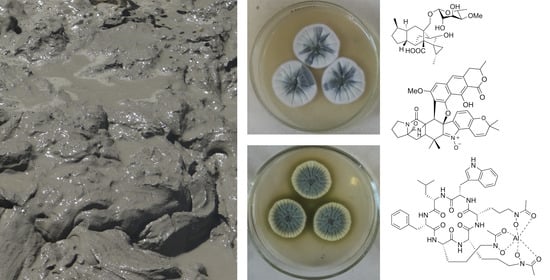
Metabolites of Marine Sediment-Derived Fungi: Actual Trends of Biological Activity Studies
Abstract
1. Introduction
2. Antiviral Activity
3. Antibacterial Activity
4. Antifungal Activity
5. Plankton Toxicity
6. Cytotoxic Activity
7. Anti-Inflammatory Activity
8. Radical Scavenging and Antioxidant Activities
9. Influence on Protein Activity and Expression
10. Other Activities
11. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
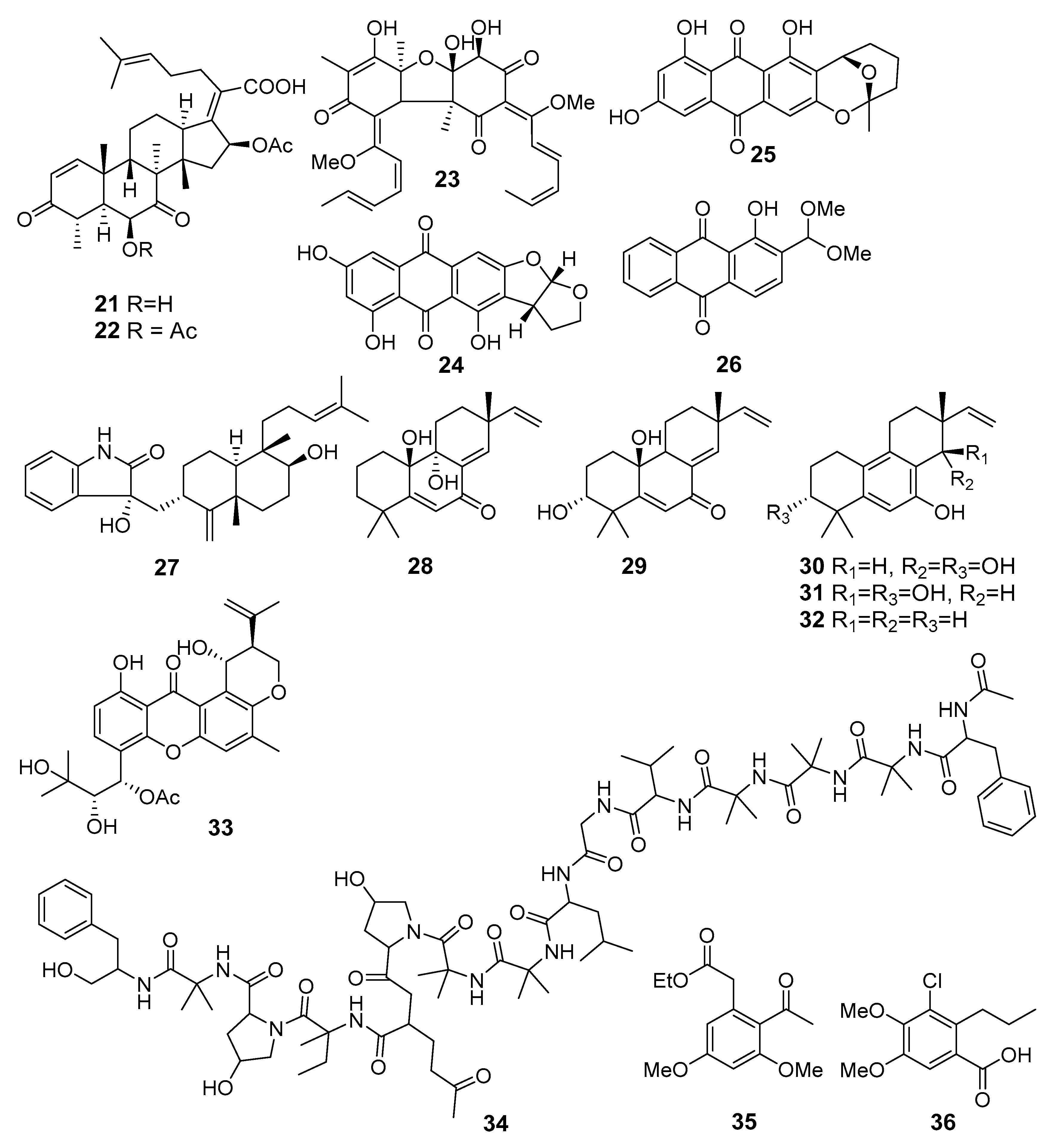
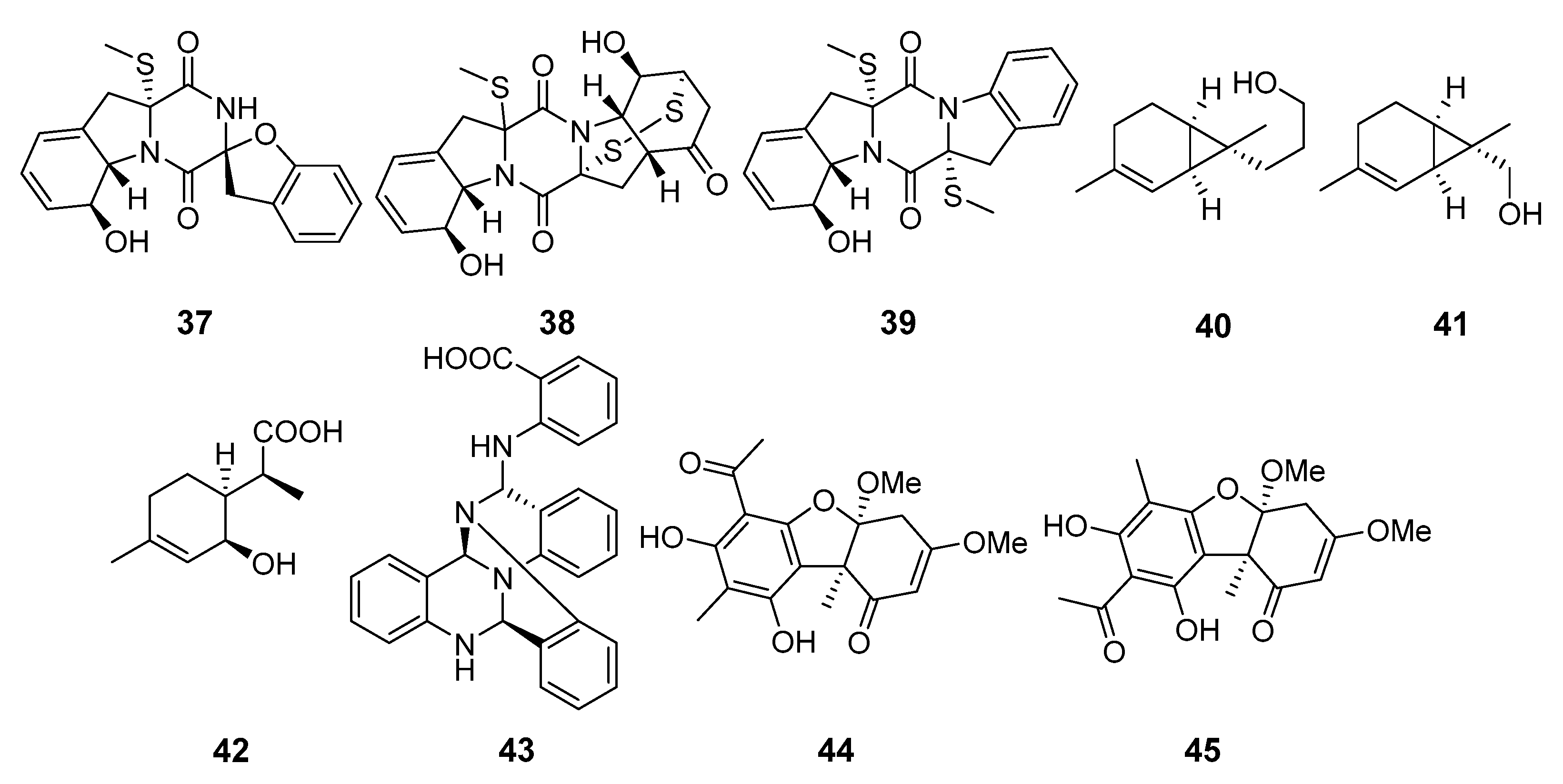
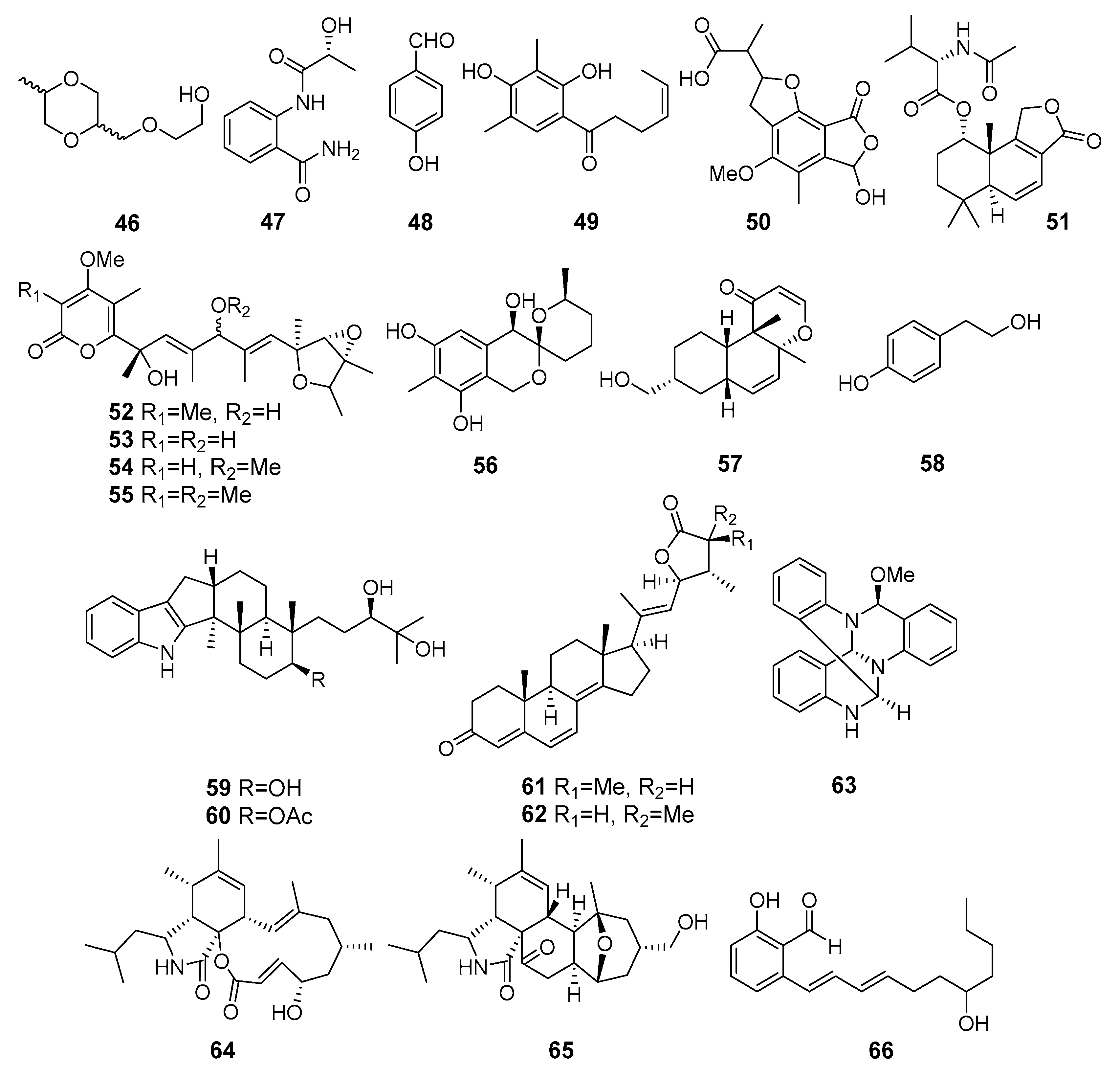
Appendix A
| Compound | Fungus | Isolation place | Activity | Ref. |
|---|---|---|---|---|
| Acremonpeptide A (1) | Acremonium persicinum SCSIO115 | The South China Sea | Antiviral (herpes simplex virus 1) | [15] |
| Acremonpeptide B (2) | ||||
| Al(III)—acremonpeptide D (3) | ||||
| Coniochaetone J (4) | Penicillium sp. SCSIO Ind16F01 | The Indian Ocean | Antiviral (enterovirus EV71), Cytotoxic (K562, MCF-7, SGC7901) | [16] |
| Epiremisporine B (5) | Antiviral (enterovirus EV71, influenza A virus subtype H3N2), Cytotoxic (K562, MCF-7, SGC7901) | |||
| Spiromastilactone D (6) | Spiromastix sp. | South of The Atlantic Ocean | Antiviral (a panel of influenza A and B viruses) | [17] |
| Diorcinol K (7) | Aspergillus sp. CUGB-F046 | The Bohai Sea, China | Antibacterial (SA, MRSA) | [19] |
| Diorcinol D (8) | ||||
| Diorcinol I (9) | ||||
| Aspergixanthone H (11) | Aspergillus sp. ZA-01 | The Bohai Sea | Antibacterial (Micrococcus lysodeikticus) | [20,21] |
| Aspergixanthone I (12) | Antibacterial (Vibrio parahemolyticus, V. anguillarum, and V. alginolyticus) | |||
| Aspergixanthone G (10) | ||||
| JG002CPA (13) | Aspergillus allahabadii | Jeju Island, Korea | Cytotoxic (A-549) | [22] |
| JG002CPB (14) | ||||
| FJ120DPA (15) | Aspergillus ochraceopetaliformis | |||
| FJ120DPB (16) | ||||
| Fiscpropionates A–D (17–20) | Aspergillus fischeri FS452 | Indian Ocean | Antibacterial (Mycobacterium tuberculosis protein tyrosine phosphatase B inhibition) | [23] |
| Helvolinic acid (21) | Aspergillus fumigatus MF071 | The Bohai Sea | Antibacterial (SA, E. coli) | [24] |
| Helvolic acid (22) | ||||
| Bisvertinolone (23) | Aspergillus protuberus MUT3638 | Hammerfest fiord, The Barents Sea | Antibacterial (SA) | [25] |
| Versicolorin B (24) | Aspergillus versicolor MF180151 | The Bohai Sea | Antibacterial (SA, MRSA) | [26] |
| Averufin (25) | ||||
| 2-(Dimethoxymethyl)-1-hydroxyanthracene-9,10-dione (26) | Aspergillus versicolor | West Pacific Ocean | Antibacterial (MRSA), inhibition of topoisomerase IV and AmpC β-lactamase enzymes activity | [27] |
| (3R,9S,12R,13S,17S,18S)-2-carbonyl-3-hydroxylemeniveol (27) | Aspergillus versicolor ZZ761 | Shengsi Island, The East China Sea | Antibacterial and antifungal (E. coli and Candida albicans) | [28] |
| Aspewentins D–H (28–32) | Aspergillus wentii SD-310 | The South China Sea | Antibacterial (Edwardsiella tarda, Micrococcus luteus, Pseudomonas aeruginosa, Vibrio harveyi, and V. parahemolyticus), antifungal (Fusarium graminearum) | [29] |
| Emerixanthone E (33) | Emericella sp. | The South China Sea | Antibacterial (SA, E. coli, Klebsiella pneumoniae, Enterococcus faecalis, Acinetobacter baumannii, Aeromonas hydrophila) | [30] |
| Emerimicin IV (34) | Emericellopsis minima | Talcahuano Bay, Chile | Bacteriostatic (MRSA, VRE) | [31] |
| Ethyl 3,5-dimethoxy-2-propionylbenzoate (35) | Engyodontium album | The Pacific Ocean | Antibacterial (MRSA and Vibrio vulnificus) | [32] |
| Engyodontiumin A (36) | Antibacterial (MRSA, Vibrio vulnificus, V. rotiferianus, and V. campbellii), antifungal (Aspergillus niger) | [33] | ||
| Eutypellazines P–R (37–39) | Eutypella sp. MCCC 3A00281 | The South Atlantic Ocean | Antibacterial (SA, VRE) | [34] |
| Eutypellol A (40) | Eutypella scoparia FS46 | The South China Sea | Antibacterial (SA) | [35] |
| Eutypellol B (41) | ||||
| 2-(2-Hydroxy-4-methylcyclohex-3-enyl)propanoic acid (42) | ||||
| Oxysporizoline (43) | Fusarium oxysporum | Suncheon Bay, South Korea, Korea Strait | Antibacterial (MRSA, MDRSA) | [36] |
| Mycousfuran A (44) | Mycosphaerella sp. | Donghae-si, Gangwon-do, South Korea, The Japanese Sea | Antibacterial (Kocuria rhizophila and SA) | [37] |
| Mycousfuran B (45) | ||||
| 2-[(5-Methyl-1,4-dioxan-2-yl)methoxy]ethanol (46) | Penicillium sp. M30 | Con Co Island, The South China Sea | Antibacterial (Enterococcus faecalis) | [38] |
| 2-[(2R-Hydroxypropanoyl)amino]benzamide (47) | Antibacterial (E. coli) | |||
| 4-Hydroxybenzandehyde (48) | ||||
| 2′,3′-Dihydrosorbicillin (49) | Inhibition of α-glucosidase activity | |||
| Penicacid D (50) | Penicillium sp. SCSIO sof101 | The South China Sea | Antibacterial (E. coli and Acinetobacter baumannii) | [39] |
| Purpuride D (51) | Penicillum sp. ZZ1283 | Karachi, Pakistan, The Arabian Sea | Antibacterial (MRSA, E. coli), antifungal (Candida albicans) | [40] |
| Penicyrone (52) | Penicillium sp. Y-50-10 | Kueishantao, Taiwan, The East China Sea | Antibacterial (Bacillus subtilis) | [41] |
| Norpenicyrone (53) | ||||
| Methyl norpenicyrone (54) | ||||
| Methyl penicyrone (55) | ||||
| 9-Dehydroxysargassopenilline A (56) | Penicillium cyclopium SD-413 | The East China Sea | Antibacterial (E. coli, E. ictaluri, Edwardsiella tarda, Micrococcus luteus, Vibrio anguillarum, and V. harveyi) | [42] |
| 1,2-Didehydropeaurantiogriseol E (57) | ||||
| Tyrosol (58) | Penicillium chrysogenum DXY-1 | Taiwan Strait | Antibacterial (inhibitor of the quorum sensing (QS) system in Chromobacterium violaceum andPseudomonas aeruginosa, biofilm formation in P. aeruginosa) | [43] |
| Penijanthines C (59) | Penicillium janthinellum | The Bohai Sea | Antibacterial (Vibrio spp.) | [44] |
| Penijanthine D (60) | ||||
| Penijanthoid A (61) | ||||
| Penijanthoid B (62) | ||||
| Thielaviazoline (63) | Thielavia sp. | Gomso Bay, The Yellow Sea | Antibacterial (MRSA, MDRSA) DPPH radical-scavenging | [45] |
| 16α-Methylaspochalasin J (64) | Westerdykella dispersa | The South China Sea | Antibacterial (Bacillus subtilis) | [46] |
| 16-Hydroxymethylaspergillin PZ (65) | Antibacterial (Proteus vulgaris, Enterobacter aerogenes) | |||
| 2-Hydroxy-6-((1E,3E)-7-hydroxyundeca-1,3-dienyl)benzaldehyde (66) | Zopfiella marina BCC 18240 (or NBRC 30420) | coast of Taiwan | Antibacterial (Mycobacterium tuberculosis H37Ra, Bacillus cereus) | [47] |
| Asperfurandione A (67) | Aspergillus versicolor | Dongji Island, The East China Sea | Antifungal (Gaeumannomyces graminis, Cryptococcus neoformans, Candida albicans) | [48] |
| Asperfurandione B (68) | ||||
| Pleosporalone A (69), | Pleosporales sp. CF09-1 | The Bohai Sea | Antigungal (Botrytis cinerea, Rhizopus oryzae, Phytophthora capsica) | [49,50] |
| Pleosporalone B (70) | Antifungal (Alternaria brassicicola, Fusarium oxysporum) | |||
| Pleosporalone C (71) | Antifungal (Botryosphaeria dothidea) | |||
| Ustusol A (72) | Antifungal (Thielaviopsis paradoxa, Pestalotia calabae, and Gloeosporium musarum) | [51] | ||
| 12R,13R)-Dihydroxylanomycinol (73), | Westerdykella dispersa | The South China Sea | Antifungal (Rhizoctorzia solani, Verticillium dahliae, Helminthosporium maydis, Fusarium tricinctum, F. oxysporum, Botryosphaeria dothidea, and Alternaria fragriae) | [52] |
| (12S,13S)-Dihydroxylanomycinol (74) | ||||
| (12R,13S)-Dihydroxylanomycinol (75) | ||||
| (12S,13R)-Dihydroxylanomycinol (76) | ||||
| (12S,13R)-N-Acetyl-dihydroxylanomycin (77) | ||||
| (12S,13S)-N-Acetyl-dihydroxylanomycin (78) | ||||
| Lanomycinol (79) | ||||
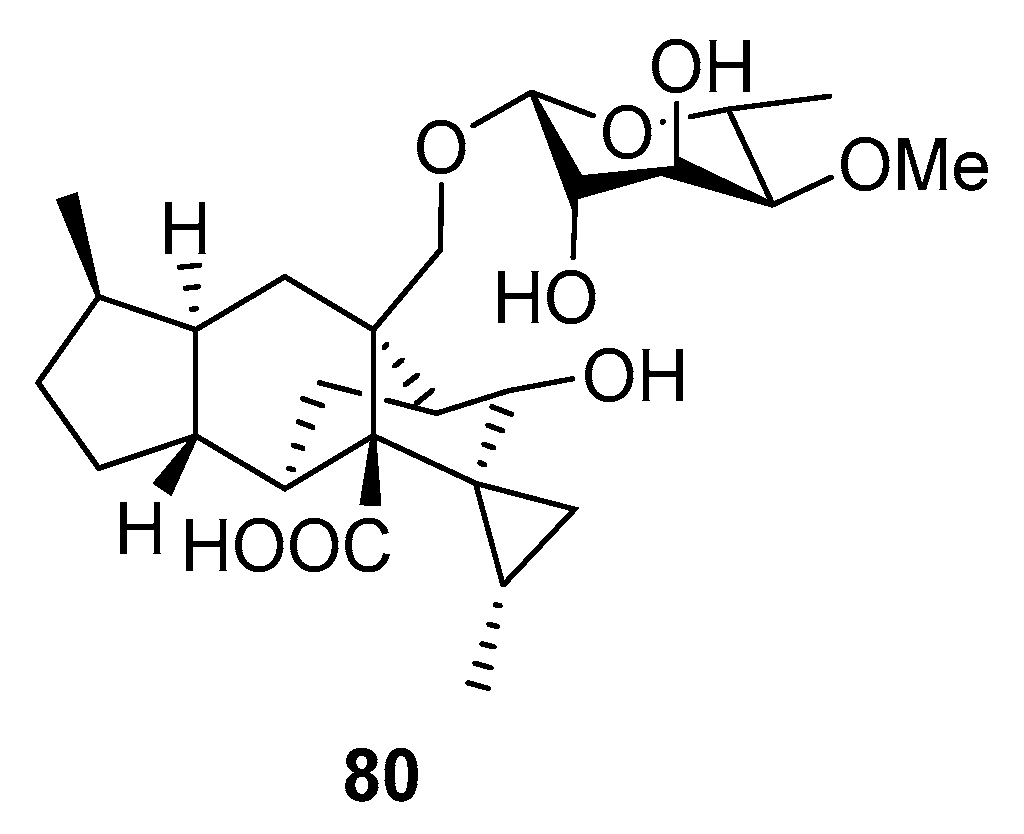
| Trichosordarin A (80) | Trichoderma harzianum R5 | The Bohai Sea | Toxicity for plankton (Artemia salina, Amphidinium carterae and Phaeocystis globose) | |
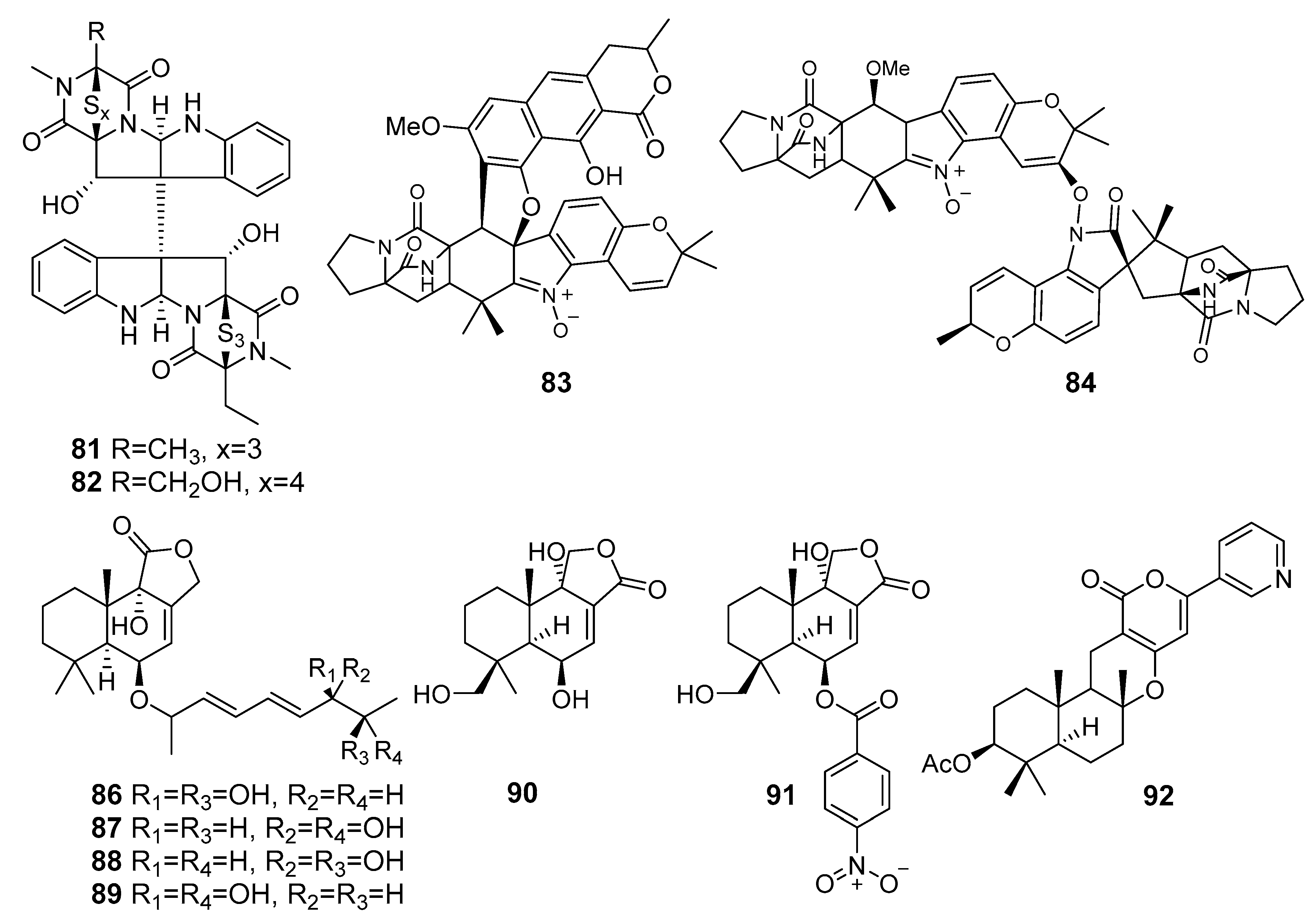
| Chetracin E (81) | Acrostalagmus luteoalbus HDN13-530 | Liaodong Bay, The Bohai Sea | Cytotoxicity, Inhibition of Hsp90 | [57] |
| Chetracin F (82) | ||||
| Waikikiamide A (83) | Aspergillus sp. FM242 | Waikiki beach, Oahu, Honolulu, Hawaii | Antiproliferative activity (HT1080, PC3, Jurkat, A2780 line cells) | [58] |
| Waikikiamide C (84) | ||||
| Asperienes A–D (86–89) | Aspergillus flavus CF13-11 | The Bohai Sea | Cytotoxicity (HeLa, MCF-7, MGC-803 and A549 cell lines) | [59] |
| 6β,9α,14-Trihydroxycinnamolide (90) | Aspergillus flocculosus | Nha Trang Bay, The South China Sea | Cytotoxicity (22Rv1, MCF-7, Neuro-2a) | [60] |
| Insulicolide A (91) | ||||
| Pyripyropene E (92) | Aspergillus fumigatus YK-7 | Yingkou, The Bohai Sea | Cytotoxicity (U937 cell line) | [61] |
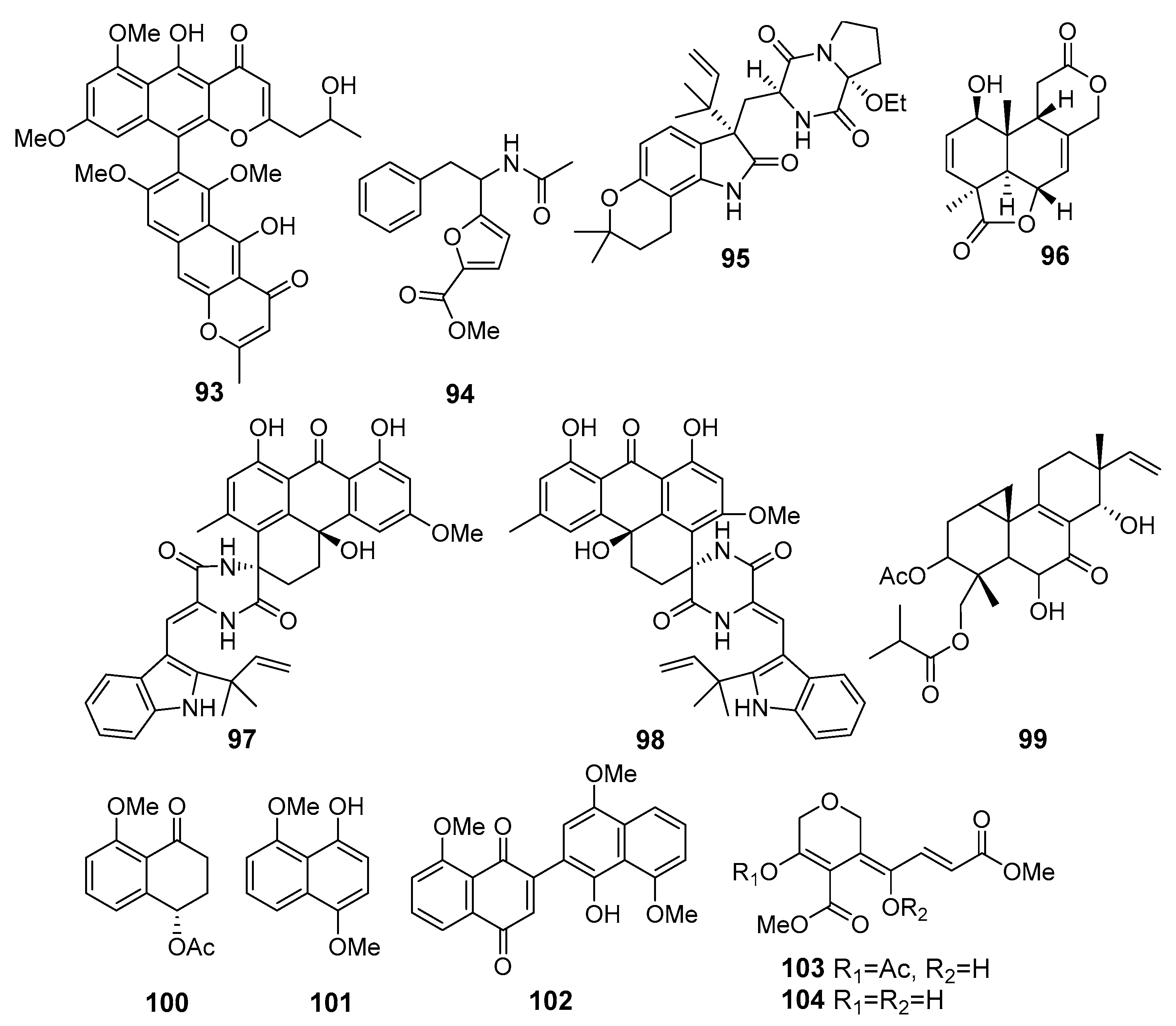
| Aurasperone H (93) | Aspergillus niger 2HL-M-8 | Northeast Brazilian coast, The Atlantic Ocean | Cytotoxicity (A549, HL-60) | [62] |
| Methyl 5-(1-acetamido-2-phenylethyl)furan-2-carboxylate (94) | Aspergillus niger | Northeast Brazilian coast, The Atlantic Ocean | Cytotoxicity (HCT-116) | [63] |
| 17-O-ethylnotoamide M (95) | Aspergillus sulphureus KMM 4640 and Isaria felina KMM 4639 | East Sakhalin shelf, The Sea of OkhotskThe South China Sea, coast of Vietnam | Inhibition of the colony formation (22Rv1 cells) | [64] |
| Asperolide E (96) | Aspergillus wentii SD-310 | The South China Sea | Cytotoxicity (HeLa, MCF-7, NCI-H446 line cells) | [65] |
| Variecolortin A (97) | Eurotium sp. SCSIO F452 | The South China Sea | Cytotoxicity (SF-268, HepG2 cell lines) | [66] |
| Variecolortin B (98) | ||||
| Scopararane I (99) | Eutypella sp. FS46 | The South China Sea | Cytotoxicity (MCF-7, NCI-H460, SF-268) - - | [67] |
| Hypoxone A (100) | Hypoxylon rubiginosum FS521 | The South China Sea | ||
| 4,8-Dimethoxy-1-naphthol (101) | ||||
| 1’-Hydroxy-4’,8,8’-trimethoxy[2,2’]binaphthalenyl-1,4-dione (102) | Cytotoxicity (SF-268, MCF-7, HepG-2, and A549) | [68] | ||
| Isariketide A (103) | Isaria felina | The South China Sea, coast of Vietnam | Cytotoxicity (human prostate cancer cells) - | [69] |
| Isariketide B (104) | ||||
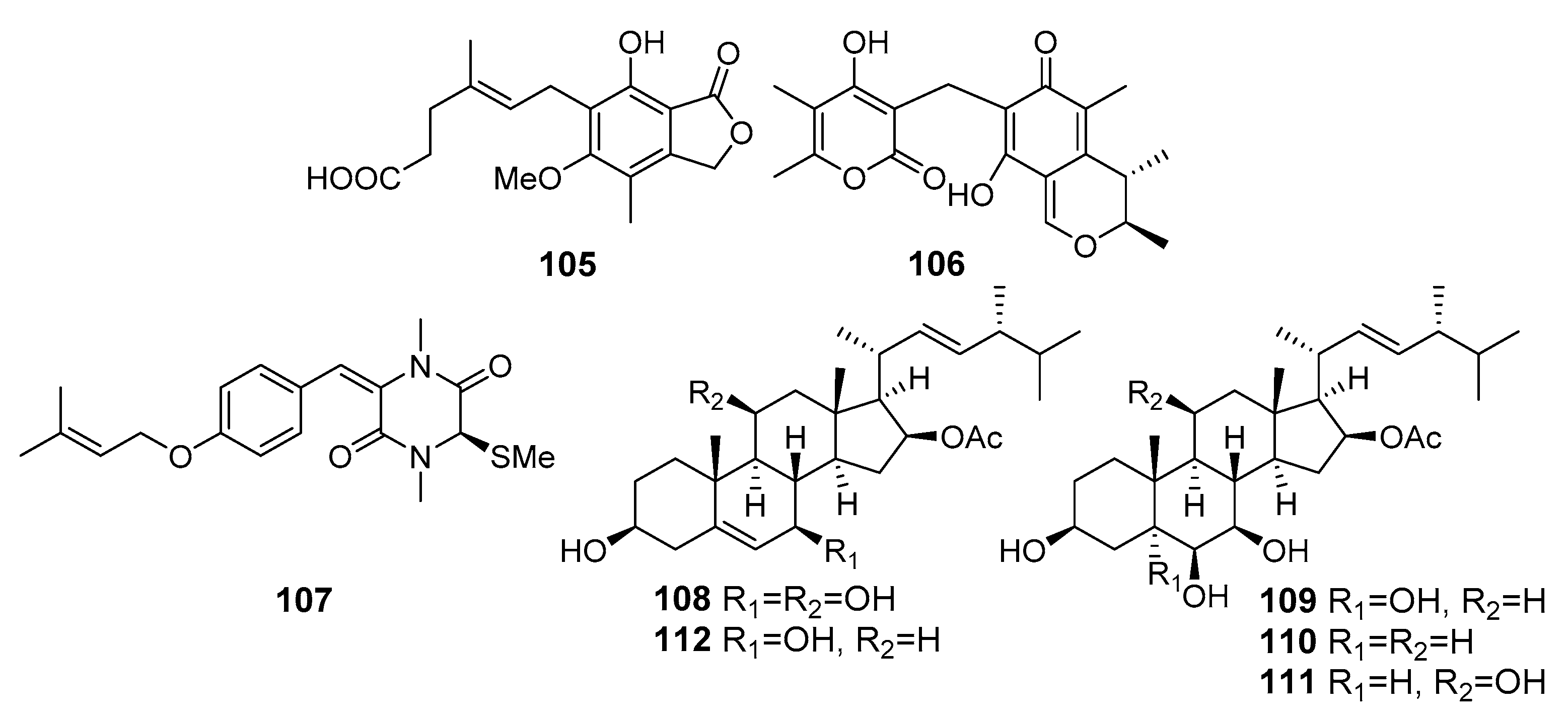
| Mycophenolic acid (105) | Penicillium brevicompactum OUCMDZ-4920 | The South China Sea | Cytotoxicity (P388, KB, HT29, MCF-7, A549) | [70] |
| Dicitrinone D (106) | Penicillium citrinum | Southeast coast of China | Cytotoxicity (SPC-A1) | [71] |
| Fusaperazine F (107) | Penicillium crustosum HDN153086 | Prydz Bay, The Antarctic Ocean | Cytotoxicity (K562) | [72] |
| Penicisteroid E (108) | Penicillium granulatum MCCC 3A00475 | Antarctica | Antiproliferative effect (12 cancer cell lines)s | [73] |
| Penicisteroid G (109) | ||||
| Penicisteroid H (110) | ||||
| Penicisteroid A (111) | ||||
| Penicisteroid C (112) | ||||
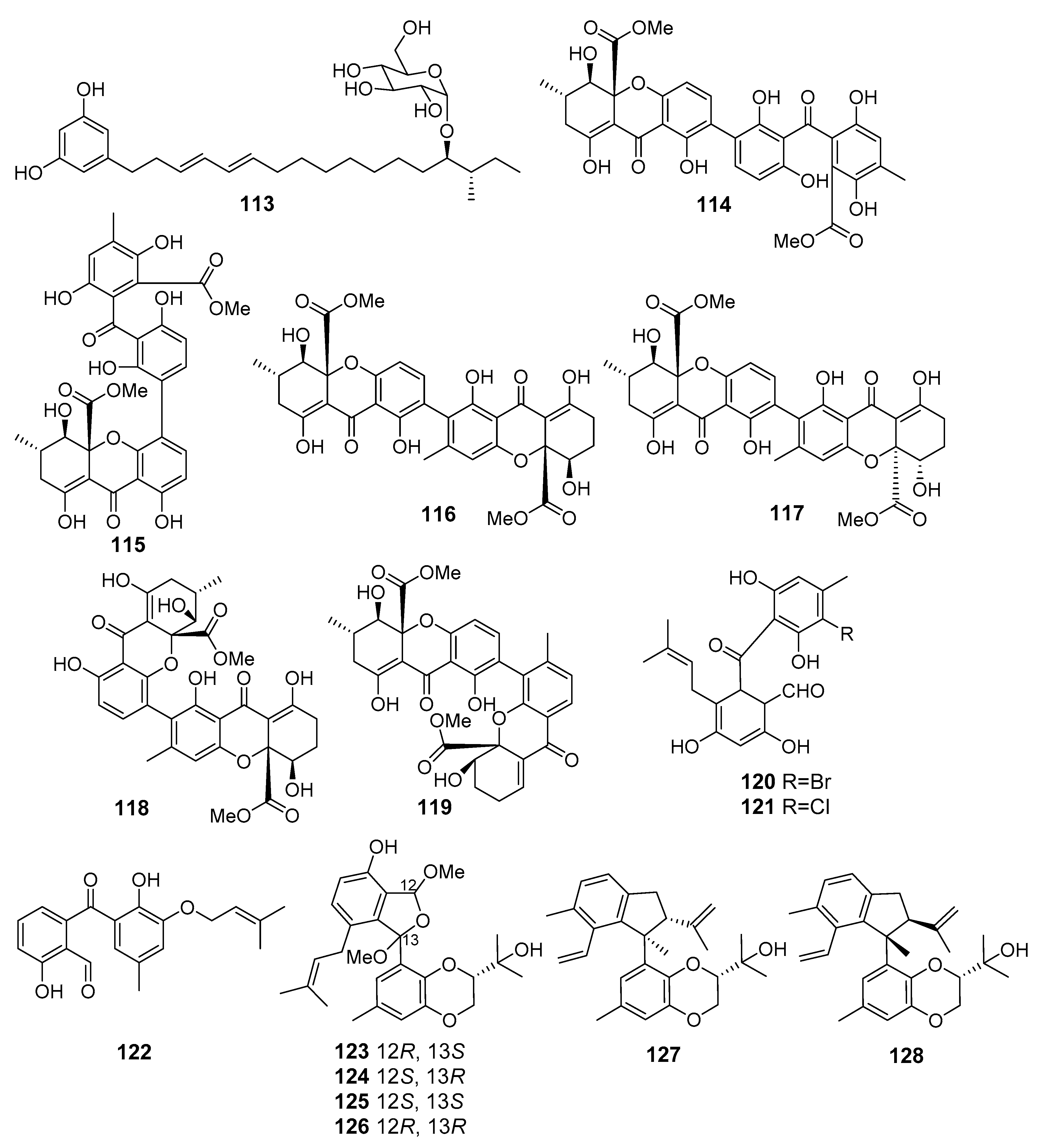
| Resorcinoside A (113) | Penicillium janthinellum | Cu Lao Cham Island, The South China Sea | Cytotoxicity (NUGC-3) | [74] |
| Secalonic acid H (114) | Penicillum oxalicum fungus (Langqi Island, Taiwan Strait) | Cytotoxicity (HCT116, KB, EC9706) | [75] | |
| Secalonic acid I (115) | [75] | |||
| Secalonic acid J (116) | Cytotoxicity (HeLa, HCT116, MCF-7, Hep-3B, A549) | [76] | ||
| Secalonic acid K (117) | ||||
| Secalonic acid L (118) | ||||
| Secalonic acid M (119) | ||||
| Pestalone C (120) Pestalone E (121) | Pestalotiopsis neglecta | Gagedo Island, The Yellow Sea | Antiproliferative and apoptotic activity (PANC-1) | [77] |
| Tenellone H (122) | Phomopsis lithocarpus FS508 | The Indian Ocean | Cytotoxicity (HepG-2, A549) | [78] |
| Lithocarols A–D (123–126) | Cytotoxicity (HepG-2, MCF-7, SF-268, and A549) | [79] | ||
| Lithocarpinols A (127) and B (128) | [80] | |||
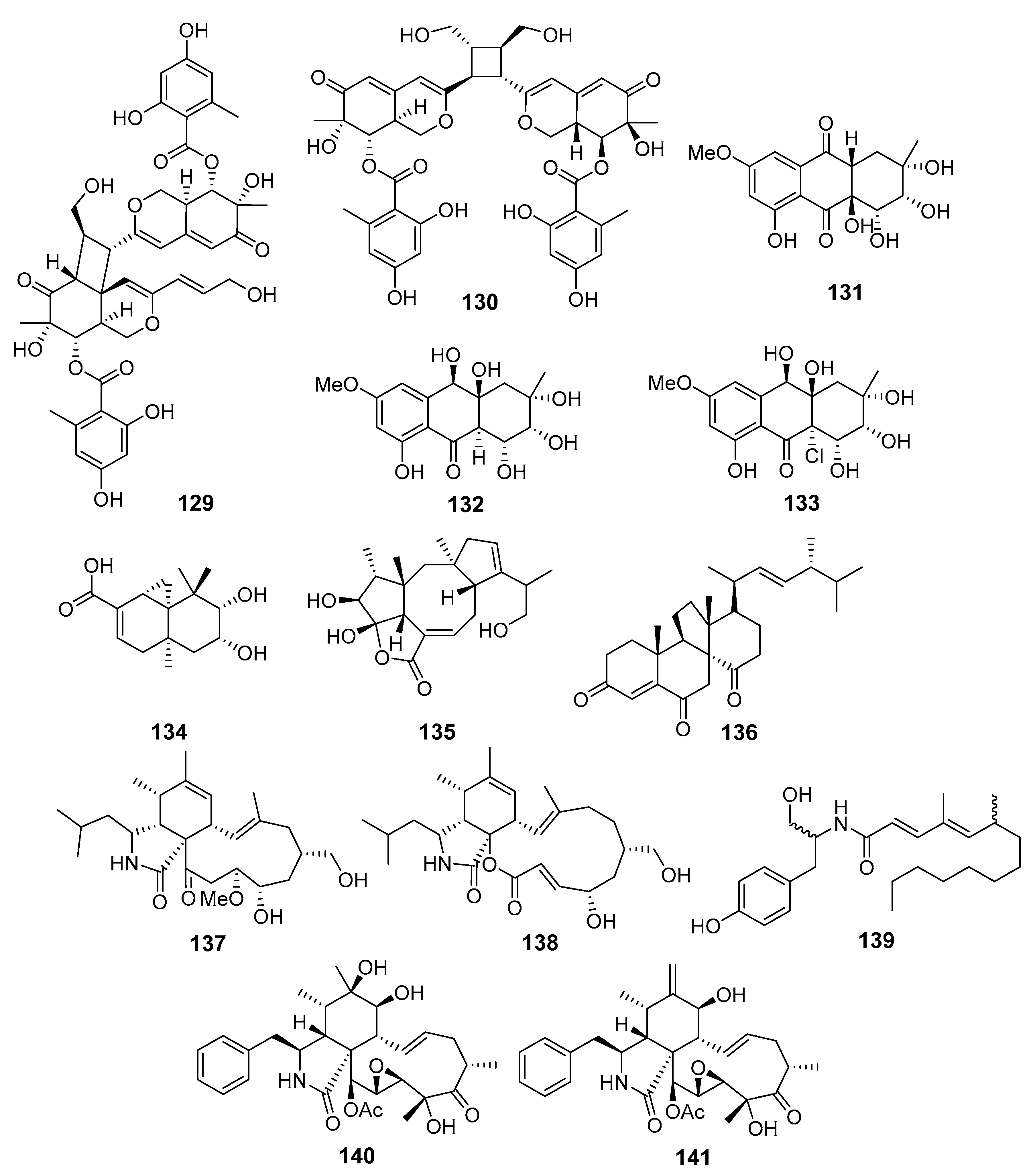
| Dipleosporalones A (129) and B (130) | Pleosporales sp. | Coast of Huanghua, The Bohai Sea | Cytotoxicity (MDA-MB-231, MCF-7, MGC-803, HeLa, A549) | [81] |
| Auxarthrols D (131) and F (132) | Sporendonema casei | Zhangzi Island, The Yellow Sea | Cytotoxicity (a few human cancer cells) | [82] |
| Altersolanol B (133) | Antibacterial (Mycobacterium phlei, Bacillus subtilis, Vibrio parahemolyticus and Pseudomonas aeruginosa) | |||
| 9,10-Diolhinokiic acid (134) | Talaromyces purpurogenus | Coast of Qinghuangdao, The Bohai Sea | Cytotoxicity (HL-60 and A549) | [83] |
| Roussoellol C (135) | Cytotoxicity (MCF-7 and HL-60) | |||
| Dankasterone (136) | Cytotoxicity (MCF-7 and HL-60) | |||
| 19-Methoxy-19,20-dihydrophomacin C (137) | Westerdykella dispersa | Guangzhou, China, The South China Sea | Cytotoxicity (MCF-7, HepG2, A549, HT-29 and SGC-7901) | [84] |
| Gymnastatin Z (138) | ||||
| Phomacin B (139) | ||||
| Cytochalasin P1 (140) | Xylaria sp. SOF11 | The South China Sea | Cytotoxicity (MCF-7, SF-268) | [85] |
| 19, 20-Epoxycytochalasin D (141) | ||||
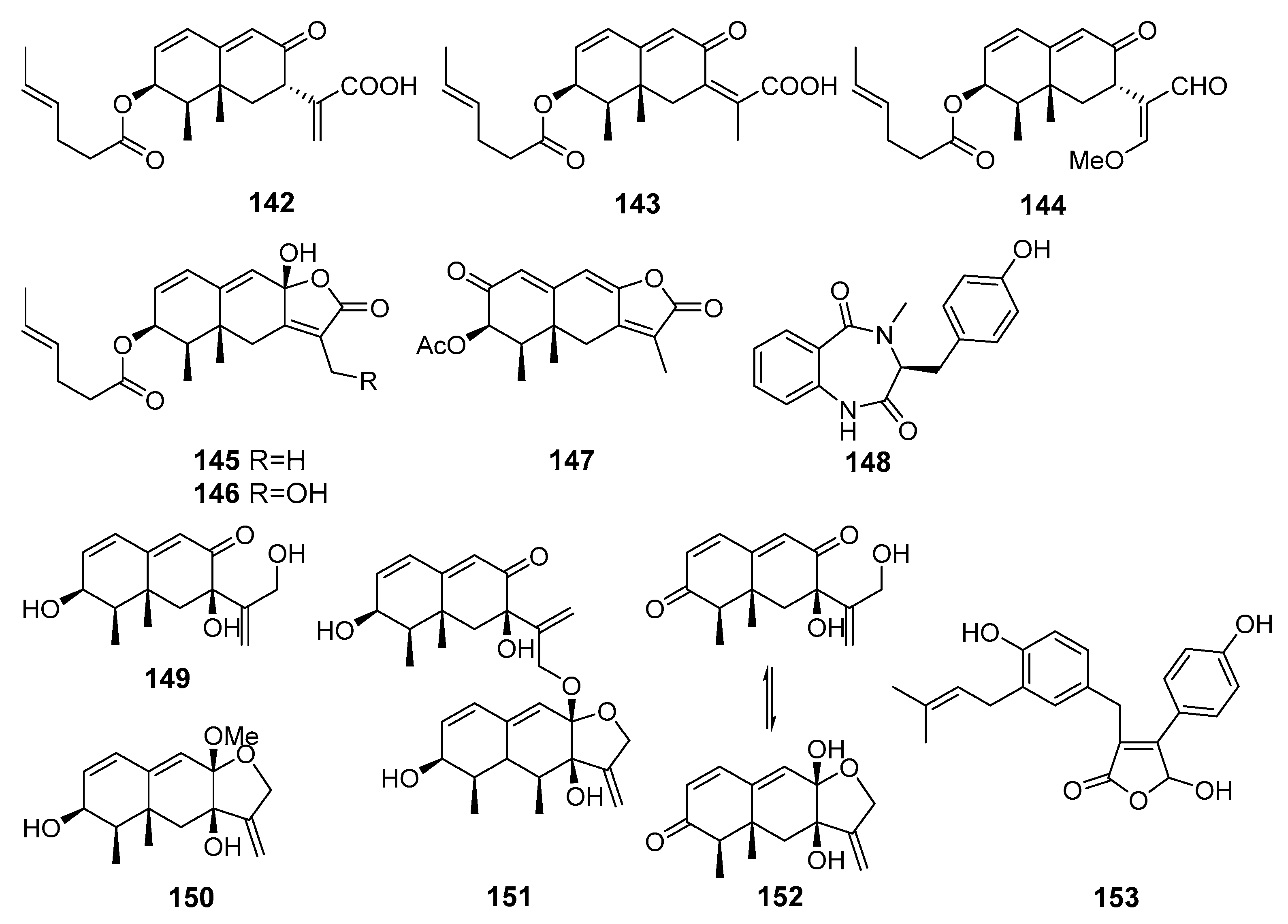
| Acremeremophilanes B–F (142–146) and N (147) | Acremonium sp. | South part of The Atlantic Ocean | Inhibition of NO production | [88] |
| 14-Hydroxy-cyclopeptine (148) | Aspergillus sp. SCSIOW2 | The South China Sea | Inhibition of NO production | [89] |
| Dihydrobipolaroxin (149) | [90] | |||
| Dihydrobipolaroxin B (150) | ||||
| Dihydrobipolaroxin C (151) | ||||
| Dihydrobipolaroxin D (152) | ||||
| Asperteretal F (153) | Aspergillus terreus Y10 | Coastal area of Hainan, The South China Sea | Inhibition of TNF-α generation | [91] |
| Asperversiamide G (154) | Aspergillus versicolor | The South China Sea | Inhibition of iNO synthase activity | [92] |
| 6-[1-(2-aminobenzoyloxy)ethyl]-1-phenazinecarboxylic acid (155) | Cystobasidium larynigs | The Indian Ocean | Inhibition of NO production | [93] |
| Saphenol (156) | ||||
| Saphenic acid (157) | ||||
| Phenazine-1-carboxylic acid (158) | ||||
| 6-(1-Hydroxyehtyl)phenazine-1-carboxylic acid (159) | ||||
| 6-Acetylphenazine-1-carboxylic acid (160) | ||||
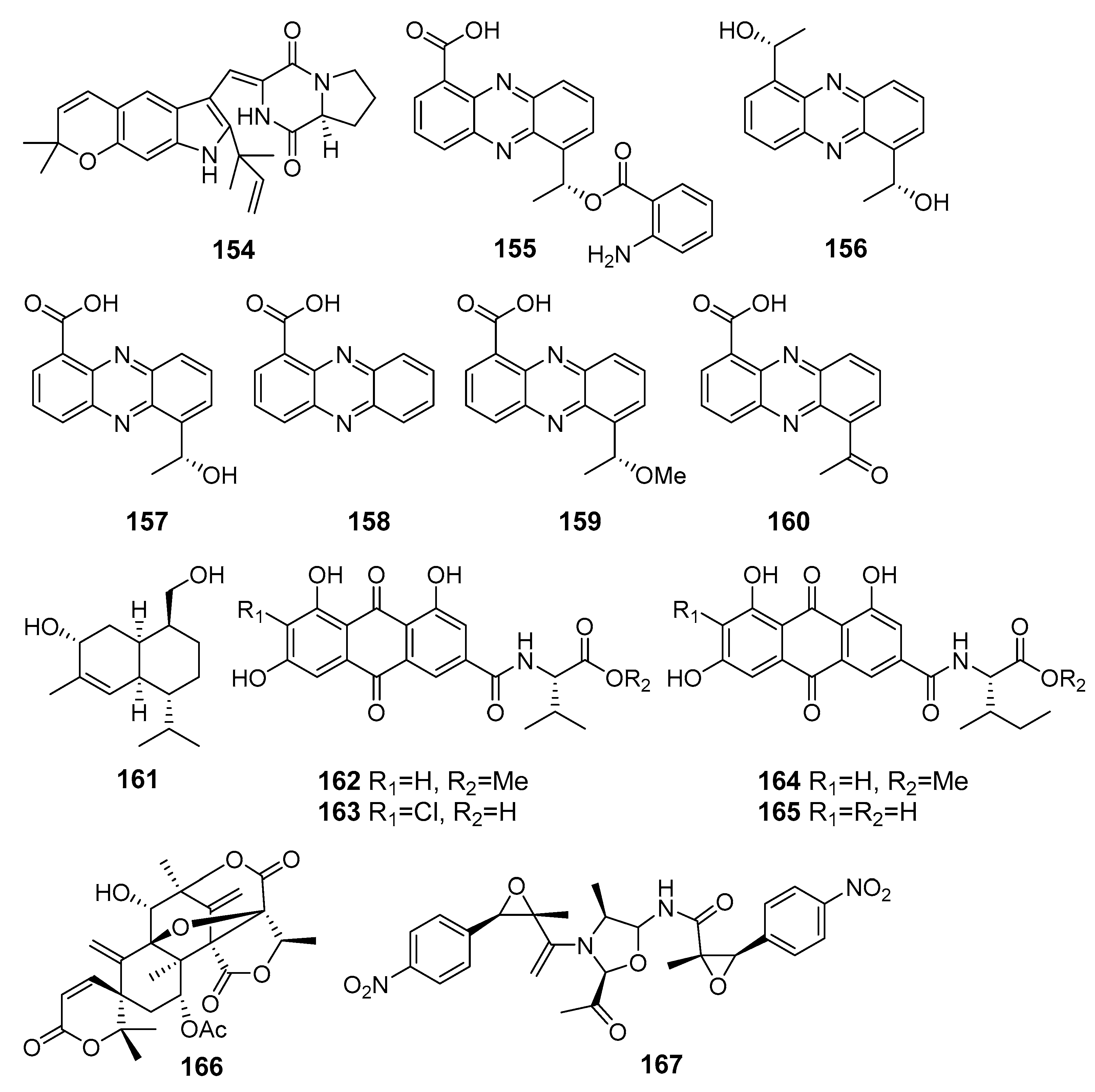
| Khusinol B (161) | Graphostroma sp. MCCC 3A00421 | The Atlantic Ocean | Inhibition of NO production | [94] |
| Emodacidamides A (162), C–E (163–165) | Penicillium sp. SCSIO sof101 | Inhibition of interleukin-2 secretion | [95] | |
| 7-Acetoxydehydroaustinol (166) | Penicillium sp. F-5497 | Busan, South Korea, Korean Strait | Suppression of NO overproduction | [96] |
| Chrysamide C (167) | Penicillium chrysogenum SCSIO41001 | The Indian Ocean | Suppression of interleukin-17 production | [97] |
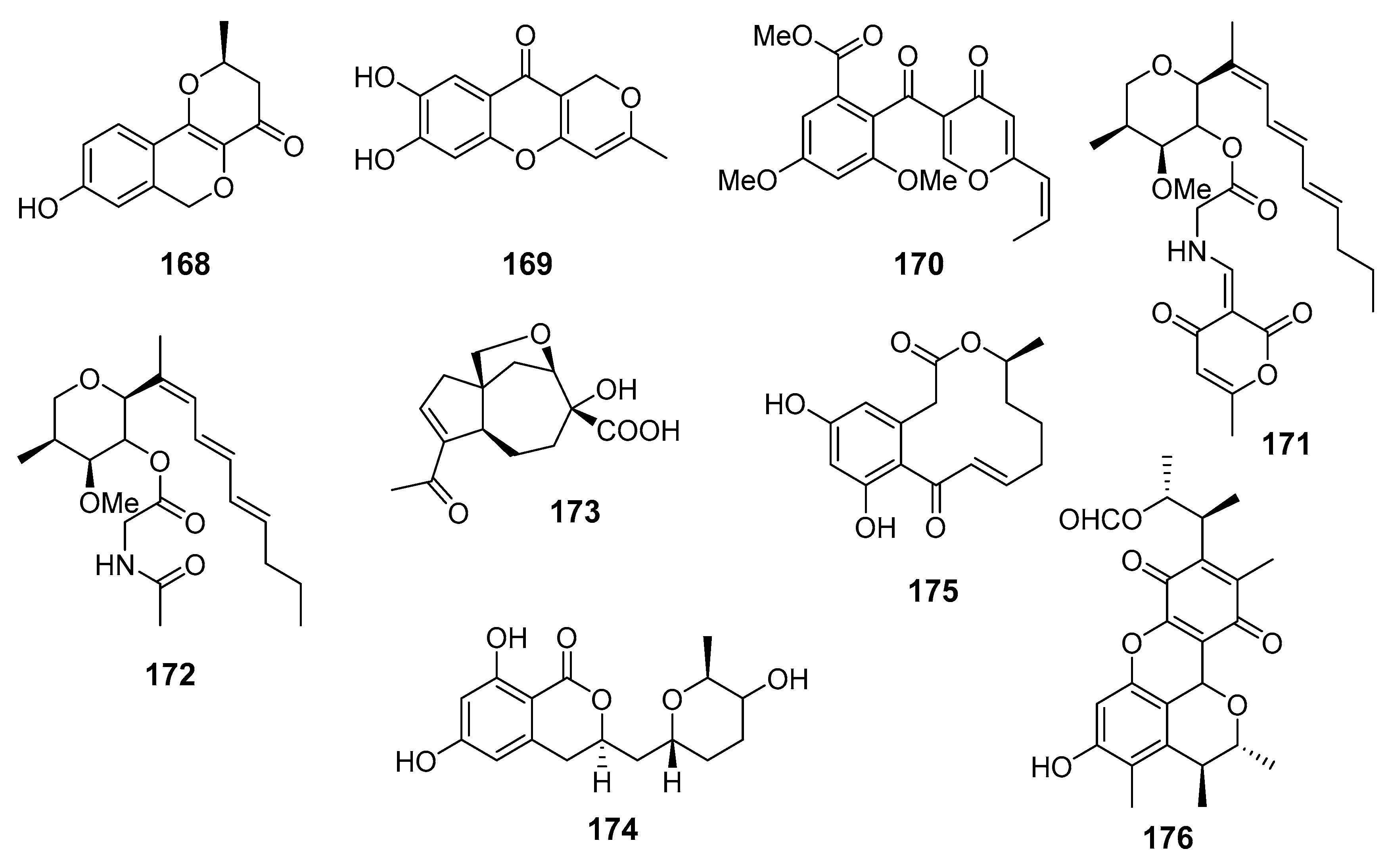
| Neuchromenin (168) | Penicillium glabrum SF-7123 | The Ross Sea, Antarctica | Inhibition of NO and prostaglandin E2 production | [98] |
| Myxotrichin C (169) | ||||
| Deoxyfunicone (170) | ||||
| Restricticin B (171) | Penicillium janthinellum | Cu Lao Cham Island, The South China Sea | Suppression of pro-inflammatory mediators production | [99] |
| N-acetyl restricticin (172) | ||||
| Piltunine E (173) | Penicillium piltunense KMM 4668 | Sakhalin Island, The Sea of Okhotsk | Downregulation of ROS production | [100] |
| 5′-Hydroxyasperentin (174) | ||||
| Decreasing of NO production | ||||
| Dehydrocurvularin (175) | Penicillium sumatrense | The Indian Ocean | Inhibition of NO production | [101] |
| Citrinin H1 (176) | Penicillium sp. SF-5629 | Ulgin, South Korea, The Sea of Japan | Inhibition of NO and prostaglandin E2 production | [102] |
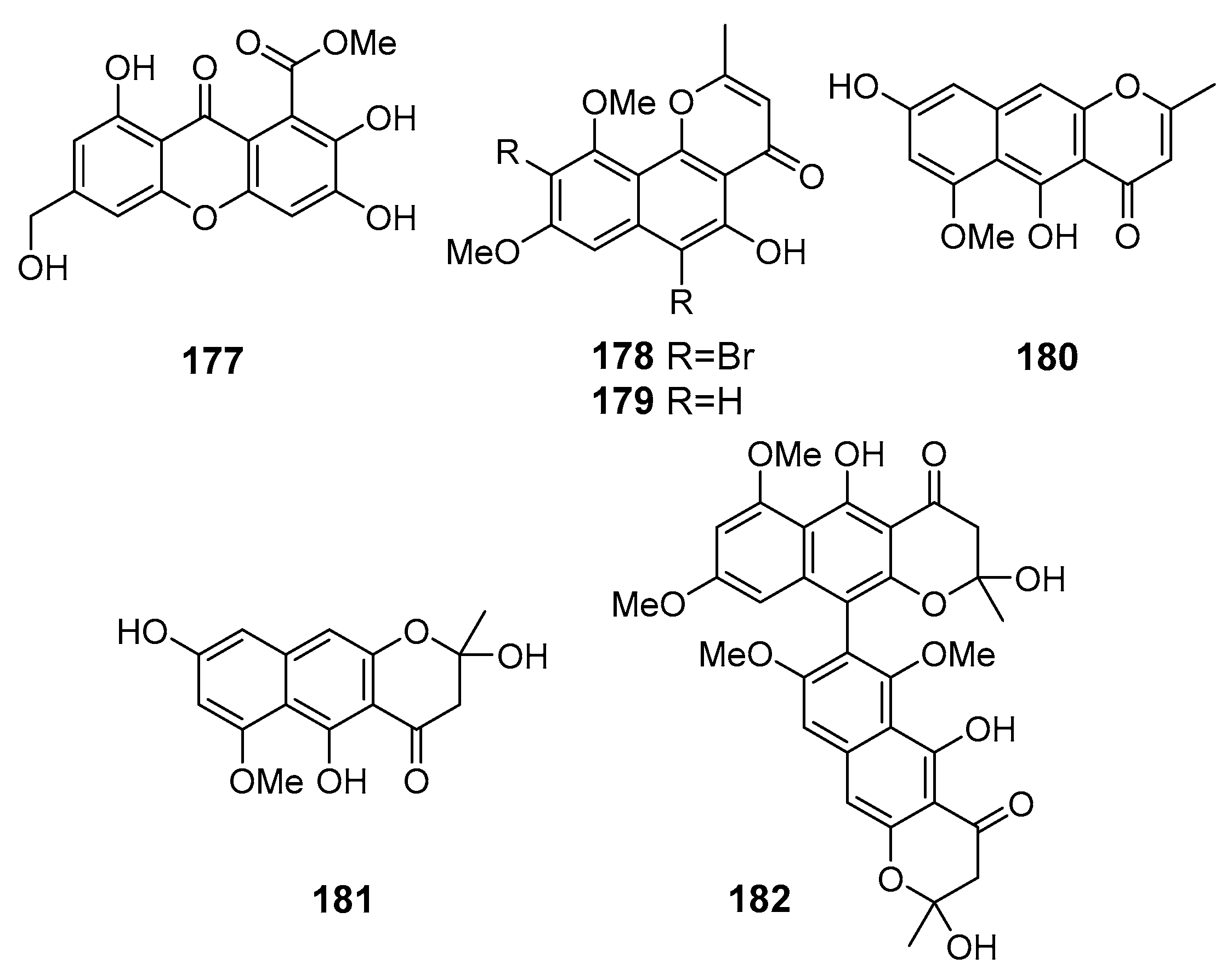
| Arthone C (177) | Arthrinium sp. UJNMF0008 | The South China Sea | DPPH- and ABTS-radical scavenging | [104] |
| 6,9-Dibromoflavasperone (178) | Aspergillus niger | Suncheon Bay, South Korea, Korean Strait | DPPH-radical scavenging | [105] |
| Flavasperone (179) | ||||
| TMC-256A1 (180) | ||||
| Fonsecin (181) | ||||
| Aurasperone B (182) | ||||
| Oxisterigmatocystin D (183) | Aspergillus versicolor | The South China Sea | Antioxidant activities in TEAC assay | [106] |
| Oxisterigmatocystin C (184) | Antioxidant activities in TEAC assay | |||
| Sterigmatocystine (185) | ||||
| Dihydrosterigmatocystine (186) | ||||
| Versicolorin B (187) | ||||
| UCT1072M1 (188) | Antioxidant activities in TEAC assay, Activation of Nrf2-regulated gene expression | |||
| Averantin (189) | ||||
| Methylaverantin (190) | Antioxidant activities in TEAC assay | |||
| Averythrin (191) | Antioxidant activities in TEAC assay, Activation of Nrf2-regulated gene expression | |||
| Averufanin (192) | Antioxidant activities in TEAC assay | |||
| Averufine (193) | ||||
| Nidurufin (194) | Antioxidant activities in TEAC assay, Activation of Nrf2-regulated gene expression | |||
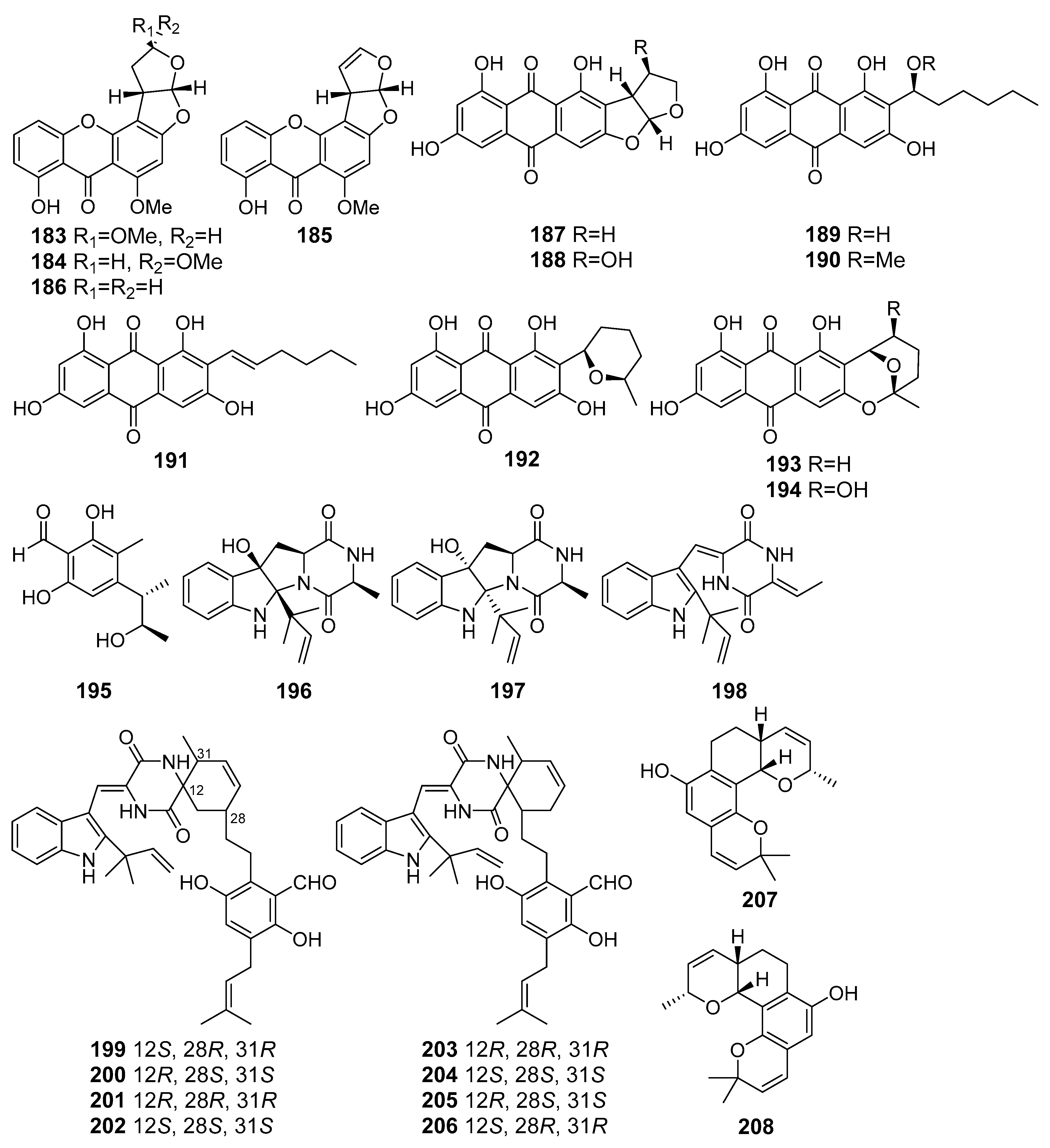
| Cladosporin D (195) | Cladosporium sp. SCSIO z015 | Okinawa Trough, The East China Sea | DPPH radical scavenging | [107] |
| Eurotiumins A–C (196–198) | Eurotium sp. SCSIO F452 | The South China Sea | DPPH-radical scavenging | [108] |
| Eurotinoids A–C (199–204) | [109] | |||
| (+)-Dihydrocryptoechinulin D (205) | Cytotoxicity (SF-268, HepG2) DPPH-radical scavenging | |||
| (-)-Dihydrocryptoechinulin D (206) | ||||
| (+)-Euroticin B (207) | Antioxidant activities | [110] | ||
| (–)-Euroticin B (208) | ||||
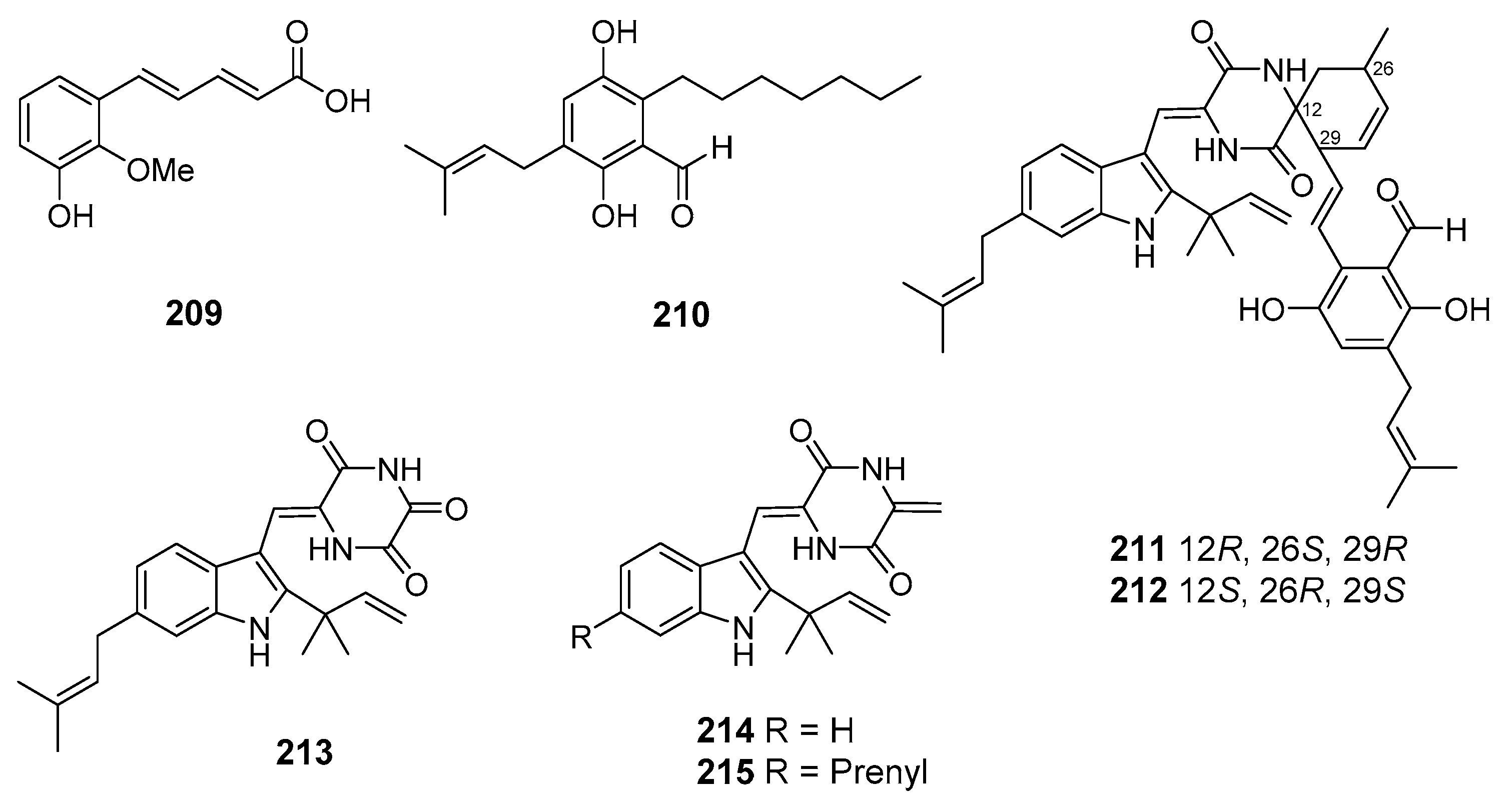
| Niveoglaucin A (209) | Aspergillus niveoglaucus | Nha Trang Bay, The South China Sea | Suppression of ROS hyperproduction, Neuroprotective activity | [111] |
| Flavoglaucin (210) | [111] | |||
| (+)-Cryptoechinuline B (211) | [112] | |||
| (–)-Cryptoechinuline B (212) | ||||
| Neoechinulin (213) | ||||
| Neoechinulines B–C (214–215) | ||||
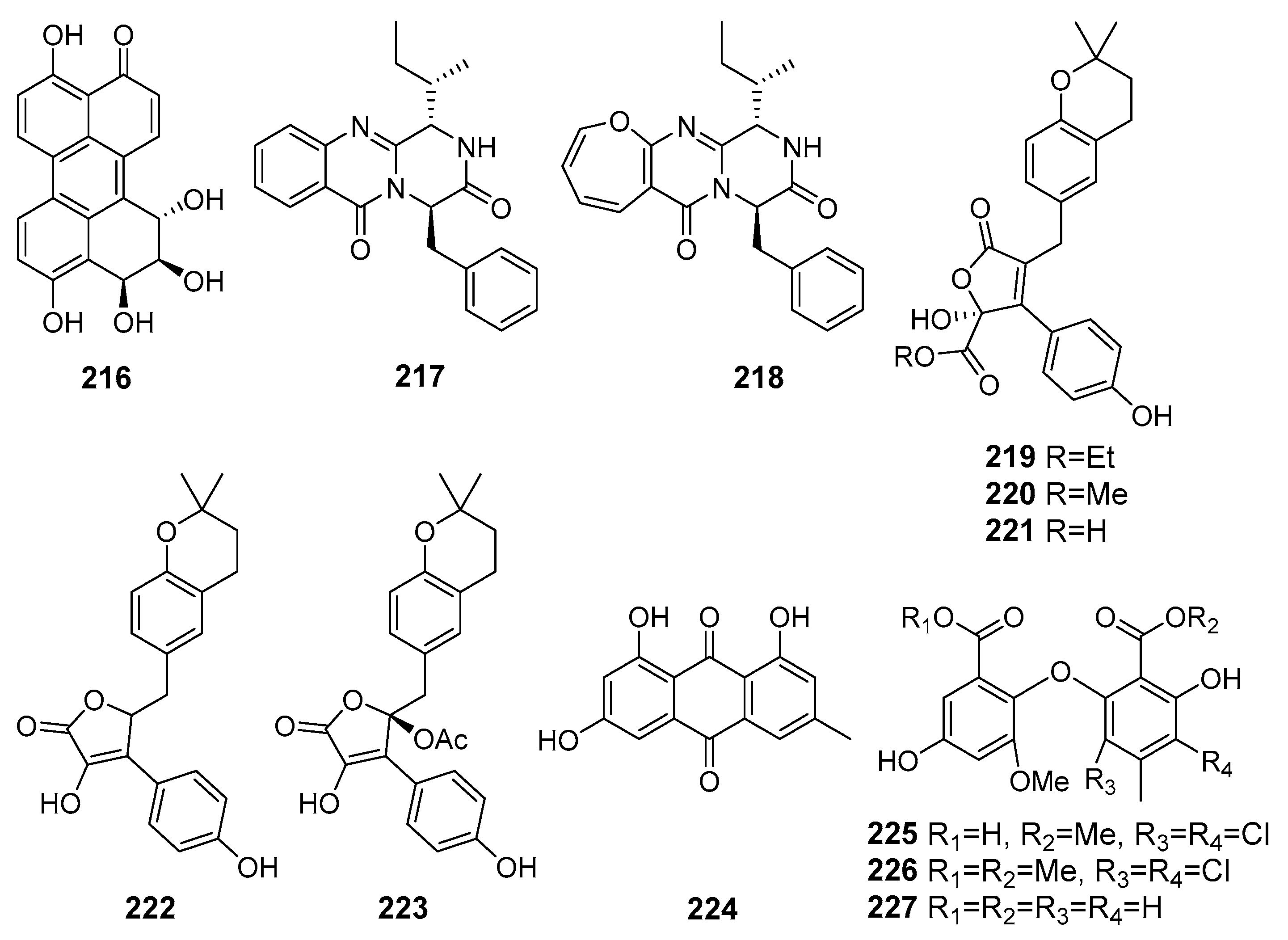
| Perylenequinone (216) | Alternaria sp. NH-F6 | The South China Sea | Inhibition of BRD4 protein | [122] |
| Protuboxepin K (217) | Aspergillus sp. BFM-0085 | Tokyo Bay, Tokyo, Japan | Inhibition of (BMP)-induced alkaline phosphatase activity | [123] |
| Protuboxepin A (218) | ||||
| Flavipesolides A–C (219–221) | Aspergillus flavipes HN4-13 | The Yellow Sea | Inhibition of α-glucosidase activity | [124] |
| 5-[(3,4-Dihydro-2,2-dimethyl-2H-1-benzopyran-6-yl)methyl]-3- hydroxy-4-(4-hydroxyphenyl)-2(5H)furanone (222) | ||||
| Aspernolide A (223) | ||||
| Emodin (224) | ||||
| Geodin hydrate (225) | ||||
| Methyl dichloroasterrate (226) | ||||
| Monomethylosoic acid (227) | ||||
| Asperversin G (228) | Aspergillus versicolor | The South China Sea | Inhibition of AChE activity | [125] |
| 7-Chlorofolipastatin (229) | Aspergillus ungui | Suruga Bay, Japan | Inhibition of SOAT1- and SOAT2-expressing | [126] |
| Biscogniauxone (230) | Biscogniauxia mediterranea | Herodotes Deep, The Mediterranean Sea | Inhibition of glycogen synthase kinase (GSK-3β) activity | [127] |
| Cyclo-(L-Phe-L-Leu-L-Val-L-Leu-L-Leu) (231) | ||||
| Preaustinoid A6 (232) | Penicillium sp. SF-5497 | Gijang-gun, Busan, South Korea, Korean Strait | Inhibition of PTP1B activity | [128] |
| Berkeleyone C (233) | ||||
| Butanolide A (234) | Penicillium sp. S-1-18 | Antarctica | [129] | |
| Austalide V (235) | Penicillium rudallense | Ga-geo Island, South Korea, The Yellow Sea | Inhibition of (RANKL)-induced osteoclast differentiation | [130] |
| Austalide W (236) | ||||
| Diorcinol J (237) | Aspergillus sulphureus KMM 4640 (The Sea of Okhotsk) and Isaria felina KMM 4639 (The South China Sea) | Hemolytic activity and cytotoxicity (Ehrlich carcinoma) | [131] | |
| Diorcinol B (238) | Enhancing of Hsp70 expression | |||
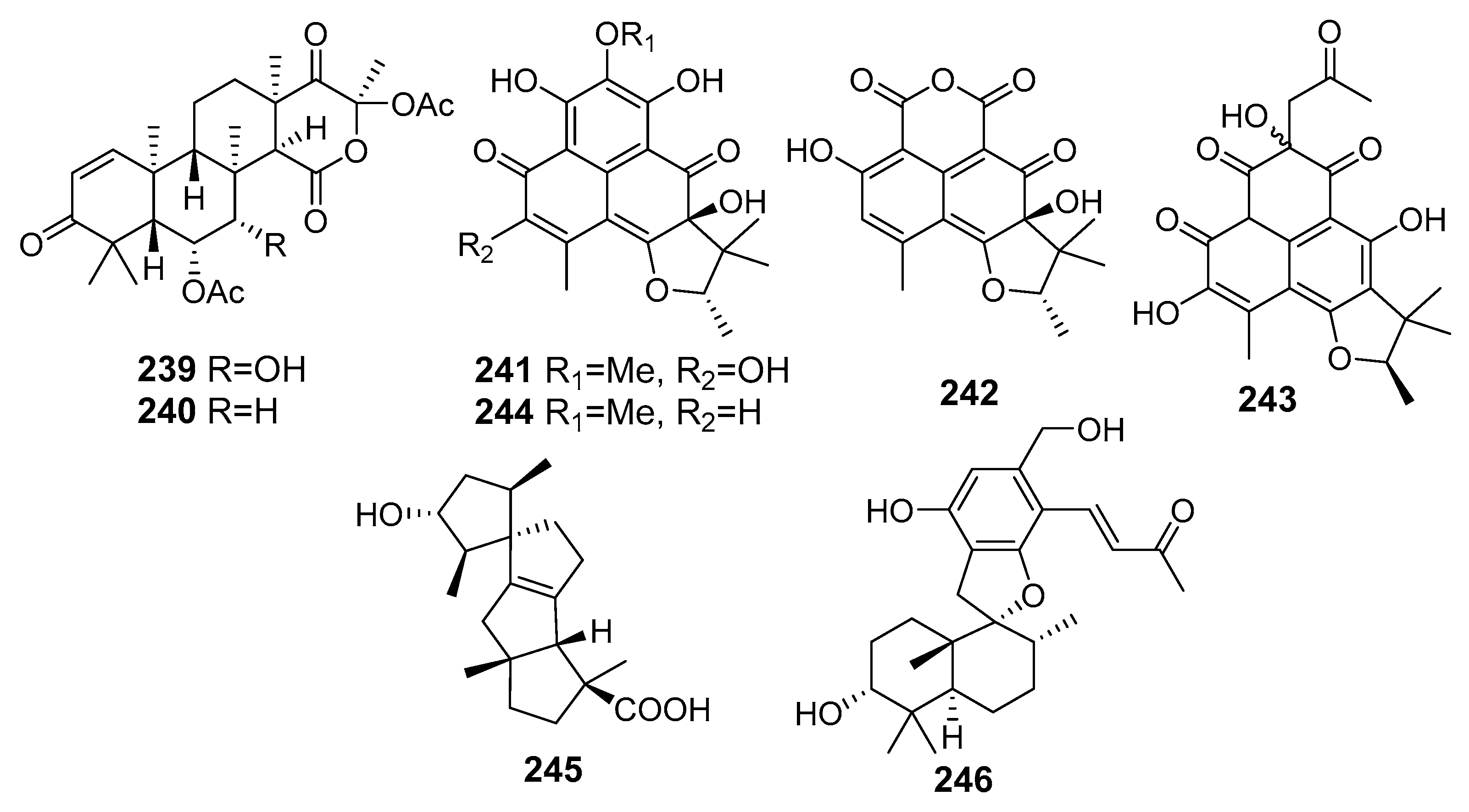
| Terretonin H (239) | Aspergillus ustus KMM 4664 | The Sea of Okhotsk | Inhibition of the ability of spermatozoa to fertilize egg-cells of the sea urchin Strongilocentrotus intermedius | [132] |
| Terretonin I (240) | ||||
| ent-Peniciherqueinone (241) | Penicillium sp. | Gagudo, South Korea | Induction of adipogenesis | [133] |
| 4-Hydroxysclerodin (242) | Anti-angiogenetic and anti-inflammatory activities, Inhibition of tube formation | |||
| (9R)-2,5,7-Trihydroxy-1,8,8,9-tetramethyl-5-(2-oxopropyl)-8,9-dihydro-3H-phenaleno[1,2-b]furan-3,4,6(3aH,5H)-trione (243) | Inhibition of NO production | |||
| Isoherqueinone (244) | Induction of adipogenesis | |||
| Spirograterpene A (245) | Penicillium granulatum MCCC 3A00475 | Antiallergic activity | [134] | |
| Stachybotrysin (246) | Stachybotrys sp. KCB13F013 | Wi Island, South Korea, The Yellow Sea | Inhibition of osteoclast differentiation | [135] |
References
- D’Hondt, S.; Rutherford, S.; Spivack, A.J. Metabolic activity of subsurface life in deep-sea sediments. Science 2002, 295, 2067–2070. [Google Scholar] [CrossRef] [PubMed]
- Parkes, R.J.; Cragg, B.; Roussel, E.; Webster, G.; Weightman, A.; Sass, H. A review of prokaryotic populations and processes in sub-seafloor sediments, including biosphere:geosphere interactions. Mar. Geol. 2014, 352, 409–425. [Google Scholar] [CrossRef]
- Bulan, D.E.; Wilantho, A.; Tongsima, S.; Viyakarn, V.; Chavanich, S.; Somboonna, N. Microbial and Small Eukaryotes Associated With Reefs in the Upper Gulf of Thailand. Front. Mar. Sci. 2018, 5, 5. [Google Scholar] [CrossRef]
- Polinski, J.M.; Bucci, J.P.; Gasser, M.; Bodnar, A.G. Metabarcoding assessment of prokaryotic and eukaryotic taxa in sediments from Stellwagen Bank National Marine Sanctuary. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Martin-Pozas, T.; Gonzalez-Pimentel, J.L.; Jurado, V.; Cuezva, S.; Dominguez-Moñino, I.; Fernandez-Cortes, A.; Cañaveras, J.C.; Sanchez-Moral, S.; Saiz-Jimenez, C. Microbial Activity in Subterranean Ecosystems: Recent Advances. Appl. Sci. 2020, 10, 8130. [Google Scholar] [CrossRef]
- Shao, M.; Sun, C.; Liu, X.; Wang, X.; Li, W.; Wei, X.; Li, Q.; Ju, J. Upregulation of a marine fungal biosynthetic gene cluster by an endobacterial symbiont. Commun. Biol. 2020, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K. Antimicrobial secondary metabolites from marine fungi: A mini review. Indian J. Geo. Mar. Sci. 2016, 45, 1067–1075. [Google Scholar]
- Frisvad, J.C.; Smedsgaard, J.; Larsen, T.O.; Samson, R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004, 49, 201–241. [Google Scholar]
- Liu, S.; Su, M.; Song, S.J.; Jung, J.H. Marine-derived Penicillium species as producers of cytotoxic metabolites. Mar. Drugs 2017, 15, 329. [Google Scholar] [CrossRef]
- Rateb, M.E.M.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef]
- Chen, G.; Wang, H.-F.; Pei, Y.-H. Secondary metabolites from marine-derived microorganisms. J. Asian Nat. Prod. Res. 2014, 16, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed]
- Liming, J.; Chunshan, Q.; Xiyan, H.; Shengdi, F. Potential pharmacological resources: Natural bioactive compounds from marine-derived fungi. Mar. Drugs 2016, 14, 76. [Google Scholar]
- Moghadamtousi, S.Z.; Nikzad, S.; Kadir, H.A.; Abubakar, S.; Zandi, K. Potential Antiviral Agents from Marine Fungi: An Overview. Mar. Drugs 2015, 13, 4520–4538. [Google Scholar] [CrossRef]
- Luo, M.; Zang, R.; Wang, X.; Chen, Z.; Song, X.; Ju, J.; Huang, H. Natural Hydroxamate-Containing Siderophore Acremonpeptides A-D and an Aluminum Complex of Acremonpeptide D from the Marine-Derived Acremonium persicinum SCSIO 115. J. Nat. Prod. 2019, 82, 2594–2600. [Google Scholar] [CrossRef]
- Liu, F.-A.; Lin, X.-P.; Zhou, X.-F.; Chen, M.; Huang, X.; Yang, B.; Tao, H. Xanthones and Quinolones Derivatives Produced by the Deep-Sea-Derived Fungus Penicillium sp. SCSIO Ind16F01. Molecules 2017, 22, 1999. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Si, L.; Liu, D.; Zhou, A.; Zhang, Z.; Shao, Z.; Wang, S.; Zhang, L.; Zhou, D.; Lin, W. Spiromastilactones: A new class of influenza virus inhibitors from deep-sea fungus. Eur. J. Med. Chem. 2016, 108, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tang, S.; Cao, S. Antimicrobial compounds from marine fungi. Phytochem. Rev. 2020, 1–33. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Xu, H.; Yin, L.; Chen, Z.; Shen, H. Diphenyl ethers from a marine-derived isolate of Aspergillus sp. CUGB-F046. Nat. Prod. Res. 2017, 32, 821–825. [Google Scholar] [CrossRef]
- Zhu, A.; Yang, M.-Y.; Zhang, Y.-H.; Shao, C.-L.; Wang, C.-Y.; Hu, L.-D.; Cao, F.; Zhu, H.-J. Absolute Configurations of 14,15-Hydroxylated Prenylxanthones from a Marine-Derived Aspergillus sp. Fungus by Chiroptical Methods. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Zhu, A.; Zhang, X.W.; Zhang, M.; Li, W.; Ma, Z.Y.; Zhu, H.J.; Cao, F. Aspergixanthones I–K, new anti-vibrio prenylxan-thones from the marine-derived fungus Aspergillus sp. ZA-01. Mar. Drugs 2018, 16, 312. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Lee, J.H.; Park, S.C.; Lee, J.; Oh, D.C.; Oh, K.B.; Shin, J. New Peptides from The Marine-Derived Fungi Aspergillus allahabadii and Aspergillus ochraceopetaliformis. Mar. Drugs 2019, 17, 488. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Q.; Li, S.; Cui, H.; Sun, Z.; Chen, D.; Lu, Y.; Liu, H.; Zhang, W. Polypropionate Derivatives with Mycobacterium tuberculosis Protein Tyrosine Phosphatase B Inhibitory Activities from the Deep-Sea-Derived Fungus Aspergillus fischeri FS452. J. Nat. Prod. 2019, 82, 3440–3449. [Google Scholar] [CrossRef]
- Han, J.; Liu, M.; Jenkins, I.; Liu, X.; Zhang, L.; Quinn, R.J.; Feng, Y. Genome-Inspired Chemical Exploration of Marine Fungus Aspergillus fumigatus MF071. Mar. Drugs 2020, 18, 352. [Google Scholar] [CrossRef] [PubMed]
- Corral, P.; Esposito, F.P.; Tedesco, P.; Falco, A.; Tortorella, E.; Tartaglione, L.; Festa, C.; D’Auria, M.V.; Gnavi, G.; Varese, G.C.; et al. Identification of a Sorbicillinoid-Producing Aspergillus Strain with Antimicrobial Activity Against Staphylococcus aureus: A New Polyextremophilic Marine Fungus from Barents Sea. Mar. Biotechnol. 2018, 20, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, Z.; Gao, J.; He, H.; Dai, H.; Xia, X.; Liu, C.H.; Zhang, L.; Song, F. New Diketopiperazines from a Marine-Derived Fungus Strain Aspergillus versicolor MF180151. Mar. Drugs 2019, 17, 262. [Google Scholar] [CrossRef]
- Wang, W.; Chen, R.; Luo, Z.; Wang, W.; Chen, J. Antimicrobial activity and molecular docking studies of a novel anthraquinone from a marine-derived fungus Aspergillus versicolor. Nat. Prod. Res. 2018, 32, 558–563. [Google Scholar] [CrossRef]
- Zhang, D.; Yi, W.; Ge, H.; Zhang, Z.; Wu, B. A new antimicrobial indoloditerpene from a marine-sourced fungus aspergillus versicolor ZZ761. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Li, X.D.; Li, X.M.; Li, X.; Xu, G.M.; Liu, Y.; Wang, B.G. Aspewentins D-H, 20-Nor-isopimarane Derivatives from the Deep Sea Sediment-Derived Fungus Aspergillus wentii SD-310. J. Nat. Prod. 2016, 79, 1347–1353. [Google Scholar] [CrossRef]
- Fredimoses, M.; Zhou, X.-F.; Ai, W.; Tian, X.; Yang, B.; Lin, X.; Liu, J.; Liu, Y. Emerixanthone E, a new xanthone derivative from deep sea fungus Emericella sp SCSIO 05240. Nat. Prod. Res. 2019, 33, 2088–2094. [Google Scholar] [CrossRef]
- Inostroza, A.; Lara, L.; Paz, C.; Perez, A.; Galleguillos, F.; Hernandez, V.; Becerra, J.; González-Rocha, G.; Silva, M. Antibiotic activity of Emerimicin IV isolated from Emericellopsis minima from Talcahuano Bay, Chile. Nat. Prod. Res. 2017, 32, 1361–1364. [Google Scholar] [CrossRef]
- Wang, W.; Chen, R.; Luo, Z.; Wang, W.; Chen, J. Two new benzoate derivatives and one new phenylacetate derivative from a marine-derived fungus Engyodontium album. Nat. Prod. Res. 2016, 31, 758–764. [Google Scholar] [CrossRef]
- Wang, W.; Li, S.; Chen, Z.; Liao, Y. Secondary Metabolites Produced by the Deep-Sea-Derived Fungus Engyodontium album. Chem. Nat. Compd. 2017, 77, 1021–1226. [Google Scholar] [CrossRef]
- Niu, S.; Liu, D.; Shao, Z.; Proksch, P.; Lin, W. Eutypellazines N−S, new thiodiketopiperazines from a deep sea sediment derived fungus Eutypella sp. with anti-VRE activities. Tetrahedron Lett. 2017, 58, 3695–3699. [Google Scholar] [CrossRef]
- Liu, H.-X.; Zhang, L.; Chen, Y.; Sun, Z.-H.; Pan, Q.; Li, H.; Zhang, W.-M. Monoterpenes and sesquiterpenes from the marine sediment-derived fungus Eutypella scoparia FS46. J. Asian Nat. Prod. Res. 2016, 19, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Nenkep, V.; Yun, K.; Son, B.W. Oxysporizoline, an antibacterial polycyclic quinazoline alkaloid from the marine-mudflat-derived fungus Fusarium oxysporum. J. Antibiot. 2016, 69, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.; Kim, G.J.; Yang, I.; Wang, W.; Nam, J.-W.; Choi, H.; Nam, S.-J.; Kang, H. Mycousfurans A and B, Antibacterial Usnic Acid Congeners from the Fungus Mycosphaerella sp., Isolated from a Marine Sediment. Mar. Drugs 2019, 17, 422. [Google Scholar] [CrossRef]
- Le, H.M.T.; Do, Q.T.; Huong, D.T.M.; Thi, Q.V.; Nguyen, M.A.; Vu, T.H.T.; Dang, N.H.; Nguyen, T.D.T.; Tran, M.H.; Chau, V.M.; et al. Chemical Composition and Biological Activities of Metabolites from the Marine Fungi Penicillium sp. Isolated from Sediments of Co To Island, Vietnam. Molecules 2019, 24, 3830. [Google Scholar] [CrossRef]
- Song, X.; Tu, R.; Mei, X.; Wu, S.; Lan, B.; Zhang, L.; Luo, M.; Liu, J.; Luo, M. A mycophenolic acid derivative from the fungus Penicillium sp. SCSIO sof101. Nat. Prod. Res. 2019, 34, 1206–1212. [Google Scholar] [CrossRef]
- Kaleem, S.; Ge, H.; Yi, W.; Zhang, Z.; Wu, B. Isolation, structural elucidation, and antimicrobial evaluation of the me-tabolites from a marine-derived fungus Penicillium sp. ZZ1283. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Pan, C.; Shi, Y.; Auckloo, B.N.; Chen, C.-T.A.; Chen, X.; Wu, X.; Wu, B. Four Verrucosidin Derivatives Isolated from the Hydrothermal Vent Sulfur-Derived Fungus Penicillium sp. Y-50-10. Chem. Nat. Compd. 2018, 54, 253–256. [Google Scholar] [CrossRef]
- Li, Y.-H.; Li, X.-M.; Li, X.; Yang, S.-Q.; Shi, X.-S.; Li, H.-L.; Wang, B.-G. Antibacterial Alkaloids and Polyketide Derivatives from the Deep Sea-Derived Fungus Penicillium cyclopium SD-413. Mar. Drugs 2020, 18, 553. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Sun, S.; Li, L.; Dai, X.; Li, H.; He, Q.; Zhu, H. Tyrosol from marine Fungi, a novel Quorum sensing inhibitor against Chromobacterium violaceum and Pseudomonas aeruginosa. Bioorganic Chem. 2019, 91, 103140. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-C.; Xu, L.-L.; Yang, R.-Y.; Yang, M.-Y.; Hu, L.-D.; Zhu, H.-J.; Cao, F. Anti-Vibrio Indole-Diterpenoids and C-25 Epimeric Steroids From the Marine-Derived Fungus Penicillium janthinellum. Front. Chem. 2019, 7, 80. [Google Scholar] [CrossRef]
- Leutou, A.S.; Yun, K.; Son, B.W. New production of antibacterial polycyclic quinazoline alkaloid, thielaviazoline, from anthranilic acid by the marine-mudflat-derived fungus Thielavia sp. Nat. Prod. Sci. 2016, 22, 216–219. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, X.; Shi, X.; Xian, P.-J.; Hong, L.; Tao, Y.-D.; Yang, X.-L. Two new cytochalasans from the marine sediment-derived fungus Westerdykella dispersa and their antibacterial activities. Phytochem. Lett. 2019, 32, 52–55. [Google Scholar] [CrossRef]
- Chokpaiboon, S.; Unagul, P.; Nithithanasilp, S.; Komwijit, S.; Somyong, W.; Ratiarpakul, T.; Isaka, M.; Bunyapaiboonsri, T. Salicylaldehyde and dihydroisobenzofuran derivatives from the marine fungus Zopfiella marina. Nat. Prod. Res. 2017, 32, 149–153. [Google Scholar] [CrossRef]
- Ding, L.; Xu, P.; Li, T.; Liao, X.; He, S.; Xu, S. Asperfurandiones A and B, two antifungal furandione analogs from a marine-derived fungus Aspergillus versicolor. Nat. Prod. Res. 2019, 33, 3404–3408. [Google Scholar] [CrossRef]
- Cao, F.; Yang, J.-K.; Liu, Y.-F.; Zhu, H.J.; Wang, C.-Y. Pleosporalone A, the first azaphilone characterized with aromatic A-ring from a marine-derived Pleosporales sp. fungus. Nat. Prod. Res. 2016, 30, 2448–2452. [Google Scholar] [CrossRef]
- Cao, F.; Meng, Z.-H.; Mu, X.; Yue, Y.-F.; Zhu, H.-J. Absolute Configuration of Bioactive Azaphilones from the Marine-Derived Fungus Pleosporales sp. CF09-1. J. Nat. Prod. 2019, 82, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Zhao, D.; Chen, X.-Y.; Liang, X.-D.; Li, W.; Zhu, H.J. Antifungal Drimane Sesquiterpenoids From a Marine-Derived Pleosporales sp. Fungus. Chem. Nat. Compd. 2017, 53, 1189–1191. [Google Scholar] [CrossRef]
- Xu, D.; Pang, X.J.; Zhao, T.; Xu, L.L.; Yang, X.L. New alkenylated tetrahydropyran derivatives from the marine sedi-ment-derived fungus Westerdykella dispersa and their bioactivities. Fitoterapia 2017, 122, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-R.; Ma, X.-Y.; Ji, N.-Y. Trichosordarin A, a norditerpene glycoside from the marine-derived fungus Trichoderma harzianum R5. Nat. Prod. Res. 2019, 34, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease 2019 Disease, Injury, and Impairment Summaries. Available online: http://www.healthdata.org/results/gbd_summaries/2019 (accessed on 25 December 2020).
- Stien, D. Marine Microbial Diversity as a Source of Bioactive Natural Products. Mar. Drugs 2020, 18, 215. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Prakash, V.; Ranjan, N. Marine Fungi: A Source of Potential Anticancer Compounds. Front. Microbiol. 2018, 8, 2536. [Google Scholar] [CrossRef]
- Yu, G.; Wang, Y.; Yu, R.; Feng, Y.; Wang, L.; Che, Q.; Gu, Q.; Li, D.; Li, J.; Zhu, T. Chetracins E and F, cytotoxic epipolythiodioxopiperazines from the marine-derived fungus Acrostalagmus luteoalbus HDN13-530. RSC Adv. 2018, 8, 53–58. [Google Scholar] [CrossRef]
- Wang, F.; Wang, F.; Sarotti, A.M.; Jiang, G.; Huguet-Tapia, J.C.; Zheng, S.L.; Wu, X.; Li, C.; Ding, Y.; Cao, S.; et al. Waikikiamides A–C: Complex Diketopiperazine Dimer and Diketopiperazine-Polyketide Hybrids from a Hawaiian Marine Fungal Strain Aspergillus sp. FM242. Org. Lett. 2020, 22, 4408–4412. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Yue, Y.-F.; Feng, L.-X.; Zhu, H.-J.; Cao, F. Asperienes A–D, Bioactive Sesquiterpenes from the Marine-Derived Fungus Aspergillus flavus. Mar. Drugs 2019, 17, 550. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Trinh, P.T.H.; Girich, E.V.; Smetanina, O.F.; Rasin, A.B.; Popov, R.S.; Dyshlovoy, S.A.; Von Amsberg, G.; Menchinskaya, E.S.; Van, T.T.T.; et al. Biologically Active Metabolites from the Marine Sediment-Derived Fungus Aspergillus flocculosus. Mar. Drugs 2019, 17, 579. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Li, Z.; Sun, Y.-J.; Hua, H.; Liu, T.; Bai, J. Terpenoids from the Marine-Derived Fungus Aspergillus fumigatus YK-7. Molecules 2015, 21, 31. [Google Scholar] [CrossRef]
- Li, D.-H.; Han, T.; Guan, L.-P.; Bai, J.; Zhao, N.; Li, Z.-L.; Wu, X.; Hua, H. New naphthopyrones from marine-derived fungus Aspergillus niger 2HL-M-8 and their in vitro antiproliferative activity. Nat. Prod. Res. 2015, 30, 1116–1122. [Google Scholar] [CrossRef]
- Uchoa, P.K.S.; Pimenta, A.T.A.; Braz-Filho, R.; Oliveira, M.D.C.F.D.; Saraiva, N.N.; Rodrigues, B.S.F.; Pfenning, L.H.; Abreu, L.M.; Wilke, D.V.; Florêncio, K.G.D.; et al. New cytotoxic furan from the marine sediment-derived fungi Aspergillus niger. Nat. Prod. Res. 2017, 31, 2599–2603. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Zhuravleva, O.I.; Antonov, A.S.; Berdyshev, D.V.; Pivkin, M.V.; Denisenko, V.A.; Popov, R.S.; Gerasimenko, A.V.; Von Amsberg, G.; Dyshlovoy, S.A.; et al. Prenylated indole alkaloids from co-culture of marine-derived fungi Aspergillus sulphureus and Isaria felina. J. Antibiot. 2018, 71, 846–853. [Google Scholar] [CrossRef]
- Li, X.D.; Li, X.; Li, X.M.; Xu, G.M.; Zhang, P.; Meng, L.H.; Wang, B.G. Tetranorlabdane Diterpenoids from the Deep Sea Sediment-Derived Fungus Aspergillus wentii SD-310. Planta Med. 2016, 82, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Wang, J.; Wei, X.; Chen, Y.; Fu, T.; Xiang, Y.; Huang, X.; Tian, X.; Xiao, Z.; Zhang, W.; et al. Variecolortins A–C, Three Pairs of Spirocyclic Diketopiperazine Enantiomers from the Marine-Derived Fungus Eurotium sp. SCSIO F452. Org. Lett. 2018, 20, 4593–4596. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, L.; Chen, Y.; Li, S.; Tan, G.; Sun, Z.; Pan, Q.; Ye, W.; Li, H.-H.; Zhang, W. Cytotoxic pimarane-type diterpenes from the marine sediment-derived fungus Eutypella sp. FS46. Nat. Prod. Res. 2016, 31, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Liu, Z.; Guo, B.; Gao, X.; Liu, H.; Zhang, W. Cytotoxic Secondary Metabolites from a Sea-Derived Fungal Strain of Hypoxylon rubiginosum FS521. Chin. J. Org. Chem. 2020, 40, 1367. [Google Scholar] [CrossRef]
- Smetanina, O.F.; Yurchenko, A.N.; Girich, E.V.; Kalinovsky, A.I.; Khudyakova, Y.V.; Dyshlovoy, S.A.; Von Amsberg, G.; Yurchenko, E.A.; Afiyatullov, S.S. Unique prostate cancer-toxic polyketides from marine sediment-derived fungus Isaria felina. J. Antibiot. 2017, 70, 856–858. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, T.; Zhu, G.; Liu, Y.; Wang, C.; Piyachaturawat, P.; Chairoungdua, A.; Zhu, W. Bioactive Natural Products from the Marine-Derived Penicillium brevicompactum OUCMDZ-4920. Chin. J. Org. Chem. 2017, 37, 2762. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Zhao, Y.-Y.; Lan, R.-F.; Du, L.; Wang, B.-S.; Zhou, T.; Li, Y.-P.; Zhang, Q.-Q.; Ying, M.-G.; Zheng, Q.-H.; et al. Dicitrinone D, an antimitotic polyketide isolated from the marine-derived fungus Penicillium citrinum. Tetrahedron 2017, 73, 5900–5911. [Google Scholar] [CrossRef]
- Liu, C.-C.; Zhang, Z.-Z.; Feng, Y.-Y.; Gu, Q.-Q.; Li, D.; Zhu, T. Secondary metabolites from Antarctic marine-derived fungus Penicillium crustosum HDN153086. Nat. Prod. Res. 2018, 33, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.L.; Zhang, D.; Xia, J.M.; Hu, C.C.; Lin, T.; Lin, Y.K.; Wang, G.H.; Tian, W.J.; Li, Z.P.; Zhang, X.K.; et al. Steroids from the Deep-Sea-Derived Fungus Penicillium granulatum MCCC 3A00475 Induced Apoptosis via Retinoid X Receptor (RXR)-α Pathway. Mar. Drugs 2019, 17, 178. [Google Scholar] [CrossRef]
- Choi, B.-K.; Phan, T.H.T.; Hwang, S.; Oh, D.-C.; Kang, J.S.; Lee, H.-S.; Ngo, T.D.N.; Van Tran, T.T.; Shin, H.J. Resorcinosides A and B, Glycosylated Alkylresorcinols from a Marine-Derived Strain of the Fungus Penicillium janthinellum. J. Nat. Prod. 2019, 82, 3186–3190. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhang, Q.-Q.; Chen, L.; Bi, Y.-X.; Li, Y.; Li, X.-X.; Liu, Q.-Y.; Ying, M.-G.; Zheng, Q.-H. Secalonic Acids H and I, Two New Secondary Metabolites from the Marine-Derived Fungus Penicillium oxalicum. Heterocycles 2017, 94, 1766. [Google Scholar] [CrossRef]
- Liu, Q.-Y.; Zhang, Q.-Q.; Chen, L.; Lu, Z.-H.; Du, L.; Zheng, Q.-H. Secalonic Acids J–M, Four New Secondary Metabolites from the Marine-derived Fungus Penicillium oxalicum. Heterocycles 2019, 98, 955. [Google Scholar] [CrossRef]
- Wang, W.; Park, C.; Oh, E.; Sung, Y.; Lee, J.; Park, K.-H.; Kang, H. Benzophenone Compounds, from a Marine-Derived Strain of the Fungus Pestalotiopsis neglecta, Inhibit Proliferation of Pancreatic Cancer Cells by Targeting the MEK/ERK Pathway. J. Nat. Prod. 2019, 82, 3357–3365. [Google Scholar] [CrossRef]
- Xu, J.-L.; Liu, H.-X.; Chen, Y.-C.; Tan, H.-B.; Guo, H.; Xu, L.-Q.; Li, S.-N.; Huang, Z.-L.; Li, H.-H.; Gao, X.-X.; et al. Highly Substituted Benzophenone Aldehydes and Eremophilane Derivatives from the Deep-Sea Derived Fungus Phomopsis lithocarpus FS508. Mar. Drugs 2018, 16, 329. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Z.; Chen, Y.; Tan, H.; Li, H.; Li, S.; Guo, H.; Huang, Z.; Gao, X.; Liu, H.; et al. Lithocarols A-F, six tenellone derivatives from the deep-sea derived fungus Phomopsis lithocarpus FS508. Bioorg. Chem. 2019, 87, 728–735. [Google Scholar] [CrossRef]
- Xu, J.; Tan, H.; Chen, Y.; Li, S.; Guo, H.; Huang, Z.; Li, H.; Gao, X.; Liu, H.; Zhang, W. Lithocarpinols A and B, a pair of diastereomeric antineoplastic tenellone derivatives from the deep-sea derived fungus Phomopsis lithocarpus FS508. Chin. Chem. Lett. 2019, 30, 439–442. [Google Scholar] [CrossRef]
- Cao, F.; Meng, Z.-H.; Wang, P.; Luo, D.-Q.; Zhu, H.-J. Dipleosporalones A and B, Dimeric Azaphilones from a Marine-Derived Pleosporales sp. Fungus. J. Nat. Prod. 2020, 83, 1283–1287. [Google Scholar] [CrossRef]
- Ge, X.; Sun, C.; Feng, Y.; Wang, L.; Peng, J.; Che, Q.; Gu, Q.-Q.; Zhu, T.; Li, D.; Zhang, G. Anthraquinone Derivatives from a Marine-Derived Fungus Sporendonema casei HDN16-802. Mar. Drugs 2019, 17, 334. [Google Scholar] [CrossRef]
- Wang, W.-J.; Wan, X.; Liu, J.; Wang, J.-P.; Zhu, H.; Chen, C.; Zhang, Y. Two New Terpenoids from Talaromyces purpurogenus. Mar. Drugs 2018, 16, 150. [Google Scholar] [CrossRef]
- Xu, D.; Luo, M.; Liu, F.; Wang, D.; Pang, X.; Zhao, T.; Xu, L.; Wu, X.; Xia, M.; Yang, X.-L. Cytochalasan and Tyrosine-Derived Alkaloids from the Marine Sediment-Derived Fungus Westerdykella dispersa and Their Bioactivities. Sci. Rep. 2017, 7, 11956. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Huang, H.; Yang, H.; Zhang, W.; Sun, Y.; Wen, J. Cytochalasin P1, a new cytochalasin from the marine-derived fungus Xylaria sp. SOF11. Z. Naturforsch. Sect. C J. Biosci. 2017, 72, 129–132. [Google Scholar] [CrossRef]
- Kishore, N.; Kumar, P.; Shanker, K.; Verma, A.K. Human disorders associated with inflammation and the evolving role of natural products to overcome. Eur. J. Med. Chem. 2019, 179, 272–309. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yi, M.; Ding, L.; He, S. A Review of Anti-Inflammatory Compounds from Marine Fungi, 2000–2018. Mar. Drugs 2019, 17, 636. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhao, J.; Liu, N.; Proksch, P.; Zhao, Z.; Lin, W. Eremophilane-Type Sesquiterpenoids from an Acremonium sp. Fungus Isolated from Deep-Sea Sediments. J. Nat. Prod. 2016, 79, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fang, P.; Tang, J.; Wu, Z.; Li, X.; Li, S.; Wang, Y.; Liu, G.; He, Z.; Gou, D.; et al. A novel cyclic dipeptide from deep marine-derived fungus Aspergillus sp. SCSIOW2. Nat. Prod. Res. 2016, 30, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, M.; Tang, J.; Li, X. Eremophilane sesquiterpenes from a deep marine-derived fungus, Aspergillus sp. SCSIOW2, cultivated in the presence of epigenetic modifying agents. Molecules 2016, 21, 473. [Google Scholar] [CrossRef]
- Yang, L.H.; Ou-Yang, H.; Yan, X.; Tang, B.W.; Fang, M.J.; Wu, Z.; Chen, J.W.; Qiu, Y.K. Open-ring butenolides from a marine-derived anti-neuroinflammatory fungus Aspergillus terreus Y10. Mar. Drugs 2018, 16, 428. [Google Scholar] [CrossRef]
- Li, H.; Sun, W.; Deng, M.; Zhou, Q.; Wang, J.; Liu, J.; Chen, C.; Qi, C.; Luo, Z.; Xue, Y.; et al. Asperversiamides, Linearly Fused Prenylated Indole Alkaloids from the Marine-Derived Fungus Aspergillus versicolor. J. Org. Chem. 2018, 83, 8483–8492. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kang, J.S.; Choi, B.K.; Lee, H.S.; Lee, Y.J.; Lee, J.; Shin, H.J. Phenazine Derivatives with Anti-Inflammatory Activity from the Deep-Sea Sediment-Derived Yeast-Like Fungus Cystobasidium laryngis IV17-028. Mar. Drugs 2019, 17, 482. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Xie, C.-L.; Zhong, T.; Xu, W.; Luo, Z.; Shao, Z.; Yang, X.-W. Sesquiterpenes from a deep-sea-derived fungus Graphostroma sp. MCCC 3A00421. Tetrahedron 2017, 73, 7267–7273. [Google Scholar] [CrossRef]
- Luo, M.; Cui, Z.; Huang, H.; Song, X.; Sun, A.; Dang, Y.; Lu, L.; Ju, J. Amino Acid Conjugated Anthraquinones from the Marine-Derived Fungus Penicillium sp. SCSIO sof101. J. Nat. Prod. 2017, 80, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Quang, T.H.; Yoon, C.-S.; Kim, H.J.; Sohn, J.H.; Oh, H.C. Furanoaustinol and 7-acetoxydehydroaustinol: New meroterpenoids from a marine-derived fungal strain Penicillium sp. SF-5497. J. Antibiot. 2018, 71, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Lin, X.; Zhao, B.; Wei, X.; Li, G.; Kaliaperumal, K.; Liao, S.; Yang, B.; Zhou, X.-F.; et al. Chrysamides A–C, Three Dimeric Nitrophenyl trans-Epoxyamides Produced by the Deep-Sea-Derived Fungus Penicillium chrysogenum SCSIO41001. Org. Lett. 2016, 18, 3650–3653. [Google Scholar] [CrossRef]
- Ha, T.M.; Kim, D.-C.; Sohn, J.H.; Yim, J.H.; Oh, H.C. Anti-Inflammatory and Protein Tyrosine Phosphatase 1B Inhibitory Metabolites from the Antarctic Marine-Derived Fungal Strain Penicillium glabrum SF-7123. Mar. Drugs 2020, 18, 247. [Google Scholar] [CrossRef]
- Choi, B.-K.; Jo, S.-H.; Choi, D.-K.; Trinh, P.T.H.; Lee, H.-S.; Van Anh, C.; Van, T.T.T.; Shin, H.J. Anti-Neuroinflammatory Agent, Restricticin B, from the Marine-Derived Fungus Penicillium janthinellum and Its Inhibitory Activity on the NO Production in BV-2 Microglia Cells. Mar. Drugs 2020, 18, 465. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Zhuravleva, O.I.; Antonov, A.S.; Leshchenko, E.V.; Pivkin, M.V.; Khudyakova, Y.V.; Denisenko, V.A.; Pislyagin, E.; Kim, N.; Berdyshev, D.V.; et al. Piltunines A–F from the Marine-Derived Fungus Penicillium piltunense KMM 4668. Mar. Drugs 2019, 17, 647. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Zhang, Z.-H.; Zhong, Y.; Huang, J.-J.; Li, X.-X.; Jiang, J.-Y.; Deng, Y.-Y.; Zhang, L.-H.; He, F. Sumalactones A–D, four new curvularin-type macrolides from a marine deep sea fungus Penicillium Sumatrense. RSC Adv. 2017, 7, 40015–40019. [Google Scholar] [CrossRef]
- Ngan, N.T.T.; Quang, T.H.; Kim, K.-W.; Kim, H.J.; Sohn, J.H.; Kang, D.G.; Lee, H.S.; Kim, Y.-C.; Oh, H. Anti-inflammatory effects of secondary metabolites isolated from the marine-derived fungal strain Penicillium sp. SF-5629. Arch. Pharmacal Res. 2017, 40, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants–An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; He, F.; Yu, J.-H.; Zhai, H.; Cheng, Z.-Q.; Jiang, C.-S.; Zhang, Y.; Zhang, Y.; Zhang, X.; Chen, G.-Y.; et al. New Chromones from a Marine-Derived Fungus, Arthrinium sp., and Their Biological Activity. Molecules 2018, 23, 1982. [Google Scholar] [CrossRef] [PubMed]
- Leutou, A.S.; Yun, K.; Son, B.W. Induced production of 6,9-dibromoflavasperone, a new radical scavenging naphthopy-ranone in the marine-mudflat-derived fungus Aspergillus niger. Arch. Pharm. Res. 2016, 39, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-H.; Liu, D.; Xu, Y.; Chen, J.-L.; Lin, W. Antioxidant xanthones and anthraquinones isolated from a marine-derived fungus Aspergillus versicolor. Chin. J. Nat. Med. 2018, 16, 219–224. [Google Scholar] [CrossRef]
- Amin, M.; Zhang, X.-Y.; Xu, X.-Y.; Qi, S.-H. New citrinin derivatives from the deep-sea-derived fungus Cladosporium sp. SCSIO z015. Nat. Prod. Res. 2019, 34, 1219–1226. [Google Scholar] [CrossRef]
- Zhong, W.-M.; Wang, J.; Shi, X.-F.; Wei, X.; Chen, Y.; Zeng, Q.; Xiang, Y.; Chen, X.-Y.; Tian, X.; Xiao, Z.-H.; et al. Eurotiumins A–E, Five New Alkaloids from the Marine-Derived Fungus Eurotium sp. SCSIO F452. Mar. Drugs 2018, 16, 136. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, J.; Wei, X.; Fu, T.; Chen, Y.; Zeng, Q.; Huang, Z.; Huang, X.; Zhang, W.; Zhang, S.; et al. Three Pairs of New Spirocyclic Alkaloid Enantiomers From the Marine-Derived Fungus Eurotium sp. SCSIO F452. Front. Chem. 2019, 7, 350. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.-M.; Chen, Y.; Mai, Z.; Wei, X.; Wang, J.; Zeng, Q.; Chen, X.; Tian, X.; Zhang, W.-M.; Wang, F.; et al. Euroticins A and B, Two Pairs of Highly Constructed Salicylaldehyde Derivative Enantiomers from a Marine-Derived Fungus Eurotium sp. SCSIO F452. J. Org. Chem. 2020, 85, 12754–12759. [Google Scholar] [CrossRef]
- Smetanina, O.F.; Yurchenko, A.N.; Girich, E.V.; Trinh, P.T.H.; Antonov, A.S.; Dyshlovoy, S.A.; Von Amsberg, G.; Kim, N.; Chingizova, E.; Pislyagin, E.; et al. Biologically Active Echinulin-Related Indolediketopiperazines from the Marine Sediment-Derived Fungus Aspergillus niveoglaucus. Molecules 2019, 25, 61. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Smetanina, O.F.; Girich, E.V.; Phan, T.T.H.; Ngo, N.T.D.; Zhuravleva, O.I.; Rasin, A.B.; Dyshlovoy, S.A.; Menchinskaya, E.S.; Pislyagin, E.A.; et al. Auroglaucin-related neuroprotective compounds from Vietnamese marine sediment-derived fungus Aspergillus niveoglaucus. Nat. Prod. Res. 2020, 34, 2589–2594. [Google Scholar] [CrossRef]
- Liang, D.; Yu, Y.; Ma, Z. Novel strategies targeting bromodomain-containing protein 4 (BRD4) for cancer drug discovery. Eur. J. Med. Chem. 2020, 200, 112426. [Google Scholar] [CrossRef]
- He, R.; Du, S.; Lei, T.; Xie, X.; Wang, Y. Glycogen synthase kinase 3 beta in tumorigenesis and oncotherapy. Oncol. Rep. 2020, 44, 2373–2385. [Google Scholar] [CrossRef]
- Rana, A.K.; Rahmatkar, S.N.; Kumar, A.; Singh, D. Glycogen synthase kinase-3: A putative target to combat severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. Cytokine Growth Factor Rev. 2020. [Google Scholar] [CrossRef]
- Usman, B.; Sharma, N.; Satija, S.; Mehta, M.; Vyas, M.; Khatik, G.L.; Khurana, N.; Hansbro, P.M.; Williams, K.A.; Dua, K. Recent Developments in Alpha-Glucosidase Inhibitors for Management of Type-2 Diabetes: An Update. Curr. Pharm. Des. 2019, 25, 2510–2525. [Google Scholar] [CrossRef]
- Ezzat, S.M.; El Bishbishy, M.H.; Habtemariam, S.; Salehi, B.; Sharifi-Rad, M.; Martins, N.; Sharifi-Rad, J. Looking at Marine-Derived Bioactive Molecules as Upcoming Anti-Diabetic Agents: A Special Emphasis on PTP1B Inhibitors. Molecules 2018, 23, 28. [Google Scholar] [CrossRef]
- Saxena, M.; Dubey, R. Target Enzyme in Alzheimer’s Disease: Acetylcholinesterase Inhibitors. Curr. Top. Med. Chem. 2019, 19, 264–275. [Google Scholar] [CrossRef]
- Guan, C.; Niu, Y.; Chen, S.-C.; Kang, Y.; Wu, J.-X.; Nishi, K.; Chang, C.C.Y.; Chang, T.-Y.; Luo, T.; Chen, L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Kim, H.J.; Lim, J.H.; Lee, S.H. Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy. Cells 2019, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Tukaj, S. Heat Shock Protein 70 as a Double Agent Acting Inside and Outside the Cell: Insights into Autoimmunity. Int. J. Mol. Sci. 2020, 21, 5298. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhang, D.; Zhou, B.; Ma, Z. Inhibitors of BRD4 Protein from a Marine-Derived Fungus Alternaria sp. NH-F6. Mar. Drugs 2017, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Ohte, S.; Shiokawa, T.; Koyama, N.; Katagiri, T.; Imada, C.; Tomoda, H. A new diketopiperazine-like inhibitor of bone morphogenetic protein-induced osteoblastic differentiation produced by marine-derived Aspergillus sp. BFM-0085. J. Antibiot. 2020, 73, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, L.; Hao, J.; Wang, L.; Zhu, W. α-Glucosidase Inhibitors from the Marine-Derived Fungus Aspergillus flavipes HN4-13. J. Nat. Prod. 2016, 79, 2977–2981. [Google Scholar] [CrossRef]
- Li, H.; Sun, W.; Deng, M.; Qi, C.; Chen, C.; Zhu, H.; Luo, Z.; Wang, J.; Xue, Y.; Zhang, Y. Asperversins A and B, two novel meroterpenoids with an unusual 5/6/6/6 ring from the marine-derived fungus Aspergillus versicolor. Mar. Drugs 2018, 16, 177. [Google Scholar] [CrossRef]
- Uchida, R.; Nakajyo, K.; Kobayashi, K.; Ohshiro, T.; Terahara, T.; Imada, C.; Tomoda, H. 7-Chlorofolipastatin, an inhibitor of sterol O-acyltransferase, produced by marine-derived Aspergillus ungui NKH-007. J. Antibiot. 2016, 69, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wiese, J.; Schmaljohann, R.; Imhoff, J.F. Biscogniauxone, a New Isopyrrolonaphthoquinone Compound from the Fungus Biscogniauxia mediterranea Isolated from Deep-Sea Sediments. Mar. Drugs 2016, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Quang, T.H.; Ngan, N.T.T.; Sohn, J.H.; Oh, H. New preaustinoids from a marine-derived fungal strain Penicillium sp. SF-5497 and their inhibitory effects against PTP1B activity. J. Antibiot. 2019, 72, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.H.; Yu, H.B.; Liu, X.Y.; Lu, X.L.; Jiao, B.H. Furanone derivative and sesquiterpene from Antarctic marine-derived fungus Penicillium sp. S-1-18. J. Asian Nat. Prod. Res. 2018, 20, 1108–1115. [Google Scholar] [CrossRef]
- Wang, W.; Lee, J.; Kim, K.-J.; Sung, Y.; Park, K.-H.; Oh, E.; Park, C.; Son, Y.-J.; Kang, H. Austalides, osteoclast differentiation inhibitors from a marine-derived strain of the fungus Penicillium rudallense. J. Nat. Prod. 2019, 82, 3083–3088. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Kirichuk, N.N.; Denisenko, V.A.; Dmitrenok, P.S.; Yurchenko, E.A.; Min′ko, E.M.; Ivanets, E.V.; Afiyatullov, S.S. New Diorcinol J Produced by Co-Cultivation of Marine Fungi Aspergillus sulphureus and Isaria felina. Chem. Nat. Compd. 2016, 52, 227–230. [Google Scholar] [CrossRef]
- Oleinikova, G.K.; Denisenko, V.A.; Berdyshev, D.V.; Pushilin, M.A.; Kirichuk, N.N.; Menzorova, N.I.; Kuzmich, A.S.; Yurchenko, E.A.; Zhuravleva, O.I.; Afiyatullov, S.S. Two new sesterterpenoids, terretonins H and I, from the marine-derived fungus Aspergillus ustus. Phytochem. Lett. 2016, 17, 135–139. [Google Scholar] [CrossRef]
- Park, S.C.; Julianti, E.; Ahn, S.; Kim, D.; Lee, S.K.; Noh, M.; Oh, D.-C.; Oh, K.-B.; Shin, A.J.; Shin, J. Phenalenones from a Marine-Derived Fungus Penicillium sp. Mar. Drugs 2019, 17, 176. [Google Scholar] [CrossRef]
- Niu, S.; Fan, Z.W.; Xie, C.L.; Liu, Q.; Luo, Z.H.; Liu, G.; Yang, X.W. Spirograterpene A, a Tetracyclic Spiro-Diterpene with a Fused 5/5/5/5 Ring System from the Deep-Sea-Derived Fungus Penicillium granulatum MCCC 3A00475. J. Nat. Prod. 2017, 80, 2174–2177. [Google Scholar] [CrossRef]
- Kim, J.W.; Ko, S.-K.; Kim, H.-M.; Kim, G.-H.; Son, S.; Kim, G.S.; Hwang, G.J.; Jeon, E.S.; Shin, K.-S.; Ryoo, I.-J.; et al. Stachybotrysin, an Osteoclast Differentiation Inhibitor from the Marine-Derived Fungus Stachybotrys sp. KCB13F013. J. Nat. Prod. 2016, 79, 2703–2708. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, J.P.; Muruganandam, V.; Suryanarayanan, T.S. Isolation and Characterization of Melanin from a Marine Fungus. Bot. Mar. 1995, 38, 413–416. [Google Scholar] [CrossRef]
- Ravishankar, J.P.; Suryanarayanan, T.S.; Muruganandam, V. Strategies for osmoregulation in the marine fungus Cirrenalia pygmea Kohl. (Hyphomycetes). Indian J. Mar. Sci. 2006, 35, 351–358. [Google Scholar]
- Girich, E.V.; Yurchenko, A.N.; Smetanina, O.F.; Trinh, P.T.; Ngoc, N.T.; Pivkin, M.V.; Popov, R.S.; Pislyagin, E.A.; Menchinskaya, E.S.; Chingizova, E.A.; et al. Neuroprotective Metabolites from Vietnamese Marine Derived Fungi of Aspergillus and Penicillium Genera. Mar. Drugs 2020, 18, 608. [Google Scholar] [CrossRef]
- Ao, J.; Bandyopadhyay, S.; Free, S.J. Characterization of the Neurospora crassa DHN melanin biosynthetic pathway in developing ascospores and peridium cells. Fungal Biol. 2019, 123, 1–9. [Google Scholar] [CrossRef]
- Mayer, K.M.; Ford, J.; MacPherson, G.R.; Padgett, D.; Volkmann-Kohlmeyer, B.; Kohlmeyer, J.; Murphy, C.; Douglas, S.E.; Wright, J.M.; Wright, J.L. Exploring the diversity of marine-derived fungal polyketide synthases. Can. J. Microbiol. 2007, 53, 291–302. [Google Scholar] [CrossRef]
- Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ (accessed on 25 December 2020).
- Plinabulin, a synthetic derivative of phenylahistine (from algicolous Aspergillus ustus)- Phase II and III of clinical trials in complex therapy of various cancers. Available online: https://www.clinicaltrials.gov (accessed on 25 December 2020).





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurchenko, A.N.; Girich, E.V.; Yurchenko, E.A. Metabolites of Marine Sediment-Derived Fungi: Actual Trends of Biological Activity Studies. Mar. Drugs 2021, 19, 88. https://doi.org/10.3390/md19020088
Yurchenko AN, Girich EV, Yurchenko EA. Metabolites of Marine Sediment-Derived Fungi: Actual Trends of Biological Activity Studies. Marine Drugs. 2021; 19(2):88. https://doi.org/10.3390/md19020088
Chicago/Turabian StyleYurchenko, Anton N., Elena V. Girich, and Ekaterina A. Yurchenko. 2021. "Metabolites of Marine Sediment-Derived Fungi: Actual Trends of Biological Activity Studies" Marine Drugs 19, no. 2: 88. https://doi.org/10.3390/md19020088
APA StyleYurchenko, A. N., Girich, E. V., & Yurchenko, E. A. (2021). Metabolites of Marine Sediment-Derived Fungi: Actual Trends of Biological Activity Studies. Marine Drugs, 19(2), 88. https://doi.org/10.3390/md19020088