In Vitro and In Vivo Anti-Inflammatory Effects of Sulfated Polysaccharides Isolated from the Edible Brown Seaweed, Sargassum fulvellum
Abstract
1. Introduction
2. Results and Discussion
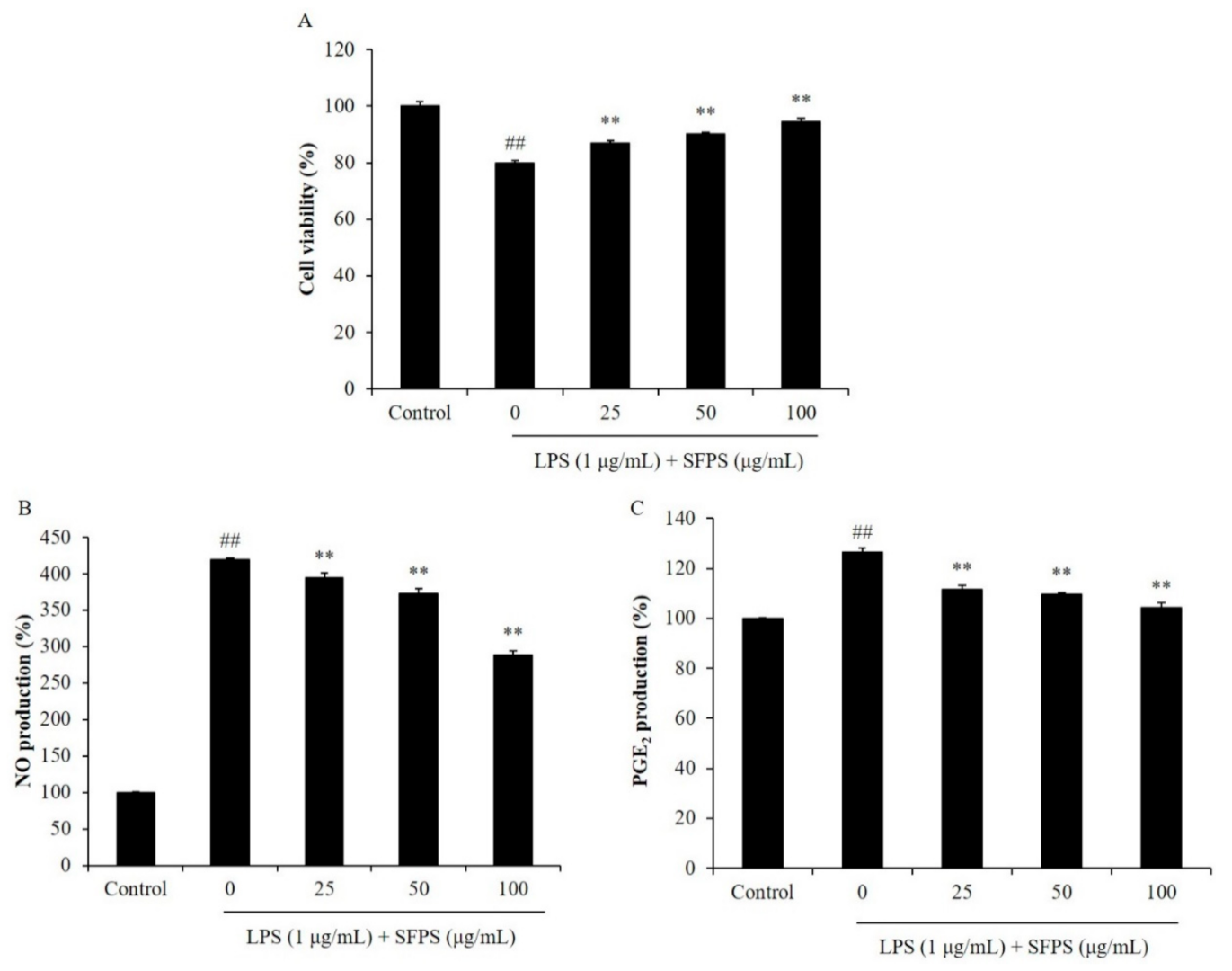
2.1. SFPS Suppresses Cytotoxicity and Inflammatory Molecules Production in LPS-Induced RAW 264.7 Macrophages
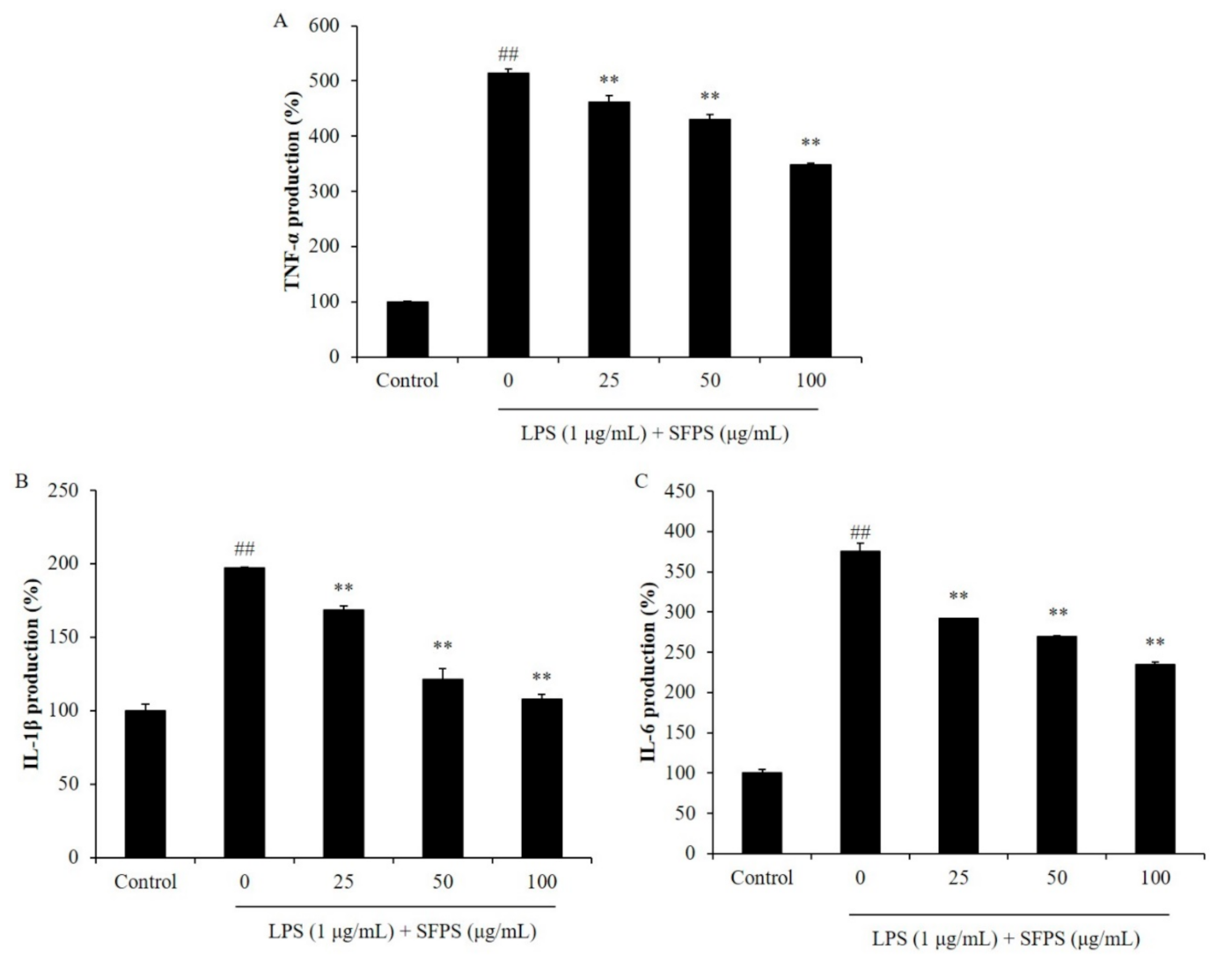
2.2. The Inhibitory Effect of SFPS against Pro-Inflammatory Cytokines, iNOS, and COX-2 Expression in LPS-Stimulated RAW 264.7 Macrophages
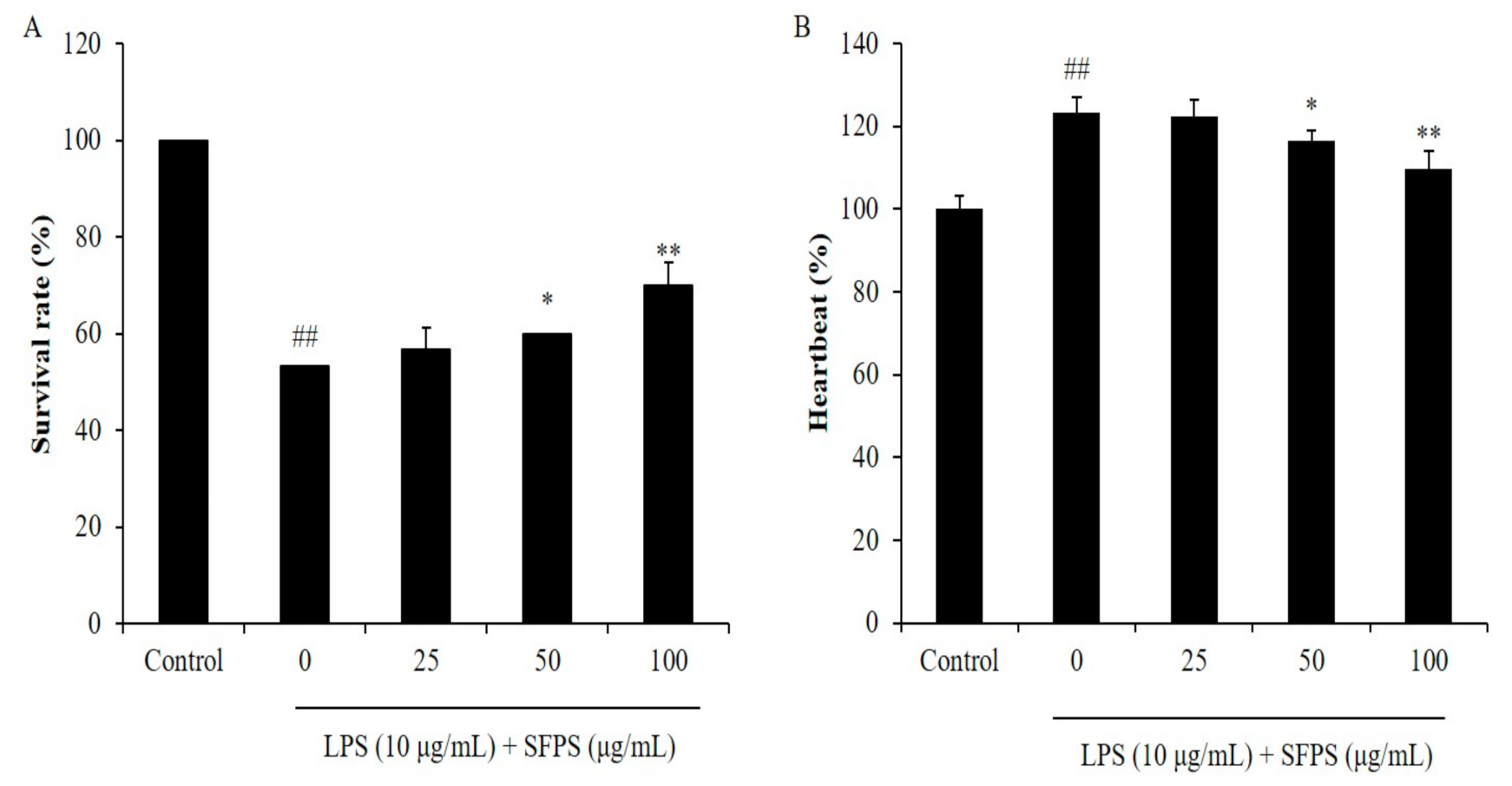
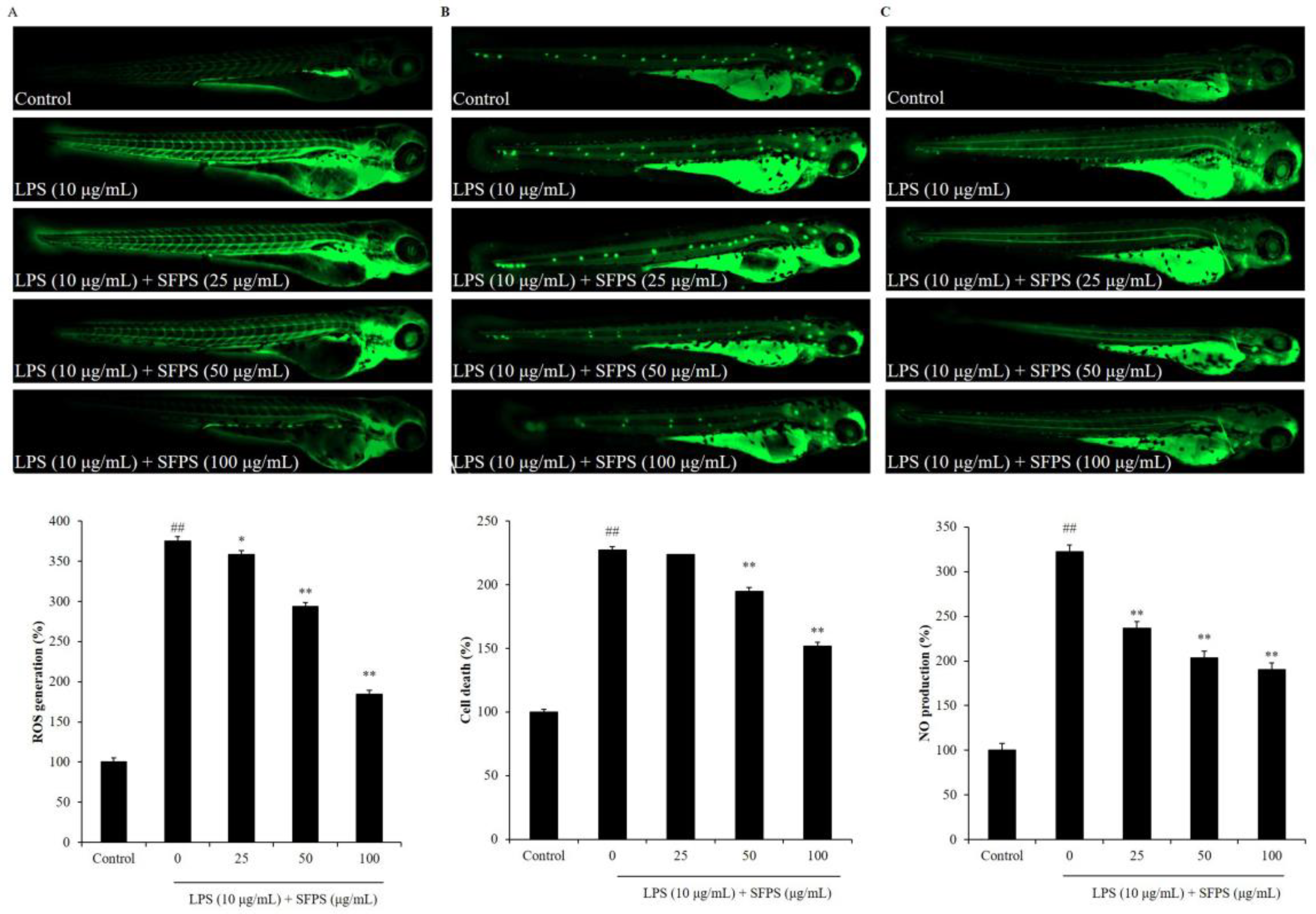
2.3. SFPS Improves LPS-Induced Inflammatory Response in Zebrafish
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Preparation of SFPS
3.3. Cell Culture
3.4. Measurement of NO Production and Cell Viability
3.5. Enzyme-Linked Immunosorbent Assay
3.6. Western Blot Analysis
3.7. Application of SFPS and LPS to Zebrafish
3.8. Measurement of Heartbeat, ROS Generation, NO Production, and Cell Death in Zebrafish
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, S.-Y.; Kim, H.-S.; Ahn, G.; Jee, Y.; Jeon, Y.-J. In vitro and in vivo anti-inflammatory activities of high molecular weight sulfated polysaccharide; containing fucose separated from Sargassum horneri: Short com-munication. Int. J. Biol. Macromol. 2018, 107, 803–807. [Google Scholar] [CrossRef] [PubMed]
- de Souza Costa, M.; de Brito, T.V.; de Oliveira, S.B.; de Souza Brauna, I.; Neto, J.C.R.M.; Teles, R.H.G.; Dutra, Y.M.; de Aguiar Magalhães, D.; Sousa, S.G.; de Sousa, J.A.; et al. Photobiomodulation exerts anti-inflammatory effects on the vascular and cellular phases of experimental inflammatory models. Lasers Med. Sci. 2021. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.-J.; Ryu, B. Anti-inflammatory and anti-melanogenesis activities of sulfated polysaccharides isolated from Hizikia fusiforme: Short communication. Int. J. Biol. Macromol. 2020, 142, 545–550. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, E.-A.; Ahn, G.; Jee, Y.; Jeon, Y.-J. Anti-inflammatory activity of a sulfated polysac-charide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells. Nutr. Res. Pract. 2017, 11, 3–10. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Hwang, J.; Ko, J.Y.; Jeon, Y.-J.; Ryu, B. In Vitro and In Vivo Antioxidant Activities of Polysaccharides Iso-lated from Celluclast-Assisted Extract of an Edible Brown Seaweed, Sargassum fulvellum. Antioxidants 2019, 8, 493. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Lee, S.J.; Kim, H.S.; Eom, J.S.; Kim, D.H.; Lee, S.S. The potential nutritive value of Sargassum fulvellum as a feed ingredient for ruminants. Algal Res. 2020, 45, 101761. [Google Scholar] [CrossRef]
- Kang, J.; Khan, M.; Park, N.; Cho, J.; Lee, M.; Fujii, H.; Hong, Y. Antipyretic, analgesic, and anti-inflammatory activities of the seaweed Sargassum fulvellum and Sargassum thunbergii in mice. J. Ethnopharmacol. 2008, 116, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luthuli, S.; Wu, Q.; Wu, M.; Choi, J.-I.; Tong, H. Pharmaceutical and Nutraceutical Potential Applications of Sargassum fulvellum. BioMed Res. Int. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Kang, B.-K.; Kim, M.-J.; Kim, K.-B.-W.-R.; Ahn, D.-H. In vivo and in vitro inhibitory activity of an ethanolic extract of Sargassum fulvellum and its component grasshopper ketone on atopic dermatitis. Int. Immunopharmacol. 2016, 40, 176–183. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeong, S.M.; Kang, B.K. Anti-Inflammatory Effects of Grasshopper Ketone from Sargassum ful-vellum Ethanol Extract on Lipopolysaccharide-Induced Inflammatory Responses in RAW 264.7 Cells. J. Microbiol. Biotechnol. 2019, 29, 820–826. [Google Scholar] [CrossRef]
- Wang, L.; Park, Y.J.; Oh, J.Y.; Fernando, I.S.; Sanjeewa, K.A.; Kang, M.C.; Cui, Y.R.; Lee, H.G.; Ko, J.Y.; Lee, W.; et al. Protective Effects of Enzyme-assistant Extracts of Sargassum fulvellum against AAPH-induced Oxidative Stress in vitro in Vero Cells. J. Chitin Chitosan 2018, 23, 113–119. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, W.; Sun, X.; Jiang, G.; Wu, S.; Xu, Y.; Song, S.; Ai, C. Sulfated polysaccharides from Undaria pinnatifida im-proved high fat diet-induced metabolic syndrome, gut microbiota dysbiosis and inflammation in BALB/c mice. Int. J. Biol. Macromol. 2021, 167, 1587–1597. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Fitton, J.H.; Park, A.Y.; Karpiniec, S.S.; Stringer, D.N. Fucoidan and Lung Function: Value in Viral Infection. Mar. Drugs 2020, 19, 4. [Google Scholar] [CrossRef]
- Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. Fucoidan Structure and Its Impact on Glucose Metabolism: Implications for Diabetes and Cancer Therapy. Mar. Drugs 2021, 19, 30. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Sea-weed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Ma, Y.; Cheung, P.C.-K.; You, L.; Liao, L.; Pedisić, S.; Kulikouskaya, V. Structural characteristics and an-ti-inflammatory activity of UV/H2O2-treated algal sulfated polysaccharide from Gracilaria lemaneiformis. Food Chem. Toxicol. 2021, 152, 112157. [Google Scholar] [CrossRef]
- Fernando, I.S.; Sanjeewa, K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kang, N.; Ranasinghe, P.; Lee, H.-S.; Jeon, Y.-J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.-J.; Xu, J.; Gao, X. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Asanka Sanjeewa, K.K.; Jayawardena, T.U.; Kim, H.-S.; Kim, S.-Y.; Shanura Fernando, I.P.; Wang, L.; Abetunga, D.T.U.; Kim, W.-S.; Lee, D.-S.; Jeon, Y.-J. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-κB signal pathway. Carbohydr. Polym. 2019, 224, 115195. [Google Scholar] [CrossRef] [PubMed]
- Rukshala, D.; de Silva, E.D.; Ranaweera, B.L.R.; Fernando, N.; Handunnetti, S.M. Anti-inflammatory effect of leaves of Vernonia zeylanica in lipopolysaccharide-stimulated RAW 264.7 macrophages and carrageenan-induced rat paw-edema model. J. Ethnopharmacol. 2021, 274, 114030. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.-Y.; Yang, H.-W.; Fu, X.; Kim, J.-I.; Jeon, Y.-J. Fucoidan isolated from the popular edible brown seaweed Sar-gassum fusiforme suppresses lipopolysaccharide-induced inflammation by blocking NF-κB signal pathway. J. Appl. Phycol. 2021, 18, 328. [Google Scholar]
- Kim, J.-H.; Kim, M.; Hong, S.; Kwon, B.; Song, M.W.; Song, K.; Kim, E.-Y.; Jung, H.-S.; Sohn, Y. Anti-inflammatory effects of Fritillaria thunbergii Miquel extracts in LPS-stimulated murine macrophage RAW 264.7 cells. Exp. Med. 2021, 21, 429. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Yoon, W.-J.; Kim, K.-N.; Ahn, G.-N.; Kang, S.-M.; Kang, D.-H.; Affan, A.; Oh, C.; Jung, W.-K.; Jeon, Y.-J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.-Y.; Cho, S.-H.; Kwon, S.-H.; Eom, C.-Y.; Jeong, M.S.; Lee, W.; Kim, S.-Y.; Heo, S.-J.; Ahn, G.; Lee, K.P.; et al. The roles of NF-κB and ROS in regulation of pro-inflammatory mediators of inflammation induction in LPS-stimulated zebrafish embryos. Fish Shellfish Immunol. 2017, 68, 525–529. [Google Scholar] [CrossRef]
- Dong, H.; Zheng, L.; Yu, P.; Jiang, Q.; Wu, Y.; Huang, C.; Yin, B. Characterization and Application of Lignin–Carbohydrate Complexes from Lignocellulosic Materials as Antioxidants for Scavenging In Vitro and In Vivo Reactive Oxygen Species. ACS Sustain. Chem. Eng. 2019, 8, 256–266. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, P.; Zhang, Y.; Wang, P.; Yan, W.; Guo, B.; Huang, C.; Jiang, Q. Evaluating the bio-application of biomacro-molecule of lignin-carbohydrate complexes (LCC) from wheat straw in bone metabolism via ROS scavenging. Int. J. Biol. Macromol. 2021, 176, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-A.; Lee, S.-H.; Ko, C.-I.; Cha, S.-H.; Kang, M.-C.; Kang, S.-M.; Ko, S.-C.; Lee, W.-W.; Ko, J.-Y.; Lee, J.-H.; et al. Protective effect of fucoidan against AAPH-induced oxidative stress in zebrafish model. Carbohydr. Polym. 2014, 102, 185–191. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Yang, H.-W.; Ahn, G.; Fu, X.; Xu, J.; Gao, X.; Jeon, Y.-J. In Vitro and In Vivo Anti-Inflammatory Effects of Sulfated Polysaccharides Isolated from the Edible Brown Seaweed, Sargassum fulvellum. Mar. Drugs 2021, 19, 277. https://doi.org/10.3390/md19050277
Wang L, Yang H-W, Ahn G, Fu X, Xu J, Gao X, Jeon Y-J. In Vitro and In Vivo Anti-Inflammatory Effects of Sulfated Polysaccharides Isolated from the Edible Brown Seaweed, Sargassum fulvellum. Marine Drugs. 2021; 19(5):277. https://doi.org/10.3390/md19050277
Chicago/Turabian StyleWang, Lei, Hye-Won Yang, Ginnae Ahn, Xiaoting Fu, Jiachao Xu, Xin Gao, and You-Jin Jeon. 2021. "In Vitro and In Vivo Anti-Inflammatory Effects of Sulfated Polysaccharides Isolated from the Edible Brown Seaweed, Sargassum fulvellum" Marine Drugs 19, no. 5: 277. https://doi.org/10.3390/md19050277
APA StyleWang, L., Yang, H.-W., Ahn, G., Fu, X., Xu, J., Gao, X., & Jeon, Y.-J. (2021). In Vitro and In Vivo Anti-Inflammatory Effects of Sulfated Polysaccharides Isolated from the Edible Brown Seaweed, Sargassum fulvellum. Marine Drugs, 19(5), 277. https://doi.org/10.3390/md19050277







