Comparative Analysis of the Functional Properties of Films Based on Carrageenans, Chitosan, and Their Polyelectrolyte Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of the Polysaccharides
2.2. Film Characteristics
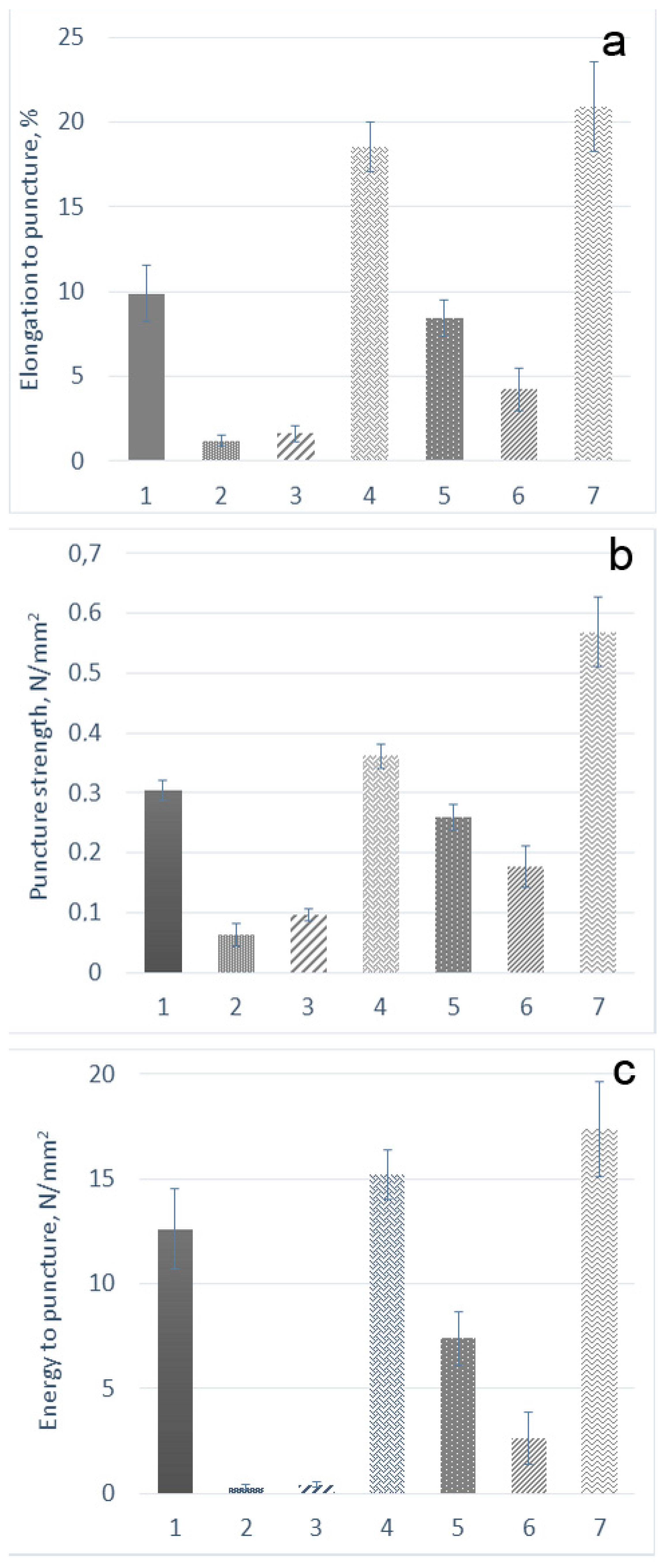
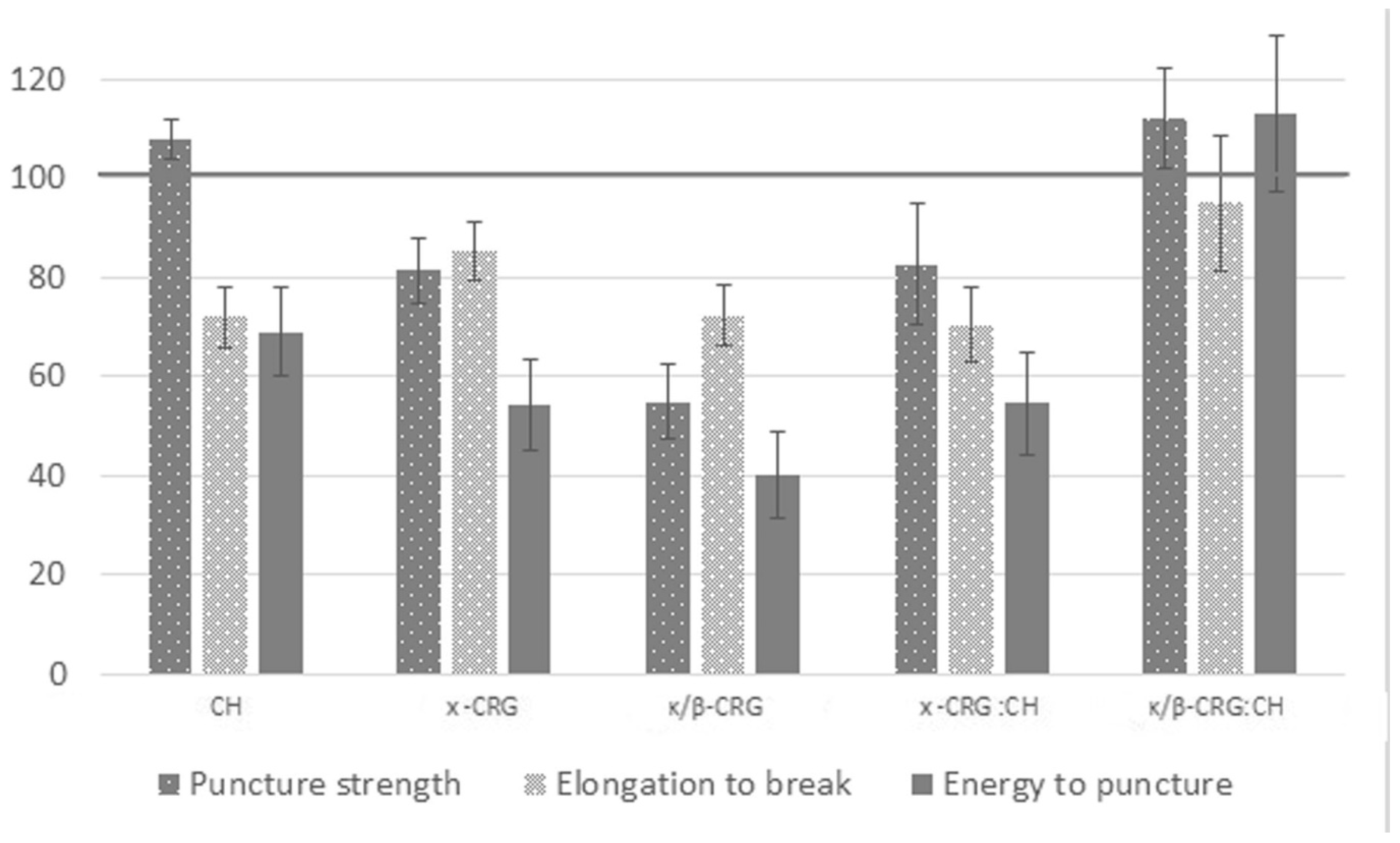
2.3. Mechanical Properties of the Films
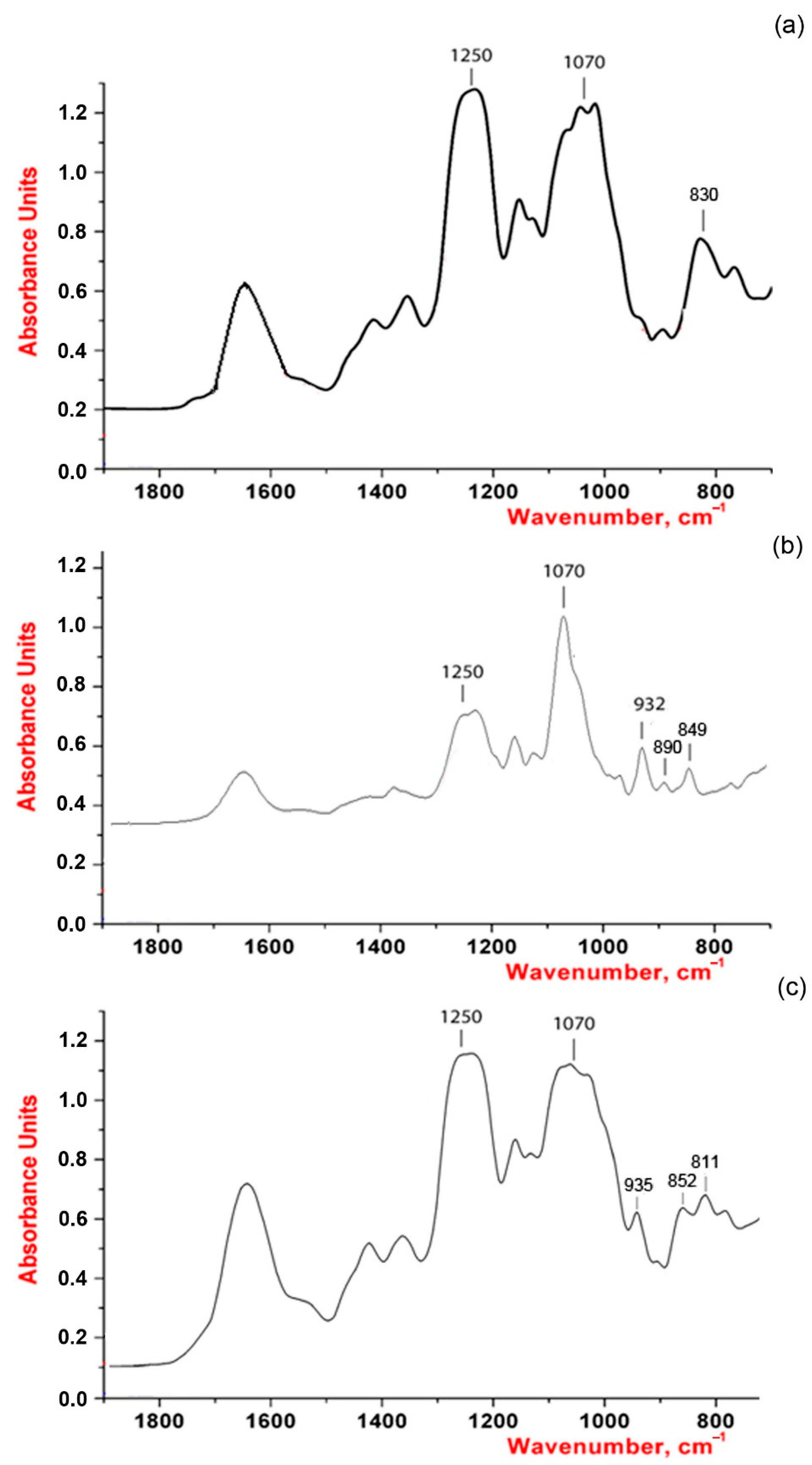
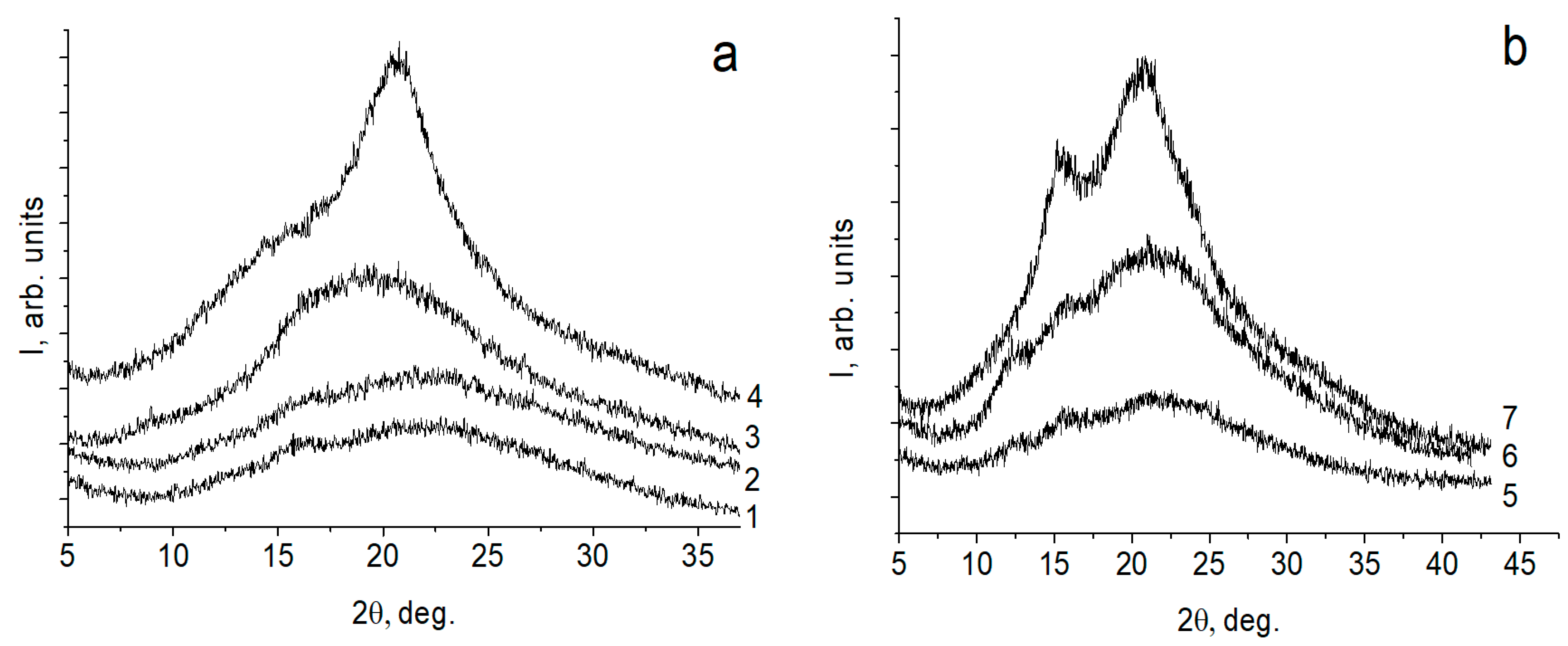
2.4. X-ray Diffraction Study
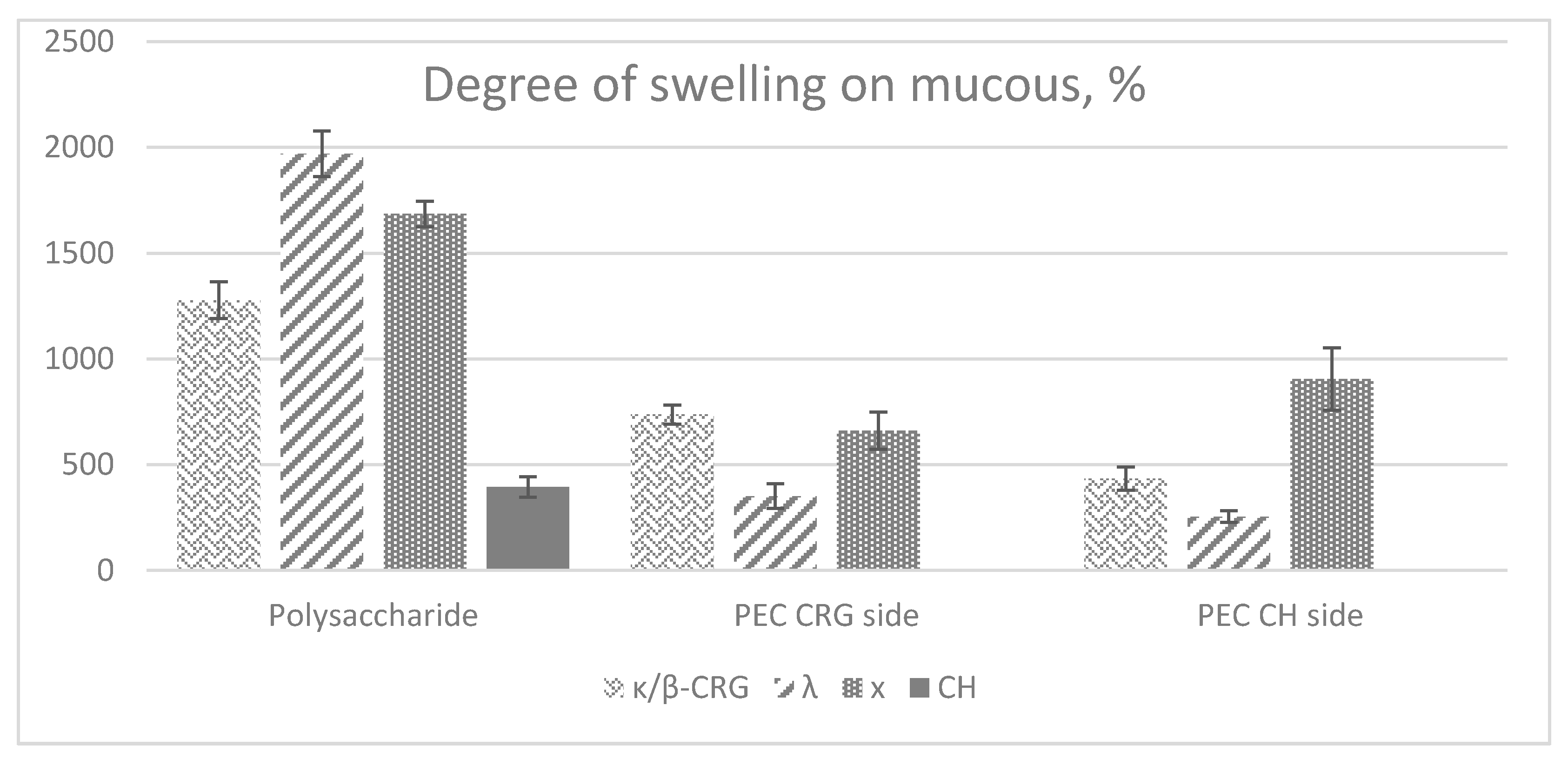
2.5. Mucoadhesive Properties of Films
2.6. Characterization of the ECH-Loaded Films
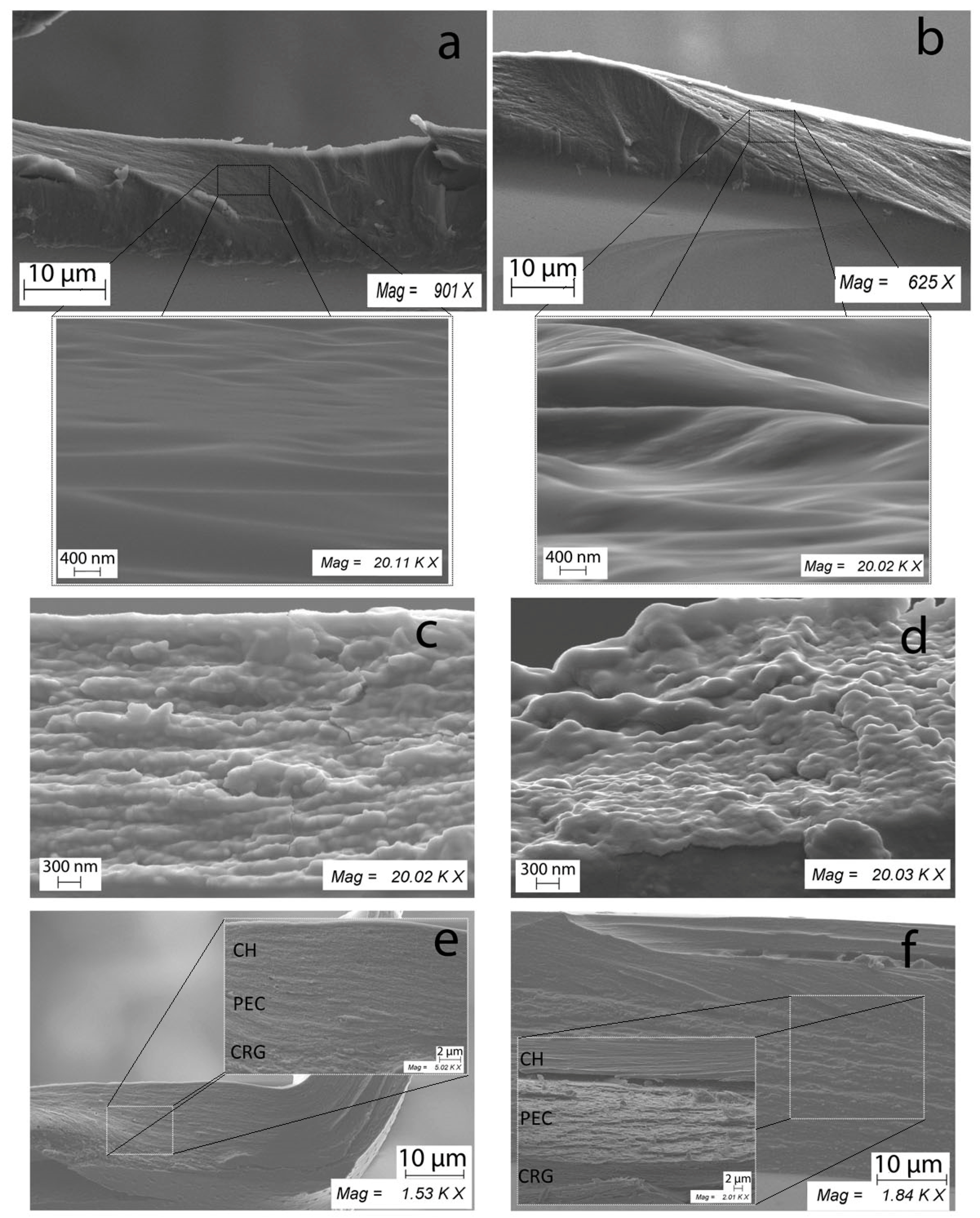
2.7. SEM
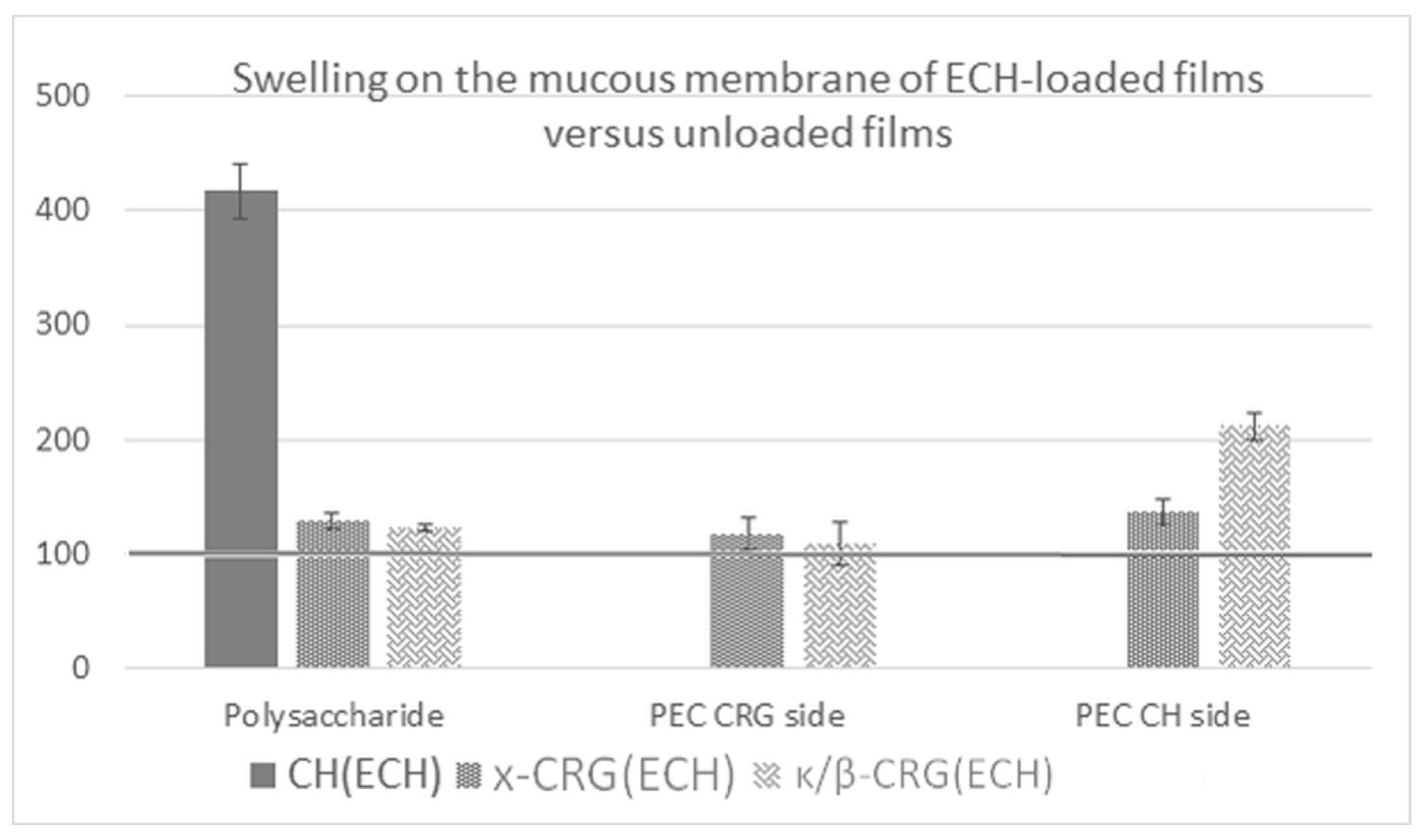
2.8. Swelling on the Mucous Membrane of ECH-Loaded Films
3. Materials and Methods
3.1. Polysaccharides
3.2. Determination of Molecular Weight
3.3. Film Preparation
3.3.1. Determination of the PEC Yield
3.3.2. ECH-Loaded Films
3.4. Film Characterization
3.4.1. Film Thickness
3.4.2. Moisture Content
3.4.3. Mechanical Strength Test of the Films
3.5. Preparation of Mucous Tissue
3.6. Swelling Test on the Mucosal Surface
3.7. Structural Studies
3.8. Scanning Electron Microscopy Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
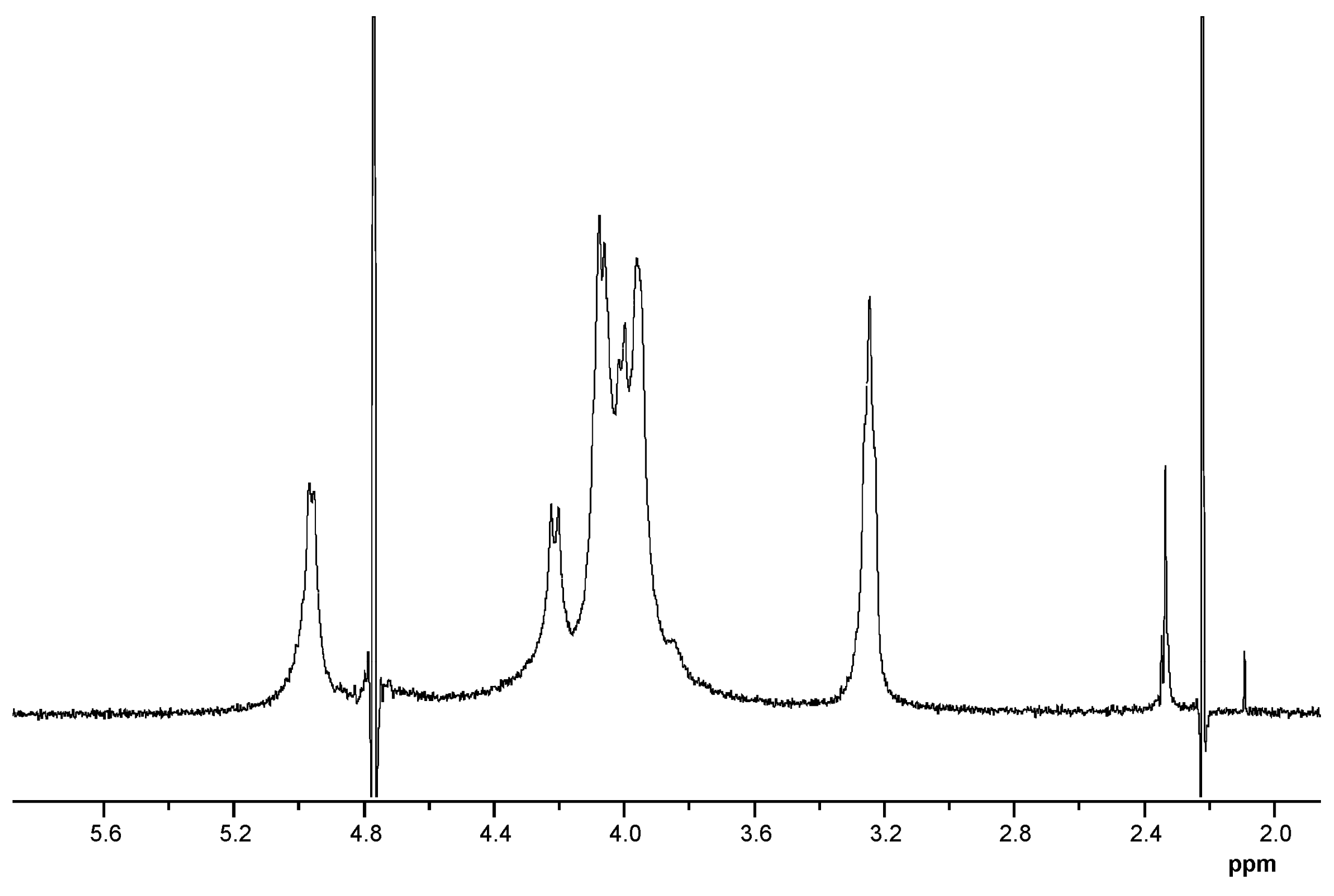
| Type of CRG | Ms Unit | 1H Chemical Shift (in ppm) | |||||
|---|---|---|---|---|---|---|---|
| H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | ||
| κ | G4S | 4.68 | 3.65 | 4.01 | 4.87 | - | 3.83 |
| DA | 5.13 | 4.01 | 4.55 | 4.62 | 4.66 | 4.23 | |
| β | G | 4.61 | 3.62 | 3.86 | 4.128 | 3.73 | 3.83 |
| DA′ | 5.11 | 4.08 | 4.53 | 4.62 | 4.66 | 4.18 | |
| 13C Chemical Shift | |||||||
| C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | ||
| κ | G4S | 103.1 | 70.0 | 79.0 | 74.0 | 73.86 | 61.17 |
| DA | 95.1 | 69.64 | 80.15 | 79.10 | 78.08 | 69.54 | |
| β | G | 102.7 | 70.1 | 80.82 | 66.15 | 75.24 | 61.17 |
| DA′ | 94.4 | 70.04 | 79.23 | 78.57 | 76.75 | 69.41 | |

References
- Karki, S.; Kim, H.; Na, S.J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Kang-Mieler, J.J.; Osswald, C.R.; Mieler, W.F. Advances in ocular drug delivery: Emphasis on the posterior segment. Expert Opin. Drug Deliv. 2014, 11, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.F.; Silva, C.; Coelho, J.F.J.; Simões, S. Oral films: Current status and future perspectives. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamróz, E.; Khachatryan, G.; Kopel, P.; Juszczak, L.; Kawecka, A.; Krzyściak, P.; Kucharek, M.; Bębenek, Z.; Zimowska, M. Furcellaran nanocomposite films: The effect of nanofillers on the structural, thermal, mechanical and antimicrobial properties of biopolymer films. Carbohydr. Polym. 2020, 240, 116244. [Google Scholar] [CrossRef]
- Yermak, I.M.; Davydova, V.N.; Kravchenko, A.O.; Chistyulin, D.A.; Pimenova, E.A.; Glazunov, V.P. Mucoadhesive properties of sulphated polysaccharides carrageenans from red seaweed families Gigartinaceae and Tichocarpaceae. Int. J. Biol. Macromol. 2020, 142, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Volod’ko, A.V.; Davydova, V.N.; Glazunov, V.P.; Likhatskaya, G.N.; Yermak, I.M. Influence of structural features of carrageenan on the formation of polyelectrolyte complexes with chitosan. Int. J. Biol. Macromol. 2016, 84, 434–441. [Google Scholar] [CrossRef]
- Knutsen, S.H.; Myslabodski, D.E.; Larsen, B.; Usov, A.I. A Modified System of Nomenclature for Red Algal Galactans. Bot. Mar. 1994, 37, 163–169. [Google Scholar] [CrossRef]
- van de Velde, F.; Knutsen, S.H.; Usov, A.I.; Rollema, H.S.; Cerezo, A.S. 1H and 13C high resolution NMR spectroscopy of carrageenans: Application in research and industry. Trends Food Sci. Technol. 2002, 13, 73–92. [Google Scholar] [CrossRef]
- Yermak, I.M.; Khotimchenko, Y.S. Chemical properties, biological activities and applications of carrageenan from red algae. In Recent advances in marine biotechnology; Fingerman, I.M., Nagabhushanam, R., Eds.; Science Publisher Inc.: New York, NY, USA, 2003; pp. 207–255. [Google Scholar]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, D.I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Geonzon, L.C.; Descallar, F.B.A.; Du, L.; Bacabac, R.G.; Matsukawa, S. Gelation mechanism and network structure in gels of carrageenans and their mixtures viewed at different length scales—A review. Food Hydrocoll. 2020, 108, 106039. [Google Scholar] [CrossRef]
- Gu, J.; Yang, S.; Ho, E.A. Biodegradable Film for the Targeted Delivery of siRNA-Loaded Nanoparticles to Vaginal Immune Cells. Mol. Pharm. 2015, 12, 2889–2903. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Chetoni, P.; Giunchedi, P.; Rossi, S.; Ferrari, F.; Burgalassi, S.; Caramella, C. Carrageenan–gelatin mucoadhesive systems for ion-exchange based ophthalmic delivery: In Vitro and preliminary in vivo studies. Eur. J. Pharm. Biopharm. 2004, 57, 465–472. [Google Scholar] [CrossRef]
- Sipahigil, O.; Dortunç, B. Preparation and in vitro evaluation of verapamil HCl and ibuprofen containing carrageenan beads. Int. J. Pharm. 2001, 228, 119–128. [Google Scholar] [CrossRef]
- Yermak, I.; Mischchenko, N.; Davydova, V.; Glazunov, V.; Tarbeeva, D.; Kravchenko, A.; Pimenova, E.; Sorokina, I. Carrageenans-Sulfated Polysaccharides from Red Seaweeds as Matrices for the Inclusion of Echinochrome. Mar. Drugs 2017, 15, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanou, M.; Verhoef, J.; Junginger, H. Oral drug absorption enhancement by chitosan and its derivatives. Adv. Drug Deliv. Rev. 2001, 52, 117–126. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Muzzarelli, C. Chitosan chemistry: Relevance to the biomedical sciences. Adv. Polym. Sci. 2005, 186, 151–209. [Google Scholar] [CrossRef]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef]
- Volod’Ko, A.V.; Davydova, V.N.; Chusovitin, E.; Sorokina, I.V.; Dolgikh, M.P.; Tolstikova, T.G.; Balagan, S.A.; Galkin, N.G.; Yermak, I.M. Soluble chitosan-carrageenan polyelectrolyte complexes and their gastroprotective activity. Carbohydr. Polym. 2014, 101. [Google Scholar] [CrossRef] [PubMed]
- Petrova, V.A.; Orekhov, A.S.; Chernyakov, D.D.; Baklagina, Y.G.; Romanov, D.P.; Kononova, S.V.; Volod’ko, A.V.; Ermak, I.M.; Klechkovskaya, V.V.; Skorik, Y.A. Preparation and analysis of multilayer composites based on polyelectrolyte complexes. Crystallogr. Reports 2016, 61, 945–953. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Perumal, V.A.; Lutchman, D.; Mackraj, I.; Govender, T. Formulation of monolayered films with drug and polymers of opposing solubilities. Int. J. Pharm. 2008, 358, 184–191. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, D.; Brar, V. Bioadhesive okra polymer based buccal patches as platform for controlled drug delivery. Int. J. Biol. Macromol. 2014, 70, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Neeraj. Polysaccharide-based component and their relevance in edible film/coating: A review. Nutr. Food Sci. 2019, 49, 793–823. [Google Scholar] [CrossRef]
- Zhuikova, Y.V.; Zhuikov, V.A.; Zubareva, A.A.; Akhmedova, S.A.; Sviridova, I.K.; Sergeeva, N.S.; Varlamov, V.P. Physicochemical and biological characteristics of chitosan/κ-carrageenan thin layer-by-layer films for surface modification of nitinol. Micron 2020, 138, 102922. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Etemadi, H. Surface modification of iron oxide nanoparticles with κ-carrageenan/carboxymethyl chitosan for effective adsorption of bovine serum albumin. Arab. J. Chem. 2019, 12, 3692–3703. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Lee, B.I.; Jung, S.T.; Park, H.J. Biopolymer composite films based on κ-carrageenan and chitosan. Mater. Res. Bull. 2001, 36, 511–519. [Google Scholar] [CrossRef]
- Shahbazi, M.; Rajabzadeh, G.; Ettelaie, R.; Rafe, A. Kinetic study of k-carrageenan degradation and its impact on mechanical and structural properties of chitosan/k-carrageenan film. Carbohydr. Polym. 2016, 142, 167–176. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Bourbon, A.I.; de S. Medeiros, B.G.; da Silva, L.H.M.; da Silva, M.C.H.; Carneiro-da-Cunha, M.G.; Coimbra, M.A.; Vicente, A.A. Interactions between κ-carrageenan and chitosan in nanolayered coatings—Structural and transport properties. Carbohydr. Polym. 2012, 87, 1081–1090. [Google Scholar] [CrossRef] [Green Version]
- Wood, K.C.; Boedicker, J.Q.; Lynn, D.M.; Hammond, P.T. Tunable Drug Release from Hydrolytically Degradable Layer-by-Layer Thin Films. Langmuir 2005, 21, 1603–1609. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Bourbon, A.I.; Quintas, M.A.C.; Coimbra, M.A.; Vicente, A.A. K-carrageenan/chitosan nanolayered coating for controlled release of a model bioactive compound. Innov. Food Sci. Emerg. Technol. 2012, 16, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Elyakov, G.B.; Maximov, O.B.; Mischenko, N.P.; Koltsova, E.A.; Fedoreev, S.A.; Glebko, L.I.; Glebko, L.I.; Krasovskaya, N.P.; Artjukov, A. Histochrome and its therapeutic use in ophthalmology //. US Patent 6,384,084.
- Yermak, I.M.; Kim, Y.H.; Titlynov, E.A.; Isakov, V.V.; Solov’eva, T.F. Chemical structure and gel properties of carrageenans from algae belonging to the Gigartinaceae and Tichocarpaceae, collected from the Russian Pacific coast. In Sixteenth International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 1999; pp. 555–562. [Google Scholar]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Ribeiro-Claro, P.J.A. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef] [Green Version]
- Barabanova, A.O.; Shashkov, A.S.; Glazunov, V.P.; Isakov, V.V.; Nebylovskaya, T.B.; Helbert, W.; Solov’eva, T.F.; Yermak, I.M. Structure and properties of carrageenan-like polysaccharide from the red alga Tichocarpus crinitus (Gmel.) Rupr. (Rhodophyta, Tichocarpaceae). J. Appl. Phycol. 2008, 20, 1013–1020. [Google Scholar] [CrossRef]
- Barabanova, A.O.; Yermak, I.M.; Glazunov, V.P.; Isakov, V.V.; Titlyanov, E.A.; Solov’Eva, T.F. Comparative study of carrageenans from reproductive and sterile forms of tichocarpus crinitus (Gmel.) Rupr (rhodophyta, tichocarpaceae). Biochem. 2005, 70, 350–356. [Google Scholar] [CrossRef]
- Sharma, S.; Swetha, K.L.; Roy, A. Chitosan-Chondroitin sulfate based polyelectrolyte complex for effective management of chronic wounds. Int. J. Biol. Macromol. 2019, 132, 97–108. [Google Scholar] [CrossRef]
- Morales, J.O.; McConville, J.T. Manufacture and characterization of mucoadhesive buccal films. Eur. J. Pharm. Biopharm. 2011, 77, 187–199. [Google Scholar] [CrossRef] [PubMed]
- van de Velde, F. Structure and function of hybrid carrageenans. Food Hydrocoll. 2008, 22, 727–734. [Google Scholar] [CrossRef]
- Stortz, C.A. Carrageenans: Structural and conformational studies. In Handbook of Carbohydrate Engineering; 2005; pp. 211–246. [Google Scholar]
- Sokolova, E.V.; Chusovitin, E.A.; Barabanova, A.O.; Balagan, S.A.; Galkin, N.G.; Yermak, I.M. Atomic force microscopy imaging of carrageenans from red algae of Gigartinaceae and Tichocarpaceae families. Carbohydr. Polym. 2013, 93, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Vodouhe, C.; Ludovic, R.; Francius, G.; Le Guen, E.; Schaaf, P.; Voegel, J.C.; Frisch, B.; Picart, C. Multifunctional polyelectrolyte multilayer films: Combining mechanical resistance, biodegradability and bioactivity. Biomacromolecules 2007, 8, 139–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Baklagina, Y.G.; Klechkovskaya, V.V.; Kononova, S.V.; Petrova, V.A.; Poshina, D.N.; Orekhov, A.S.; Skorik, Y.A. Polymorphic Modifications of Chitosan. Crystallogr. Reports 2018, 63, 303–313. [Google Scholar] [CrossRef]
- Kononova, S.V.; Volod’ko, A.V.; Petrova, V.A.; Kruchinina, E.V.; Baklagina, Y.G.; Chusovitin, E.A.; Skorik, Y.A. Pervaporation multilayer membranes based on a polyelectrolyte complex of λ-carrageenan and chitosan. Carbohydr. Polym. 2018, 181, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Prabhakar, B. Bioadhesive polymeric platforms for transmucosal drug delivery systems—A review. Trop. J. Pharm. Res. 2010, 9. [Google Scholar] [CrossRef] [Green Version]
- Aburahma, M.H.; Mahmoud, A.A. Biodegradable Ocular Inserts for Sustained Delivery of Brimonidine Tartarate: Preparation and In Vitro/In Vivo Evaluation. AAPS PharmSciTech 2011, 12, 1335–1347. [Google Scholar] [CrossRef] [Green Version]
- Philippova, O.E.; Volkov, E.V.; Sitnikova, N.L.; Khokhlov, A.R.; Desbrieres, J.; Rinaudo, M. Two types of hydrophobic aggregates in aqueous solutions of chitosan and its hydrophobic derivative. Biomacromolecules 2001, 2, 483–490. [Google Scholar] [CrossRef]
- Davydova, V.N.; Ermak, I.M.; Gorbach, V.I.; Drozdov, A.L.; Solov’eva, T.F. Comparative study of the physico-chemical properties of chitosans with varying degree of polymerization in neutral aqueous solutions. Biofizika 2000, 45, 641–647. [Google Scholar]
- Mortazavi, S.A.; Smart, J.D. An investigation into the role of water movement and mucus gel dehydration in mucoadhesion. J. Control. Release 1993, 25, 197–203. [Google Scholar] [CrossRef]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, B.; Delorme, N.; Doench, I.; Sukhorukov, G.B.; Fery, A.; Glinel, K. Polyelectrolyte films based on polysaccharides of different conformations: Effects on multilayer structure and mechanical properties. Biomacromolecules 2006, 7, 2065–2071. [Google Scholar] [CrossRef]
- Reynaers, H. Light scattering study of polyelectrolyte polysaccharides—The carrageenans. Fibres Text. East. Eur. 2003, 11, 88–96. [Google Scholar]
- Vanneste, K.; Slootmaekers, D.; Reynaers, H. Light scattering studies of the dilute solution behaviour of κ-, ι- and λ-carrageenan. Food Hydrocoll. 1996, 10, 99–107. [Google Scholar] [CrossRef]
- Sokolova, E.; Menzorova, N.; Davydova, V.; Kuz’mich, A.; Kravchenko, A.; Mishchenko, N.; Yermak, I. Effects of Carrageenans on Biological Properties of Echinochrome. Mar. Drugs 2018, 16, 419. [Google Scholar] [CrossRef] [Green Version]
- Baranowski, P.; Karolewicz, B.; Gajda, M.; Pluta, J. Ophthalmic Drug Dosage Forms: Characterisation and Research Methods. Sci. World J. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfrom, M.L.; Han, T.M.S. The Sulfonation of Chitosan 1,2. J. Am. Chem. Soc. 1959, 81, 1764–1766. [Google Scholar] [CrossRef]
- Domard, A.; Gey, C.; Rinaudo, M.; Terrassin, C. 13C and 1H n.m.r. spectroscopy of chitosan and N-trimethyl chloride derivatives. Int. J. Biol. Macromol. 1987, 9, 233–237. [Google Scholar] [CrossRef]
- Rochas, C.; Rinaudo, M.; Landry, S. Role of the molecular weight on the mechanical properties of kappa carrageenan gels. Carbohydr. Polym. 1990, 12, 255–266. [Google Scholar] [CrossRef]
- Roberts, G.G.; Maghami, G.A.F. Evaluation of the viscometric constants for chitosan. Die Makromol. Chemie Macromol. Chem. Phys. 1988, 189, 195–200. [Google Scholar]
| Red Algae | Type of CRG | Structure of the Repeating Disaccharide Unit | MW, kDa | Elemental Composition, % | ||
|---|---|---|---|---|---|---|
| C | H | S | ||||
| Chondrus armatus | λ | 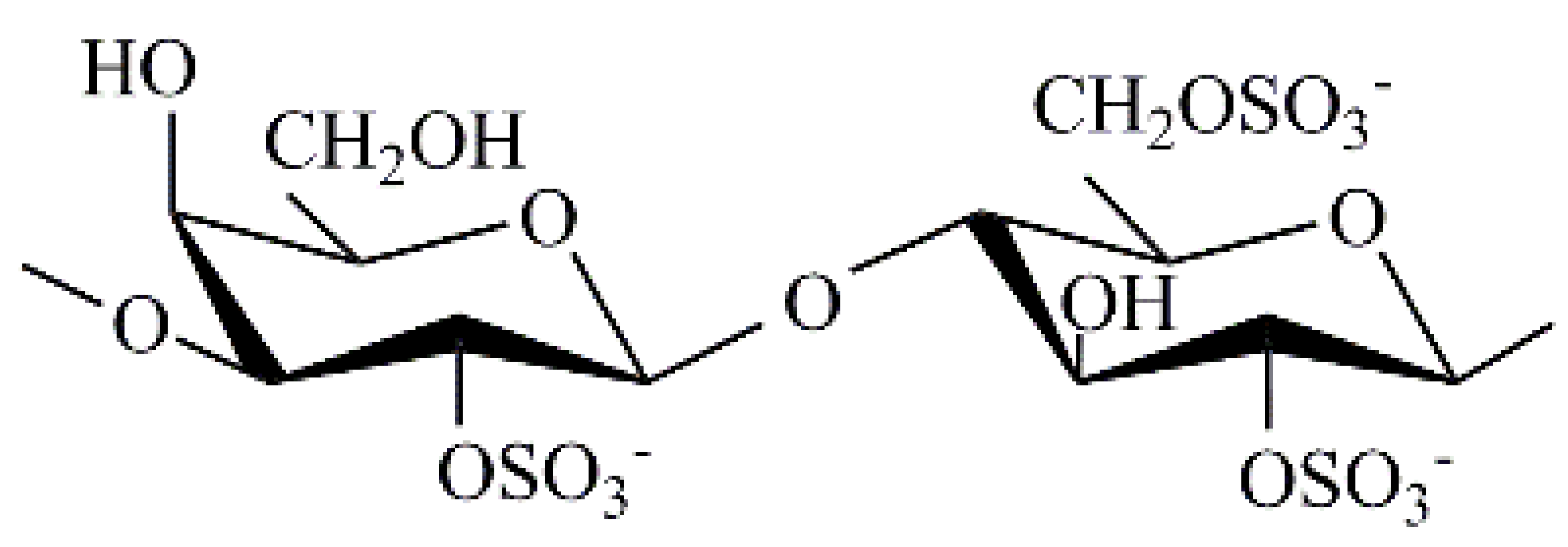 | 350 | 23.0 | 3.8 | 13.2 |
| Tichocarpus crinitus | κ/β | 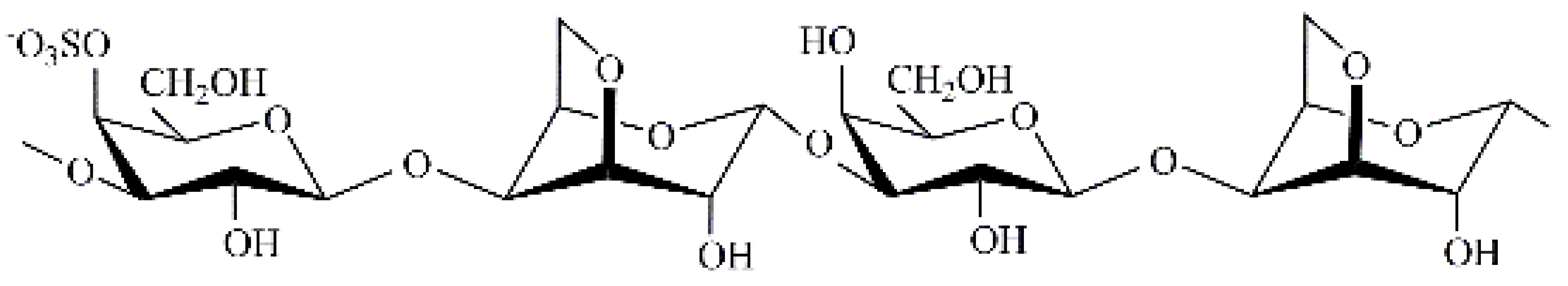 | 592 | 28.7 | 5.0 | 9.4 |
| x | 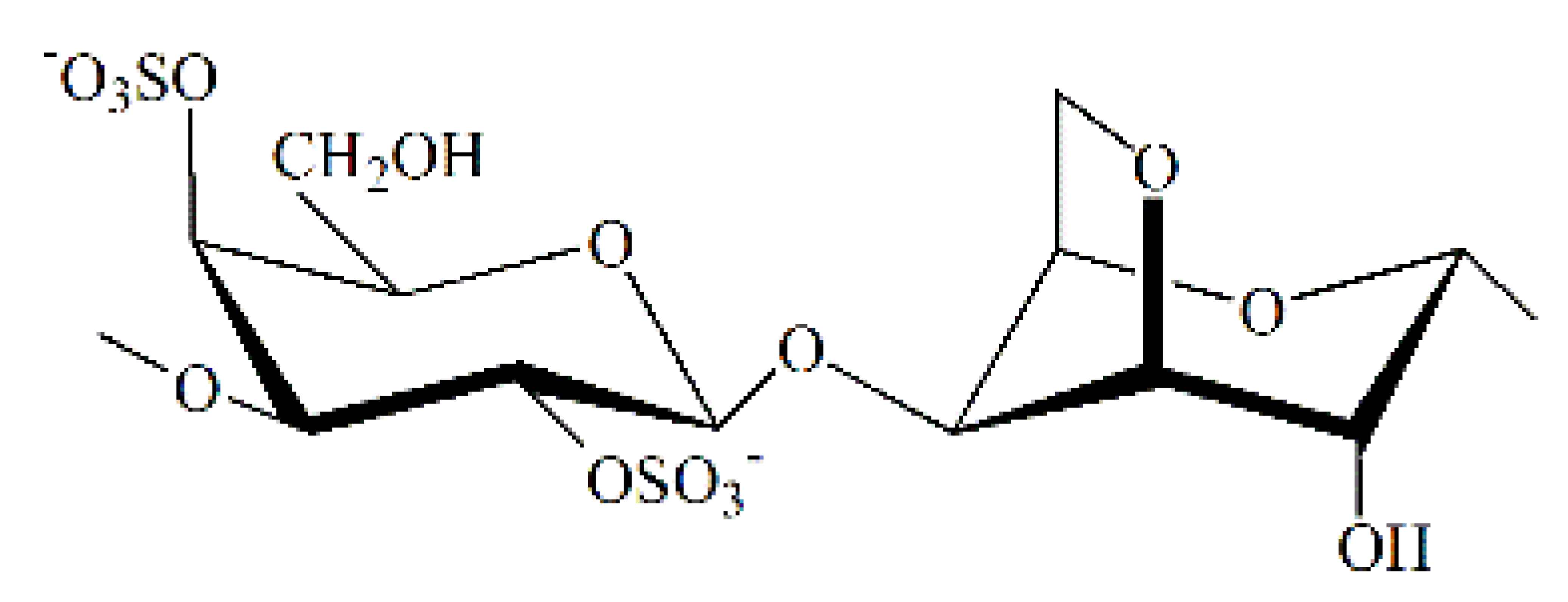 | 950 | 25.2 | 4.1 | 12.3 | |
| PEC | Elemental Composition, % | PEC Content in the Three-Layer Films, % | |||
|---|---|---|---|---|---|
| C | H | S | N | ||
| κ/β-CRG:CH | 33.9 | 5.17 | 5.60 | 2.66 | 35.2 |
| x-CRG:CH | 34.1 | 5.52 | 5.93 | 3.75 | 29.5 |
| λ-CRG:CH | 31.9 | 5.47 | 6.85 | 3.05 | 22.2 |
| Sample | ||
|---|---|---|
| λ-CRG | 90.7 ± 1.3 | |
| x-CRG | 128.2 ± 1.9 | |
| κ/β-CRG | 116.8 ± 2.8 | |
| CH | 108.0 ± 4.3 | |
| λ-CRG:PEC:CH | CRG side | 94.5 ± 2.0 |
| CH side | 106.2 ± 0.3 | |
| x-CRG:PEC:CH PEC | CRG side | 102.6 ± 1.7 |
| CH side | 160.3 ± 0.1 | |
| κ/β-CRG:PEC:CH | CRG side | 144.2 ± 12.2 |
| CH side | 126.0 ± 1.6 | |
| Sample | ||
|---|---|---|
| CH(ECH) | 176.3 ± 9.9 | |
| x-CRG(ECH) | 142.7 ± 0.6 | |
| κ/β-CRG(ECH) | 128.9 ± 3.9 | |
| x-CRG(ECH):PEC:CH | CRG side | 103.2 ± 5.8 |
| CH side | 135.6 ±1.2 | |
| κ/β-CRG (ECH):PEC:CH | CRG side | 120.1 ± 2.0 |
| CH side | 124.6 ± 4.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volod’ko, A.V.; Davydova, V.N.; Petrova, V.A.; Romanov, D.P.; Pimenova, E.A.; Yermak, I.M. Comparative Analysis of the Functional Properties of Films Based on Carrageenans, Chitosan, and Their Polyelectrolyte Complexes. Mar. Drugs 2021, 19, 704. https://doi.org/10.3390/md19120704
Volod’ko AV, Davydova VN, Petrova VA, Romanov DP, Pimenova EA, Yermak IM. Comparative Analysis of the Functional Properties of Films Based on Carrageenans, Chitosan, and Their Polyelectrolyte Complexes. Marine Drugs. 2021; 19(12):704. https://doi.org/10.3390/md19120704
Chicago/Turabian StyleVolod’ko, Aleksandra V., Viktoriya N. Davydova, Valentina A. Petrova, Dmitry P. Romanov, Evgeniya A. Pimenova, and Irina M. Yermak. 2021. "Comparative Analysis of the Functional Properties of Films Based on Carrageenans, Chitosan, and Their Polyelectrolyte Complexes" Marine Drugs 19, no. 12: 704. https://doi.org/10.3390/md19120704
APA StyleVolod’ko, A. V., Davydova, V. N., Petrova, V. A., Romanov, D. P., Pimenova, E. A., & Yermak, I. M. (2021). Comparative Analysis of the Functional Properties of Films Based on Carrageenans, Chitosan, and Their Polyelectrolyte Complexes. Marine Drugs, 19(12), 704. https://doi.org/10.3390/md19120704





