Chemical Diversity of Soft Coral Steroids and Their Pharmacological Activities
Abstract
1. Introduction
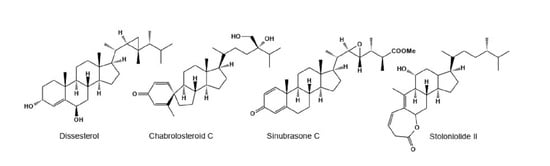
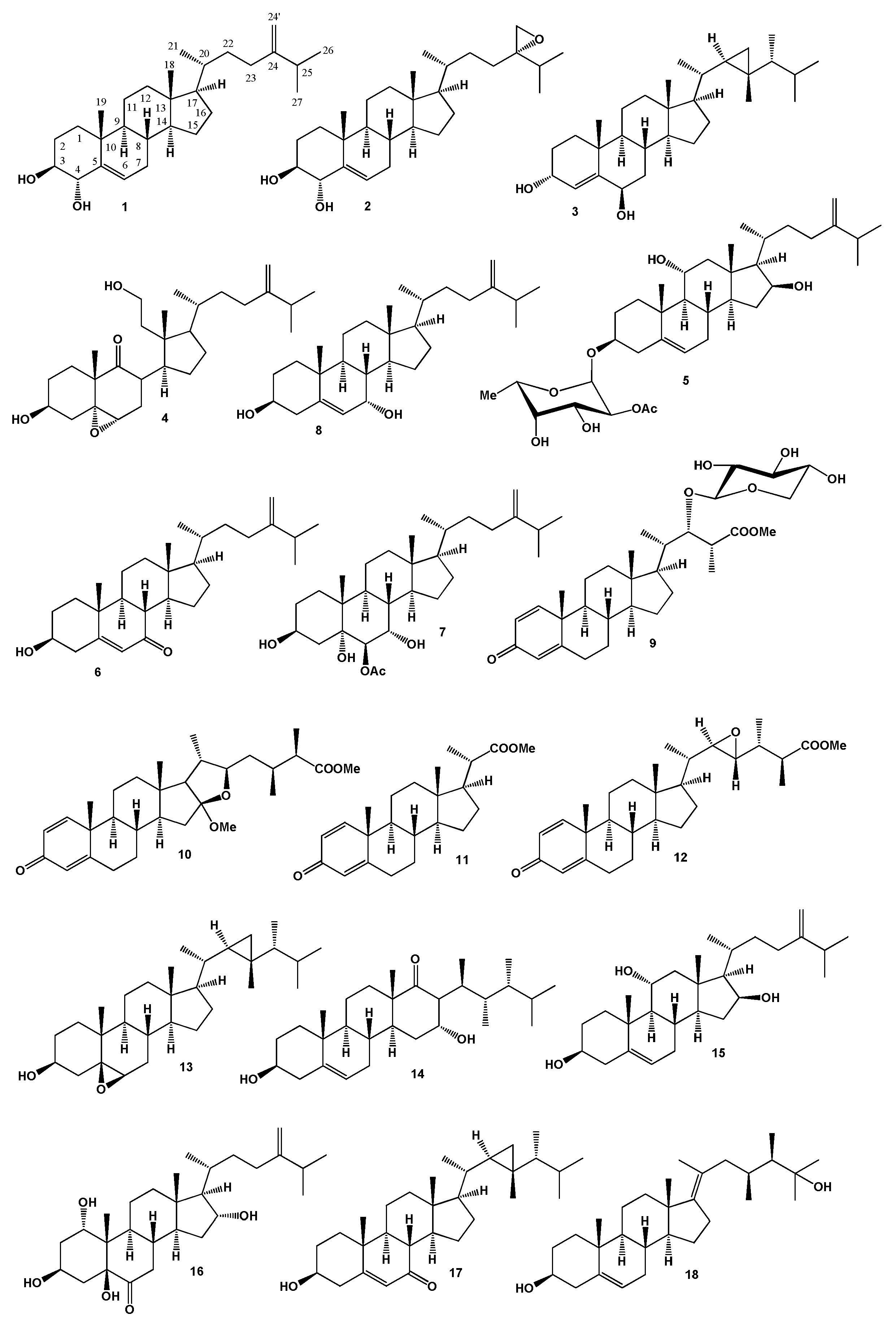
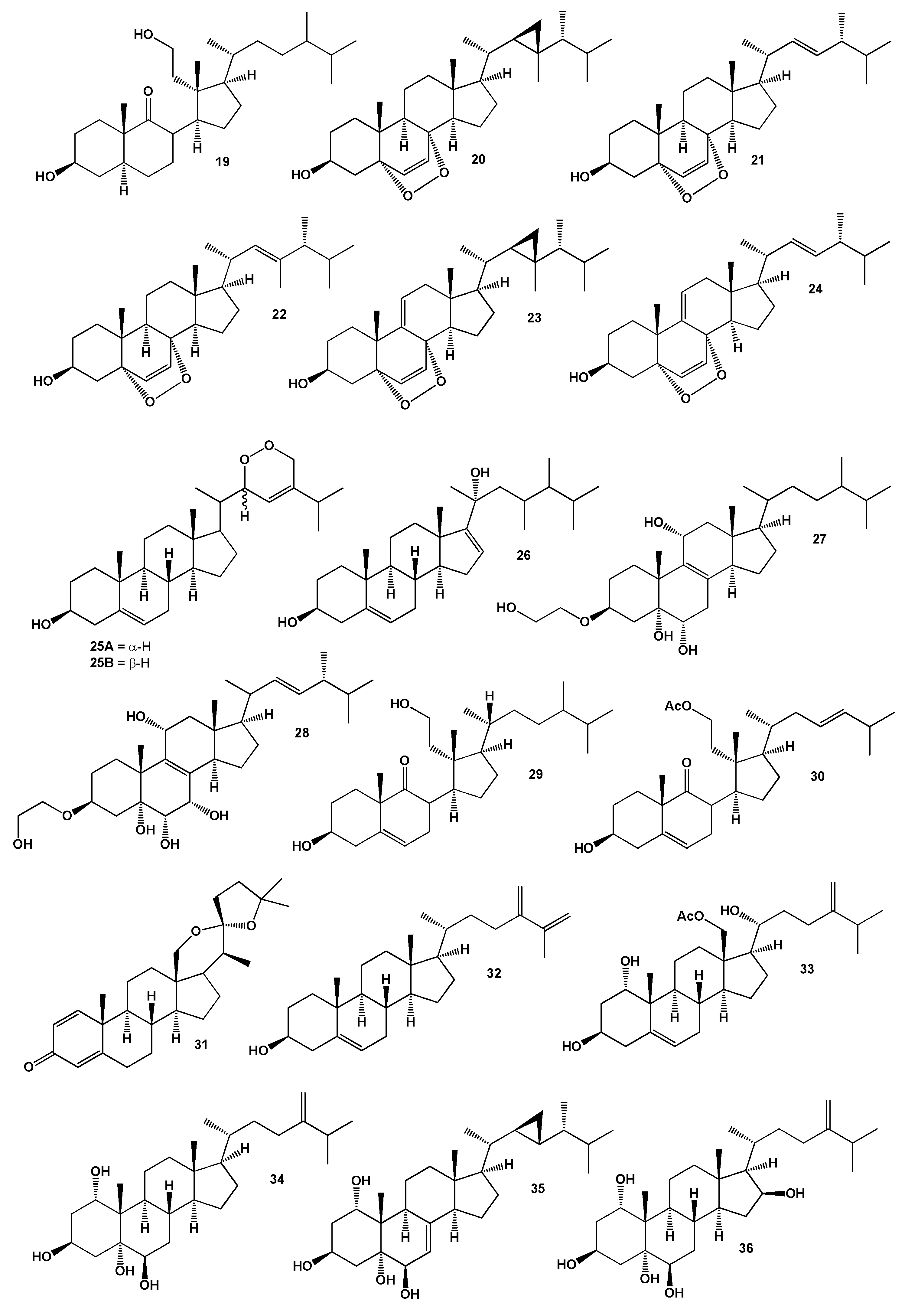
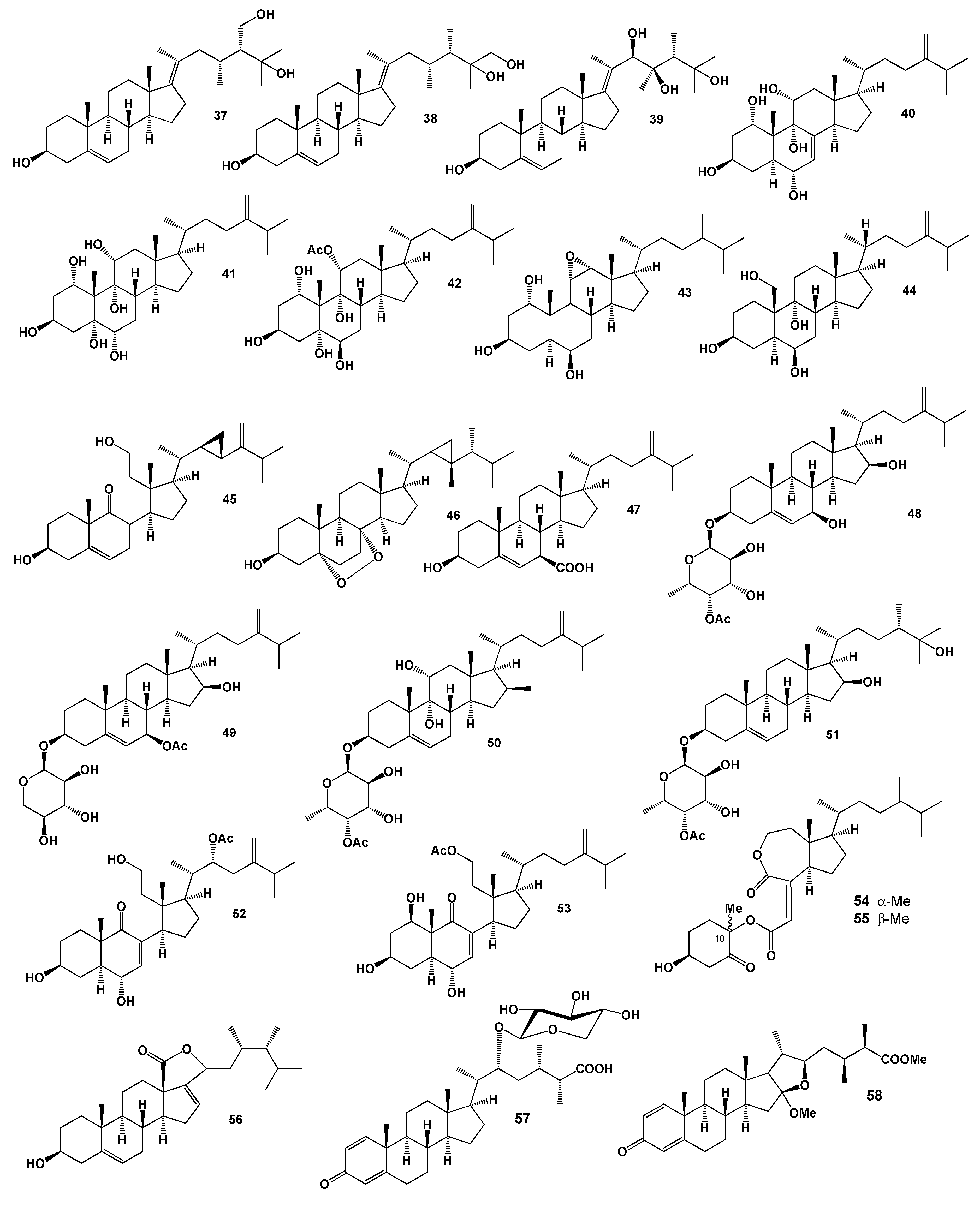
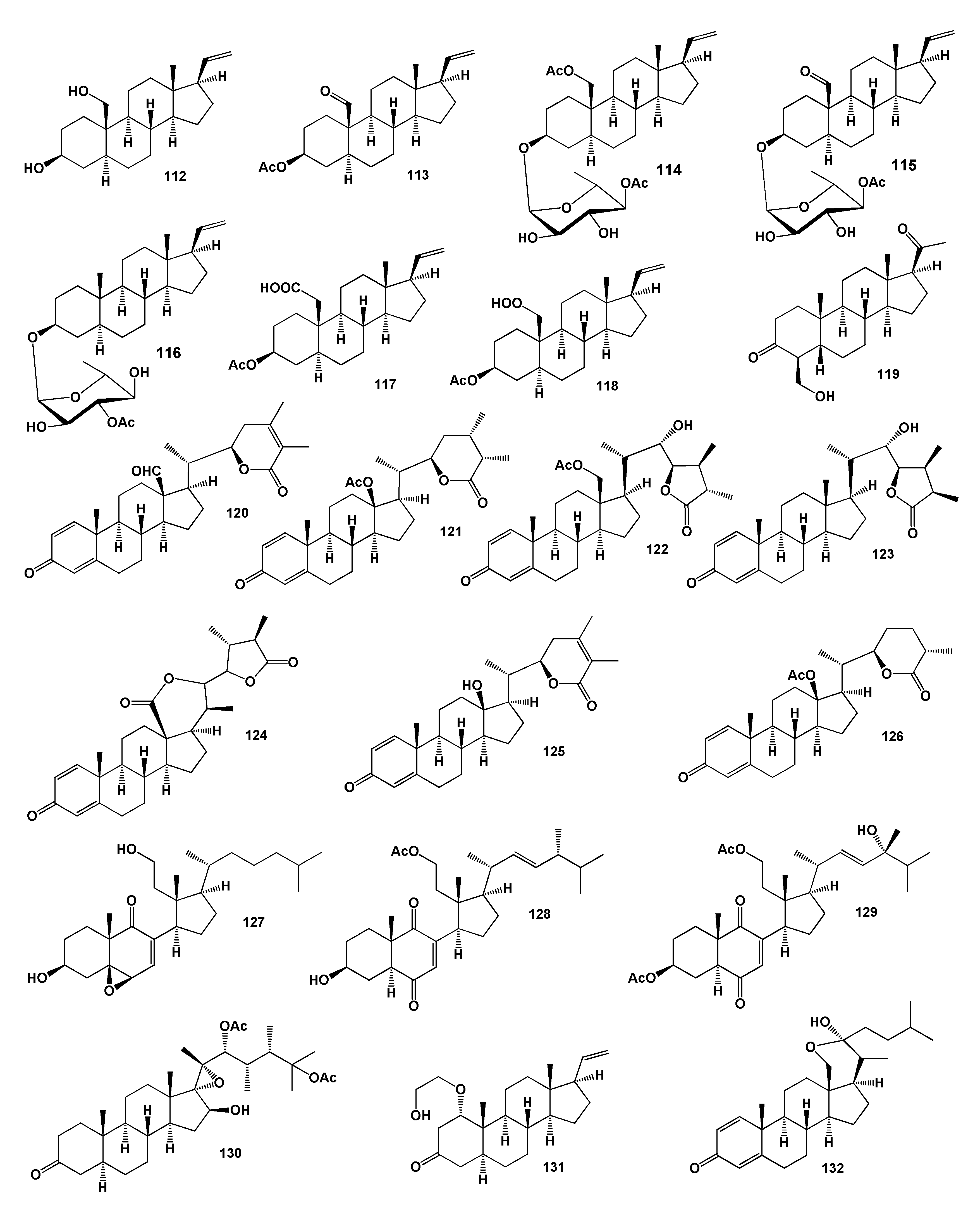
2. Steroids Derived from the Genus Sinularia
3. Steroids Derived from the other Coral’s Genera
4. Comparison of Biological Activities of Natural Soft Coral Steroids
5. Conclusions
Funding
Conflicts of Interest
References
- Horvath, E.A. A review of gorgonian coral species (Cnidaria, Octocorallia, Alcyonacea) held in the Santa Barbara museum of natural history research collection: Focus on species from Scleraxonia, Holaxonia, and Calcaxonia—Part I: Introduction, species of Scleraxonia and Holaxonia (Family Acanthogorgiidae). ZooKeys 2019, 860, 1–66. [Google Scholar]
- Jankowski, T.; Anokhin, B. Phylum Cnidaria. Thorp. Covichs Freshwater Invertebrat. 2019, 4, 93–111. [Google Scholar]
- Boström-Einarsson, L.; Babcock, R.C.; Bayraktarov, E.; Ceccarelli, D.; Cook, N.; Ferse, S.C.A. Coral restoration—A systematic review of current methods, successes, failures and future directions. PLoS ONE 2020, 15, e0226631. [Google Scholar] [CrossRef]
- Leal, M.C.; Ferrier-Pages, C.; Petersen, D.; Osinga, R. Coral aquaculture: Applying scientific knowledge to ex situ production. Rev. Aquacult. 2014, 6, 1–18. [Google Scholar]
- Li, G.; Li, P.; Tang, X. Natural products from corals. In Symbiotic Microbiomes of Coral Reefs Sponges and Corals; Li, Z., Ed.; Springer: Dordrecht, The Netherlands, 2019. [Google Scholar]
- Imbs, A.B.; Maliotin, A.N.; Huyen, L.V.; Long, P.Q. Study of fatty acid composition of 17 coral species of Vietnam. Vietnam. J. Sci. Technol. 2005, 43, 84–91. [Google Scholar]
- Imbs, A.B.; Demina, O.A.; Demidkova, D.A. Lipid class and fatty acid composition of the boreal soft coral Gersemia rubiformis. Lipids 2006, 41, 721–725. [Google Scholar] [CrossRef]
- Imbs, A.B.; Luu, H.V.; Long, P.Q. Lipids of soft coral Sinularia species. Chem. Nat. Comp. 2007, 43, 610–611. [Google Scholar] [CrossRef]
- Imbs, A.B.; Dautova, T.N. Use of lipids for chemotaxonomy of octocorals (Cnidaria: Alcyonaria). Russ. J. Mar. Biol. 2008, 34, 205–209. [Google Scholar] [CrossRef]
- Imbs, A.B.; Yakovleva, I.M.; Pham, L.Q. Distribution of lipids and fatty acids in the zooxanthellae and host of the soft coral Sinularia sp. Fish. Sci. 2010, 76, 375–380. [Google Scholar] [CrossRef]
- Imbs, A.B. Prostaglandins and oxylipins of corals. Russ. J. Mar. Biol. 2011, 37, 325–334. [Google Scholar] [CrossRef]
- Imbs, A.B.; Yakovleva, I.M. Dynamics of lipid and fatty acid composition of shallow-water corals under thermal stress: An experimental approach. Coral Reefs 2012, 31, 41–53. [Google Scholar] [CrossRef]
- Imbs, A.B. Fatty acids and other lipids of corals: Composition, distribution, and biosynthesis. Russ. J. Mar. Biol. 2013, 39, 153–168. [Google Scholar] [CrossRef]
- Elkhawas, Y.A.; Elissawy, A.M.; Elnaggar, M.S.; Mostafa, N.M.; Al-Sayed, E.; Bishr, M.M.; Singa, A.N.B.; Salama, O.M. Chemical diversity in species belonging to soft coral genus Sacrophyton and its impact on biological activity: A review. Mar. Drugs 2020, 18, 41. [Google Scholar] [CrossRef]
- Sheu, J.H.; Chen, Y.H.; Chen, Y.-H.; Su, Y.-D.; Chang, Y.-C. Briarane Diterpenoids isolated from gorgonian corals between 2011 and 2013. Mar. Drugs 2014, 12, 2164–2181. [Google Scholar] [CrossRef]
- Sikorsky, T.V.; Ermolenko, E.V.; Gloriozova, T.A.; Dembitsky, V.M. Mini Review: Anticancer activity of diterpenoid peroxides. Vietnam J. Chem. 2020, 58, 273–280. [Google Scholar] [CrossRef]
- Sunassee, S.N.; Davies-Coleman, M.T. Cytotoxic and antioxidant marine prenylated quinones and hydroquinone. Nat. Prod. Rep. 2012, 29, 513–589. [Google Scholar] [CrossRef]
- Hou, X.-M.; Yang, H.; Gu, Y.-C.; Wang, C.-Y.; Shao, C.-L. Chemical and bioactive marine natural products of coral-derived microorganisms (2015-2017). Current Med. Chem. 2019, 26, 6930–6941. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Bioactive cyclobutane-containing alkaloids. J. Nat. Med. 2008, 62, 1–33. [Google Scholar] [CrossRef]
- Ismail, F.M.D.; Levitsky, D.O.; Dembitsky, V.M. Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem. 2009, 44, 3373–3387. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Naturally occurring bioactive cyclobutane-containing (CBC) alkaloids in fungi, fungal endophytes, and plants. Phytomedicine 2014, 21, 1559–1581. [Google Scholar] [CrossRef]
- Souza, C.R.M.; Bezerra, W.P.; Sout, J.T. Marine alkaloids with anti-inflammatory activity: Current knowledge and future perspectives. Mar. Drugs 2020, 18, 147. [Google Scholar] [CrossRef]
- Xu, J.; Yi, M.; Ding, L.; He, S. A review of anti-inflammatory compounds from marine fungi, 2000–2018. Mar. Drugs 2019, 17, 636. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Chemistry and biodiversity of the biologically active natural glycosides. Chem. Biodivers. 2004, 1, 673–781. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 4. Fatty acid amide glycosides, their analogs and derivatives. Lipids 2005, 40, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 5. Biologically active glycosides of aromatic metabolites. Lipids 2005, 40, 869–900. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 6. Biologically active marine and terrestrial alkaloid glycosides. Lipids 2005, 40, 1081–1114. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 7. Biologically active hemi-and monoterpenoid glycosides. Lipids 2006, 41, 1–27. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 3. Carotenoid glycosides and isoprenoid glycolipids. Lipids 2005, 40, 535–557. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 1. Glycosides of fatty acids and alcohols. Lipids 2004, 39, 933–953. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 2. Polyether glycosidic ionophores and macrocyclic glycosides. Lipids 2005, 40, 219–248. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Antitumor and hepatoprotective activity of natural and synthetic neo steroids. Prog. Lipid Res. 2020, 79, 101048. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Savidov, N.; Gloriozova, T.A. Natural sulphur-containing steroids: Origin and biological activities. Vietnam J. Chem. 2018, 56, 533–541. [Google Scholar] [CrossRef]
- Vil, V.A.; Gloriozova, T.A.; Poroikov, V.V.; Terent’ev, A.O.; Savidov, N.; Dembitsky, V.M. Peroxy steroids derived from plant and fungi and their biological activities. Appl. Microbiol. Biotechnol. 2018, 102, 7657–7667. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Naturally occurring marine α,β-epoxy steroids: Origin and biological activities. Vietnam J. Chem. 2018, 56, 409–433. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Savidov, N.; Poroikov, V.V.; Gloriozova, T.A.; Imbs, A.B. Naturally occurring aromatic steroids and their biological activities. Appl. Microbiol. Biotechnol. 2018, 102, 4663–4674. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Gloriozova, T.A.; Poroikov, V.V.; Dembitsky, V.M. Halogenated (Cl, Br and I) marine steroids and their biological activities: A brief review. Pharma Innov. J. 2017, 6, 456–462. [Google Scholar]
- Siddiq, A.; Dembitsky, V. Acetylenic anticancer agents. AntiCancer Agents Med. Chem. 2008, 8, 132–170. [Google Scholar] [CrossRef]
- Kuklev, D.V.; Domb, A.J.; Dembitsky, V.M. Bioactive acetylenic metabolites. Phytomedicine 2013, 20, 1145–1159. [Google Scholar] [CrossRef]
- Kuklev, D.V.; Dembitsky, V.M. Chemistry, origin, antitumor and other activities of fungal homo-dimeric alkaloids. Mathews J. Pharm. Sci. 2016, 1, 004. [Google Scholar]
- Kilimnik, A.; Kuklev, D.V.; Dembitsky, V.M. Antitumor Acetylenic Lipids. Mathews J. Pharmaceut. Sci. 2016, 1, 005. [Google Scholar]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Novel antitumor agents: Marine sponge alkaloids, their synthetic analogs and derivatives. Mini Rev. Med. Chem. 2005, 5, 319–336. [Google Scholar] [CrossRef]
- Wali, A.F.; Majid, S.; Rasool, S.; Shehada, S.B.; Abdulkareem, S.K.; Firdous, A.; Beigh, S.; Shakeel, S.; Mushtaq, S.; Akbar, I.; et al. Natural products against cancer: Review on phytochemicals from marine sources in preventing cancer. Saudi Pharm. J. 2019, 27, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Matulja, D.; Wittine, K.; Malatesti, N.; Laclef, S.; Turks, M.; Markovic, M.K.; Ambrožić, G.; Marković, D. Marine natural products with high anticancer activities. Curr. Med. Chem. 2020, 27, 1243–1307. [Google Scholar] [CrossRef]
- Di Costanzo, F.; Di Dato, V.; Ianora, A.; Romano, G. Prostaglandins in marine organisms: A review. Mar. Drugs 2019, 17, 428. [Google Scholar] [CrossRef]
- McFadden, C.S.; Van Ofwegen, L.P.; Beckman, E.J.; Benayahu, Y.; Alderslade, P. Molecular systematics of the speciose Indo-Pacific soft coral genus, Sinularia (Anthozoa: Octocorallia). Invertebr. Biol. 2009, 128, 303–323. [Google Scholar] [CrossRef]
- Quattrini, A.M.; Wu, T.; Soong, K.; Jeng, M.S.; Benayahu, Y.; McFadden, C.S. A next generation approach to species delimitation reveals the role of hybridization in a cryptic species complex of corals. BMC Evol. Biol. 2019, 19, 116–132. [Google Scholar] [CrossRef]
- Lakshmi, V.; Kumar, R. Metabolites from Sinularia species. Nat. Prod. Res. 2009, 23, 801–850. [Google Scholar] [CrossRef]
- Chen, W.-T.; Li, Y.; Guo, Y.-W. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta Pharm. Sin. B 2012, 2, 227–237. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Huong, P.T.M.; Thanh, N.V.; Cuong, N.X. Steroid Constituents from the Soft Coral Sinularia nanolobata. Chem. Pharm. Bull. 2016, 64, 1417–1419. [Google Scholar] [CrossRef]
- Chao, C.H.; Chou, K.J.; Huang, C.Y.; Wen, Z.H.; Hsu, C.H.; Wu, Y.C.; Dai, C.F.; Sheu, J.H. Steroids from the Soft Coral Sinularia crassa. Mar. Drugs 2012, 10, 439–450. [Google Scholar] [CrossRef]
- Mariottini, G.L. The role of cnidaria in drug discovery. In The Cnidaria, Past, Present and Future; Goffredo, S., Dubinsky, Z., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Ilhan, H.A.; Pulat, C.C. Cytotoxic and antitumor compounds from marine invertebrates. Encycl. Mar. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Huong, P.T.M.; Van Thanh, N.; Chia, N.T.P.C. Cytotoxic steroids from the Vietnamese soft coral Sinularia conferta. Chem. Pharm. Bull. 2017. [Google Scholar] [CrossRef]
- Cường, N.X.; Nhiệm, N.X.; NV Thanh, N.V. Review of chemistry and biological activity studies some marine species in Vietnam in the period 2013-2017. Vietnam J. Chem. 2018, 56, 1–19. [Google Scholar] [CrossRef]
- Huang, C.Y.; Su, J.S.; Liaw, C.C.; Sung, P.J.; Chiang, P.L.; Hwang, T.L.; Chang-Feng Dai, J.-H.; Sheu, H. Bioactive steroids with methyl ester group in the side chain from a reef soft coral Sinularia brassica cultured in a tank. Mar. Drugs 2017, 15, 280. [Google Scholar] [CrossRef]
- Wu, M.J.; Wang, H.; Jiang, C.S.; Guo, Y.W. New cembrane-type diterpenoids from the South China Sea soft coral Sinularia crassa and their α-glucosidase inhibitory activity. Bioorg. Chem. 2020, 104, 104281. [Google Scholar] [CrossRef]
- Chen, K.H.; Dai, C.F.; Hwang, T.L.; Chen, C.Y.; Li, J.J.; Chen, J.J. Discovery of novel diterpenoids from Sinularia arborea. Mar. Drugs 2014, 12, 385–393. [Google Scholar] [CrossRef]
- Chen, W.T.; Liu, H.L.; Yao, L.G.; Guo, Y.W. 9,11-Secosteroids and polyhydroxylated steroids from two South China Sea soft corals Sarcophyton trocheliophorum and Sinularia flexibilis. Steroids 2014, 92, 56–61. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. 1α,3β,5β-Trihydroxy-24-methyl-enecholestan-6-one: A novel steroid from a soft coral Sinularia gibberosa. Steroids 2003, 68, 377–381. [Google Scholar] [CrossRef]
- Tseng, Y.-J.; Wang, S.K.; Duh, C.Y. Secosteroids and norcembranoids from the soft coral Sinularia nanolobata. Mar. Drugs 2013, 11, 3288–3296. [Google Scholar] [CrossRef]
- Thanh, N.V.; Ngoc, N.T.; Anh, H.L.T.; Thung, D.C.; Thao, D.T.; Cuong, N.X. Steroid constituents from the soft coral Sinularia microspiculata. J. Asian Nat. Prod. Res. 2016, 18, 938–944. [Google Scholar] [CrossRef]
- Su, J.H.; Tseng, Y.J.; Huang, H.H.; Ahmed, A.F.; Lu, C.K.; Wu, Y.C.; Sheu, J.H. 9,11-Secosterols from the soft corals Sinularia lochmodes and Sinularia leptoclados. J. Nat. Prod. 2006, 69, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. 5, 8-Epidioxysterols and related derivatives from a Chinese soft coral Sinularia flexibilis. Steroids 2006, 71, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, N.T.; Van Thanh, N.; Cuong, N.X.; Nam, N.H. Structure elucidation of four steroids from the soft coral Sinularia nanolobata. Vietnam J. Sci. Technol. 2017, 55, 258–262. [Google Scholar] [CrossRef]
- Qia, S.H.; Gao, C.H.; Qian, P.Y.; Zhang, S. Steroids from the South China Sea Gorgonian Subergorgia suberosa. Nat. Prod. Commun. 2010, 5, 201–204. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Ravi, B.N.; Sanders, R.L.; Wells, R.J. Spermidine derivatives and 9,11-secosteroids from a soft coral (Sinularia sp.). Austral. J. Chem. 1982, 35, 69–75. [Google Scholar] [CrossRef]
- Bonini, C.; Cooper, C.B.; Kazlauskas, R.; Wells, R.J.; Djerassi, C. Minor and trace sterols in marine invertebrates. 41. Structure and stereochemistry of naturally occurring 9,11-seco sterols. J. Org. Chem. 1983, 48, 2108–2111. [Google Scholar] [CrossRef]
- Ciminiello, P.; Fenical, W.; Paul, V.J. Structure assignments of two new C-18-oxygenated steroidal ketals isolated from a pacific soft coral of the genus Sinularia. Experientia 1990, 46, 980–982. [Google Scholar] [CrossRef]
- Gebreyesus, T.; Stoilov, I.; Luo, F.T.; Djerassi, C. Minor and trace sterols in marine invertebrates 55. The isolation, structure elucidation and synthesis of ergosta-5,24(28),25-trien-3β-ol. Steroids 1985, 45, 447–452. [Google Scholar] [CrossRef]
- Jagodzinska, B.M.; Trimmer, J.S.; Fenical, W.; Djerassi, C. Sterols in marine invertebrates. 49. Isolation and structure elucidation of eight new polyhydroxylated sterols from the soft coral Sinularia dissecta. J. Org. Chem. 1985, 50, 1435–1439. [Google Scholar]
- Jagodzinska, B.M.; Trimmer, J.S.; Fenical, W.; Djerassi, C. Sterols in marine invertebrates. 51. Isolation and structure elucidation of C-18 functionalized sterols from the soft coral Sinularia dissecta. J. Org. Chem. 1985, 50, 2988–2992. [Google Scholar]
- Su, J.; Yu, X.; Zeng, L.; Mak, T.C.W. Noval Polyhydroxylated sterols from the Soft Coral Sinularia numerose. J. Nat. Prod. 1989, 52, 934–940. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ishizaka, T.; Miura, N.; Mitsuhashi, H. Marine terpenes and terpenoids. III. Isolation and structures of two cembrane diols from the soft coral Sinularia mayi. Chem. Pharm. Bull. 1987, 35, 2314–2318. [Google Scholar]
- Kobayashi, M.; Krishna, M.M.; Anjaneyulu, V. Marine Sterols. XXIV. Isolation of 24-Methylenecholestane-1α, 3β, 3α, 6β, 16β-pentol from Sinularia sp. of soft coral. Chem. Pharm. Bull. 1992, 40, 2845–2846. [Google Scholar] [CrossRef]
- Kobayashi, M.; Haribabu, B.; Anjaneyulu, V. Marine Sterol. XXV. Isolation of 23-demethylgorgost-7-ene-3β, 5α,6β-triol and(24S)-ergostane-3β,5α,6β,7β,15β-pentol from soft corals of the Andaman and Nicobar Coasts. Chem. Pharm. Bull. 1993, 41, 87–89. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Sagar, K.S.; Venugopal, M.J.R.V. Terpenoid and steroid constituents of the Indian Ocean soft coral Sinularia maxima. Tetrahedron 1995, 51, 10997–11010. [Google Scholar] [CrossRef]
- Kobayashi, M. Marine sterols. 27. 25-hydroxy derivative of sarcosterol, a novel marine sterol with a 23-methyl and a 17(20)E-double bond, from the soft coral Sinularia mayi. Steroids 1994, 59, 27–29. [Google Scholar]
- Ramesh, P.; Venkateswarlu, Y. Novel steroid constituents of the soft coral Sinularia dissecta. Steroids 1999, 64, 785–789. [Google Scholar] [CrossRef]
- Xu, S.; Guo, S.; Liu, Y.; Zeng, L. Isolation and identification of two steroids from Sinularia inexplicita. Zhong Yao Cai 2001, 24, 34–35. [Google Scholar]
- Weng, J.-R.; Chiu, C.F.; Sheu, J.H. A sterol from soft coral induces apoptosis and autophagy in MCF-7 breast cancer cells. Mar. Drugs 2018, 16, 238. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lai, K.H.; Kumar, S.; Chen, P.J.; Wu, Y.H.; Lai, C.L. 1H NMR-Based Isolation of Anti-Inflammatory 9,11-secosteroids from the octocoral Sinularia leptoclados. Mar. Drugs 2020, 18, 271. [Google Scholar] [CrossRef]
- Sheu, J.H.; Chang, K.C.; Sung, P.J.; Duh, C.Y. Chemical constituents of a formosan soft coral Sinularia sp. J. Chin. Chem. Soc. 1999, 46, 253–257. [Google Scholar] [CrossRef]
- Sheu, J.H.; Chang, K.C.; Duh, C.Y. A Cytotoxic 5α,8α-epidioxysterol from a soft coral Sinularia species. J. Nat. Prod. 2000, 63, 149–151. [Google Scholar] [CrossRef]
- Tillekeratne, L.M.V.; Liyanage, G.K.; Ratnasooriya, W.D.; Ksebati, M.B.; Schmitz, F.J. A new spermatostatic glycoside from the soft coral Sinularia crispa. J. Nat. Prod. 1989, 52, 1143–1145. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Kulatheeswaran, R. Bioactive compounds from a new species of Sinularia soft coral. Indian J. Chem. 1999, 38B, 1388–1390. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2005, 22, 15–61. [Google Scholar] [CrossRef]
- Kawagishi, H.; Kawagishi, H.; Choi, J.H.; Choi, J.H.; Ogawa, A.; Ogawa, A.; Yazawa, K. Chaxines B, C, D, and E from the edible mushroom Agrocybe chaxingu. Tetrahedron 2009, 65, 9850–9853. [Google Scholar]
- Liu, J.; Wu, X.; Yang, M.; Gu, Y.C.; Yao, L.G.; Huan, X.J.; Miao, Z.H.; Luo, H.; Guo, Y.W. Erectsterates A and B, a pair of novel highly degraded steroid derivatives from the South China Sea soft coral Sinularia erecta. Steroids 2020, 161, 108681. [Google Scholar] [CrossRef]
- Jin, P.; Deng, Z.; Pei, Y.; Fu, H.; Lin, W. Polyhydroxylated steroids from the soft coral Sinularia dissecta. Steroids 2005, 70, 487–493. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Wang, K.L.; Liao, X.J.; Deng, Z.; Xu, S.H. Antifouling steroids from the South China Sea gorgonian coral Subergorgia suberosa. Steroids 2014, 79, 1–6. [Google Scholar] [CrossRef]
- Xiao, J.; Gao, M.; Fei, B.; Huang, G.; Diao, Q. Nature-derived anticancer steroids outside cardica glycosides. Fitoterapia 2020, 147, 104757. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Krishna Murthy, M.V.R.; Gowri, P.M. Novel epoxy steroids from the Indian ocean soft coral Sarcophyton crassocaule. J. Nat. Prod. 2000, 63, 112–118. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Dai, C.F.; Duh, C.Y. Sesquiterpenoids and artificial 19-oxygenated steroids from the Formosan soft coral Nephthea erecta. J. Nat. Prod. 2007, 70, 1449–1453. [Google Scholar] [CrossRef]
- Wang, S.K.; Puu, S.Y.; Duh, C.Y. Novel steroids from the soft coral Nephthea chabrolii. Tetrahedron 2007, 63, 703–707. [Google Scholar]
- Huang, C.Y.; Chang, C.W.; Sheu, J.H. Bioactive steroids from the Formosan soft coral Umbellulifera petasites. Mar. Drugs 2016, 14, 180. [Google Scholar] [CrossRef]
- Seo, Y.; Jung, J.H.; Rho, J.R.; Shin, J. Isolation of novel bioactive steroids from the soft coral Alcyonium gracillimum. Tetrahedron 1995, 51, 2497–2506. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L.; Gopichand, Y.; Schmitz, F.J.; Djerassi, C. Minor and trace sterols in marine invertebrates. Isolation and structure elucidation of nine new 5α,8α-epidioxy sterols from four marine organisms. J. Org. Chem. 1981, 46, 3860–3866. [Google Scholar] [CrossRef]
- Tomono, Y.; Hirota, H.; Fusetani, N. Isogosterones A-D, antifouling 13,17-secosteroids from an octocoral Dendronephthya sp. J. Org. Chem. 1999, 64, 2272–2275. [Google Scholar] [CrossRef]
- Chung, H.M.; Hong, P.H.; Su, J.H. Bioactive compounds from a gorgonian coral Echinomuricea sp. (Plexauridae). Mar. Drugs 2012, 10, 1169–1179. [Google Scholar] [CrossRef]
- Duh, C.Y.; Lo, I.W.; Wang, S.K.; Dai, C.F. New cytotoxic steroids from the soft coral Clavularia viridis. Steroids 2007, 72, 573–579. [Google Scholar] [CrossRef]
- Kobayashi, M.; Lee, N.K.; Byeng, W.; Kazunori, S.; Kyogoku, Y.; Kitagawa, I. Stoloniferone-a, -b, -c, and -d, four new cytotoxic steroids from the Okinawan soft coral Clavularia viridis. Tetrahedron Lett. 1984, 25, 5925–5928. [Google Scholar] [CrossRef]
- Kazuo, I.; Makoto, I.; Kinzo, W. Stoloniolide I and II, new marine lactonic steroids with an unprecedented 1,10-secoergostane skeleton, isolated from the Okinawan Soft Coral, Clavularia viridis. Chem. Lett. 1995, 24, 1109–1110. [Google Scholar]
- Iwashima, M.; Nara, K.; Iguchi, K. New marine steroids, yonarasterols, isolated from the Okinawan soft coral. Clavularia viridis. Steroids 2000, 65, 130–137. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, S.K.; Duh, C.Y. Polyhydroxylated steroids from the bamboo coral Isis hippuris. Mar. Drugs 2011, 9, 1829–1839. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Dai, C.-F.; Duh, C.-Y. New 4-methylated and 19-oxygenated steroids from the Formosan soft coral Nephthea erecta. Steroids 2007, 72, 653–659. [Google Scholar] [CrossRef]
- Amir, F.; Koay, Y.C.; Yam, W.S. Chemical constituents and biological properties of the marine soft coral Nephthea: A review (Part 1). Trop. J. Pharm. Res. 2012, 11, 485–498. [Google Scholar]
- Cheng, S.-Y.; Wen, Z.-H.; Wang, S.-K.; Chiang, M.Y.; El Gamal, A.A.H.; Dai, C.-F.; Duh, C.-Y. Revision of the absolute configuration at C(23) of lanostanoids and isolation of secondary metabolites from Formosan soft coral Nephthea erecta. Chem. Biodiver. 2009, 6, 86–95. [Google Scholar] [CrossRef]
- Tsai, Y.Y.; Huang, C.Y.; Tseng, W.R.; Chiang, P.L.; Hwang, T.L.; Su, J.H.; Sung, P.J.; Dai, C.F.; Sheu, J.H. Klyflaccisteroids K-M, bioactive steroidal derivatives from a soft coral Klyxum flaccidum. Bioorg. Med. Chem. Lett. 2017, 27, 1220–1224. [Google Scholar] [CrossRef]
- Chang, Y.C.; Kuo, L.M.; Hwang, T.L.; Yeh, J.; Wen, J.H.; Fang, L.S.; Wu, Y.C.; Lin, C.S.; Sheu, J.H.; Sung, P.J. Pinnisterols A–C, new 9,11-secosterols from a Gorgonian Pinnigorgia sp. Mar. Drugs 2016, 14, 12. [Google Scholar] [CrossRef]
- Quang, T.H.; Ha, T.T.; Minh, C.V.; Kiem, P.V.; Huong, H.T.; Ngan, N.T.; Nhiem, N.X.; Tung, N.H.; Thao, N.P.; Thuy, D.T. Cytotoxic and PPARs transcriptional activities of sterols from the Vietnamese soft coral Lobophytum laevigatum. Bioorg. Med. Chem. Lett. 2011, 21, 2845–2849. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Kuo, L.-M.; Su, J.-H.; Hwang, T.-L.; Kuo, Y.-H.; Lin, C.-S.; Wu, Y.-C.; Sheu, J.-H.; Sung, P.-J. Pinnigorgiols A–C, 9,11-secosterols with a rare ring arrangement from a gorgonian coral Pinnigorgia sp. Tetrahedron 2016, 72, 999–1004. [Google Scholar] [CrossRef]
- Chang, Y.C.; Hwang, T.L.; Sheu, J.H.; Wu, Y.C.; Sung, P.J. New anti-inflammatory 9,11-secosterols with a rare tricyclo[5,2,1,1]decane ring from a Formosan Gorgonian Pinnigorgia sp. Mar. Drugs 2016, 14, 218. [Google Scholar] [CrossRef]
- Nam, N.H.; Ninh, N.N.; Ngoc, T.T.; Hong, H.S.; Chau, V.M. Cytotoxic steroids from the Vietnamese gorgonian Verrucella corona. Steroids 2018, 138, 57–63. [Google Scholar] [CrossRef]
- Chen, W.C.; Sheu, J.H.; Fang, L.S.; Hu, W.P.; Sung, P.J. 3α,7α,12α-Triacetoxy-5β-cholanic acid, a steroid from the Formosan soft coral Alcyonium sp. (Alcyoniidae). Nat. Prod. Res. 2006, 20, 748–753. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Alarif, W.M.; Alburae, N.A.; Algandaby, M.M. Alcyonium octocorals: Potential source of diverse. Bioactive terpenoids. Molecules 2019, 24, 1370. [Google Scholar] [CrossRef]
- Häder, D.-P. Natural bioactive compounds technological advancements. In Bioreactive Substances from Coral Reefs and Gorgonians; Sinha, R.P., Häder, D.P., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 381–391. [Google Scholar]
- Tomono, Y.; Hirota, H.; Imahara, Y.; Fusetani, N. Four new steroids from two octocorals. J. Nat. Prod. 1999, 62, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Manzo, E.; Ciavatta, M.L.; Nuzzo, G.; Gavagnin, M. Terpenoid content of the Antarctic soft coral Alcyonium antarcticum. Nat. Prod. Commun. 2009, 4, 1615–1619. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kiyota, Y.; Orito, S.; Kyogoku, Y.; Kitagawa, I. Five new steroidal glycosides, pregnedioside-A, B, and their three monoacetates, from an Okinawan soft coral of Alcyonium sp. Tetrahedron Lett. 1984, 25, 3731–3734. [Google Scholar] [CrossRef]
- Wang, S.K.; Dai, C.F.; Duh, C.Y. Cytotoxic pregnane steroids from the Formosan Soft Coral Stereonephthya crystalliana. J. Nat. Prod. 2006, 69, 103–106. [Google Scholar] [CrossRef]
- Fang, H.Y.; Liaw, C.C.; Chao, C.H.; Wen, Z.H.; Wu, Y.C. Bioactive pregnane-type steroids from the soft coral Scleronephthya gracillimum. Tetrahedron 2012, 68, 9694–9700. [Google Scholar] [CrossRef]
- Chen, X.Q.; Xing, N.; Yang, B.; Zhou, X.; Gao, C.; Liu, Y. Two novel sesquiterpenes and a new pregnane derivative from the South China Sea Gorgonian Subergorgia suberosa. Rec. Nat. Prod. 2020, 14, 57–64. [Google Scholar] [CrossRef]
- Chao, C.H.; Chou, K.J.; Wen, Z.H.; Wang, G.H.; Wu, Y.C.; Dai, C.F.; Sheu, J.H. Paraminabeolides A-F, cytotoxic and anti-inflammatory marine withanolides from the Soft Coral Paraminabea acronocephala. J. Nat. Prod. 2011, 74, 1132–1141. [Google Scholar] [CrossRef]
- Ksebati, M.B.; Schmitz, F.J. Minabeolides: A group of withanolides from a Soft Coral, Minabea sp. J. Org. Chem. 1988, 53, 3926–3929. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Li, C.-H.; Wang, S.-K.; Dai, C.-F. Diterpenoids, norditerpenoids, and secosteroids from the Formosan soft coral Cespitularia hypotentaculata. J. Nat. Prod. 2006, 69, 1188–1192. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Hwang, T.L.; Sung, P.J. Pinnisterols D–J, new 11-acetoxy-9,11-secosterols with a 1,4-quinone moiety from Formosan Gorgonian coral Pinnigorgia sp. (Gorgoniidae). Mar. Drugs 2017, 15, 11. [Google Scholar] [CrossRef]
- Sheu, J.H.; Huang, L.F.; Chen, S.P.; Yang, Y.L. Hippuristerones E−I, new polyoxygenated steroids from the Gorgonian coral Isis hippuris. J. Nat. Prod. 2003, 66, 917–921. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Chen, S.P.; Sung, P.J.; Chiang, M.Y.; Dai, C.F. Hippuristerone A, a novel polyoxygenated steroid from the gorgonian Isis hippuris. Tetrahedron Lett. 2000, 41, 7885–7888. [Google Scholar] [CrossRef]
- Chao, C.H.; Wen, Z.H.; Su, J.H.; Chen, I.M.; Huang, H.C.; Dai, C.F.; Sheu, J.H. Further study on anti-inflammatory oxygenated steroids from the octocoral Dendronephthya griffini. Steroids 2008, 73, 1353–1358. [Google Scholar] [CrossRef]
- Zhang, G.W.; Ma, X.Q.; Kurihara, H.; Zhang, C.X.; Yao, X.S. New hemiketal steroid from the soft coral Cladiella sp. Organic Lett. 2005, 7, 991–994. [Google Scholar] [CrossRef]
- Fleury, B.G.; Lages, B.G.; Barbosa, J.P.; Kaiser, C.R.; Pinto, A.C. New hemiketal steroid from the introduced soft coral Chromonephthea braziliensis is a chemical defense against predatory fishes. J. Chem. Ecol. 2008, 34, 987–993. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.-C.; Wang, K.-L.; Liao, X.-J.; Deng, Z.; Xu, S.-H. Pentacyclic hemiacetal sterol with antifouling and cytotoxic activities from the soft coral Nephthea sp. , Bioorg. Med. Chem. Lett. 2013, 23, 1079–1082. [Google Scholar] [CrossRef]
- Shanura Fernando, I.P.; Asanka Sanjeewa, K.K.; Kim, H.S.; Wang, L.; Lee, W.W.; Jeon, Y.J. Apoptotic and antiproliferative properties of 3β-hydroxy-Δ5-steroidal congeners from a partially purified column fraction of Dendronephthya gigantea against HL-60 and MCF-7 cancer cells. J. Appl. Toxicol. 2017, 11, 1–10. [Google Scholar]
- Huang, X.; Deng, Z.; Zhu, X.; van Ofwegen, L.; Proksch, P.; Lin, W. Krempenes A–D: A series of unprecedented pregnane-type steroids from the marine soft coral Cladiella krempfi. Helv. Chim. Acta 2006, 89, 2020–2026. [Google Scholar] [CrossRef]
- Ghandourah, M.A.; Alarif, W.M.; Abdel-Lateff, A.; Al-Lihaibi, S.S.; Ayyad, S.-E.N.; Basaif, S.A.; Badria, F.A. Two new terpenoidal derivatives: A himachalene-type sesquiterpene and 13,14-secosteroid from the soft coral Litophyton arboretum. Med. Chem. Res. 2015, 24, 4070–4077. [Google Scholar] [CrossRef]
- Fusetani, N.; Yasukawa, K.; Matsunaga, S.; Hashimoto, K. Dimorphosides A and B, novel steroids glycosides from the gorgonian Anthoplexaura dimorpha. Tetrahedron Lett. 1987, 28, 1187–1190. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, Z.; Yu, Y.; van Ofwegen, L.; Proksch, P.; Yu, S.; Lin, W. An unusual spinaceamine-bearing pregnane from a soft coral Scleronephthya sp. inhibits the migration of tumor cells. Bioorg. Med. Chem. Lett. 2017, 27, 2736–2741. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Cheng, Y.-B.; Kobayashi, J.; Kubota, T.; Takahashi, Y.; Mikami, Y.; Ito, J.; Lin, Y.-S. Nitrogen-containing verticillene diterpenoids from the Taiwanese soft coral Cespitularia taeniata. J. Nat. Prod. 2007, 70, 1961–1965. [Google Scholar] [CrossRef]
- Hegazy, M.F.; Mohamed, T.A.; Alhammady, M.A.; Shaheen, A.M.; Reda, E.R.; Elshamy, A.I. Molecular architecture and biomedical leads of terpenes from red sea marine invertebrates. Mar. Drugs 2015, 13, 3154–3181. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Nassara, M.I.; Mohamed, T.A.; Hegazy, M.E.F. Chemical and biological profile of Cespitularia species: A mini review. J. Adv. Res. 2016, 7, 209–224. [Google Scholar] [CrossRef]
- Díaz-Marrero, A.R.; Porras, G.; Aragón, Z.; de la Rosa, J.M.; Dorta, E.; Cueto, M.; D’Croz, L.; Maté, J.; Darias, J. Carijodienone from the octocoral Carijoa multiflora. A spiropregnane-based steroid. J. Nat. Prod. 2011, 74, 292–295. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Shao, C.L.; Li, Z.Y.; Han, L. Bioactive pregnane steroids from a South China Sea Gorgonian Carijoa sp. Molecules 2013, 18, 3458–3466. [Google Scholar] [CrossRef]
- Dorta, E.; Diaz-Marrero, A.R.; Cueto, M.; D’Croz, L.; Maté, J.L.; San-Martín, A.; Darías, J. Two unique chloro-pregnane steroids have been isolated from the eastern Pacific octocoral Carijoa Multiflora. Tetrahedron Lett. 2004, 45, 915–917. [Google Scholar] [CrossRef]
- Han, L.; Wang, C.Y.; Huang, H.; Shao, C.L.; Liu, Q.A.; Qi, J.; Sun, X.P.; Zhai, P.; Gu, Y.C. A new pregnane analogue from Hainan soft coral Scleronephthya gracillimum Kukenthal. Biochem. Syst. Ecol. 2010, 38, 243–246. [Google Scholar] [CrossRef]
- Iwashima, M.; Nara, K.; Nakamichi, Y.; Iguchi, K. Three new chlorinated marine steroids, yonarasterols G, H and I, isolated from the Okinawan soft coral, Clavularia viridis. Steroids 2001, 66, 25–32. [Google Scholar] [CrossRef]
- Poza, J.; Fernández, R.; Reyes, F.; Reyes, F.; Jiménez, C.; Jiménez, C. Isolation, biological significance, synthesis, and cytotoxic evaluation of new natural parathiosteroids A-C and analogues from the soft coral Paragorgia sp. J. Org. Chem. 2008, 73, 7978–7984. [Google Scholar] [CrossRef]
- Huang, C.Y.; Tseng, W.R.; Ahmed, A.F.; Chiang, P.L.; Tai, C.J.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. Anti-inflammatory polyoxygenated steroids from the soft coral Lobophytum michaelae. Mar. Drugs 2018, 16, 93. [Google Scholar] [CrossRef]
- Kapustina, I.I.; Makarieva, T.N.; Guzii, A.G.; Kalinovsky, A.I.; Popov, R.S.; Dyshlovoy, S.A.; Grebnev, B.B.; von Amsberg, G.; Stonik, V.A. Leptogorgins A–C, humulane sesquiterpenoids from the Vietnamese gorgonian Leptogorgia sp. Mar. Drugs 2020, 18, 310. [Google Scholar] [CrossRef]
- Fusetani, N.; Nagata, H.; Hirota, H.; Tsuyuki, T. Astrogorgiadiol and astrogorgin, inhibitors of cell division in fertilized starfish eggs, from a gorgonian Astrogorgia sp. Tetrahedron Lett. 1989, 30, 7079–7082. [Google Scholar] [CrossRef]
- Seo, Y.; Shin, J.; Song, J.I. New secosteroids from an undescribed gorgonian of the genus Muricella. J. Nat. Prod. 1995, 58, 1291–1295. [Google Scholar] [CrossRef]
- Masamitsu, O.; Koji, Y.; Hiyoshizo, K.; Kozo, S. Calicoferols A and B, two novel secosterols possessing brine-shrimp lethality from the gorgonian Calicogorgia sp. Chem. Lett. 1991, 20, 427–430. [Google Scholar]
- Lu, Y.-N.; Ping, C.; Tian, X.-Q.; Lou, L.-G.; Fan, C.-Q. Unusual cytotoxic steroidal saponins from the gorgonian Astrogorgia dumbea. Planta Med. 2016, 82, 882–887. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Kumar, S.R.; Reddy, G.D. A new pregnane derivative from the Indian ocean gorgonian Subergorgia suberosa (Pallas). Indian J. Chem. 2003, 42, 219–220. [Google Scholar] [CrossRef]
- Seo, Y.; Rho, J.-R.; Cho, K.W.; Shin, J. Isolation of new steroidal hemiacetals from the gorgonian Euplexaura anastomosans. J. Nat. Prod. 1996, 59, 1196–1199. [Google Scholar] [CrossRef]
- Yang, J.; Qi, S.-H.; Zhang, S.; Xiao, Z.-H.; Li, Q.-X. Bebrycoside, a new steroidal glycoside from the Chinese gorgonian coral Bebryce indica. Pharmazie 2007, 62, 154–155. [Google Scholar] [CrossRef]
- Wang, P.; Qi, S.H.; Liu, K.S.; Huang, L.S.; He, F.; Wang, Y.F. Steroids from the South China sea gorgonian coral Muricella Flexuosa. Z. Naturforsch. 2011, 66, 635–640. [Google Scholar] [CrossRef][Green Version]
- Anjaneyulu, A.S.R.; Rao, V.L.; Sastry, V.G. A new spiroketal steroid from gorgonella Umbraculum. Nat. Prod. Res. 2003, 17, 149–152. [Google Scholar] [CrossRef]
- Murillo-Alvarez, J.; Encarnacion-Dimayuga, R. New bioactive pregnadiene-derived glycosides from the gulf of California gorgonian Muricea cf. Austera. Pharm. Biol. 2003, 41, 531–535. [Google Scholar] [CrossRef]
- Cardoso-Martínez, F.; de la Rosa, J.M.; Díaz-Marrero, A.R.; Darias, J.; D’Croz, L.; Jiménez-Antón, M.D.; Corral, M.J.; Cueto, M. Oxysterols from an octocoral of the genus Gorgonia from the eastern Pacific of Panama. J. RSC Adv. 2013, 6, 1–15. [Google Scholar] [CrossRef]
- Fabricius, K.; Alderslade, P. Soft Corals and Sea Fans: A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea; AIMS: Townsville, Australia, 2001; p. 264. [Google Scholar]
- Sorokin, Y.I. Biomass, metabolic rates and feeding of some common zoantharians and octocorals. Aust. J. Mar. Freshw. Res. 1991, 42, 729–741. [Google Scholar] [CrossRef]
- Chao, C.H.; Huang, L.F.; Wu, S.L.; Su, J.H.; Huang, H.C.; Sheu, J.H. Steroids from the gorgonian Isis Hippuris. J. Nat. Prod. 2005, 68, 1366–1370. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, S.K.; Duh, C.Y. Polyhydroxylated steroids from the bamboo coral Isis Hippuris. Tetrahedron 2002, 58, 6259–6266. [Google Scholar] [CrossRef]
- Sheu, J.H.; Chao, C.H.; Wang, G.H.; Hung, K.C.; Duh, C.Y. The first A-nor-hippuristanol and two novel 4,5-secosuberosanoids from the gorgonian Isis Hippuris. Tetrahedron Lett. 2004, 45, 6413–6416. [Google Scholar] [CrossRef]
- Rao, C.B.; Ramana, K.V.; Venkata Rao, D.; Fahy, E.; Faulkner, D.J. Metabolites of the Gorgonian Isis hippuris from India. J. Nat. Prod. 1988, 51, 954–958. [Google Scholar] [CrossRef]
- Anisatusholihah, A. The Effects of Gorgonian Isis hippuris on Histologic Grade Score of Adenocarcinoma Mammae Tissue in C3H Mice Strain. Ph.D. Dissertation, Diponegoro University, Semarang, Indonesia, 2009. [Google Scholar]
- Liang, C.H.; Chou, T.H.; Yang, C.C.; Hung, W.J.; Chan, L.C.; Cheng, D.L.; Wang, G.H. Cytotoxic effect of Discosoma sp., Isis hippuris and Nephthea chabrolii on human oral SCC25 cells. J. Taiwan Inst. Chem. Eng. 2010, 41, 333–337. [Google Scholar] [CrossRef]
- Putra, M.Y.; Wibowo, J.T.; Murniasih, T. A review of chemistry and biological activities of the Indonesian Octocorallia. J. Appl. Pharm. Sci. 2017, 7, 219–227. [Google Scholar]
- Sayuti, M.; Rukmi, W.D.; Yunianta, P. Secondary metabolites and antioxidant potentials of axis sea Bamboo (Isis hippuris). Dhaka Univ. J. Pharm. Sci. 2019, 18, 13–20. [Google Scholar] [CrossRef]
- Chao, C.H.; Huang, L.F.; Yang, Y.L.; Su, J.H.; Wang, G.H.; Chiang, M.Y.; Wu, Y.C.; Dai, C.F.; Sheu, J.H. Polyoxygenated steroids from the Gorgonian Isis hippuris. J. Nat. Prod. 2005, 68, 880–885. [Google Scholar] [CrossRef]
- Brown, A.C.; Fraser, T.R. The connection of chemical constitution and physiological action. Trans. Roy. Soc. Edinburg 1868, 25, 224–242. [Google Scholar]
- Parascandola, J. The controversy over structure-activity relationships in the early twentieth century. Pharm. Hist. 1974, 16, 54–63. [Google Scholar]
- Barlow, R.B. Structure-activity relationships. Trends Pharm. Sci. 1979, 1, 109–111. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A. Substituent Constants for Correlation Analysis in Chemistry and Biology; John Wiley & Sons: New York, NY, USA, 1979. [Google Scholar]
- Schultz, T.W.; Cronin, M.T.D.; Walker, J.D.; Aptula, A.O. Quantitative structure–activity relationships (QSARs) in toxicology: A historical perspective. J. Mol. Struct. Theochem. 2003, 622, 1–22. [Google Scholar] [CrossRef]
- Allen, T.E.H.; Goodman, J.M.; Gutsell, S.; Russell, P.J. Quantitative predictions for molecular initiating events using three-dimensional quantitative structure–activity relationships. Chem. Res. Toxicol. 2020, 33, 324–333. [Google Scholar] [CrossRef]
- Sliwoski, G.; Kothiwale, S.; Meiler, J.; Lowe, E.W., Jr. Computational methods in drug discovery. Pharm. Rev. 2014, 66, 334–395. [Google Scholar] [CrossRef]
- Leelananda, S.P.; Lindert, S. Computational methods in drug discovery. Beilstein J. Org. Chem. 2016, 12, 2694–2718. [Google Scholar] [CrossRef]
- Kokh, D.B.; Amaral, M.; Bomke, J.; Grädler, U.; Musil, D. Estimation of drug-target residence times by τ-random acceleration molecular dynamics simulations. J. Chem. Theor. Comput. 2018, 14, 3859–3869. [Google Scholar] [CrossRef]
- Cherkasov, A.M.; Muratov, E.N.; Fourches, D.; Varnek, A.; Baskin, I.I.; Cronin, M.; Dearden, J. QSAR modeling: Where have you been? Where are you going to? J. Med. Chem. 2014, 57, 4977–5010. [Google Scholar] [CrossRef]
- Burov, Y.V.; Poroikov, V.V.; Korolchenko, L.V. National system for registration and biological testing of chemical compounds: Facilities for new drugs search. Bull. Natl. Center Biol. Active Comp. 1990, 1, 4–25. [Google Scholar]
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.; Filimonov, D.; Poroikov, V.; Oprea, T. QSAR without Borders. Chem. Soc. Rev. 2020, 49, 3525–3564. [Google Scholar] [CrossRef]
- Poroikov, V.V.; Filimonov, D.A.; Gloriozova, T.A.; Lagunin, A.A.; Druzhilovskiy, D.S.; Rudik, A.V. Computer-aided prediction of biological activity spectra for organic compounds: The possibilities and limitations. Russ. Chem. Bull. 2019, 68, 2143–2154. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Druzhilovskiy, D.S.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Dmitriev, A.V.; Pogodin, P.V.; Poroikov, V.V. Computer aided prediction of biological activity spectra for chemical compounds: Opportunities and limitations. Biom. Chem. Res. Method 2018, 1, e00004. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskiy, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Anusevicius, K.; Mickevicius, V.; Stasevych, M.; Zvarych, V.; Komarovska-Porokhnyavets, O.; Novikov, V.; Tarasova, O.; Gloriozova, T.; Poroikov, V. Design, synthesis, in vitro antimicrobial activity evaluation and computational studies of new N-(4-iodophenyl)-β-alanine derivatives. Res. Chem. Intermed. 2015, 41, 7517–7540. [Google Scholar] [CrossRef]
- Druzhilovskiy, D.S.; Rudik, A.V.; Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Poroikov, V.V. Online resources for the prediction of biological activity of organic compounds. Rus. Chem. Bull. 2016, 65, 384–393. [Google Scholar] [CrossRef]
- PASS Online URL. Available online: http://www.way2drug.com/passonline/ (accessed on 20 October 2020).
- Lagunin, A.A.; Goel, R.K.; Gawande, D.Y.; Priynka, P.; Gloriozova, T.A.; Dmitriev, A.V.; Ivanov, S.M.; Rudik, A.V.; Konova, V.I.; Pogodin, P.V.; et al. Chemo- and bioinformatics resources for in silico drug discovery from medicinal plants beyond their traditional use: A critical review. Nat. Prod. Rep. 2014, 31, 1585–1611. [Google Scholar] [CrossRef]
- Savidov, N.; Gloriozova, T.A.; Poroikov, V.V.; Dembitsky, V.M. Highly oxygenated isoprenoid lipids derived from fungi and fungal endophytes: Origin and biological activities. Steroids 2018, 140, 114–124. [Google Scholar] [CrossRef]
- Gawande, D.Y.; Druzhilovsky, D.; Gupta, R.C.; Poroikov, V.; Goel, R.K. Anticonvulsant activity and acute neurotoxic profile of Achyranthes aspera Linn. J. Ethnopharmacol. 2017, 202, 97–102. [Google Scholar] [CrossRef]
- Goel, R.K.; Poroikov, V.; Gawande, D.; Lagunin, A.; Randhawa, P.; Mishra, A. Revealing medicinal plants useful for comprehensive management of epilepsy and associated co-morbidities through in silico mining of their phytochemical diversity. Planta Med. 2015, 81, 495–506. [Google Scholar]
- Vil, V.; Terent’ev, A.O.; Al Quntar, A.A.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M. Oxetane-containing metabolites: Origin, structures and biological activities. Appl. Microbiol. Biotechnol. 2019, 103, 2449–2467. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Dzhemileva, L.; Gloriozova, T.; D’yakonov, V. Natural and synthetic drugs used for the treatment of the dementia. Biochem. Biophys. Res. Commun. 2020, 523, 772–783. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Naturally occurring isocyano/isothiocyanato compounds: Their pharmacological and SAR activities. Mathews J. Pharm. Sci. 2016, 1, 003. [Google Scholar]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Biological activities of organometalloid (As, At, B, Ge, Si, Se, Te) steroids. J. Appl. Pharm. Sci. 2017, 7, 184–202. [Google Scholar]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Pharmacological profile of natural and synthetic compounds with rigid adamantane-based scaffolds as potential agents for the treatment of neurodegenerative diseases. Biochem. Biophys. Res. Commun. 2020, 529, 1225–1241. [Google Scholar] [CrossRef]
- Herbey, I.I.; Ivankova, N.V.; Katkoori, V.R.; Mamaeva, O.A. Colorectal cancer and hypercholesterolemia: Review of current research. Exp. Oncol. 2005, 27, 166–178. [Google Scholar]
- Papanagnou, P.; Stivarou, T.; Papageorgiou, I.; Papadopoulos, G.E.; Pappas, A. Marketed drugs used for the management of hypercholesterolemia as anticancer armament. Onco Targets Ther. 2017, 10, 4393–4411. [Google Scholar] [CrossRef]
- Levitsky, D.O.; Dembitsky, V.M. Anti-breast cancer agents derived from plants. Nat. Prod. Bioprospect. 2015, 5, 1–16. [Google Scholar] [CrossRef]
- Cho, J.H.; Weinstein, D.A.; Lee, Y.M. Emerging roles of autophagy in hepatic tumorigenesis and therapeutic strategies in glycogen storage disease type IA: A review. J. Inherit. Metab. 2020. [Google Scholar] [CrossRef]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and breast cancer: A literature review on prevention, treatment and recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef]
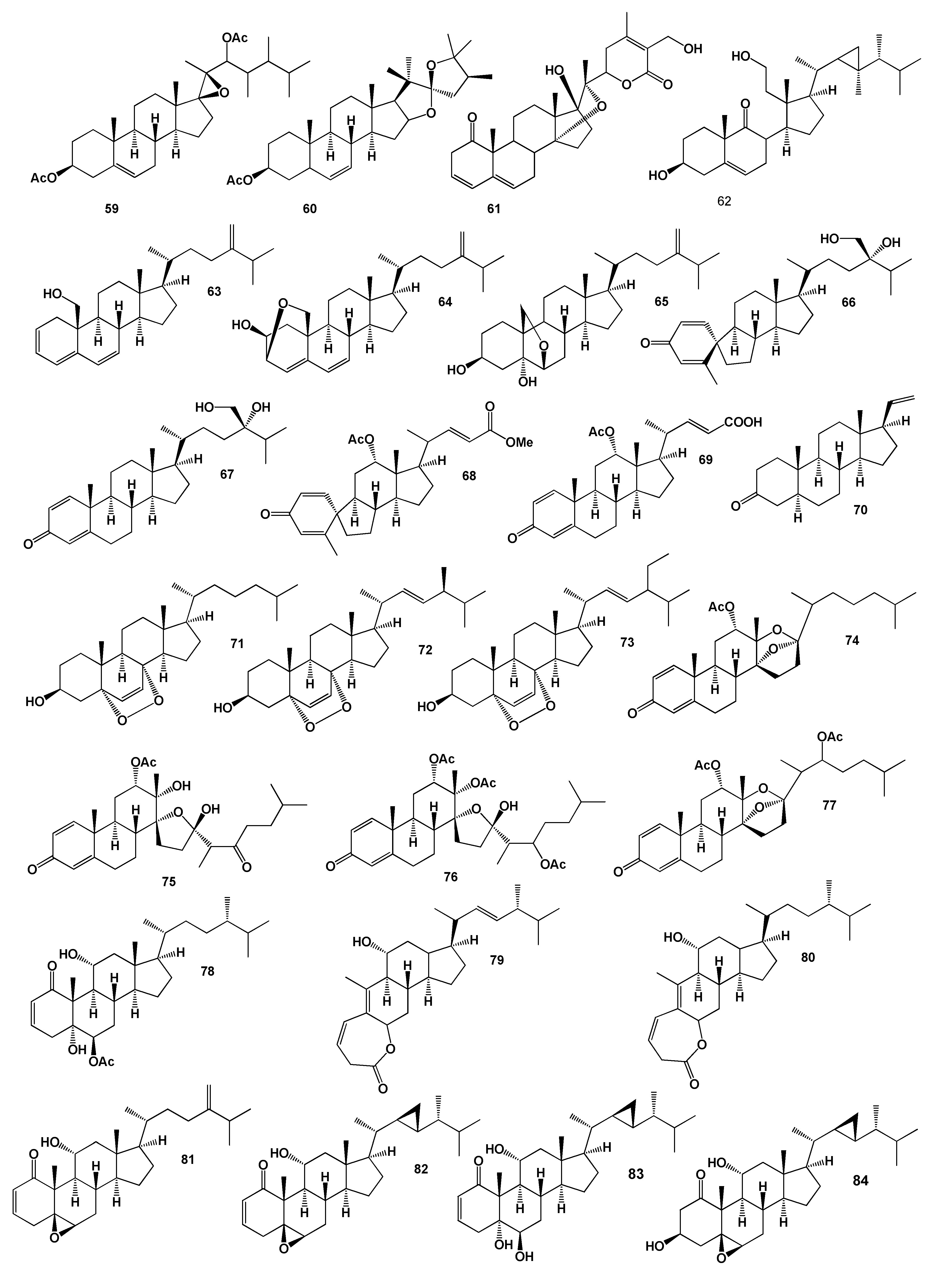
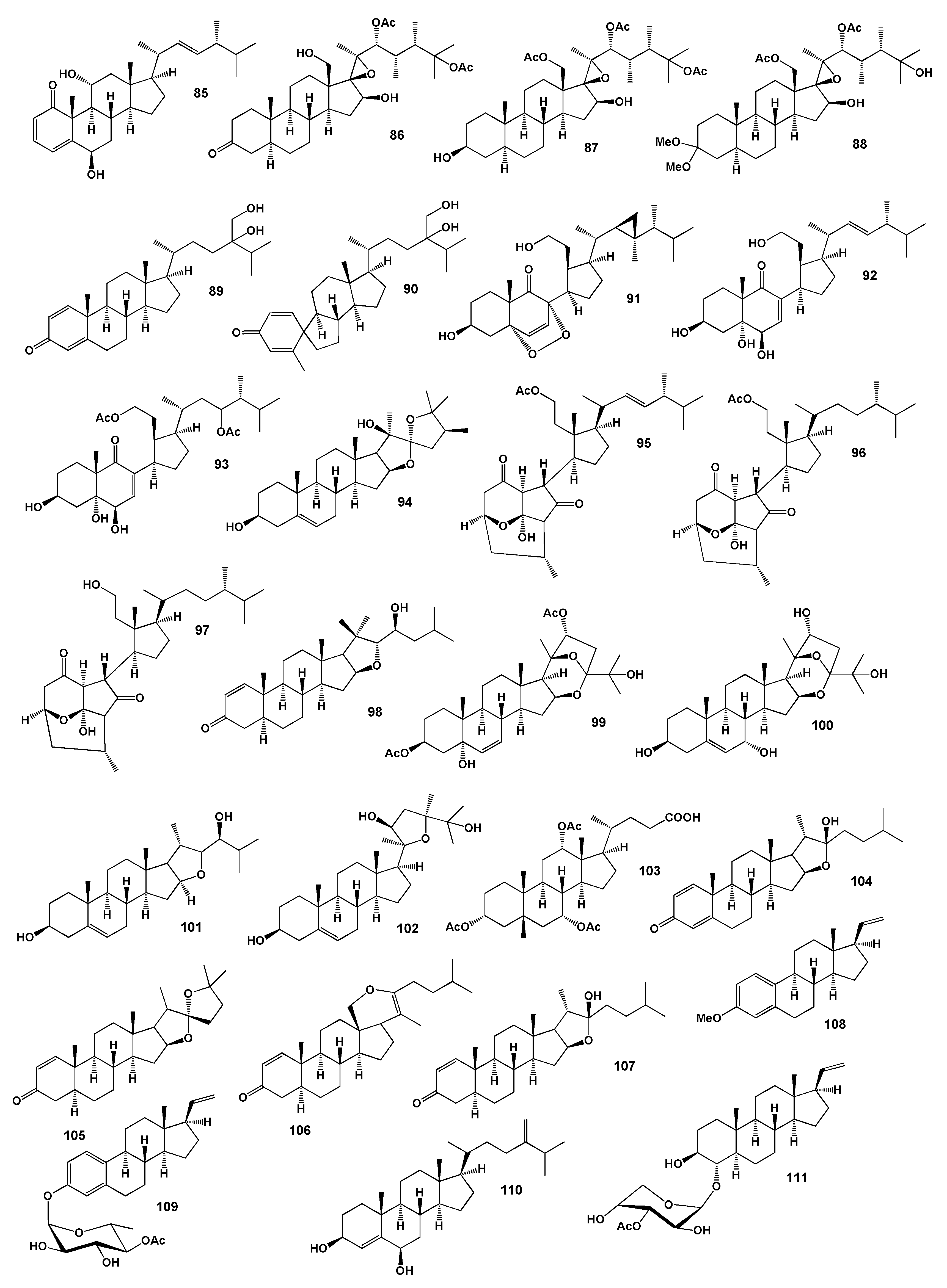
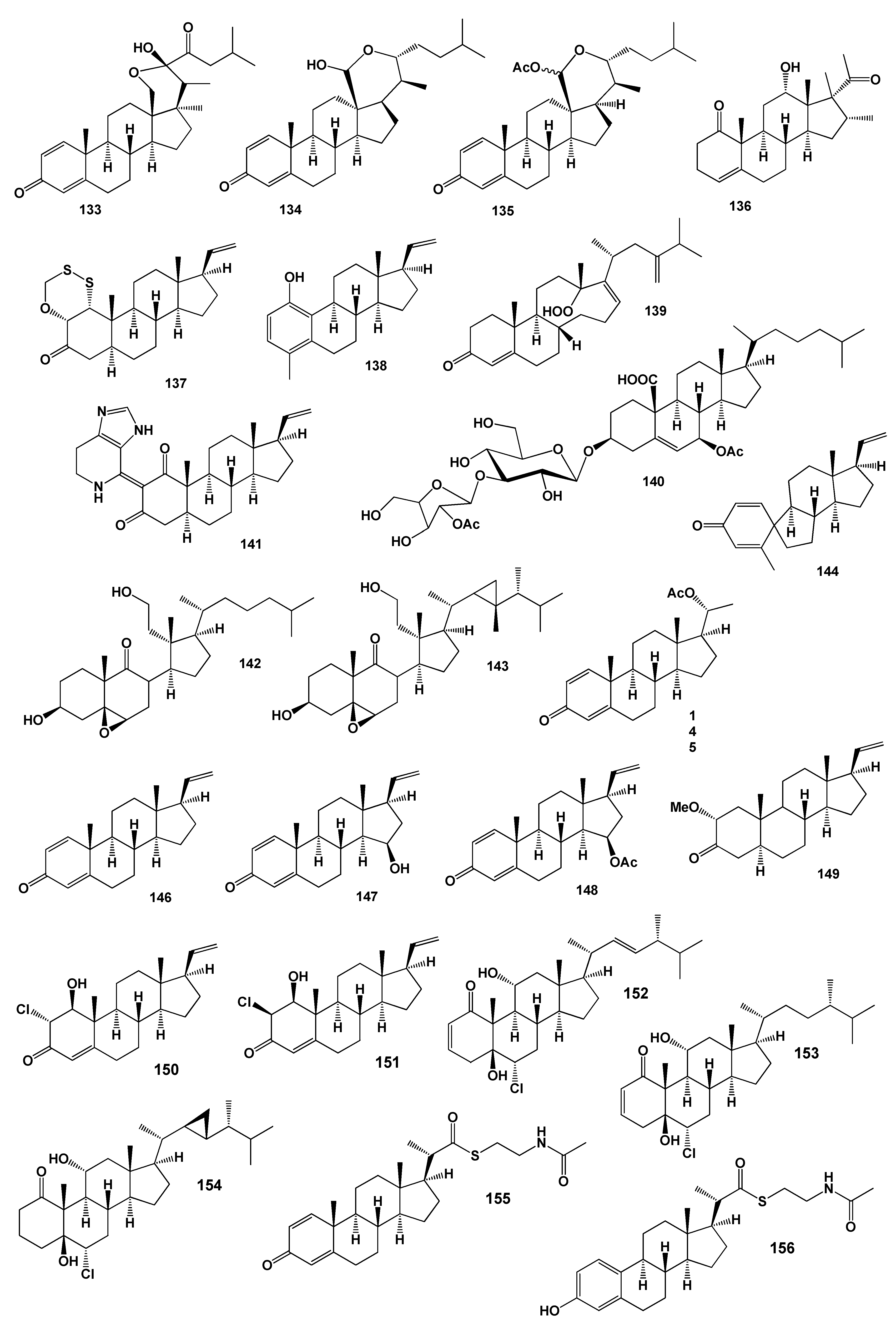
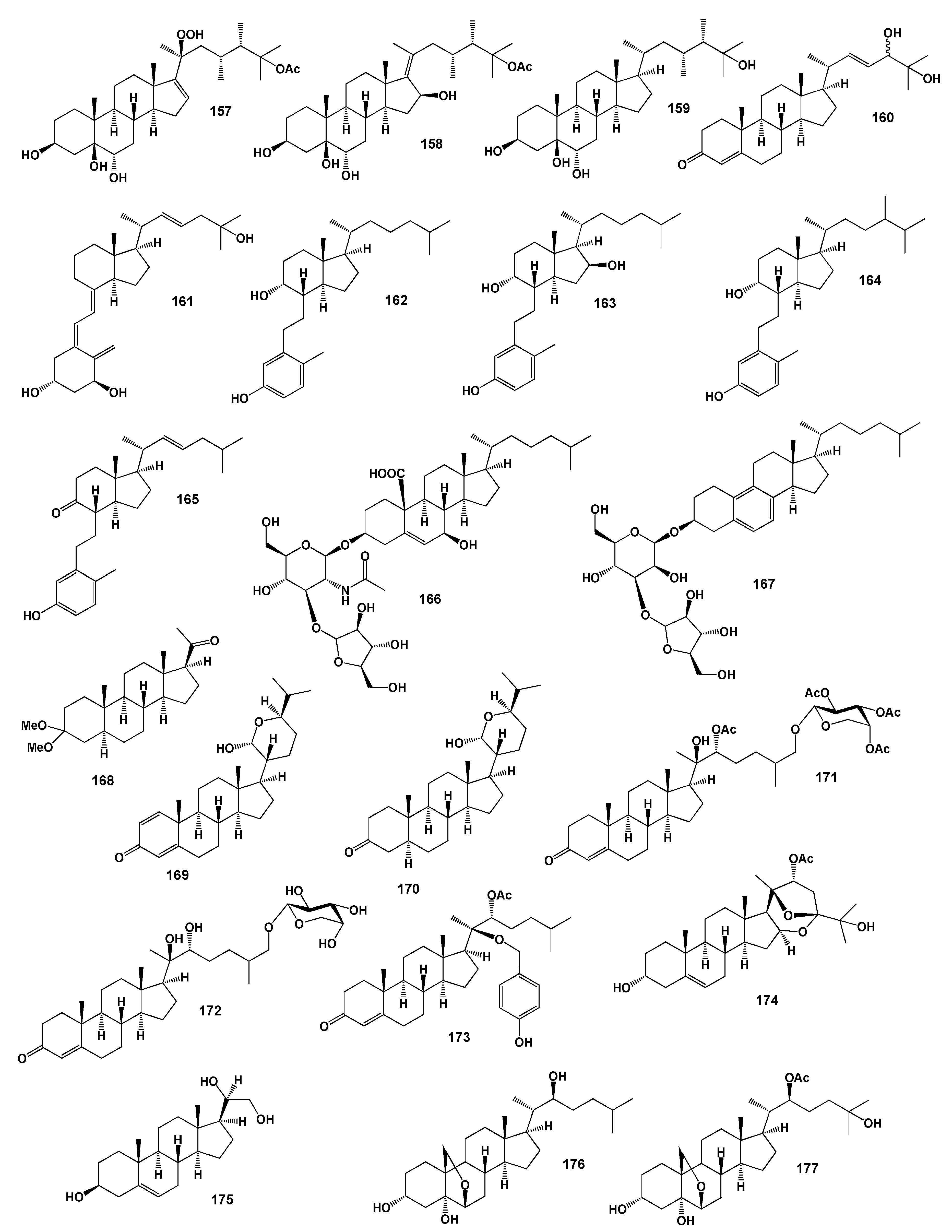
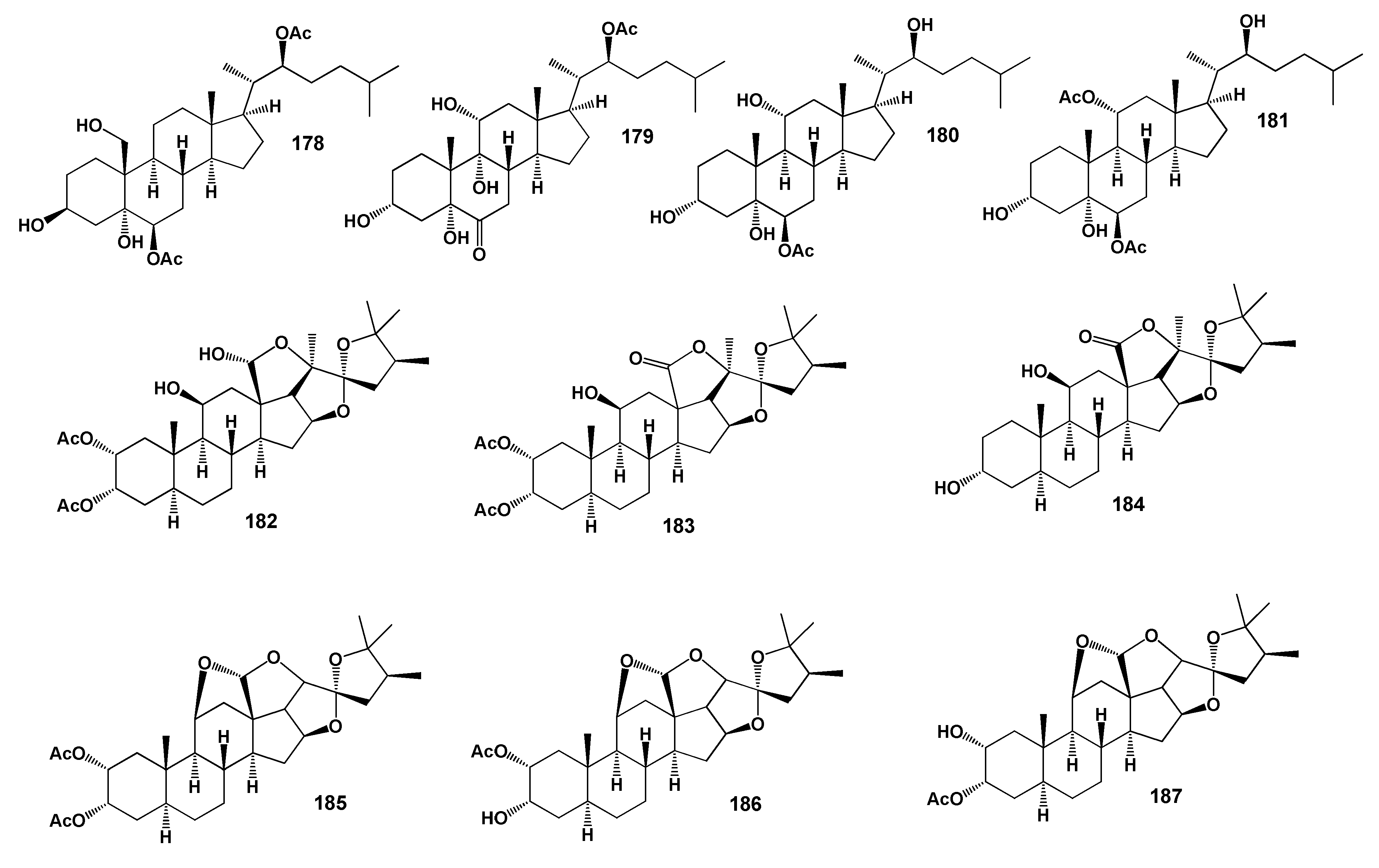
| No. | Antitumor and Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Biological Activity, (Pa) * |
|---|---|---|---|
| 1 | Antineoplastic (0.840) Chemopreventive (0.806) Proliferative diseases treatment (0.705) Antimetastatic (0.562) Anticarcinogenic (0.542) | Anti-hypercholesterolemic (0.920) Hypolipemic (0.798) Atherosclerosis treatment (0.637) Hyperparathyroidism treatment (0.523) | Respiratory analeptic (0.879) Anesthetic general (0.851) Antifungal (0.792) Anti-inflammatory (0.754) Antibacterial (0.545) |
| 2 | Antineoplastic (0.833) Chemopreventive (0.738) Proliferative diseases treatment (0.643) Antimetastatic (0.626) | Anti-hypercholesterolemic (0.840) Hypolipemic (0.646) Atherosclerosis treatment (0.634) | Anti-inflammatory (0.806) Anesthetic general (0.800) Respiratory analeptic (0.789) Antifungal (0.616) |
| 3 | Antineoplastic (0.765) Dementia treatment (0.512) | Anti-hypercholesterolemic (0.655) Hypolipemic (0.530) | Immunosuppressant (0.735) Anti-inflammatory (0.667) |
| 4 | Antineoplastic (0.798) Apoptosis agonist (0.751) Antimetastatic (0.524) | Hypolipemic (0.732) Cholesterol synthesis inhibitor (0.545) Antileukemic (0.539) | Immunosuppressant (0.759) Antifungal (0.748) Antibacterial (0.642) |
| 5 | Proliferative diseases treatment (0.967) Chemopreventive (0.958) Anticarcinogenic (0.907) Antineoplastic (0.902) Dementia treatment (0.508) | Anti-hypercholesterolemic (0.947) Hypolipemic (0.781) Atherosclerosis treatment (0.609) | Respiratory analeptic (0.992) Anti-inflammatory (0.900) Antifungal (0.880) Neuroprotector (0.849) Antibacterial (0.797) |
| 6 | Chemopreventive (0.839) Antineoplastic (0.834) Proliferative diseases treatment (0.716) Antimetastatic (0.573) | Anti-hypercholesterolemic (0.937) Hypolipemic (0.809) Neuroprotector (0.767) Atherosclerosis treatment (0.592) | Respiratory analeptic (0.896) Anesthetic general (0.828) Anti-inflammatory (0.778) Antifungal (0.768) |
| 7 | Antineoplastic (0.861) Chemopreventive (0.827) Proliferative diseases treatment (0.779) Anticarcinogenic (0.565) Antimetastatic (0.560) | Anti-hypercholesterolemic (0.883) Hypolipemic (0.771) Atherosclerosis treatment (0.675) | Respiratory analeptic (0.868) Antifungal (0.821) Anti-inflammatory (0.755) Antibacterial (0.603) Antimycobacterial (0.569) |
| 8 | Antineoplastic (0.852) Apoptosis agonist (0.801) Proliferative diseases treatment (0.766) Anticarcinogenic (0.732) | Anti-hypercholesterolemic (0.955) Hypolipemic (0.878) Neuroprotector (0.788) Atherosclerosis treatment (0.658) | Respiratory analeptic (0.856) Antifungal (0.780) Anti-inflammatory (0.750) Antimycobacterial (0.653) |
| 9 | Chemopreventive (0.902) Antineoplastic (0.858) Proliferative diseases treatment (0.821) | Anti-hypercholesterolemic (0.911) Atherosclerosis treatment (0.548) Hypolipemic (0.548) | Anti-inflammatory (0.896) Antifungal (0.765) |
| 10 | Antineoplastic (0.776) Apoptosis agonist (0.601) | Antiprotozoal (Plasmodium) (0.778) Anti-inflammatory (0.765) | |
| 11 | Antineoplastic (0.807) Apoptosis agonist (0.747) Proliferative diseases treatment (0.596) | Anti-hypercholesterolemic (0.630) | Anti-inflammatory (0.930) Anti-asthmatic (0.627) |
| 12 | Antineoplastic (0.838) | Anti-inflammatory (0.792) | |
| 13 | Apoptosis agonist (0.790) Antineoplastic (0.781) | Respiratory analeptic (0.772) Immunosuppressant (0.744) Antifungal (0.708) | |
| 14 | Antineoplastic (0.824) Proliferative diseases treatment (0.610) | Anti-hypercholesterolemic (0.909) Lipid metabolism regulator (0.672) | Respiratory analeptic (0.907) Anti-inflammatory (0.673) |
| 15 | Antineoplastic (0.865) Proliferative diseases treatment (0.812) Apoptosis agonist (0.767) Anticarcinogenic (0.730) | Anti-hypercholesterolemic (0.930) Hypolipemic (0.827) Atherosclerosis treatment (0.646) | Respiratory analeptic (0.970) Anti-inflammatory (0.904) Anesthetic general (0.850) Antibacterial (0.586) |
| 16 | Antineoplastic (0.838) Proliferative diseases treatment (0.729) Antimetastatic (0.651) | Anti-hypercholesterolemic (0.909) Hypolipemic (0.786) | Respiratory analeptic (0.937) Antifungal (0.789) Cardiotonic (0.626) |
| 17 | Antineoplastic (0.730) | Anti-hypercholesterolemic (0.679) | Immunosuppressant (0.722) |
| 18 | Antineoplastic (0.925) Proliferative diseases treatment (0.787) Prostate cancer treatment (0.670) | Anti-hypercholesterolemic (0.913) Hypolipemic (0.626) Lipid metabolism regulator (0.566) | Autoimmune disorders treatment (0.908) Respiratory analeptic (0.780) Antifungal (0.525) |
| No. | Antitumor and Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Biological Activity, (Pa) * |
|---|---|---|---|
| 19 | Antineoplastic (0.759) Antimetastatic (0.661) | Anti-hypercholesterolemic (0.800) Hypolipemic (0.653) | Anesthetic general (0.768) Antibacterial (0.615) |
| 20 | Apoptosis agonist (0.941) Antineoplastic (0.761) Chemopreventive (0.687) | Atherosclerosis treatment (0.747) Lipoprotein disorders treatment (0.567) | Antiprotozoal (Plasmodium) (0.791) Antioxidant (0.650) Antifungal (0.634) |
| 21 | Apoptosis agonist (0.982) Chemopreventive (0.945) Antineoplastic (0.920) Prostate cancer treatment (0.680) | Atherosclerosis treatment (0.909) Hypolipemic (0.831) Lipoprotein disorders treatment (0.816) Anti-hypercholesterolemic (0.799) | Antiprotozoal (Plasmodium) (0.845) Immunosuppressant (0.733) Antiparkinsonian, rigidity relieving (0.730) |
| 22 | Apoptosis agonist (0.969) Antineoplastic (0.897) Chemopreventive (0.880) Antimetastatic (0.645) Proliferative diseases treatment (0.513) | Atherosclerosis treatment (0.841) Lipoprotein disorders treatment (0.701) Hypolipemic (0.645) Anti-hypercholesterolemic (0.559) | Antiprotozoal (Plasmodium) (0.828) Antioxidant (0.670) Immunosuppressant (0.653) Antifungal (0.619) Anti-inflammatory (0.523) |
| 23 | Apoptosis agonist (0.962) Chemopreventive (0.751) Antineoplastic (0.740) | Atherosclerosis treatment (0.626) | Antifungal (0.630) Antiprotozoal (Plasmodium) (0.623) Antileukemic (0.552) |
| 24 | Apoptosis agonist (0.994) Chemopreventive (0.966) Antineoplastic (0.905) Prostate cancer treatment (0.546) | Atherosclerosis treatment (0.833) Hypolipemic (0.785) Anti-hypercholesterolemic (0.746) Lipoprotein disorders treatment (0.594) | Antiprotozoal (Plasmodium) (0.693) Immunosuppressant (0.692) Antiparkinsonian, rigidity relieving (0.672) |
| 25A | Antineoplastic (0.816) Proliferative diseases treatment (0.577) | Anti-hypercholesterolemic (0.788) Hypolipemic (0.655) | Antiprotozoal (Plasmodium) (0.868) Immunosuppressant (0.766) |
| 25B | Antineoplastic (0.819) Proliferative diseases treatment (0.581) | Anti-hypercholesterolemic (0.784) Hypolipemic (0.635) | Antiprotozoal (Plasmodium) (0.861) Immunosuppressant (0.756) |
| 26 | Antineoplastic (0.758) Chemopreventive (0.757) Proliferative diseases treatment (0.642) Prostate cancer treatment (0.598) | Anti-hypercholesterolemic (0.867) Anxiolytic (0.736) Hypolipemic (0.640) Atherosclerosis treatment (0.526) | Respiratory analeptic (0.809) Immunosuppressant (0.711) Anti-inflammatory (0.689) Antifungal (0.560) |
| 27 | Antineoplastic (0.886) Apoptosis agonist (0.856) | Anti-hypercholesterolemic (0.686) Atherosclerosis treatment (0.630) | Antifungal (0.739) Anti-inflammatory (0.696) |
| 28 | Antineoplastic (0.876) Apoptosis agonist (0.874) Chemopreventive (0.696) | Hypolipemic (0.724) Anti-hypercholesterolemic (0.677) Atherosclerosis treatment (0.620) | Immunosuppressant (0.669) Anesthetic (0.570) Anti-inflammatory (0.553) |
| 29 | Antineoplastic (0.763) Chemopreventive (0.651) Proliferative diseases treatment (0.541) | Anti-hypercholesterolemic (0.831) Hypolipemic (0.625) Prostate disorders treatment (0.536) | Respiratory analeptic (0.625) Antibacterial (0.573) Antifungal (0.558) |
| 30 | Antineoplastic (0.807) Proliferative diseases treatment (0.651) | Anti-hypercholesterolemic (0.915) Lipid metabolism regulator (0.768) | Anti-inflammatory (0.736) Antibacterial (0.582) |
| 31 | Antineoplastic (0.885) | Hypolipemic (0.616) | Anti-inflammatory (0.911) |
| 32 | Antineoplastic (0.910) Proliferative diseases treatment (0.882) | Anti-hypercholesterolemic (0.955) Atherosclerosis treatment (0.596) | Respiratory analeptic (0.959) Antiprotozoal (Plasmodium) (0.667) |
| 33 | Antineoplastic (0.831) Proliferative diseases treatment (0.698) Apoptosis agonist (0.593) | Anti-hypercholesterolemic (0.903) Hypolipemic (0.685) Lipid metabolism regulator (0.546) | Anti-inflammatory (0.870) Antifungal (0.783) Neuroprotector (0.636) |
| 34 | Apoptosis agonist (0.864) Antineoplastic (0.860) Proliferative diseases treatment (0.700) | Anti-hypercholesterolemic (0.889) Atherosclerosis treatment (0.699) Lipoprotein disorders treatment (0.587) | Antifungal (0.769) Anti-inflammatory (0.735) Antibacterial (0.550) |
| 35 | Antineoplastic (0.805) | Hypolipemic (0.529) | Immunosuppressant (0.640) |
| 36 | Antineoplastic (0.883) Apoptosis agonist (0.848) Proliferative diseases treatment (0.655) | Hypolipemic (0.795) Anti-hypercholesterolemic (0.772) Atherosclerosis treatment (0.666) | Anti-inflammatory (0.763) Antifungal (0.753) Antibacterial (0.588) |
| No. | Antitumor and Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Biological Activity, (Pa) * |
|---|---|---|---|
| 37 | Antineoplastic (0.915) Proliferative diseases treatment (0.722) | Anti-hypercholesterolemic (0.910) Lipid metabolism regulator (0.675) | Autoimmune disorders treatment (0.889) Neuroprotector (0.700) |
| 38 | Antineoplastic (0.895) Proliferative diseases treatment (0.673) | Anti-hypercholesterolemic (0.910) Atherosclerosis treatment (0.695) Lipid metabolism regulator (0.575) | Autoimmune disorders treatment (0.874) Anti-inflammatory (0.717) Neuroprotector (0.700) |
| 39 | Antineoplastic (0.903) Proliferative diseases treatment (0.768) Dementia treatment (0.554) | Anti-hypercholesterolemic (0.947) Lipid metabolism regulator (0.768) Hypolipemic (0.713) | Autoimmune disorders treatment (0.829) Neuroprotector (0.835) Anti-inflammatory (0.786) |
| 40 | Apoptosis agonist (0.823) Antineoplastic (0.815) Proliferative diseases treatment (0.610) | Hypolipemic (0.901) Anti-hypercholesterolemic (0.754) Atherosclerosis treatment (0.582) | Antifungal (0.737) Anti-inflammatory (0.705) Antibacterial (0.597) |
| 41 | Apoptosis agonist (0.845) Antineoplastic (0.823) Proliferative diseases treatment (0.678) | Hypolipemic (0.790) Anti-hypercholesterolemic (0.687) Atherosclerosis treatment (0.659) | Anti-fungal (0.761) Anti-inflammatory (0.735) |
| 42 | Apoptosis agonist (0.851) Antineoplastic (0.824) Proliferative diseases treatment (0.739) | Hypolipemic (0.769) Anti-hypercholesterolemic (0.733) Atherosclerosis treatment (0.631) | Antifungal (0.783) Anti-inflammatory (0.775) Antibacterial (0.543) |
| 43 | Antineoplastic (0.890) Apoptosis agonist (0.743) Proliferative diseases treatment (0.551) | Anti-hypercholesterolemic (0.793) Hypolipemic (0.767) Atherosclerosis treatment (0.638) | Anti-inflammatory (0.728) Antifungal (0.725) Immunosuppressant (0.704) |
| 44 | Antineoplastic (0.841) Apoptosis agonist (0.770) Proliferative diseases treatment (0.760) | Anti-hypercholesterolemic (0.900) Hypolipemic (0.843) Atherosclerosis treatment (0.620) | Cardiotonic (0.872) Anesthetic general (0.847) Antifungal (0.787) |
| 45 | Antineoplastic (0.838) Proliferative diseases treatment (0.729) Antimetastatic (0.551) | Anti-hypercholesterolemic (0.909) Hypolipemic (0.786) | Antifungal (0.789) Anti-inflammatory (0.674) Cardiotonic (0.626) |
| 46 | Antineoplastic (0.665) Antimetastatic (0.626) | Antiprotozoal (Plasmodium) (0.854) Antifungal (0.642) | |
| 47 | Antineoplastic (0.790) Proliferative diseases treatment (0.652) | Anti-hypercholesterolemic (0.963) Atherosclerosis treatment (0.742) | Antifungal (0.798) Anti-inflammatory (0.722) |
| 48 | Proliferative diseases treatment (0.953) Anticarcinogenic (0.922) Antineoplastic (0.911) | Anti-hypercholesterolemic (0.962) Hypolipemic (0.825) Atherosclerosis treatment (0.588) | Neuroprotector (0.976) Antimycobacterial (0.929) Antifungal (0.885) |
| 49 | Chemopreventive (0.960) Proliferative diseases treatment (0.945) Anticarcinogenic (0.881) Antineoplastic (0.877) | Anti-hypercholesterolemic (0.964) Hypolipemic (0.797) | Antimycobacterial (0.936) Antifungal (0.847) Anti-inflammatory (0.823) Neuroprotector (0.757) |
| 50 | Chemopreventive (0.953) Proliferative diseases treatment (0.944) Antineoplastic (0.858) | Anti-hypercholesterolemic (0.943) Hypolipemic (0.846) Atherosclerosis treatment (0.510) | Respiratory analeptic (0.957) Neuroprotector (0.892) Antifungal (0.873) |
| 51 | Proliferative diseases treatment (0.976) Chemopreventive (0.959) Antineoplastic (0.906) Dementia treatment (0.631) | Anti-hypercholesterolemic (0.976) Hypolipemic (0.735) Lipid metabolism regulator (0.707) | Respiratory analeptic (0.992) Neuroprotector (0.953) Antifungal (0.838) Antiviral (Influenza) (0.653) |
| 52 | Antineoplastic (0.840) Apoptosis agonist (0.581) | Hypolipemic (0.682) Cholesterol synthesis inhibitor (0.508) | Immunosuppressant (0.671) Nootropic (0.548) |
| 53 | Antineoplastic (0.790) Proliferative diseases treatment (0.667) | Anti-hypercholesterolemic (0.729) Cholesterol synthesis inhibitor (0.664) | Anti-osteoporotic (0.965) Anti-eczematic (0.910) |
| 54 | Antineoplastic (0.790) Proliferative diseases treatment (0.767) | Anti-hypercholesterolemic (0.729) Cholesterol synthesis inhibitor (0.614) | Anti-osteoporotic (0.965) Anti-eczematic (0.910) |
| 55 | Antineoplastic (0.948) Alzheimer’s disease treatment (0.851) Apoptosis agonist (0.696) | Anti-hypercholesterolemic (0.821) Neurodegenerative diseases treatment (0.815) Cholesterol synthesis inhibitor (0.741) | Immunomodulator (HIV) (0.893) Antioxidant (0.821) |
| 56 | Chemopreventive (0.918) Proliferative diseases treatment (0.874) Antineoplastic (0.845) | Anti-hypercholesterolemic (0.914) Acute neurologic disorders treatment (0.632) Atherosclerosis treatment (0.612) | Anti-inflammatory (0.870) Immunosuppressant (0.818) |
| 57 | Antineoplastic (0.784) Apoptosis agonist (0.654) Prostate disorders treatment (0.625) | Antiprotozoal (Plasmodium) (0.766) Anti-inflammatory (0.752) Immunosuppressant (0.705) | |
| 58 | Antineoplastic (0.795) Apoptosis agonist (0.711) Prostate disorders treatment (0.633) | Antiprotozoal (Plasmodium) (0.754) Anti-inflammatory (0.732) Immunosuppressant (0.712) |
| No. | Antitumor and Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional biological activity, (Pa) * |
|---|---|---|---|
| 59 | Antineoplastic (0.886) Apoptosis agonist (0.648) | Anti-hypercholesterolemic (0.819) Cholesterol synthesis inhibitor (0.570) | Respiratory analeptic (0.888) Immunomodulator (HIV) (0.850) |
| 60 | Antineoplastic (0.843) Apoptosis agonist (0.780) Dementia treatment (0.528) | Neuroprotector (0.611) Nootropic (0.587) Hypolipemic (0.536) | Respiratory analeptic (0.835) |
| 61 | Antineoplastic (0.892) Apoptosis agonist (0.639) | Anti-inflammatory (0.599) | |
| 62 | Antineoplastic (skin cancer) (0.650) Antineoplastic (0.650) | Anti-inflammatory (0.608) Immunomodulator (HIV) (0.568) | |
| 63 | Antineoplastic (0.788) Apoptosis agonist (0.628) | Hypolipemic (0.738) Cholesterol synthesis inhibitor (0.660) | Antifungal (0.699) Anti-inflammatory (0.623) |
| 64 | Antineoplastic (0.824) Apoptosis agonist (0.634) | Hypolipemic (0.707) Cholesterol synthesis inhibitor (0.533) | Antifungal (0.777) |
| 65 | Antineoplastic (0.841) Proliferative diseases treatment (0.732) Apoptosis agonist (0.713) | Anti-hypercholesterolemic (0.848) Hypolipemic (0.762) Cholesterol synthesis inhibitor (0.615) | Respiratory analeptic (0.947) Immunosuppressant (0.834) |
| 66 | Antineoplastic (0.761) Proliferative diseases treatment (0.575) | Anti-hypercholesterolemic (0.824) Atherosclerosis treatment (0.636) | Respiratory analeptic (0.833) |
| 67 | Chemopreventive (0.882) Antineoplastic (0.830) Proliferative diseases treatment (0.738) | Anti-hypercholesterolemic (0.884) Atherosclerosis treatment (0.679) | Anti-inflammatory (0.860) Respiratory analeptic (0.848) |
| 68 | Antineoplastic (0.744) | Anti-inflammatory (0.691) | |
| 69 | Antineoplastic (0.875) Proliferative diseases treatment (0.856) | Anti-hypercholesterolemic (0.918) Atherosclerosis treatment (0.618) | Anti-inflammatory (0.903) Nootropic (0.654) |
| 70 | Antineoplastic (0.822) Prostate disorders treatment (0.796) | Anti-hypercholesterolemic (0.634) | Erythropoiesis stimulant (0.858) Immunomodulator (HIV) (0.858) |
| 71 | Apoptosis agonist (0.964) Chemopreventive (0.906) Antineoplastic (0.854) | Atherosclerosis treatment (0.865) Lipoprotein disorders treatment (0.761) Anti-hypercholesterolemic (0.694) | Antiprotozoal (Plasmodium) (0.876) Respiratory analeptic (0.766) |
| 72 | Apoptosis agonist (0.982) Chemopreventive (0.945) Antineoplastic (0.920) Antiparkinsonian, rigidity relieving (0.730) | Atherosclerosis treatment (0.909) Hypolipemic (0.831) Lipoprotein disorders treatment (0.816) Anti-hypercholesterolemic (0.799) | Antiprotozoal (Plasmodium) (0.845) |
| 73 | Apoptosis agonist (0.970) Chemopreventive (0.929) Antineoplastic (0.883) Antiparkinsonian, rigidity relieving (0.614) | Atherosclerosis treatment (0.904) Anti-hypercholesterolemic (0.856) Hypolipemic (0.838) Lipoprotein disorders treatment (0.733) | Antiprotozoal (Plasmodium) (0.828) |
| 74 | Antineoplastic (0.891) Proliferative diseases treatment (0.600) | Hypolipemic (0.683) | Anti-inflammatory (0.816) Antiprotozoal (Plasmodium) (0.719) |
| 75 | Antineoplastic (0.932) Proliferative diseases treatment (0.611) | Anti-hypercholesterolemic (0.612) Autoimmune disorders treatment (0.587) | Anti-inflammatory (0.886) |
| 76 | Antineoplastic (0.933) Proliferative diseases treatment (0.623) | Anti-inflammatory (0.902) Respiratory analeptic (0.823) | |
| 77 | Antineoplastic (0.905) Chemopreventive (0.617) | Autoimmune disorders treatment (0.640) | Anti-inflammatory (0.863) Antiprotozoal (Plasmodium) (0.653) |
| 78 | Apoptosis agonist (0.854) Antineoplastic (0.787) | Anti-hypercholesterolemic (0.769) Atherosclerosis treatment (0.635) | Respiratory analeptic (0.941) |
| 79 | Antineoplastic (0.887) Apoptosis agonist (0.880) Chemopreventive (0.605) | Anti-hypercholesterolemic (0.808) Hypolipemic (0.787) Atherosclerosis treatment (0.689) | Anti-inflammatory (0.682) |
| 80 | Antineoplastic (0.799) Apoptosis agonist (0.701) | Atherosclerosis treatment (0.611) Cholesterol synthesis inhibitor (0.589) | Antifungal (0.751) Antithrombotic (0.558) |
| 81 | Antineoplastic (0.877) Apoptosis agonist (0.838) | Anti-hypercholesterolemic (0.638) Cholesterol synthesis inhibitor (0.628) | Respiratory analeptic (0.945) |
| 82 | Antineoplastic (0.857) | Antifungal (0.786) | |
| 83 | Antineoplastic (0.753) Alzheimer’s disease treatment (0.674) | Anti-inflammatory (0.733) Immunosuppressant (0.723) | |
| 84 | Antineoplastic (0.852) Apoptosis agonist (0.760) | Respiratory analeptic (0.871) Immunosuppressant (0.781) |
| No. | Antitumor and Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Biological Activity, (Pa) * |
|---|---|---|---|
| 85 | Apoptosis agonist (0.902) Antineoplastic (0.878) Antiparkinsonian, rigidity relieving (0.507) | Anti-hypercholesterolemic (0.894) Hypolipemic (0.785) Atherosclerosis treatment (0.650) | Anti-inflammatory (0.829) Immunosuppressant (0.799) |
| 86 | Antineoplastic (0.891) | Anti-hypercholesterolemic (0.841) Neuroprotector (0.727) | Angiogenesis inhibitor (0.910) Respiratory analeptic (0.848) |
| 87 | Antineoplastic (0.864) | Anti-hypercholesterolemic (0.836) Neuroprotector (0.806) | Anesthetic general (0.910) Angiogenesis inhibitor (0.895) |
| 88 | Antineoplastic (0.868) | Anti-hypercholesterolemic (0.623) Hypolipemic (0.521) | Angiogenesis inhibitor (0.765) Anti-asthmatic (0.630) |
| 89 | Chemopreventive (0.882) Antineoplastic (0.830) Apoptosis agonist (0.668) | Anti-hypercholesterolemic (0.884) Atherosclerosis treatment (0.679) Hypolipemic (0.623) | Anti-inflammatory (0.860) Respiratory analeptic (0.848) Anti-asthmatic (0.542) |
| 90 | Chemopreventive (0.761) Antineoplastic (0.761) | Anti-hypercholesterolemic (0.824) Atherosclerosis treatment (0.636) | Respiratory analeptic (0.833) Immunosuppressant (0.740) |
| 91 | Apoptosis agonist (0.767) Antineoplastic (0.597) | Hypolipemic (0.520) Atherosclerosis treatment (0.500) | Antiprotozoal (0.723) Immunosuppressant (0.534) |
| 92 | Antineoplastic (0.871) Apoptosis agonist (0.859) | Anti-hypercholesterolemic (0.832) Atherosclerosis treatment (0.687) | Nootropic (0.744) Immunosuppressant (0.733) |
| 93 | Antineoplastic (0.812) Apoptosis agonist (0.737) Chemopreventive (0.650) | Hypolipemic (0.764) Anti-hypercholesterolemic (0.686) Atherosclerosis treatment (0.575) | Immunosuppressant (0.652) Nootropic (0.602) |
| 94 | Antineoplastic (0.935) Apoptosis agonist (0.833) | Anti-hypercholesterolemic (0.789) | Immunomodulator (HIV) (0.958) Respiratory analeptic (0.923) |
| 95 | Antineoplastic (0.860) Apoptosis agonist (0.844) | Hypolipemic (0.748) Anti-hypercholesterolemic (0.536) | Immunosuppressant (0.773) Allergic conjunctivitis treatment (0.608) |
| 96 | Antineoplastic (0.773) | Hypolipemic (0.595) | Anti-eczematic (0.864) |
| 97 | Antineoplastic (0.776) | Hypolipemic (0.559) | Anti-eczematic (0.856) |
| 98 | Antineoplastic (0.809) | Anti-hypercholesterolemic (0.672) | Respiratory analeptic (0.935) |
| No. | Antitumor and Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Biological Activity, (Pa) * |
|---|---|---|---|
| 99 | Antineoplastic (0.893) Apoptosis agonist (0.791) | Hypolipemic (0.736) Atherosclerosis treatment (0.717) | Respiratory analeptic (0.842) Antifungal (0.821) |
| 100 | Antineoplastic (0.895) Apoptosis agonist (0.793) | Hypolipemic (0.738) Atherosclerosis treatment (0.712) | Respiratory analeptic (0.849) Antifungal (0.825) |
| 101 | Antineoplastic (0.887) Apoptosis agonist (0.764) Proliferative diseases treatment (0.712) Dementia treatment (0.629) | Anti-hypercholesterolemic (0.906) Neuroprotector (0.892) Hypolipemic (0.733) Autoimmune disorders treatment (0.675) | Respiratory analeptic (0.934) Immunomodulator (HIV) (0.925) Anesthetic general (0.786) Antiviral (Influenza) (0.664) |
| 102 | Antineoplastic (0.870) Prostate cancer treatment (0.786) Apoptosis agonist (0.642) | Atherosclerosis treatment (0.879) Hypolipemic (0.823) Cholesterol synthesis inhibitor (0.736) | Immunomodulator (HIV) (0.939) Respiratory analeptic (0.932) |
| 103 | Proliferative diseases treatment (0.834) Antineoplastic (0.805) | Anti-hypercholesterolemic (0.918) Hypolipemic (0.818) Atherosclerosis treatment (0.655) Cholesterol synthesis inhibitor (0.650) | Analeptic (0.864) Antiviral (Influenza) (0.789) Antiprotozoal (0.691) |
| 104 | Proliferative diseases treatment (0.886) Antineoplastic (0.886) Anticarcinogenic (0.832) Dementia treatment (0.752) Alzheimer’s disease treatment (0.700) | Neurodegenerative diseases treatment (0.566) | Anti-inflammatory (0.849) Antiprotozoal (0.828) |
| 105 | Apoptosis agonist (0.931) Antineoplastic (0.892) Chemopreventive (0.793) | Hypolipemic (0.807) Atherosclerosis treatment (0.607) Autoimmune disorders treatment (0.531) | Nootropic (0.895) Anti-inflammatory (0.854) Antiprotozoal (Plasmodium) (0.633) |
| 106 | Antineoplastic (0.760) | Antiprotozoal (0.593) | |
| 107 | Antineoplastic (0.881) Proliferative diseases treatment (0.823) Dementia treatment (0.795) Alzheimer’s disease treatment (0.688) | Neurodegenerative diseases treatment (0.562) Anti-hypercholesterolemic (0.539) Andropause treatment (0.527) | Antiprotozoal (0.862) Respiratory analeptic (0.846) Nootropic (0.671) |
| 108 | Antineoplastic (0.743) Prostate disorders treatment (0.719) | Anti-hypercholesterolemic (0.613) | Immunomodulator (HIV) (0.776) |
| 109 | Antineoplastic (0.871) Apoptosis agonist (0.649) Prostate disorders treatment (0.634) | Neuroprotector (0.981) Anti-hypercholesterolemic (0.946) Acute neurologic disorders treatment (0.749) | Respiratory analeptic (0.941) Vasoprotector (0.883) Antiprotozoal (Leishmania) (0.870) |
| 110 | Antineoplastic (0.860) Chemopreventive (0.812) Proliferative diseases treatment (0.694) | Anti-hypercholesterolemic (0.934) Hypolipemic (0.852) Cholesterol synthesis inhibitor (0.746) Atherosclerosis treatment (0.645) | Nootropic (0.810) Respiratory analeptic (0.785) Immunomodulator (HIV) (0.730) |
| 111 | Chemopreventive (0.873) Antineoplastic (0.853) Proliferative diseases treatment (0.814) | Anti-hypercholesterolemic (0.809) Neuroprotector (0.731) Hypolipemic (0.612) | Respiratory analeptic (0.941) Hepatoprotectant (0.891) Antithrombotic (0.618) |
| No. | Antitumor and Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Biological Activity, (Pa) * |
|---|---|---|---|
| 112 | Antineoplastic (0.864) Apoptosis agonist (0.850) Prostate disorders treatment (0.705) Dementia treatment (0.629) | Neuroprotector (0.786) Anti-hypercholesterolemic (0.774) Cholesterol synthesis inhibitor (0.524) | Immunomodulator (HIV) (0.895) Erythropoiesis stimulant (0.894) Anesthetic general (0.865) |
| 113 | Antineoplastic (0.848) Apoptosis agonist (0.826) Proliferative diseases treatment (0.608) | Anti-hypercholesterolemic (0.811) Lipid metabolism regulator (0.673) | Respiratory analeptic (0.911) Neuroprotector (0.825) Immunomodulator (HIV) (0.820) |
| 114 | Proliferative diseases treatment (0.923) Antineoplastic (0.889) Chemopreventive (0.853) Apoptosis agonist (0.741) Dementia treatment (0.673) | Neuroprotector (0.950) Anti-hypercholesterolemic (0.821) Spasmolytic (0.684) Hypolipemic (0.621) | Respiratory analeptic (0.981) Anesthetic general (0.918) Cardiotonic (0.813) Antithrombotic (0.567) |
| 115 | Chemopreventive (0.942) Antineoplastic (0.901) Apoptosis agonist (0.837) Proliferative diseases treatment (0.788) Dementia treatment (0.665) | Neuroprotector (0.971) Anti-hypercholesterolemic (0.877) | Respiratory analeptic (0.987) Anesthetic general (0.938) Antiprotozoal (Leishmania) (0.927) Cardiotonic (0.791) Antithrombotic (0.557) |
| 116 | Chemopreventive (0.918) Antineoplastic (0.893) Proliferative diseases treatment (0.890) Dementia treatment (0.738) | Neuroprotector (0.983) Anti-hypercholesterolemic (0.919) Acute neurologic disorders treatment (0.636) Hypolipemic (0.626) | Respiratory analeptic (0.989) Anesthetic general (0.949) Antiprotozoal (Leishmania) (0.936) |
| 117 | Antineoplastic (0.757) Prostate disorders treatment (0.678) Proliferative diseases treatment (0.599) | Neuroprotector (0.852) Anti-hypercholesterolemic (0.828) Lipid metabolism regulator (0.732) | Respiratory analeptic (0.853) Erythropoiesis stimulant (0.851) Immunomodulator (HIV) (0.829) |
| 118 | Antineoplastic (0.869) Apoptosis agonist (0.775) Alzheimer’s disease treatment (0.571) | Anti-eczematic (0.924) Immunosuppressant (0.790) | |
| 119 | Antineoplastic (0.900) Prostate disorders treatment (0.741) | Anti-eczematic (0.842) Erythropoiesis stimulant (0.827) | |
| 120 | Antineoplastic (0.869) Apoptosis agonist (0.775) Alzheimer’s disease treatment (0.671) | Anti-eczematic (0.924) Immunosuppressant (0.790) Anti-asthmatic (0.644) | |
| 121 | Antineoplastic (0.863) Apoptosis agonist (0.724) | Anti-inflammatory (0.834) Immunosuppressant (0.798) | |
| 122 | Antineoplastic (0.747) Proliferative diseases treatment (0.641) | Lipid metabolism regulator (0.507) | Anti-inflammatory (0.788) Immunosuppressant (0.735) |
| 123 | Antineoplastic (0.762) Chemopreventive (0.643) Proliferative diseases treatment (0.619) | Anti-hypercholesterolemic (0.671) Cholesterol synthesis inhibitor (0.543) | Anti-eczematic (0.868) Immunosuppressant (0.755) Anesthetic general (0.549) |
| 124 | Antineoplastic (0.882) Prostate disorders treatment (0.648) | Anti-inflammatory (0.904) Antiprotozoal (0.818) | |
| 125 | Antineoplastic (0.877) Apoptosis agonist (0.773) Alzheimer’s disease treatment (0.721) | Neurodegenerative diseases treatment (0.718) | Spasmolytic, urinary (0.959) Anti-eczematic (0.924) Immunosuppressant (0.794) |
| 126 | Antineoplastic (0.870) Apoptosis agonist (0.683) | Immunosuppressant (0.788) Growth stimulant (0.537) | |
| 127 | Antineoplastic (skin cancer) (0.650) Antineoplastic (0.650) | Antibacterial (0.639) Immunomodulator (HIV) (0.568) | |
| 128 | Antineoplastic (0.813) Apoptosis agonist (0.685) Chemopreventive (0.542) | Hypolipemic (0.781) Anti-hypercholesterolemic (0.594) Cholesterol synthesis inhibitor (0.552) | Nootropic (0.610) Immunosuppressant (0.601) |
| 129 | Apoptosis agonist (0.844) Antineoplastic (0.796) Chemopreventive (0.631) | Hypolipemic (0.575) Anti-hypercholesterolemic (0.551) Cholesterol synthesis inhibitor (0.507) | Anesthetic (0.689) Anti-inflammatory (0.581) |
| 130 | Antineoplastic (0.858) Apoptosis agonist (0.853) Chemopreventive (0.840) Prostate disorders treatment (0.539) | Anti-hypercholesterolemic (0.894) Hypolipemic (0.762) Lipid metabolism regulator (0.615) Neuroprotector (0.611) | Anti-psoriatic (0.762) Anti-eczematic (0.750) Nootropic (0.647) Anesthetic (0.602) |
| 131 | Antineoplastic (0.906) Chemopreventive (0.826) Apoptosis agonist (0.785) | Anti-hypercholesterolemic (0.711) Lipid metabolism regulator (0.695) Hypolipemic (0.599) | Immunosuppressant (0.735) Anti-inflammatory (0.724) Urolithiasis treatment (0.722) |
| 132 | Antineoplastic (0.901) Prostate disorders treatment (0.670) Apoptosis agonist (0.627) | Anti-hypercholesterolemic (0.770) Neuroprotector (0.700) Hypolipemic (0.597) | Angiogenesis inhibitor (0.920) Respiratory analeptic (0.901) Anesthetic general (0.807) |
| No. | Antitumor and Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Biological Activity, (Pa) * |
|---|---|---|---|
| 133 | Antineoplastic (0.819) Prostate disorders treatment (0.701) | Respiratory analeptic (0.793) Immunomodulator (HIV) (0.743) | |
| 134 | Antineoplastic (0.888) Proliferative diseases treatment (0.699) Anticarcinogenic (0.618) | Ankylosing spondylitis treatment (0.839) Antiprotozoal (0.773) Antiprotozoal (Plasmodium) (0.697) | |
| 135 | Antineoplastic (0.900) | Neurodegenerative diseases treatment (0.519) | Anti-inflammatory (0.861) |
| 136 | Antineoplastic (0.871) Apoptosis agonist (0.585) | Antiprotozoal (0.763) Antiprotozoal (Plasmodium) (0.683) | |
| 137 | Antineoplastic (0.880) Proliferative diseases treatment (0.607) | Antiprotozoal (0.821) Antiprotozoal (Plasmodium) (0.700) | |
| 138 | Antineoplastic (0.856) Prostate disorders treatment (0.651) Proliferative diseases treatment (0.586) | Anti-hypercholesterolemic (0.813) Neuroprotector (0.734) | Anti-inflammatory (0.929) Immunomodulator (HIV) (0.868) Anesthetic general (0.813) |
| 139 | Antineoplastic (0.842) | Neuroprotector (0.558) | Immunomodulator (HIV) (0.751) |
| 140 | Antineoplastic (0.780) Apoptosis agonist (0.562) | Neuroprotector (0.722) Anti-hypercholesterolemic (0.609) | Immunomodulator (HIV) (0.861) |
| 141 | Antineoplastic (0.862) Chemoprotective (0.694) | Hypolipemic (0.554) Cholesterol synthesis inhibitor (0.509) | Immunosuppressant (0.753) Antiprotozoal (Plasmodium) (0.658) |
| 142 | Chemopreventive (0.989) Proliferative diseases treatment (0.969) Antineoplastic (0.874) Alzheimer’s disease treatment (0.570) | Anti-hypercholesterolemic (0.977) Neuroprotector (0.895) Atherosclerosis treatment (0.601) Neurodegenerative diseases treatment (0.590) | Hepatoprotectant (0.986) Respiratory analeptic (0.978) Antimycobacterial (0.939) Antiprotozoal (Leishmania) (0.772) |
| 143 | Antineoplastic (0.749) | Anti-eczematic (0.729) Dermatologic (0.651) Anti-psoriatic (0.570) | |
| 144 | Antineoplastic (0.796) Apoptosis agonist (0.750) | Hypolipemic (0.660) Cholesterol synthesis inhibitor (0.510) | Hepatoprotectant (0.748) Anti-eczematic (0.739) |
| 145 | Antineoplastic (0.697) Antineoplastic (bladder cancer) (0.568) | Antibacterial (0.688) Antifungal (0.620) | |
| 146 | Antineoplastic (0.735) Prostate disorders treatment (0.696) | Neuroprotector (0.580) | Immunomodulator (HIV) (0.817) Anti-eczematic (0.808) |
| 147 | Antineoplastic (0.828) Proliferative diseases treatment (0.699) | Neuroprotector (0.640) Acute neurologic disorders treatment (0.626) | Anti-inflammatory (0.920) Respiratory analeptic (0.838) |
| 148 | Antineoplastic (0.837) Apoptosis agonist (0.607) | Neuroprotector (0.684) Autoimmune disorders treatment (0.611) | Anti-inflammatory (0.889) Antiprotozoal (Leishmania) (0.571) |
| 149 | Antineoplastic (0.851) Proliferative diseases treatment (0.687) | Neuroprotector (0.771) Acute neurologic disorders treatment (0.600) | Anti-inflammatory (0.923) Immunomodulator (HIV) (0.859) |
| 150 | Antineoplastic (0.849) Proliferative diseases treatment (0.709) | Neuroprotector (0.736) Anti-hypercholesterolemic (0.634) Acute neurologic disorders treatment (0.615) | Anti-inflammatory (0.910) Antiprotozoal (Leishmania) (0.584) |
| 151 | Antineoplastic (0.818) Apoptosis agonist (0.571) | Respiratory analeptic (0.858) Cardiotonic (0.612) | |
| 152 | Antineoplastic (0.832) Proliferative diseases treatment (0.578) | Anti-hypercholesterolemic (0.789) | Respiratory analeptic (0.894) Immunomodulator (HIV) (0.857) |
| 153 | Antineoplastic (0.837) Proliferative diseases treatment (0.598) | Anti-hypercholesterolemic (0.789) | Respiratory analeptic (0.897) Immunomodulator (HIV) (0.858) |
| 154 | Apoptosis agonist (0.862) Antineoplastic (0.846) Proliferative diseases treatment (0.623) | Anti-hypercholesterolemic (0.911) Hypolipemic (0.751) Atherosclerosis treatment (0.611) | Antidiabetic (type 2) (0.617) Antifungal (0.584) |
| 155 | Antineoplastic (0.738) Proliferative diseases treatment (0.647) | Anti-hypercholesterolemic (0.845) Cholesterol synthesis inhibitor (0.562) | Respiratory analeptic (0.911) Myocardial infarction treatment (0.906) |
| 156 | Antineoplastic (0.737) | Anti-hypercholesterolemic (0.538) | Myocardial infarction treatment (0.889) Respiratory analeptic (0.813) |
| No. | Antitumor and Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Biological Activity, (Pa) * |
|---|---|---|---|
| 157 | Antineoplastic (0.749) | Anti-hypercholesterolemic (0.825) | Anti-inflammatory (0.881) |
| 158 | Antineoplastic (0.701) Prostate disorders treatment (0.629) | Anti-hypercholesterolemic (0.929) Hypolipemic (0.645) | Anti-seborrheic (0.868) Radioprotector (0.801) |
| 159 | Apoptosis agonist (0.791) Antineoplastic (0.787) | Hypolipemic (0.680) | Anti-psoriatic (0.795) Immunosuppressant (0.726) |
| 160 | Antineoplastic (0.913) Apoptosis agonist (0.862) Proliferative diseases treatment (0.687) | Atherosclerosis treatment (0.628) Anti-hypercholesterolemic (0.587) | Anti-eczematic (0.844) Anti-psoriatic (0.820) |
| 161 | Antineoplastic (0.860) Apoptosis agonist (0.851) Proliferative diseases treatment (0.700) | Anti-hypercholesterolemic (0.789) Atherosclerosis treatment (0.724) Cholesterol synthesis inhibitor (0.617) | Anti-osteoporotic (0.913) Nootropic (0.870) |
| 162 | Antineoplastic (0.872) Apoptosis agonist (0.811) Proliferative diseases treatment (0.786) | Anti-hypercholesterolemic (0.943) Hypolipemic (0.730) Atherosclerosis treatment (0.648) | Anesthetic general (0.917) Respiratory analeptic (0.869) Nootropic (0.698) |
| 163 | Antineoplastic (0.937) Apoptosis agonist (0.906) Proliferative diseases treatment (0.886) Antiparkinsonian, rigidity relieving (0.729) Multiple sclerosis treatment (0.565) | Anti-eczematic (0.973) Anti-psoriatic (0.963) Anti-osteoporotic (0.961) Hyperparathyroidism treatment (0.842) Hypercalcemia treatment (0.509) | |
| 164 | Antineoplastic (0.706) Proliferative diseases treatment (0.608) | Anti-hypercholesterolemic (0.882) Atherosclerosis treatment (0.723) Cholesterol synthesis inhibitor (0.632) | Hepatic disorders treatment (0.824) Anti-osteoporotic (0.665) |
| 165 | Antineoplastic (0.750) | Anti-hypercholesterolemic (0.791) Atherosclerosis treatment (0.700) Acute neurologic disorders treatment (0.635) | Hepatic disorders treatment (0.816) Anesthetic general (0.774) Anti-osteoporotic (0.663) |
| 166 | Antineoplastic (0.694) Apoptosis agonist (0.578) Proliferative diseases treatment (0.542) | Anti-hypercholesterolemic (0.852) Hypolipemic (0.788) Atherosclerosis treatment (0.744) | Anti-eczematic (0.870) Hepatic disorders treatment (0.817) Anti-osteoporotic (0.678) |
| 167 | Antineoplastic (0.789) Apoptosis agonist (0.726) Proliferative diseases treatment (0.608) | Anti-hypercholesterolemic (0.891) Atherosclerosis treatment (0.630) Acute neurologic disorders treatment (0.560) | Anti-eczematic (0.894) Anti-psoriatic (0.777) Anti-osteoporotic (0.736) |
| 168 | Proliferative diseases treatment (0.947) Dementia treatment (0.657) | Neuroprotector (0.981) Anti-hypercholesterolemic (0.971) Hypolipemic (0.778) | Respiratory analeptic (0.987) Hepatoprotectant (0.945) Anesthetic general (0.903) |
| 169 | Proliferative diseases treatment (0.920) Antineoplastic (0.841) Dementia treatment (0.661) | Anti-hypercholesterolemic (0.962) Neuroprotector (0.952) Acute neurologic disorders treatment (0.783) | Respiratory analeptic (0.991) Anesthetic general (0.922) Spasmolytic (0.719) |
| 170 | Antineoplastic (0.793) Proliferative diseases treatment (0.605) | Anesthetic general (0.815) Cardiotonic (0.770) | |
| 171 | Antineoplastic (0.872) Alzheimer’s disease treatment (0.634) Proliferative diseases treatment (0.625) | Neuroprotector (0.732) Hypolipemic (0.592) Acute neurologic disorders treatment (0.557) | Anti-inflammatory (0.923) Nootropic (0.672) |
| 172 | Antineoplastic (0.871) Alzheimer’s disease treatment (0.681) Dementia treatment (0.576) | Neuroprotector (0.762) Anti-hypercholesterolemic (0.633) Neurodegenerative diseases treatment (0.618) | Respiratory analeptic (0.894) Anesthetic general (0.890) |
| 173 | Proliferative diseases treatment (0.922) Antineoplastic (0.842) | Anti-hypercholesterolemic (0.859) Neuroprotector (0.706) | Respiratory analeptic (0.962) Anti-ischemic, cerebral (0.864) |
| 174 | Proliferative diseases treatment (0.935) Antineoplastic (0.837) Dementia treatment (0.616) | Anti-hypercholesterolemic (0.927) Neuroprotector (0.773) Hypolipemic (0.584) | Anti-ischemic, cerebral (0.944) Respiratory analeptic (0.940) Antithrombotic (0.720) |
| 175 | Antineoplastic (0.793) | Anti-hypercholesterolemic (0.954) Neuroprotector (0.754) Hypolipemic (0.704) | Respiratory analeptic (0.979) Anesthetic general (0.927) Anti-ischemic, cerebral (0.679) |
| 176 | Antineoplastic (0.893) Proliferative diseases treatment (0.768) | Anti-hypercholesterolemic (0.899) Atherosclerosis treatment (0.754) | Respiratory analeptic (0.979) Immunomodulator (HIV) (0.912) |
| 177 | Antineoplastic (0.812) Dementia treatment (0.505) | Neuroprotector (0.938) Anti-hypercholesterolemic (0.912) Atherosclerosis treatment (0.548) | Respiratory analeptic (0.984) Immunomodulator (HIV) (0.933) Anesthetic general (0.917) |
| No. | Antitumor and Related Activity, (Pa) * | Lipid Metabolism Regulators, (Pa) * | Additional Biological Activity, (Pa) * |
|---|---|---|---|
| 178 | Antineoplastic (0.886) Apoptosis agonist (0.829) Proliferative diseases treatment (0.710) | Anti-hypercholesterolemic (0.716) Hypolipemic (0.596) Atherosclerosis treatment (0.588) | Respiratory analeptic (0.966) Immunosuppressant (0.837) Anesthetic general (0.775) |
| 179 | Antineoplastic (0.901) Proliferative diseases treatment (0.832) | Anti-hypercholesterolemic (0.839) Atherosclerosis treatment (0.597) | Respiratory analeptic (0.979) Anesthetic general (0.841) |
| 180 | Antineoplastic (0.897) Apoptosis agonist (0.858) Proliferative diseases treatment (0.778) | Anti-hypercholesterolemic (0.865) Atherosclerosis treatment (0.639) | Respiratory analeptic (0.974) Anesthetic (0.904) Anesthetic general (0.731) |
| 181 | Antineoplastic (0.842) Apoptosis agonist (0.779) Proliferative diseases treatment (0.711) | Anti-hypercholesterolemic (0.658) Hypolipemic (0.633) | Respiratory analeptic (0.941) Anesthetic (0.842) Cardiotonic (0.730) |
| 182 | Apoptosis agonist (0.828) Antineoplastic (0.827) Proliferative diseases treatment (0.536) | Hypolipemic (0.636) Atherosclerosis treatment (0.630) Cholesterol synthesis inhibitor (0.519) | Anesthetic (0.902) Antithrombotic (0.573) Spasmolytic (0.538) |
| 183 | Apoptosis agonist (0.898) Antineoplastic (0.852) Proliferative diseases treatment (0.519) | Hypolipemic (0.660) Atherosclerosis treatment (0.618) Lipid metabolism regulator (0.535) | Anesthetic (0.869) Immunosuppressant (0.798) Anti-inflammatory (0.792) |
| 184 | Antineoplastic (0.881) Apoptosis agonist (0.797) Proliferative diseases treatment (0.611) | Neuroprotector (0.603) Hypolipemic (0.568) | Respiratory analeptic (0.915) Antifungal (0.828) Antiprotozoal (0.718) |
| 185 | Antineoplastic (0.937) Alzheimer’s disease treatment (0.633) | Anti-inflammatory (0.939) Antiprotozoal (0.663) | |
| 186 | Antineoplastic (0.934) Apoptosis agonist (0.792) Antimetastatic (0502) | Anti-inflammatory (0.934) Anti-asthmatic (0.645) Antiprotozoal (Plasmodium) (0.601) | |
| 187 | Antineoplastic (0.934) Apoptosis agonist (0.792) Antimetastatic (0.502) | Anti-inflammatory (0.934) Anti-asthmatic (0.645) Antiprotozoal (Plasmodium) (0.601) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermolenko, E.V.; Imbs, A.B.; Gloriozova, T.A.; Poroikov, V.V.; Sikorskaya, T.V.; Dembitsky, V.M. Chemical Diversity of Soft Coral Steroids and Their Pharmacological Activities. Mar. Drugs 2020, 18, 613. https://doi.org/10.3390/md18120613
Ermolenko EV, Imbs AB, Gloriozova TA, Poroikov VV, Sikorskaya TV, Dembitsky VM. Chemical Diversity of Soft Coral Steroids and Their Pharmacological Activities. Marine Drugs. 2020; 18(12):613. https://doi.org/10.3390/md18120613
Chicago/Turabian StyleErmolenko, Ekaterina V., Andrey B. Imbs, Tatyana A. Gloriozova, Vladimir V. Poroikov, Tatyana V. Sikorskaya, and Valery M. Dembitsky. 2020. "Chemical Diversity of Soft Coral Steroids and Their Pharmacological Activities" Marine Drugs 18, no. 12: 613. https://doi.org/10.3390/md18120613
APA StyleErmolenko, E. V., Imbs, A. B., Gloriozova, T. A., Poroikov, V. V., Sikorskaya, T. V., & Dembitsky, V. M. (2020). Chemical Diversity of Soft Coral Steroids and Their Pharmacological Activities. Marine Drugs, 18(12), 613. https://doi.org/10.3390/md18120613