Surface Glucan Structures in Aeromonas spp.
Abstract
1. Introduction
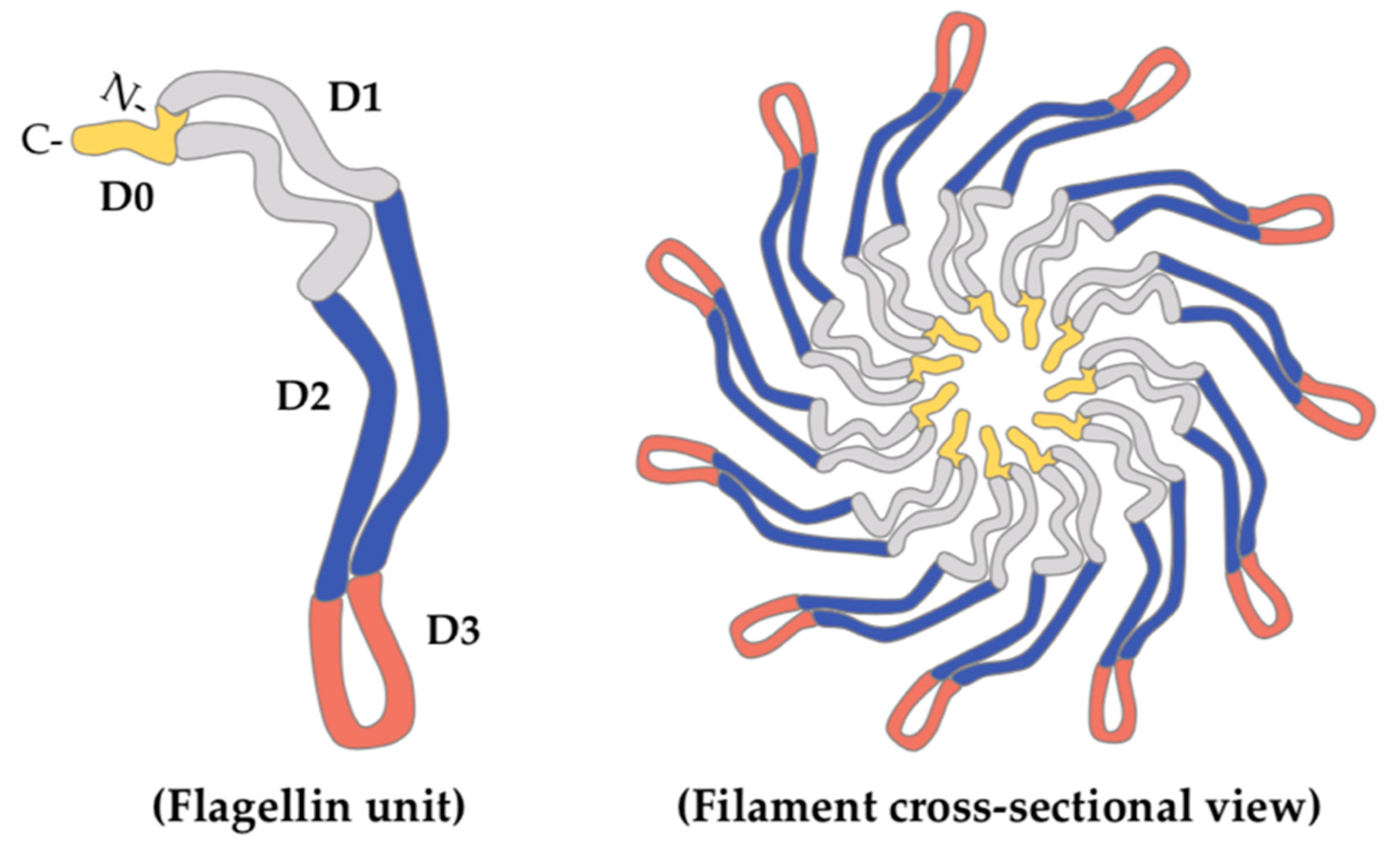
2. Glycosylated Flagella
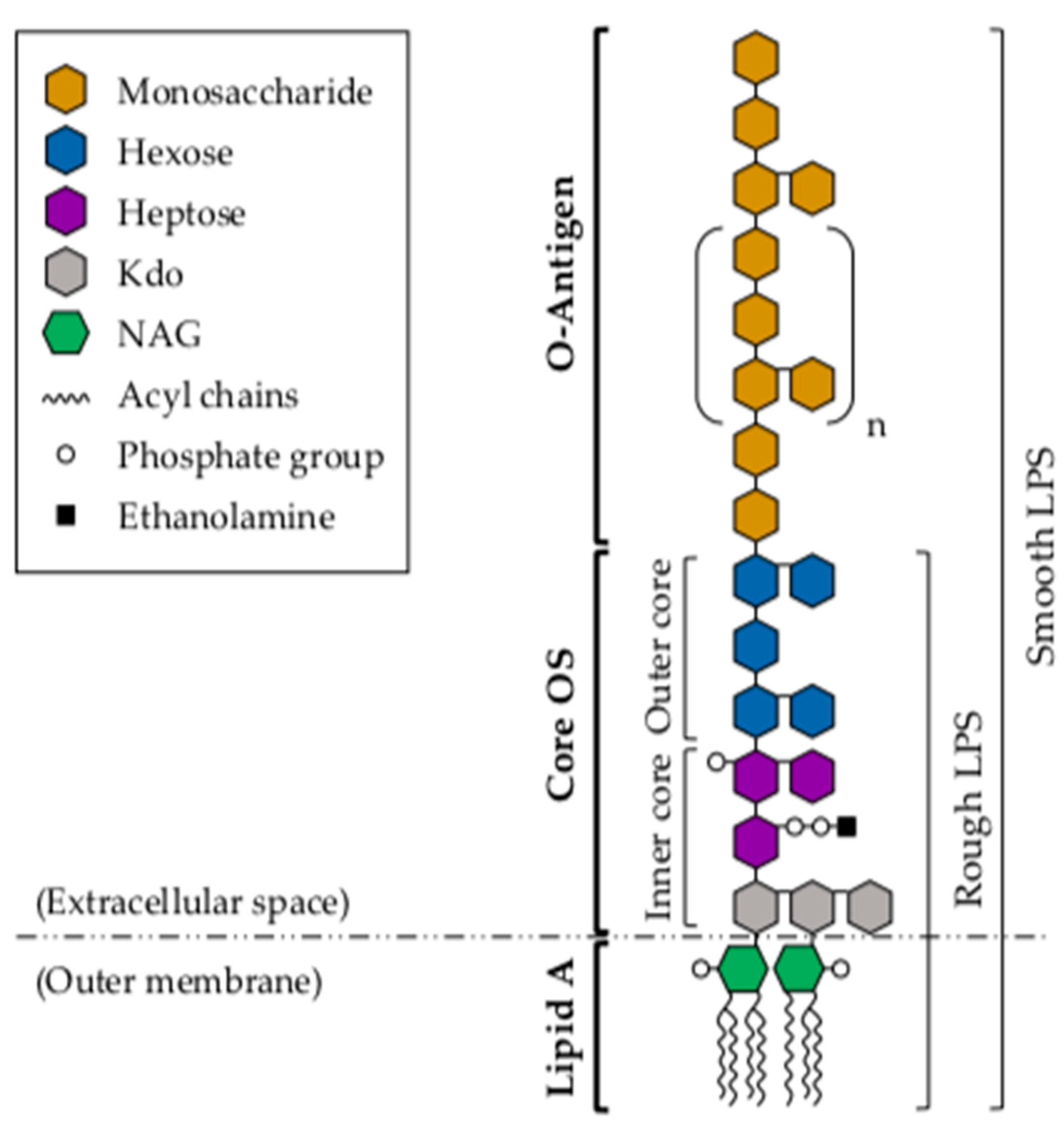
3. Lipopolysaccharide
3.1. Lipid A
3.2. Core Oligosaccharide
3.3. O-Antigen
4. Capsular Polysaccharide
5. α-Glucan
6. Future Perspectives and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Martin-Carnahan, A.; Joseph, S.W. Order XII. Aeromonadales ord. nov. In Bergey’s Manual® of Systematic Bacteriology, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.T., Eds.; Springer: Boston, MA, USA, 2005; Volume 2, Part B; pp. 556–587. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef]
- Majeed, K.N.; Egan, A.F.; Rae, I.C. Mac. Production of exotoxins by Aeromonas spp. at 5 °C. J. Appl. Bacteriol. 1990, 69, 332–337. [Google Scholar] [CrossRef]
- Galindo, C.L.; Chopra, A.K. Aeromonas and Plesiomonas species. In Food Microbiology: Fundamentals and Frontiers, 3rd ed.; Doyle, M.P., Beuchat, L.R., Montville, T.J., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 381–400. [Google Scholar]
- Joseph, S.W.; Carnahan, A. The isolation, identification, and systematics of the motile Aeromonas species. Annu Rev. Fish. Dis. 1994, 4, 315–343. [Google Scholar] [CrossRef]
- Beaz-Hidalgo, R.; Alperi, A.; Buján, N.; Romalde, J.L.; Figueras, M.J. Comparison of phenotypical and genetic identification of Aeromonas strains isolated from diseased fish. Syst. Appl. Microbiol. 2010, 33, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Beaz-Hidalgo, R.; Alperi, A.; Figueras, M.J.; Romalde, J.L. Aeromonas piscicola sp. nov., isolated from diseased fish. Syst. Appl. Microbiol. 2009, 32, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Monette, S.; Dallaire, A.D.; Mingelbier, M.; Groman, D.; Uhland, C.; Richard, J.P.; Paillard, G.; Johannson, L.M.; Chivers, D.P.; Ferguson, H.W.; et al. Massive mortality of common carp (Cyprinus carpio carpio) in the St. Lawrence River in 2001: Diagnostic investigation and experimental induction of lymphocytic encephalitis. Vet. Pathol. 2006, 43, 302–310. [Google Scholar] [CrossRef]
- Rahman, M.; Colque-Navarro, P.; Kühn, I.; Huys, G.; Swings, J.; Möllby, R. Identification and Characterization of Pathogenic Aeromonas veronii Biovar Sobria Associated with Epizootic Ulcerative Syndrome in Fish in Bangladesh. Appl. Environ. Microbiol. 2002, 68, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. Evolving Concepts Regarding the Genus Aeromonas: An Expanding Panorama of Species, Disease Presentations, and Unanswered Questions. Clin. Infect. Dis. 1998, 27, 332–344. [Google Scholar] [CrossRef]
- Teunis, P.; Figueras, M.J. Reassessment of the Enteropathogenicity of Mesophilic Aeromonas Species. Front. Microbiol. 2016, 7, 1395. [Google Scholar] [CrossRef]
- Fernández-Bravo, A.; Figueras, M.J. An update on the genus Aeromonas: Taxonomy, epidemiology, and pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef]
- Pal, M.; Ayele, Y.; Durglishvili, N. Emerging Role of Aeromonas hydrophila as a Foodborne Pathogen of Public Health Concern. EC Microbiol. 2020, 16, 55–58. [Google Scholar]
- Granum, P.E.; O’Sullivan, K.; Tomás, J.M.; Ørmen, Ø. Possible virulence factors of Aeromonas spp. from food and water. FEMS Immunol. Med. Microbiol. 1998, 21, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Krovacek, K.; Baloda, S.B.; Dumontet, S.; Eriksson, E. Isolation, and Virulence Profiles, of Aeromonas hydrophila Implicated in an Outbreak of Food Poisoning in Sweden. Microbiol. Immunol. 1995, 39, 655–661. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, G.-Q.; Tiang, G.-P.; Zou, Z.-T.; Yao, G.-H.; Zeng, G. A foodborne outbreak of Aeromonas hydrophila in a college, Xingyi City, Guizhou, China, 2012. West. Pacific Surveill Response J. 2012, 3, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Tsheten, T.; Tshering, D.; Gyem, K.; Dorji, S.; Wangchuk, S.; Tenzin, T.; Norbu, L.; Jamtsho, T. An Outbreak of Aeromonas hydrophila Food Poisoning in Deptsang Village, Samdrup Jongkhar, Bhutan, 2016. J. Res. Health Sci. 2016, 16, 224–227. [Google Scholar]
- Hiransuthikul, N.; Tantisiriwat, W.; Lertutsahakul, K.; Vibhagool, A.; Boonma, P. Skin and soft-tissue infections among tsunami survivors in southern Thailand. Clin. Infect. Dis. 2005, 41, e93–e96. [Google Scholar] [CrossRef]
- Presley, S.M.; Rainwater, T.R.; Austin, G.P.; Platt, S.G.; Zak, J.C.; Cobb, G.P.; Marsland, E.J.; Tian, K.; Zhang, B.; Anderson, T.A.; et al. Assessment of pathogens and toxicants in New Orleans, LA following Hurricane Katrina. Environ. Sci. Technol. 2006, 40, 468–474. [Google Scholar] [CrossRef]
- Wimalasena, S.H.M.P.; Shin, G.W.; Hossain, S.; Heo, G.J. Potential enterotoxicity and antimicrobial resistance pattern of Aeromonas species isolated from pet turtles and their environment. J. Vet. Med. Sci. 2017, 79, 921–926. [Google Scholar] [CrossRef]
- Dias, C.; Borges, A.; Saavedra, M.J.; Simões, M. Biofilm formation and multidrug-resistant Aeromonas spp. from wild animals. J. Glob. Antimicrob Resist. 2018, 12, 227–234. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, L.; Nan, Z.; Zhang, P.; Kan, B.; Yan, D.; Su, J. Taxonomy, virulence genes and antimicrobial resistance of Aeromonas isolated from extra-intestinal and intestinal infections. BMC Infect. Dis. 2019, 19, 158. [Google Scholar] [CrossRef]
- Parker, J.L.; Shaw, J.G. Aeromonas spp. clinical microbiology and disease. J. Infect. 2011, 62, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Schäffer, C.; Messner, P. Emerging facets of prokaryotic glycosylation. FEMS Microbiol. Rev. 2017, 41, 49–91. [Google Scholar] [CrossRef] [PubMed]
- Wilhelms, M.; Fulton, K.M.; Twine, S.M.; Tomás, J.M.; Merino, S. Differential glycosylation of polar and lateral flagellins in Aeromonas hydrophila AH-3. J. Biol. Chem. 2012, 287, 27851–27862. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.L.; Day-Williams, M.J.; Tomas, J.M.; Stafford, G.P.; Shaw, J.G. Identification of a putative glycosyltransferase responsible for the transfer of pseudaminic acid onto the polar flagellin of Aeromonas caviae Sch3N. Microbiol. Open 2012, 1, 149–160. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Gryllos, I.; Tomás, J.M.; Shaw, J.G. Motility and the polar flagellum are required for Aeromonas caviae adherence to HEp-2 cells. Infect. Immun. 2001, 69, 4257–4267. [Google Scholar] [CrossRef]
- Merino, S.; Wilhelms, M.; Tomás, J.M. Role of Aeromonas hydrophila flagella glycosylation in adhesion to Hep-2 cells, biofilm formation and immune stimulation. Int. J. Mol. Sci. 2014, 15, 21935–21946. [Google Scholar] [CrossRef]
- Fulton, K.M.; Mendoza-Barberá, E.; Twine, S.M.; Tomas, J.; Merino, S. Polar Glycosylated and Lateral Non-Glycosylated Flagella from Aeromonas hydrophila Strain AH-1 (Serotype O11). Int. J. Mol. Sci. 2015, 16, 28255–28269. [Google Scholar] [CrossRef]
- Zamyatina, A.; Heine, H. Lipopolysaccharide Recognition in the Crossroads of TLR4 and Caspase-4/11 Mediated Inflammatory Pathways. Front. Immunol. 2020, 11, 585146. [Google Scholar] [CrossRef]
- Gumenscheimer, M.; Mitov, I.; Galanos, C.; Freudenberg, M.A. Beneficial or deleterious effects of a preexisting hypersensitivity to bacterial components on the course and outcome of infection. Infect. Immun. 2002, 70, 5596–5603. [Google Scholar] [CrossRef][Green Version]
- Martínez, M.J.; Simon-Pujol, D.; Congregado, F.; Merino, S.; Rubires, X.; Tomás, J.M. The presence of capsular polysaccharide in mesophilic Aeromonas hydrophila serotypes O:11 and O:34. FEMS Microbiol. Lett. 1995, 128, 69–73. [Google Scholar] [CrossRef]
- Garrote, A.; Bonet, R.; Merino, S.; Simon-Pujol, M.D.; Congregado, F. Occurrence of a capsule in Aeromonas salmonicida. FEMS Microbiol. Lett. 1992, 74, 127–131. [Google Scholar] [CrossRef]
- Merino, S.; Bouamama, L.; Knirel, Y.A.; Senchenkova, S.N.; Regué, M.; Tomás, J.M. Aeromonas Surface Glucan Attached through the O-Antigen Ligase Represents a New Way to Obtain UDP-Glucose. PLoS ONE 2012, 7, e35707. [Google Scholar] [CrossRef] [PubMed]
- Kirov, S.M.; Tassell, B.C.; Semmler, A.B.T.; O’Donovan, L.A.; Rabaan, A.A.; Shaw, J.G. Lateral flagella and swarming motility in Aeromonas species. J. Bacteriol. 2002, 184, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Macnab, R.M. How Bacteria Assemble Flagella. Annu. Rev. Microbiol. 2003, 57, 77–100. [Google Scholar] [CrossRef]
- McCarter, L.L. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 2001, 65, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Macnab, R.M. Flagella and motility. In Escherichia coli and Salmonella: Cellular and Molecular Biology, 2nd ed.; Neidhardt, F.C., Curtiss, R., III, Ingraham, J.L., Lin, E.C.C., Low, K.B., Magasanik, B., Reznikoff, W.S., Riley, M., Schaechter, M., Eds.; ASM Press: Washington, DC, USA, 1996; Volume 1, pp. 123–145. ISBN 9781555810845, 9781555810849. [Google Scholar]
- Canals, R.; Ramirez, S.; Vilches, S.; Horsburgh, G.; Shaw, J.G.; Tomás, J.M.; Merino, S. Polar Flagellum Biogenesis in Aeromonas hydrophila. J. Bacteriol. 2006, 188, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Canals, R.; Altarriba, M.; Vilches, S.; Horsburgh, G.; Shaw, J.G.; Tomás, J.M.; Merino, S. Analysis of the lateral flagellar gene system of Aeromonas hydrophila AH-3. J. Bacteriol. 2006, 188, 852–862. [Google Scholar] [CrossRef]
- Gavín, R.; Rabaan, A.A.; Merino, S.; Tomás, J.M.; Gryllos, I.; Shaw, J.G. Lateral flagella of Aeromonas species are essential for epithelial cell adherence and biofilm formation. Mol. Microbiol. 2002, 43, 383–397. [Google Scholar] [CrossRef]
- Yonekura, K.; Maki-Yonekura, S.; Namba, K. Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature 2003, 424, 643–650. [Google Scholar] [CrossRef]
- Tabei, S.M.B.; Hitchen, P.G.; Day-Williams, M.J.; Merino, S.; Vart, R.; Pang, P.-C.; Horsburgh, G.J.; Viches, S.; Wilhelms, M.; Tomás, J.M.; et al. An Aeromonas caviae genomic island is required for both O-antigen lipopolysaccharide biosynthesis and flagellin glycosylation. J. Bacteriol. 2009, 191, 2851–2863. [Google Scholar] [CrossRef]
- Schirm, M.; Soo, E.C.; Aubry, A.J.; Austin, J.; Thibault, P.; Logan, S.M. Structural, genetic and functional characterization of the flagellin glycosylation process in Helicobacter pylori. Mol. Microbiol. 2003, 48, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Thibault, P.; Logan, S.M.; Kelly, J.F.; Brisson, J.-R.; Ewing, C.P.; Trust, T.J.; Guerry, P. Identification of the Carbohydrate Moieties and Glycosylation Motifs in Campylobacter jejuni Flagellin. J. Biol. Chem. 2001, 276, 34862–34870. [Google Scholar] [CrossRef] [PubMed]
- Moens, S.; Vanderleyden, J. Glycoproteins in prokaryotes. Arch. Microbiol. 1997, 168, 169–175. [Google Scholar] [CrossRef][Green Version]
- Nothaft, H.; Szymanski, C.M. Protein glycosylation in bacteria: Sweeter than ever. Nat. Rev. Microbiol. 2010, 8, 765–778. [Google Scholar] [CrossRef]
- Guerry, P.; Ewing, C.P.; Schirm, M.; Lorenzo, M.; Kelly, J.; Pattarini, D.; Majam, G.; Thibault, P.; Logan, S. Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol. Microbiol. 2006, 60, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, C.M.; Logan, S.M.; Linton, D.; Wren, B.W. Campylobacter—A tale of two protein glycosylation systems. Trends Microbiol. 2003, 11, 233–238. [Google Scholar] [CrossRef]
- Asakura, H.; Churin, Y.; Bauer, B.; Boettcher, J.P.; Bartfeld, S.; Hashii, N.; Kawasaki, N.; Mollenkopf, H.J.; Jungblut, P.R.; Brinkmann, V.; et al. Helicobacter pylori HP0518 affects flagellin glycosylation to alter bacterial motility. Mol. Microbiol. 2010, 78, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Arora, S.K.; Kuravi, S.K.; Ramphal, R. Roles of specific amino acids in the N terminus of Pseudomonas aeruginosa flagellin and of flagellin glycosylation in the innate immune response. Infect. Immun. 2005, 73, 8237–8246. [Google Scholar] [CrossRef]
- Arora, S.K.; Neely, A.N.; Blair, B.; Lory, S.; Ramphal, R. Role of motility and flagellin glycosylation in the pathogenesis of Pseudomonas aeruginosa burn wound infections. Infect. Immun. 2005, 73, 4395–4398. [Google Scholar] [CrossRef]
- Szymanski, C.M.; Yao, R.; Ewing, C.P.; Trust, T.J.; Guerry, P. Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol. Microbiol. 1999, 32, 1022–1030. [Google Scholar] [CrossRef]
- Logan, S.M. Flagellar glycosylation—A new component of the motility repertoire? Microbiology 2006, 152, 1249–1262. [Google Scholar] [CrossRef]
- Canals, R.; Vilches, S.; Wilhelms, M.; Shaw, J.G.; Merino, S.; Tomás, J.M. Non-structural flagella genes affecting both polar and lateral flagella-mediated motility in Aeromonas hydrophila. Microbiology 2007, 153, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Fulton, K.M.; Twine, S.M.; Wilhelms, M.; Molero, R.; Tomás, J.M. Aeromonas hydrophila flagella glycosylation: Involvement of a lipid carrier. PLoS ONE 2014, 9, e89630. [Google Scholar] [CrossRef]
- Karlyshev, A.V.; Linton, D.; Gregson, N.A.; Wren, B.W. A novel paralogous gene family involved in phase-variable flagella-mediated motility in Campylobacter jejuni. Microbiology 2002, 148, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, R.; Joseph, S.W.; Chopra, A.K.; Sha, J.; Shaw, J.; Graf, J.; Haft, D.; Wu, M.; Ren, Q.; Rosovitz, M.J.; et al. Genome sequence of Aeromonas hydrophila ATCC 7966T: Jack of all trades. J. Bacteriol. 2006, 188, 8272–8282. [Google Scholar] [CrossRef] [PubMed]
- Forn-Cuní, G.; Tomás, J.M.; Merino, S. Whole-genome sequence of Aeromonas hydrophila strain AH-1 (serotype O11). Genome Announc. 2016, 4, e00920-16. [Google Scholar] [CrossRef]
- Forn-Cuní, G.; Tomás, J.M.; Merino, S. Genome sequence of Aeromonas hydrophila strain AH-3 (serotype O34). Genome Announc. 2016, 4, e00919-16. [Google Scholar] [CrossRef]
- Forn-Cuní, G.; Fulton, K.M.; Smith, J.C.; Twine, S.M.; Mendoza-Barberà, E.; Tomás, J.M.; Merino, S. Polar Flagella Glycosylation in Aeromonas: Genomic Characterization and Involvement of a Specific Glycosyltransferase (Fgi-1) in Heterogeneous Flagella Glycosylation. Front. Microbiol. 2021, 11, 595697. [Google Scholar] [CrossRef]
- Nagai, Y.; Akashi, S.; Nagafuku, M.; Ogata, M.; Iwakura, Y.; Akira, S.; Kitamura, T.; Kosugi, A.; Kimoto, M.; Miyake, K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 2002, 3, 667–672. [Google Scholar] [CrossRef]
- Whitfield, C.; Valvano, M.A. Biosynthesis and expression of cell-surface polysaccharides in Gram-negative bacteria. Adv. Microb. Physiol. 1993, 35, 135–246. [Google Scholar] [CrossRef]
- Steimle, A.; Autenrieth, I.B.; Frick, J.S. Structure and function: Lipid A modifications in commensals and pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef]
- Samuel, G.; Reeves, P. Biosynthesis of O-antigens: Genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydr. Res. 2003, 338, 2503–2519. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Lüderitz, O.; Galanos, C.; Lehmann, V.; Mayer, H.; Rietschel, E.T.; Weckesser, J. Chemical structure and biological activities of lipid A’s from various bacterial families. Naturwissenschaften 1978, 65, 578–585. [Google Scholar] [CrossRef]
- Merino, S.; Rubires, X.; Knochel, S.; Tomas, J.M. Emerging pathogens: Aeromonas spp. Int. J. Food Microbiol. 1995, 28, 157–168. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Altman, E. Structural characterization of the lipid A region of Aeromonas salmonicida subsp. salmonicida lipopolysaccharide. Carbohydr. Res. 2006, 341, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Hagar, J.A.; Powell, D.A.; Aachoui, Y.; Ernst, R.K.; Miao, E.A. Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science 2013, 341, 1250–1253. [Google Scholar] [CrossRef]
- Lagrange, B.; Benaoudia, S.; Wallet, P.; Magnotti, F.; Provost, A.; Michal, F.; Martin, A.; Di Lorenzo, F.; Py, B.F.; Molinaro, A.; et al. Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine caspase-11. Nat. Commun. 2018, 9, 242. [Google Scholar] [CrossRef]
- Jimenez, N.; Lacasta, A.; Vilches, S.; Reyes, M.; Vazquez, J.; Aquillini, E.; Merino, S.; Regué, M.; Tomás, J.M. Genetics and proteomics of Aeromonas salmonicida lipopolysaccharide core biosynthesis. J. Bacteriol. 2009, 191, 2228–2236. [Google Scholar] [CrossRef]
- Jimenez, N.; Canals, R.; Lacasta, A.; Kondakova, A.N.; Lindner, B.; Knirel, Y.A.; Merino, S.; Regué, M.; Tomás, J.M. Molecular analysis of three Aeromonas hydrophila AH-3 (serotype O34) lipopolysaccharide core biosynthesis gene clusters. J. Bacteriol. 2008, 190, 3176–3184. [Google Scholar] [CrossRef]
- Merino, S.; Canals, R.; Knirel, Y.A.; Tomás, J.M. Molecular and chemical analysis of the lipopolysaccharide from Aeromonas hydrophila strain AH-1 (Serotype O11). Mar. Drugs. 2015, 13, 2233–2249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, J.; Vinogradov, E.; Altman, E. Structural studies of the core region of Aeromonas salmonicida subsp. <salmonicida> lipopolysaccharide. Carbohydr Res. 2006, 341, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Knirel, Y.A.; Vinogradov, E.; Jimenez, N.; Merino, S.; Tomás, J.M. Structural studies on the R-type lipopolysaccharide of Aeromonas hydrophila. Carbohydr. Res. 2004, 339, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Turska-Szewczuk, A.; Duda, K.A.; Schwudke, D.; Pekala, A.; Kozinska, A.; Holst, O. Structural studies of the lipopolysaccharide from the fish pathogen Aeromonas veronii strain Bs19, serotype O16. Mar. Drugs. 2014, 12, 1298–1316. [Google Scholar] [CrossRef]
- Turska-Szewczuk, A.; Lindner, B.; Komaniecka, I.; Kozinska, A.; Pekala, A.; Choma, A.; Holst, O. Structural and immunochemical studies of the lipopolysaccharide from the fish pathogen, Aeromonas bestiarum strain K296, serotype O18. Mar. Drugs. 2013, 11, 1235–1255. [Google Scholar] [CrossRef]
- Merino, S.; Tomás, J.M. The Aeromonas salmonicida Lipopolysaccharide Core from Different Subspecies: The Unusual subsp. pectinolytica. Front. Microbiol. 2016, 7, 125. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide endotoxins. Annu Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef]
- Raetz, C.R.H. Bacterial lipopolysaccharides: A remarkable family of bioactive macroamphiphiles. In Escherichia Coli and Salmonella: Cellular and Molecular Biology, 2nd ed.; Neidhardt, F.C., Curtiss, R., III, Ingraham, J.L., Lin, E.C.C., Low, K.B., Magasanik, B., Reznikoff, W.S., Riley, M., Schaechter, M., Eds.; ASM Press: Washington, DC, USA, 1996; Volume 1, pp. 1035–1063. ISBN 9781555810845, 9781555810849. [Google Scholar]
- Sakazaki, R.; Shimada, T. O-serogrouping scheme for mesophilic Aeromonas strains. Jpn J. Med. Sci. Biol. 1984, 37, 247–255. [Google Scholar] [CrossRef]
- Thomas, L.V.; Gross, R.J.; Cheasty, T.; Rowe, B. Extended serogrouping scheme for motile, mesophilic Aeromonas species. J. Clin. Microbiol. 1990, 28, 980–984. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L.; Khashe, S.; Kellogg, G.H.; Shimada, T. Further studies on biochemical characteristics and serologic properties of the genus Aeromonas. J. Clin. Microbiol. 1996, 34, 1930–1933. [Google Scholar] [CrossRef]
- Hasunuma, R.; Morita, H.; Tanaka, S.; Ryll, R.; Freudenberg, M.A.; Galanos, C.; Kumazawa, Y. Differential clearance and induction of host responses by various administered or released lipopolysaccharides. J. Endotoxin Res. 2001, 7, 421–429. [Google Scholar] [CrossRef]
- Kapp, A.; Freudenberg, M.; Galanos, C. Induction of human granulocyte chemiluminescence by bacterial lipopolysaccharides. Infect. Immun. 1987, 55, 758–761. [Google Scholar] [CrossRef]
- Huber, M.; Kalis, C.; Keck, S.; Jiang, Z.; Georgel, P.; Du, X.; Shamel, L.; Sovath, S.; Mudd, S.; Beutler, B.; et al. R-form LPS, the master key to the activation ofTLR4/MD-2-positive cells. Eur. J. Immunol. 2006, 36, 701–711. [Google Scholar] [CrossRef]
- Gomes, N.E.; Brunialti, M.K.; Mendes, M.E.; Freudenberg, M.; Galanos, C.; Salomão, R. Lipopolysaccharide-induced expression of cell surface receptors and cell activation of neutrophils and monocytes in whole human blood. Brazilian J. Med. Biol. Res. 2010, 43, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.; Merino, S.; Rubires, X.; Tomas, J.M. Influence of osmolarity on lipopolysaccharides and virulence of Aeromonas hydrophila serotype O:34 strains grown at 37 degrees C. Infect. Immun. 1997, 65, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Camprubí, S.; Tomás, J.M. Effect of growth temperature on outer membrane components and virulence of Aeromonas hydrophila strains of serotype O:34. Infect. Immun. 1992, 60, 4343–4349. [Google Scholar] [CrossRef]
- Merino, S.; Rubires, X.; Aguilar, A.; Albert, S.; Hernandez-Allé, S.; Bened, V.J.; Tomás, J.M. Mesophilic Aeromonas sp. serogroup O:11 resistance to complement-mediated killing. Infect. Immun. 1996, 64, 5302–5309. [Google Scholar] [CrossRef] [PubMed]
- Knirel, Y.A.; Shashkov, A.S.; Senchenkova, S.N.; Merino, S.; Tomás, J.M. Structure of the O-polysaccharide of Aeromonas hydrophila O:34; a case of random O-acetylation of 6-deoxy-L-talose. Carbohydr. Res. 2002, 337, 1381–1386. [Google Scholar] [CrossRef]
- Wang, Z.; Vinogradov, E.; Larocque, S.; Harrison, B.A.; Li, J.; Altman, E. Structural and serological characterization of the O-chain polysaccharide of Aeromonas salmonicida strains A449, 80204 and 80204-1. Carbohydr. Res. 2005, 340, 693–700. [Google Scholar] [CrossRef]
- Merino, S.; de Mendoza, E.; Canals, R.; Tomás, J.M. Functional Genomics of the Aeromonas salmonicida Lipopolysaccharide O-Antigen and A-Layer from Typical and Atypical Strains. Mar. Drugs. 2015, 13, 3791–3808. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Li, J.; Altman, E. Structural characterization of the O-chain polysaccharide of Aeromonas caviae ATCC 15468 lipopolysaccharide. Carbohydr. Res. 2008, 343, 483–488. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Arakawa, E.; Leung, K.Y. Novel Aeromonas hydrophila PPD134/91 genes involved in O-antigen and capsule biosynthesis. Infect. Immun. 2002, 70, 2326–2335. [Google Scholar] [CrossRef][Green Version]
- Jimenez, N.; Canals, R.; Saló, M.T.; Vilches, S.; Merino, S.; Tomás, J.M. The Aeromonas hydrophila wb*O34 gene cluster: Genetics and temperature regulation. J. Bacteriol. 2008, 190, 4198–4209. [Google Scholar] [CrossRef]
- Hossain, M.J.; Waldbieser, G.C.; Sun, D.; Capps, N.K.; Hemstreet, W.B.; Carlisle, K.; Griffin, M.J.; Khoo, L.; Goodwin, A.E.; Sonstegard, T.S.; et al. Implication of Lateral Genetic Transfer in the Emergence of Aeromonas hydrophila Isolates of Epidemic Outbreaks in Channel Catfish. PLoS ONE 2013, 8, e80943. [Google Scholar] [CrossRef]
- Abeyrathne, P.D.; Daniels, C.; Poon, K.K.H.; Matewish, M.J.; Lam, J.S. Functional characterization of WaaL, a ligase associated with linking O-antigen polysaccharide to the core of Pseudomonas aeruginosa lipopolysaccharide. J. Bacteriol. 2005, 187, 3002–3012. [Google Scholar] [CrossRef] [PubMed]
- Valvano, M. O Antigen Biosynthesis. In Comprehensive Natural Products II: Chemistry and Biology, 1st ed.; Mander, L., Liu, H.-W., Eds.; Elsevier: Oxford, UK, 2010; Volume 6, pp. 297–314. [Google Scholar]
- Price, N.P.; Momany, F.A. Modeling bacterial UDP-HexNAc: Polyprenol-P HexNAc-1-P transferases. Glycobiology 2005, 15, 29R–42R. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Jimenez, N.; Molero, R.; Bouamama, L.; Regué, M.; Tomás, J.M. A UDP-HexNAc:polyprenol-P GalNAc-1-P transferase (WecP) representing a new subgroup of the enzyme family. J. Bacteriol. 2011, 193, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.T.; Lam, J.S. Wzx flippase-mediated membrane translocation of sugar polymer precursors in bacteria. Environ. Microbiol. 2013, 15, 1001–1015. [Google Scholar] [CrossRef]
- Collins, L.V.; Hackett, J. Molecular cloning, characterization, and nucleotide sequence of the rfc gene, which encodes an O-antigen polymerase of Salmonella typhimurium. J. Bacteriol. 1991, 173, 2521–2529. [Google Scholar] [CrossRef]
- Kalynych, S.; Valvano, M.A.; Cygler, M. Polysaccharide co-polymerases: The enigmatic conductors of the O-antigen assembly orchestra. Protein Eng Des. Sel. 2012, 25, 797–802. [Google Scholar] [CrossRef]
- Islam, S.T.; Lam, J.S. Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway. Can. J. Microbiol. 2014, 60, 697–716. [Google Scholar] [CrossRef]
- Hug, I.; Feldman, M.F. Analogies and homologies in lipopolysaccharide and glycoprotein biosynthesis in bacteria. Glycobiology 2011, 21, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Gonzalez, V.; Tomás, J.M. The first sugar of the repeat units is essential for the Wzy polymerase activity and elongation of the O-antigen lipopolysaccharide. Future Microbiol. 2016, 11, 903–918. [Google Scholar] [CrossRef]
- Merino, S.; Gonzalez, V.; Tomás, J.M. The Polymerization of Aeromonas hydrophila AH-3 O-Antigen LPS: Concerted Action of WecP and Wzy. PLoS ONE 2015, 10, e0131905. [Google Scholar] [CrossRef]
- Merino, S.; Tomás, J.M. Bacterial Capsules and Evasion of Immune Responses. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Roberts, I.S. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu. Rev. Microbiol. 1996, 50, 285–315. [Google Scholar] [CrossRef]
- Robbins, J.B.; McCracken, G.H.J.; Gotschlich, E.C.; Ørskov, F.; Ørskov, I.; Hanson, L.A. Escherichia coli K1 Capsular Polysaccharide Associated with Neonatal Meningitis. N. Engl. J. Med. 1974, 290, 1216–1220. [Google Scholar] [CrossRef]
- Grados, O.; Ewing, W.H. Antigenic relationship between Escherichia coli and Neisseria meningitidis. J. Infect. Dis. 1970, 122, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Larocque, S.; Vinogradov, E.; Brisson, J.R.; Dacanay, A.; Greenwell, M.; Brown, L.L.; Li, J.; Altman, E. Structural studies of the capsular polysaccharide and lipopolysaccharide O-antigen of Aeromonas salmonicida strain 80204-1 produced under in vitro and in vivo growth conditions. Eur. J. Biochem. 2004, 271, 4507–4516. [Google Scholar] [CrossRef] [PubMed]
- Sadovskaya, I.; Brisson, J.R.; Altman, E.; Mutharia, L.M. Structural studies of the lipopolysaccharide O-antigen and capsular polysaccharide of Vibrio anguillarum serotype O:2. Carbohydr Res. 1996, 283, 111–127. [Google Scholar] [CrossRef]
- Sadovskaya, I.; Brisson, J.R.; Khieu, N.H.; Mutharia, L.M.; Altman, E. Structural characterization of the lipopolysaccharide O-antigen and capsular polysaccharide of Vibrio ordalii serotype O:2. Eur. J. Biochem. 1998, 253, 319–327. [Google Scholar] [CrossRef]
- Contreras Sánchez-Matamoros, R.; Gil-Serrano, A.M.; Espuny, M.R.; Ollero, F.J.; Megías, M.; Rodríguez-Carvajal, M.A. Structure of surface polysaccharides from Aeromonas sp. AMG272, a plant-growth promoting rhizobacterium isolated from rice rhizosphere. Carbohydr. Res. 2018, 462, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.S.; Whitfield, C. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 1999, 31, 1307–1319. [Google Scholar] [CrossRef]
- Whitfield, C. Biosynthesis and Assembly of Capsular Polysaccharides in Escherichia coli. Annu. Rev. Biochem. 2006, 75, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Lau, Y.L.; Arakawa, E.; Leung, K.Y. Detection and genetic analysis of group II capsules in Aeromonas hydrophila. Microbiology 2003, 149, 1051–1060. [Google Scholar] [CrossRef][Green Version]
- Aguilar, A.; Merino, S.; Nogueras, M.M.; Regué, M.; Tomás, J.M. Two genes from the capsule of Aeromonas hydrophila (serogroup O:34) confer serum resistance to Escherichia coli K12 strains. Res. Microbiol. 1999, 150, 395–402. [Google Scholar] [CrossRef]
- Garduño, R.A.; Thornton, J.C.; Kay, W.W. Aeromonas salmonicida grown in vivo. Infect. Immun. 1993, 61, 3854–3862. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Aguilar, A.; Rubires, X.; Simon-Pujol, D.; Congregado, F.; Tomás, J.M. The role of the capsular polysaccharide of Aeromonas salmonicida in the adherence and invasion of fish cell lines. FEMS Microbiol. Lett. 1996, 142, 185–189. [Google Scholar] [CrossRef]
- Merino, S.; Aguilar, A.; Rubires, X.; Abitiu, N.; Regué, M.; Tomás, J.M. The role of the capsular polysaccharide of Aeromonas hydrophila serogroup O:34 in the adherence to and invasion of fish cell lines. Res. Microbiol. 1997, 148, 625–631. [Google Scholar] [CrossRef]
- Preiss, J.; Romeo, T. Molecular biology and regulatory aspects of glycogen biosynthesis in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 1994, 47, 299–329. [Google Scholar] [CrossRef]
- Preiss, J. Bacterial Glycogen Synthesis and its Regulation. Annu. Rev. Microbiol. 1984, 38, 419–458. [Google Scholar] [CrossRef]
- Wang, L.; Wise, M.J. Glycogen with short average chain length enhances bacterial durability. Naturwissenschaften 2011, 98, 719–729. [Google Scholar] [CrossRef]
- Vilches, S.; Canals, R.; Wilhelms, M.; Saló, M.T.; Knirel, Y.A.; Vinogradog, E.; Merino, S.; Tomás, J.M. Mesophilic Aeromonas UDP-glucose pyrophosphorylase (GalU) mutants show two types of lipopolysaccharide structures and reduced virulence. Microbiology 2007, 153, 2393–2404. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.G.; Sutherland, I.W. The Role of Exopolysaccharides in Adhesion of Freshwater Bacteria. Microbiology 1987, 133, 1319–1327. [Google Scholar] [CrossRef][Green Version]
- Sutherland, I.W. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Agladze, K.; Wang, X.; Romeo, T. Spatial periodicity of Escherichia coli K-12 biofilm microstructure initiates during a reversible, polar attachment phase of development and requires the polysaccharide adhesin PGA. J. Bacteriol. 2005, 187, 8237–8246. [Google Scholar] [CrossRef]
- Bornemann, S. α-Glucan biosynthesis and the GlgE pathway in Mycobacterium tuberculosis. Biochem. Soc. Trans. 2016, 44, 68–73. [Google Scholar] [CrossRef]
- Price, N.P.; Goyette-Desjardins, G.; Nothaft, H.; Valguarnera, E.; Szymanski, C.M.; Segura, M.; Feldman, M.F. Glycoengineered Outer Membrane Vesicles: A Novel Platform for Bacterial Vaccines. Sci. Rep. 2016, 6, 24931. [Google Scholar] [CrossRef]
- Chen, L.; Valentine, J.L.; Huang, C.J.; Endicott, C.E.; Moeller, T.D.; Rasmussen, J.A.; Fletcher, J.R.; Boll, J.M.; Rosenthal, J.A.; Dobruchowska, J.; et al. Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies. Proc. Natl. Acad. Sci. USA 2016, 113, E3609–E3618. [Google Scholar] [CrossRef]
- Valentine, J.L.; Chen, L.; Perregaux, E.C.; Weyant, K.B.; Rosenthal, J.A.; Heiss, C.; Azadi, P.; Fisher, A.C.; Putnam, D.; Moe, G.R.; et al. Immunization with outer membrane vesicles displaying designer glycotopes yields class-switched, glycan-specific antibodies. Cell Chem. Biol. 2016, 23, 655–665. [Google Scholar] [CrossRef]
- Casella, C.R.; Mitchell, T.C. Putting endotoxin to work for us: Monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol. Life Sci. 2008, 65, 3231–3240. [Google Scholar] [CrossRef]
- Needham, B.D.; Carroll, S.M.; Giles, D.K.; Georgiou, G.; Whiteley, M.; Trent, M.S. Modulating the innate immune response by combinatorial engineering of endotoxin. Proc. Natl. Acad. Sci. USA 2013, 110, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Gregg, K.A.; Harberts, E.; Gardner, F.M.; Pelletier, M.R.; Cayatte, C.; Yu, L.; McCarthy, M.P.; Marshall, J.D.; Ernst, R.K. Rationally Designed TLR4 Ligands for Vaccine Adjuvant Discovery. MBio 2017, 8, e00492-17. [Google Scholar] [CrossRef]
- Zariri, A.; Pupo, E.; van Riet, E.; van Putten, J.P.M.; van der Ley, P. Modulating endotoxin activity by combinatorial bioengineering of meningococcal lipopolysaccharide. Sci. Rep. 2016, 6, 36575. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Prevnar 13. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar-13 (accessed on 24 October 2021).
- U.S. Food & Drug Administration. PNEUMOVAX 23—Pneumococcal Vaccine, Polyvalent. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/pneumovax-23-pneumococcal-vaccine-polyvalent (accessed on 24 October 2021).
- Rodríguez, I.; Chamorro, R.; Novoa, B.; Figueras, A. β-Glucan administration enhances disease resistance and some innate immune responses in zebrafish (Danio rerio). Fish Shellfish Immunol. 2009, 27, 369–373. [Google Scholar] [CrossRef]
- Kumari, J.; Sahoo, P.K. Dietary β-1,3 glucan potentiates innate immunity and disease resistance of Asian catfish, Clarias batrachus (L.). J. Fish. Dis. 2006, 29, 95–101. [Google Scholar] [CrossRef] [PubMed]

| Capsule Composition | Species (Strains) | Ref. |
|---|---|---|
| D-Glc, D-Man, L-Rha, ManNAc, D-ManA | A. salmonicida (A449, A450, A894) | [33] |
| D-Glc, D-Man, L-Rha, D-ManA, Acetic acid | A. hydrophila (TF7, LL1, Ba5) | [32] |
| A. piscicola (AH3) | [32] | |
| QuiNAc, Qui3NAlaNAc, GalNAcA | A. salmonicida (80204-1) | [114] |
| D-Gal, GlcNAc, GalNAc, D-Fuc3NAc4Ac | Aeromonas sp. (AMG272) | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Barberá, E.; Merino, S.; Tomás, J. Surface Glucan Structures in Aeromonas spp. Mar. Drugs 2021, 19, 649. https://doi.org/10.3390/md19110649
Mendoza-Barberá E, Merino S, Tomás J. Surface Glucan Structures in Aeromonas spp. Marine Drugs. 2021; 19(11):649. https://doi.org/10.3390/md19110649
Chicago/Turabian StyleMendoza-Barberá, Elena, Susana Merino, and Juan Tomás. 2021. "Surface Glucan Structures in Aeromonas spp." Marine Drugs 19, no. 11: 649. https://doi.org/10.3390/md19110649
APA StyleMendoza-Barberá, E., Merino, S., & Tomás, J. (2021). Surface Glucan Structures in Aeromonas spp. Marine Drugs, 19(11), 649. https://doi.org/10.3390/md19110649








