1. Introduction
Chitosan (CH) is the deacetylated derivative of chitin with a degree of deacetylation of at least 50% and is regularly used in the food industry as an edible, renewably sourced food preservative. CH has shown antimicrobial effects against bacterial strains including
Escherichia coli and
Staphylococcus aureus (
S. aureus) [
1,
2]. CH hydrogels are easily produced by combining acid-solubilized CH with beta-glycerol phosphate (β-GP), the neutralizing, biocompatible salt that makes CH solutions thermosensitive [
3]. Once the CH solution is chilled, the β-GP stabilizes the CH through ion and hydrogen bonding [
4]. As hydrogen bonds are weak, physiological temperatures (37 °C) are sufficient to break them, allowing the CH to precipitate and forming a hydrogel [
4]. The gels can be modified to create a composite material, or loaded with antibiotic or chemotherapy drug, for a variety of applications [
5].
Osteomyelitis, a bone infection, is difficult to treat with traditional antibiotics alone, a challenge further compounded by the rise in antibiotic resistant bacteria [
6], most commonly
S. aureus [
7]. Prolonged systemic (IV) or oral administration of an antibiotic in a high enough concentration to be effective may cause kidney and/or liver toxicity [
8]. Localized treatment via a biomaterial carrier would allow for tunable release of drug, keeping the concentration high at the infection site while avoiding systemic toxicity. Combining localized treatment with the use of non-traditional antimicrobials is expected to combat antibiotic resistant bacterial infections more effectively, such as
S. aureus osteomyelitis.
Fosfomycin (FOS) is a unique antibiotic that has traditionally been used in Europe for uncomplicated urinary tract infections in women [
9]. FOS is first internalized by the bacteria, and then it inhibits the function of MurA, which is necessary for the first step in peptidoglycan biosynthesis [
10]. Due to the unique mechanism of action, little to no cross resistance with other common antibiotics, such as vancomycin or methicillin, have been observed [
11]. Thus, FOS is a good candidate to treat antibiotic-resistant bacteria such as methicillin- or vancomycin-resistant
S. aureus (MRSA or VRSA). Against both MRSA and methicillin sensitive
S. aureus, FOS’s minimum inhibitory concentration (MIC) was 6 to 10 times lower than that of vancomycin [
6,
12]. FOS was also reported to have extensive tissue penetration for reaching infections of the bone tissue compared to other antibiotics, likely owing at least in part to its smaller size [
9,
13].
Due to the relatively limited use of FOS [
11,
14], the interactions between FOS and CH have yet to be explored. FOS must have an epoxide ring to be biologically active [
9], such that when the ring is hydrolyzed, the antibiotic is no longer effective [
15]. Ring-opened FOS, also known as FOS impurity A, is structurally similar to β-GP and can be generated in the CH gel system via two mechanisms: hydrolysis or nucleophilic attack by an amine group [
16]. Loading the CH hydrogel with additional FOS may function to saturate the amine groups in the CH, overcoming any loss of antimicrobial efficacy due to the conversion of some of the FOS to its inactive, ring-opened form.
The objective of this work was to elucidate the effects of FOS on the physicochemical and antimicrobial properties of CH gels. We hypothesized: (i) the CH gel would inactivate some FOS, but that effect would be saturated at 9 mg/mL FOS so that antimicrobial efficacy would not be reduced at 9 mg/mL FOS, (ii) the addition of FOS to CH hydrogels would increase the storage (G′) and loss (G″) moduli and reduce the gelation temperature compared to CH alone, and (iii) the CH would retain FOS in the gel matrix by the reaction between FOS and CH.
3. Discussion
It has been previously established that CH and FOS are effective in inhibiting
S. aureus growth independently [
2,
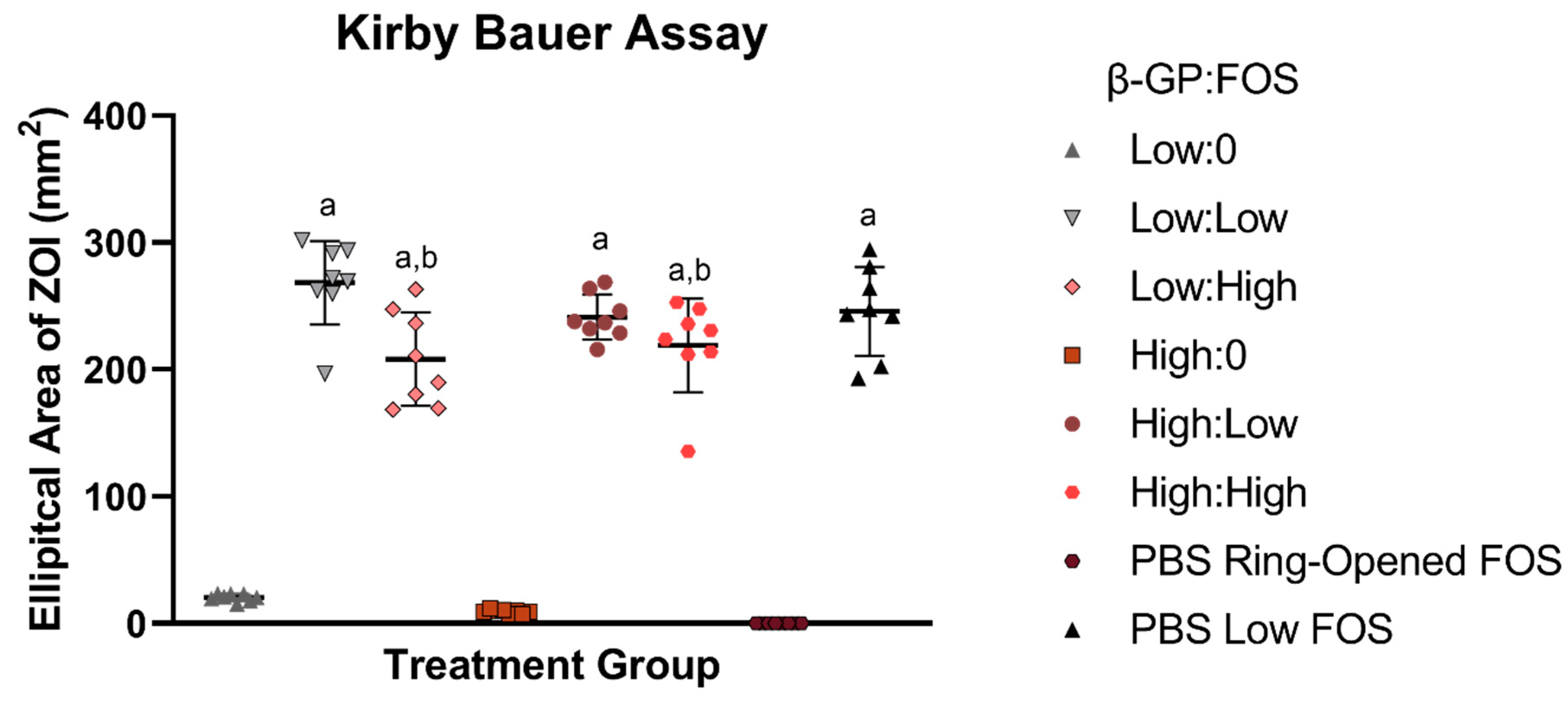
9]. Here, FOS had a positive effect on the antimicrobial properties of CH hydrogels, as demonstrated by the decrease in bacterial growth in both the planktonic assay and the Kirby Bauer assay. In the Kirby Bauer assay, antimicrobial must first be released from its delivery vehicle, traditionally saturated paper disks, but here, the samples were pipetted directly onto the agar plate. Then, the antibiotic must diffuse through the agar to reach bacteria and prevent growth, which is likely why increasing the volume of CH did not increase the ZOI (
Figure S1). While keeping the dose of FOS constant, an effect of FOS concentration was expected, whereby the high FOS concentration groups (Low:High and High:High) were expected to have larger ZOIs compared to their lower concentration FOS counterparts (Low:Low and High:Low, respectively). Surprisingly, the converse was observed, but only at the low β-GP concentration. In other words, the ZOI for Low:Low was greater than that for Low:High, likely due to differences in release/availability of the antibiotic from the gel as a function of gel stiffness. This effect was not observed at the high β-GP concentration, likely due to the decrease in ZOI from reduced spreading of the high β-GP concentration (FOS-free) gels on the agar, regardless of volume delivered (
Figure S1). It was noted that in placing the gel onto the agar plate, the low β-GP groups spread out more, suggesting a lower modulus. This effect of β-GP on physical properties was quantified via rheology and may explain the higher antimicrobial efficacy with the low β-GP concentration (compared to high β-GP) in the absence of FOS (
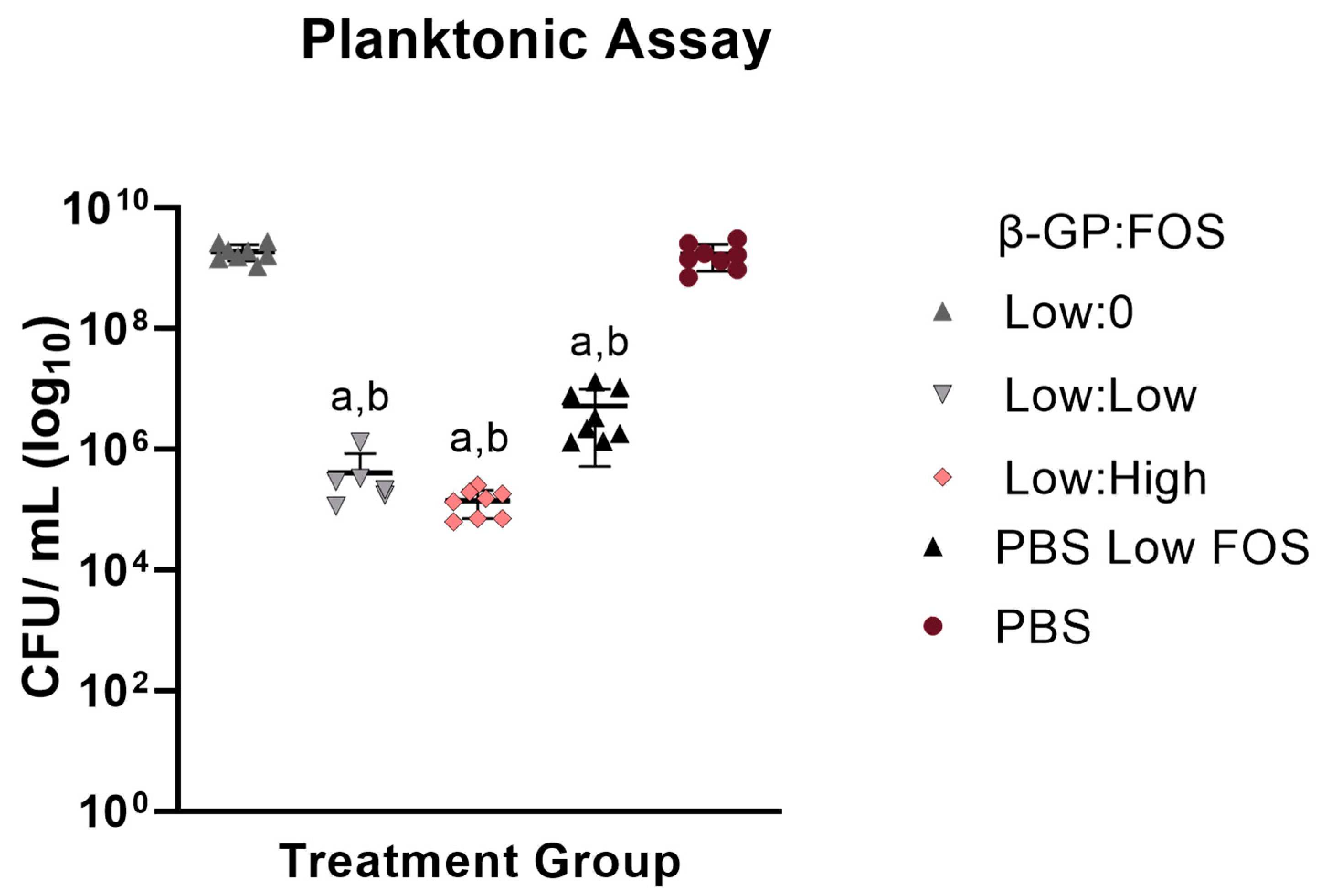
Figure S1). As differences were observed between the low β-GP (but not between the high β-GP) concentration groups in the Kirby Bauer assay, only the low β-GP concentration groups were evaluated in the planktonic assay.
S. aureus has a planktonic stage in its pathogenesis [
25]; therefore, of the two assays, the planktonic assay served to more closely mimic
S. aureus pathogenesis in vivo. Due to the fluid environment of the assay, the diffusivity of the material was believed to have less of an effect (relative to the Kirby Bauer assay) on the inhibition of the bacteria. Despite no antimicrobial effect of the CH itself, notably, the bacterial colonies were smaller for the CH groups compared to those for the PBS groups. This could be due to CH’s ability to act as a flocculant [
26], a chemical that induces particle sedimentation. To minimize the flocculant effect of CH [
2] and reduce the risk of this effect impeding accurate quantification of bacterial loads, NaCl was added to the planktonic solution. At the low NaCl concentration used (5%
w/v), no effect of NaCl on bacterial growth was observed (
Figure S2). Though minimal, ZOIs for groups without FOS were nonzero, indicating chitosan’s innate antimicrobial inhibition of
S. aureus, an enhanced functionality other hydrogel systems such as poloxamer 407, which did not offer any antimicrobial activity [
27]. Future biological testing will include antibiofilm assays to challenge the CH biomaterial system with the difficult to treat biofilm stage of
S. aureus pathogenesis.
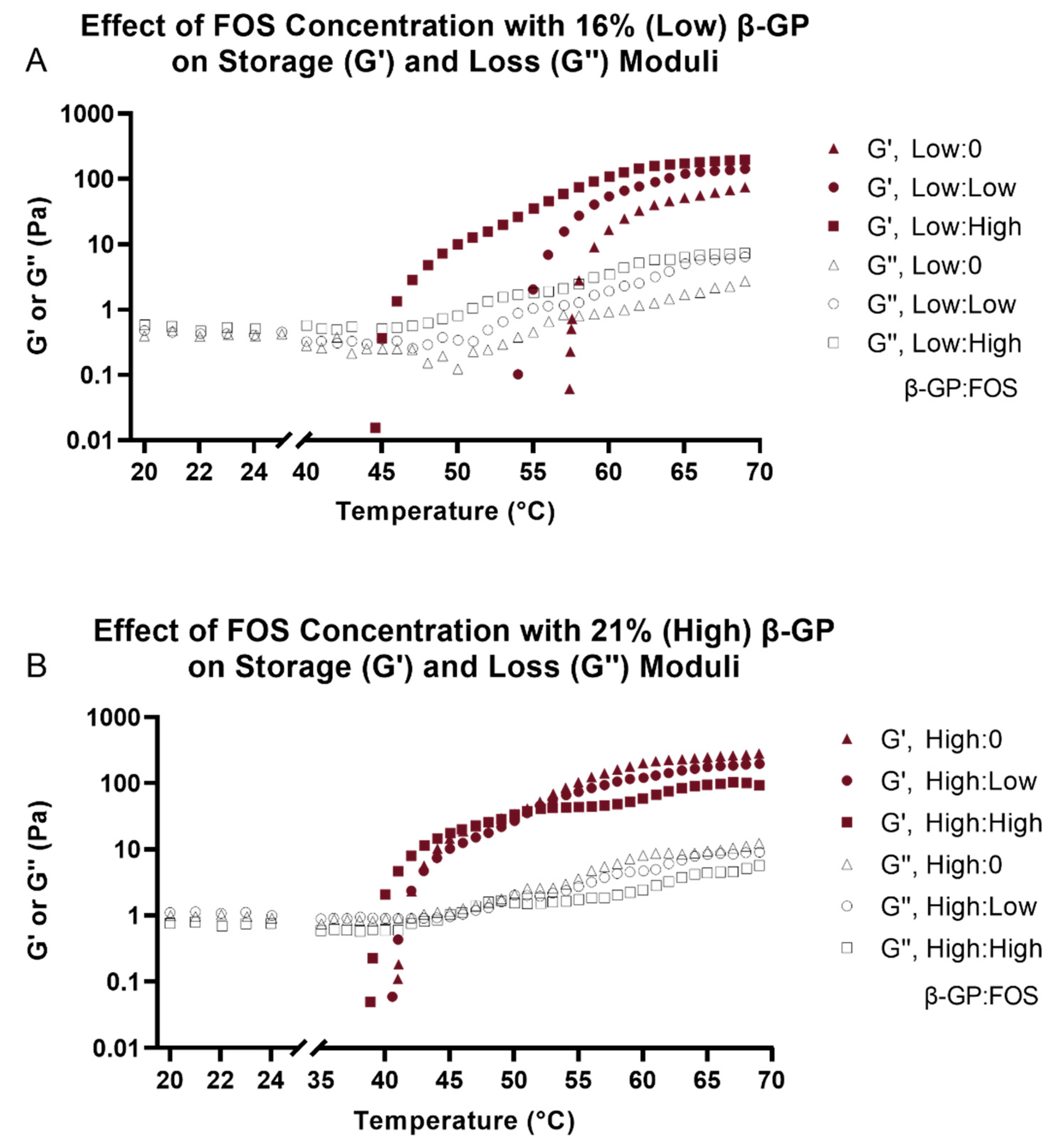
Evaluation of the physical properties of CH hydrogels showed that both FOS and β-GP influenced gelation temperature, storage modulus (G′), and porosity. Similar increases in G′ and G′′ with higher β-GP concentrations have been observed previously [
28]. Although differences in G′ and G′′ were more apparent at the low β-GP concentration, the effects of FOS were not negligible at the high β-GP concentration. It has been shown that increasing β-GP concentration can mask the effect of molecular weight on gel rheology [
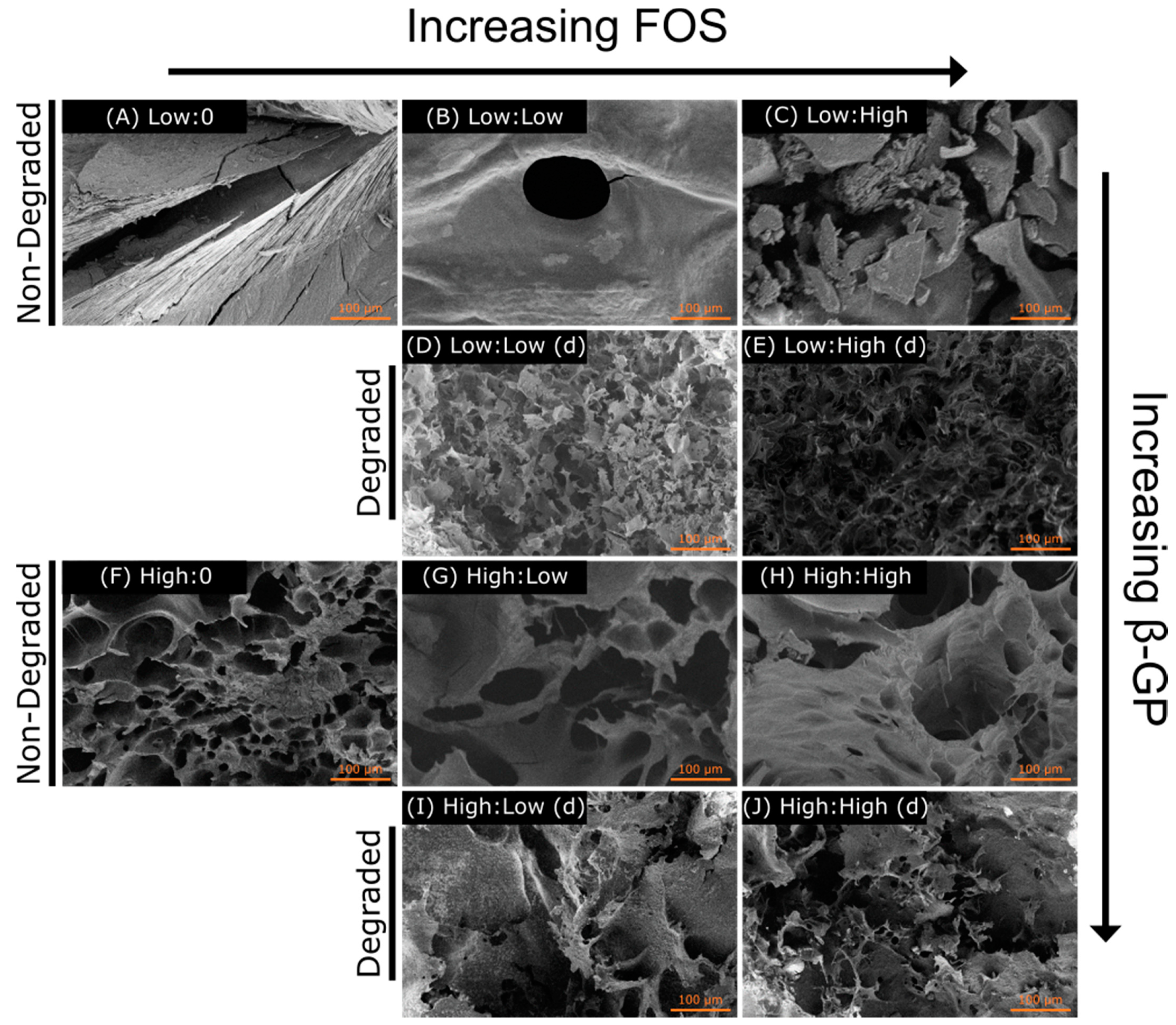
29], suggesting the effect of β-GP concentration may have overpowered the effect of FOS concentration on moduli observed here. There was a reduction in gelation temperature with increasing β-GP concentration, an effect that was observed for all concentrations of FOS. Future rheological testing will include frequency and amplitude sweeps to further understand the interaction of CH and FOS on gel mechanics. The SEM images supported the rheological findings, whereby a more porous structure in the CH matrix was observed with increasing β-GP and FOS concentrations. This agrees with prior results that demonstrated increasing the salt composition decreased the homogeneity of the hydrogel density profile, thus increasing the porosity of the freeze-dried architecture [
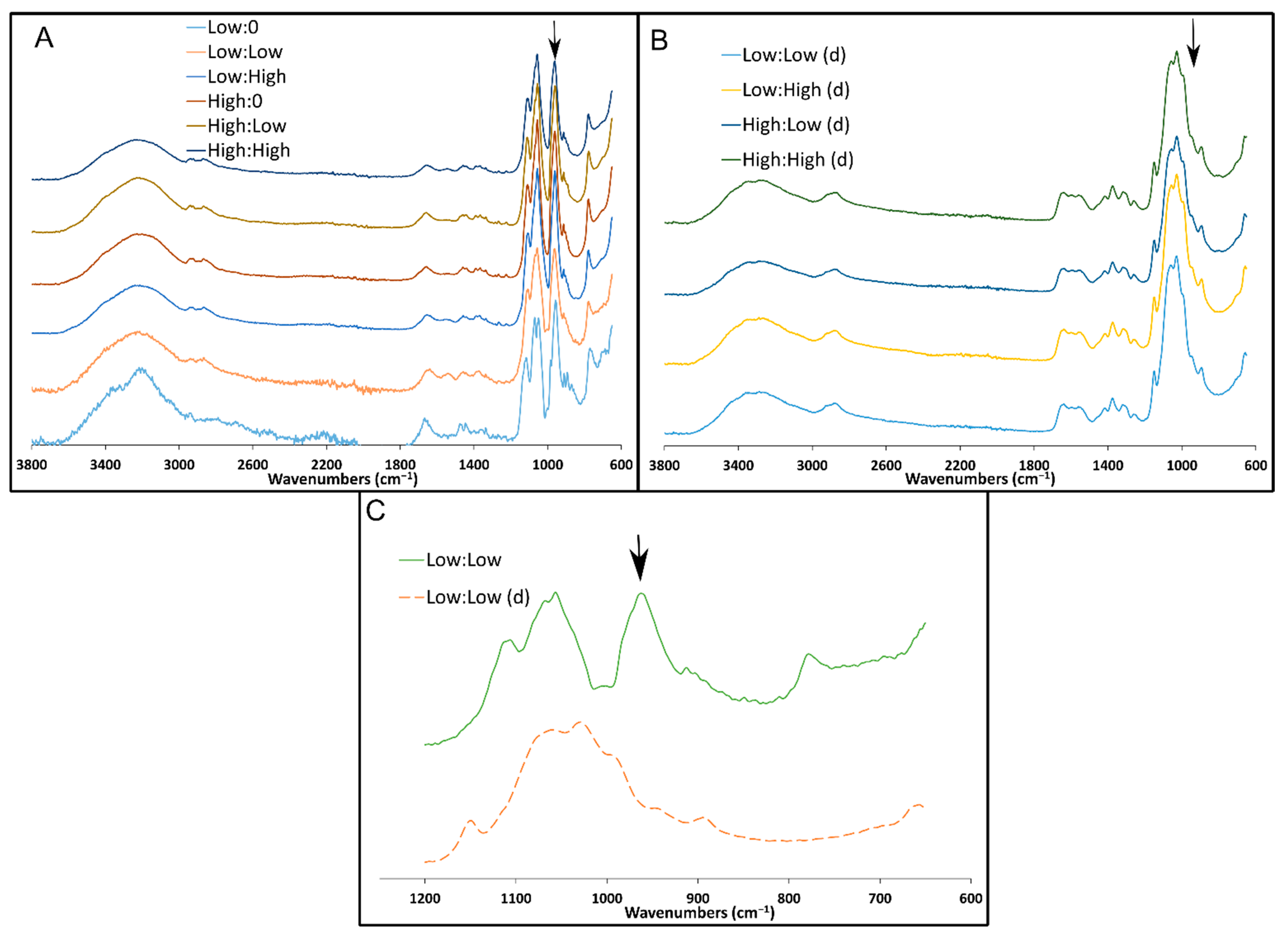
28]. Overall, the porous structure of these scaffolds indicates their suitability for drug delivery and tissue engineering applications by allowing for cell infiltration and biomolecule delivery. Sample architecture was altered following degradation in PBS for 5 days, most likely due to the low molecular weight of the CH allowing the monomer and oligomer particles to be suspended in solution and the change in concentration of salts during degradation. Mass loss over time should be measured to predict the longevity of the material in vivo.
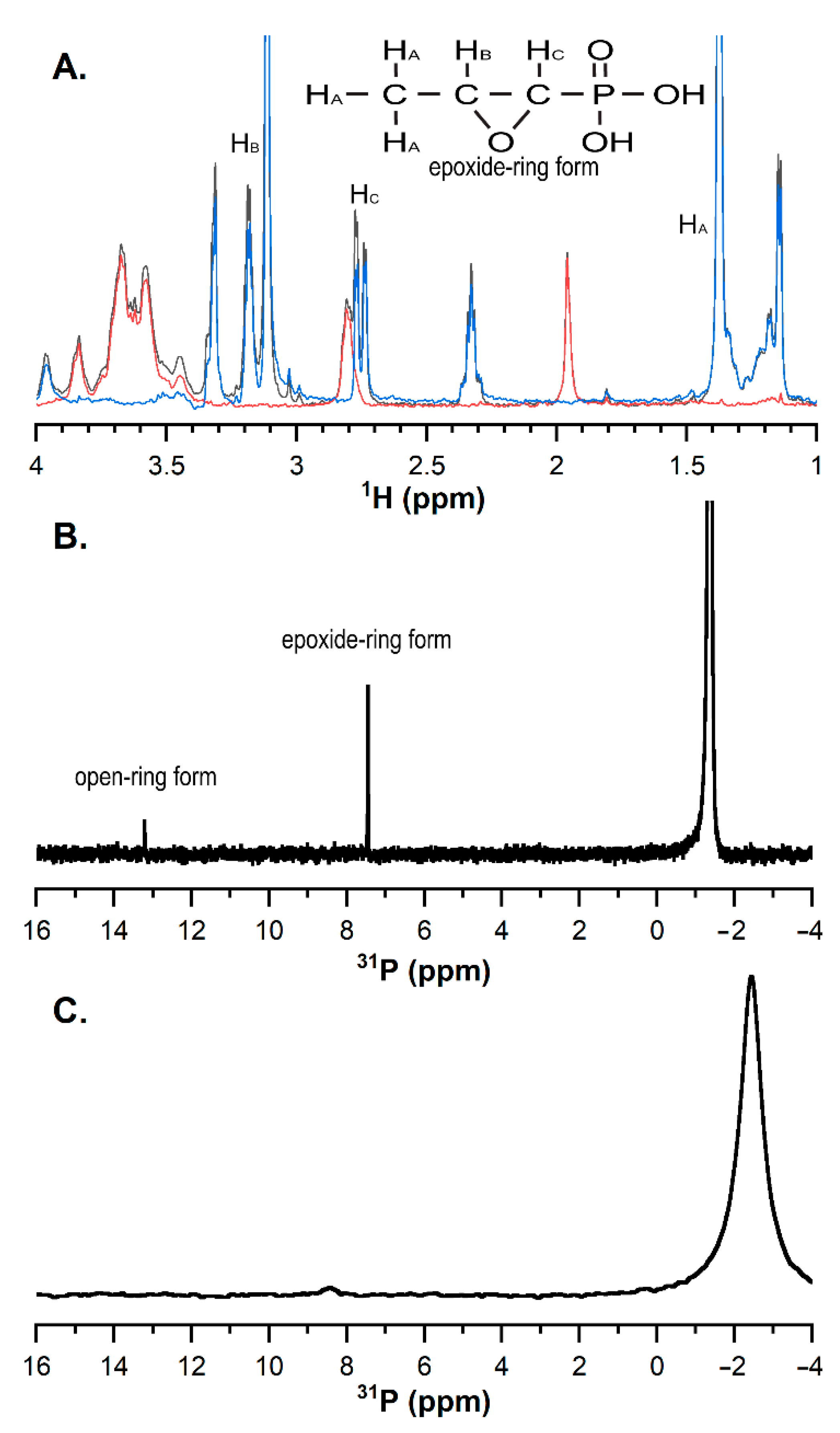
Finally, the chemical testing confirmed that, during hydrogel formation, some of the FOS is taken out of the liquid phase of the hydrogel and rendered chemically unavailable. Quantifying signals in
31P spectra is challenging because of nuclear Overhauser enhancement that occurs during decoupling, but a clear peak was observed for the native FOS in the presence of a low concentration (3 mM) of CH (
Figure 6B). This experiment established a baseline FOS signal for comparing samples with higher amounts of CH. The 1D
1H and
31P NMR spectra of FOS did not change over at least 6 days at low concentrations of CH, and at low concentrations of CH, no reactions appeared to occur. Hydrogel formation at the higher CH concentration complicated NMR measurements because the sample largely behaved like a solid. Moreover, the integral of the FOS signal relative to the phosphate buffer decreased over time, as a 5% reduction was seen over 12 h, supporting the hypothesis that FOS and CH are reacting. FOS and CH reactions would be expected if the FOS reacted with any of the large number of amine groups in the high-concentration CH sample, forming a covalent bond as a result of the nucleophilic attack and epoxide ring opening [
16]. Under these circumstances, FOS would adopt the rotational diffusion time of the CH hydrogel, leading to a fast R
2 relaxation rate and extremely broad peaks [
30]. Though challenges with the hydrogel sample preclude an absolute quantitation of
31P signal originating from FOS, the decrease in intensity from the P
31 NMR spectra relative to the phosphate peak suggests that over time, FOS became immobilized in the CH matrix, rendering it chemically unavailable, as confirmed with the lower apparent translational diffusivity coefficient compared to CH alone. Thus, a combination of factors is likely at play to immobilize FOS in the CH matrix, including chemical reactivity with the CH matrix itself, and slowed translational and rotational diffusion within the matrix. The slowed diffusion is likely mediated by hydrogen bonding and other non-covalent interactions between the FOS and the CH matrix. FTIR of gels after 5 days of degradation revealed the FOS and β-GP signals decreased in strength, suggesting the FOS and β-GP were released as the CH gels degraded. Additional experiments including solid phase extraction of the chitosan degradation products for quantification of released FOS by liquid chromatography-mass spectrometry (LC-MS) are needed to fully understand the specific chemical interactions between FOS and CH. Due to fosfomycin’s optical inactivity, direct quantification is difficult to achieve. A method for quantification via LC-MS has been reported [
31]. From a preliminary experiment using these methods, it is believed the low molecular weight chitosan (degradation products, i.e., chitosan monomer/oligomer) used here may have interfered with the hydrophilic interaction liquid chromatography column, causing retention time peaks of the eluent to broaden and vary drastically compared to the standard curve samples, preventing accurate quantification of the released FOS.
4. Materials and Methods
4.1. Materials
Low molecular weight CH with degree of deacetylation of 95.5%, viscosity of 39 cps 1% CH in 1% acetic acid and sourced from blue water crab shell from China (information provided by the supplier) was used for all experiments (Sigma-Aldrich, #448869, Saint Louis, MO, USA). Phosphate buffered saline (PBS) prepared from tablets (Sigma-Aldrich, P4417, Saint Louis, MO, USA) was used for all experiments except for the gel degradation experiment, in which PBS with calcium and magnesium ions was used (ThermoFisher, #14040-117, Waltham, MA, USA). Fosfomycin (FOS) disodium salt was sourced from MP Biomedicals, LLC, #151876 (Santa Ana, CA, USA). Beta-glycerol phosphate (β-GP) was sourced from EMD Millipore Corp, #35675 (Temecula, CA, USA). Hydrochloric acid (1 M) was sourced from Supelco Titripur®, #1.09057.1000 (Kenilworth, NY, USA). Brain Heart Infusion (BHI) #211059 and DifcoTM Agar Bacteriological #214530 were purchased from BD (Franklin Lakes, NJ, USA). Sodium chloride was purchased from Sigma-Aldrich (Saint Louis, MO, USA).
4.2. Chitosan Gel Creation
Two hundred mg of CH was measured into a 20 mL glass scintillation vial. Nine mL of 0.1 M HCl was slowly added and constantly stirred for 3 h. In a separate tube, 1 mL of water was added to 1.12 g of β-GP and vortexed until homogenous. Both the CH and β-GP solutions were autoclaved at 121°C for 30 min, cooled to room temperature, and placed on ice. While the CH solution was stirring in the ice bath, either 1.88 or 1.12 g of β-GP powder was slowly added to the CH solution. Once the solution was homogeneous, 1 mL of the β-GP solution was added dropwise into the CH solution to create either 156 or 212 mg/mL w/v β-GP concentrations. The solutions were stored at 4 °C up to one month. The FOS was added by resuspending 69.4 or 125 mg in 1 mL PBS, then taking 36 μL of the solution and adding it to 464 μL of the CH + β-GP solution in a single 1 mL Luer Lock syringe. The solutions were mixed by a dual-syringe system. The syringes were stored at 4 °C for up to 24 h before being used. The FOS solution was stored at −80 °C for up to 6 months.
4.3. Kirby Bauer Antibacterial Assay
A modified Kirby Bauer assay was performed as previously reported [
6]. A colony of
S. aureus (ATCC 6538-GFP) was grown overnight at 37 °C with shaking (150 rpm) for 18 h. A sterile cotton swab was used to spread the culture onto BHI agar plates. Then, the treatments were directly pipetted onto the plates (without the use of disks as traditionally performed). All nine treatment groups outlined in
Table 1 were evaluated. An additional four treatments (
Table S1) were evaluated to determine whether volume of chitosan affected ZOI. The plates were incubated statically for 24 h at 37 °C. The elliptical zone of inhibition (ZOI) was calculated by measuring the major and minor diameters (d1 and d2, respectively) from the equation for the area of an ellipse: ZOI = π(d1/2) ∗ (d2/2).
4.4. Planktonic Antibacterial Assay
This assay was modified from a previous planktonic antibacterial assay [
27] to include sodium chloride in the growth medium. Briefly, a colony of
S. aureus modified to express green fluorescent protein (ATCC 6538-GFP) [
6] was grown in 4 mL of Brain Heart Infusion (BHI) media overnight at 37 °C with 150 rpm shaking. Aliquots (200 µL each) of the overnight culture were added to five culture tubes containing 4 mL of BHI. These cultures were grown to an optical density (OD) at 600 nm of 0.2 (µQuant, BioTek Instruments, Inc., Winooski, VT, USA) [
32]. The cultures were centrifuged at 4000×
g rpm for 4 min; then 20 mL of culture were resuspended in fresh BHI containing 1% glucose (
w/v) and 5% NaCl (
w/v). Then, 500 μL of culture in BHI with glucose and NaCl was pipetted into non-tissue culture treated 24-well plates. Five treatment groups (n = 6–8) were investigated: low β-GP CH gel groups containing 0, 5, or 9 mg/mL FOS; PBS with 5 mg/mL FOS; PBS. A control group of PBS with no additional NaCl was used to determine the effects of NaCl on bacterial growth. For groups containing FOS, twice the volume listed in
Table 1 was delivered (indicated by *), to double the dose of FOS compared to the dose used in the Kirby Bauer assay. Treated cultures were incubated at 37 °C with shaking at 150 rpm for 24 h. Solutions were collected, serially diluted, and plated for bacterial counting.
4.5. Rheology
Rheological experiments were conducted using a TA Instruments Discovery HR-2 Hybrid Rheometer (TA Instruments, New Castle, DE, USA). Then, 20 mm parallel plate geometry with a solvent trap was used and the experimental temperature was controlled using a Peltier system. Six groups were evaluated in triplicate: 158 mg/mL (Low) β-GP or 212 mg/mL (High) β-GP with 0, 5, or 9 mg/mL FOS. Samples were prepared using the dual-syringe system and ejected onto the bottom plate of the rheometer maintained at 20 °C. The samples were transported in an icebox and stored there until loaded into the rheometer. The top plate was lowered to 500 µm, and the excess material was wiped away. Samples were subjected to a two-minute soak time to reach thermal equilibrium. A temperature sweep oscillation experiment was conducted with 2 °C/min ramp from 20 to 70 °C with a strain of 1% and a frequency of 1 Hz. The intersection between the storage and loss moduli was considered the gelation temperature.
4.6. Chitosan Gel Degradation and Freeze-Drying
The same 6 groups from rheology were evaluated in a degradation experiment: 158 mg/mL (Low) β-GP or 212 mg/mL (High) β-GP with 0, 5, or 9 mg/mL FOS. Then, 100 μL of each gel was pipetted into a 48-well plate, submerged in 700 μL of PBS (with calcium and magnesium ions), and placed on a shaker plate set to 100 rpm in a 37 °C incubator. Solutions were collected and replaced at 6, 12, 24, 48, 72, 96, and 120 h. Then, the gel was collected by carefully scoping from the bottom of the well. Degraded and non-degraded (fresh) samples were placed in microcentrifuge tubes, frozen in a −80 °C freezer overnight, and freeze dried (Labconco FreeZone1, Labconco, Kansas City, MO, USA).
4.7. Scanning Electron Microscopy (SEM)
The morphology of the freeze-dried samples (n = 3) was accessed by scanning electron microscopy (SEM). A SUPRA 40 scanning electron microscope (Carl Zeiss, Thornwood, NY, USA) was used. The samples were sputter-coated with Au/Pd prior to scanning and were scanned at 5 kV. SEM images were captured at 500× magnification to reveal the key morphological features.
4.8. Nuclear Magnetic Resonance (NMR)
All the experiments were carried out using Bruker Avance (San Jose, CA, USA) III QCI cryoprobe-equipped NMR spectrophotometer operating at a
1H frequency of 600 MHz. To observe the interactions between FOS and CH, several NMR samples were prepared, using low (3 mM) and high (104 mM) concentrations of CH, either with or without 1 mM FOS. No samples contained β-GP as it would further complicate
31P NMR spectrograms. Additionally, all samples contained 5 % D
2O and 50 mM sodium phosphate buffer. For the combined FOS and CH samples, the pH was 6.5, and the pH was confirmed after mixing. All samples were prepared as 600 μL solutions before being transferred to NMR tubes. Each
1H-NMR spectrum was recorded at 298 K with 128 scans using a perfect-echo WATERGATE sequence to refocus scalar couplings and to suppress solvent [
33]. Each
31P-NMR spectrum was recorded at 298 K using an inverse-gated decoupling pulse sequence, with 128 scans, a spectral width of 38 ppm, an acquisition time of 1 s, and a recycle delay of 4 s. The native and ring-opened
31P signals of FOS were assigned by monitoring the NMR spectra of a pH 2.5 sample by adding 1 M HCl to lower the pH. Diffusion ordered spectroscopy (DOSY) experiments were performed in triplicate, with samples taken from independent peaks in the spectra, and recorded as an interleaved set of 25 experiments, with gradient powers ranging from 1–47 G/cm [
18,
19]. Water suppression in DOSY experiments was obtained with 4 s of presaturation during the recycle delay. For the free FOS (without CH) DOSY experiments, the diffusion and gradient encoding times (Δ and δ) were 120 ms and 1.3 ms, respectively. For the FOS in CH matrix DOSY sample, Δ and δ were 85 ms and 1.9 ms. The apparent translational diffusion coefficient was found using the Stejskal-Tanner equation. The hydrogel DOSY sample required 512 scans for each gradient strength (approximately 15 h total acquisition time) to obtain sufficient signal to noise for fitting the broadened FOS peaks.
4.9. Fourier-Transform Infrared Spectroscopy (FTIR)
The chemical composition of the freeze-dried samples (n = 3) was assessed by attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy, and a representative sample was chosen for each group. A Spectrum 100 FTIR spectrophotometer (PerkinElmer, Waltham, MA, USA) was used. Spectra were collected from 600 to 4000 cm−1 at 4 cm−1 resolution and averaged between four scans.
4.10. Statistical Analyses
Data from the Kirby Bauer and planktonic assays, as well as gelation temperature, were analyzed with one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test with alpha = 0.05, except for the planktonic assay of PBS with and without NaCl, which used a Student’s t-test with alpha = 0.05. All aforementioned data are presented as mean ± standard deviation. The apparent translational diffusion coefficients from DOSY were analyzed using a Student’s t-test with alpha = 0.01 and are presented as mean ± standard error of the mean.