Abstract
Neurodegenerative diseases are among the most widespread diseases affecting humans, and the number of patients is only rising. Seaweed polysaccharide extracts show significant neuroprotective and reparative activities. Seaweed polysaccharides might provide the next big breakthrough in neurodegenerative disease treatment. This paper reviews the applications of seaweed polysaccharides as potential treatments of neurodegenerative diseases. The particular focus is on fucoidan, ulvan, and their derivatives as potential agents to treat Alzheimer’s disease. This review provides a critical update on the progress in this important research area.
1. Introduction
Neurodegenerative diseases are characterized by the progressive loss of cognitive and physical function. The most common neurodegenerative diseases are Alzheimer’s disease (AD), Parkinson’s disease, Huntington’s disease, and Amyotrophic Lateral Sclerosis (ALS) [1]. There are two main types of neurodegenerative diseases: movement disorders and degeneration/dementia disorders. AD is the most common degenerative disorder [2].
AD is an irreversible progressive neurodegenerative disease leading to memory loss and cognitive deficit [3]. The etiology of AD is not fully understood, as many factors impact disease progression and presentation [4]. Alois Alzheimer first discovered this disease in 1907, which was when the two main characteristics of Alzheimer’s were first discussed, namely, amyloid-beta plague and neurofibrillary tangles [5]. There are several contributing factors to AD development and progression, including apoptosis, oxidative stress, neuroinflammation, mitochondrial dysfunction, cholinic dysfunction, and abnormal protein development [6]. An excess of D-galactose can be an early sign of Alzheimer’s disease progression. This sugar conjugates with glucose to form lactose, and when there is too much D-galactose vs. the amount of glucose present, metabolic dysfunction and oxidative stress occur [7].
As medical science progresses the average human lifespan increases, leading to a rising number of AD cases [8]. Almost 6 million people exhibited AD symptoms in 2018; of these cases, 200,000 were early onset, occurring in people less than 65 years old [9]. The number of AD patients is projected to increase to over 100 million by 2050 [10]. The cases of Alzheimer’s disease in the world have been doubling every 20 years, with projections for 2040 at around 80 million [11].
Alzheimer’s is known as a tauopathy, meaning that in Alzheimer’s patients there is a high concentration of misfolded and insoluble tau protein. The first location where this insoluble protein builds up is in the hippocampus [12]. This is what forms the trademark neurofibrillary tangles found in Alzheimer’s patients. This misfolding of protein is common in all neurodegenerative diseases as these proteins control brain function. The concentration of tau in the human body is determined by two systems: the autophagy-lysosome pathway and the ubiquitin-proteasome pathway. When autophagy is suppressed in mice models of Alzheimer’s, neurodegeneration quickly follows indicating that failure in this pathway can contribute to the neurodegeneration found in Alzheimer’s. There is also a high concentration of unubiquitinated tau found in models where the autophagy pathway has been compromised, indicating these systems may be linked. When these systems function properly, the tau protein is soluble and moves throughout the brain and body causing no damage, but when they fail, tau becomes insoluble due to misfolding and can build up due to the blood–brain barrier preventing the release of such insoluble particles. Amyloid-beta is another protein commonly found in the human body; it is usually soluble and passes through the body without causing harm. Amyloid-beta becomes neurotoxic when it aggregates and misfolds, the most toxic species being amyloid-beta42 [5]. The true nature of what makes hyperphosphorylated tau and aggregated amyloid-beta neurotoxic is still not understood and requires more research. These two misfolded proteins are the ideal targets for potential Alzheimer’s disease treatments [5].
Mitochondria are energy-producing organelles that control cell survival and neuronal cell death [13]. Mitochondria produce the energy molecule ATP, with reactive oxygen species as byproducts. When mitochondrial DNA is mutated, the mitochondria can produce excess reactive oxygen species, leading to oxidative stress. This starts a vicious cycle in the mitochondria in which mDNA mutates, leading to the overproduction of ROS, resulting in oxidative stress and DNA mutations (Figure 1) [9]. Brain tissue is very sensitive to oxidative stress due to its high concentration of unsaturated fatty acids and the inability of the brain to regulate itself like other organs [13].
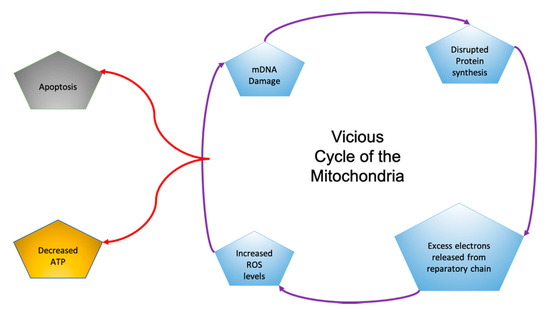
Figure 1.
In the “Vicious Cycle of the Mitochondria”, each aspect of damage leads to subsequent dysfunction and affects other aspects of the cycle [9].
Approximately 90% of the Earth’s biomass is found in the ocean, and marine organisms represent about half of the world’s known species [14]. Marine macroalgae, or seaweed, make up a large portion of this biomass with over 10,000 species found globally [15]. Due to the ready availability of seaweed globally, it has been used in medicines for millennia, ever since the year 3000 BC [9].
Seaweed is classed into three categories based on pigmentation: green seaweed (Chlorophyta), red seaweed (Rhodophyta), and brown seaweed (Phaeophyta). There are over 4000 species of red seaweed, over 900 species of green seaweed, and over 1500 species of brown seaweed worldwide. Brown seaweed can be found in temperate water, while red and green seaweeds grow exclusively in tropical waters [4].
Polysaccharides serve as energy reserves and as structural components and are found in all organisms. Many types of seaweed contain over 80 wt.% polysaccharides [16]. Seaweeds frequently rely on sulfated polysaccharides as cell wall material to aid in their flexibility to prevent tidal damage [17]. Sulfated polysaccharides, such as those found in seaweed, have been shown to exhibit high anti-inflammatory and antioxidant activities (Table 1) as well as the ability to scavenge free radicals [16]. Oxidative stress and inflammation are some of the leading agents in the progression of AD [3]. Nutraceuticals protect against the formation of reactive oxygen species and subsequent tissue damage; thus, seaweed polysaccharides may be a good source of nutraceuticals [18].

Table 1.
A compilation of seaweed species used in recent Alzheimer’s disease (AD) research.
Sulfation has been shown to directly impact the bioactivity of polysaccharides. In ROS-compromised cells, cell viability increased by 40% when treated with sulfated polysaccharides and only by 10% when treated with the same non-sulfated polysaccharides. This is probably due to the increases in free radical scavenging associated with sulfated polysaccharides [7].
Unfractionated heparin was used as a reference for the study shown in Table 2, which has an IC50 of 0.29 ± 0.02 μg/mL [19]. The most sulfated sample A09-SP had a degree of sulfation (DS) value of 0.81 and had a lower IC50 value than the reference sample. The increase in sulfation showed a significant improvement in IC50 value between fraction 2 and fraction 3. This demonstrates that increasing sulfation drastically lowers the IC50 [19].

Table 2.
A comparison of sulfation content and IC50. Adapted from [19].
2. Application of Polysaccharides from Brown Algae in Treating AD
2.1. Phaeophyta
The sulfated polysaccharide found in brown seaweed is fucoidan. There are many variations in fucoidan structure depending on the species of seaweed, but their overall general structures are quite similar. Fucoidans (Figure 2) primarily contain sulfated L-fucose residues [22]. Some species of brown seaweed also contain a compound known as lamaran. Lamaran consists of a β-(1-3)-glucan with β-(1-6)-linkages of 20–25 units [27]. Another main polysaccharide found in brown seaweed is alginate, found in the cell wall of some seaweed species [28].
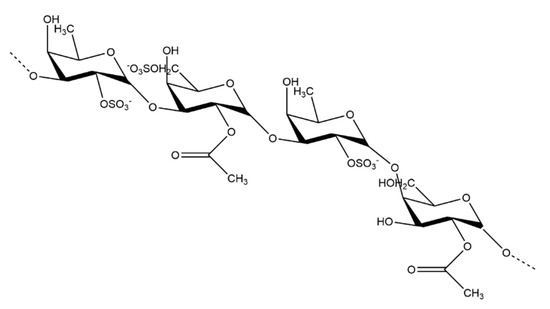
Figure 2.
Generalized structure of the fucoidan polysaccharide found in brown seaweed.
2.2. Findings of Recent Studies
An in vitro study found that polysaccharide extracts from Ecklonia radiata (a brown seaweed) prevented apoptosis and amyloid-beta toxicity, and revived compromised cells after oxidative damage [10]. Fucoidans have been found to inhibit ROS production [22], as well as to inhibit the formation of nitric oxide (NO) and prostaglandins [29]. In a 2018 study, polysaccharide extracts from Sargassum muticum (a brown seaweed native to Japan) were added to 6-OHDA comprised cells. Because it increases hydrogen peroxide concentration leading to oxidative stress and decreased cell viability, 6-OHDA has been used to study neurodegenerative damage. All known fucoidan extracts scavenge DPPH, ABTS, and FRAB used in monitoring toxic free radicals [2]. Ecklonia cava polysaccharide extracts also lowered mitochondrial-mediated protein expression and protein aggregation [13].
Polysaccharides from brown seaweeds show many bioactive properties (Figure 3). These properties include anti-inflammatory, antioxidant, anticholinic, and regulatory activities. Studies suggest their potential use in the treatment of neurodegenerative disease. Mice exhibiting signs of neurodegeneration showed improved memory and learning when treated with fucoidan extracts [14], suggesting great promise for similar sulfated polysaccharides in future human trials.
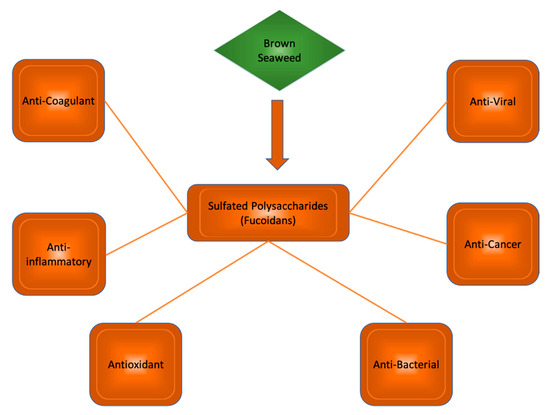
Figure 3.
Sulfated polysaccharides in brown seaweed species and their bioactive properties.
Another recent study details a trial in which neurologically compromised mice were fed polysaccharide extracts from the brown seaweed Sargassum fusiforme [20]. LXRβ and LXRα are liver X receptors (LXRs) in the brain. The activation of LXRβ improves cognition and reduces amyloid-beta plaques in AD patients; however, activation of LXRα can lead to hypertriglyceridemia and hepatic steatosis, making this a difficult treatment route [20]. Phytosterols found in plants/foods common to Western diets do not activate LXRs. A series of plants used in Eastern diets were tested for their in vitro ability to activate LXRs. Sargassum fusiforme extracts showed the best activation of LXRβ, with limited activation of LXRα, and resulted in cell death at 5 µg/mL. This concentration was then tested on AD mice, with a control group not receiving treatment. The mice treated with Sargassum fusiforme polysaccharide extracts showed a significant reduction in amyloid-beta plaques, improved memory, and improved cognition, as compared to both the baseline and the control group [20]. A series of tests were performed on the cardiovascular systems of mice using alginate oligosaccharides. The tests all showed that the pretreatment with alginate oligosaccharides vastly improved the amount of myocardial infarction and cell apoptosis. This study also looked at the ability of a pretreatment with active oxygen species (AOS) to reduce oxidative stress in mice tissue. The generation of ROS and the expression of protein were measured after reperfusion and myocardial infarction, events that would normally drastically increase the levels of ROS, especially the NO, present in cardiac cells. Pretreatment with AOS not only mitigated this reaction but lowered the overall ROS content in the cardiac cells. This suggests a similar reaction is possible in brain tissues suffering oxidative stress [30].
3. Application of Polysaccharides from Green Algae in Treating AD
3.1. Chlorophyta
Green seaweed, known as Chlorophyta, has slightly fewer polysaccharides per dry weight than brown seaweed, with 77% of its mass being polysaccharides; these polysaccharides contain 21% sulfate [31]. The primary sulfated polysaccharide found in green seaweed is ulvan. The sulfate content of each polysaccharide has been directly correlated to its antioxidant and neuroprotective potential.
3.2. Findings of Recent Studies
Green seaweed has also been investigated for its nutraceutical potential. A study was conducted to test the in vivo as well as the in vitro protective effects of sulfated polysaccharides from green seaweed [32]. In hydrogen peroxide-compromised cells, the cell viability and amount of apoptosis both improved after treatment with polysaccharide extracts from green seaweed. Zebrafish were treated with high levels of hydrogen peroxide to lead to oxidative stress in order to test the neuroreparative effects in animals. The survival rate for the fish treated in this way was very low; when the second group of zebrafish was treated with hydrogen peroxide and green seaweed polysaccharide extracts, the survival rate increased due to a decreased level of ROS. This led to a recovery in some of the zebrafish, demonstrating the potential for neurorepair in animals [32].
4. Application of Polysaccharides from Red Algae in Treating AD
4.1. Rhodophyta
Red seaweeds (Rhodophyta) provide many bioactive constituents, such as proteins, polysaccharides, pigments, polyunsaturated fatty acids, and phenolic compounds. Polysaccharides account for 40–50% of their dry weight. Agar and carrageenan are the two main types of cell wall polysaccharides from red seaweed, and are important in nutritional, medical, and industrial products [33].
4.2. Findings Findings of Recent Studies
Sulfated polysaccharides from the red alga Gelidium pristoides incubated with Aβ1–42 showed disappearance of Aβ1–42 fibrils, suggesting the activity of disaggregation and inhibition of aggregation of the fibrils [26]. к-carrageenan oligosaccharides were reported to exhibit immunomodulatory function by acting on LPS-activated microglial cells, resulting in their biological activity for preventing inflammation-related neurodegenerative diseases [34]. Another study showed that к-carrageenan-derived pentasaccharide can attenuate Aβ 25–35-induced apoptosis through the JNK pathway [35]. The scavenging activity of the polysaccharide carrageenan was determined. IC50 values of 36.6 and 32.8 mg/mL were measured for naturally extracted carrageenan and commercially available carrageenan, respectively [36]. This suggests a similar scavenging activity for carrageenans from different sources [35].
5. The Mechanism of Polysaccharides from Seaweeds for the Treatment of AD
Fucoidan has been shown to increase GPX levels and prevent ROS production [37]. High levels of acetylcholinesterase cause cholinergic dysfunction, which leads to memory loss in AD patients. Seaweed polysaccharide extracts inhibit both acetylcholinesterase and butyl cholinesterase, and this could improve cholinergic deficit in patients [31]. Fucoidan extracts inhibit the production of ROS, inhibit the aggregation of amyloid-beta leading to toxicity, and significantly improve hydrogen peroxide toxicity in mice models. Apoptosis due to oxidative damage is also decreased [10]. The body’s natural defense against oxidative stress is antioxidants; sulfated polysaccharides from brown seaweed show more antioxidant activity than any other known compound, perhaps due to their high content of sulfation [38]. Seaweed polysaccharides reduce ROS production and scavenge O2− and OH, leading to increased cell viability and decreased apoptosis, and improving memory and learning function [39]. By increasing SOD1 and SOD2, oxidative stress can be avoided or reduced in cells treated with seaweed polysaccharide extracts [40]. Lamaran has also been found to inhibit the production of hydrogen peroxide, the most damaging ROS [29].
Neuroinflammation is a controlling factor for both damage and repair of brain tissue [37]. Inflammatory responses are present around amyloid-beta plaques in AD [41]. Seaweed polysaccharides decreased lipid peroxidation and erythrocyte hemolysis, leading to decreased inflammation of cells [39].
BACE-1 is a protease that regulates the production of amyloid-beta, and fucoidan extracts inhibit BACE-1, leading to decreased production of amyloid-beta. By limiting the aggregation of amyloid-beta, seaweed polysaccharide extracts decrease the cytotoxicity of amyloid-beta [31]. Fucoidan can ameliorate spatial learning or memory defects by preventing the formation of amyloid-beta plaques [37].
In summary (Figure 4), the possible mechanisms for the treatment of Alzheimer’s disease using seaweed extracts (poly/oligo saccharides) include: (i) anti-inflammatory and antioxidant activities; (ii) scavenging free radicals; (iii) inhibition of ROS production and inhibition of the formation of nitric oxide (NO) and prostaglandins; (iv) lowering mitochondrial-mediated protein expression and protein aggregation; (v) directly interacting with the aggregated peptide, preventing oligomerization and fibrillation of Aβ; (vi) attenuation of Aβ-induced apoptosis through the JNK pathway; and (vii) impacting gut microbial processing and subsequent neuroinflammation.
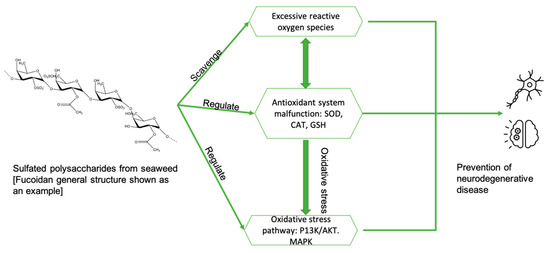
Figure 4.
The possible mechanisms of sulfated polysaccharides in regard to neurodegenerative disease progression. Adapted from [22].
6. Challenges and Opportunities
China has a long history of using herbal medicine to treat a variety of illnesses including dementia. In 2017, a retroactive cohort study was conducted to see if the addition of herbal medicine to conventional treatment options for AD could improve the cognitive ability of the patients or even slow the progression of the deterioration. Patients showed an improvement in their mini-mental state examination (MMSE) scores compared to the initial baseline and the expected untreated progression of AD (Figure 5). The normal decline in MMSE scores was significantly delayed when treated with both conventional treatments and herbal treatments [42].
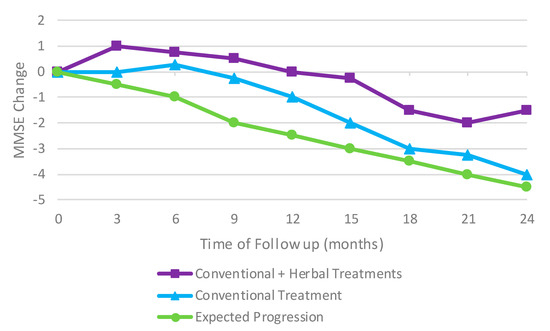
Figure 5.
An improvement in mini-mental state examination (MMSE) scores compared to the initial baseline and the expected untreated progression of AD. Results adapted from [42].
Seaweed is not the only source of natural compounds being explored as an option in the treatment of AD. A Korean group looked at the impact that long-term treatment with red ginseng extracts could have on Alzheimer’s patients. This study was 24 weeks long, with 61 patients undergoing treatment. During the 24-week trial, the patients’ AD assessment scale score improved markedly when compared with the control group. More important than the initial 12-week and 24-week scores were the scores that were seen over the next two years: these scores showed no deterioration once treatment ceased [11,43].
Some species of seaweed have a large quantity of non-sulfated polysaccharides along with their sulfated polysaccharides; this is true for other medicinal plants as well. Non-sulfated polysaccharides that had undergone chemical sulfation, in place of their naturally sulfated counterparts, were also shown to reduce oxidative stress [7]. This addition of sulfate groups onto polysaccharides extracted from Rhodiola sachalinensis using chlorosulfonic acid-pyridine suggests the same practice could be applied to other seaweed polysaccharides to increase their level of sulfation.
Seaweed is currently widely available globally but because of climate change, the availability of seaweed could change. A study was conducted to see if polysaccharides from seaweed grown in bacterial hosts could be sulfated, affording sulfated polysaccharides with the same bioactivity as naturally occurring sulfated polysaccharides [44]. This study found that in cells pretreated with the synthetic sulfated polysaccharides, the level of hydrogen peroxide required to induce oxidative stress was decreased. The survival rate of cells treated with only hydrogen peroxide was less than 60%, while the cells that were pretreated with 0.5 µg/mL sulfated polysaccharides showed a survival rate of nearly 100%. The highest concentration, 2.0 µg/mL, was the least effective concentration to ameliorate the oxidative damage; this concentration still resulted in a cell survival rate of nearly 90%. This demonstrates that chemical modification is a useful method for mass-producing sulfated polysaccharides that could provide a potential solution to oxidative stress injury and subsequent apoptosis in neuronal cells [44].
One of the main challenges in AD treatment is a therapeutic’s ability to navigate the blood–brain barrier. One solution to overcome the blood–brain barrier may be to attach these molecules to nanoparticles, relying on nanotechnology to overcome this obstacle [45]. A nanotechnological approach demonstrated that curcumin attached to nanoparticles could cross the blood–brain barrier. Curcumin has also been proposed as a possible treatment for AD due to its ability to bind to amyloid-beta, preventing aggregation [46]. This study found that a relatively low dose of nanoparticles (23 mg/week) when delivered across the blood–brain barrier showed improvements in memory and cognition in mice with AD symptoms [46]. A similar study using seaweed polysaccharides immobilized to nanoparticles could represent a new approach for patients suffering from AD.
Currently, the only treatment options for AD are cholinesterase inhibitory medications. These medications cause liver damage and do not block AD progression and, in some cases, they even exacerbate this progression [47]. Current research suggests that polysaccharide extracts from brown seaweed show low cytotoxicity, relatively high bioavailability, and low production costs and, thus, might offer alternative therapeutics [48]. These polysaccharides may represent ideal candidates for drug research and discovery. As of this publication, there have been no positive results in human trials to confirm the utility of seaweed polysaccharides in treating AD [45]. As with any possible treatment, the difficulties in undertaking human trials still represent a large barrier to success.
China has particularly high rates of AD, with nearly one third of the population over 90 being affected. China has issued conditional approval for a new AD drug, GV-971, to address this growing problem [49]. GV-971 stems from a study performed to test the impact of sodium oligomannate on gut microbial processing and subsequent neuroinflammation [50]. The 5XFAD transgenic mouse model, a standard mouse model commonly used in AD studies, mimics amyloid-beta formation as well as cognitive deficits. After one month of receiving oral GV-971, the affected mice showed a different gut microbiome as well as improved cognition [50].
This breakthrough suggests the possible use of seaweed polysaccharide extracts in treatment options for AD. Testing the efficacy of seaweed polysaccharides will require careful monitoring and data tracking to confirm the utility of such treatments in humans.
In conclusion, polysaccharides from seaweed could very well be an effective treatment for AD and other neurodegenerative diseases. The current data are very positive and show great potential, but additional research is required to establish efficacy, low toxicity, and beneficial human responses, as well as to examine other possible interactions that could undermine the neuroprotective capability of these polysaccharides. Additional studies on a wider variety of seaweeds, as well as human efficacy trials, are required. Finally, additional studies on the mechanism of seaweed polysaccharides in relation to AD symptoms and progression are needed to further the understanding of the effects of these polysaccharides.
Author Contributions
Conceptualization, W.J., F.Z., and R.J.L.; writing, S.B. and W.J.; review and editing, F.Z. and R.J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, C.H.; Chen, P.W. Compressional-Puffing Pretreatment Enhances Neuroprotective Effects of Fucoidans from the Brown Seaweed Sargassum Hemiphyllum on 6-Hydroxydopamine-Induced Apoptosis in SH-SY5Y Cells. Molecules 2018, 23, 78. [Google Scholar] [CrossRef]
- Manigandan, V.; Karthik, R.; Saravanan, R. Marine Carbohydrate Based Therapeutics for Alzheimer Disease-Mini Review. J. Neurol. Neurosci. 2015, 6. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Macroalgae as a Valuable Source of Naturally Occurring Bioactive Compounds for the Treatment of Alzheimer’s Disease. Mar. Drugs 2019, 17, 609. [Google Scholar] [CrossRef]
- Bertsch, M.; Franchi, B.; Meschini, V.; Tesi, M.C.; Tosin, A. A Sensitivity Analysis of a Mathematical Model for the Synergistic Interplay of Amyloid Beta and Tau on the Dynamics of Alzheimer’s Disease. Brain Multiphys. 2021, 2, 100020. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-Inflammatory Activities of Marine Algae in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wu, Y.; Jiang, G.; Feng, L.; Wang, Z.; Yuan, G.; Tong, H. Sulfated Polysaccharides from Rhodiola Sachalinensis Reduce D-Gal-Induced Oxidative Stress in NIH 3T3 Cells. Int. J. Biol. Macromol. 2019, 140, 288–293. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Pinteus, S.; Mendes, S.; Pedrosa, R. Neuroprotective Effects of Seaweeds against 6-Hydroxidopamine-Induced Cell Death on an in Vitro Human Neuroblastoma Model. BMC Complement. Altern. Med. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Elfawy, H.A.; Das, B. Crosstalk between Mitochondrial Dysfunction, Oxidative Stress, and Age Related Neurodegenerative Disease: Etiologies and Therapeutic Strategies. Life Sci. 2019, 218, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Alghazwi, M.; Charoensiddhi, S.; Smid, S.; Zhang, W. Impact of Ecklonia Radiata Extracts on the Neuroprotective Activities against Amyloid Beta (Aβ1-42) Toxicity and Aggregation. J. Funct. Foods 2020, 68. [Google Scholar] [CrossRef]
- Heo, J.-H.; Lee, S.-T.; Oh, M.-J.; Park, H.-J.; Shim, J.-Y.; Chu, K.; Kim, M.-H. Improvement of Cognitive Deficit in Alzheimer’s Disease Patients by Long Term Treatment with Korean Red Ginseng. J. Ginseng Res. 2011, 35, 457–461. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Zhu, J.; Liang, H.; He, X.; Qian, J.; Lin, H.; Tao, Y.; Zhu, K. Quantitative Assessment of Hippocampal Tau Pathology in AD and PART. J. Mol. Neurosci. 2020, 70, 1808–1811. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Park, S.H.; Kwon, B.S.; Kim, G.H.; Heo, H.J. Protective Effect of Fucoidan Extract from Ecklonia Cava on Hydrogen Peroxide-Induced Neurotoxicity. J. Microbiol. Biotechnol. 2018, 28, 40–49. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Chung, D.H.; Kim, D.O.; Kim, G.H.; Heo, H.J. Fucoidan-Rich Substances from Ecklonia Cava Improve Trimethyltin-Induced Cognitive Dysfunction via Down-Regulation of Amyloid β Production/Tau Hyperphosphorylation. Mar. Drugs 2019, 17, 591. [Google Scholar] [CrossRef]
- Collins, K.G.; Fitzgerald, G.F.; Stanton, C.; Ross, R.P. Looking beyond the Terrestrial: The Potential of Seaweed Derived Bioactives to Treat Non-Communicable Diseases. Mar. Drugs 2016, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed Nutraceuticals and Their Therapeutic Role in Disease Prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Tasdemir, D. Seaweeds to the Rescue of Forgotten Diseases: A Review. Bot. Mar. 2019, 62, 211–226. [Google Scholar] [CrossRef]
- Jutur, P.P.; Nesamma, A.A.; Shaikh, K.M. Algae-Derived Marine Oligosaccharides and Their Biological Applications. Front. Mar. Sci. 2016, 3, 83. [Google Scholar] [CrossRef]
- Ehrig, K.; Alban, S. Sulfated Galactofucan from the Brown Alga Saccharina Latissima-Variability of Yield, Structural Composition and Bioactivity. Mar. Drugs 2015, 13, 76–101. [Google Scholar] [CrossRef] [PubMed]
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum Fusiforme Improves Memory and Reduces Amyloid Plaque Load in an Alzheimer’s Disease Mouse Model. Sci. Rep. 2019, 9, 4908. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kang, N.; Ahn, G.; Jee, Y.; Kim, Y.T.; Jeon, Y.J. Bioactive Potentials of Sulfated Polysaccharides Isolated from Brown Seaweed Sargassum Spp in Related to Human Health Applications: A Review. Food Hydrocoll. 2018, 81, 200–208. [Google Scholar] [CrossRef]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The Antioxidant Activity of Polysaccharides Derived from Marine Organisms: An Overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef]
- Gao, Y.; Li, C.; Yin, J.; Shen, J.; Wang, H.; Wu, Y.; Jin, H. Fucoidan, a sulfated polysaccharide from brown algae, improves cognitive impairment induced by infusion of Aβ peptide in rats. Environ. Toxicol. Pharmacol. 2012, 33, 304–311. [Google Scholar] [CrossRef]
- Rizki, I.F.; Sinurat, E.; Fajriah, S.; Saefudin, E. Antioxidant Activity of Sulfated Polysaccharide Extract from Green Seaweed (Caulerpa Lentillifera) Makassar, Indonesia. Key Eng. Mater. 2020, 840, 214–220. [Google Scholar] [CrossRef]
- Barzkar, N.; Jahromi, S.T.; Poorsaheli, H.B.; Vianello, F. Metabolites from Marine Microorganisms, Micro, and Macroalgae: Immense Scope for Pharmacology. Mar. Drugs 2019, 17, 464. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical characterization, antioxidant properties, cholinesterase inhibitory and anti-amyloidogenic activities of sulfated polysaccharides from some seaweeds. Bioactive. Carbohydr. Diet. Fibre 2019, 18, 100182. [Google Scholar] [CrossRef]
- Déléris, P.; Nazih, H.; Bard, J.M. Seaweeds in Human Health. In Seaweed in Health and Disease Prevention; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 319–367. ISBN 978-0-12-802793-6. [Google Scholar]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.T.; Jiang, Z. Alginate Oligosaccharides: Production, Biological Activities, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Mishra, A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-J.; Xu, F.-Q.; Li, Y.-H.; Li, J.; Liu, X.; Wang, X.-F.; Hu, L.-G.; An, Y. Alginate Oligosaccharide Alleviates Myocardial Reperfusion Injury by Inhibiting Nitrative and Oxidative Stress and Endoplasmic Reticulum Stress-Mediated Apoptosis. Drug Des. Devel. Ther. 2017, 11, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical Characterization of Sulfated Polysaccharides from Gracilaria Gracilis and Ulva Lactuca and Their Radical Scavenging, Metal Chelating, and Cholinesterase Inhibitory Activities. Int. J. Food Prop. 2019, 22, 100–110. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Je, J.G.; Jayawardena, T.U.; Kim, Y.-S.; Ko, J.Y.; Fu, X.; Jeon, Y.-J. Protective Effects of Sulfated Polysaccharides Isolated from the Enzymatic Digest of Codium Fragile against Hydrogen Peroxide-Induced Oxidative Stress in in Vitro and in Vivo Models. Algal Res. 2020, 48, 101891. [Google Scholar] [CrossRef]
- Ismail, M.M.; Alotaibi, B.S.; EL-Sheekh, M.M. Therapeutic Uses of Red Macroalgae. Molecules 2020, 25, 4411. [Google Scholar] [CrossRef]
- Yao, Z.A.; Xu, L.; Wu, H.G. Immunomodulatory function of κ-carrageenan oligosaccharides acting on LPS-activated microglial cells. Neurochem. Res. 2014, 39, 333–343. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, L.; Li, X. κ-Carrageenan-Derived Pentasaccharide Attenuates Aβ25–35-Induced Apoptosis in SH-SY5Y Cells via Suppression of the JNK Signaling Pathway. Mol. Med. Rep. 2017, 15, 285–290. [Google Scholar] [CrossRef]
- Suganya, A.M.; Sanjivkumar, M.; Chandran, M.N.; Palavesam, A.; Immanuel, G. Pharmacological Importance of Sulphated Polysaccharide Carrageenan from Red Seaweed Kappaphycus Alvarezii in Comparison with Commercial Carrageenan. Biomed. Pharmacother. 2016, 84, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Schepers, M.; Martens, N.; Tiane, A.; Vanbrabant, K.; Liu, H.B.; Lütjohann, D.; Mulder, M.; Vanmierlo, T. Edible Seaweed-Derived Constituents: An Undisclosed Source of Neuroprotective Compounds. Neural Regen. Res. 2020, 15, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Mariya Jose, G.; Muraleedhara Kurup, G. In Vitro Antioxidant Properties of Edible Marine Algae Sargassum Swartzii, Ulva Fasciata and Chaetomorpha Antennina of Kerala Coast. J. Pharmacol. Rep. 2016, 4, 100–108. [Google Scholar]
- Li, H.; Ding, F.; Xiao, L.; Shi, R.; Wang, H.; Han, W.; Huang, Z. Food-Derived Antioxidant Polysaccharides and Their Pharmacological Potential in Neurodegenerative Diseases. Nutrients 2017, 9, 778. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Hwang, J.-H.; Kim, K.-J.; Lee, B.-Y. Ecklonia Cava-Derived Polysaccharide Prevent Hydro Peroxide-Induced Oxidative Stress and Neurotoxicity in Human Microglial HMO6 Cells. J. Food Nutr. Res. 2017, 5, 187–190. [Google Scholar] [CrossRef]
- Watson, P.M.D.; Kavanagh, E.; Allenby, G.; Vassey, M. Bioengineered 3D Glial Cell Culture Systems and Applications for Neurodegeneration and Neuroinflammation. SLAS Discov. 2017, 22, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ni, J.; Lu, T.; Zhang, X.; Wei, M.; Li, T.; Liu, W.; Wang, Y.; Shi, Y.; Tian, J. Adding Chinese Herbal Medicine to Conventional Therapy Brings Cognitive Benefits to Patients with Alzheimer’s Disease: A Retrospective Analysis. BMC Complement. Altern. Med. 2017, 17, 533. [Google Scholar] [CrossRef]
- Heo, J.-H.; Lee, S.-T.; Chu, K.; Oh, M.J.; Park, H.-J.; Shim, J.-Y.; Kim, M. Heat-Processed Ginseng Enhances the Cognitive Function in Patients with Moderately Severe Alzheimer’s Disease. Nutr. Neurosci. 2012, 15, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhao, X.; Wang, F.; Lu, Z.; Wang, Y.; Jin, M. Proteomic Study of Sulfated Polysaccharide from Enterobacter Cloacae Z0206 against H2O2-Induced Oxidative Damage in Murine Macrophages. Carbohydr. Polym. 2020, 237, 116147. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid. Based Complement. Alternat. Med. 2020, 2020. [Google Scholar] [CrossRef]
- Cheng, K.K.; Yeung, C.F.; Ho, S.W.; Chow, S.F.; Chow, A.H.L.; Baum, L. Highly Stabilized Curcumin Nanoparticles Tested in an In Vitro Blood–Brain Barrier Model and in Alzheimer’s Disease Tg2576 Mice. AAPS J. 2013, 15, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, G.T. Cholinesterase Inhibitors for the Treatment of Alzheimer’s Disease: Getting on and Staying On. Curr. Ther. Res. Clin. Exp. 2003, 64, 216–235. [Google Scholar] [CrossRef]
- Nagarajan, S.; Mathaiyan, M. Emerging Novel Anti HIV Biomolecules from Marine Algae: An Overview. J. Appl. Pharm. Sci. 2015, 5, 153–158. [Google Scholar] [CrossRef]
- Poo, M. New Light on the Horizon of Alzheimer’s Disease. Natl. Sci. Rev. 2020, 7, 831. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium Oligomannate Therapeutically Remodels Gut Microbiota and Suppresses Gut Bacterial Amino Acids-Shaped Neuroinflammation to Inhibit Alzheimer’s Disease Progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).