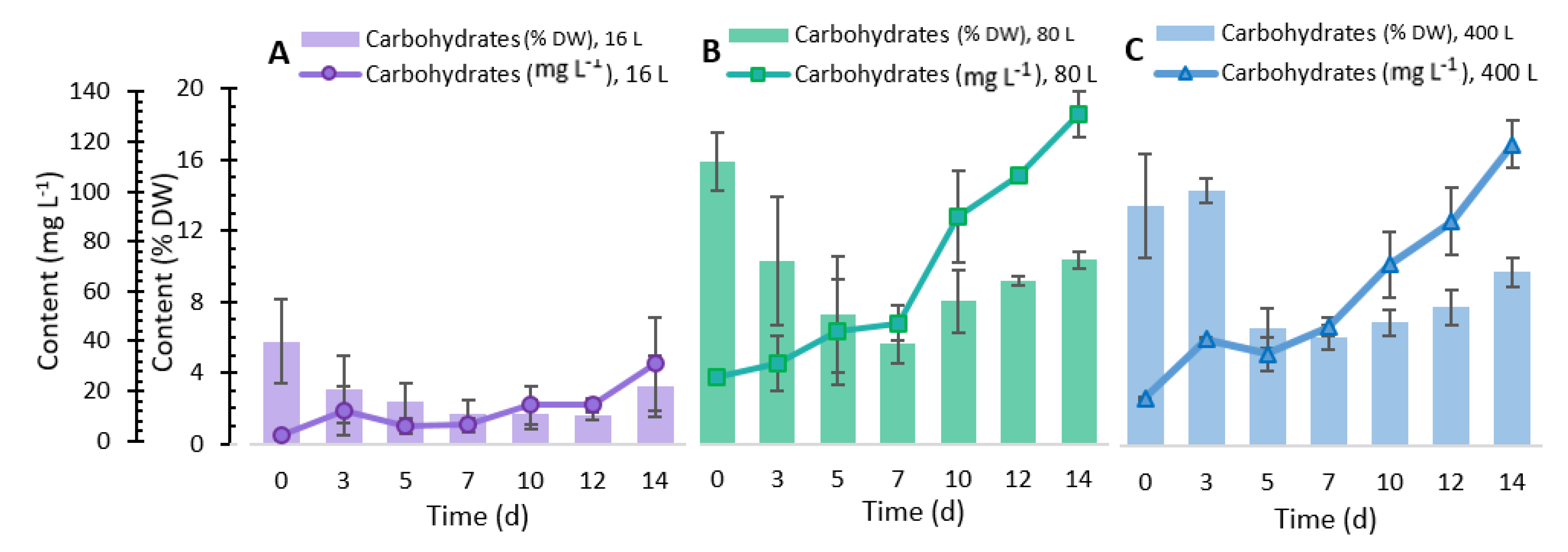
2.3. Total Carbohydrate Content
2.4. Lipid Analysis
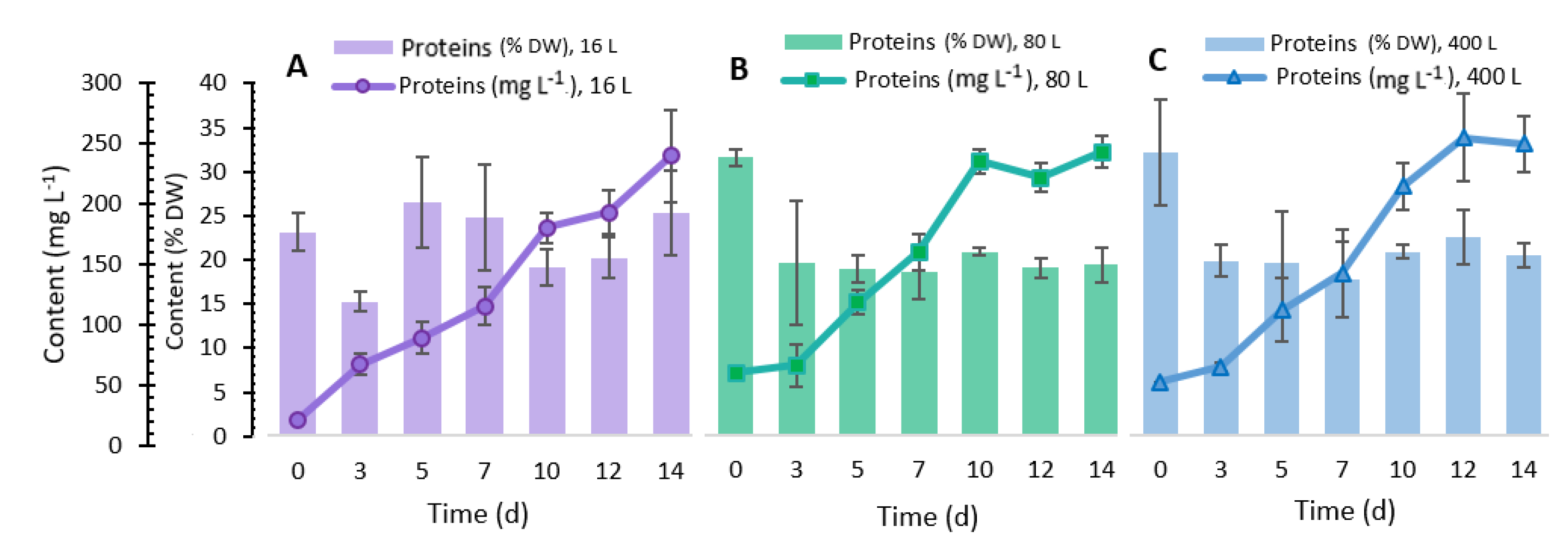
2.5. Total Protein Content
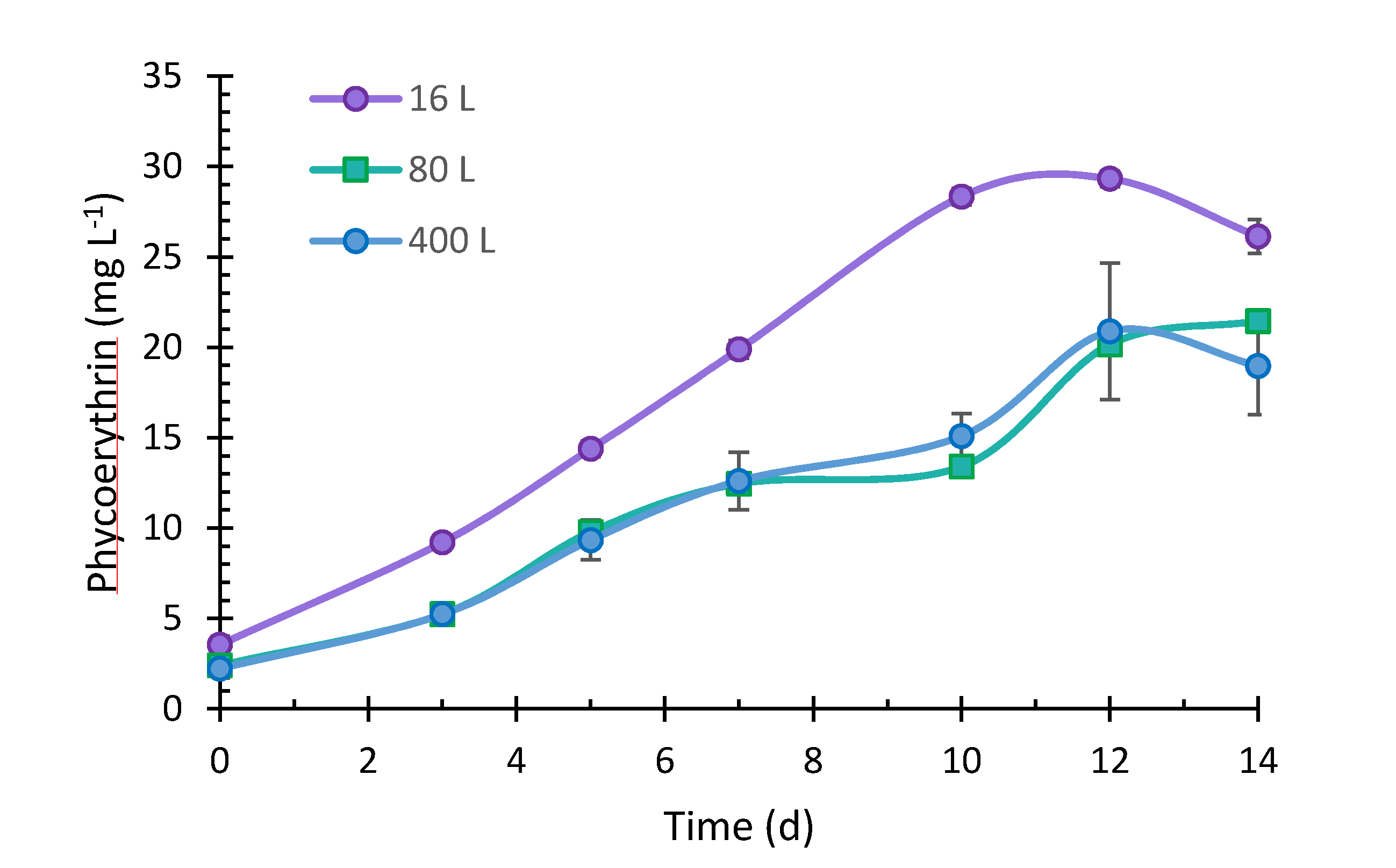
2.6. Phycoerythrin Content
3. Material and Methods
3.1. Microalgal Strain and Culture Conditions
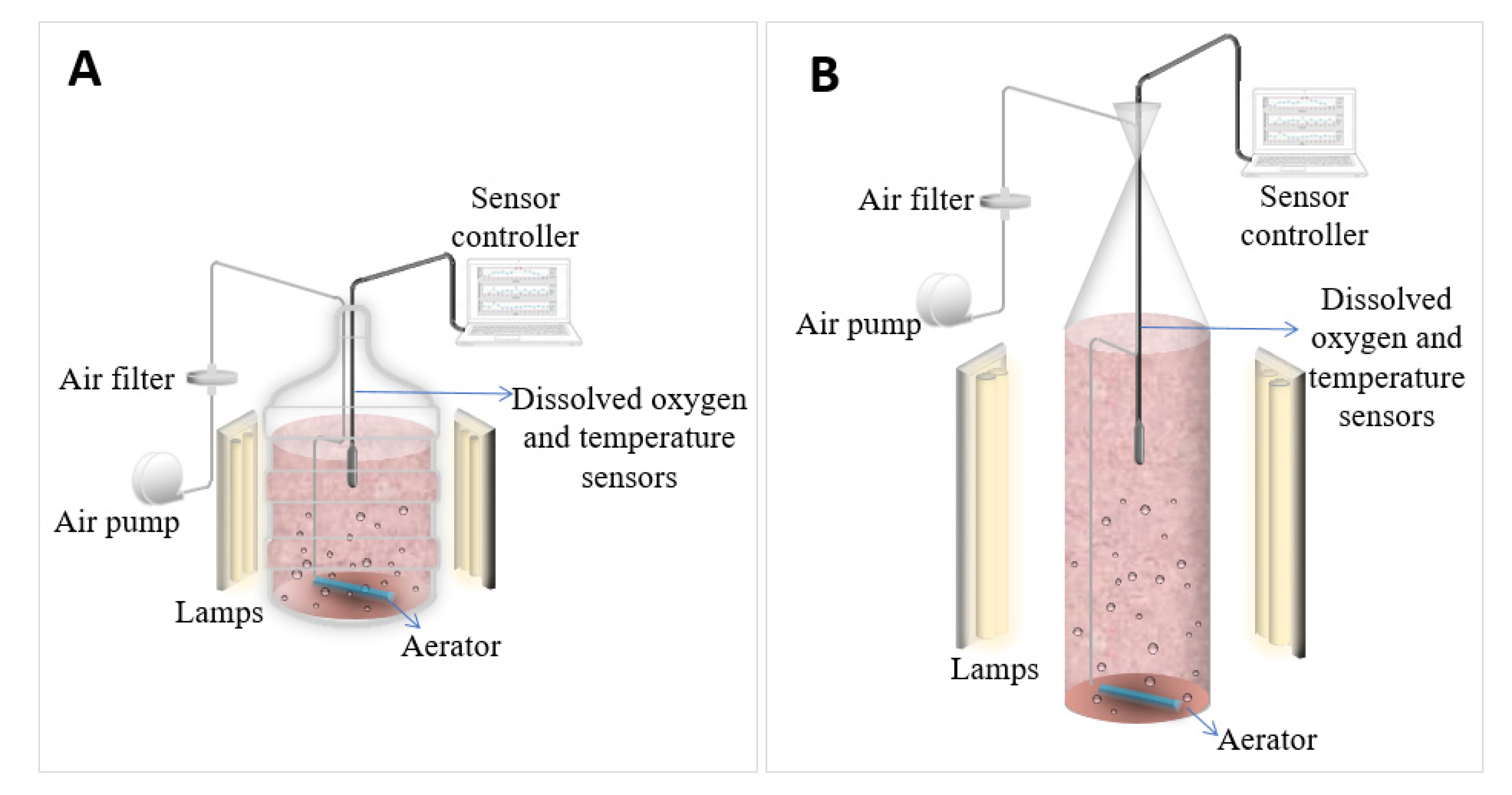
3.2. Real-Time Oxygen and Temperature Monitoring
3.3. Volumetric Mass Transfer Coefficients
3.4. Biomass Analyses
3.4.1. Cell Growth
3.4.2. Total Carbohydrates Content
3.4.3. Total Lipids and Fatty Acids Analysis
3.4.4. Total Protein Content
3.4.5. Phycoerythrin Extraction and Purification
3.5. Statistical Analysis
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, S.; Ji, L.; Chen, C.; Zhao, S.; Sun, M.; Gao, Z.; Wu, H.; Fan, J. Efficient accumulation of high-value bioactive substances by carbon to nitrogen ratio regulation in marine microalgae Porphyridium purpureum. Bioresour. Technol. 2020, 309, 123362. [Google Scholar] [CrossRef] [PubMed]
- Gaignard, C.; Gargouch, N.; Dubessay, P.; Delattre, C.; Pierre, G.; Laroche, C.; Fendri, I.; Abdelkafi, S.; Michaud, P. New horizons in culture and valorization of red microalgae. Biotechnol. Adv. 2019, 37, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Dragan, I.; Ishrat, S.; Gregory, J.P.; Edward, S.Y. Formation of fluorescent proteins by the attachment of phycoerythrobilin to R-phycoerythrin alpha and beta apo-subunits. Anal. Biochem. 2007, 358, 38–50. [Google Scholar]
- Bermejo, R.; Tobaruela, D.J.; Talavera, E.M.; Orte, A.; Alvarez-Pez, J.M. Fluorescent behavior of B-phycoerythrin in microemulsions of aerosol OT/water/isooctane. J. Colloid Interface Sci. 2003, 263, 616–624. [Google Scholar] [CrossRef]
- Bermejo, R.; Ruiz, E.; Acien, F.; Fernandez, F.G.A. Recovery of B-phycoerythrin using expanded bed adsorption chromatography: Scale-up of the process. Enzym. Microb. Technol. 2007, 40, 927–933. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Hernandez-Mireles, T.; Rito-Palomares, M. Improved recovery of B-phycoerythrin produced by the red microalga Porphyridium cruentum. J. Chem. Technol. Biotechnol. 2006, 81, 989–996. [Google Scholar] [CrossRef]
- Kathiresan, S.; Sarada, R.; Bhattacharya, S.; Ravishankar, G. Culture media optimization for growth and phycoerythrin production from Porphyridium purpureum. Biotechnol. Bioeng. 2007, 96, 456–463. [Google Scholar] [CrossRef]
- Cohen, E.; Arad, S.M. A closed system for outdoor cultivation of Porphyridium. Biomass 1989, 18, 59–67. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Vonshak, A. Scaling up microalgal cultures to commercial scale. Eur. J. Phycol. 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Ugwu, C.; Aoyagi, H.; Uchiyama, H. Photobioreactors for mass cultivation of algae. Bioresour. Technol. 2008, 99, 4021–4028. [Google Scholar] [CrossRef]
- Takors, R. Scale-up of microbial processes: Impacts, tools and open questions. J. Biotechnol. 2012, 160, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Vernerey, A.; Albiol, J.; Lasseur, C.; Godia, F. Scale-Up and Design of a Pilot-Plant Photobioreactor for the Continuous Culture of Spirulina platensis. Biotechnol. Prog. 2001, 17, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Enzmann, F.; Stöckl, M.; Zeng, A.-P.; Holtmann, D. Same but different-Scale up and numbering up in electrobiotechnology and photobiotechnology. Eng. Life Sci. 2019, 19, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Kubar, A.A.; Cheng, J.; Kumar, S.; Shuzheng, L.; Chen, S.; Tian, J. Strengthening mass transfer with the Tesla-valve baffles to increase the biomass yield of Arthrospira platensis in a column photobioreactor. Bioresour. Technol. 2020, 320, 124337. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.G.; Lam, M.K.; Lee, K.T. Pilot-scale semi-continuous cultivation of microalgae Chlorella vulgaris in bubble column photobioreactor (BC-PBR): Hydrodynamics and gas–liquid mass transfer study. Algal Res. 2016, 15, 65–76. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, J.; Ye, Q.; Lai, X.; Zhang, X.; Zhou, J. Strengthening mass transfer of carbon dioxide microbubbles dissolver in a horizontal tubular photo-bioreactor for improving microalgae growth. Bioresour. Technol. 2019, 277, 11–17. [Google Scholar] [CrossRef]
- Inostroza, C.; Solimeno, A.; García, J.; Fernández-Sevilla, J.M.; Acién, F.G. Improvement of real-scale raceway bioreactors for microalgae production using Computational Fluid Dynamics (CFD). Algal Res. 2021, 54, 102207. [Google Scholar] [CrossRef]
- Lone, S.R.; Kumar, V.; Seay, J.R.; Englert, D.L.; Hwang, H.T. Evaluation of Volumetric Mass Transfer Coefficient in a Stirred Tank Bioreactor Using Response Surface Methodology. Environ. Prog. Sustain. Energy. 2018. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E.; Santos, V.E.; Merchuk, J.C. Oxygen uptake rate in microbial processes: An overview. Biochem. Eng. J. 2010, 49, 289–307. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y. Process effect of microalgal-carbon dioxide fixation and biomass production: A review. Renew. Sustain. Energy Rev. 2014, 31, 121–132. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Ginzberg, A.; Korin, E.; Arad, S. (Malis) Effect of drying on the biological activities of a red microalgal polysaccharide. Biotechnol. Bioeng. 2008, 99, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Merchuk, J.C.; Gluz, M.; Mukmenev, I. Comparison of photobioreactors for cultivation of the red microalga Porphyridium sp. J. Chem. Technol. Biotechnol. 2000, 75, 1119–1126. [Google Scholar] [CrossRef]
- Shastik, E.; Romanova, A.; Laurinavichene, T.; Petushkova, E.; Sakurai, H.; Tsygankov, A. Plastic bags as simple photobioreactors for cyanobacterial hydrogen production outdoors in Moscow region. Int. J. Energy Environ. Eng. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Nagarajan, D.; Cheah, W.Y. Eicosapentaenoic acid production from Nannochloropsis oceanica CY2 using deep sea water in outdoor plastic-bag type photobioreactors. Bioresour. Technol. 2018, 253, 1–7. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Stengel, D.B. Towards the biorefinery concept: Interaction of light, temperature and nitrogen for optimizing the co-production of high-value compounds in Porphyridium purpureum. Algal Res. 2015, 10, 152–163. [Google Scholar] [CrossRef]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and cyanobacterial cultivation: The supply of nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef]
- Li, S.; Ji, L.; Shi, Q.; Wu, H.; Fan, J. Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Bioresour. Technol. 2019, 292, 122048. [Google Scholar] [CrossRef]
- Razzak, S.A.; Al-Aslani, I.; Hossain, M.M. Hydrodynamics and mass transfer of CO2 in water in a tubular photobioreactor. Eng. Life Sci. 2016, 16, 355–363. [Google Scholar] [CrossRef]
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Show, P.L. Determination of Dissolved CO2 Concentration in Culture Media: Evaluation of pH Value and Mathematical Data. Processes 2020, 8, 1373. [Google Scholar] [CrossRef]
- Fuentes, M.R.; Sánchez, J.G.; Sevilla, J.F.; Fernández, F.A.; Pérez, J.A.S.; Grima, E.M. Outdoor continuous culture of Porphyridium cruentum in a tubular photobioreactor: Quantitative analysis of the daily cyclic variation of culture parameters. J. Biotechnol. 1999, 70, 271–288. [Google Scholar] [CrossRef]
- Ju, J.-H.; Oh, B.-R.; Ko, D.-J.; Heo, S.-Y.; Lee, J.-J.; Kim, Y.-M.; Yang, K.; Seo, J.-W.; Hong, W.-K.; Kim, C.-H. Boosting productivity of heterotrophic microalgae by efficient control of the oxygen transfer coefficient using a microbubble sparger. Algal Res. 2019, 41, 101474. [Google Scholar] [CrossRef]
- Qu, L.; Ren, L.-J.; Huang, H. Scale-up of docosahexaenoic acid production in fed-batch fermentation by Schizochytrium sp. based on volumetric oxygen-transfer coefficient. Biochem. Eng. J. 2013, 77, 82–87. [Google Scholar] [CrossRef]
- Deniz, I. Scaling-up of Haematococcus pluvialis production in stirred tank photobioreactor. Bioresour. Technol. 2020, 310, 123434. [Google Scholar] [CrossRef] [PubMed]
- Assunção, M.F.; Varejão, J.M.; Santos, L.M. Nutritional characterization of the microalga Ruttnera lamellosa compared to Porphyridium purpureum. Algal Res. 2017, 26, 8–14. [Google Scholar] [CrossRef]
- Granata, T. Dependency of Microalgal Production on Biomass and the Relationship to Yield and Bioreactor Scale-up for Biofuels: A Statistical Analysis of 60+ Years of Algal Bioreactor Data. BioEnergy Res. 2017, 10, 267–287. [Google Scholar] [CrossRef]
- Kavitha, M.D.; Kathiresan, S.; Bhattacharya, S.; Sarada, R. Culture media optimization of Porphyridium purpureum: Production potential of biomass, total lipids, arachidonic and eicosapentaenoic acid. J. Food Sci. Technol. 2016, 53, 2270–2278. [Google Scholar] [CrossRef]
- Razaghi, A.; Godhe, A.; Albers, E. Effects of nitrogen on growth and carbohydrate formation in Porphyridium cruentum. Open Life Sci. 2014, 9, 156–162. [Google Scholar] [CrossRef]
- Fuentes-Grünewald, C.; Bayliss, C.; Zanain, M.; Pooley, C.; Scolamacchia, M.; Silkina, A. Evaluation of batch and semi-continuous culture of Porphyridium purpureum in a photobioreactor in high latitudes using Fourier Transform Infrared spectroscopy for monitoring biomass composition and metabolites production. Bioresour. Technol. 2015, 189, 357–363. [Google Scholar] [CrossRef]
- Cohen, E.; Koren, A.; Arad, S.M. A closed system for outdoor cultivation of microalgae. Biomass Bioenergy 1991, 1, 83–88. [Google Scholar] [CrossRef]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol. 2012, 96, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Saavedra, M.D.P.; Castro-Ochoa, F.Y.; Nava-Ruiz, V.M.; Ruiz-Güereca, D.A.; Villagómez-Aranda, A.L.; Siqueiros-Vargas, F.; Molina-Cárdenas, C.A. Effects of nitrogen source and irradiance on Porphyridium cruentum. Environ. Boil. Fishes 2017, 30, 783–792. [Google Scholar] [CrossRef]
- Schulze, C.; Strehle, A.; Merdivan, S.; Mundt, S. Carbohydrates in microalgae: Comparative determination by TLC, LC-MS without derivatization, and the photometric thymol-sulfuric acid method. Algal Res. 2017, 25, 372–380. [Google Scholar] [CrossRef]
- Oh, S.H.; Han, J.G.; Kim, Y.; Ha, J.H.; Kim, S.S.; Jeong, M.H.; Jeong, H.S.; Kim, N.Y.; Cho, J.S.; Yoon, W.B.; et al. Lipid production in Porphyridium cruentum grown under different culture conditions. J. Biosci. Bioeng. 2009, 108, 429–434. [Google Scholar] [CrossRef]
- Coward, T.; Fuentes-Grünewald, C.; Silkina, A.; Oatley-Radcliffe, D.L.; Llewellyn, G.; Lovitt, R.W. Utilising light-emitting diodes of specific narrow wavelengths for the optimization and co-production of multiple high-value compounds in Porphyridium purpureum. Bioresour. Technol. 2016, 221, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Cheirsilp, B.; Torpee, S. Enhanced growth and lipid production of microalgae under mixotrophic culture condition: Effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol. 2012, 110, 510–516. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Mathimani, T.; Rene, E.R.; Shanmugam, S.; Chi, N.T.L.; Pugazhendhi, A. Impact of cultivation conditions on the biomass and lipid in microalgae with an emphasis on biodiesel. Fuel 2021, 284, 119058. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Wu, H.; Jiang, P.; Chen, Z.; Xiang, W. Growth and Biochemical Composition of Porphyridium purpureum SCS-02 under Different Nitrogen Concentrations. Mar. Drugs 2019, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.R. Biomass nutrient profiles of the microalga Porphyridium cruentum. Food Chem. 2000, 70, 345–353. [Google Scholar] [CrossRef]
- Moretti, A.; Musacchio, A. Chemotaxonomic notes on Porphyridium Näg. (Rhodophyta, Porphyridiales. Plant Biosyst. 1982, 116, 269–274. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. Environ. Boil. Fishes 2015, 27, 1109–1119. [Google Scholar] [CrossRef]
- Safi, C.; Charton, M.; Pignolet, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Evaluation of the protein quality of Porphyridium cruentum. Environ. Boil. Fishes 2012, 25, 497–501. [Google Scholar] [CrossRef]
- Wang, B.; Lan, C.Q.; Horsman, M. Closed photobioreactors for production of microalgal biomasses. Biotechnol. Adv. 2012, 30, 904–912. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Rodas-Zuluaga, L.I.; Castillo-Zacarías, C.; Rostro-Alanís, M.; De La Cruz, R.; Carrillo-Nieves, D.; Salinas-Salazar, C.; Grunewald, C.F.; Llewellyn, C.A.; Olguín, E.J.; et al. Light Intensity and Nitrogen Concentration Impact on the Biomass and Phycoerythrin Production by Porphyridium purpureum. Mar. Drugs 2019, 17, 460. [Google Scholar] [CrossRef]
- Marcati, A.; Ursu, A.V.; Laroche, C.; Soanen, N.; Marchal, L.; Jubeau, S.; Djelveh, G.; Michaud, P. Extraction and fractionation of polysaccharides and B-phycoerythrin from the microalga Porphyridium cruentum by membrane technology. Algal Res. 2014, 5, 258–263. [Google Scholar] [CrossRef]
- Hemlata; Afreen, S.; Fatma, T. Extraction, purification and characterization of phycoerythrin from Michrochaete and its biological activities. Biocatal. Agric. Biotechnol. 2018, 13, 84–89. [Google Scholar] [CrossRef]
- Gargouch, N.; Karkouch, I.; Elleuch, J.; Elkahoui, S.; Michaud, P.; Abdelkafi, S.; Laroche, C.; Fendri, I. Enhanced B-phycoerythrin production by the red microalga Porphyridium marinum: A powerful agent in industrial applications. Int. J. Biol. Macromol. 2018, 120, 2106–2114. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; El-Sheekh, M.; Hanelt, D. Pilot cultivation of the chlorophyte microalga Scenedesmus obliquus as a promising feedstock for biofuel. Biomass Bioenergy 2014, 64, 237–244. [Google Scholar] [CrossRef]
- Fuentes-Grünewald, C.; Bayliss, C.; Fonlut, F.; Chapuli, E. Long-term dinoflagellate culture performance in a commercial photobioreactor: Amphidinium carterae case. Bioresour. Technol. 2016, 218, 533–540. [Google Scholar] [CrossRef]
- Zittelli, G.C.; Pastorelli, R.; Tredici, M.R. A Modular Flat Panel Photobioreactor (MFPP) for indoor mass cultivation of Nannochloropsis sp. under artificial illumination. Environ. Boil. Fishes 2000, 12, 521–526. [Google Scholar] [CrossRef]
- Valiorgue, P.; Ben Hadid, H.; El Hajem, M.; Rimbaud, L.; Muller-Feuga, A.; Champagne, J. CO2 mass transfer and conversion to biomass in a horizontal gas–liquid photobioreactor. Chem. Eng. Res. Des. 2014, 92, 1891–1897. [Google Scholar] [CrossRef]
- Pérez, L.; Salgueiro, J.L.; González, J.; Parralejo, A.I.; Maceiras, R.; Cancela, Á. Scaled up from indoor to outdoor cultures of Chaetoceros gracilis and Skeletonema costatum microalgae for biomass and oil production. Biochem. Eng. J. 2017, 127, 180–187. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; López, V.H.; Rodríguez-Rodríguez, J.; Alemán-Nava, G.S.; Cuéllar-Bermúdez, S.P.; Rostro-Alanis, M.; Parra-Saldívar, R. Supercritical Carbon Dioxide and Microwave-Assisted Extraction of Functional Lipophilic Compounds from Arthrospira platensis. Int. J. Mol. Sci. 2016, 17, 658. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field permeabilization and extraction of phycoerythrin from Porphyridium cruentum. Algal Res. 2019, 37, 51–56. [Google Scholar] [CrossRef]
| Volume (L) | Aeration (L min−1) | kLa (CO2) (s−1) |
|---|---|---|
| 16 | 25 | 0.0052 ± 0.0005 |
| 80 | 50 | 0.0007 ± 0.0001 |
| 70 | 0.0022 ± 0.0003 | |
| 80 | 0.0024 ± 0.0005 |
| Culture Volume (L) | Final Biomass Concentration (g L−1) | Biomass Productivity (mg L−1 d−1) | Specific Growth Rate µmax (d−1) | Doubling Time (d) |
|---|---|---|---|---|
| 16 * | 0.95 ± 0.052 | 68.64 ± 0.004 | 0.20 ± 0.011 | 3.45 ± 0.187 |
| 80 * | 1.25 ± 0.081 | 92.25 ± 0.006 | 0.14 ± 0.008 | 4.85 ± 0.286 |
| 400 ** | 1.22 ± 0.049 | 85.42 ± 0.001 | 0.14 ± 0.002 | 5.10 ± 1.000 |
| Component | Lipids (%) | ||
|---|---|---|---|
| 16 L | 80 L | 400 L | |
| Total lipids | 23.59 ± 2.77 | 15.16 ± 0.79 | 17.75 ± 2.58 |
| (C8:0) Caprylic acid | 3.05 ±1.30 | 11.83 ± 1.96 | 11.17± 1.85 |
| (C16:0) Palmitic acid | 40.41 ± 3.92 | 44.66 ± 4.12 | 46.03 ± 3.89 |
| (C18:0) Stearic acid | 2.28 ± 0.35 | 6.49 ± 0.71 | 6.25 ± 0.67 |
| (C18:1n9c) Oleic acid | 2.20 ± 0.11 | 4.25 ± 2.87 | 3.29 ± 2.70 |
| (C18:2n6c) Linoleic acid | 13.34 ± 0.60 | 7.93 ± 0.30 | 8.03 ± 0.29 |
| (C20:4n6) Arachidonic acid | 32.57 ± 5.11 | 22.34 ± 0.97 | 22.67 ± 0.91 |
| (C20:5n3) Eicosapentaenoic acid | 6.14 ± 0.28 | 2.50 ± 0.16 | 2.55 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodas-Zuluaga, L.I.; Castillo-Zacarías, C.; Núñez-Goitia, G.; Martínez-Prado, M.A.; Rodríguez-Rodríguez, J.; López-Pacheco, I.Y.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Parra-Saldívar, R. Implementation of kLa-Based Strategy for Scaling Up Porphyridium purpureum (Red Marine Microalga) to Produce High-Value Phycoerythrin, Fatty Acids, and Proteins. Mar. Drugs 2021, 19, 290. https://doi.org/10.3390/md19060290
Rodas-Zuluaga LI, Castillo-Zacarías C, Núñez-Goitia G, Martínez-Prado MA, Rodríguez-Rodríguez J, López-Pacheco IY, Sosa-Hernández JE, Iqbal HMN, Parra-Saldívar R. Implementation of kLa-Based Strategy for Scaling Up Porphyridium purpureum (Red Marine Microalga) to Produce High-Value Phycoerythrin, Fatty Acids, and Proteins. Marine Drugs. 2021; 19(6):290. https://doi.org/10.3390/md19060290
Chicago/Turabian StyleRodas-Zuluaga, Laura Isabel, Carlos Castillo-Zacarías, Gabriela Núñez-Goitia, María Adriana Martínez-Prado, José Rodríguez-Rodríguez, Itzel Y. López-Pacheco, Juan Eduardo Sosa-Hernández, Hafiz M. N. Iqbal, and Roberto Parra-Saldívar. 2021. "Implementation of kLa-Based Strategy for Scaling Up Porphyridium purpureum (Red Marine Microalga) to Produce High-Value Phycoerythrin, Fatty Acids, and Proteins" Marine Drugs 19, no. 6: 290. https://doi.org/10.3390/md19060290
APA StyleRodas-Zuluaga, L. I., Castillo-Zacarías, C., Núñez-Goitia, G., Martínez-Prado, M. A., Rodríguez-Rodríguez, J., López-Pacheco, I. Y., Sosa-Hernández, J. E., Iqbal, H. M. N., & Parra-Saldívar, R. (2021). Implementation of kLa-Based Strategy for Scaling Up Porphyridium purpureum (Red Marine Microalga) to Produce High-Value Phycoerythrin, Fatty Acids, and Proteins. Marine Drugs, 19(6), 290. https://doi.org/10.3390/md19060290









