24(S)-Saringosterol Prevents Cognitive Decline in a Mouse Model for Alzheimer’s Disease
Abstract
1. Introduction
2. Results
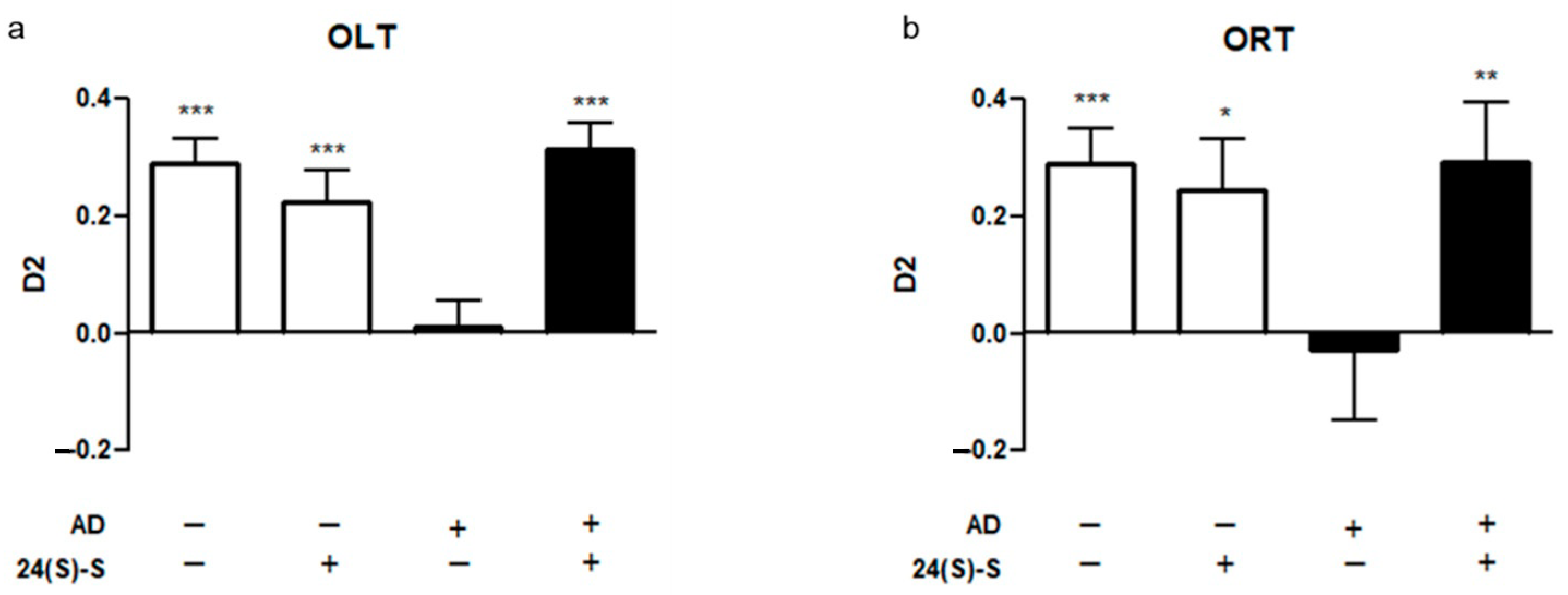
2.1. 24(S)-Saringosterol Prevents Cognitive Decline in APPswePS1ΔE9 Mice
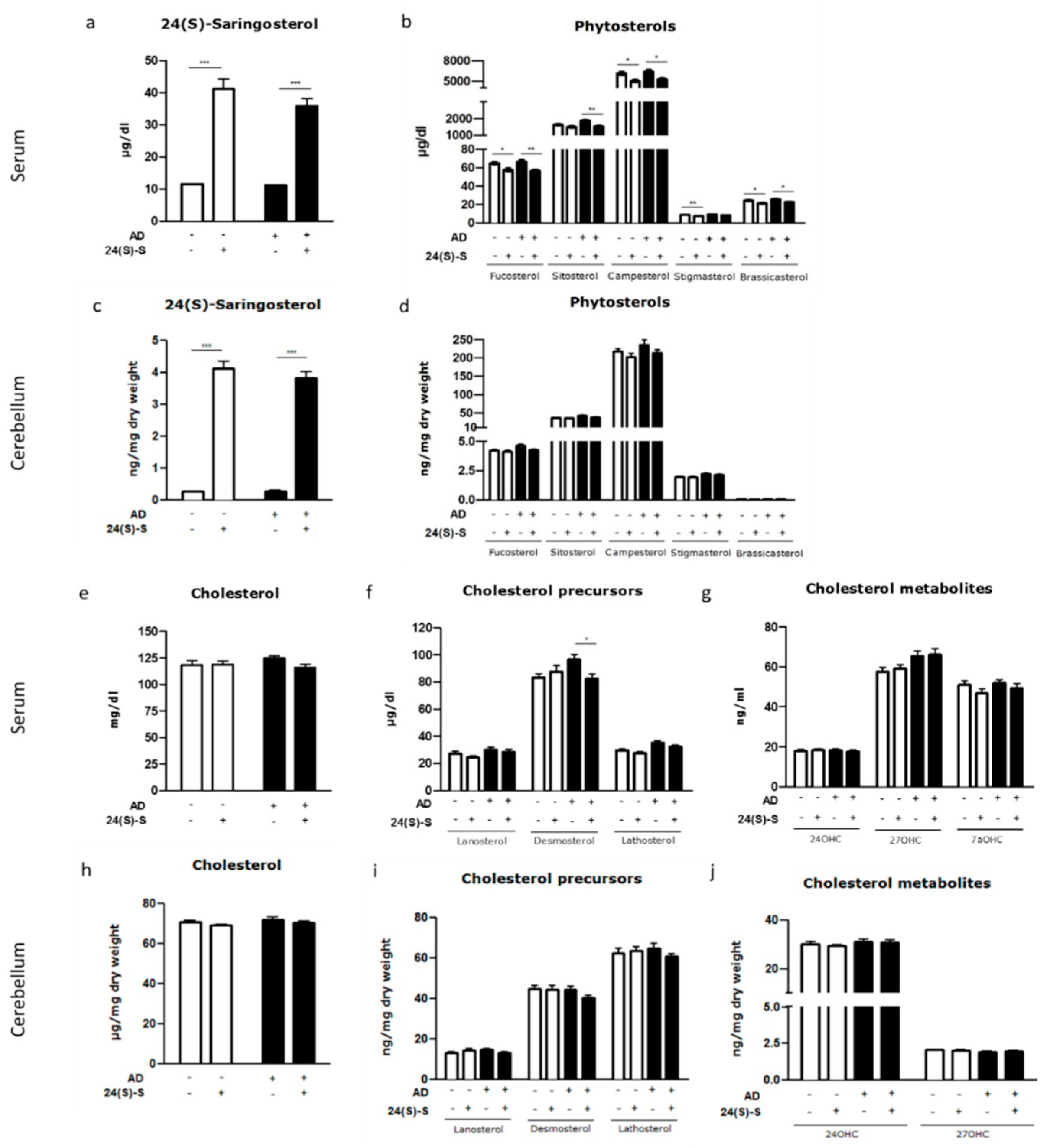
2.2. 24(S)-Saringosterol Detectable in the Circulation and the Brain after Administration
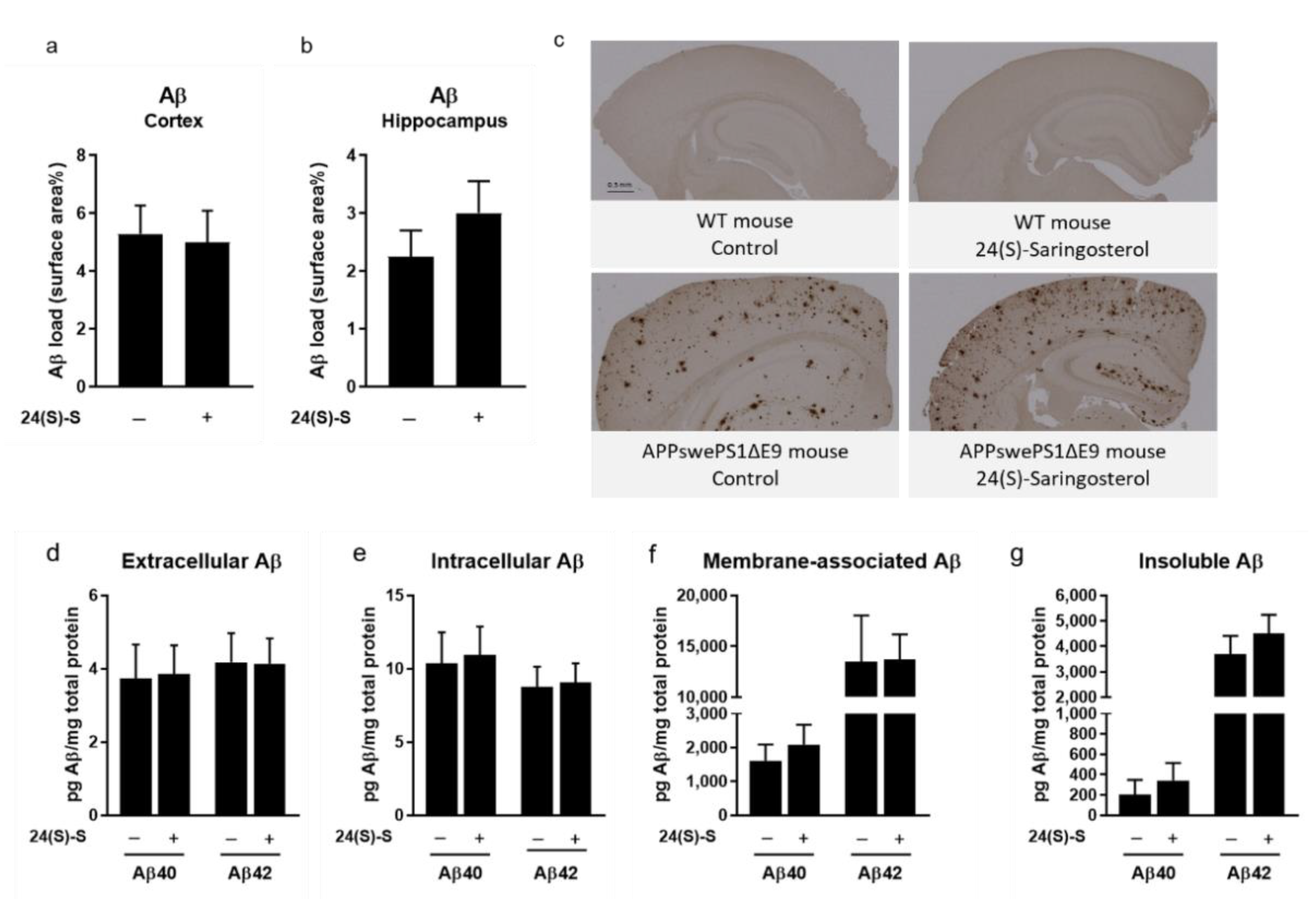
2.3. 24(S)-Saringosterol Does Not Affect the Aβ Plaque Load in the Cortex and the Hippocampus
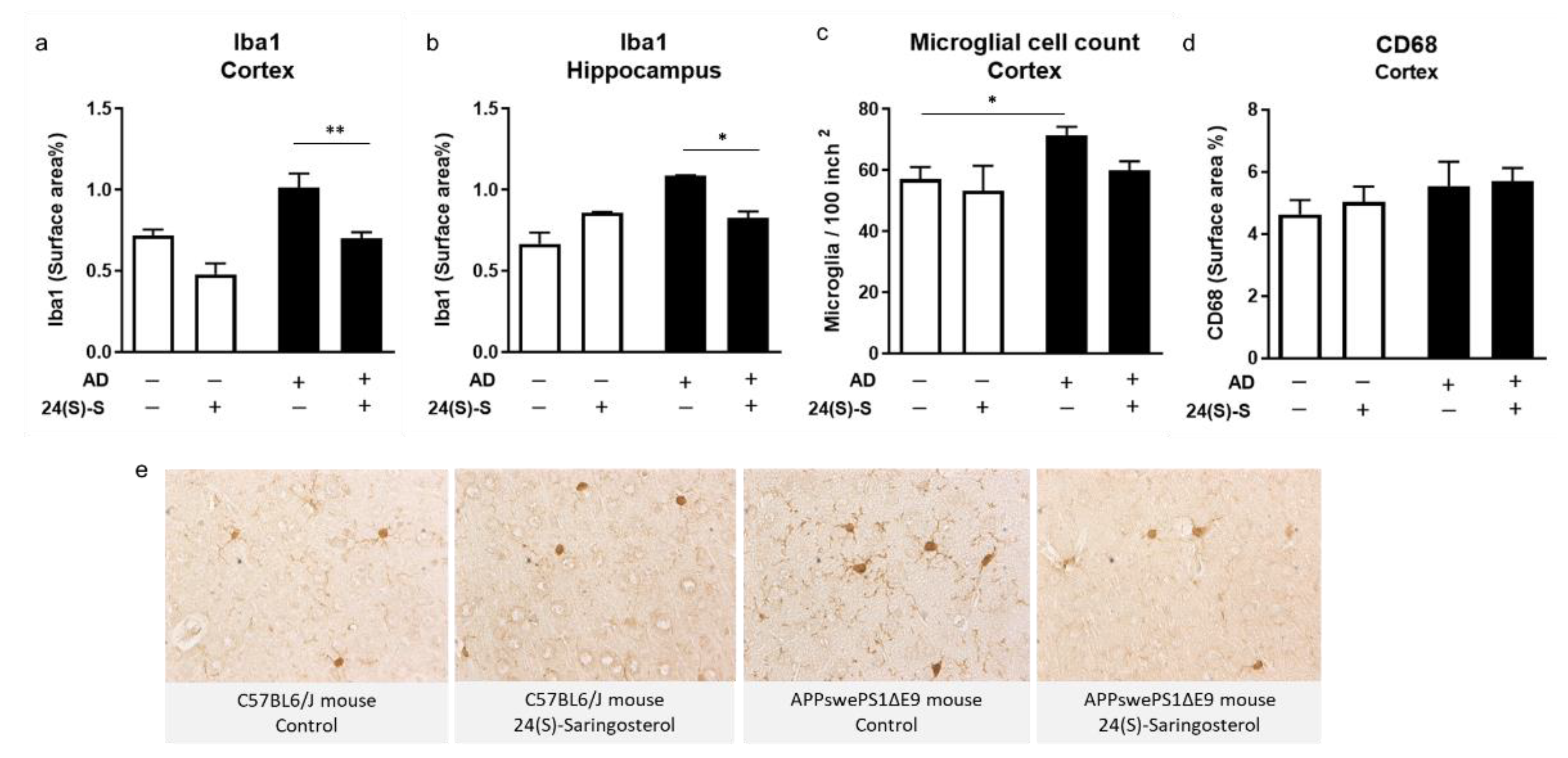
2.4. 24(S)-Saringosterol Prevents the Increase in the Expression of Microglia Activation Marker Iba1 in APPswePS1ΔE9 Mice
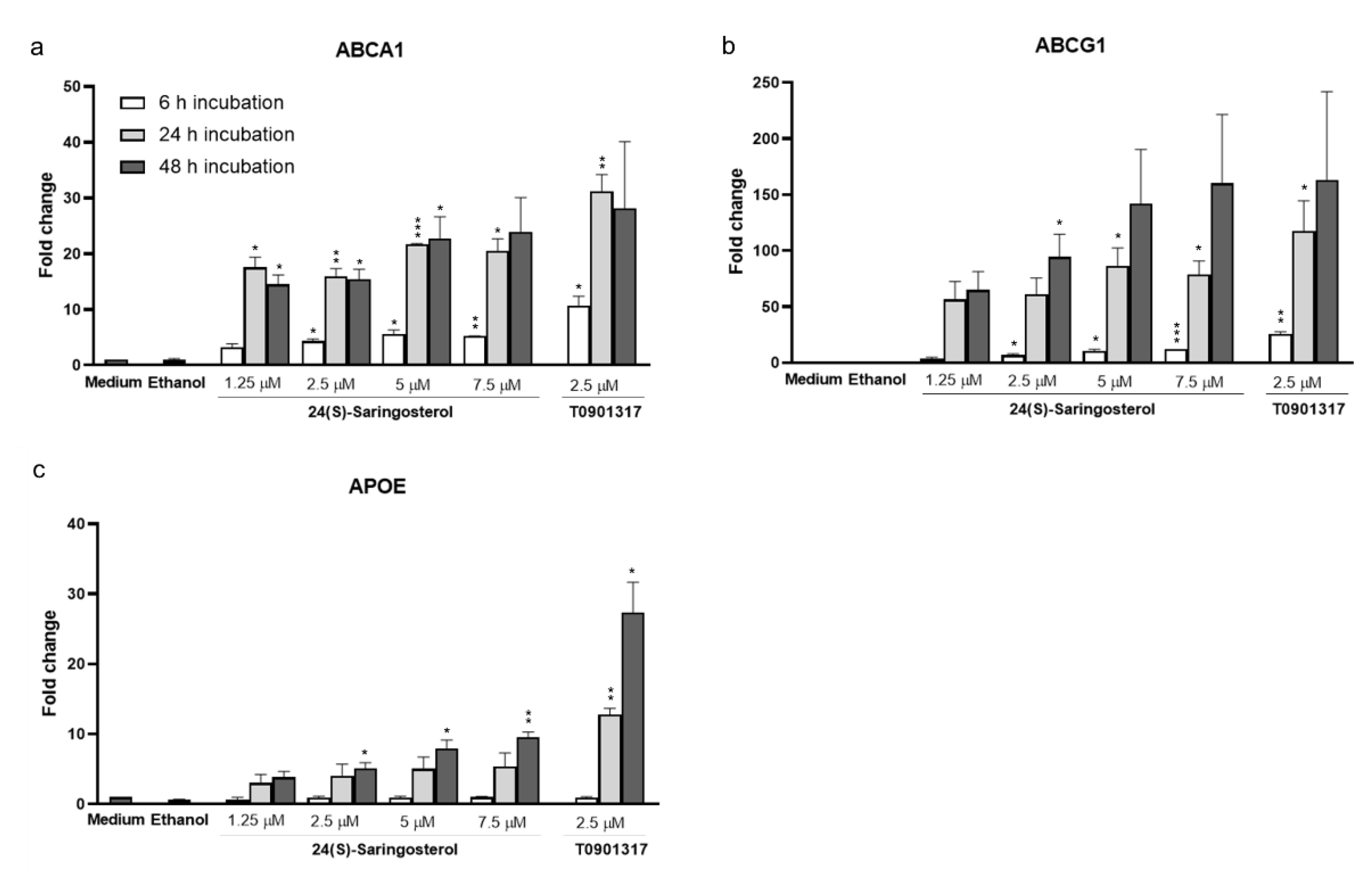
2.5. 24(S)-Saringosterol Affects the Expression of LXR Target Genes In Vitro, But Not In Vivo
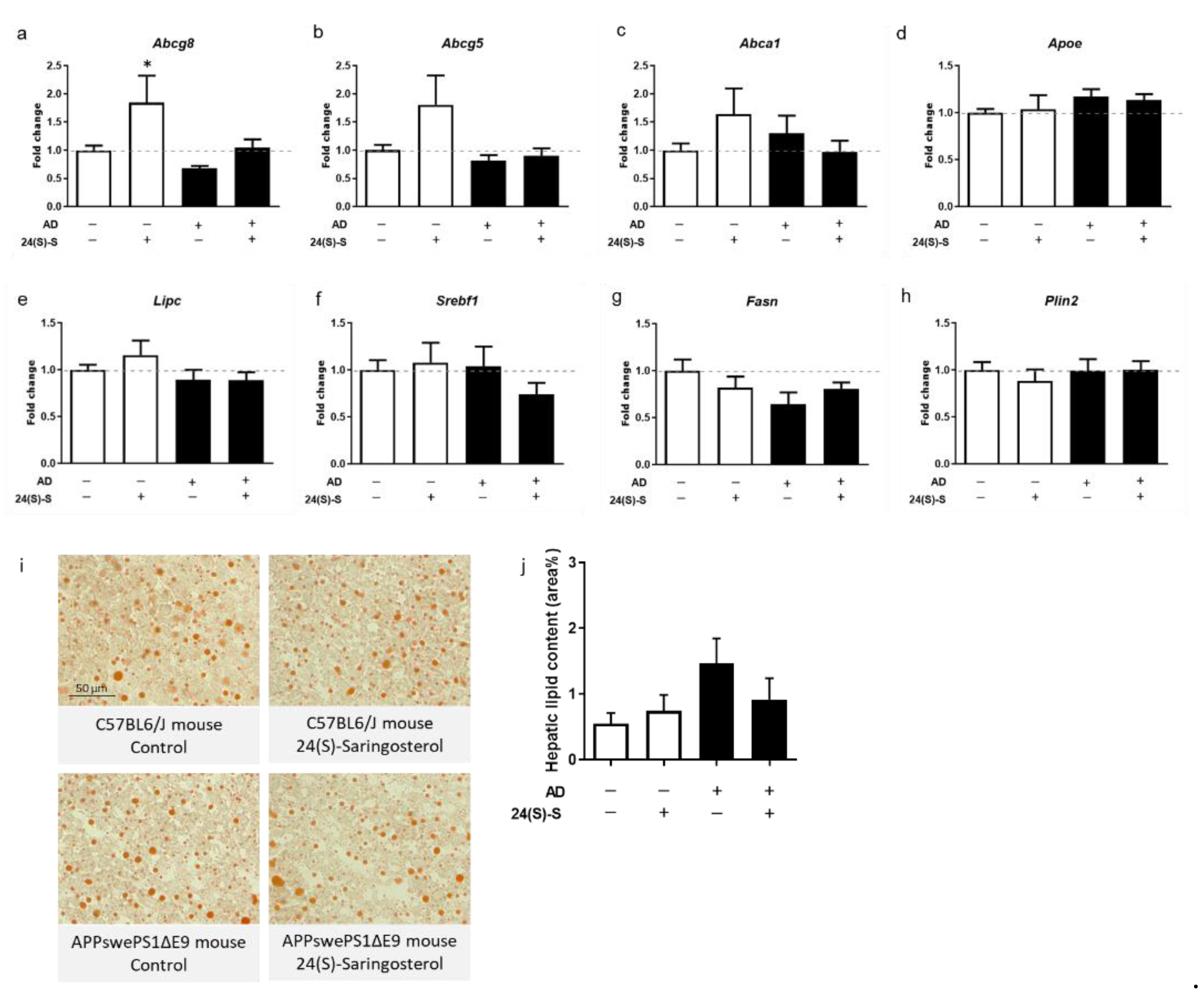
2.6. 24(S)-Saringosterol Administration Does Not Induce Accumulation of Triglycerides in the Liver or in Serum
3. Discussion
4. Materials and Methods
4.1. Route of 24(S)-Saringosterol Administration
4.2. Animals and Diet
4.3. Cognitive Testing
4.4. Tissue Sample Preparation
4.5. Determination of Lipid Profile
4.6. Immunohistochemistry—Quantification of Aβ, Iba1 and, CD68
4.7. ELISA—Quantification of Aβ
4.8. Cell Culture—CCF-STTG1 Cells
4.9. RNA Isolation and RT-Q-PCR
4.10. Hepatic Neutral Lipid Quantification
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swerdlow, R.H. Pathogenesis of Alzheimer’s disease. Clin. Interv. Aging 2007, 2, 347. [Google Scholar]
- Cummings, J.; Aisen, P.S.; Dubois, B.; Frölich, L.; Jack, C.R.; Jones, R.W.; Morris, J.C.; Raskin, J.; Dowsett, S.A.; Scheltens, P. Drug development in Alzheimer’s disease: The path to 2025. Alzheimers Res. Ther. 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Jansen, D.; Janssen, C.I.; Vanmierlo, T.; Dederen, P.J.; van Rooij, D.; Zinnhardt, B.; Nobelen, C.L.M.; Janssen, A.-L.; Hafkemeijer, A.; Mutsaers, M.P.C.; et al. Cholesterol and synaptic compensatory mechanisms in Alzheimer’s disease mice brain during aging. J. Alzheimers Dis. 2012, 31, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Holmans, P.A.; Hamshere, M.L.; Harold, D.; Moskvina, V.; Ivanov, D.; Pocklington, A.; Abraham, R.; Hollingworth, P.; Sims, R.; et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS ONE 2010, 5, e13950. [Google Scholar] [CrossRef] [PubMed]
- Kölsch, H.; Heun, R.; Jessen, F.; Popp, J.; Hentschel, F.; Maier, W.; Lütjohann, D. Alterations of cholesterol precursor levels in Alzheimer’s disease. Biochim. Biophys. Acta 2010, 1801, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M. Sterols in the central nervous system. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Loera-Valencia, R.; Goikolea, J.; Parrado-Fernandez, C.; Merino-Serrais, P.; Maioli, S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: Potential novel targets for treatment. J. Steroid Biochem. Mol. Biol. 2019, 190, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Vanmierlo, T.; Bloks, V.W.; Zee, L.C.V.V.-V.D.; Rutten, K.; Kerksiek, A.; Friedrichs, S.; Sijbrands, E.; Steinbusch, H.W.; Kuipers, F.; Lütjohann, D.; et al. Alterations in Brain Cholesterol Metabolism in the APPSLxPS1mut mouse, a Model for Alzheimer’s Disease. J. Alzheimers Dis. 2010, 19, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Verghese, P.B.; Castellano, J.M.; Holtzman, D.M. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011, 10, 241–252. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Zelcer, N.; Tontonoz, P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Investig. 2006, 116, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xiao, X.; Yan, Y.; Zhang, T. Activation of liver X receptors prevents emotional and cognitive dysfunction by suppressing microglial M1-polarization and restoring synaptic plasticity in the hippocampus of mice. Brain Behav. Immun. 2021. [Google Scholar] [CrossRef]
- Jiang, Q.; Lee, C.D.; Mandrekar, S.; Wilkinson, B.; Cramer, P.; Zelcer, N.; Mann, K.; Lamb, B.; Willson, T.M.; Collins, J.L.; et al. ApoE promotes the proteolytic degradation of Abeta. Neuron 2008, 58, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Vanmierlo, T.; Rutten, K.; Dederen, J.; Bloks, V.W.; Zee, L.C.V.V.-V.D.; Kuipers, F.; Kiliaan, A.; Blokland, A.; Sijbrands, E.J.; Steinbusch, H.; et al. Liver X receptor activation restores memory in aged AD mice without reducing amyloid. Neurobiol. Aging 2011, 32, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Riddell, D.R.; Zhou, H.; Comery, T.A.; Kouranova, E.; Lo, C.F.; Warwick, H.K.; Ring, R.H.; Kirksey, Y.; Aschmies, S.; Xu, J.; et al. The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer’s disease. Mol. Cell. Neurosci. 2007, 34, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Navas Guimaraes, M.E.; Lopez-Blanco, R.; Correa, J.; Fernandez-Villamarin, M.; Bistué, M.B.; Martino-Adami, P.; Morelli, L.; Kumar, V.; Wempe, M.F.; Cuello, A.C.; et al. Liver X Receptor Activation with an Intranasal Polymer Therapeutic Prevents Cognitive Decline without Altering Lipid Levels. ACS Nano 2021, 15, 4678–4687. [Google Scholar] [CrossRef] [PubMed]
- Lefterov, I.; Bookout, A.; Wang, Z.; Staufenbiel, M.; Mangelsdorf, D.; Koldamova, R. Expression profiling in APP23 mouse brain: Inhibition of Abeta amyloidosis and inflammation in response to LXR agonist treatment. Mol. Neurodegener. 2007, 2, 20. [Google Scholar] [CrossRef]
- Grefhorst, A.; Elzinga, B.M.; Voshol, P.J.; Plo, T.; Kok, T.; Bloks, V.W.; van der Sluijs, F.H.; Havekes, L.M.; Romijn, J.A.; Verkade, H.J.; et al. Stimulation of Lipogenesis by Pharmacological Activation of the Liver X Receptor Leads to Production of Large, Triglyceride-rich Very Low Density Lipoprotein Particles. J. Biol. Chem. 2002, 277, 34182–34190. [Google Scholar] [CrossRef] [PubMed]
- Repa, J.J.; Liang, G.; Ou, J.; Bashmakov, Y.; Lobaccaro, J.-M.A.; Shimomura, I.; Shan, B.; Brown, M.S.; Goldstein, J.L.; Mangelsdorf, D.J. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000, 14, 2819–2830. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.R.; Tu, H.; Luk, A.; Repa, J.J.; Medina, J.C.; Li, L.; Schwendner, S.; Wang, S.; Thoolen, M.; Mangelsdorf, D.J.; et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000, 14, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum fusiforme improves memory and reduces amyloid plaque load in an Alzheimer’s disease mouse model. Sci. Rep. 2019, 9, 4908. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Wijesekara, I.; Kim, S.-K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef]
- Yende, S.R.; Harle, U.N.; Chaugule, B.B. Therapeutic potential and health benefits of Sargassum species. Pharmacogn. Rev. 2014, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schepers, M.; Martens, N.; Tiane, A.; Vanbrabant, K.; Liu, H.-B.; Lütjohann, D.; Mulder, M.; Vanmierlo, T. Edible seaweed-derived constituents: An undisclosed source of neuroprotective compounds. Neural Regen. Res. 2020, 15, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, C.; Yin, J.; Shen, J.; Wang, H.; Wu, Y.; Jin, H. Fucoidan, a sulfated polysaccharide from brown algae, improves cognitive impairment induced by infusion of Aβ peptide in rats. Environ. Toxicol. Pharmacol. 2012, 33, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Li, Z.; Chen, M.; Sun, Z.; Ling, Y.; Jiang, J.; Huang, C. Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice. Carbohydr. Polym. 2016, 139, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Ali, M.Y.; Choi, R.J.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Kinetics and molecular docking studies of fucosterol and fucoxanthin, BACE1 inhibitors from brown algae Undaria pinnatifida and Ecklonia stolonifera. Food Chem. Toxicol. 2016, 89, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Choi, J.S.; Nam, T.-J. Fucosterol from an Edible Brown Alga Ecklonia stolonifera Prevents Soluble Amyloid Beta-Induced Cognitive Dysfunction in Aging Rats. Mar. Drugs 2018, 16, 368. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.B.; Barbosa, M.; Matos, R.P.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Liu, F.; Lin, J.; Chen, H.; Huang, C.; Chen, L.; Zhou, Y.; Ye, L.; Zhang, K.; Jin, J.; et al. Fucoxanthin Inhibits beta-Amyloid Assembly and Attenuates beta-Amyloid Oligomer-Induced Cognitive Impairments. J. Agric. Food Chem. 2017, 65, 4092–4102. [Google Scholar] [CrossRef] [PubMed]
- Alghazwi, M.; Smid, S.; Musgrave, I.; Zhang, W. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (Abeta1-42) toxicity and aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, S.; An, C.; Zhang, H.; Sun, Y.; Li, Y.; Pu, X. Neuroprotective effect of fucoxanthin on β-amyloid-induced cell death. J. Chin. Pharm. Sci. 2015, 24, 467–474. [Google Scholar]
- Jang, E.J.; Kim, S.C.; Lee, J.H.; Lee, J.R.; Kim, I.K.; Baek, S.Y.; Kim, Y.W. Fucoxanthin, the constituent of Laminaria japonica, triggers AMPK-mediated cytoprotection and autophagy in hepatocytes under oxidative stress. BMC Complementary Altern. Med. 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, R.K.; Bhaskar, N.; Baskaran, V. Comparative effects of beta-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol. Cell. Biochem. 2009, 331, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, J.; Fu, Z.; Ye, C.; Zhang, R.; Song, Y.; Zhang, Y.; Li, H.; Ying, H.; Liu, H. 24(S)-Saringosterol from edible marine seaweed Sargassum fusiforme is a novel selective LXRbeta agonist. J. Agric. Food Chem. 2014, 62, 6130–6137. [Google Scholar] [CrossRef]
- Ye, J.Y.; Li, L.; Hao, Q.M.; Qin, Y.; Ma, C.S. beta-Sitosterol treatment attenuates cognitive deficits and prevents amyloid plaque deposition in amyloid protein precursor/presenilin 1 mice. Korean J. Physiol. Pharmacol. 2020, 24, 39–46. [Google Scholar] [CrossRef]
- Wang, J.; Wu, F.; Shi, C. Substitution of membrane cholesterol with β-sitosterol promotes nonamyloidogenic cleavage of endogenous amyloid precursor protein. Neuroscience 2013, 247, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Burg, V.K.; Grimm, H.S.; Rothhaar, T.L.; Grösgen, S.; Hundsdörfer, B.; Haupenthal, V.J.; Zimmer, V.C.; Mett, J.; Weingärtner, O.; Laufs, U.; et al. Plant sterols the better cholesterol in Alzheimer’s disease? A mechanistical study. J. Neurosci. 2013, 33, 16072–16087. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, H.; Grimm, M.O.; Rothhaar, T.L.; Berkecz, R.; Lütjohann, D.; Giniatullina, R.; Takalo, M.; Miettinen, P.O.; Lahtinen, H.-M.; Giniatullin, R.; et al. Special lipid-based diets alleviate cognitive deficits in the APPswe/PS1dE9 transgenic mouse model of Alzheimer’s disease independent of brain amyloid deposition. J. Nutr. Biochem. 2014, 25, 157–169. [Google Scholar] [CrossRef]
- Kruit, J.K.; Plösch, T.; Havinga, R.; Boverhof, R.; Groot, P.H.; Groen, A.K.; Kuipers, F. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology 2005, 128, 147–156. [Google Scholar] [CrossRef]
- Yu, L.; York, J.; von Bergmann, K.; Lutjohann, D.; Cohen, J.C.; Hobbs, H.H. Stimulation of Cholesterol Excretion by the Liver X Receptor Agonist Requires ATP-binding Cassette Transporters G5 and G8. J. Biol. Chem. 2003, 278, 15565–15570. [Google Scholar] [CrossRef] [PubMed]
- Ghisletti, S.; Huang, W.; Ogawa, S.; Pascual, G.; Lin, M.-E.; Willson, T.M.; Rosenfeld, M.G.; Glass, C.K. Parallel SUMOylation-Dependent Pathways Mediate Gene- and Signal-Specific Transrepression by LXRs and PPARγ. Mol. Cell 2007, 25, 57–70. [Google Scholar] [CrossRef]
- Minett, T.; Cfas, M.; Classey, J.; Matthews, F.E.; Fahrenhold, M.; Taga, M.; Brayne, C.; Ince, P.G.; Nicoll, J.A.R.; Boche, D. Microglial immunophenotype in dementia with Alzheimer’s pathology. J. Neuroinflamm. 2016, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.E.; Ince, P.G.; Higham, C.E.; Gelsthorpe, C.H.; Fernando, M.S.; Matthews, F.; Forster, G.; O’Brien, J.T.; Barber, R.; Kalaria, R.N.; et al. Microglial activation in white matter lesions and nonlesional white matter of ageing brains. Neuropathol. Appl. Neurobiol. 2007, 33, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Hopperton, K.E.; Mohammad, D.; Trépanier, M.O.; Giuliano, V.; Bazinet, R.P. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: A systematic review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ladu, M.J.; Van Eldik, L.J. A Dual Role for Apolipoprotein E in Neuroinflammation: Anti- and Pro-Inflammatory Activity. J. Mol. Neurosci. 2004, 23, 205–212. [Google Scholar] [CrossRef]
- Liu, R.; Hao, D.; Xu, W.; Li, J.; Li, X.; Shen, D.; Sheng, K.; Zhao, L.; Xu, W.; Gao, Z.; et al. β-Sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharm. Biol. 2019, 57, 161–168. [Google Scholar] [CrossRef]
- Yang, E.-J.; Ahn, S.; Ryu, J.; Choi, M.-S.; Choi, S.; Chong, Y.H.; Hyun, J.-W.; Chang, M.-J.; Kim, H.-S. Phloroglucinol Attenuates the Cognitive Deficits of the 5XFAD Mouse Model of Alzheimer’s Disease. PLoS ONE 2015, 10, e0135686. [Google Scholar] [CrossRef]
- Philippou, E.; Constantinou, M. The Influence of Glycemic Index on Cognitive Functioning: A Systematic Review of the Evidence. Adv. Nutr. 2014, 5, 119–130. [Google Scholar] [CrossRef]
- Haskell-Ramsay, C.F.; Jackson, P.A.; Dodd, F.L.; Forster, J.S.; Bérubé, J.; Levinton, C.; Kennedy, D.O. Acute Post-Prandial Cognitive Effects of Brown Seaweed Extract in Humans. Nutrients 2018, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Lütjohann, D.; Brzezinka, A.; Barth, E.; Abramowski, D.; Staufenbiel, M.; von Bergmann, K.; Beyreuther, K.; Multhaup, G.; Bayer, T.A. Profile of cholesterol-related sterols in aged amyloid precursor protein transgenic mouse brain. J. Lipid Res. 2002, 43, 1078–1085. [Google Scholar] [CrossRef]
- Şık, A.; van Nieuwehuyzen, P.; Prickaerts, J.; Blokland, A. Performance of different mouse strains in an object recognition task. Behav. Brain Res. 2003, 147, 49–54. [Google Scholar] [CrossRef]
- Batenburg, W.W.; van den Heuvel, M.; van Esch, J.H.; van Veghel, R.; Garrelds, I.M.; Leijten, F.; Danser, A.H. The (pro)renin receptor blocker handle region peptide upregulates endothelium-derived contractile factors in aliskiren-treated diabetic transgenic (mREN2)27 rats. J. Hypertens. 2013, 31, 292–302. [Google Scholar] [CrossRef]
- Dixon, W.J. Ratios Involving Extreme Values. Ann. Math. Stat. 1951, 22, 68–78. [Google Scholar] [CrossRef]
- Dixon, W.J. Analysis of Extreme Values. Ann. Math. Stat. 1950, 21, 488–506. [Google Scholar] [CrossRef]
| Gene Symbol | Gene Name | Forward and Reverse Primer Sequence |
|---|---|---|
| Mouse Organ Samples | ||
| Abca1 | ATP-binding cassette, sub-family A, member 1 | F: ACA TGA GTG CCA CTT TCC GA R: AGC AGG GGT TGT TGG CAT TA |
| Abcg1 | ATP binding cassette, subfamily G, member 1 | F: AAG GTC TCC AAT CTC GTG CC R: TCC ATG ACA AAG TCT GCT GGG |
| Abcg5 | ATP binding cassette, subfamily G, member 5 | F: CCT GCT GAG GCG AGT AAC AA R: GGA CGC GGA GAA GGT AGA AA |
| Abcg8 | ATP binding cassette, subfamily G, member 8 | F: ACA ACC TGT GGA TAG TGC CTG R: TTG AAT CTG CAT CAG CCC CG |
| Actb | Actin Beta | F: TTC TTG GGT ATG GAA TCC TGT GG R: GTC TTT ACG GAT GTC AAC GTC AC |
| Apoe | Apolipoprotein E | F: CAA GAA CTG ACG GCA CTG ATG R: TGT TCC TCC AGC TCC TTT TTG T |
| App | Amyloid beta (A4) precursor protein | F: GTC ATG ACT ATC CTC CTG GTG G R: GTG GAT ACC CCC TCC CCC AGC CTA GAC C |
| B2m | Beta-2-Microglobulin | F: CAT GGC TCG CTC GGT GAC C R: AAT GTG AGG CGG GTG GAA CTG |
| Fasn | Fatty Acid Synthase | F: CCC CTC TGT TAA TTG GCT CC R: TTG TGG AAG TGC AGG TTA GG |
| Hprt1 | Hypoxanthine guanine phosphoribosyl transferase | F: CCT AAG ATG AGC GCA AGT TGA A R: CCA CAG GAC TAG AAC ACC TGC TAA |
| Lipc | Lipase C, hepatic type | F: ACG GGT GGT CGG TGG AT R: ATT CAC AGG TTG GGA CTG TCG |
| Nr1h3 (Lxra) | Nuclear receptor subfamily 1, group H, member 3 (Oxysterols receptor LXR-alpha) | F: AAC AGC TCC CTG GCT TCC TA R: CAG AAG CAT GAC CTC GAT TGC |
| Plin2 | Perilipin 2 | F: AGC CAA CGT CCG AGA TTG TT R: CTC CAG CCA TGG TAG TCG TC |
| Scd1 | Stearoyl-Coenzyme A desaturase 1 | F: GGC CTG TAC GGG ATC ATA CTG R: GGT CAT GTA GTA GAA AAT CCC GAA G |
| Sdha | Succinate dehydrogenase complex flavoprotein subunit A | F: CTT GAA TGA GGC TGA CTG TG R: ATC ACA TAA GCT GGT CCT GT |
| Srebf1 | Sterol regulatory element-binding transcription factor 1 | F: CAC ACA AAA GCA AAT CAC TGA AGG R: TCT CCA CCA CTT CGG GTT TC |
| Human Caucasian astrocytoma cells (CCF-STTG1) | ||
| ABCA1 | ATP-binding cassette, subfamily A, member 1 | F: TCT CTG TTC GGC TGA GCT AC R: TGC AGA GGG CAT GGC TTT AT |
| ABCG1 | ATP-binding cassette, subfamily G, member 1 | F: GGT CGC TCC ATC ATT TGC AC R: GCA GAC TTT TCC CCG GTA CA |
| APOE | Apolipoprotein E | F: ACC CAG GAA CTG AGG GC R: CTC CTT GGA CAG CCG TG |
| SDHA | Succinate dehydrogenase complex flavoprotein subunit A | F: TGG GAA CAA GAG GGC ATC TG R: CCA CCA CTG CAT CAA ATT CAT G |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, N.; Schepers, M.; Zhan, N.; Leijten, F.; Voortman, G.; Tiane, A.; Rombaut, B.; Poisquet, J.; Sande, N.v.d.; Kerksiek, A.; et al. 24(S)-Saringosterol Prevents Cognitive Decline in a Mouse Model for Alzheimer’s Disease. Mar. Drugs 2021, 19, 190. https://doi.org/10.3390/md19040190
Martens N, Schepers M, Zhan N, Leijten F, Voortman G, Tiane A, Rombaut B, Poisquet J, Sande Nvd, Kerksiek A, et al. 24(S)-Saringosterol Prevents Cognitive Decline in a Mouse Model for Alzheimer’s Disease. Marine Drugs. 2021; 19(4):190. https://doi.org/10.3390/md19040190
Chicago/Turabian StyleMartens, Nikita, Melissa Schepers, Na Zhan, Frank Leijten, Gardi Voortman, Assia Tiane, Ben Rombaut, Janne Poisquet, Nienke van de Sande, Anja Kerksiek, and et al. 2021. "24(S)-Saringosterol Prevents Cognitive Decline in a Mouse Model for Alzheimer’s Disease" Marine Drugs 19, no. 4: 190. https://doi.org/10.3390/md19040190
APA StyleMartens, N., Schepers, M., Zhan, N., Leijten, F., Voortman, G., Tiane, A., Rombaut, B., Poisquet, J., Sande, N. v. d., Kerksiek, A., Kuipers, F., Jonker, J. W., Liu, H., Lütjohann, D., Vanmierlo, T., & Mulder, M. T. (2021). 24(S)-Saringosterol Prevents Cognitive Decline in a Mouse Model for Alzheimer’s Disease. Marine Drugs, 19(4), 190. https://doi.org/10.3390/md19040190







