Recent Advancements and Future Perspectives of Microalgae-Derived Pharmaceuticals
Abstract
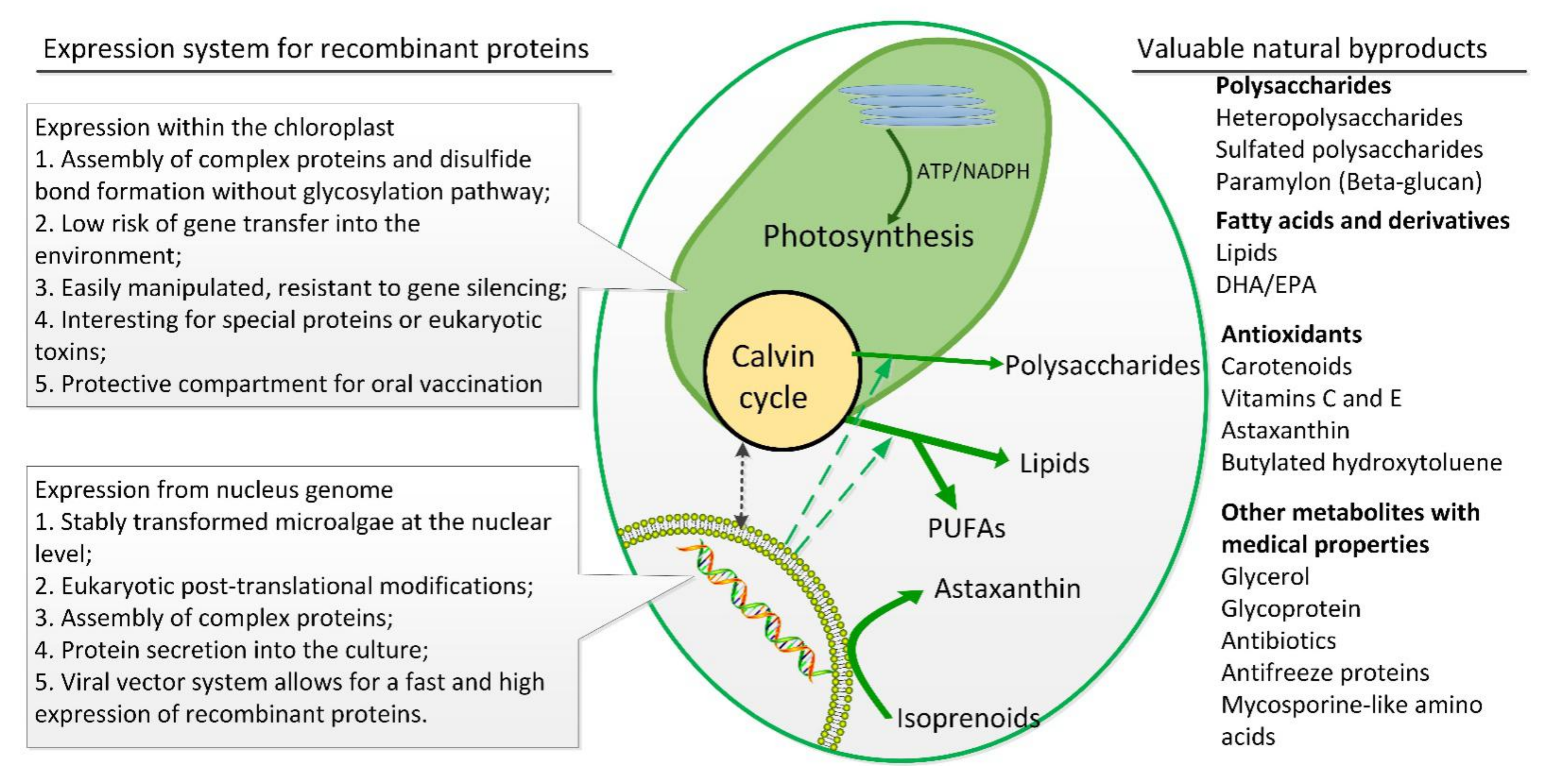
:1. Introduction
2. Bioactive Compounds
2.1. PUFAs
2.2. Polysaccharides
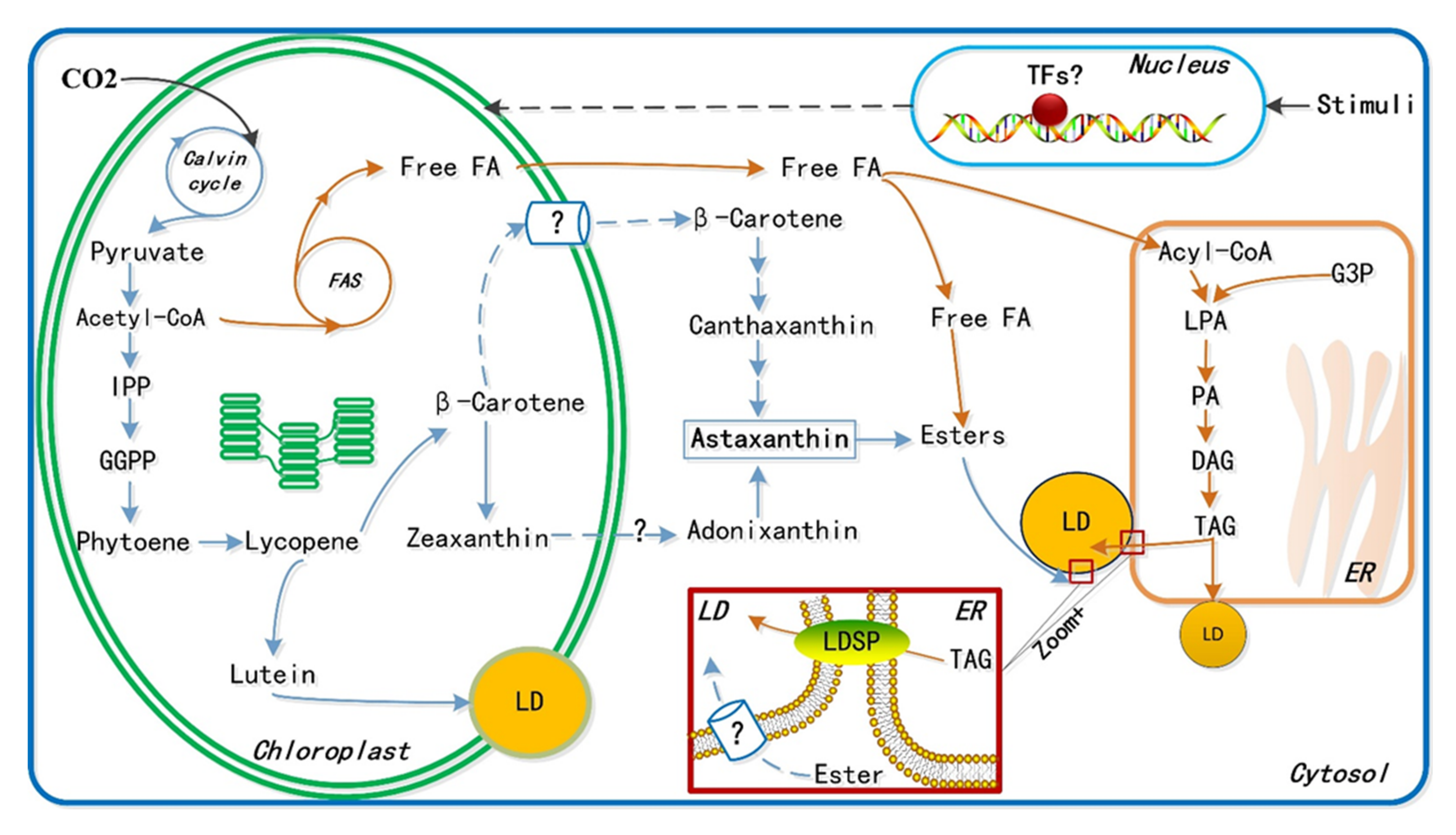
2.3. Astaxanthin
- (1)
- Astaxanthin may alleviate neurodegenerative injuries in Alzheimer’s disease. One of the most common forms of dementia, Alzheimer’s disease is widely spread and cannot be ignored [63]. More than 46 million humans currently live with dementia, and over 130 million estimated cases are expected worldwide by 2050 [10]. While the etiology of Alzheimer’s disease is not fully understood [10], astaxanthin exhibits neuroprotective effects in retinal ganglion cells and can regulate the AKT/GSK-3b signaling pathway [76]. Collectively, these properties, along with the antioxidative activity of astaxanthin, may protect against Alzheimer’s disease.
- (2)
- Astaxanthin performs multiple biological activities. For example, it can alleviate neurotoxicity in young and aged rat models of Parkinson’s disease (PD) after exposure to the neurotoxin MPTP through which neurons in the substantial nigra of aged mice groups are preserved [66]. Moreover, astaxanthin has more potent effects in young animals, as the loss of tyrosine hydroxylase through the nigrostriatal circuit induced by MPTP in aged mice cannot be reduced.
- (3)
- Researchers have suggested that astaxanthin may enhance the expression of a brain-derived neurotrophic factor in rats with a subarachnoid hemorrhage (SAH), a serious clinical disease that makes its victims comatose. In contrast, neuronal differentiation factors were reduced after SAH [81]. Although the mechanism underlying clinical symptoms is still not fully understood, the mechanism underlying the inhibition of astaxanthin in mitochondria-associated neuron apoptosis has been proposed to be (1) increased mitochondrial membrane potential; (2) decreased Bax/Bcl-2 ratio; (3) inhibition of the release of cytochrome C to the cytoplasm; and (4) suppression of caspase-3 enzyme activity [79].
- (4)
- Astaxanthin exhibits protective activity in the central nervous system by inhibiting neuronal damage induced by H2O2. Additionally, astaxanthin may act as a neuroprotective agent against spinal cord injury-induced neuronal damage by attenuating oxidative damage and inhibiting apoptosis [77]. Furthermore, it has been confirmed that astaxanthin can reduce the effects of ischemic brain injury in adult rats by inhibiting glutamate overflow. However, the direct and indirect effects of astaxanthin on the expression of aquaporins and Na+-K+-2Cl− co-transporters during traumatic brain injury remain to be determined [73]. The proposed underlying mechanism is as follows: (1) the antioxidative properties of astaxanthin protect against oxidative stress in neurological diseases by activating the signaling pathway of extracellular signal-regulated protein kinase and upregulating the expression of Nrf2-regulated enzymes [76]; and (2) the anti-inflammatory properties of astaxanthin allow it to inhibit inflammatory molecule expression by suppressing the degradation of IκB-α and the translocation, or nuclear expression, of nuclear factor kappa B, thus lowering the expression level of proinflammatory cytokines [82]. The multiple neuroprotective properties of astaxanthin have been explored using various neurological disease models, indicating its potential ability to serve as a future neuroprotective candidate for treating chronic neurodegenerative disorders. However, it is still challenging to comprehensively evaluate the underlying mechanisms [36].
- (5)
- The antioxidant characteristics of astaxanthin have been applied in treating other human diseases, such as CVDs, alcoholic liver disease, and acute lung injury. CVDs accounted for approximately 16.7 million deaths worldwide in 2010. The financial burden for CVD prevention and treatment is speculated to reach up to USD 47 trillion on a global scale in the next 25 years [60]. Astaxanthin shows excellent potential for reducing atherosclerosis occurrence, but its potential must be further examined, as it has not been studied in humans [60]. Apart from CVDs, researchers have found that astaxanthin can reduce hepatic inflammation and lipid dysmetabolism in alcoholic liver diseases, providing new insight into a promising nutrition-related therapy for alcoholic liver disease [83]. Furthermore, astaxanthin treatment significantly decreased cecal ligation and puncture-induced lung damage, as well as the resulting mortality rate in rats [84]. The putative mechanisms underlying the protective effects against lung injury suppress inflammatory responses and inhibit NF-κBP65 expression [73]. Additionally, astaxanthin supplementation may help healthy people recover from the mental fatigue induced by oxidative stress [85]. Therefore, the antioxidant properties of astaxanthin have broad and promising applicability in inflammation-associated human diseases. Moreover, the combination of astaxanthin with PUFAs may shed new light on reducing inflammation-driven infections in humans [86].
2.4. Beta-Glucan
3. Discussion
3.1. Algae-Based Antibodies and Vaccines
3.2. Current Commercialization Hurdles
4. Current Technological Difficulties of Microalgal Platforms
4.1. Genetic and Metabolic Engineering
4.2. Harvesting and Purification
4.3. Scale-Up Technology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilal, M.; Rasheed, T.; Ahmed, I.; Iqbal, H. High-value compounds from microalgae with industrial exploitability—A review. Front. Biosci. 2017, 9, 319–342. [Google Scholar]
- Sharma, N.; Sharma, P. Industrial and biotechnological applications of algae: A review. J. Adv. Plant Biol. 2018, 1, 1–25. [Google Scholar] [CrossRef]
- Fu, W.; Chaiboonchoe, A.; Khraiwesh, B.; Nelson, D.R.; Al-Khairy, D.; Mystikou, A.; Alzahmi, A.; Salehi-Ashtiani, K. Algal cell factories: Approaches, applications, and potentials. Mar. Drugs 2016, 14, 225. [Google Scholar] [CrossRef] [Green Version]
- Hempel, F.; Maier, U.G. Microalgae as solar-powered protein factories. In Advanced Technologies for Protein Complex Production and Characterization; Vega, M.C., Ed.; Springer International Publishing: Cham, Germany, 2016; pp. 241–262. [Google Scholar]
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From marine origin to therapeutics: The antitumor potential of marine algae-derived compounds. Front. Pharmacol. 2018, 9, 777. [Google Scholar] [CrossRef] [Green Version]
- Jha, D.; Jain, V.; Sharma, B.; Kant, A.; Garlapati, V.K. Microalgae-based pharmaceuticals and nutraceuticals: An emerging field with immense market potentia. ChemBioEng Rev. 2017, 4, 257–272. [Google Scholar] [CrossRef]
- Khan, M.; Rahman, M.M.; Zaman, S.; Jahangir, T.A.; Razu, M.H. Omega-3 polyunsaturated fatty acids from algae. In Recent Advances in Microalgal Biotechnology; Liu, J.L., Sun, Z., Gerken, H., Eds.; OMICS Group eBooks: Oster City, CA, USA, 2016. [Google Scholar]
- Ramana, K.; Xavier, J.; Sharma, R. Recent trends in pharmaceutical biotechnology. Pharm. Biotechnol. Curr. Res. 2017, 1, 1. [Google Scholar]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Therapeutic potentials of microalgae in the treatment of Alzheimer’s disease. Molecules 2017, 22, 480. [Google Scholar] [CrossRef] [Green Version]
- Virginie, M.; Lionel, U.; Virginie, P.; Marie, M.; Laurent, P.; Gael, B.; Jean-Paul, C.; Annick, M.-M.; Benoit, S. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as sustainable bio-factories of healthy lipids: Evaluating fatty acid content and antioxidant activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef]
- Lopes, D.; Melo, T.; Meneses, J.; Abreu, M.H.; Pereira, R.; Domingues, P.; Lillebø, A.I.; Calado, R.; Domingues, M.R. A new look for the red macroalga Palmaria palmata: A seafood with polar lipids rich in EPA and with antioxidant properties. Mar. Drugs 2019, 17, 533. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, E.; Melo, T.; Reis, M.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Polar lipids composition, antioxidant and anti-inflammatory activities of the atlantic red seaweed Grateloupia turuturu. Mar. Drugs 2021, 19, 414. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, D.Y.; Lu, T.; Liu, Z.Y.; Zhao, Q.; Liu, Y.X.; Hu, X.P.; Zhang, J.H.; Shahidi, F. Characterization of lipids in three species of sea urchin. Food Chem. 2018, 241, 97–103. [Google Scholar] [CrossRef]
- Shikov, A.N.; Laakso, I.; Pozharitskaya, O.N.; Seppänen-Laakso, T.; Krishtopina, A.S.; Makarova, M.N.; Vuorela, H.; Makarov, V. Chemical profiling and bioactivity of body wall lipids from Strongylocentrotus droebachiensis. Mar. Drugs 2017, 15, 365. [Google Scholar] [CrossRef] [Green Version]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Ramos-Vega, A.; Rosales-Mendoza, S.; Bañuelos-Hernández, B.; Angulo, C. Prospects on the use of Schizochytrium sp. to develop oral vaccines. Front. Microbiol. 2018, 9, 2506. [Google Scholar] [CrossRef]
- Ortiz-Viedma, J.; Aguilera, J.M.; Flores, M.; Lemus-Mondaca, R.; Larrazabal, M.J.; Miranda, J.M.; Aubourg, S.P. Protective effect of red algae (Rhodophyta) extracts on essential dietary components of heat-treated salmon. Antioxidants 2021, 10, 1108. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Barranco, A.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Development of new green processes for the recovery of bioactives from Phaeodactylum tricornutum. Food Res. Int. 2017, 99 Pt 3, 1056–1065. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, A.; Costa, E.D.; Silva, J.; Domingues, M.R.; Domingues, P. The effects of different extraction methods of lipids from Nannochloropsis oceanica on the contents of omega-3 fatty acids. Algal Res. 2019, 41, 101556. [Google Scholar] [CrossRef]
- Meenakshi, B. Pharmaceutically valuable bioactive compounds of algae. Asian J. Pharm. Clin. Res. 2016, 9, 6. [Google Scholar]
- Raposo, M.F.D.J.; Morais, A.; Morais, R. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci. 2014, 101, 56–63. [Google Scholar] [CrossRef]
- Yang, S.; Wan, H.; Wang, R.; Hao, D. Sulfated polysaccharides from Phaeodactylum tricornutum: Isolation, structural characteristics, and inhibiting HepG2 growth activity in vitro. PeerJ 2019, 7, e6409. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Shao, Q.; Xu, W.; Rui, L.; Sumi, R.; Eguchi, F.; Li, Z. Immunomodulatory and anti-IBDV activities of the polysaccharide AEX from Coccomyxa gloeobotrydiformis. Mar. Drugs 2017, 15, 36. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, H.; Qian, J.; He, Y.; Zheng, J.; Lu, Z.; Xu, Z.; Shi, J. Structural and immunological activity characterization of a polysaccharide isolated from Meretrix meretrix Linnaeus. Mar. Drugs 2016, 14, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Chen, M.; Gui, J.; Huang, S.; Liu, Y.; Shentu, H.; He, J.; Fang, Z.; Wang, W.; Zhang, Y. Preparation of Chlorella vulgaris polysaccharides and their antioxidant activity in vitro and in vivo. Int. J. Biol. Macromol. 2019, 137, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, F.; Yang, H.; Wang, G. Water-soluble polysaccharide isolated with alkali from the stem of Physalis alkekengi L.: Structural characterization and immunologic enhancement in DNA vaccine. Carbohydr. Polym. 2015, 121, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; He, M.; Gu, C.; Wei, D.; Liang, Y.; Yan, J.; Wang, C. Extraction optimization, purification, antioxidant activity, and preliminary structural characterization of crude polysaccharide from an arctic Chlorella sp. Polymers 2018, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Su, L.; Chen, S.; Zhao, L.; Wang, H.; Ding, F.; Chen, H.; Shi, R.; Wang, Y.; Huang, Z. Physicochemical characterization and functional analysis of the polysaccharide from the edible microalga Nostoc sphaeroides. Molecules 2018, 23, 508. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, M.; Liu, H.; Zhou, A.; Cao, Y.; Liu, X. Preliminary characterization of the structure and immunostimulatory and anti-aging properties of the polysaccharide fraction of Haematococcus pluvialis. RSC Adv. 2018, 8, 9243–9252. [Google Scholar] [CrossRef] [Green Version]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the barents sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, D.; Wu, J.; Chen, Y.; Wang, S. In vitro antioxidant activities of sulfated polysaccharide fractions extracted from Corallina officinalis. Int. J. Biol. Macromol. 2011, 49, 1031–1037. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef]
- Oka, S.; Okabe, M.; Tsubura, S.; Mikami, M.; Imai, A. Properties of fucoidans beneficial to oral healthcare. Odontology 2020, 108, 34–42. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Chen, H.; Sun, P. Extraction, structural characterization, and potential antioxidant activity of the polysaccharides from four seaweeds. Int. J. Mol. Sci. 2016, 17, 1988. [Google Scholar] [CrossRef]
- Phull, A.R.; Kim, S. Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Funct. Foods 2017, 38, 415–426. [Google Scholar] [CrossRef]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.J.; Xu, J.; Gao, X. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef]
- Reys, L.L.; Silva, S.S.; Oliveira, C.; Neves, N.M.; Martins, A.; Reis, R.L.; Silva, T.H. Angiogenic potential of airbrushed fucoidan/polycaprolactone nanofibrous meshes. Int. J. Biol. Macromol. 2021, 183, 695–706. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Mani, S.; Malarvizhi, R.; Sali, V.K.; Vasanthi, H.R. Immunomodulatory activity of brown algae Turbinaria ornata derived sulfated polysaccharide on LPS induced systemic inflammation. Phytomedicine 2021, 89, 153615. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Padmavathy, T.K.; Mani, S.; Malarvizhi, R.; Sali, V.K.; Vasanthi, H.R. Sulfated polysaccharide from Turbinaria ornata suppress lipopolysaccharide-induced inflammatory response in RAW 264.7 macrophages. Int. J. Biol. Macromol. 2020, 164, 4299–4305. [Google Scholar] [CrossRef]
- Je, J.-G.; Lee, H.-G.; Fernando, K.; Jeon, Y.-J.; Ryu, B. Purification and structural characterization of sulfated polysaccharides derived from brown algae, Sargassum binderi: Inhibitory mechanism of inos and cox-2 pathway interaction. Antioxidants 2021, 10, 822. [Google Scholar] [CrossRef]
- Komatsu, T.; Kido, N.; Sugiyama, T.; Yokochi, T. Antiviral activity of acidic polysaccharides from Coccomyxa gloeobotrydiformi, a green alga, against an in vitro human influenza A virus infection. Immunopharmacol. Immunotoxicol. 2013, 35, 1–7. [Google Scholar] [CrossRef]
- Song, L.; Chen, X.; Liu, X.; Zhang, F.; Hu, L.; Yue, Y.; Li, K.; Li, P. Characterization and comparison of the structural features, immune-modulatory and anti-avian influenza virus activities conferred by three algal sulfated polysaccharides. Mar. Drugs 2015, 14, 4. [Google Scholar] [CrossRef]
- Qi, X.; Mao, W.; Gao, Y.; Chen, Y.; Zhao, C.; Li, N.; Wang, C.; Yan, M.; Lin, C.; Shan, J. Chemical characteristic of an anticoagulant-active sulfated polysaccharide from Enteromorpha clathrata. Carbohydr. Polym. 2012, 90, 1804–1810. [Google Scholar] [CrossRef]
- Dore, C.M.P.G.; Alves, M.G.D.C.F.; Will, L.S.E.P.; Costa, T.G.; Sabry, D.A.; de Souza Rêgo, L.A.; Accardo, C.M.; Rocha, H.A.O.; Filgueira, L.G.A.; Leite, E.L. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef]
- Shao, P.; Liu, J.; Chen, X.; Fang, Z.; Sun, P. Structural features and antitumor activity of a purified polysaccharide extracted from Sargassum horneri. Int. J. Biol. Macromol. 2015, 73, 124–130. [Google Scholar] [CrossRef]
- Murad, H.; Ghannam, A.; Al-Ktaifani, M.; Abbas, A.; Hawat, M. Algal sulfated carrageenan inhibits proliferation of MDA-MB-231 cells via apoptosis regulatory genes. Mol. Med. Rep. 2015, 11, 2153–2158. [Google Scholar] [CrossRef]
- Yim, J.H.; Son, E.; Pyo, S.; Lee, H.K. Novel sulfated polysaccharide derived from red-tide microalga Gyrodinium impudicum strain KG03 with immunostimulating activity in vivo. Mar. Biotechnol. 2005, 7, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular modification of polysaccharides and resulting bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Mourão, P.A.; Pereira, M.S.; Pavão, M.S.; Mulloy, B.; Tollefsen, D.M.; Mowinckel, M.C.; Abildgaard, U. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm. Sulfated fucose branches on the polysaccharide account for its high anticoagulant action. J. Biol. Chem. 1996, 271, 23973–23984. [Google Scholar] [CrossRef] [Green Version]
- Toida, T.; Chaidedgumjorn, A.; Linhardt, R.J. Structure and bioactivity of sulfated polysaccharides. Trends Glycosci. Glyc. 2003, 15, 29–46. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Gao, R.L.; Xiong, Y.K.; Huang, Q.C.; Xu, M. Antitumor and immunomodulatory effects of a water-soluble polysaccharide from Lilii Bulbus in mice. Carbohydr. Polym. 2014, 102, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sun, Y.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring ultrasound, microwave and ultrasound-microwave assisted extraction technologies to increase the extraction of bioactive compounds and antioxidants from brown macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohyd. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Zuluaga, M.; Gueguen, V.; Letourneur, D.; Pavon-Djavid, G. Astaxanthin-antioxidant impact on excessive Reactive Oxygen Species generation induced by ischemia and reperfusion injury. Chem.-Biol. Interact. 2018, 279, 145–158. [Google Scholar] [CrossRef]
- Feng, Y.; Chu, A.; Luo, Q.; Wu, M.; Shi, X.; Chen, Y. The protective effect of astaxanthin on cognitive function via inhibition of oxidative stress and inflammation in the brains of chronic T2DM rats. Front. Pharmacol. 2018, 9, 748. [Google Scholar] [CrossRef]
- Visioli, F.; Artaria, C. Astaxanthin in cardiovascular health and disease: Mechanisms of action, therapeutic merits, and knowledge gaps. Food Funct. 2017, 8, 39–63. [Google Scholar] [CrossRef]
- Yang, X.; Guo, A.-L.; Pang, Y.-P.; Cheng, X.-J.; Xu, T.; Li, X.-R.; Liu, J.; Zhang, Y.-Y.; Liu, Y. Astaxanthin attenuates environmental tobacco smoke-induced cognitive deficits: A critical role of p38 MAPK. Mar. Drugs 2019, 17, 24. [Google Scholar] [CrossRef] [Green Version]
- Fassett, R.G.; Coombes, J.S. Astaxanthin in cardiovascular health and disease. Molecules 2012, 17, 2030–2048. [Google Scholar] [CrossRef]
- Fanaee-Danesh, E.; Gali, C.C.; Tadic, J.; Zandl-Lang, M.; Carmen Kober, A.; Agujetas, V.R.; de Dios, C.; Tam-Amersdorfer, C.; Stracke, A.; Albrecher, N.M.; et al. Astaxanthin exerts protective effects similar to bexarotene in Alzheimer’s disease by modulating amyloid-beta and cholesterol homeostasis in blood-brain barrier endothelial cells. Biochim. Et Biophys. Acta -Mol. Basis Dis. 2019, 1865, 2224–2245. [Google Scholar] [CrossRef]
- Lobos, P.; Bruna Jara, B.; Córdova, A.; Barattini, P.; Galaz, J.; Adasme, T.; Hidalgo, C.; Muñoz, P.; Paula-Lima, A. Astaxanthin protects primary hippocampal neurons against noxious effects of Aβ-oligomers. Neural Plast. 2016, 2016, 3456783. [Google Scholar] [CrossRef] [Green Version]
- Yook, J.S.; Okamoto, M.; Rakwal, R.; Shibato, J.; Lee, M.C.; Matsui, T.; Chang, H.; Cho, J.Y.; Soya, H. Astaxanthin supplementation enhances adult hippocampal neurogenesis and spatial memory in mice. Mol. Nutr. Food Res. 2016, 60, 589–599. [Google Scholar] [CrossRef]
- Grimmig, B.; Daly, L.; Subbarayan, M.; Hudson, C.; Williamson, R.; Nash, K.; Bickford, P.C. Astaxanthin is neuroprotective in an aged mouse model of Parkinson’s disease. Oncotarget 2017, 9, 10388–10401. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lu, Y.; Wu, Q.; Dai, H.; Li, W.; Lv, S.; Zhou, X.; Zhang, X.; Hang, C.; Wang, J. Astaxanthin mitigates subarachnoid hemorrhage injury primarily by increasing sirtuin 1 and inhibiting the Toll-like receptor 4 signaling pathway. FASEB J. 2019, 33, 722–737. [Google Scholar] [CrossRef]
- Wang, X.J.; Tian, D.C.; Wang, F.W.; Zhang, M.H.; Fan, C.D.; Chen, W.; Wang, M.H.; Fu, X.Y.; Ma, J.-K. Astaxanthin inhibits homocysteine-induced endothelial cell dysfunction via the regulation of the reactive oxygen species-dependent VEGF-VEGFR2-FAK signaling pathway. Mol. Med. Rep. 2019, 19, 4753–4760. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-S.; Zhang, X.; Wu, Q.; Li, W.; Wang, C.-X.; Xie, G.-B.; Zhou, X.-M.; Shi, J.-X.; Zhou, M.-L. Astaxanthin offers neuroprotection and reduces neuroinflammation in experimental subarachnoid hemorrhage. J. Surg. Res. 2014, 192, 206–213. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Zhang, X.; Zhang, Q.-R.; Wu, Q.; Li, W.; Jiang, T.-W.; Hang, C.-H. Astaxanthin reduces matrix metalloproteinase-9 expression and activity in the brain after experimental subarachnoid hemorrhage in rats. Brain Res. 2015, 1624, 113–124. [Google Scholar] [CrossRef]
- Xue, Y.; Qu, Z.; Fu, J.; Zhen, J.; Wang, W.; Cai, Y.; Wang, W. The protective effect of astaxanthin on learning and memory deficits and oxidative stress in a mouse model of repeated cerebral ischemia/reperfusion. Brain Res. Bull. 2017, 131, 221–228. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, Z.; Cui, H.; Wang, Y.; Zhong, C. Astaxanthin protects astrocytes against trauma-induced apoptosis through inhibition of NKCC1 expression via the NF-κB signaling pathway. BMC Neurosci. 2017, 18, 42. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Cui, Z.; Cui, H.; Cao, Y.; Wang, Y.; Zhong, C. Astaxanthin alleviates cerebral edema by modulating NKCC1 and AQP4 expression after traumatic brain injury in mice. BMC Neurosci. 2016, 17, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, Y.; Kobara, N.; Higashino, S.; Giddings, J.C.; Yamamoto, J. Astaxanthin inhibits thrombosis in cerebral vessels of stroke-prone spontaneously hypertensive rats. Nutr. Res. 2011, 31, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Nai, Y.; Liu, H.; Bi, X.; Gao, H.; Ren, C. Protective effect of astaxanthin on acute cerebral infarction in rats. Hum. Exp. Toxicol. 2018, 37, 929–936. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Masoudi, A.; Dargahi, L.; Abbaszadeh, F.; Pourgholami, M.H.; Asgari, A.; Manoochehri, M.; Jorjani, M. Neuroprotective effects of astaxanthin in a rat model of spinal cord injury. Behav. Brain Res. 2017, 329, 104–110. [Google Scholar] [CrossRef]
- Ji, X.; Peng, D.; Zhang, Y.; Zhang, J.; Wang, Y.; Gao, Y.; Lu, N.; Tang, P. Astaxanthin improves cognitive performance in mice following mild traumatic brain injury. Brain Res. 2017, 1659, 88–95. [Google Scholar] [CrossRef]
- Lu, Y.; Xie, T.; He, X.-X.; Mao, Z.-F.; Jia, L.-J.; Wang, W.-P.; Zhen, J.-L.; Liu, L.-M. Astaxanthin rescues neuron loss and attenuates oxidative stress induced by amygdala kindling in adult rat hippocampus. Neurosci. Lett. 2015, 597, 49–53. [Google Scholar] [CrossRef]
- Grimmig, B.; Hudson, C.; Moss, L.; Peters, M.; Subbarayan, M.; Weeber, E.J.; Bickford, P.C. Astaxanthin supplementation modulates cognitive function and synaptic plasticity in young and aged mice. GeroScience 2019, 41, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Li, Y.; Liu, B.; Wu, P.; Xu, S.; Shi, H. Protective effects of astaxanthin on subarachnoid hemorrhage-induced early brain injury: Reduction of cerebral vasospasm and improvement of neuron survival and mitochondrial function. Acta Histochem. 2019, 121, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Niu, H.; Shao, A.; Wu, C.; Dixon, B.; Zhang, J.; Yang, S.; Wang, Y. Astaxanthin as a potential neuroprotective agent for neurological diseases. Mar. Drugs 2015, 13, 5750–5766. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Liu, H.; Zhu, L.; Zhang, Z.; Zheng, X.; Liu, J.; Fu, X. Comparative transcriptome analyses provide potential insights into the molecular mechanisms of astaxanthin in the protection against alcoholic liver disease in mice. Mar. Drugs 2019, 17, 181. [Google Scholar] [CrossRef] [Green Version]
- Nagendraprabhu, P.; Sudhandiran, G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NFkB and COX-2. Investig. New Drugs 2011, 29, 207–224. [Google Scholar] [CrossRef]
- Imai, A.; Oda, Y.; Ito, N.; Seki, S.; Nakagawa, K.; Miyazawa, T.; Ueda, F. Effects of dietary supplementation of astaxanthin and sesamin on daily fatigue: A randomized, double-blind, placebo-controlled, two-way crossover study. Nutrients 2018, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Iwata, S.; Imai, T.; Shimazawa, M.; Ishibashi, T.; Hayashi, M.; Hara, H.; Nakamura, S. Protective effects of the astaxanthin derivative, adonixanthin, on brain hemorrhagic injury. Brain Res. 2018, 1698, 130–138. [Google Scholar] [CrossRef]
- Gissibl, A.; Sun, A.; Care, A.; Nevalainen, H.; Sunna, A. Bioproducts From Euglena gracilis: Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 108. [Google Scholar] [CrossRef]
- Guo, Q.; Bi, D.; Wu, M.; Yu, B.; Hu, L.; Liu, C.; Gu, L.; Zhu, H.; Lei, A.; Xu, X.; et al. Immune activation of murine RAW264.7 macrophages by sonicated and alkalized paramylon from Euglena gracilis. BMC Microbiol. 2020, 20, 171. [Google Scholar] [CrossRef]
- Yasuda, K.; Nakashima, A.; Murata, A.; Suzuki, K.; Adachi, T. Euglena Gracilis and beta-Glucan Paramylon Induce Ca(2+) Signaling in Intestinal Tract Epithelial, Immune, and Neural Cells. Nutrients 2020, 12, 2293. [Google Scholar] [CrossRef]
- Lohr, M.; Schwender, J.; Polle, J.E.W. Isoprenoid biosynthesis in eukaryotic phototrophs: A spotlight on algae. Plant Sci. 2012, 185–186, 9–22. [Google Scholar] [CrossRef]
- Schulze, C.; Wetzel, M.; Reinhardt, J.; Schmidt, M.; Felten, L.; Mundt, S. Screening of microalgae for primary metabolites including β-glucans and the influence of nitrate starvation and irradiance on β-glucan production. J. Appl. Phycol. 2016, 28, 2719–2725. [Google Scholar] [CrossRef]
- Nakashima, A.; Suzuki, K.; Asayama, Y.; Konno, M.; Saito, K.; Yamazaki, N.; Takimoto, H. Oral administration of Euglena gracilis Z and its carbohydrate storage substance provides survival protection against influenza virus infection in mice. Biochem. Biophys. Res. Commun. 2017, 494, 379–383. [Google Scholar] [CrossRef]
- Cui, Y.; Thomas-Hall, S.R.; Chua, E.T.; Schenk, P.M. Development of high-level omega-3 eicosapentaenoic acid (EPA) production from Phaeodactylum tricornutum. J. Phycol. 2021, 57, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Falcone, P.H.; Crowley, D.C.; Sulley, A.M.; Campbell, M.; Zakaria, N.; Lasrado, J.A.; Fritz, E.P.; Herrlinger, K.A. Effect of a Euglena gracilis fermentate on immune function in healthy, active adults: A randomized, double-blind, placebo-controlled trial. Nutrients 2019, 11, 2926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Ehrlich, A.; Perng, V.; Chase, J.A.; Raybould, H.; Li, X.; Atwill, E.R.; Whelan, R.; Sokale, A.; Liu, Y. Algae-derived β-glucan enhanced gut health and immune responses of weaned pigs experimentally infected with a pathogenic E. coli. Anim. Feed Sci. Technol. 2019, 248, 114–125. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, L.; Li, Y.; Jiang, S.; Sun, Q.; Xie, E.; Chen, H.; Zhao, Z.; Qiao, W.; Xu, J.; et al. Structure of a laminarin-type β-(1→3)-glucan from brown algae Sargassum henslowianum and its potential on regulating gut microbiota. Carbohydr. Polym. 2021, 255, 117389. [Google Scholar] [CrossRef]
- Ferreira Virginio, G., Jr.; Reis, M.E.; da Silva, A.P.; de Toledo, A.F.; Cezar, A.M.; Mendes, L.W.; Greco, L.; Montenegro, H.; Coutinho, L.L.; Bittar, C.M.M. Does algae β-glucan affect the fecal bacteriome in dairy calves? PLoS ONE 2021, 16, e0258069. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Kumar, S.P.; Rao, H.C.Y.; Chelliah, J. Synthesis of β-glucan nanoparticles from red algae-derived β-glucan for potential biomedical applications. Appl. Biochem. Biotechnol. 2021, 193, 3983–3995. [Google Scholar] [CrossRef]
- Criscuolo, E.; Caputo, V.; Diotti, R.A.; Sautto, G.A.; Kirchenbaum, G.A.; Clementi, N. Alternative methods of vaccine delivery: An overview of edible and intradermal vaccines. J. Immunol. Res. 2019, 2019, 13. [Google Scholar] [CrossRef] [Green Version]
- Mayfield, S.P.; Franklin, S.E.; Lerner, R.A. Expression and assembly of a fully active antibody in algae. Proc. Natl. Acad. Sci. USA 2003, 100, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Shamriz, S.; Ofoghi, H. Expression of recombinant PfCelTOS antigen in the chloroplast of Chlamydomonas reinhardtii and its potential use in detection of Malaria. Mol. Biotechnol. 2019, 61, 102–110. [Google Scholar] [CrossRef]
- Shahriari, A.G.; Afsharifar, A.; Habibi-Pirkoohi, M. Expression of hemagglutinin-neuraminidase (HN) and fusion (f) epitopes of newcastle disease virus (NDV) in Chlamydomonas reinhardtii. Plant Omics 2019, 12, 63–69. [Google Scholar] [CrossRef]
- Bañuelos-Hernández, B.; Monreal-Escalante, E.; González-Ortega, O.; Angulo, C.; Rosales-Mendoza, S. Algevir: An expression system for microalgae based on viral vectors. Front. Microbiol. 2017, 8, 1100. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Berlanga, B.; Bañuelos-Hernández, B.; Rosales-Mendoza, S. Efficient expression of an Alzheimer’s disease vaccine candidate in the microalga Schizochytrium sp. using the Algevir system. Mol. Biotechnol. 2018, 60, 362–368. [Google Scholar] [CrossRef]
- Li, S.-S.; Tsai, H.-J. Transgenic microalgae as a non-antibiotic bactericide producer to defend against bacterial pathogen infection in the fish digestive tract. Fish Shellfish. Immunol. 2009, 26, 316–325. [Google Scholar] [CrossRef]
- Feng, S.; Feng, W.; Zhao, L.; Gu, H.; Li, Q.; Shi, K.; Guo, S.; Zhang, N. Preparation of transgenic Dunaliella salina for immunization against white spot syndrome virus in crayfish. Arch. Virol. 2014, 159, 519–525. [Google Scholar] [CrossRef]
- Geng, D.; Wang, Y.; Wang, P.; Li, W.; Sun, Y. Stable expression of hepatitis B surface antigen gene in Dunaliella salina (Chlorophyta). J. Appl. Phycol. 2003, 15, 451–456. [Google Scholar] [CrossRef]
- Vanier, G.; Hempel, F.; Chan, P.; Rodamer, M.; Vaudry, D.; Maier, U.G.; Lerouge, P.; Bardor, M. Biochemical characterization of human anti-hepatitis B monoclonal antibody produced in the microalgae Phaeodactylum tricornutum. PLoS ONE 2015, 10, e0139282. [Google Scholar] [CrossRef]
- Kwon, K.-C.; Lamb, A.; Fox, D.; Porphy Jegathese, S.J. An evaluation of microalgae as a recombinant protein oral delivery platform for fish using green fluorescent protein (GFP). Fish Shellfish Immunol. 2019, 87, 414–420. [Google Scholar] [CrossRef]
- Embregts, C.W.E.; Forlenza, M. Oral vaccination of fish: Lessons from humans and veterinary species. Dev. Comp. Immunol. 2016, 64, 118–137. [Google Scholar] [CrossRef] [Green Version]
- Specht, E.; Mayfield, S. Algae-based oral recombinant vaccines. Front. Microbiol. 2014, 5, 60. [Google Scholar] [CrossRef] [Green Version]
- Yan, N.; Fan, C.; Chen, Y.; Hu, Z. The potential for microalgae as bioreactors to produce pharmaceuticals. Int. J. Mol. Sci. 2016, 17, 962. [Google Scholar] [CrossRef] [Green Version]
- Dadar, M.; Dhama, K.; Vakharia, V.N.; Hoseinifar, S.H.; Karthik, K.; Tiwari, R.; Khandia, R.; Munjal, A.; Salgado-Miranda, C.; Joshi, S.K. Advances in aquaculture vaccines against fish pathogens: Global status and current trends. Rev. Fish. Sci. Aquac. 2017, 25, 184–217. [Google Scholar] [CrossRef]
- Grimm, P.; Risse, J.M.; Cholewa, D.; Müller, J.M.; Beshay, U.; Friehs, K.; Flaschel, E. Applicability of Euglena gracilis for biorefineries demonstrated by the production of α-tocopherol and paramylon followed by anaerobic digestion. J. Biotechnol. 2015, 215, 72–79. [Google Scholar] [CrossRef]
- Hasan, M.T.; Sun, A.; Mirzaei, M.; Te’O, J.; Hobba, G.; Sunna, A.; Nevalainen, H. A comprehensive assessment of the biosynthetic pathways of ascorbate, α-tocopherol and free amino acids in Euglena gracilis var. saccharophila. Algal Res. 2017, 27, 140–151. [Google Scholar] [CrossRef]
- Beacham, T.A.; Sweet, J.B.; Allen, M.J. Large scale cultivation of genetically modified microalgae: A new era for environmental risk assessment. Algal Res. 2017, 25, 90–100. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [Green Version]
- Gerngross, T.U. Advances in the production of human therapeutic proteins in yeasts and filamentous fungi. Nat. Biotechnol. 2004, 22, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Nymark, M.; Sharma, A.K.; Sparstad, T.; Bones, A.M.; Winge, P. A CRISPR/Cas9 system adapted for gene editing in marine algae. Sci. Rep. 2016, 6, 24951. [Google Scholar] [CrossRef]
- Shin, S.-E.; Lim, J.-M.; Koh, H.G.; Kim, E.K.; Kang, N.K.; Jeon, S.; Kwon, S.; Shin, W.-S.; Lee, B.; Hwangbo, K.; et al. CRISPR/Cas9-induced knockout and knock-in mutations in Chlamydomonas reinhardtii. Sci. Rep. 2016, 6, 27810. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, H.C.; Laurens, L.M.L.; Shields, R.J.; Lovitt, R.W.; Flynn, K.J. Placing microalgae on the biofuels priority list: A review of the technological challenges. J. R. Soc. Interface 2010, 7, 703–726. [Google Scholar] [CrossRef] [Green Version]
- Gangl, D.; Zedler, J.A.Z.; Rajakumar, P.D.; Martinez, E.M.R.; Riseley, A.; Włodarczyk, A.; Purton, S.; Sakuragi, Y.; Howe, C.J.; Jensen, P.E.; et al. Biotechnological exploitation of microalgae. J. Exp. Bot. 2015, 66, 6975–6990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, E.; Corchero, J.L.; Villaverde, A. Post-production protein stability: Trouble beyond the cell factory. Microb. Cell Factories 2011, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Elena, G.-F.; Esther, V.; Nuria, G.-M.; Neus, F.-M.; Antonio, V. Analytical approaches for assessing aggregation of protein biopharmaceuticals. Curr. Pharm. Biotechnol. 2011, 12, 1530–1536. [Google Scholar] [CrossRef]
- Antosova, Z.; Mackova, M.; Kral, V.; Macek, T. Therapeutic application of peptides and proteins: Parenteral forever? Trends Biotechnol. 2009, 27, 628–635. [Google Scholar] [CrossRef]
- El-Ayouty, Y.; El-Manawy, I.; Nasih, S.; Hamdy, E.; Kebeish, R. Engineering Chlamydomonas reinhardtii for expression of functionally active human interferon-α. Mol. Biotechnol. 2019, 61, 134–144. [Google Scholar] [CrossRef]
- Reddy, P.H.; Johnson, A.M.A.; Kumar, J.K.; Naveen, T.; Devi, M.C. Heterologous expression of Infectious bursal disease virus VP2 gene in Chlorella pyrenoidosa as a model system for molecular farming. Plant Cell Tissue Organ Cult. 2017, 131, 119–126. [Google Scholar] [CrossRef]
- Márquez-Escobar, V.A.; Bañuelos-Hernández, B.; Rosales-Mendoza, S. Expression of a Zika virus antigen in microalgae: Towards mucosal vaccine development. J. Biotechnol. 2018, 282, 86–91. [Google Scholar] [CrossRef]

| Name | Algae/Other | Study Model | Positive/Negative Control | Dose/Concentration Range | Effect | Ref. | |
|---|---|---|---|---|---|---|---|
| ABTS+ (µg/mL), IC50 | DPPH (µg/mL), IC20 | ||||||
| Algae | Chlorella vulgaris | ABTS+, DPPH | control lipid: replace ABTS+ with ethanol | 25, 125, 250, 500 µg/mL | 51.1 | 50.5 | [12] |
| Chlorococcum amblystomatis | 52.6 | 58.4 | |||||
| Scenedesmus obliquus | 29.4 | 89.1 | |||||
| Tetraselmis chui | 40.9 | 225.7 | |||||
| Phaeodactylum tricornutum | 57.3 | 75.4 | |||||
| Spirulina sp. | 38.7 | 96.6 | |||||
| Nannochloropsis oceanica | 101.9 | 175.6 | |||||
| Palmaria palmata | ABTS+, DPPH | control lipid: replace ABTS+ with ethanol | 25, 50, 100, 250 µg/mL | 26.2 | IC30 = 171 | [13] | |
| Grateloupia turuturu Yamada | ABTS+, DPPH; COX-2 | control lipid: replace ABTS+ with ethanol | 12.5, 250 µg/mL | 130.4 | IC50 = 130.4 | [14] | |
| 33 * | |||||||
| Sea urchin | Glyptocidaris crenularis | - | - | 11.4% of DW | - | [15] | |
| Strongylocentrotus intermedius | - | - | 20.0% of DW | - | |||
| Strongylocentrotus nudus | - | - | 9.3% of DW | - | |||
| Sea urchin | Strongylocentrotus droebachiensis | human mononuclear U937 cell | Escherichia coli LPS | 15.2 µg/mg, 9.6% of DW | anti-inflammatory activity: 88% of MAPK p38 inhibition at a dose of 0.033 µg/mL, COX-1, and COX-2. | [16] | |
| Name | Algae | Monosaccharide Composition | Molecular Weight | Study Model | Positive/Negative Control | Dose/Concentration Range | Effect | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fucoidan (sulfate content 27.0%) | Fucus vesiculosus | fucose 73.5 mol%, galactose 3.7 mol%, glucose 11.8 mol%, xylose 6.6 mol%, mannose 0.2 mol%, arabinose 0.2 mol% | 735 kDa ultrasound-assisted extraction | human mononuclear U937 cells | Escherichia coli LPS | 10 mg/mL | inhibit hyaluronidase and DPP-IV. IC50 for DPPH 35 μg/mL, AA 0.32, BHA 0.59 μg/mL | [32] |
| human DPP-IV | sitagliptin | 0.2–200 μg/mL | ||||||
| platelet-poor plasma | - | 1.6, 3.2, 4.8, 6.3, 9.1, 10 μg/mL; 80, 310, 380, 450 μg/mL | ||||||
| Fucoidan (sulfate content 27.1%) | Ascophyllum nodosum | fucose 41.2 mol%, galactose 6 mol%, glucose 6 mol%, xylose 15 mol%, mannose 11.3 mol%, uronic acid 24.6 mol% | 34.4 kDa | - | - | - | 30.4% scavenging of DPPH at 10 mg/mL | [33] |
| Sulfated polysaccharides (F1, F2) | Corallina officinalis | galactose, xylose | - | - | - | - | - | [34] |
| Crude fucoidan (sulfate content 22.8%) | Undaria pinnatifida | fucose 29 mol%, xylose 30 mol%, galactose 23 mol%, glucose 3 mol%, uronic acid 4 mol% | >300 kDa | - | - | AA 244 μg/mL, BHA 235 μg/mL, Trolox equivalent | 7.4 μg/mL Trolox equivalent | [35] |
| Purified fucoidan (sulfate content 20.0%) | fucose 27 mol%, xylose 3 mol%, galactose 18 mol%, glucose 2 mol%, uronic acid 4.6 mol% | 300 kDa | - | - | 9.0 μg/mL Trolox equivalent | |||
| Standard fucoidan (sulfate content 30.0%) | - | lower than 10 kDa | 8.8 μg/mL Trolox equivalent | |||||
| Sulfated α-L-fucan | 9072-19-9, Cayman Chemical, Ann Arbor, MI, USA | unknown | oral pathogens: Candida albicans (JCM1537), Streptococcus mutans (JCM5705), Porphyromonas gingivalis (JCM8525) | antibiotic (positive control), PBS (negative control). Endotoxin-neutralizing of LPS | 100 mg/mL | strong antimicrobial activity, crude fucoidan showed stronger inhibition effect than purified fucoidan | [36] | |
| Fucoidan extract with a low molecular weight prepared by glycosidase digestion (sulfation 14.5%) | Cladosiphon novae-caledoniae | fucose (73%), xylose (12%), and mannose (7%) | digested low-molecular-weight fraction (72% <500 kDa) and non-digested fraction (>28%, 800 kDa peak) | |||||
| Crude fucoidan sulfate (23%) | Fucus vesiculosus | fucose (33%), uronic acid (8%), | 20–200 kDa | |||||
| Purified (>95%) fucoidan | Fucus vesiculosus | - | 68.6 kDa | |||||
| Fucoidan | Durvillaea antarctica | mole ratio: 1.1 fucose, 26.2 glucose, 0.9 xylose, 2.9 mannose, 2.7 sorbose | 482 kDa | - | - | - | - | [37] |
| Sarcodia ceylonensis | mole ratio: 5.3 glucose, 1.2 arabinose, 14.4 mannose, 2.8 sorbose | 466 kDa | - | - | - | - | ||
| Ulva lactuca L. | mole ratio: 0.2 fucose, 1.9 glucose, 0.2 galactose, 0.5 arabinose, 0.3 xylose, 6.7 mannose, 0.5 sorbose | 404 kDa | - | - | - | - | ||
| Gracilaria lemaneiformis | mole ratio: 4.5 glucose, 1.8 xylose, 18.8 galactose, 6.0 frucose | 591 kDa | - | - | - | - | ||
| macroalga Ulva lactuca | molar rate 1.1, 1.9, 0.2, 0.5, 0.3, 6.7, and 0.5 for fucose, glucose, galactose, arabinose, xylose, mannose, and sorbose | 466 kDa | - | - | - | - | [38] | |
| Fucoidan (LJSF4) (sulfate 30.7%, yield 7.5%) | Saccharina-japonica | fucose, galactose, rhamnose, xylose, mannose | 104.3 kDa | zebrafish | LPS-induced toxicity | 12.5–50 μg/mL | reduces the cell death rate, decreases the production of nitric oxide, ROS and cytokines, including TNF-α, IL-1β, and IL-6. LJSF4 pre-treatment significantly decreased the heart rates of zebrafish larvae and even reduced to 103.2% at 50 μg/mL | [39] |
| Fucoidan (Fu) | Fucus vesiculosus | - | - | human pulmonary microvascular endothelial (HPMEC-ST1.6R) cells/chick chorioallantoic membrane | - | - | enhanced viability of endothelial cells and vascularization | [40] |
| Sulfated polysaccharide (PS) | Turbinaria ornata | glucopyranose, fucopyranose | unknown | rat | LPS, LPS + dexamethasone, LPS + PS/normal control | 2.5, 5, 10 mg/kg body weight | prevents LPS-induced systemic inflammation in the cardiac tissue, PS mitigates inflammation by repressing and/or inhibiting iNOS, NFκB, and PI3K/Akt pathway | [41] |
| Sulfated polysaccharide (PS) | Turbinaria ornata | - | unknown | RAW 264.7 macrophages | DMEM medium, LPS | 10, 20, 40 μg/mL | increases the antioxidants GSH and SOD, significantly reduces mRNA levels of IL6 and TNFα | [42] |
| SBPs (sulfate, 24.1%) | Sargassum binderi | fucose, galactose, glucose, mannose, arabinose, rhamnose | average 2.867 × 105 g/mol for SBP-fraction 4 | macrophages (RAW 264.7)/zebrafish | LPS + SBPs/control (neither LPS nor SBP) | 25, 50, 100, 200 µg/mL | inhibits COX-2 and iNOS protein levels in LPS-activated macrophages and reduces cell death and NO production in LPS-treated zebrafish larvae | [43] |
| lambda-carrageenan (λ-CGN), commercial | - | α-galactose | - | influenza A and B viruses, d severe respiratory syndrome coronavirus 2 (SARS-CoV-2)/mice | virus infected MDCK or Vero cells/mock-infected; λ-CGN/p-KG03 or EGCG | 10, 100, 300 µg/mL | targets viral attachment to cell surface receptors and prevents virus entry | |
| acidic polysaccharide of the Coccomyxa gloeobotrydiformis Nikken strain (AEX) | Coccomyxa gloeobotrydiformis | galactose, mannose, glucose, arabinose, xylose, rhamnose | - | MDCK cells; human influenza A virus, | MDCK cells inoculated with human influenza A virus/uninfected living cells | 26–70 µg/mL | prevents the cell attachment and/or penetration of influenza virus; prevents the interaction of virus and host cells | [44] |
| acidic polysaccharide of the Coccomyxa gloeobotrydiformis Nikken strain (AEX), Nikken Sohonsha Corporation (Hashima, Gifu, Japan) | Coccomyxa gloeobotrydiformis Nikken strain | galactose, mannose, glucose, arabinose, xylose, rhamnose | - | chicken immune cells; IBDV | Vero cells incubate with IBDV Ts strain and AEX, IBDV live vaccine; Con A as positive control | 12.5, 25, 50, 100 mg/mL | represses IBDV replication by the deactivation of viral particles or by interfering with adsorption in vitro, and reduces the IBDV viral titer in the chicken bursa of Fabricius | |
| GFP (sulfate, 19.9%) | Grateloupia filicina (19.7% yield) | molar ratio: 0.01 Man, 0.02 Glc A, 0.07 Glc, 1 Gal, 0.1 Xyl, 0.05 Fuc | unknown | avian influenza virus (AIV)/MDCK cells, mice | MDCK cells in DMEM as a control, MDCK cells in sulfated polysaccharides dissolved in DMEM | 50, 100, 500 µg/mL | stimulation of IFN-γ production, IL-4 stimulation | [45] |
| UPP (sulfate, 13.5%) | Ulva pertusa (12.1% yield) | molar ratio: 0.06 Man, 1 Rha, 0.53 Glc A, 0.19 Glc, 0.09 Gal, 0.39 Xyl, 0.02 Fuc | - | - | ||||
| SQP (sulfate, 5.6%) | Sargassum qingdaoense (7.1% yield) | molar ratio: 0.56 man, 0.13 Glc A, 0.37 Glc, 0.6 Gal, 1 Fuc | ||||||
| (Sulfate, 31.0%) (1→4)-linked β-L-arabinopyranose | Enteromorpha clathrata | arabinose (80.5%), rhamnose (10.7%), galactose (4.8%), glucuronic acid (4.0%) | 511 kDa | human plasma samples | heparin as a reference | 10, 20, 50, 100 µg/mL | stimulates TNF-α expression in serum and induces lymphocyte proliferation | [46] |
| Fucan SV1 (sulfate 22.6%) | Sargassum horneri | fucose 36.8%, galactose 17.1%, xylose 8.1%, glucuronic acid 11.1%, mannose 12.4% | unknown | rat, RAW 264.7 (mouse leukemic monocyte macrophage cell line) | DMEM medium | 0.3–2.5 mg/mL | reduces edema and cellular infiltration | [47] |
| Glucan | Sargassum horneri | T-D-Glcp, 1,3-D-Glcp, 1,6-D-Glcp and 1,3,6-D-Glcp | 578 kDa | human colon cancer DLD cells | - | - | inhibits human colon cancer DLD cell growth | [48] |
| Extracted sulfated carrageenan (ESC) | Laurencia papillosa | ι-carrageenan | - | MDA-MB-231 cancer cell line | - | 50 µM | inhibits breast cancer cells (MDA-MB-231) via apoptosis regulatory genes | [49] |
| p-KG103 | Gyrodinium impudium | - | - | mice | - | 100 or 200 mg/kg body weight | activates NO production to stimulate the production of cytokines and prevent tumor cell growth | [50] |
| Disease Type | Model | Effect | Significant Findings | Reference |
|---|---|---|---|---|
| Alzheimer’s disease | primary porcine brain capillary endothelial cells (pBCEC), and in 3xTg AD mice | neuroprotective effect | astaxanthin reduces BACE-1 (activity) and Aβ/oligomers in mBCEC and deeper regions of the brain, affecting not only the clearance but also the generation of Aβ | [63] |
| primary hippocampal neurons | neuroprotective effect | astaxanthin protects neurons from the harmful effects of A𝛽Os on mitochondrial ROS production, NFATc4 activation, and RyR2 gene expression downregulation | [64] | |
| adult hippocampal neurogenesis (AHN) and spatial memory using a mouse model | neuroprotective effect | novel insights into the neurogenic effect of astaxanthin on hippocampus-dependent cognitive function by preventing cognitive impairment | [65] | |
| Parkinson’s disease | an aged mouse model | multiple biological activities | astaxanthin attenuates neurotoxicity of Parkinson’s disease in both young and aged mice | [66] |
| Subarachnoid hemorrhage injury | adult male Sprague Dawley rats | anti-inflammation | astaxanthin increases sirtuin one levels and inhibits the TLR4 signaling pathway, then reduces the proinflammatory response and second brain injury | [67] |
| adult male SD rats | neuroprotective effect | astaxanthin attenuated SAH-induced cerebral vasospasm and reduced neuronal apoptosis | [68] | |
| adult male SD rats, prechiasmatic cistern SAH model | antineuroinflammation | astaxanthin shows neuroprotective effect with the possible mechanism of suppression of cerebral inflammation | [69] | |
| male Sprague Dawley rats, prechiasmatic cistern SAH model | neurovascular protection | astaxanthin reduces the expression and activity of MMP-9 and ameliorates brain edema, BBB impairment, neurological deficits, and TUNEL-positive cells | [70] | |
| adult male SD rats | neuroprotective effects | astaxanthin attenuates SAH-induced EBI by enhancing neuronal survival and mitochondrial function | [68] | |
| male ICR mice | antioxidant activity | astaxanthin can suppress learning and memory impairment and attenuate oxidative stress | [71] | |
| astrocytes isolated from the cerebral cortices of neonatal C57BL/6 mouse pups | anti-inflammatory, neuroprotective | astaxanthin inhibits NKCC1 expression and reduces the expression of NF-κB-mediated proinflammatory factors | [72] | |
| Acute cerebral infarction (stroke) | male C57BL/6 mice | anti-inflammatory, neuroprotective | astaxanthin ameliorates AQP4/NKCC1-mediated cerebral edema and then reduces TBI-related injury in brain tissue | [73] |
| stroke-prone spontaneously hypertensive rats | antithrombotic, antihypertensive | antihypertensive and antithrombotic properties of astaxanthin | [74] | |
| male Sprague Dawley rats | neuroprotective effect | astaxanthin ameliorates ACI via the suppression of oxidative stress and upregulation of BDNF and NGF mRNA | [75] | |
| Spinal cord injury | adult male Wistar rats | antineuroinflammation | Astaxanthin inhibits glutamate-initiated signaling pathway and inflammatory reactions in the secondary phase of SCI | [76] |
| adult male Wistar rats | anti-inflammatory, neuroprotective | astaxanthin can reduce neuronal apoptosis and improves functional recovery after SCI | [77] | |
| Traumatic brain injury | male adult ICR mice | neuroprotective action | astaxanthin reduces cortical lesion volume, neuronal cell loss, and neurodegeneration in the cortex by simulating neurotrophic factors and promoting synaptic survival | [78] |
| Cognitive disease | adult male Sprague Dawley rats, amygdala kindling, epilepsy | neuroprotective effects | astaxanthin attenuates oxidative damage and lipid peroxidation and inhibits the mitochondrion-related apoptotic pathway | [79] |
| male C57BL/6J | neurodegenerative disease | astaxanthin modulates cognitive function and synaptic plasticity | [80] | |
| male Wistar rats | antioxidant | astaxanthin inhibits oxidative stress and inflammatory responses | [59] | |
| Peripheral vascular disease | human umbilical vein endothelial cells (HuVecs) | antioxidant | astaxanthin inhibits Hcy-induced endothelial dysfunction via the suppression of Hcy-induced activation of the VEGF-VeGFr2-FaK signaling axis | [68] |
| Name | Algae | Effect | Host | Ref. |
|---|---|---|---|---|
| β-glucan | Euglena gracilis | alleviates diarrhea of F18 E. coli-infected pigs by enhancing gut integrity, stimulates T cell activation, and reduces inflammation | pig | [95] |
| Paramylon (storage β-1,3-glucan) | Eu. gracilis | directly stimulates intestinal epithelial cells via Ca2+ signaling, stimulates dendritic cells (DCs) in Peyer’s patches | mice | [89] |
| β-1,3-glucan | Eu. gracilis Kelbs var. bacillaris ATCC PTA-123017 strain | reduces and prevents upper respiratory tract infection (URTI) symptoms in humans including incidence, duration, and severity: fewer sick days, URTI symptoms, URTI symptom days, URTI episodes, and lower global severity | humans | [94] |
| Paramylon | Eu. gracilis Z | higher survival rates from influenza virus infection, significantly lower virus titer in the lung, increased inflammatory cytokines (higher amount of IL-1β, IL-6, IL-12 (p70), IFN-γ, IL-10). Induces CD8+ T cells and/or NK cells | mice | [92] |
| β-1,3-glucan | Eu. gracilis | upregulates inducible nitric oxide synthase (iNOS) and increases secretion of nitric oxide (NO), interleukin (IL)-6, and tumor necrosis factor (TNF)-α, activates the nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways | murine RAW264.7 macrophages | [88] |
| Laminarin-type β-(1→3)-glucan | Sargassum henslowianum | regulates the intestinal microbiota composition by stimulating the growth of species belonging to Enterobacteriaceae while depleting Haemophilus parainfluenzae and Gemmiger formicilis | gut microbiota via in vitro fermentation with human fecal cultures | [96] |
| β-glucans (commercial) | 2 g/d Aleta™, Kemin Industries, Inc., Des Moines, IA, USA | promotes a higher abundance of Alloprevotella and Holdemanella; beneficial to fecal bacteriome and consequently to the health and performance of dairy calves (affects the fecal bacterial community with possible consequences on animal growth and health) | newborn Holstein calves | [97] |
| β-glucan nanoparticles (β-GluNPs) | Gracilaria corticata | Fabrication of the water-soluble β-GluNPs using β-glucan. β-GluNPs have potential antibacterial activity against Gram-positive bacterial isolates. Stronger cytotoxicity efficacies of β-GluNPs than free β-Glu for breast cancer cells | bacterial Staphylococcus aureus MTCC 96, Bacillus subtilis MTCC-2387, Pseudomonas aeruginosa MTCC 424, Proteus vulgaris MTCC 426human, MCF-7 breast cancer cell line | [98] |
| Properties | Bacteria | Yeast | Plant | Insect | Human Cell Line | Algae |
|---|---|---|---|---|---|---|
| Production cost | inexpensive | inexpensive | inexpensive (€0.0045/g) | expensive | expensive (€70–140/g) | inexpensive (€0.0022/g) |
| Human pathogen | susceptible | non-susceptible | non-susceptible | susceptible | non-susceptible | |
| Contamination | high risk | high risk | moderate risk | low risk | high risk | low risk |
| Glycosylation | nonglycosylation | glycosylation | nonglycosylation | non-human glycosylation | glycosylation | nonglycosylation |
| Therapeutic efficacy | low | good | high | good | good | high |
| Half-life | n/a | low | high | n/a | low | high |
| Production time | a few weeks to a month | short | a few months to years | short | a few months to a year | a few weeks to months |
| Current market | 32% | 15% | less than 10% | less than 10% | 43% | less than 10% |
| Product | Target Disease | Microalgae | Vector/Transformation | Expression Level | Reference |
|---|---|---|---|---|---|
| Antigen | Newcastle disease virus | Chlamydomonas reinhardtii | pGH vector/Agrobacterium | N/A | [102] |
| Oral vaccine | malaria | Chlamydomonas reinhardtii | SapI/HindIII pASapI vector/chloroplast | 1.5% TSP | [101] |
| Vaccine | Alzheimer’s disease | Schizochytrium sp. | Algevir system/nuclear | 380 µg LTB:RAGE/g | [104] |
| bursal disease virus | Chlorella pyrenoidosa | pART27 binary vector/Agrobacterium | N/A | [127] | |
| Antigen | Zika virus (ZIKV) | Schizochytrium sp. | Algevir | 365 μg g−1 | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, D.; Qiu, W.; Wang, X.; Liu, J. Recent Advancements and Future Perspectives of Microalgae-Derived Pharmaceuticals. Mar. Drugs 2021, 19, 703. https://doi.org/10.3390/md19120703
Xia D, Qiu W, Wang X, Liu J. Recent Advancements and Future Perspectives of Microalgae-Derived Pharmaceuticals. Marine Drugs. 2021; 19(12):703. https://doi.org/10.3390/md19120703
Chicago/Turabian StyleXia, Donghua, Wen Qiu, Xianxian Wang, and Junying Liu. 2021. "Recent Advancements and Future Perspectives of Microalgae-Derived Pharmaceuticals" Marine Drugs 19, no. 12: 703. https://doi.org/10.3390/md19120703
APA StyleXia, D., Qiu, W., Wang, X., & Liu, J. (2021). Recent Advancements and Future Perspectives of Microalgae-Derived Pharmaceuticals. Marine Drugs, 19(12), 703. https://doi.org/10.3390/md19120703





