Localization and Bioreactivity of Cysteine-Rich Secretions in the Marine Gastropod Nucella lapillus
Abstract
1. Introduction
2. Results
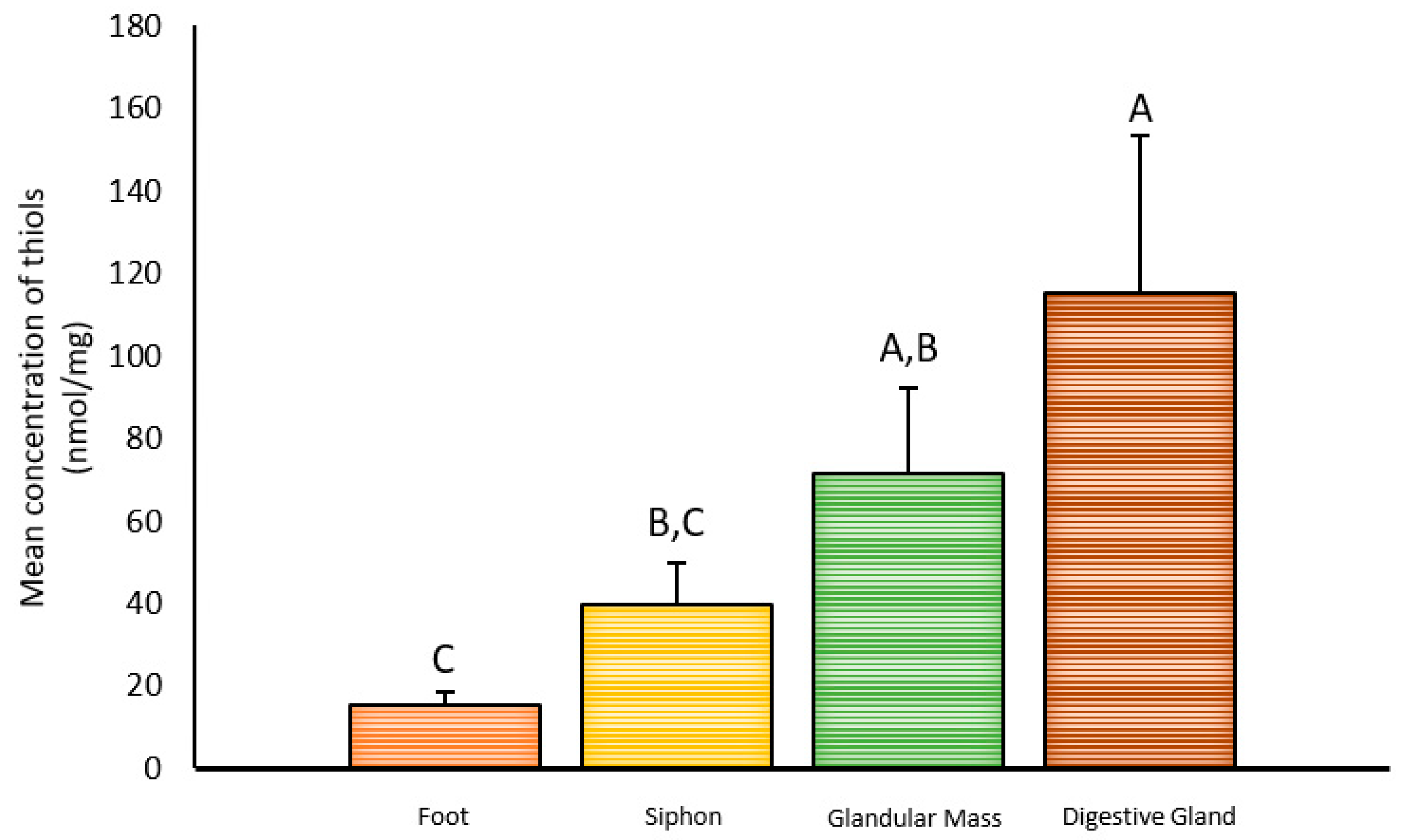
2.1. Inter-Organ Concentrations of Thiols
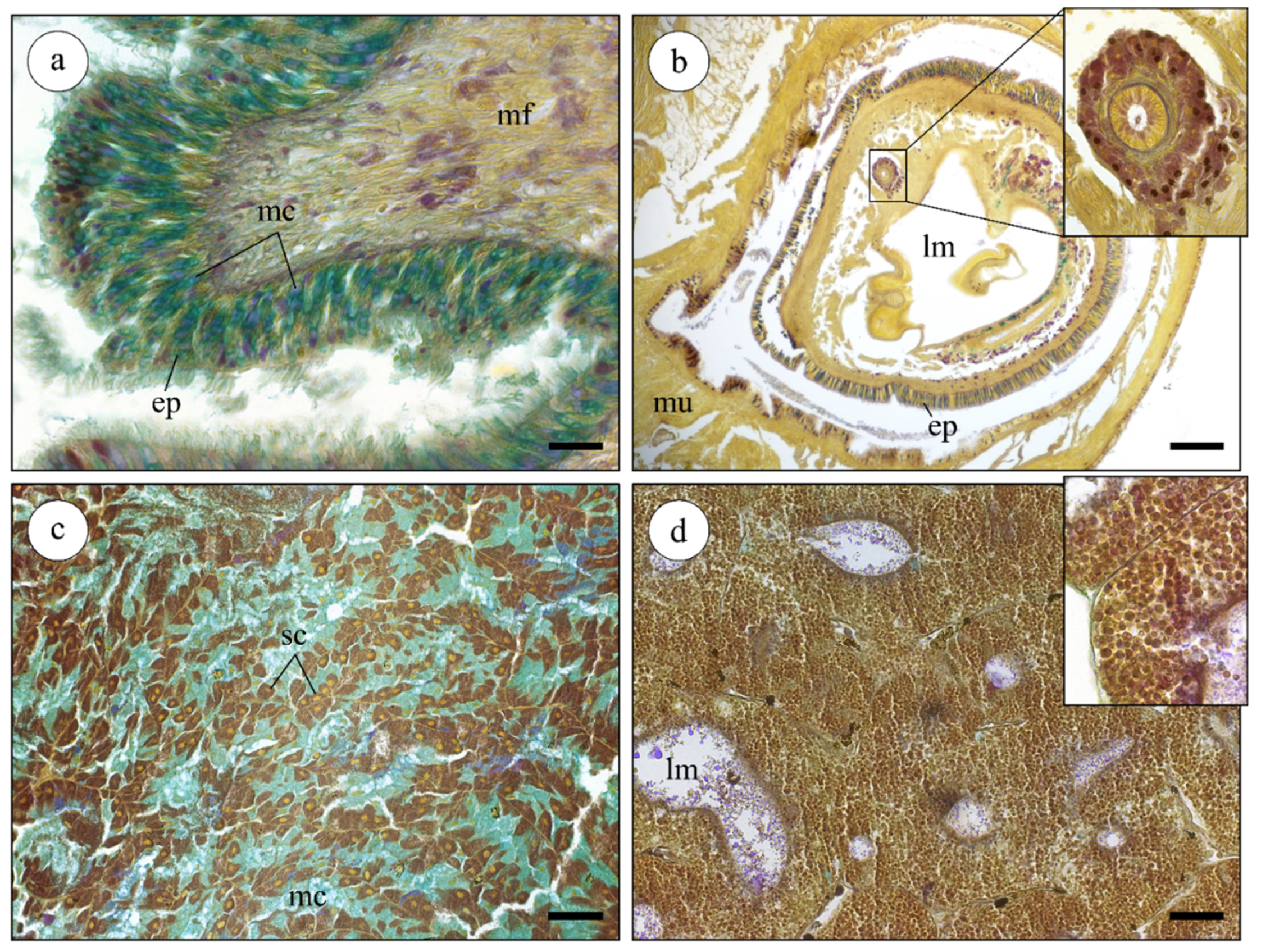
2.2. Histological Analysis of Nucella lapillus Glandular Systems
2.3. Toxicological Effects of Extracts
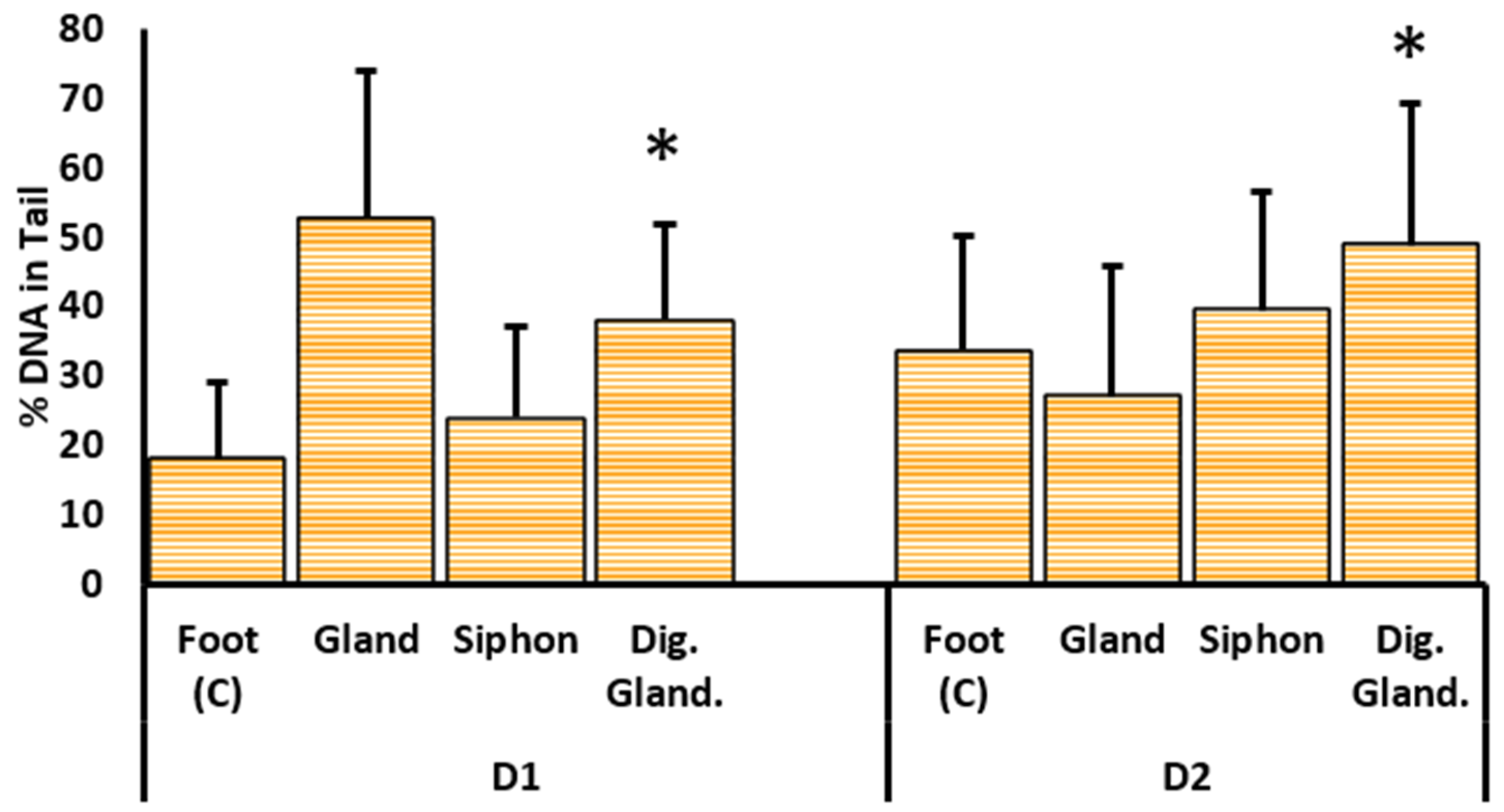
2.3.1. DNA Damage
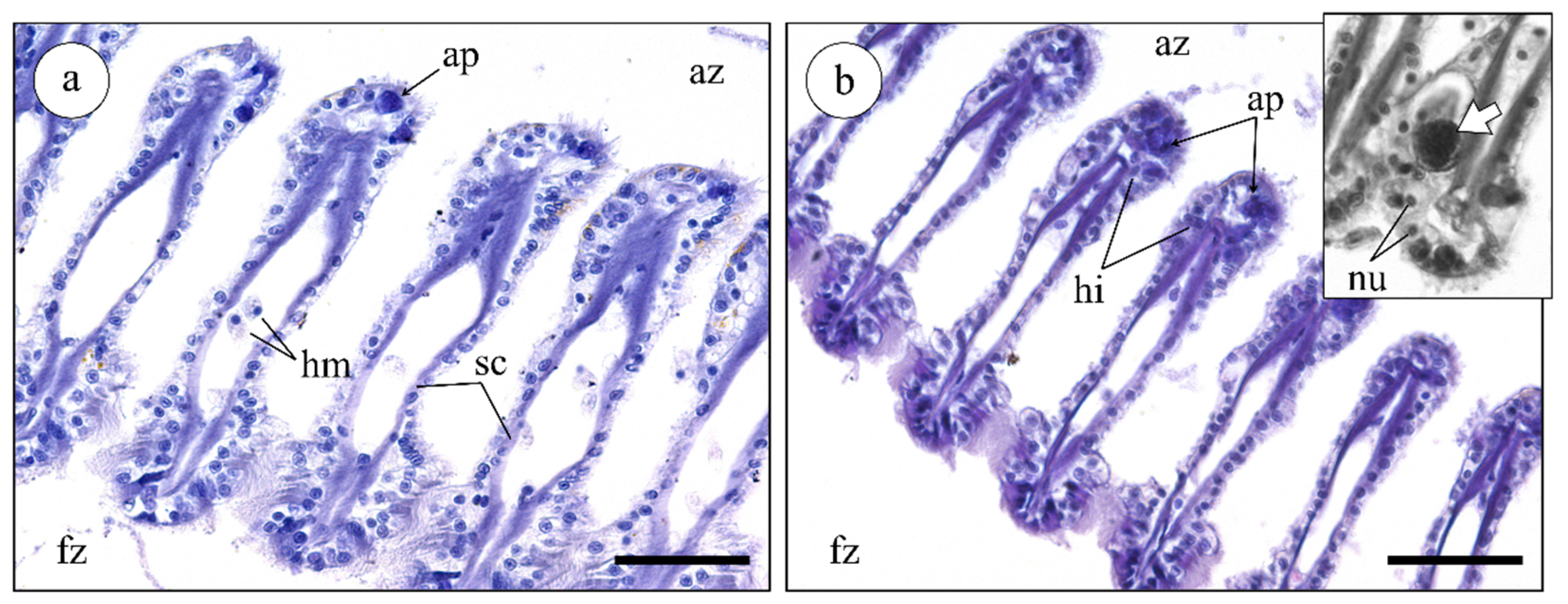
2.3.2. Histological Analysis of Mussel Gills
3. Discussion
4. Materials and Methods
4.1. Animal Collection
4.2. Preparation of Protein Extracts
4.3. Quantification of Protein Thiols
4.4. Histology and Histochemistry
4.5. Assays with Mytilus Gills
4.5.1. Experimental Design
4.5.2. Comet Assay
4.5.3. Histopathology
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelsen, D.R.; Nisani, Z.; Cooper, A.M.; Fox, G.A.; Gren, E.C.K.; Corbit, A.G.; Hayes, W.K. Poisons, toxungens, and venoms: Redefining and classifying toxic biological secretions and the organisms that employ them. Biol. Rev. 2014, 89, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.A.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef]
- Kem, W.R. Marine Venoms and Toxins. In Encyclopedia of Toxicology, 3rd ed.; Elsevier Inc.: Gainesville, FL, USA, 2014; pp. 160–163. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Mebs, D. Venomous and Poisonous Animals: A Handbook for Biologists, Toxicologists and Toxinologists, Physicians and Pharmacists; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar] [CrossRef]
- King, G.F. Venoms as a platform for human drugs: Translating toxins into therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef]
- Rodrigo, A.P.; Costa, P.M. The hidden biotechnological potential of marine invertebrates: The Polychaeta case study. Environ. Res. 2019, 173, 270–280. [Google Scholar] [CrossRef]
- Gonçalves, C.; Costa, P.M. Cephalotoxins: A Hotspot for Marine Bioprospecting? Front. Mar. Sci. 2021, 8, 1–7. [Google Scholar] [CrossRef]
- D’Ambra, I.; Lauritano, C. A Review of Toxins from Cnidaria. Mar. Drugs 2020, 18, 507. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Morita, T. Structure and function of snake venom cysteine-rich secretory proteins. Toxicon 2004, 44, 227–231. [Google Scholar] [CrossRef]
- Tadokoro, T.; Modahl, C.; Maenaka, K.; Aoki-Shioi, N. Cysteine-rich secretory proteins (CRISPs) from venomous snakes: An overview of the functional diversity in a large and underappreciated superfamily. Toxins 2020, 12, 175. [Google Scholar] [CrossRef]
- Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef]
- Ito, N.; Mita, M.; Takahashi, Y.; Matsushima, A.; Watanabe, Y.G.; Hirano, S.; Odani, S. Novel cysteine-rich secretory protein in the buccal gland secretion of the parasitic lamprey, Lethenteron japonicum. Biochem. Biophys. Res. Commun. 2007, 358, 35–40. [Google Scholar] [CrossRef]
- Rodrigo, A.P.; Grosso, A.R.; Baptista, P.V.; Fernandes, A.R.; Costa, P.M. A transcriptomic approach to the recruitment of venom proteins in a marine annelid. Toxins 2021, 13, 97. [Google Scholar] [CrossRef]
- Cuevas, N.; Martins, M.; Rodrigo, A.P.; Martins, C.; Costa, P.M. Explorations on the ecological role of toxin secretion and delivery in jawless predatory Polychaeta. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Terlau, H.; Olivera, B.M. Conus Venoms: A Rich Source of Novel Ion Channel-Targeted Peptides. Physiol. Rev. 2004, 84, 41–68. [Google Scholar] [CrossRef] [PubMed]
- Cusick, K.D.; Sayler, G.S. An overview on the marine neurotoxin, saxitoxin: Genetics, molecular targets, methods of detection and ecological functions. Mar. Drugs 2013, 11, 991–1018. [Google Scholar] [CrossRef]
- Prashanth, J.R.; Dutertre, S.; Lewis, R.J. Pharmacology of predatory and defensive venom peptides in cone snails. Mol. Biosyst. 2017, 13, 2453–2465. [Google Scholar] [CrossRef]
- Rigo, F.K.; Dalmolin, G.D.; Trevisan, G.; Tonello, R.; Silva, M.A.; Rossato, M.F.; Klafke, J.Z.; Cordeiro, M.D.N.; Castro Junior, C.J.; Montijo, D.; et al. Effect of ω-conotoxin MVIIA and Phα1β on paclitaxel-induced acute and chronic pain. Pharmacol. Biochem. Behav. 2013, 114–115, 16–22. [Google Scholar] [CrossRef]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Futur. Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Layer, R.T.; McIntosh, J.M. Conotoxins: Therapeutic potential and application. Mar. Drugs 2006, 4, 119–142. [Google Scholar] [CrossRef]
- Oehlmann, J.; Bauer, B.; Minchin, D.; Schulte-Oehlmann, U.; Fioroni, P.; Markert, B. Imposex in Nucella lapillus and intersex in Littorina littorea: Interspecific comparison of two TBT-induced effects and their geographical uniformity. Hydrobiologia 1998, 378, 199–213. [Google Scholar] [CrossRef]
- Andrews, E.B. The fine stucture and function of the salivary glands of Nucella lapillus (Gastropoda: Muricidae). J. Molluscan Stud. 1991, 57, 111–126. [Google Scholar] [CrossRef]
- Ponder, W.F. The origin and evolution of the Neogastropoda. Malacologia 1973, 12, 295–338. [Google Scholar] [PubMed]
- West, D.J.; Andrews, E.B.; Bowman, D.; McVean, A.R.; Thorndyke, M.C. Toxins from some poisonous and venomous marine snails. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1996, 113, 1–10. [Google Scholar] [CrossRef]
- Martoja, M.; Thiriot-Quiévreux, C. Données histologiques sur l’appareil digestif et la digestion des atlantidae (prosobranchia: Heteropoda). Malacologia 1975, 1215, 1–27. [Google Scholar]
- Lavergne, V.; Taft, R.J.; Alewood, P.F. Cysteine-rich mini-proteins in human biology. Curr. Top. Med. Chem. 2012, 12, 1514–1533. [Google Scholar] [CrossRef]
- Simone, L. Gastropod pallial siphons and siphonal canals. Malacopedia 2020, 3, 1–9. [Google Scholar]
- Ruder, T.; Sunagar, K.; Undheim, E.A.B.; Ali, S.A.; Wai, T.C.; Low, D.H.W.; Jackson, T.N.W.; King, G.F.; Antunes, A.; Fry, B.G. Molecular phylogeny and evolution of the proteins encoded by coleoid (cuttlefish, octopus, and squid) posterior venom glands. J. Mol. Evol. 2013, 76, 192–204. [Google Scholar] [CrossRef]
- West, D.J.; Andrews, E.B.; McVean, A.R.; Osborne, D.J.; Thorndyke, M.C. Isolation of serotonin from the accessory salivary glands of the marine snail Nucella lapillus. Toxicon 1994, 32, 1261–1264. [Google Scholar] [CrossRef]
- Sher, D.; Zlotkin, E. A hydra with many heads: Protein and polypeptide toxins from hydra and their biological roles. Toxicon 2009, 54, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, J.M.; Foderaro, T.A.; Li, W.; Ireland, C.M.; Olivera, B.M. Presence of serotonin in the venom of Conus imperialis. Toxicon 1993, 31, 1561–1566. [Google Scholar] [CrossRef]
- Ponte, G.; Modica, M.V. Salivary glands in predatory mollusks: Evolutionary considerations. Front. Physiol. 2017, 8, 580. [Google Scholar] [CrossRef]
- Welsh, J.H. Composition and Mode of Action of Some Invertebrate Venoms. Annu. Rev. Pharmacol. 1964, 4, 293–304. [Google Scholar] [CrossRef]
- Endean, R.; Rudkin, C. Studies of the Venoms of Some Conidae. Toxicon 1963, 1, 49–64. [Google Scholar] [CrossRef]
- Dobson, R.; Collodoro, M.; Gilles, N.; Turtoi, A.; De Pauw, E.; Quinton, L. Secretion and maturation of conotoxins in the venom ducts of Conus textile. Toxicon 2012, 60, 1370–1379. [Google Scholar] [CrossRef]
- Rodrigo, A.P.; Costa, P.M. The Role of the Cephalopod Digestive Gland in the Storage and Detoxification of Marine Pollutants. Front. Physiol. 2017, 8, 232. [Google Scholar] [CrossRef]
- Costa, P.M.; Costa, M.H. Development and application of a novel histological multichrome technique for clam histopathology. J. Invertebr. Pathol. 2012, 110, 411–414. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Santos, A.C.; Alejo-Armijo, A.; Parola, A.J.; Costa, P.M. Light-Mediated Toxicity of Porphyrin-Like Pigments from a Marine Polychaeta. Mar. Drugs 2020, 18, 302. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Raimundo, J.; Costa, P.M.; Vale, C.; Costa, M.H.; Moura, I. DNA damage and metal accumulation in four tissues of feral Octopus vulgaris from two coastal areas in Portugal. Ecotoxicol. Environ. Saf. 2010, 73, 1543–1547. [Google Scholar] [CrossRef]
- Martins, C.; Costa, P.M. Technical updates to the Comet assay in vivo for assessing DNA damage in zebrafish embryos from fresh and frozen cell suspensions. Zebrafish 2020, 1–9. [Google Scholar] [CrossRef]
- Costa, P.M. The Handbook of Histopathological Practices in Aquatic Environments: Guide to Histology for Environmental Toxicology; Academic Press: Cambridge, MA, USA, 2017; ISBN 978-0-12-812032-3. [Google Scholar]
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ambrosio, M.; Gonçalves, C.; Calmão, M.; Rodrigues, M.; Costa, P.M. Localization and Bioreactivity of Cysteine-Rich Secretions in the Marine Gastropod Nucella lapillus. Mar. Drugs 2021, 19, 276. https://doi.org/10.3390/md19050276
D’Ambrosio M, Gonçalves C, Calmão M, Rodrigues M, Costa PM. Localization and Bioreactivity of Cysteine-Rich Secretions in the Marine Gastropod Nucella lapillus. Marine Drugs. 2021; 19(5):276. https://doi.org/10.3390/md19050276
Chicago/Turabian StyleD’Ambrosio, Mariaelena, Cátia Gonçalves, Mariana Calmão, Maria Rodrigues, and Pedro M. Costa. 2021. "Localization and Bioreactivity of Cysteine-Rich Secretions in the Marine Gastropod Nucella lapillus" Marine Drugs 19, no. 5: 276. https://doi.org/10.3390/md19050276
APA StyleD’Ambrosio, M., Gonçalves, C., Calmão, M., Rodrigues, M., & Costa, P. M. (2021). Localization and Bioreactivity of Cysteine-Rich Secretions in the Marine Gastropod Nucella lapillus. Marine Drugs, 19(5), 276. https://doi.org/10.3390/md19050276






