In Silico Prediction of Steroids and Triterpenoids as Potential Regulators of Lipid Metabolism
Abstract
1. Introduction
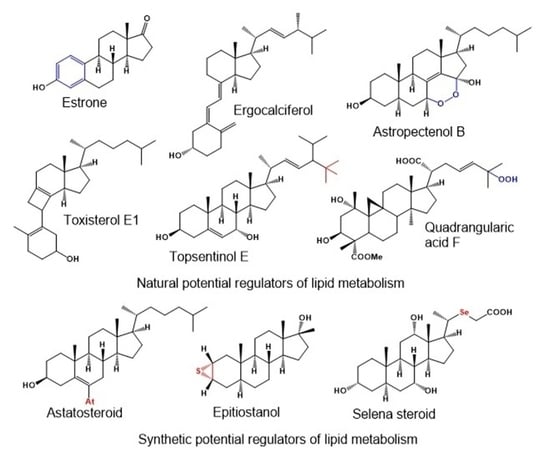
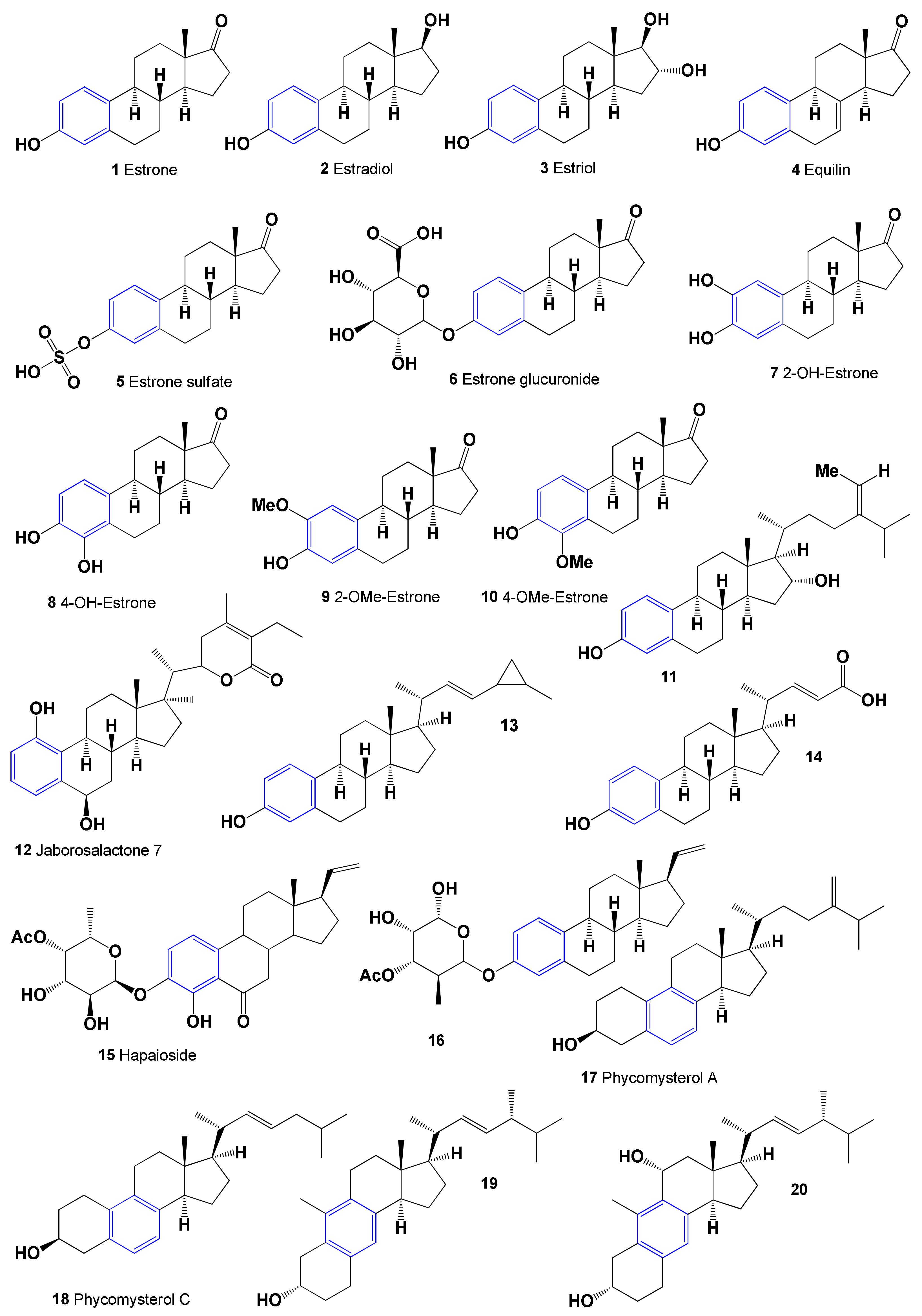
2. Aromatic Steroids Derived from Natural Sources
Comparison of Biological Activities of Natural Aromatic Steroids
3. Natural, Semi-, and Synthetic Steroid Phosphate Esters
Comparison of Biological Activities of Steroid Phosphate Esters
4. Highly Oxygenated Natural Steroids and Triterpenoids
4.1. Secosteroids Derived from Marine and Terrestrial Sources
Comparison of Biological Activities of Secosteroids
4.2. Natural Epoxy Steroids Derived from Marine Sources
Comparison of Biological Activities of α,β-Epoxy Steroids Derived from Marine Sources
4.3. Peroxy-Type Steroids Derived from Natural Sources
4.3.1. Steroid Endoperoxides
4.3.2. Steroid and Triterpenoid Hydroperoxides
4.3.3. Comparison of Biological Activities of Peroxy Steroids Derived from Natural Sources
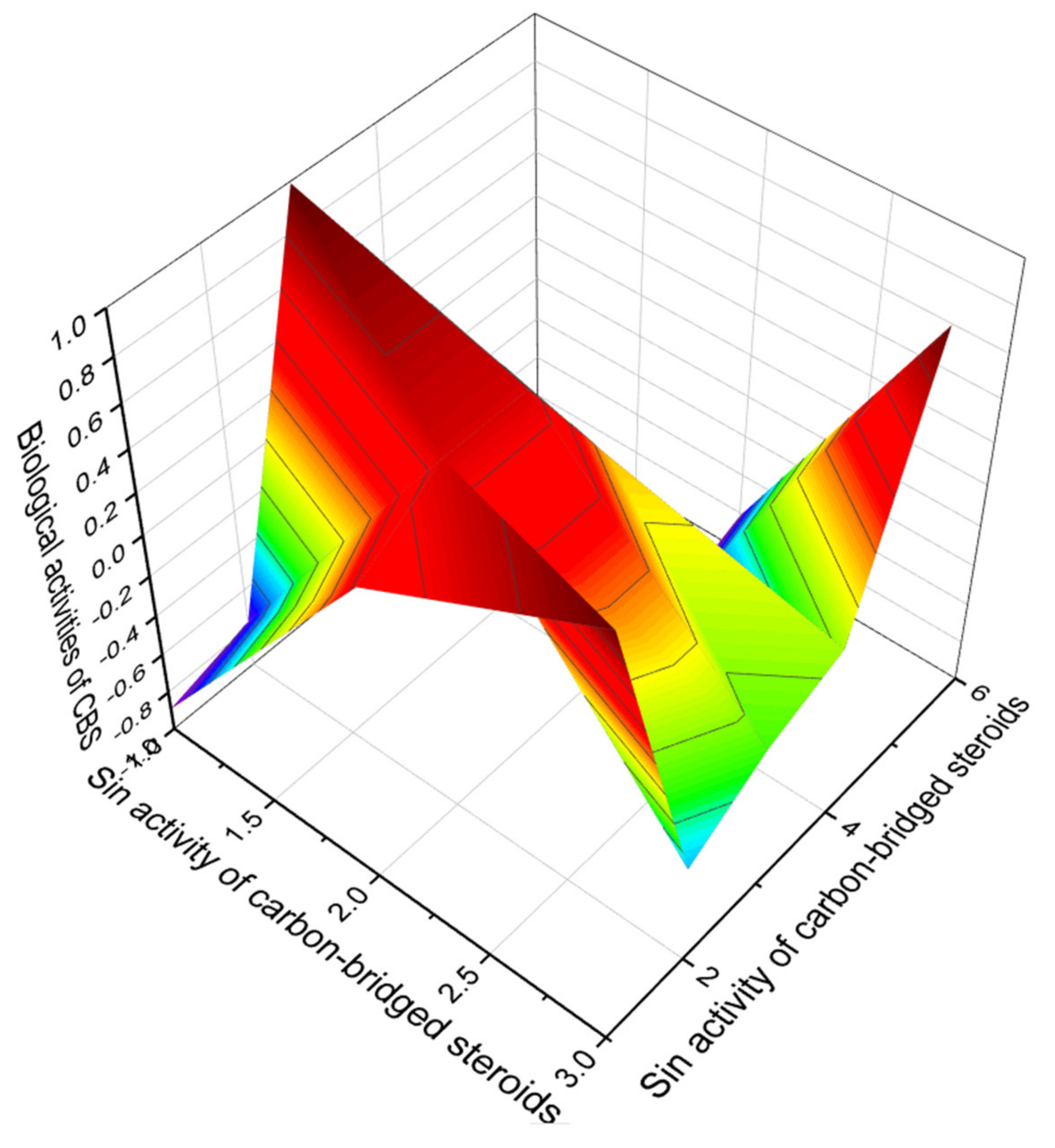
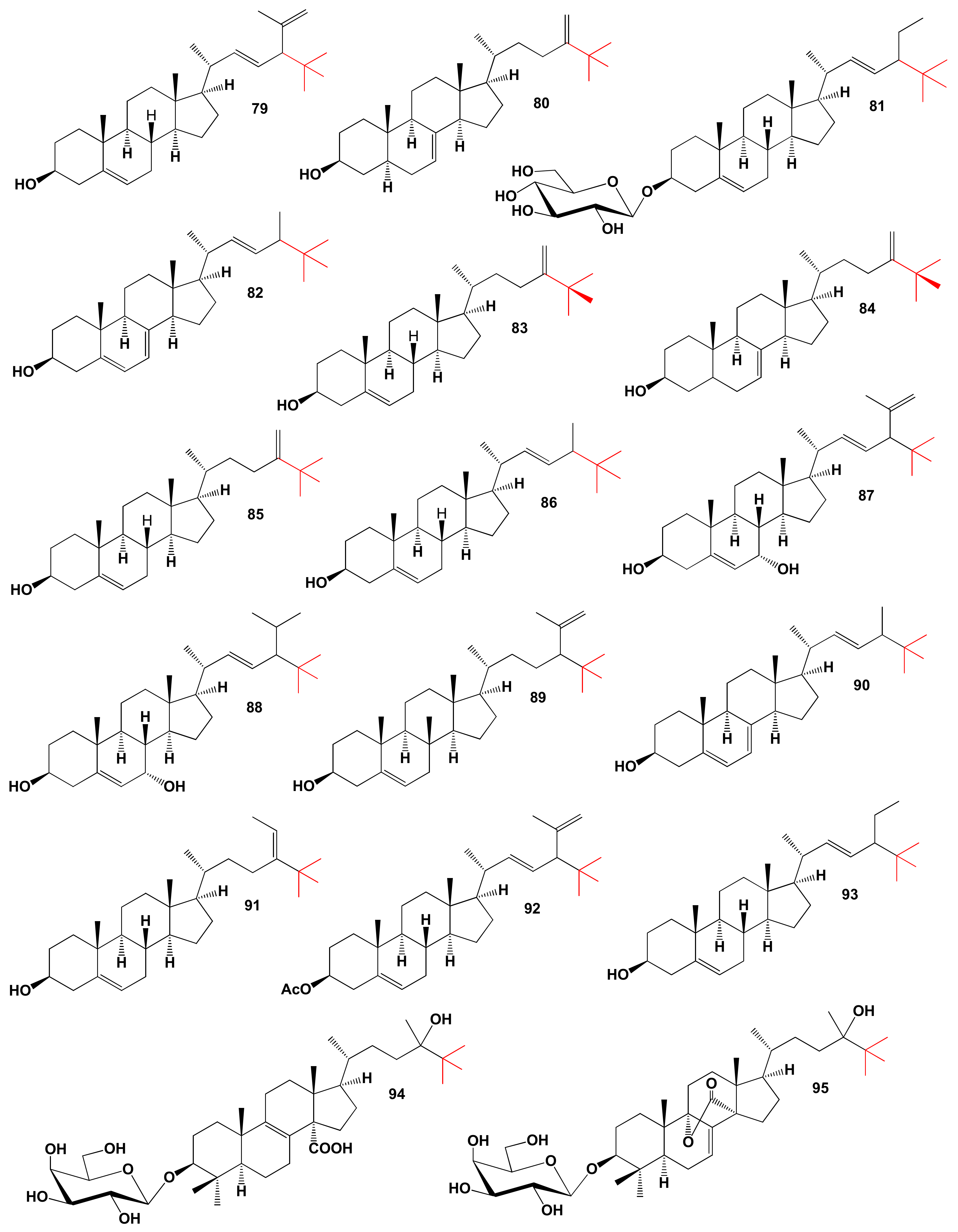
5. Carbon-Bridged Steroids (CBS) and Triterpenoids
Comparison of Biological Activities of CBS Steroids and Triterpenoids
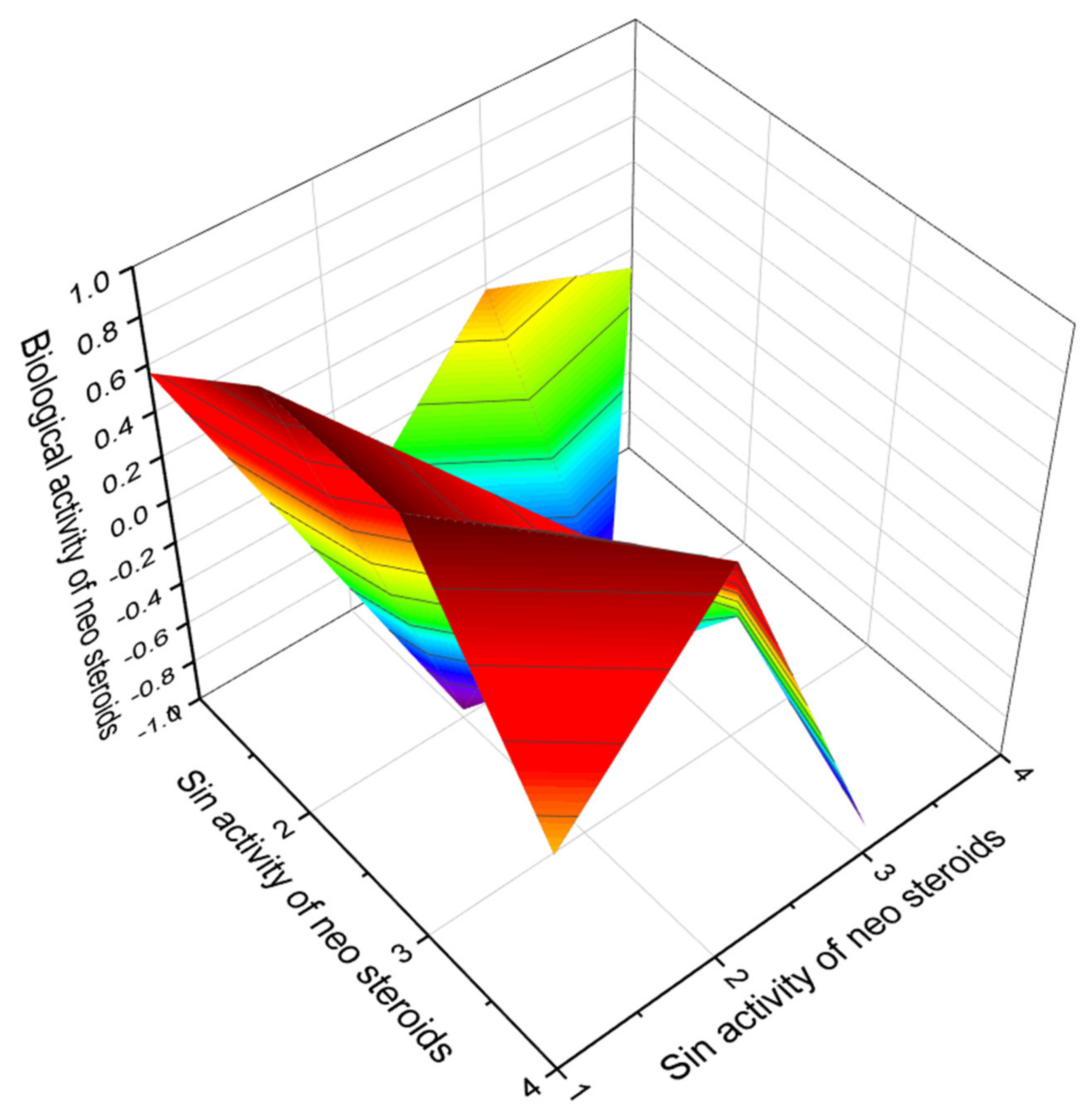
6. Neo Steroids Derived from Terrestrial and Marine Sources
Comparison of Biological Activities of Neo Steroids
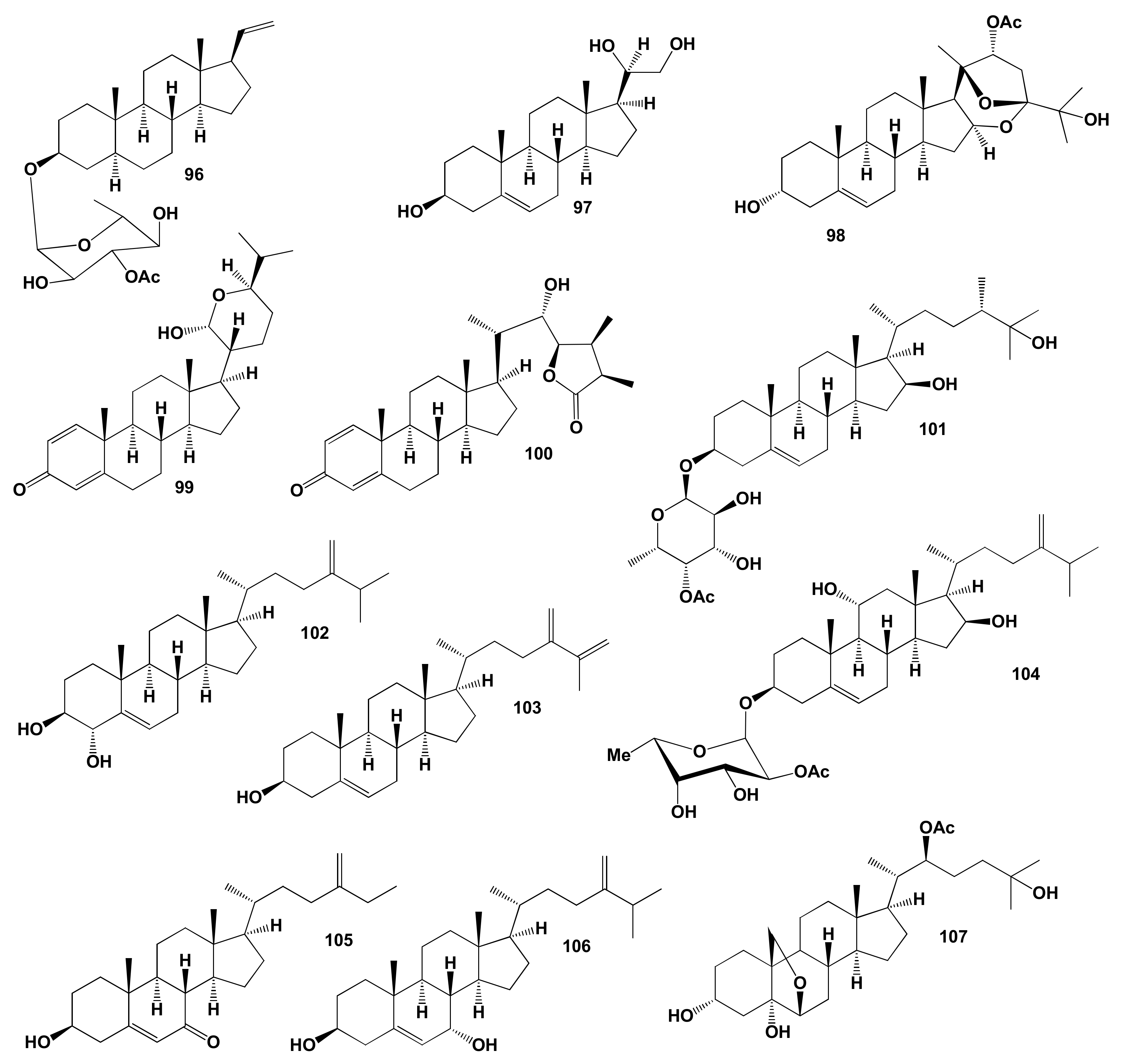
7. Miscellaneous Steroids and Triterpenoids Derived from Marine Sources
Comparison of Biological Activities of Soft Coral Steroids
8. Synthetic and Semi-Synthetic Steroids and Triterpenoids and Comparison of Their Biological Activities
8.1. Bioactive Epithio Steroids and Triterpenoids
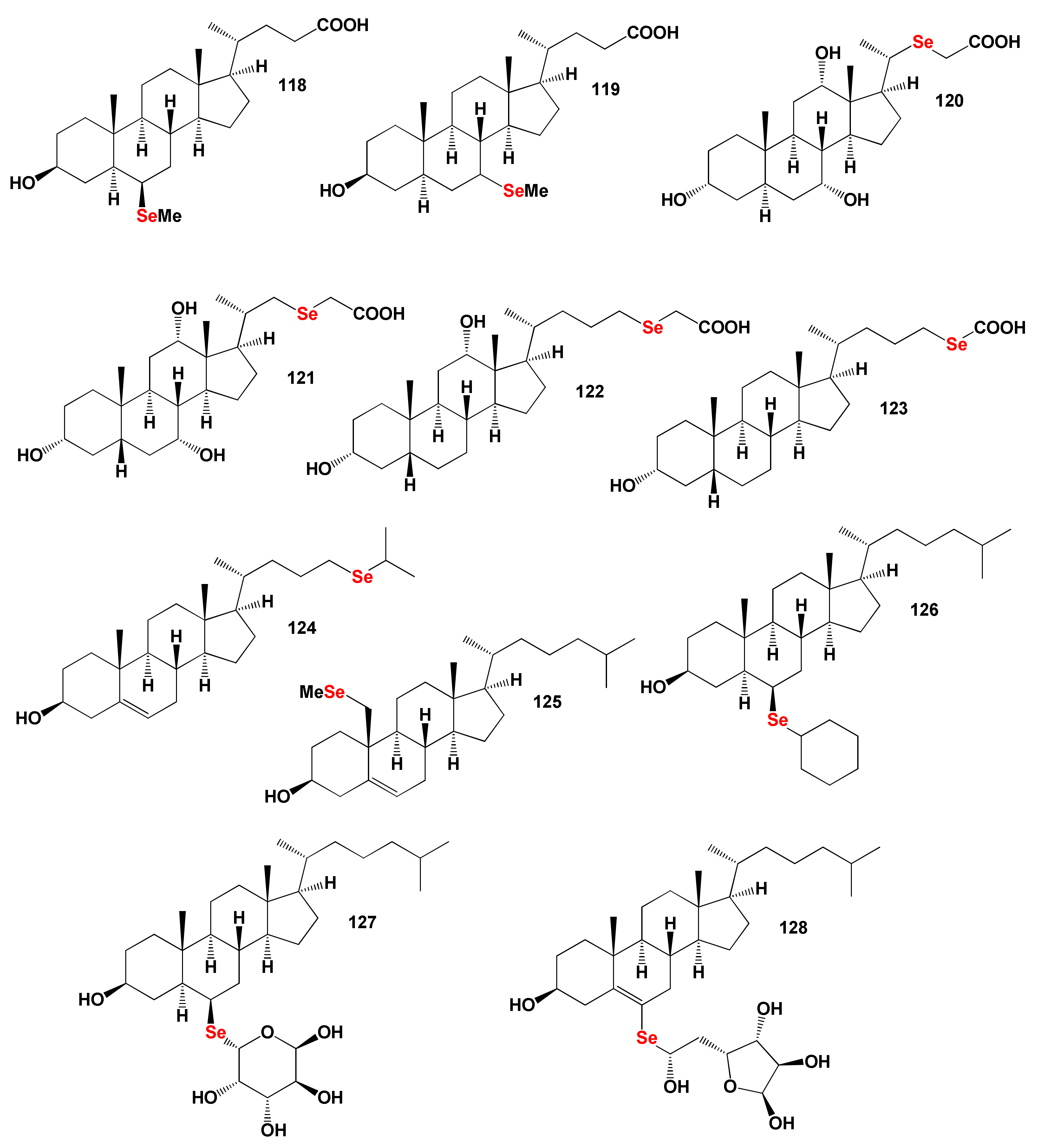
8.2. Bioactive Selena Steroids
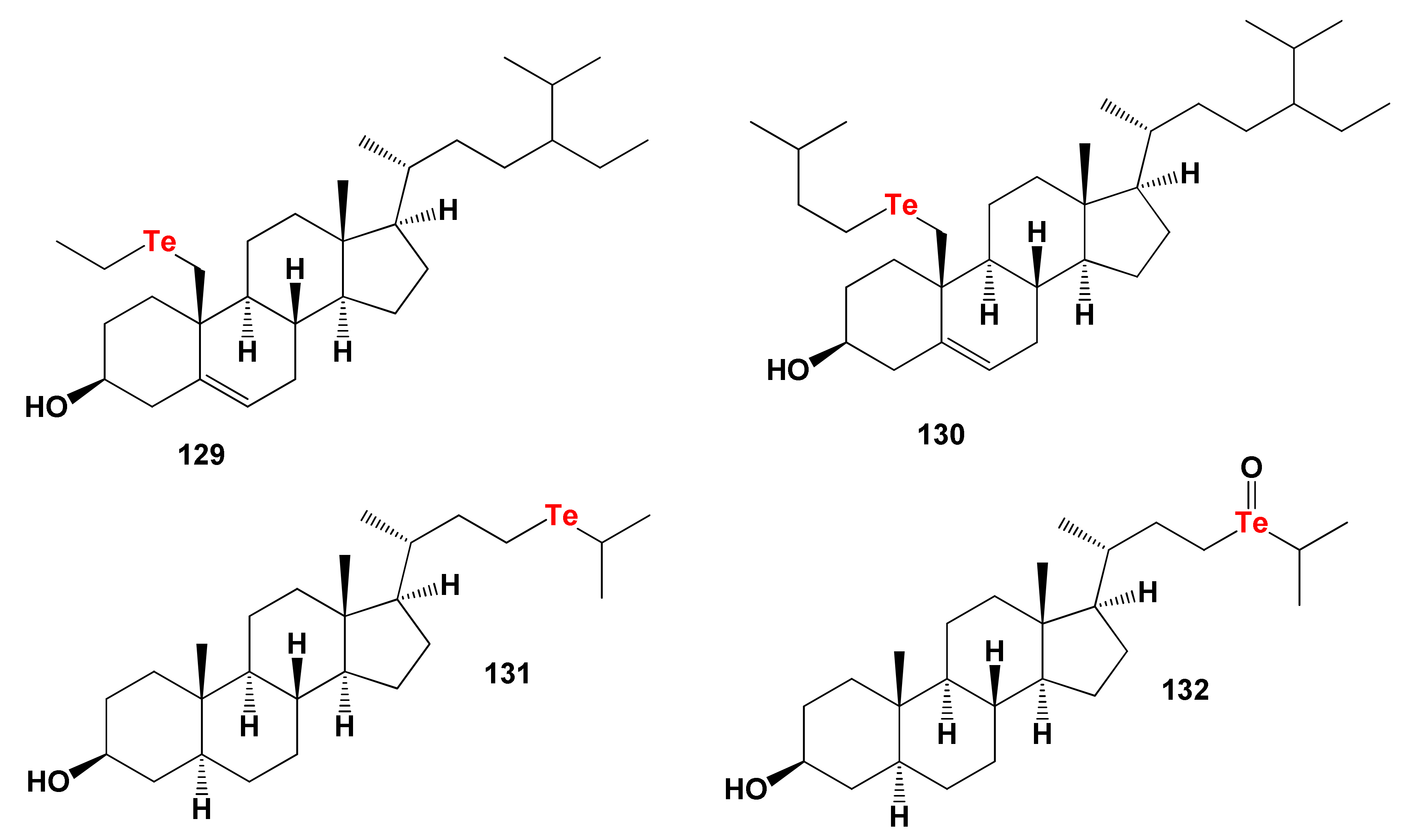
8.3. Bioactive Tellura Steroids
8.4. Bioactive Astatosteroids
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, X.; Smith, J.C. Biological membrane organization and cellular signaling. Chem. Rev. 2019, 119, 5849–5880. [Google Scholar] [CrossRef]
- Jensen, M.Ø.; Mouritsen, O.G. Lipids do influence protein function—The hydrophobic matching hypothesis revisited. Biochim. Biophys. Acta Biomembr. 2004, 1666, 205–226. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, J.L.; Tieleman, D.P. Hydrophobicity scales: A thermodynamic looking glass into lipid–protein interactions. Trends Biochem. Sci. 2011, 36, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Lundstedt, E.; Kahne, D.; Ruiz, N. Assembly, and maintenance of lipids at the bacterial outer membrane. Chem. Rev. 2021, 121, 5098–5123. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, M. Phospholipids in marine environments: A review. Talanta 2005, 66, 422–434. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Dowhan, W. Molecular basis for membrane phospholipid diversity: Why are there so many lipids? Ann. Rev. Biochem. 1997, 66, 199–232. [Google Scholar] [CrossRef] [PubMed]
- Berne, B.J.; Weeks, J.D.; Zhou, R. Dewetting and hydrophobic interaction in physical and biological systems. Ann. Rev. Phys. Chem. 2009, 60, 85–103. [Google Scholar] [CrossRef]
- Gisterå, A.; Hansson, G. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef]
- Alexopoulos, N.; Raggi, P. Calcification in atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 681–688. [Google Scholar] [CrossRef]
- Faulds, M.H.; Zhao, C.; Dahlman-Wright, K.; Gustafsson, J.Å. The diversity of sex steroid action: Regulation of metabolism by estrogen signaling. J. Endocrinol. 2011, 212, 3–12. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Antitumor and hepatoprotective activity of natural and synthetic neo steroids. Prog. Lipid Res. 2020, 79, 101048. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Antitumor profile of carbon-bridged steroids (CBS) and triterpenoids. Mar. Drugs 2021, 19, 324. [Google Scholar] [CrossRef]
- Pounina, T.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M. Sulfated and sulfur-containing steroids and their pharmacological profile. Mar. Drugs 2021, 19, 240. [Google Scholar] [CrossRef]
- Zagoskin, P.P.; Erlykina, E.I. Bile acids as a new type of steroid hormones regulating nonspecific energy expenditure of the body (review). Sovremen. Tehnol. Med. 2020, 12, 114. [Google Scholar] [CrossRef]
- Ko, C.W.; Qu, J.; Black, D.D.; Tso, P. Regulation of intestinal lipid metabolism: Current concepts and relevance to disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 169–183. [Google Scholar] [CrossRef]
- De Bose-Boyd, R.A. Significance, and regulation of lipid metabolism. Semin. Cell Dev. Biol. 2018, 81, 97. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms, and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota, and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Matsushita, Y.; Nakagawa, H.; Koike, K. Lipid metabolism in oncology: Why it matters, how to research, and How to treat. Cancers 2021, 13, 474. [Google Scholar] [CrossRef]
- Edgar, A.; Doisy, E.A. An ovarian hormone: Preliminary report on its localization, extraction and partial purification, and action in test animals. J. Am. Med. Assoc. 1923, 81, 819–821. [Google Scholar]
- Doisy, E.A.; Clement, D.V.; Sidney, T. Folliculin from urine of pregnant women. Am. J. Phys. 1929, 90, 329–330. [Google Scholar]
- Butenandt, A.B. Über, Progynonein krystallisiertes weibliches Sexualhormon. Die Naturwissenschaften 1929, 17, 879. [Google Scholar] [CrossRef]
- Butenandt, A. Über physikalische und chemische Eigenschaften des krystallisierten Follikelhormons. Untersuchungen über das weibliche Sexualhormon. Hoppe-Seyler’s Zeit. Physiol. Chem. 1930, 191, 140–156. [Google Scholar] [CrossRef]
- Butenandt, A. Über die chemische Untersuchung der Sexualhormone. Zeit Angew. Chem. 1931, 44, 905–908. [Google Scholar] [CrossRef]
- Butenandt, A.; Jacobi, H. Über die Darstellung eines krystallisierten pflanzlichen Tokokinins (Thelykinins) und seine Identifizierung mit dem α-Follikelhormon. Untersuchungen über das weibliche Sexualhormon. Hoppe Seyler’s Z. Physiol. Chem. 1933, 218, 104–112. [Google Scholar] [CrossRef]
- Ramirez, M.P.; Haas, S. Hormone replacement therapy for women: The benefits, risks, and considerations for use in 2003. Curr. Opinion Endocrin. Diabet. 2003, 10, 400–418. [Google Scholar] [CrossRef]
- Fluhmann, C.F. Estrogenic hormones: Their clinical usage. Cal. West Med. 1938, 49, 362–366. [Google Scholar]
- Younglai, E.V.; Solomon, S. Formation of estra-1,3,5(10)-triene-3,15a,16a,17b-tetrol (estetrol) and estra-1,3,5(10)-triene-3,15a,17btriol from neutral precursors. J. Clin. Endocrinol. Metab. 1968, 28, 1611–1617. [Google Scholar] [CrossRef]
- Raeside, J.I. A brief account of the discovery of the fetal/placental unit for estrogen production in equine and human pregnancies: Relation to human medicine. Yale J. Biol. Med. 2017, 90, 449–461. [Google Scholar]
- Trifunović, J.; Borčić, V.; Vukmirović, S.; Mikov, M. Structural insights into anticancer activity of D-ring modified estrone derivatives using their lipophilicity in estimation of SAR and molecular docking studies. Drug Test Anal. 2017, 9, 1650. [Google Scholar] [CrossRef]
- Dohrn, M.; Faure, W.; Poll, H.; Blotevogel, W. Tokokinine, Stoff mit sexualhormonartiger Wirkung aus Pflanzenzellen. Med. Klin. 1926, 22, 1417–1419. [Google Scholar]
- Skarzynski, B. An oestrogenic substance from plant material. Nature 1933, 131, 766. [Google Scholar]
- Janeczko, A.; Skoczowski, A. Mammalian sex hormones in plants. Folia Histochem. Cytobiol. 2005, 43, 71–79. [Google Scholar]
- Jailer, J.W. The metabolism of the estrogens: A review. J. Clin. Endocrinol. 1949, 9, 557–572. [Google Scholar] [CrossRef]
- Xu, Y.; López, M. Central regulation of energy metabolism by estrogens. Mol. Metabol. 2018, 15, 104–115. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Piquereau, J.; Veksler, V.; Garnier, A. Estrogens, estrogen receptors effects on cardiac and skeletal muscle mitochondria. Front. Endocrinol. 2019, 10, 557. [Google Scholar] [CrossRef]
- Le, J.; Thomas, N.; Gurvich, C. Cognition, the menstrual cycle, and premenstrual disorders: A Review. Brain Sci. 2020, 10, 198. [Google Scholar] [CrossRef]
- Su, Z.; Yuan, W.; Wang, P.; Li, S. Ethnobotany, phytochemistry, and biological activities of Taxodium Rich. Pharm. Crops 2013, 4, 1–14. [Google Scholar]
- Sukandar, E.Y.; Suganda, A.G.; Pertiwi, G.U. Aktivitas sediaan yang mengandung ekstrak daun ketapang pada kulit kelinci yang diinfeksi dengan ephidermophyton floccosum dan Candida albicans. Acta Pharm. Ind. 2007, 32, 45–49. [Google Scholar]
- Suganda, A.G.; Sukandar, E.Y.; Hardhiko, R.S. Aktivitas antimikroba ekstrak etanol daun yang dipetik dan ekstrak air daun gugur pohon ketapang (Terminalia catappa L.). Acta Pharm. Ind. 2004, 29, 129–133. [Google Scholar]
- Suganda, A.G.; Sukandar, E.Y.; Ratna, L. Aktivitas antimikroba ekstrak etanol daun dua belas jenis Tumbuhan marga terminalia (Combretaceae). Acta Pharm. Ind. 2006, 31, 18–23. [Google Scholar]
- Pranjali, C.; Lokesh, R. A review on medicinal potential of Terminalia catappa. Int. J. Green Pharm. 2020, 14, 229–234. [Google Scholar]
- Misico, R.I.; Veleiro, A.S.; Burton, G.; Oberti, J.C. Withanolides from Jaborosa leucotricha. Phytochemistry 1997, 45, 1045–1048. [Google Scholar] [CrossRef]
- Yan, X.-H.; Liu, H.-L.; Huang, H.; Li, X.-B.; Guo, Y.-W. Steroids with aromatic A rings from the Hainan soft coral Dendronephthya studeri Ridley. J. Nat. Prod. 2011, 74, 175–180. [Google Scholar] [CrossRef]
- Lu, Z.; Van Wagoner, R.M.; Harper, M.K.; Hooper, J.N.A.; Ireland, C.M. Two ring-A aromatized bile acids from the marine sponge Sollasella moretonensis. Nat. Prod. Commun. 2010, 5, 1571–1574. [Google Scholar] [CrossRef]
- Yeung, B.K.S.; Hamann, M.T.; Scheuer, P.J.; Kelly-Borges, M. Hapaioside: A 19-norpregnane glycoside from the sponge Cribrochalina olemda. Tetrahedron 1994, 50, 12593–12598. [Google Scholar] [CrossRef]
- Nakao, Y.; Kuo, J.; Yoshida, W.Y.; Kelly, M.; Scheuer, P.J. More kapakahines from the marine sponge Cribrochalina olemda. Org. Lett. 2003, 5, 1387–1390. [Google Scholar] [CrossRef]
- Tomono, Y.; Hirota, H.; Imahara, Y.; Fusetani, N. Four new steroids from two octocorals. J. Nat. Prod. 1999, 62, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Oltra, J.E.; Poyatos, J.A.; Jiménez, D.; Oliver, E. Phycomysterols and other sterols from the fungus Phycomyces blakesleeanus. J. Nat. Prod. 1998, 61, 1491–1496. [Google Scholar] [CrossRef]
- Liu, X.H.; Tang, X.Z.; Miao, F.P.; Ji, N.Y. A new pyrrolidine derivative and steroids from an algicolous Gibberella zeae strain. Nat. Prod. Commun. 2011, 6, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, F.; Shinde, P.B.; Hong, J.; Lee, C.-O.; Im, K.S.; Jung, J.H. 26,27-Cyclosterols and other polyoxygenated sterols from marine sponge Topsentia sp. J. Nat. Prod. 2006, 69, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Fraser, T.R. The connection of chemical constitution and physiological action. Trans. Roy. Soc. Edinb. 1868, 25, 224–242. [Google Scholar]
- Cros, A.F.A. Action de L’alcohol Amylique Sur L’organisme. Ph.D. Thesis, University of Strasbourg, Strasbourg, France, 1863. [Google Scholar]
- Richet, M.C. Note sur le rapport entre la toxicité et les propriétes physiques des corps. Compt. Rend. Soc. Biol. 1893, 45, 775–776. [Google Scholar]
- Meyer, H. Zur Theorie der AIkoholnarkose. Arch. Exp. Path. Pharm. 1899, 42, 109–118. [Google Scholar] [CrossRef]
- Overton, C.E. Studien über Die Narkose; Fischer: Jena, Germany, 1901. [Google Scholar]
- Hammett, L.P. Some relations between reaction rates and equilibrium constants. Chem. Rev. 1935, 17, 125–136. [Google Scholar] [CrossRef]
- Hammett, L.P. The effect of structure upon the reactions of organic compounds. Benzene derivatives. J. Am. Chem. Soc. 1937, 59, 96–103. [Google Scholar] [CrossRef]
- Taft, R.W. Separation of polar, steric and resonance effects in reactivity. In Steric Effects in Organic Chemistry; Newman, M.S., Ed.; Wiley: Hoboken, NJ, USA, 1956; pp. 556–675. [Google Scholar]
- Hansch, C.; Fujita, T. π-τ-π Analysis. A method for the correlation of biological activity and chemical structure. J. Am. Chem. Soc. 1964, 86, 1616–1626. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A. Exploring QSAR; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Sliwoski, G.; Kothiwale, S.; Meiler, J.; Lowe, E.W., Jr. Computational methods in drug discovery. Pharm. Rev. 2014, 66, 334–395. [Google Scholar] [CrossRef]
- Leelananda, S.P.; Lindert, S. Computational methods in drug discovery. Beilstein J. Org. Chem. 2016, 12, 2694–2718. [Google Scholar] [CrossRef]
- Kokh, D.B.; Amaral, M.; Bomke, J.; Grädler, U.; Musil, D. Estimation of drug-target residence times by τ-random acceleration molecular dynamics simulations. J. Chem. Theor. Comput. 2018, 14, 3859–3869. [Google Scholar] [CrossRef]
- Cherkasov, A.M.; Muratov, E.N.; Fourches, D.; Varnek, A.; Baskin, I.I. QSAR modeling: Where have you been? Where are you going to? J. Med. Chem. 2014, 57, 4977–5010. [Google Scholar] [CrossRef]
- Burov, Y.V.; Poroikov, V.V.; Korolchenko, L.V. National system for registration and biological testing of chemical compounds: Facilities for new drugs search. Bull. Natl. Cent. Biol. Act. Comp. 1990, 1, 4–25. [Google Scholar]
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.; Filimonov, D.; Poroikov, V.; Oprea, T. QSAR without borders. Chem. Soc. Rev. 2020, 49, 3525–3564. [Google Scholar] [CrossRef]
- Poroikov, V.V.; Filimonov, D.A.; Gloriozova, T.A.; Lagunin, A.A.; Druzhilovskiy, D.S.; Rudik, A.V. Computer-aided prediction of biological activity spectra for organic compounds: The possibilities and limitations. Russ. Chem. Bull. 2019, 68, 2143–2154. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Druzhilovskiy, D.S.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Dmitriev, P.V.; Poroikov, V.V. Computer-aided prediction of biological activity spectra for chemical compounds: Opportunities and limitations. Biom. Chem. Res. Method. 2018, 1, e00004. [Google Scholar] [CrossRef]
- Anusevicius, K.; Mickevicius, V.; Stasevych, M.; Zvarych, V.; Komarovska-Porokhnyavets, O.; Novikov, V.; Tarasova, O.; Gloriozova, T.; Poroikov, V. Design, synthesis, in vitro antimicrobial activity evaluation and computational studies of new N-(4-iodophenyl)-Alanine derivatives. Res. Chem. Intermed. 2015, 41, 7517–7540. [Google Scholar] [CrossRef]
- Murtazalieva, K.A.; Druzhilovskiy, D.S.; Goel, R.K.; Sastry, G.N.; Poroikov, V.V. How good are publicly available web services that predict bioactivity profiles for drug repurposing? SAR QSAR Environ. Res. 2017, 28, 843–862. [Google Scholar] [CrossRef]
- PASS Online. Available online: http://www.way2drug.com/passonline/ (accessed on 4 June 2021).
- Lagunin, A.A.; Goel, R.K.; Gawande, D.Y.; Priynka, P.; Gloriozova, T.A.; Dmitriev, A.V.; Ivanov, S.M.; Rudik, A.V.; Konova, V.I.; Pogodin, P.V. Chemo- and bioinformatics resources for in silico drug discovery from medicinal plants beyond their traditional use: A critical review. Nat. Prod. Rep. 2014, 31, 1585–1611. [Google Scholar] [CrossRef]
- Goel, R.K.; Poroikov, V.; Gawande, D.; Lagunin, A.; Randhawa, P.; Mishra, A. Revealing medicinal plants useful for comprehensive management of epilepsy and associated co-morbidities through in silico mining of their phytochemical diversity. Planta Med. 2015, 81, 495–506. [Google Scholar]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Naturally occurring plant isoquinoline N-oxide alkaloids: Their pharmacological and SAR activities. Phytomedicine 2015, 22, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Gawande, D.Y.; Druzhilovsky, D.; Gupta, R.C.; Poroikov, V.; Goel, R.K. Anticonvulsant activity and acute neurotoxic profile of Achyranthes aspera Linn. J. Ethnopharmacol. 2017, 202, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Dzhemileva, L.; Gloriozova, T.; D’yakonov, V. Natural and synthetic drugs used for the treatment of the dementia. Biochem. Biophys. Res. Commun. 2020, 524, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.; Povydysh, M.; Ivkin, D.; Luzhanin, V.; Krasnova, M. Antihypoxic action of Panax japonicus, Tribulus terrestris and Dioscorea deltoidea cell cultures: In silico and animal studies. Mol. Inform. 2020, 39, 2000093. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Ermolenko, E.; Savidov, N.; Gloriozova, T.A.; Poroikov, V.V. Antiprotozoal and antitumor activity of natural polycyclic endoperoxides: Origin, structures, and biological activity. Molecules 2021, 19, 686. [Google Scholar] [CrossRef]
- Qureshi, R.; Picon-Ruiz, M.; Aurrekoetxea-Rodriguez, I.; Kesmodel, S.; del Mar Vivanco, M.; Slinger, J.M. The major pre- and postmenopausal estrogens play opposing roles in obesity-driven mammary inflammation and breast cancer development. Cell Metab. 2020, 31, 1154–1172. [Google Scholar] [CrossRef]
- Ahmed, S.; Owen, C.P.; James, K.; Sampson, L.; Patel, C.K. Review of estrone sulfatase and its inhibitors—An important new target against hormone dependent breast cancer. Curr. Med. Chem. 2002, 9, 263–273. [Google Scholar] [CrossRef]
- Neilson, H.K.; Friedenreich, C.M.; Brockton, N.T.; Millikan, R.C. Physical activity and postmenopausal breast cancer: Proposed biologic mechanisms and areas for future research. Cancer Epidemiol. Biomark. Prev. 2009, 18, 11–27. [Google Scholar] [CrossRef]
- Bassol, S.; Garza-Flores, J. Review of ovulation return upon discontinuation of once-a-month injectable contraceptives. Contraception 1994, 49, 441–453. [Google Scholar] [CrossRef]
- McCarthy, M.M. Estradiol, and the developing brain. Physiol. Rev. 2008, 88, 91–134. [Google Scholar] [CrossRef]
- Soltysik, K.; Czekaj, P. Membrane estrogen receptors—Is it an alternative way of estrogen action? J. Physiol. Pharmacol. 2013, 64, 129–142. [Google Scholar]
- Mauvais-Jarvis, F.; Klein, S.L.; Levin, E.R. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology 2020, 161, 127. [Google Scholar] [CrossRef]
- Lappano, R.; Rosano, C.; De Marco, P.; De Francesco, E.M.; Pezzi, V.; Maggiolini, M. Estriol acts as a GPR30 antagonist in estrogen receptor-negative breast cancer cells. Mol. Cell. Endocrinol. 2010, 320, 162–170. [Google Scholar] [CrossRef]
- Ali, E.S.; Mangold, C.; Peiris, A.N. Estriol: Emerging clinical benefits. Menopause 2017, 24, 1081–1085. [Google Scholar] [CrossRef]
- Li, M.; Scott, R.Y. A review on structural elucidation of metabolites of environmental steroid hormones via liquid chromatography–mass spectrometry. Trends Anal. Chem. 2018, 109, 142–153. [Google Scholar]
- Brandán, S.A. Structural and vibrational studies of equilenin, equilin and estrone steroids. Biointerface Res. Appl. Chem. 2019, 9, 4502–4516. [Google Scholar]
- Bhavnani, B.R.; Stanczyk, F.Z. Pharmacology of conjugated equine estrogens: Efficacy, safety and mechanism of action. J. Steroid Biochem. Mol. Biol. 2014, 142, 16–29. [Google Scholar] [CrossRef]
- Ruder, H.J.; Loriaux, L.; Lipsett, M.B. Lipsett, Estrone sulfate: Production rate and metabolism in man. J. Clin. Investig. 1972, 51, 1020–1033. [Google Scholar] [CrossRef]
- Banjare, L.; Jain, A.K.; Thareja, A. Dual aromatase-sulphatase inhibitors (DASIs) for the treatment of hormone dependent breast cancer. Mini Rev. Med. Chem. 2021, 21, 10. [Google Scholar] [CrossRef]
- Blackwell, L.F.; Cooke, D.G.; Brown, S. The use of estrone-3-glucuronide and pregnanediol-3-glucuronide excretion rates to navigate the continuum of ovarian activity. Front. Public Health 2018, 6, 153. [Google Scholar] [CrossRef]
- Katayama, S.; Fishman, J. 2-Hydroxyestrone suppresses and 2-methoxyestrone augments the preovulatory prolactin surge in the cycling rat. Endocrinology 1982, 110, 1448–1450. [Google Scholar] [CrossRef]
- Gupta, M.; McDougal, A.; Safe, S. Estrogenic and antiestrogenic activities of 16α- and 2-hydroxy metabolites of 17β-estradiol in MCF-7 and T47D human breast cancer cells. J. Steroid Biochem. Mol. Biol. 1998, 67, 413–419. [Google Scholar] [CrossRef]
- Bradlow, H.L.; Telang, N.T.; Sepkovic, D.W.; Osborne, M.P. 2-Hydroxyestrone: The good estrogen. J. Endocrinol. 1996, 150, S259–S265. [Google Scholar]
- Williams, J.G.; Longcope, C.; Williams, K.I.H. 4-Hydroxyestrone: A new metabolite of estradiol-17β from humans. Steroids 1974, 24, 687–701. [Google Scholar] [CrossRef]
- Emons, G.; Hoppen, H.O.; Ball, P.; Knuppen, R. 4-Hydroxyestrone, isolation and identification in human urine. Steroids 1980, 36, 73–79. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, A.J.; Kang, K.S. 4-Hydroxyestrone, an endogenous estrogen metabolite. Can strongly protect neuronal cells against oxidative damage. Sci. Rep. 2020, 10, 7283. [Google Scholar] [CrossRef]
- Mueck, A.O.; Seeger, H.; Lippert, T.H. Estradiol metabolism and malignant disease. Maturitas 2002, 43, 1–10. [Google Scholar] [CrossRef]
- Anh, N.H.; Long, N.P.; Kim, S.J.; Min, J.E. Steroidomics for the prevention, assessment, and management of cancers: A systematic review and functional analysis. Metabolites 2019, 9, 199. [Google Scholar] [CrossRef]
- Seeger, H.; Deuringer, F.-U.; Wallwiener, D.; Mueck, A.O. Breast cancer risk during HRT: Influence of estradiol metabolites on breast cancer and endothelial cell proliferation. Maturitas 2004, 49, 235–240. [Google Scholar] [CrossRef]
- Fuhrman, B.J.; Schairer, C.; Gail, M.H.; Boyd-Morin, J. Estrogen metabolism and risk of breast cancer in postmenopausal women. J. Nat. Cancer Inst. 2012, 104, 326–339. [Google Scholar] [CrossRef]
- Fishman, J.; Cox, R.I.; Gallagher, T.F. 2-Hydroxyestrone: A new metabolite of estradiol in man. Archiv. Biochem. Biophys. 1960, 90, 318–319. [Google Scholar] [CrossRef]
- Taylor, H.C. The present status of gynecologic endocrine therapy. Bull. N. Y. Acad. Med. 1938, 14, 608–634. [Google Scholar] [PubMed]
- Marrian, G.F. The conjugated estrogens. Cold Spring Harb. Symp. Quant. Biol. 1937, 5, 16–24. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Savidov, N.; Poroikov, V.V.; Gloriozova, T.A.; Imbs, A.B. Naturally occurring aromatic steroids and their biological activities. Appl. Microbiol. Biotechnol. 2018, 102, 4663–4674. [Google Scholar] [CrossRef]
- De Riccardis, F.; Minale, L.; Riccio, R.; Giovannitti, B.; Iorizzi, M.; Debitus, C. Phosphated and sulfated marine polyhydroxylated steroids fromthe starfish Tremaster novaecaledoniae. Gazz. Chim. Ital. 1993, 123, 79–86. [Google Scholar]
- Dembitsky, V.M.; Gloriozova, T.A.; Savidov, N. Steroid phosphate esters and phosphonosteroids and their biological activities. Appl. Microbiol. Biotechnol. 2018, 102, 7679–7692. [Google Scholar] [CrossRef]
- Delrio, G.; Botte, V. Testosterone 17-phosphate and 19-nortestosterone 17-phosphate as substrate for rabbit prostate phosphatases. Biochim. Biophys. Acta 1970, 218, 327–332. [Google Scholar] [CrossRef]
- Kokado, A.; Tsuji, A.; Maeda, M. Chemiluminescence assay of alkaline phosphatase using cortisol-21-phosphate as substrate and its application to enzyme immunoassays. Anal. Chim. Acta 1997, 337, 335–340. [Google Scholar] [CrossRef]
- Ellam, T.J.; Chico, T.J. Phosphate: The new cholesterol? The role of the phosphate axis in non-uremic vascular disease. Atherosclerosis 2012, 220, 310–318. [Google Scholar] [CrossRef]
- Davis, S.C.; Szoka, F.C., Jr. Cholesterol phosphate derivatives: Synthesis and incorporation into a phosphatase and calcium-sensitive triggered release liposome. Bioconjug. Chem. 1998, 9, 783–792. [Google Scholar] [CrossRef]
- Sachs-Barrable, K.; Darlington, J.W.; Wasan, K.M. The effect of two novel cholesterol-lowering agents, disodium ascorbyl phytostanol phosphate and nanostructured aluminosilicate on the expression and activity of P-glycoprotein within Caco-2 cells. Lipids Health Dis. 2014, 13, 153–163. [Google Scholar] [CrossRef]
- Kutney, J.P.; Pritchard, H.P.; Lukic, T. Novel Compounds and Compositions Comprising Sterols and/or Stanols and Cholesterol Biosynthesis Inhibitors and Use Thereof in Treating or Preventing a Variety of Diseases and Conditions. Europe Patent EP1644399A2, 7 September 2003. [Google Scholar]
- Gunnarsson, P.O.; Norlén, B.J. Clinical pharmacology of polyestradiol phosphate. Prostate 1988, 13, 299–304. [Google Scholar] [CrossRef]
- Vil, V.; Gloriozova, T.A.; Terentev, A.O.; Zhukova, N.V.; Dembitsky, V.M. Highly oxygenated isoprenoid lipids derived from terrestrial and aquatic sources: Origin, structures, and biological activities. Vietnam J. Chem. 2019, 57, 1–15. [Google Scholar] [CrossRef]
- Vil, V.A.; Terentev, A.O.; Savidov, N.; Gloriozova, T.A.; Poroikov, V.V.; Pounina, T.A.; Dembitsky, V.M. Hydroperoxy steroids and triterpenoids derived from plant and fungi: Origin, structures and biological activities. J. Steroid Biochem. Mol. Biol. 2019, 190, 76–87. [Google Scholar] [CrossRef]
- Savidov, N.; Gloriozova, T.A.; Poroikov, V.V.; Dembitsky, V.M. Highly oxygenated isoprenoid lipids derived from fungi and fungal endophytes: Origin and biological activities. Steroids 2018, 140, 114–124. [Google Scholar] [CrossRef]
- Vil, V.A.; Gloriozova, T.A.; Terentev, A.O.; Savidov, N.; Dembitsky, V.M. Hydroperoxides derived from marine sources: Origin and biological activities. Appl. Microbiol. Biotechnol. 2019, 103, 1627–1642. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Naturally occurring marine α,β-epoxy steroids: Origin and biological activities. Vietnam J. Chem. 2018, 56, 409–433. [Google Scholar] [CrossRef]
- Rashidi, B.; Hoseini, Z.; Sahebkar, A.; Mirzaei, H. Anti-atherosclerotic effects of vitamins D and E in suppression of atherogenesis. J. Cell. Physiol. 2017, 232, 2968–2976. [Google Scholar] [CrossRef]
- Kutner, A.; Brown, G. Vitamins D: Relationship between structure and biological activity. Int. J. Mol. Sci. 2018, 19, 2119. [Google Scholar] [CrossRef]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Renal. Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef]
- Perez-Lopez, F.R. Vitamin D: The secosteroid hormone and human reproduction. Gynecolog. Endocrinol. 2007, 23, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Ermolenko, E.V.; Imbs, A.B.; Gloriozova, T.A.; Poroikov, V.V.; Sikorskaya, T.V.; Dembitsky, V.M. Chemical diversity of soft coral steroids and their pharmacological activities. Mar. Drugs 2020, 20, 613. [Google Scholar] [CrossRef] [PubMed]
- Grishko, V.V.; Galaiko, N.V. Structural diversity, natural sources and pharmacological potential of naturally occurring A-seco-triterpenoids studies. Nat. Prod. Chem. 2016, 51, 51–149. [Google Scholar]
- Sica, D.; Musumeci, D. Secosteroids of marine origin. Steroids 2004, 69, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Luo, X.D.; Ma, Y.B.; Liu, Y.K.; Wu, D.G.; Zhao, B.; Lu, Y.; Zheng, Q.T. Two novel secoergosterols from the fungus Tylopilus plumbeoviolaceus. J. Nat. Prod. 2000, 63, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Z.; Chen, H.P.; Huang, Y.; Zhang, S.B.; Li, Z.H.; Feng, T.; Liu, J.K. Bioactive polyketides and 8,14-seco-ergosterol from fruiting bodies of the ascomycete Daldinia childiae. Phytochemistry 2017, 142, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.M.; Li, X.M.; Meng, L.; Li, C.S.; Huang, C.G.; Wang, B.G. 7-Nor-ergosterolide, a pentalactone-containing norsteroid and related steroids from the marine-derived endophytic Aspergillus ochraceus EN-31. J. Nat. Prod. 2010, 73, 1780–1784. [Google Scholar] [CrossRef]
- Onodera, H.; Ichimura, M.; Baba, K.; Agatsuma, T.; Sasho, S.; Suzuki, M.; Iwamoto, S.; Kakita, S. PCT Int. Appl. WO 2009096445, Nerve Trunk Cell Propagation Accelerator, 06.08.2009; Kyowa Hakko Kirin Co., Ltd.: Tokyo, Japan.
- Kazlauskas, R.; Murphy, P.T.; Ravi, B.N.; Sanders, R.L.; Wells, R.J. Spermidine derivatives and 9,11-secosteroids from a soft coral (Sinularia sp.). Austral. J. Chem. 1982, 35, 69–75. [Google Scholar] [CrossRef]
- Bonini, C.; Cooper, C.B.; Kazlauskas, R.; Wells, R.J.; Djerassi, C. Minor and trace sterols in marine invertebrates. 41. Structure and stereochemistry of naturally occurring 9,11-seco sterols. J. Org. Chem. 1983, 48, 2108–2111. [Google Scholar] [CrossRef]
- Weng, J.R.; Chiu, C.F.; Sheu, J.H. A sterol from soft coral induces apoptosis and autophagy in MCF-7 breast cancer cells. Mar. Drugs 2018, 16, 238. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lai, K.H.; Kumar, S.; Chen, P.J.; Wu, Y.H.; Lai, C.L. 1HNMR-Based Isolation of Anti-Inflammatory 9,11-secosteroids from the octocoral Sinularia leptoclados. Mar. Drugs 2020, 18, 271. [Google Scholar] [CrossRef]
- Hirsch, A.L. Industrial aspects of vitamin D. In Vitamin, D., 3rd ed.; Feldman, D., Pike, J.W., Adams, J.S., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 73–93. [Google Scholar]
- Teichmann, A.; Dutta, P.C.; Staffas, A.; Jagerstäd, M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: Effect of UV radiation. LWT Food Sci. Technol. 2007, 40, 815–822. [Google Scholar] [CrossRef]
- Koyyalamudi, S.R.; Jeong, S.C.; Song, C.H.; Cho, K.Y.; Pang, G. Vitamin D2 formation and bioavailability from Agaricus bisporus button mushrooms treated with ultraviolet irradiation. J. Agric. Food Chem. 2009, 57, 3351–3355. [Google Scholar] [CrossRef]
- Koyyalamudi, S.R.; Jeong, S.C.; Pang, G.; Teal, A.; Biggs, T. Concentration of vitamin D2 in white button mushrooms (Agaricus bisporus) exposed to pulsed UV light. J. Food Comp. Anal. 2011, 24, 976–979. [Google Scholar] [CrossRef]
- Phillips, K.M.; Ruggio, D.M.; Horst, R.L.; Minor, B.; Simon, R.R.; Feeney, M.J.; Byrdwell, W.C.; Haytowitz, D.B. Vitamin D and sterol composition of 10 types of mushrooms from retail suppliers in the United States. J. Agric. Food Chem. 2011, 59, 7841–7853. [Google Scholar] [CrossRef]
- Jasinghe, V.J.; Perera, C.O. Ultraviolet irradiation: The generator of vitamin D2 in edible mushrooms. Food Chem. 2006, 95, 638–643. [Google Scholar] [CrossRef]
- Roberts, J.S.; Teichert, A.; McHugh, T.H. Vitamin D2 formation from post-harvest UV-B treatment of mushrooms (Agaricus bisporus) and retention during storage. J. Agric. Food Chem. 2008, 56, 4541–4544. [Google Scholar] [CrossRef]
- Shen, Y.C.; Cheng, Y.B.; Kobayashi, J.; Kubota, T.; Takahashi, Y.; Mikami, Y.; Ito, J.; Lin, Y.S. Nitrogen-containing verticillene diterpenoids from the Taiwanese soft coral Cespitularia taeniata. J. Nat. Prod. 2007, 70, 1961–1965. [Google Scholar] [CrossRef]
- Lakshmi, V.; Kumar, R. Metabolites from Sinularia species. Nat. Prod. Res. 2009, 23, 801–850. [Google Scholar] [CrossRef]
- Kulda, V. Vitamin D metabolism. Vnitr Lek. 2012, 58, 400–404. [Google Scholar]
- Windaus, A.; Schenck, F.R.; Werder, F.V. The anti-rachitically active irradiation product from 7-dehydro-cholesterol. Hoppe-Seyler’s Zeitsch. Physiol. Chem. 1936, 241, 100–103. [Google Scholar] [CrossRef]
- Wanga, T.; Bengtsson, G.; Kärnefeltd, I.; Björn, L.O. Provitamins and vitamins D2 and D3 in Cladina spp. over a latitudinal gradient: Possible correlation with UV levels. J. Photochem. Photobiol. 2001, 62B, 118–122. [Google Scholar] [CrossRef]
- Horst, R.L.; Reinhardt, T.A.; Russell, J.R.; Napoli, J.L. The isolation and identification of vitamin D2 and vitamin D3 from Medicago sativa (Alfalfa plant). Arch. Biochem. Biophys. 1984, 231, 67–71. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Model List of Essential Medicines: 21st List 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Trump, D.L.; Aragon-Ching, J.B. Vitamin D in prostate cancer. Asian J. Androl. 2018, 20, 244–252. [Google Scholar] [CrossRef]
- Laaksi, I. Vitamins, infectious and chronic disease during adulthood and aging Vitamin D and respiratory infection in adults. Proceed. Nutr. Soc. 2012, 71, 90–97. [Google Scholar] [CrossRef]
- Saikia, S.; Kolita, B.; Dutta, P.P.; Dutta, D.J.; Neipihoi, S.; Nath, S. Marine steroids as potential anticancer drug candidates: In silico investigation in search of inhibitors of Bcl-2 and CDK-4/Cyclin D1. Steroids 2015, 102, 7–16. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Anticancer activity of natural and synthetic acetylenic lipids. Lipids 2006, 41, 883–924. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Wang, H. Cytotoxic natural products from marine sponge-derived microorganisms. Mar. Drugs 2017, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Mioso, R.; Marante, F.J.T.; Bezerra, R.D.S.; Borges, F.V.P.; Santos, B.V.; Laguna, I.H.B.D. Cytotoxic compounds derived from marine sponges, A review (2010–2012). Molecules 2017, 22, 208. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Rezanka, T.; Srebnik, M. Lipid compounds of freshwater sponges: Family Spongillidae, class Demospongiae. Chem. Phys. Lipids 2003, 123, 117–155. [Google Scholar] [CrossRef]
- Xu, S.; Liao, X.; Du, B.; Zhou, X.; Huang, Q.; Wu, C. A series of new 5,6-epoxysterols from a Chinese sponge Ircinia aruensis. Steroids 2008, 73, 568–573. [Google Scholar]
- Li, T.; Wang, N.; Zhang, T.; Zhang, B.; Sajeevan, T.P.; Valsamma, J.; Armstrong, L.; He, S.; Yan, X.; Naman, C.B. A systematic review of recently reported marine derived natural product kinase inhibitors. Mar. Drugs 2019, 17, 493. [Google Scholar] [CrossRef]
- Zhang, H.J.; Yi, Y.H.; Yang, F.; Chen, W.S.; Lin, H.W. Sesterterpenes and a new sterol from the marine sponge Phyllospongia foliascens. Molecules 2010, 15, 834–841. [Google Scholar]
- Afiyatullov, S.S.; Kalinovsky, A.I.; Antonov, A.S.; Ponomarenko, L.P. Isolation and structures of erylosides from the Carribean sponge Erylus goffrilleri. J. Nat. Prod. 2007, 70, 1871–1877. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Krishna Murthy, M.V.R.; Gowri, P.M. Novel epoxy steroids from the Indian ocean soft coral Sarcophyton crassocaule. J. Nat. Prod. 2000, 63, 112–118. [Google Scholar]
- Funel, C.; Berrué, F.; Roussakis, C.; Fernandez Rodriguez, R.; Amade, P. New cytotoxic steroids from the Indian ocean sponge Axinella cf. bidderi. J. Nat. Prod. 2004, 67, 491–494. [Google Scholar]
- Dembitsky, V.M. Bioactive fungal endoperoxides. Med. Mycol. 2015, 1, 1–7. [Google Scholar]
- Dembitsky, V.M. Astonishing diversity of natural peroxides as potential therapeutic agents. J. Mol. Genet. Med. 2015, 9, 1000163. [Google Scholar]
- Dembitsky, V.M. Bioactive peroxides as potential therapeutic agents. Eur. J. Med. Chem. 2008, 43, 223–251. [Google Scholar]
- Kyasa, S.K. New Methods for Synthesis of Organic Peroxides and Application of Peroxide Electrophiles to Synthesis of Functionalized Ethers. Ph.D. Thesis, University of Nebraska-Lincoln, Lincoln, NE, USA, 2005. [Google Scholar]
- Klussmann, M. Alkenyl and aryl peroxides. Chemistry 2018, 24, 4480–4496. [Google Scholar]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Natural peroxy anticancer agents. Mini-Rev. Med. Chem. 2007, 7, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.; Shkrob, I.; Hanus, L.O. Ascaridole and related peroxides from the genus Chenopodium. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2008, 152, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Z.; Liu, J.K. Peroxy natural products. Nat. Prod. Bioprospect. 2013, 3, 161–206. [Google Scholar] [CrossRef]
- Thao, N.P.; Cuong, N.X.; Luyen, B.T.T.; Nam, N.H. Steroidal constituents from the starfish Astropecten polyacanthus and their anticancer effects. Chem. Pharm. Bull. 2013, 61, 1044–1051. [Google Scholar] [CrossRef]
- Seo, Y.W.; Rho, J.R.; Cho, K.W.; Sim, C.J.; Shin, J.H. Isolation of epidioxysteroids from a sponge of the genus Tethya. Bull. Korean Chem. Soc. 1997, 18, 631–635. [Google Scholar]
- Gunatilaka, A.A.L.; Gopichand, Y.; Schmitz, F.J.; Djerassi, C. Minor and trace sterols in marine invertebrates. 26. Isolation and structure elucidation of nine new 5,8-epidoxy sterols from four marine organisms. J. Org. Chem. 1981, 46, 3860–3866. [Google Scholar] [CrossRef]
- Gauvin, A.; Smadja, J.; Aknin, M.; Faure, R.; Gaydou, E.M. Isolation of bioactive 5α,8α-epidioxy sterols from the marine sponge Luffariella cf. variabilis. Can. J. Chem. 2000, 78, 986–992. [Google Scholar] [CrossRef]
- Sheikh, Y.M.; Djerassi, C. Steroids from sponges. Tetrahedron 1974, 30, 4095–4103. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, T.; Xiang, X.; Gu, Q. Sterol composition in field-grown and cultured mycelia of Inonotus obliquus. Yaoxue Xuebao 2007, 42, 750–756. [Google Scholar]
- Zhang, Y.; Pei, L.; Gao, L.; Huang, Q.; Qi, J. A neuritogenic compound from Tremella fuciformis. Zhongguo Zhong Yao Za Zhi 2011, 36, 2358–2360. [Google Scholar]
- Shi, X.W.; Li, X.J.; Gao, J.M.; Zhang, X.C. Fasciculols H and I, two lanostane derivatives from Chinese mushroom Naematoloma fasciculare. Chem. Biodivers. 2011, 8, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Yaoita, Y.; Amemiya, K.; Ohnuma, H.; Furumura, K.; Masaki, A.; Matasuki, T.; Kikuchi, M. Sterol constituents from five edible mushrooms. Chem. Pharm. Bull. 1998, 46, 944–950. [Google Scholar] [CrossRef]
- Yue, J.M.; Chen, C.N.; Lin, Z.W.; Sun, H.D. Sterols from the fungus Lactarium volemus. Phytochemistry 2001, 56, 801–806. [Google Scholar] [CrossRef]
- Zang, M.; Ying, J.Z. Economic Fungi in the South West of China; Scientific Press: Beijing, China, 1994. [Google Scholar]
- Greca, M.D.; Fiorentino, A.; Molinaro, A.; Monaco, P.; Previtera, L. Hydroperoxysterols in Arum italicum. Nat. Prod. Lett. 1994, 5, 7–14. [Google Scholar] [CrossRef]
- Wu, S.B.; Bao, Q.Y.; Wang, W.X.; Zhao, Y.; Xia, G. Cytotoxic triterpenoids and steroids from the bark of Melia azedarach. Planta Med. 2011, 77, 922–928. [Google Scholar] [CrossRef]
- Ponce, M.A.; Ramirez, J.A.; Galagovsky, L.R.; Gros, E.G.; Erra-Balsells, R. A new look into the reaction between ergosterol and singlet oxygen in vitro. Photochem. Photobiol. Sci. 2002, 1, 749–756. [Google Scholar] [CrossRef]
- Bocking, T.; Barrow, K.D.; Netting, A.G.; Chilcott, T.C.; Coster, H.G.L.; Hofer, M. Effects of singlet oxygen on membrane sterols in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 2000, 267, 1607–1618. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Phung, L.K.; Tran, K.Q.; Saiki, I. Cytotoxic cycloartane-type triterpenes from Combretum quadrangulare. Bioorg. Med. Chem. Lett. 1998, 8, 3519–3524. [Google Scholar] [CrossRef]
- Bankota, A.H.; Tezuka, Y.; Tran, K.Q.; Tanaka, K.; Saiki, I.; Kadota, S. Thirteen novel cycloartane-type triterpenes from Combretum quadrangulare. J. Nat. Prod. 2000, 63, 57–64. [Google Scholar] [CrossRef]
- Roy, R.; Raj, K.; Singh, K.; Jash, S.K.; Sarkar, A.; Gorai, D. Combretum quadrangulare (Combretaceae): Phytochemical constituents and biological activity. Indo Amer. J. Pharm. Res. 2014, 4, 3416–3427. [Google Scholar]
- Asai, T.; Hara, N.; Fujimoto, Y. Fatty acid derivatives and dammarane triterpenes from the glandular trichome exudates of Ibicella lutea and Proboscidea louisiana. Phytochemistry 2010, 71, 877–894. [Google Scholar] [CrossRef]
- Lee, I.S.; Oh, S.R.; Ahn, K.S.; Lee, H.K. Semialactone, isofouquierone peroxide and fouquierone, three new dammarane triterpenes from Rhus javanica. Chem. Pharm. Bull. 2001, 49, 1024–1029. [Google Scholar] [CrossRef]
- Abdel Bar, F.M.; Zaghloul, A.M.; Bachawal, S.V.; Sylvester, P.W.; Ahmad, K.F.; El Sayed, K.A. Antiproliferative triterpenes from Melaleuca ericifolia. J. Nat. Prod. 2008, 71, 1787–1790. [Google Scholar] [CrossRef]
- Chiamg, Y.M.; Kuo, Y.H. New peroxy triterpenes from the aerial roots of Ficus macrocarpa. J. Nat. Prod. 2001, 64, 436–439. [Google Scholar] [CrossRef]
- Sikorsky, T.V.; Ermolenko, E.V.; Gloriozova, T.A.; Dembitsky, V.M. Mini Review: Anticancer activity of diterpenoid peroxides. Vietnam J. Chem. 2020, 58, 273–280. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Yaremenko, I.A. Stable and unstable 1,2-dioxolanes: Origin, synthesis, and biological activities. Sci. Synth. Knowl. Updates 2020, 38, 277–321. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Vil, V.A. Medicinal chemistry of stable and unstable 1,2-dioxetanes: Origin, formation, and biological activities. Sci. Synth. Knowl. Updates 2020, 38, 333–381. [Google Scholar] [CrossRef]
- Phillipson, J.D.; Wright, C.W. Antiprotozoal agents from plant sources. Planta Med. 1991, 57, S53–S59. [Google Scholar] [CrossRef] [PubMed]
- Moss, G.P. The nomenclature of steroids. Eur. J. Biochem. 1989, 186, 429–458. [Google Scholar]
- Burger, A. Cyclopropane compounds of biological interest. Prog. Drug Res. 1971, 15, 227–270. [Google Scholar]
- Schoenheimer, R.; Evans, E.A., Jr. The chemistry of the steroids. Ann. Rev. Biochem. 1937, 6, 139–162. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A. Astonishing diversity of carbon-bridged steroids and their biological activities: A brief review. Eur. J. Biotechnol. Biosci. 2018, 6, 6–23. [Google Scholar]
- Jacobs, H.J.C. Photochemistry of conjugated trienes: Vitamin D revisited. Pure Appl. Chem. 1995, 67, 63–70. [Google Scholar] [CrossRef][Green Version]
- Kalaras, M.D. Production of Ergocalciferol (Vitamin D2) and Related Sterols in Mushrooms with Exposure to Pulsed Ultraviolet Light. Ph.D. Thesis, Pennsylvania State University, State College, PA, USA, January 2012. [Google Scholar]
- Mitome, H.; Shirato, N.; Hoshino, A.; Miyaoka, H.; Yamada, Y.; Yamada, Y.; Van Soest, R.W.M. New polyhydroxylated sterols stylisterols A–C and a novel 5, 19-cyclosterol hatomasterol from the Okinawan marine sponge Stylissa sp. Steroids 2005, 70, 63–70. [Google Scholar] [CrossRef]
- Calabro, K.; Kalahroodi, E.L.; Rodrigues, D.; Díaz, C.; Cruz, M.D.L.; Cautain, B.; Laville, R. Poecillastrosides, steroidal saponins from the Mediterranean deep-sea sponge Poecillastra compressa (Bowerbank, 1866). Mar. Drugs 2017, 15, 199. [Google Scholar] [CrossRef]
- Giner, J.-L.; Gunasekera, S.P.; Pomponi, S.A. Sterols of the marine sponge Petrosia weinbergi: Implications for the absolute configurations of the antiviral orthoesterols and weinbersterols. Steroids 1999, 64, 820–824. [Google Scholar] [CrossRef]
- HXinping, H.; Xiaobin, Z.; Liping, D.; Zhiwei, D.; Wenhan, L. Cycloartane triterpenes from marine green alga Cladophora fascicularis. Chin. J. Ocean. Limnol. 2006, 24, 443–448. [Google Scholar] [CrossRef]
- Tung, N.H.; Van Minh, C.; Ha, T.T.; Van Kiem, P.; Huong, H.T. C29-Sterols with a cyclopropane ring at C-25 and 26 from the Vietnamese marine sponge Ianthella sp. and their anticancer properties. Bioorg. Med. Chem. Lett. 2009, 19, 4584–4588. [Google Scholar] [CrossRef]
- Gauvin, A.; Smadja, J.; Aknin, M.; Gaydou, E.M. Cyclopropane-containing sterols in the marine sponge Petrosia spheroïda. Comp. Biochem. Physiol. 1998, 121B, 451–456. [Google Scholar] [CrossRef]
- Abdjul, D.B.; Yamazaki, H.; Takahashi, O.; Kirikoshi, R.; Ukai, K.; Namikoshi, M. Isopetrosynol, a new protein tyrosine phosphatase 1B inhibitor, from the marine sponge Halichondria cf. panicea collected at Iriomote Island. Chem. Pharm. Bull. 2016, 64, 733–736. [Google Scholar] [CrossRef][Green Version]
- Umeyama, A.; Ito, S.; Yoshigaki, A.; Arihara, S. Two new 26,27-cyclosterols from the marine sponge Strongylophora corticate. J. Nat. Prod. 2000, 63, 1540–1542. [Google Scholar] [CrossRef]
- Minale, L.; Riccio, R.; Scalona, O.; Sodano, G.; Fattorusso, E.; Magno, S.; Mayol, L.; Santacroce, C. Metabolism in Porifera. VII. Conversion of [7,7-3H2]-fucosterol into calysterol by the sponge Calyx niceaensis. Experientia 1977, 33, 1550–1552. [Google Scholar] [CrossRef]
- Fatturosso, E.; Magno, S.; Mayol, L.; Santacrove, C.; Sioa, D. Calysterol: A C-29 cyclopropene-containing marine sterol from the sponge Calyx nicaensis. Tetrahedron 1975, 31, 1715–1716. [Google Scholar] [CrossRef]
- O’Connor, J.M.; Pu, L.; Chadha, R.K. Metallacycle annelation: Reaction of a metallacycle alpha-substituent and a vinylidene ligand to give a bicyclic metallalactone complex. J. Am. Chem. Soc. 1990, 112, 9627–9628. [Google Scholar] [CrossRef]
- Li, L.N.; Li, H.T.; Lang, R.W.; Itoh, T.; Sica, D.; Djerassi, C. Minor and trace sterols in marine invertebrates. 31. Isolation and structure elucidation of 23H-isocalysterol, a naturally occurring cyclopropene. Some comparative observations on the course of hydrogenolytic ring opening of steroidal cyclopropenes and cyclopropanes. J. Am. Chem. Soc. 1982, 104, 6726–6732. [Google Scholar]
- Gunasekera, S.P.; Cranick, S.; Pomponi, S.A. Pomponi, New sterol ester from a deep-water marine sponge, Xestospongia sp. J. Nat. Prod. 1991, 54, 1119–1122. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Natural neo acids and neo alkanes: Their analogs and derivatives. Lipids 2006, 41, 309–340. [Google Scholar] [CrossRef]
- Bisel, P.; Al-Momani, L.; Müller, M. The tert-butyl group in chemistry and biology. Org. Biomol. Chem. 2008, 6, 2655–2665. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Alkoxylipids of the Organic World. Chemistry and Biology. Ph.D. Thesis, Lomonosov University of Fine Chemical Technology, Moscow, Russia, 1996. [Google Scholar]
- Akihisa, A.; Inada, Y.; Ghosh, P. Compositions of triterpene alcohols of seeds and mature plants of family Cucurbitaceae. J. Am. Oil Chem. Soc. 1988, 65, 607–610. [Google Scholar] [CrossRef]
- Akihisa, T.; Tamura, T.; Matsumoto, T. 24-Methylene-25-methyllathosterol: A sterol from Sicyos angulatus. Phytochemistry 1987, 26, 575–577. [Google Scholar] [CrossRef]
- Rahimi, R.; Shams-Ardekani, M.R.; Abdollahi, M. A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J. Gastroenterol. 2010, 16, 4504–4514. [Google Scholar] [CrossRef]
- Bojić, M.; Maleš, Ž.; Antolić, A.; Babić, I.; Tomičić, M. Antithrombotic activity of flavonoids and polyphenols rich plant species. Acta Pharma. 2019, 69, 483–495. [Google Scholar] [CrossRef]
- Sestili, P.; Ismail, T.; Calcabrini, C.; Guescini, M.; Catanzaro, E.; Turrini, E. The potential effects of Ocimum basilicum on health: A review of pharmacological and toxicological studies. Expert Opin. Drug Metab. Toxicol. 2018, 14, 679–692. [Google Scholar] [CrossRef]
- Devi, P.U. Radioprotective, anticarcinogenic and antioxidant properties of the Indian holy basil, Ocimum sanctum (Tulasi). Indian J. Exp. Biol. 2001, 39, 185–190. [Google Scholar]
- Ch, M.A.; Naz, S.B.; Sharif, A.; Akram, M.; Saeed, M.A. Biological and pharmacological properties of the sweet basil (Ocimum basilicum). Br. J. Pharm Res. 2015, 7, 330–339. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Aslam, H.; Ali, S.T. Two new triterpenoids and a steroidal glycoside from the aerial parts of Ocimum basilicum. Chem. Pharm Bull. 2007, 55, 516–519. [Google Scholar] [CrossRef]
- Li, Y. Stereo Chemical Studies on the Metabolism of Sterols by Saccharomyces Cerevisiae Strain GL7. Ph.D. Thesis, Texas Tech University, Lubbock, TX, USA, 1996. [Google Scholar]
- Liu, J.; Nes, W.D. Steroidal triterpenes: Design of substrate-based inhibitors of ergosterol and sitosterol synthesis. Molecules 2009, 14, 4690–4706. [Google Scholar] [CrossRef]
- Ishibashi, M.; Yamagishi, E.; Kobayashi, J. Topsentinols A-J, new sterols with highly branched side chains from marine sponge Topsentia sp. Chem. Pharm Bull. 1997, 45, 1435–1438. [Google Scholar] [CrossRef]
- Nojo, R.; Echigo, S.; Hara, N.; Fujimoto, Y. C-24 stereochemistry of marine sterols: (22E)-25,28-dimethylstigmasta-5,22,28-trien-3β-ol and 25,28-dimethylstigmasta-5,28-dien-3β-ol. Nat. Prod. Commun. 2014, 9, 1699–1704. [Google Scholar] [CrossRef]
- Li, X.; Djerassi, C. Minor and trace sterols in marine invertebrates 40. Structure and synthesis of axinyssasterol, 25-methylfucosterol and 24-ethyl-24-methylcholesterol—Novel sponge sterols with highly branched side chains. Tetrahedron Lett. 1983, 24, 665–668. [Google Scholar] [CrossRef]
- Shubina, L.K.; Makar’eva, T.N.; Stonik, V.A. Steroidal compounds of marine sponges. III. 24-Ethyl-25-methylcholesta-5,22-dien-3b-ol—A novel marine sterol from the sponge Halichondria sp. Khim. Prir. Soedin. 1984, 4, 464–467. [Google Scholar]
- Shubina, L.K.; Makar’eva, T.N.; Stonik, V.A. Steroidal compounds of marine sponges. VI. Sterols and their derivatives from Trachyopsis aplysinoides. Khim. Prir. Soedin. 1985, 5, 715–716. [Google Scholar]
- Fusetani, N.; Matsunaga, S.; Konosu, S. Bioactive marine metabolites. II. Halistanol sulfate, an antimicrobial novel steroid sulfate from the marine sponge Halichondria cf. moorei Bergquist. Tetrahedron Lett. 1981, 22, 1985–1988. [Google Scholar] [CrossRef]
- Gulavita, N.K.; Wright, A.E.; Kelly-Borges, M.; Longley, R.E. Eryloside E from an Atlantic sponge Erylus goffrilleri. Tetrahedron Lett. 1994, 35, 4299–4302. [Google Scholar] [CrossRef]
- Ebada, S.S.; Lin, W.H.; Proksch, P. Bioactive sesterterpenes and triterpenes from marine sponges: Occurrence and pharmacological significance. Marine Drugs 2010, 8, 313–346. [Google Scholar] [CrossRef]
- Morrison-Gardiner, S. Dominant fungi from Australian reefs. Fungal Divers. 2002, 9, 105–121. [Google Scholar]
- Jones, E.B.G.; Sakayaroj, J.; Suetrong, S.; Somrithipol, S.; Pang, K.L. Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Divers. 2009, 35, 1–187. [Google Scholar]
- El-Bondkly, E.A.M.; El-Bondkly, A.A.M.; El-Bondkly, A.A.M. Marine endophytic fungal metabolites: A whole new world of pharmaceutical therapy exploration. Heliyon 2021, 7, e06362. [Google Scholar] [CrossRef]
- Wang, S.K.; Dai, C.F.; Duh, C.Y. Cytotoxic pregnane steroids from the Formosan Soft Coral Stereonephthya crystalliana. J. Nat. Prod. 2006, 69, 103–106. [Google Scholar] [CrossRef]
- Murillo-Alvarez, J.; Encarnacion-Dimayuga, R. New bioactive pregnadiene-derived glycosides from the gulf of California gorgonian Muricea cf. austera. Pharm. Biol. 2003, 41, 531–535. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Rao, V.L.; Sastry, V.G. A new spiroketal steroid from Gorgonella umbraculum. Nat. Prod. Res. 2003, 17, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Rho, J.R.; Cho, K.W.; Shin, J. Isolation of new steroidal hemiacetals from the gorgonian Euplexaura anastomosans. J. Nat. Prod. 1996, 59, 1196–1199. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chang, C.W.; Sheu, J.H. Bioactive steroids from the Formosan soft coral Umbellulifera petasites. Mar. Drugs 2016, 14, 180. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2005, 22, 15–61. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Huong, P.T.M.; Thanh, N.V.; Cuong, N.X. Steroid constituents from the soft coral Sinularia nanolobata. Chem. Pharm. Bull. 2016, 64, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Gebreyesus, T.; Stoilov, I.; Luo, F.T.; Djerassi, C. Minor and trace sterols in marine invertebrates 55. The isolation, structure elucidation and synthesis of ergosta-5,24(28),25-trien-3-ol. Steroids 1985, 45, 447–452. [Google Scholar] [CrossRef]
- Chao, C.H.; Chou, K.J.; Huang, C.Y.; Wen, Z.H.; Hsu, C.H. Steroids from the soft coral Sinularia crassa. Mar. Drugs 2012, 10, 439–450. [Google Scholar] [CrossRef]
- Mariottini, G.L. The role of cnidaria in drug discovery. In The Cnidaria, Past, Present and Future; Goredo, S., Dubinsky, Z., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Ilhan, H.A.; Pulat, C.C. Cytotoxic and antitumor compounds from marine invertebrates. Encycl. Mar. Biotechnol. 2020, 4, 2529–2584. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Huong, P.T.M.; Van Thanh, N.; Chia, N.T.P.C. Cytotoxic steroids from the Vietnamese soft coral Sinularia conferta. Chem. Pharm. Bull. 2017, 65, 300–305. [Google Scholar] [CrossRef]
- Cuíng, N.X.; Nhiem, N.X.; Thanh, N.V. Review of chemistry and biological activity studies some marine species in Vietnam in the period 2013–2017. Vietnam J. Chem. 2018, 56, 1–19. [Google Scholar]
- Cardoso-Martínez, F.; de la Rosa, J.M.; Díaz-Marrero, A.R.; Darias, J. Oxysterols from an octocoral of the genus Gorgonia from the eastern Pacific of Panama. J. RSC Adv. 2013, 6, 38579–38591. [Google Scholar] [CrossRef]
- Yan, X.; Liu, J.; Leng, X.; Ouyang, H. Chemical diversity and biological activity of secondary metabolites from soft coral genus Sinularia since 2013. Mar. Drugs 2021, 19, 335. [Google Scholar] [CrossRef]
- Radhika, P. Chemical constituents and biological activities of the soft corals of genus Cladiella: A review. Biochem. Syst. Ecol. 2006, 34, 781–789. [Google Scholar] [CrossRef]
- Zubair, M.S.; Al-Footy, K.O.; Ayyad, S.-E.N.; Al-Lihaibi, S.S.; Alarif, W.M. A review of steroids from Sarcophyton species. Nat. Prod. Res. 2016, 30, 869–879. [Google Scholar] [CrossRef]
- Amir, F.; Koay, Y.C.; Yam, W.S. Chemical constituents, and biological properties of the marine soft coral Nephthea: A review (Part 1). Trop. J. Pharm. Res. 2012, 11, 485–498. [Google Scholar]
- Amir, F.; Koay, Y.C.; Yam, W.S. Chemical constituents, and biological properties of the marine soft coral Nephthea: A review (Part 2). Trop. J. Pharm. Res. 2012, 11, 499–517. [Google Scholar]
- Baulieu, E.E. Steroid hormones in the brain: Several mechanisms? Steroid hormone regulation of the brain. In Proceedings of the International Symposium Held at the Wenner-Gren Center, Stockholm, Sweden, 27–28 October 1980; pp. 3–14. [Google Scholar]
- Vest, R.S.; Pike, C.J. Gender, sex steroid hormones, and Alzheimer’s disease. Horm Behav. 2013, 63, 301–307. [Google Scholar] [CrossRef]
- Lenz, K.M.; McCarthy, M.M. Organized for sex–steroid hormones and the developing hypothalamus. Eur. J. Neurosci. 2010, 32, 2096–2104. [Google Scholar] [CrossRef]
- Tchernof, A.; Després, J.P. Sex steroid hormones, sex hormone-binding globulin, and obesity in men and women. Horm. Metab. Res. 2000, 32, 526–536. [Google Scholar] [CrossRef]
- Luine, V.N. Sex steroids and cognitive function. J. Neuroendocrinol. 2008, 20, 866–872. [Google Scholar] [CrossRef]
- Rubinow, K.B. An intracrine view of sex steroids, immunity, and metabolic regulation. Mol. Metab. 2018, 15, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Owens, S.J.; Murphy, C.E.; Purves-Tyson, T.D.; Weickert, T.W.; Weickert, S.C. Considering the role of adolescent sex steroids in schizophrenia. J. Neuroendocrinol. 2018, 30, e12538. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dong, G.; Sheng, C. Structural simplification of natural products. Chem. Rev. 2019, 119, 4180–4220. [Google Scholar] [CrossRef] [PubMed]
- Nahar, L.; Sarker, S.D. A review on steroid dimers: 2011–2019. Steroids 2020, 164, 108736. [Google Scholar] [CrossRef]
- Sahu, P.; Gidwani, B.; Dhongade, H.J. Pharmacological activities of dehydroepiandrosterone: A review. Steroids 2020, 153, 108507. [Google Scholar] [CrossRef]
- Biellmann, J.-F. Enantiomeric steroids: synthesis, physical, and biological properties. Chem. Rev. 2003, 103, 2019–2034. [Google Scholar] [CrossRef]
- Skoda-Földes, R.; Kollár, L. Transition-metal-catalyzed reactions in steroid synthesis. Chem. Rev. 2003, 103, 4095–4130. [Google Scholar] [CrossRef]
- Albano, G.D.; Amico, F.; Cocimano, G.; Liberto, A.; Maglietta, F. Adverse effects of anabolic-androgenic steroids: A literature review. Healthcare 2021, 9, 97. [Google Scholar] [CrossRef]
- Pope, H.G., Jr.; Kanayama, G.; Hudson, J.I.; Kaufman, M.J. Anabolic-androgenic steroids, violence, and crime: Two cases and literature review. Am. J. Addict. 2021, 30, 423–432. [Google Scholar] [CrossRef]
- Hearne, E.; Wazaify, M.; Van Hout, M.C.; Atkinson, A.; McVeigh, J. Anabolic-androgenic steroid use in the Eastern Mediterranean region: A scoping review of extant empirical literature. Int. J. Ment. Health Addict. 2021, 19, 1162–1189. [Google Scholar] [CrossRef]
- Shamsaei, N.; Ahmadian, N. Investigation of psychological relationship between the level of religiosity and doping susceptibility among bodybuilding athletes. Relig. Health Spring Summer 2020, 8, 30–38. [Google Scholar]
- Hoseini, M.; Yousefi, B.; Khazaei, A. The prevalence of anabolic-androgenic steroids abuse, knowledge and attitude of their side effects, and attitude toward them among the female bodybuilding athletes in kermanshah. J. Fasa Univ. Med. Sci. 2020, 10, 2436–2446. [Google Scholar]
- Murtha, R.; Heffernan, C.; Hunt, T. Definition diets and deteriorating masculinity? Bodybuilding diets in Mid-Century America. Glob. Food Hist. 2021, 7, 71–91. [Google Scholar] [CrossRef]
- Okano, M.; Sato, M.; Ikekita, A. Analysis of non-ketoic steroids 17α-methylepithiostanol and desoxymethyl-testosterone in dietary supplements. Drug Test. Anal. 2009, 1, 518–525. [Google Scholar]
- Akram, O.N.; Bursill, C.; Desai, R.; Heather, A.K.; Kazlauskas, R.; Handelsman, D.J.; Lambert, G. Evaluation of androgenic activity of nutraceutical-derived steroids using mammalian and yeast in vitro androgen bioassays. Anal. Chem. 2011, 83, 2065–2072. [Google Scholar] [CrossRef]
- Díaz, F.C.; Sáez-González, E.; Benlloch, S.; Álvarez-Sotomayor, D. Albumin dialysis with MARS for the treatment of anabolic steroid-induced cholestasis. Ann. Hepatol. 2016, 15, 939–943. [Google Scholar]
- Okuno, Y.; Nakabou, Y.; Suzuki, S.; Ichiba, S.; Sugiyama, H. Complete remission by mepitiostane in hypoplastic leukemia. Rinsho Ketsueki 1989, 30, 1280–1283. [Google Scholar]
- Komeno, T. Steromal 2,3-Diol Cyclic Trithiocarbonate. U.S. Patent 3,139,128, 30 June 1964. [Google Scholar]
- Korneno, T.; Kawanami, E. 11,12-Epithio Steroids of Pregnane Series. U.S. Patent 3160627, 25 December 1964. [Google Scholar]
- Komeno, T. 2,3-Epithio-Steroids and Production Thereof. U.S. Patent 3,230,215, 18 January 1966. [Google Scholar]
- Klimstra, P.D. Optionally 17-Hydrocarbon (Substituted), 17-Oxygenated-2,3-Epithio-5a-Androstanes. U.S. Patent 3,405,124, 25 December 1968. [Google Scholar]
- Hirata, M. Process for Stabilization of a Composition of 2,3-Epithio-Androstanes and Composition Obtained Thereby. U.S. Patent 3,670,080, 13 June 1972. [Google Scholar]
- Miyake, T.; Uchida, K.; Kakushi, H.; Nomura, Y.; Kadowaki, M. 2a,3a-epithio-5a-androstan-17b-yl 1-methoxycyclopentyl ether (10361-S), a new orally active anabolic-androgenic steroid. Jpn. J. Pharmacol. 1974, 24, 551–558. [Google Scholar] [CrossRef]
- Kurachi, K.; Aono, T.; Tomoyama, J.; Matsumoto, K.; Nakasima, A. Effects of 2,3-epithio-5-androstan-17-ol (epitiostanol) on hypothalamo-pituitary-gonadal axis in humans. Acta Obstet. Gynaecol. Jpn. 1975, 22, 42–48. [Google Scholar]
- Li, X.; Rhee, D.K.; Malhotra, R.; Mayeur, C.; Hurst, L.A.; Ager, E. Progesterone receptor membrane component-1 regulates hepcidin biosynthesis. J. Clin. Investig. 2016, 126, 389–401. [Google Scholar] [CrossRef]
- Kang, L.; Li, X.Q.; Chen, C.X.; Wang, F.R. Research progress on structure modification and biological activity of 18β-glycyrrhetinic acid. Curr. Opin. Compl. Alternat. Med. 2014, 1, e00008. [Google Scholar]
- Wu, S.; Wang, W.; Dou, J.H. Research progress on the protective effects of licorice-derived 18β-glycyrrhetinic acid against liver injury. Acta Pharmacol. Sin. 2021, 42, 18–26. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Pharmacological activities of epithio steroids. J. Pharm. Res. Int. 2017, 18, 1–19. [Google Scholar] [CrossRef]
- Huang, M.; Xie, X.; Gong, P.; Wei, Y.; Du, H.; Xu, Y. A 18β-glycyrrhetinic acid conjugate with Vorinostat degrades HDAC3 and HDAC6 with improved antitumor effects. Eur. J. Med. Chem. 2020, 188, 111991. [Google Scholar] [CrossRef]
- Conor, R. Selenium in Food and Health; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Kang, D.; Lee, J.; Wu, C. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208. [Google Scholar] [CrossRef]
- Terry, N.; Zayed, A.M.; De Souza, M.P.; Tarun, A.S. Selenium in higher plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Sentkowska, A. Selenium in plant foods: Speciation analysis, bioavailability, and factors affecting composition. Crit. Rev. Food Sci. Nutrit. 2021, 61, 1340–1352. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H. Selenium in plants. Prog. Bot. 2015, 76, 93–106. [Google Scholar]
- Nogueira, C.W.; Barbosa, N.V.; Rocha, J.B.T. Toxicology and pharmacology of synthetic organoselenium compounds: An update. Arch. Toxicol 2021, 95, 1179–1226. [Google Scholar] [CrossRef]
- Ranu, B.C.; Banerjee, B. Organoselenium Chemistry; De Gruyter: Berlin, Germany; Boston, MA, USA, 2020. [Google Scholar]
- Li, Q.S.; Wu, D.M.; Zhu, B.C.; Wang, Y.G. Organic selenium resin in solid phase synthesis and its application in constructing medicinally relevant small organic molecules. Mini Rev. Med. Chem. 2013, 13, 854–869. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Biological activities of organometalloid (As, At, B, Ge, Si, Se, Te) steroids. J. App. Pharm. Sci. 2017, 7, 184–202. [Google Scholar]
- Wirth, T. Organoselenium Chemistry: Synthesis and Reactions; John Wiley & Sons, Inc.: Weinheim, Germany, 2011. [Google Scholar]
- Ibrahim-Ouali, M. Total synthesis of steroids and heterosteroids from BISTRO. Steroids 2015, 98, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim-Ouali, M. First total synthesis of 11-selena steroids. Tetrahedron Lett. 2009, 50, 1607–1609. [Google Scholar] [CrossRef]
- Ibrahim-Ouali, M. First total synthesis of 11-tellura steroids. Tetrahedron Lett. 2010, 51, 3610–3612. [Google Scholar] [CrossRef]
- Ibrahim-Ouali, M.; Romero, E.; Bouleghlem, H. First total syntheses of (±)-3-aza-11-selena and (±)-3-aza-11-tellura steroids. Tetrahedron 2011, 67, 3668–3676. [Google Scholar] [CrossRef]
- Santi, C. Organoselenium Chemistry: Between Synthesis and Biochemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014. [Google Scholar]
- Knapp, F.F. The synthesis of 123Te-labeled 17β-hydroxy-2-tellura-A-nor-5α-androstane. J. Label. Comp. Radiopharm. 1980, 17, 81–91. [Google Scholar] [CrossRef]
- Leimbach, D.; Karls, J.; Guo, Y.; Ahmed, R.; Ballof, J.; Bengtsson, L.; Pamies, F.B.; Borschevsky, A. The electron affinity of astatine. Nat. Commun. 2020, 11, 3824. [Google Scholar] [CrossRef]
- Kugler, H.K.; Keller, C. At Astatine; Springer: Berlin/Heidelberg, Germany, 1985. [Google Scholar]
- Eychenne, R.; Bouvry, C.; Bourgeois, M.; Loyer, P.; Benoist, E.; Lepareur, N. Overview of radiolabeled somatostatin analogs for cancer imaging and therapy. Molecules 2020, 25, 4012. [Google Scholar] [CrossRef]
- Visser, G.W.M.; Diemer, E.L.; Kaspersen, F.M. The preparation of aromatic astatine compounds through aromatic mercury compound’s part II: Astatination of pyrimidines and steroids. J. Label. Comp. Radiopharm. 1981, 18, 799–807. [Google Scholar] [CrossRef]
- Liu, B.L.; Jin, Y.T.; Liu, Z.H.; Luo, C.; Kojima, M.; Maeda, M. Halogen exchanges using crown ethers: Synthesis and preliminary biodistribution of 6-(211At)-astatomethyl-19-norcholest-5(10)-en-3beta-ol. Int. J. Appl. Radiat. Isot. 1985, 36, 561–563. [Google Scholar]





| No. | Discovered Activity, (Pa) * | Reported Activity | References |
|---|---|---|---|
| 1 | Ovulation inhibitor (0.942) Cardiovascular analeptic (0.924) Antihypercholesterolemic (0.871) Lipid metabolism regulator (0.730) | Inhibitor aromatase Sulfatase inhibitor Estrogenic Promotor breast cancer | [81,82,83,84] |
| 2 | Antihypercholesterolemic (0.904) Ovulation inhibitor (0.889) Neuroprotector (0.870) Anesthetic general (0.868) Acute neurologic disorders treatment (0.793) Prostate disorders treatment (0.729) Anti-inflammatory (0.713) | Antioxidant Anti-inflammatory Uterotrophic RNA polymerase Promoter of breast, ovarian and endometrial cancers Neuroprotective properties | [81,85,86,87] |
| 3 | Ovulation inhibitor (0.900) Acute neurologic disorders treatment (0.822) Antihypercholesterolemic (0.791) | Estrogenic Agonist of the ERs RNA polymerase | [88,89] |
| 4 | Antihypercholesterolemic (0.856) Ovulation inhibitor (0.847) Cardiovascular analeptic (0.842) Lipid metabolism regulator (0.788) | Estrogenic Estrogen agonist | [90,91,92] |
| 5 | Acute neurologic disorders treatment (0.912) Male reproductive disfunction treatment (0.847) Ovulation inhibitor (0.786) Postmenopausal disorders treatment (0.643) Antihypercholesterolemic (0.640) Menopausal disorders treatment (0.579) | Inhibitor aromatase Sulfatase inhibitor Inhibitor of human breast cancer Concentration Cardiovascular agent Postmenopausal disorders | [93,94] |
| 6 | Antihypercholesterolemic (0.973) Acute neurologic disorders treatment (0.922) Lipid metabolism regulator (0.907) Antithrombotic (0.714) Ovulation inhibitor (0.662) Dementia treatment (0.616) Hypolipemic (0.613) Male reproductive disfunction treatment (0.587) Menopausal disorders treatment (0.582) | Estrogenic Ovarian activity Urine production | [95] |
| 7 | Ovulation inhibitor (0.956) Cardiovascular analeptic (0.927) Antihypercholesterolemic (0.868) Male reproductive disfunction treatment (0.847) Menopausal disorders treatment (0.842) Acute neurologic disorders treatment (0.745) Lipid metabolism regulator (0.701) Menstruation disorders treatment (0.639) Postmenopausal disorders treatment (0.605) Muscular dystrophy treatment (0.601) Contraceptive female (0.570) Anti-infertility, female (0.567) | Estrogenic Antiestrogenic effects Anticancer | [96,97,98] |
| 8 | Ovulation inhibitor (0.930) Cardiovascular analeptic (0.925) Antihypercholesterolemic (0.855) Male reproductive disfunction treatment (0.843) Acute neurologic disorders treatment (0.780) Menopausal disorders treatment (0.747) Lipid metabolism regulator (0.601) Muscular dystrophy treatment (0.579) Postmenopausal disorders treatment (0.561) Menstruation disorders treatment (0.533) | Estrogenic Strongest neuroprotective effect UDP-glucuronosyltransferase Anti-breast cancer | [99,100,101] |
| 9 | Ovulation inhibitor (0.953) Cardiovascular analeptic (0.928) Antihypercholesterolemic (0.857) Menopausal disorders treatment (0.807) Male reproductive disfunction treatment (0.805) Acute neurologic disorders treatment (0.660) Contraceptive (0.655) Lipid metabolism regulator (0.645) Contraceptive female (0.558) Menstruation disorders treatment (0.542) Anti-infertility, female (0.503) Postmenopausal disorders treatment (0.500) | Antioxidant Estrogenic Anti-breast cancer | [102] |
| 10 | Ovulation inhibitor (0.925) Cardiovascular analeptic (0.922) Antihypercholesterolemic (0.833) Male reproductive disfunction treatment (0.821) Menopausal disorders treatment (0.712) Acute neurologic disorders treatment (0.662) Contraceptive (0.602) Lipid metabolism regulator (0.571) Muscular dystrophy treatment (0.548) | Estrogenic Proliferation of human breast cancer | [103,104,105] |
| 11 | Antihypercholesterolemic (0.959) Hypolipemic (0.808) | Activity not studied | |
| 12 | Lipid metabolism regulator (0.913) Antihypercholesterolemic (0.767) | Activity not studied | |
| 13 | Antihypercholesterolemic (0.961) Hypolipemic (0.711) | Activity not studied | |
| 14 | Antihypercholesterolemic (0.953) | Activity not studied | |
| 15 | Antihypercholesterolemic (0.946) | Activity not studied | |
| 16 | Antihypercholesterolemic (0.907) | Activity not studied | |
| 17 | Antihypercholesterolemic (0.929) | Activity not studied | |
| 18 | Antihypercholesterolemic (0.935) | Activity not studied | |
| 19 | Antihypercholesterolemic (0.950) | Activity not studied | |
| 20 | Antihypercholesterolemic (0.914) | Anticancer | [52] |
| No. | Lipid Metabolism Regulators, (Pa) * | Reported Activity | Ref. |
|---|---|---|---|
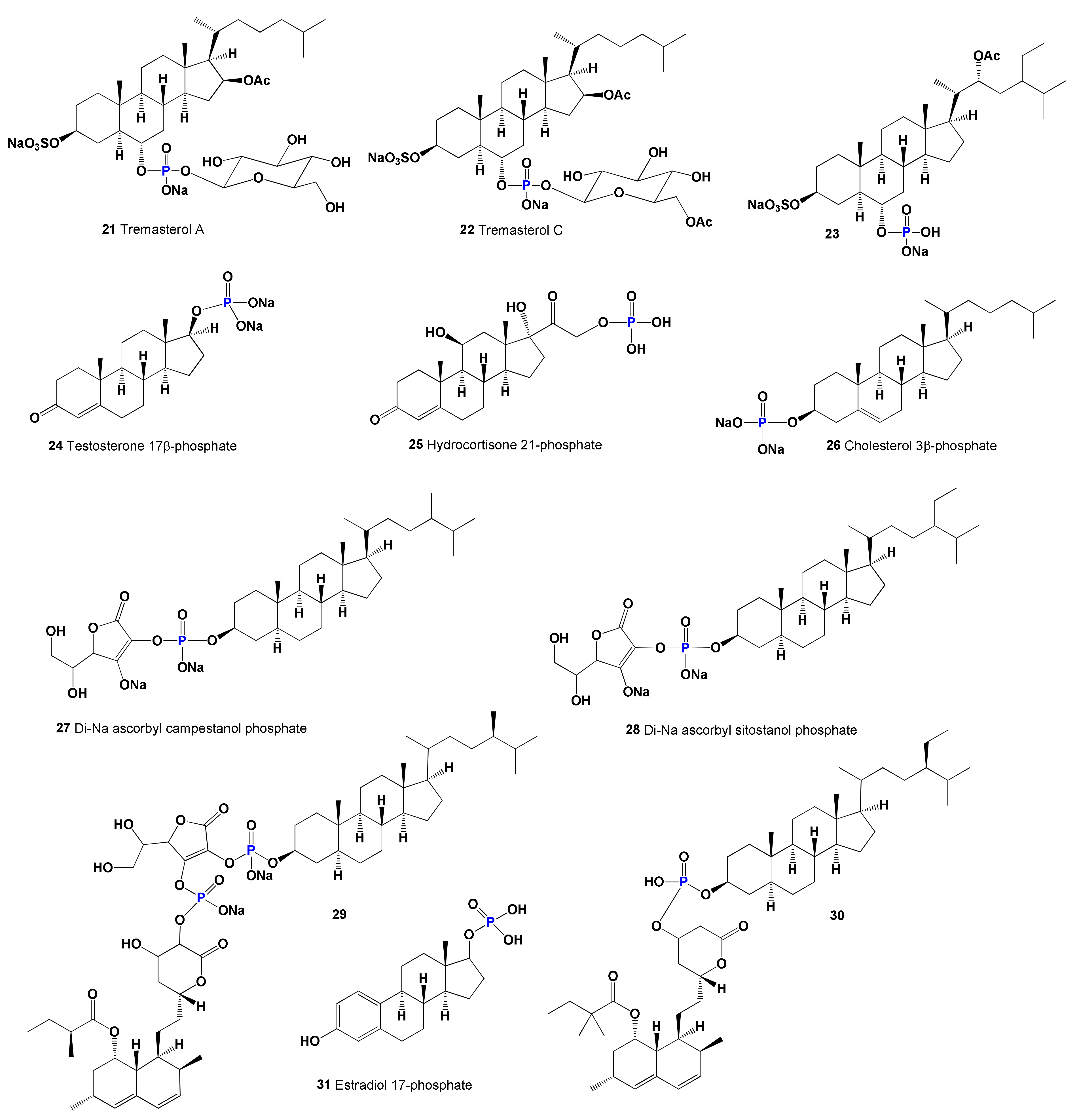
| 21 | Wound healing agent (0.975) Hepatoprotectant (0.961) Analeptic (0.952) Antihypercholesterolemic (0.926) Cholesterol synthesis inhibitor (0.799) | Activity not studied | |
| 22 | Wound healing agent (0.947) Analeptic (0.941) Hepatoprotectant (0.932) Anticarcinogenic (0.915) Antihypercholesterolemic (0.912) Cholesterol synthesis inhibitor (0.778) | Activity not studied | |
| 23 | Antihypercholesterolemic (0.900) Hepatoprotectant (0.853) Wound healing agent (0.844) Antineoplastic (0.816) Antiinflammatory (0.782) Cholesterol synthesis inhibitor (0.778) Atherosclerosis treatment (0.675) | Activity not studied | |
| 24 | Neuroprotector (0.987) Anesthetic general (0.959) Respiratory analeptic (0.944) Antihypercholesterolemic (0.909) | Substrate for phosphatases | [112] |
| 25 | Anesthetic general (0.991) Respiratory analeptic (0.990) Neuroprotector (0.976) Antiinflammatory (0.906) Antihypercholesterolemic (0.900) | Increases blood sugar levels | [113] |
| 26 | Respiratory analeptic (0.979) Anesthetic general (0.973) Neuroprotector (0.972) Antihypercholesterolemic (0.971) Wound healing agent (0.913) Antineoplastic (0.826) Cholesterol synthesis inhibitor (0.801) | Stabilizes blood pressure | [114] |
| 27 | Respiratory analeptic (0.995) Anesthetic general (0.948) Antihypercholesterolemic (0.945) Neuroprotector (0.932) Hemostatic (0.910) Wound healing agent (0.897) Cholesterol synthesis inhibitor (0.867) Acute neurologic disorders treatment (0.827) | Reduces cholesterol levels | [116] |
| 28 | Antihypercholesterolemic (0.967) Wound healing agent (0.921) Neuroprotector (0.909) Cholesterol synthesis inhibitor (0.872) | Reduces cholesterol levels | [116] |
| 29 | Antihypercholesterolemic (0.996) Cholesterol absorption inhibitor (0.976) Cholesterol synthesis inhibitor (0.952) Lipid metabolism regulator (0.952) Lipoprotein disorders treatment (0.893) | Cholesterol biosynthesis inhibitor | [117] |
| 30 | Antihypercholesterolemic (0.999) Antihyperlipoproteinemic (0.986) Hypolipemic (0.974) Cholesterol absorption inhibitor (0.957) Lipid metabolism regulator (0.954) Cholesterol synthesis inhibitor (0.916) Lipoprotein disorders treatment (0.782) Acute neurologic disorders treatment (0.751) Atherosclerosis treatment (0.729) | Cholesterol biosynthesis inhibitor | [117] |
| 31 | Neuroprotector (0.982) Anesthetic general (0.931) Antihypercholesterolemic (0.909) Acute neurologic disorders treatment (0.831) Prostate disorders treatment (0.640) | Remedy for the treatment of prostate cancer | [118] |
| No. | Discovered Activity, (Pa) * | Reported Activity | Ref. |
|---|---|---|---|
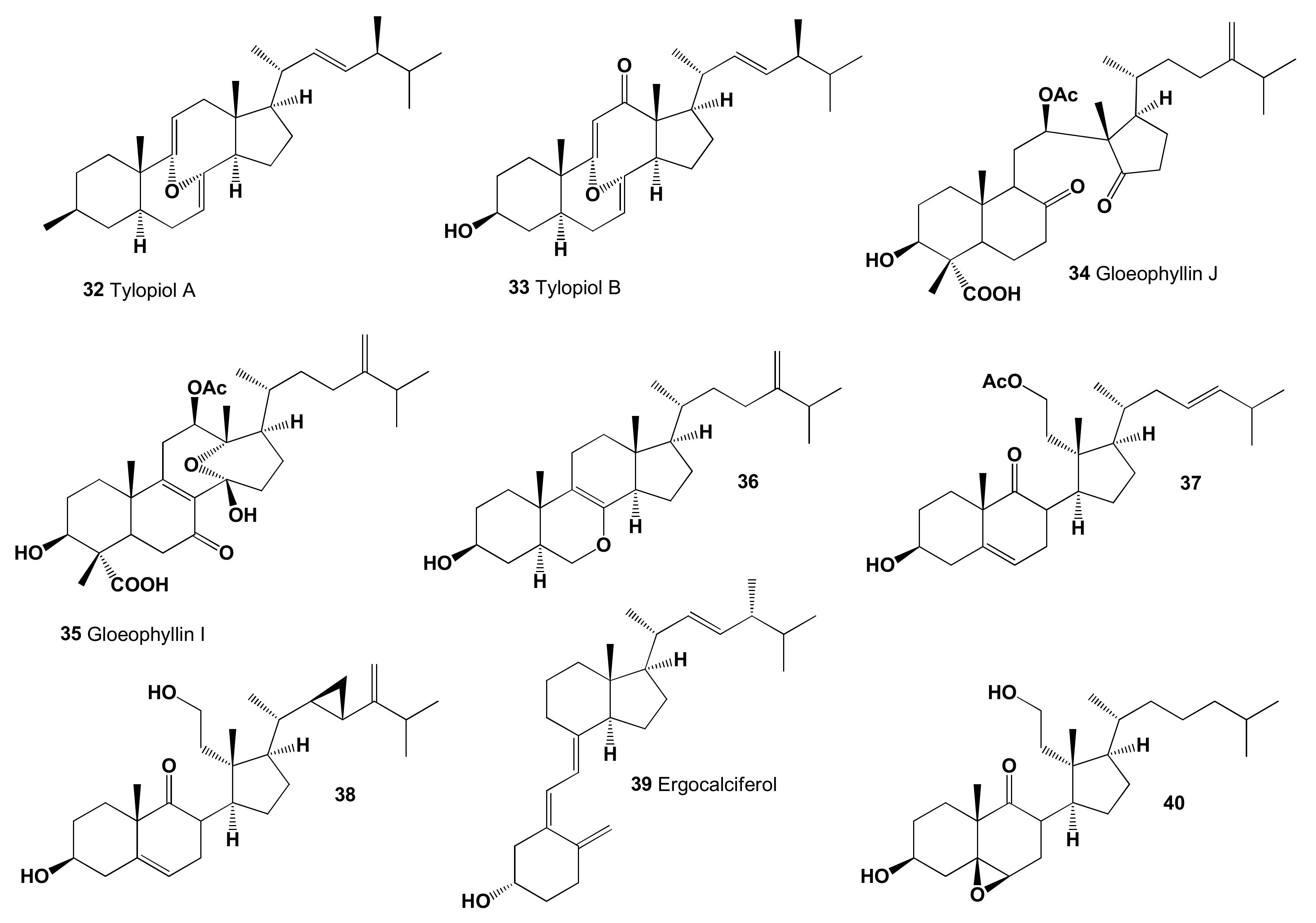
| 32 | Antihypercholesterolemic (0.912) Hypolipemic (0.802) Atherosclerosis treatment (0.643) Antiparkinsonian, rigidity relieving (0.562) | Activity not studied | |
| 33 | Antihypercholesterolemic (0.905) Hypolipemic (0.753) Atherosclerosis treatment (0.559) | Activity not studied | |
| 34 | Antihypercholesterolemic (0.908) Antineoplastic (0.785) Hypolipemic (0.764) Apoptosis agonist (0.747) Cholesterol synthesis inhibitor (0.744) | Anticancer | [131] |
| 35 | Antihypercholesterolemic (0.916) Antineoplastic (0.833) Hypolipemic (0.827) Apoptosis agonist (0.771) Cholesterol synthesis inhibitor (0.685) Atherosclerosis treatment (0.633) | Anticancer | [131] |
| 36 | Antihypercholesterolemic (0.904) Hypolipemic (0.767) Antineoplastic (0.743) Apoptosis agonist (0.676) Proliferative diseases treatment (0.625) Atherosclerosis treatment (0.539) | Anticancer | [133] |
| 37 | Antihypercholesterolemic (0.915) Lipid metabolism regulator (0.768) | Activity not studied | |
| 38 | Antihypercholesterolemic (0.909) Hypolipemic (0.786) | Anticancer | [147] |
| 39 | Antiparkinsonian, rigidity relieving (0.960) Hyperparathyroidism treatment (0.892) Antihypercholesterolemic (0.845) Hypolipemic (0.790) Atherosclerosis treatment (0.628) | Calcium and phosphates metabolism regulator | [148] |
| 40 | Chemopreventive (0.989) Hepatoprotectant (0.986) Respiratory analeptic (0.978) Antihypercholesterolemic (0.977) Proliferative diseases treatment (0.969) Antimycobacterial (0.939) Neuroprotector (0.895) Antineoplastic (0.874) Antiprotozoal (Leishmania) (0.772) Atherosclerosis treatment (0.601) Neurodegenerative diseases treatment (0.590) Alzheimer’s disease treatment (0.570) | Activity not studied previously |
| No. | Discovered Activity, (Pa) * | Reported Activity | Ref. |
|---|---|---|---|
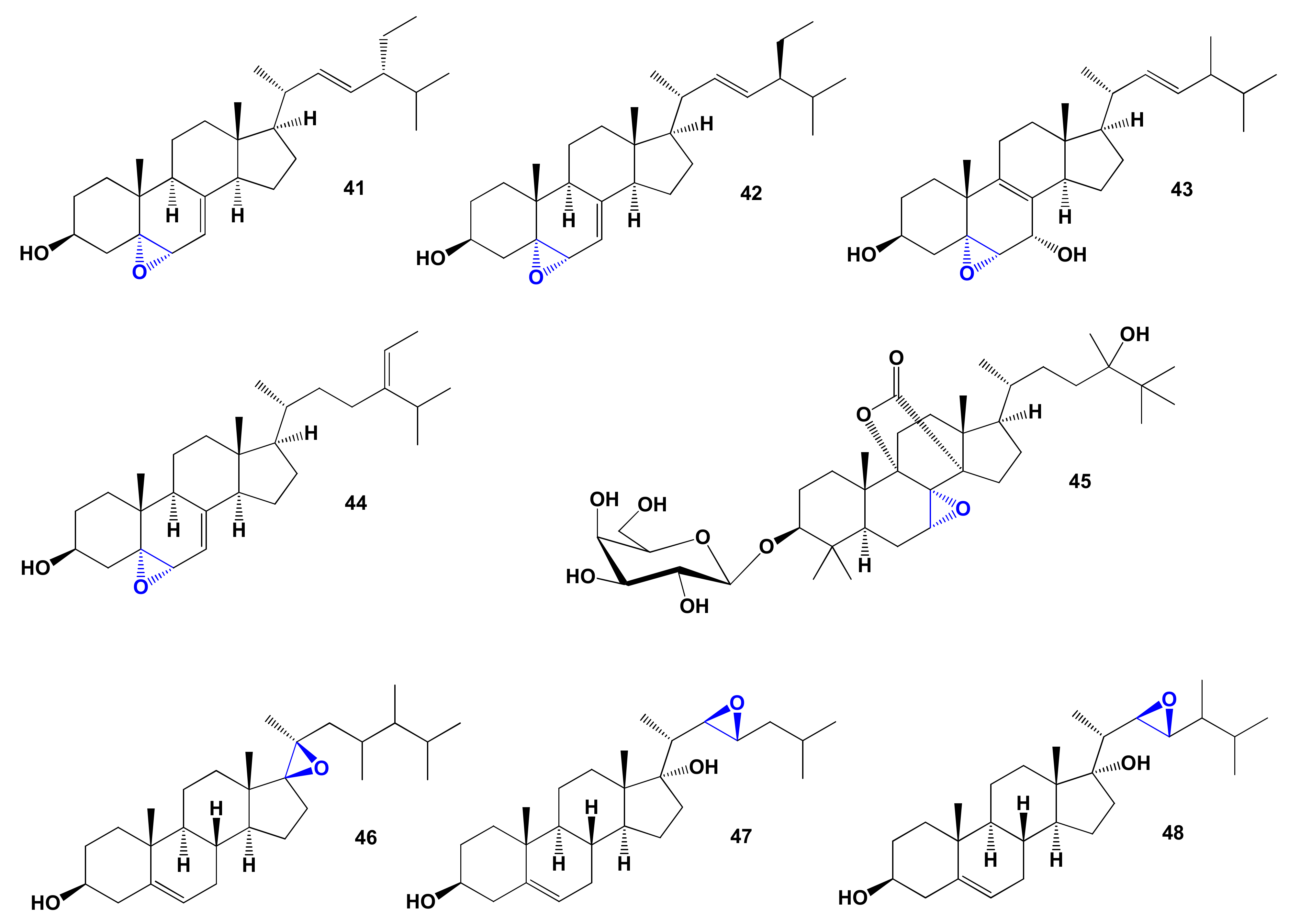
| 41 | Apoptosis agonist (0.950) Antihypercholesterolemic (0.931) Antineoplastic (0.886) Antieczematic (0.842) Atherosclerosis treatment (0.712) | Cytotoxic | [160] |
| 42 | Apoptosis agonist (0.950) Antihypercholesterolemic (0.931) Antineoplastic (0.886) Antieczematic (0.842) Atherosclerosis treatment (0.712) | Cytotoxic | [160] |
| 43 | Apoptosis agonist (0.954) Antineoplastic (0.914) Antihypercholesterolemic (0.906) Atherosclerosis treatment (0.741) | Activity not studied | |
| 44 | Antihypercholesterolemic (0.934) Apoptosis agonist (0.929) Hypolipemic (0.864) Antineoplastic (0.861) | Activity not studied | |
| 45 | Hepatoprotectant (0.994) Respiratory analeptic (0.990) Antihypercholesterolemic (0.897) | Activity not studied | |
| 46 | Antihypercholesterolemic (0.900) Neuroprotector (0.749) Cholesterol synthesis inhibitor (0.636) | Activity not studied | |
| 47 | Antihypercholesterolemic (0.901) Lipid metabolism regulator (0.833) Prostate disorders treatment (0.714) | Anticancer | [165] |
| 48 | Antihypercholesterolemic (0.924) Lipid metabolism regulator (0.820) Neuroprotector (0.728) | Anticancer | [165] |
| No. | Lipid Metabolism Regulators, (Pa) * | Reported Activity | Ref. |
|---|---|---|---|
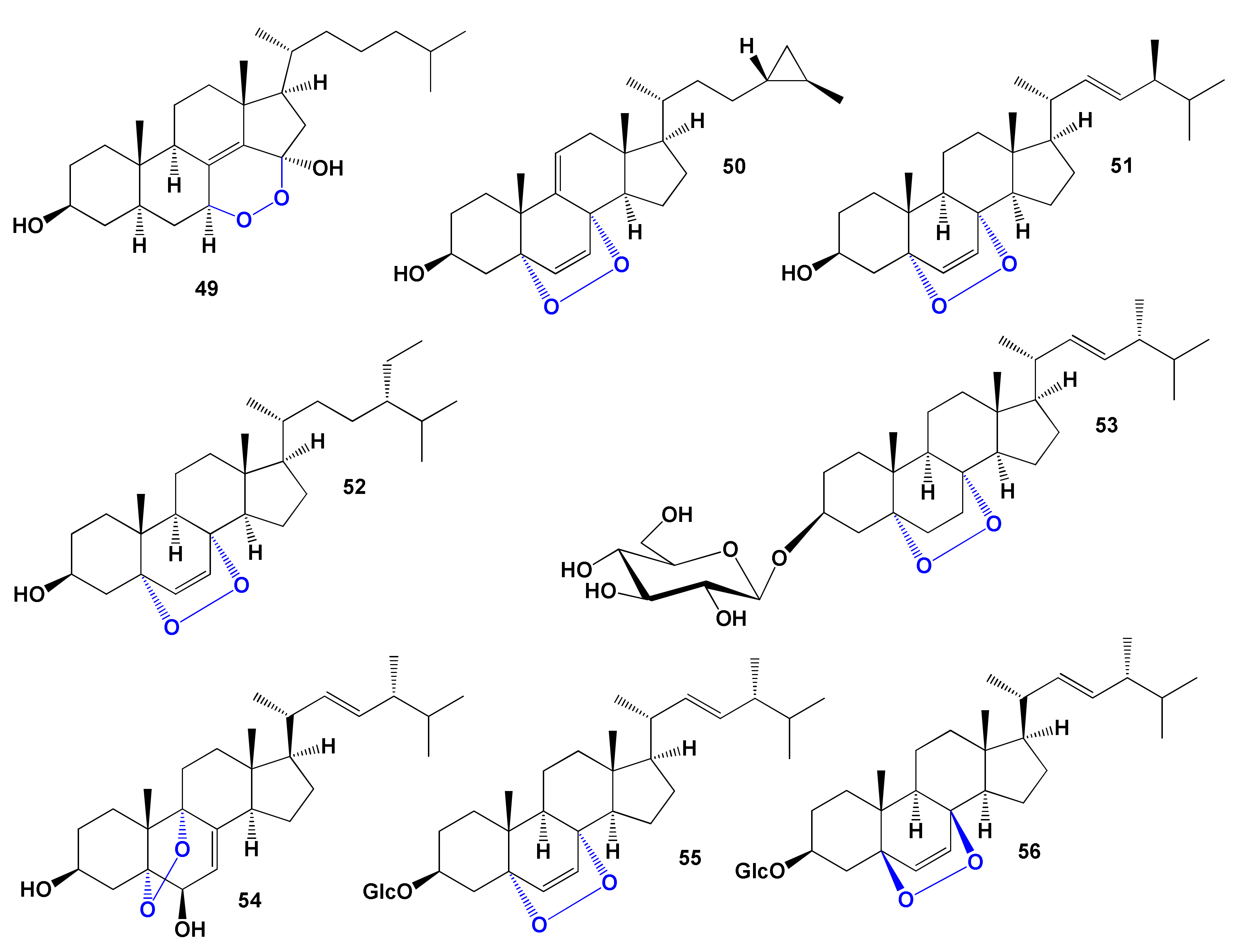
| 49 | Antihypercholesterolemic (0.914) Hypolipemic (0.635) | Activity not studied | |
| 50 | Atherosclerosis treatment (0.911) Hypolipemic (0.836) Lipoprotein disorders treatment (0.826) Antihypercholesterolemic (0.802) | Activity not studied | |
| 51 | Atherosclerosis treatment (0.907) Antihypercholesterolemic (0.788) | Activity not studied | |
| 52 | Atherosclerosis treatment (0.919) Hypolipemic (0.822) Lipoprotein disorders treatment (0.814) | Activity not studied | |
| 53 | Antihypercholesterolemic (0.926) Hypolipemic (0.800) Atherosclerosis treatment (0.709) | Activity not studied | |
| 54 | Antihypercholesterolemic (0.900) Hypolipemic (0.827) Atherosclerosis treatment (0.659) Hyperparathyroidism treatment (0.502) | Activity not studied | |
| 55 | Antihypercholesterolemic (0.917) Hypolipemic (0.786) | Anticancer | [183] |
| 56 | Antihypercholesterolemic (0.917) Atherosclerosis treatment (0.858) Hypolipemic (0.786) | Anticancer | [183] |
| No. | Discovered Activity, (Pa) * | Reported Activity | Ref. |
|---|---|---|---|
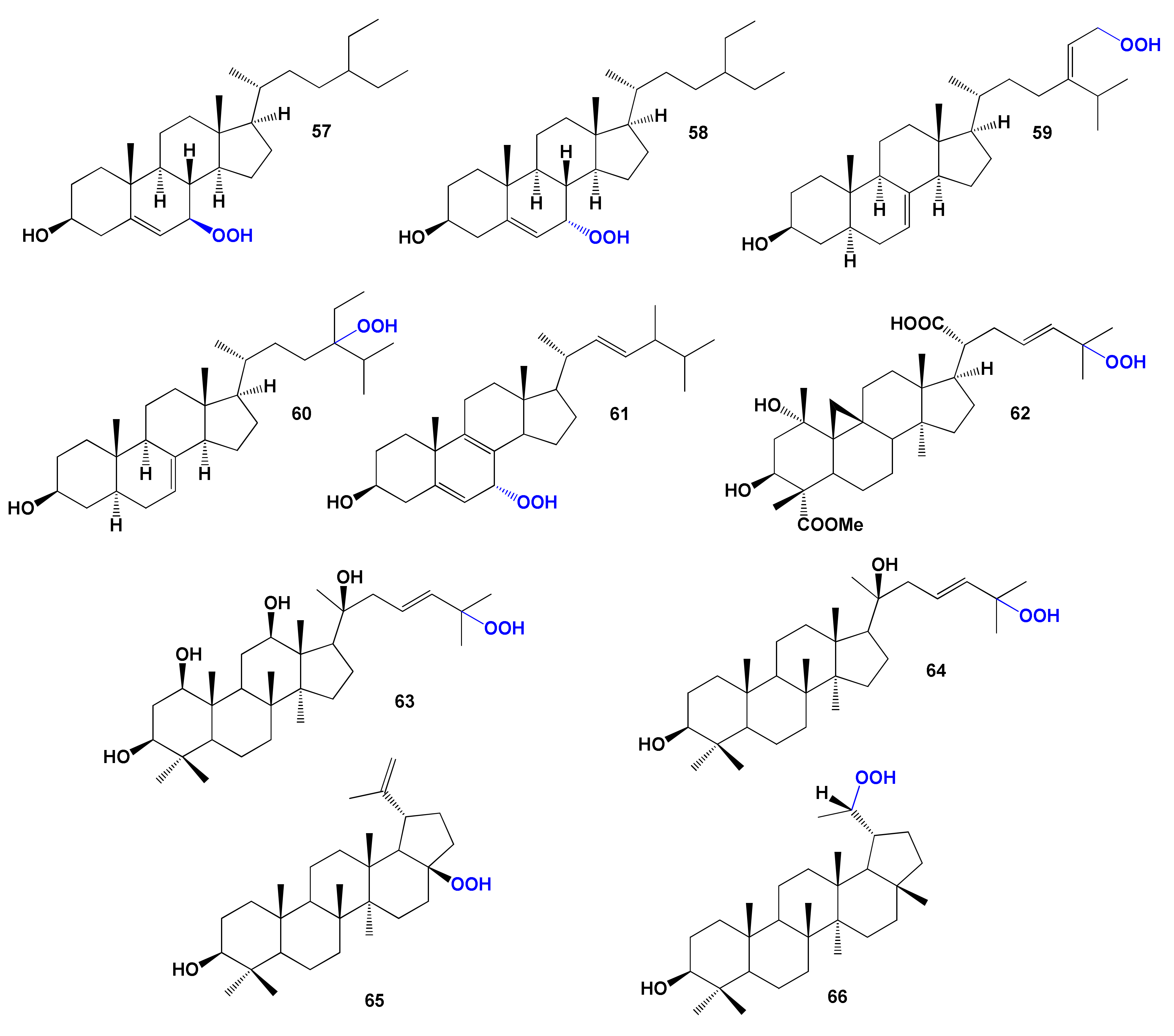
| 57 | Antihypercholesterolemic (0.905) Cholesterol synthesis inhibitor (0.799) | Activity not studied | |
| 58 | Antihypercholesterolemic (0.905) Hypolipemic (0.802) Cholesterol synthesis inhibitor (0.621) Atherosclerosis treatment (0.542) | Activity not studied | |
| 59 | Antihypercholesterolemic (0.933) Hypolipemic (0.877) Cholesterol synthesis inhibitor (0.644) Atherosclerosis treatment (0.675) Prostate disorders treatment (0.645) | Cytotoxic | [186] |
| 60 | Antihypercholesterolemic (0.933) Hypolipemic (0.877) Antineoplastic (0.835) Cholesterol synthesis inhibitor (0.650) Atherosclerosis treatment (0.554) | Cytotoxic | [186] |
| 61 | Antihypercholesterolemic (0.922) Hypolipemic (0.868) Atherosclerosis treatment (0.678) Antiparkinsonian, rigidity relieving (0.516) | Activity not studied | |
| 62 | Antihypercholesterolemic (0.913) Antineoplastic (0.802) Hypolipemic (0.795) | Hepatoprotective Cytotoxic | [191] |
| 63 | Antihypercholesterolemic (0.933) Hypolipemic (0.877) Cholesterol synthesis inhibitor (0.644) | Activity not studied | |
| 64 | Antihypercholesterolemic (0.923) Hypolipemic (0.774) Cholesterol synthesis inhibitor (0.604) Biliary tract disorders treatment (0.577) | Activity not studied | |
| 65 | Antihypercholesterolemic (0.918) Hypolipemic (0.779) Biliary tract disorders treatment (0.655) | Antiproliferative | [194] |
| 66 | Antihypercholesterolemic (0.930) Hypolipemic (0.767) Biliary tract disorders treatment (0.717) Atherosclerosis treatment (0.590) | Activity not studied |
| No. | Discovered Activity, (Pa) * | Reported Activity | Ref. |
|---|---|---|---|
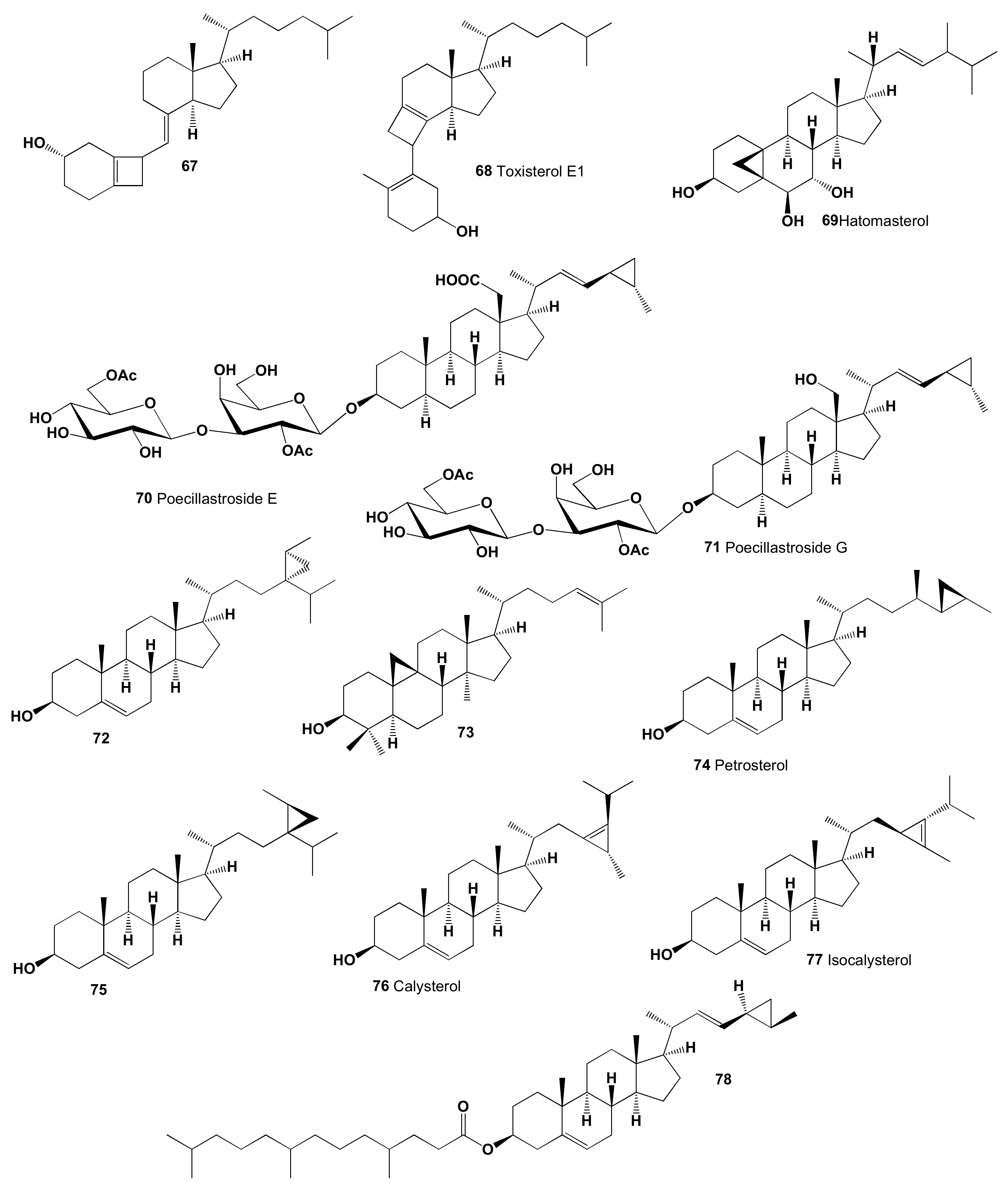
| 67 | Antihypercholesterolemic (0.902) Hypolipemic (0.721) Cholesterol synthesis inhibitor (0.534) | Activity not studied | |
| 68 | Antihypercholesterolemic (0.932) Hypolipemic (0.695) Cholesterol synthesis inhibitor (0.588) | Activity not studied | |
| 69 | Antineoplastic (0.915) Antihypercholesterolemic (0.900) Hypolipemic (0.897) Apoptosis agonist (0.892) Antineoplastic (liver cancer) (0.822) Chemopreventive (0.776) Atherosclerosis treatment (0.690) Cytoprotectant (0.611) Prostate cancer treatment (0.557) Antimetastatic (0.528) | Cytotoxic | [206] |
| 70 | Antihypercholesterolemic (0.953) Hypolipemic (0.758) Lipid metabolism regulator (0.674) Atherosclerosis treatment (0.513) | Antifungal | [207] |
| 71 | Antihypercholesterolemic (0.939) Hypolipemic (0.746) Lipid metabolism regulator (0.599) | No activity detected | [207] |
| 72 | Antihypercholesterolemic (0.923) Hypolipemic (0.732) Atherosclerosis treatment (0.643) Cholesterol synthesis inhibitor (0.640) | Activity not studied | |
| 73 | Hypolipemic (0.900) Atherosclerosis treatment (0.689) Cholesterol synthesis inhibitor (0.671) Antihypercholesterolemic (0.662) Lipid metabolism regulator (0.529) | Activity not studied | |
| 74 | Antihypercholesterolemic (0.964) Hypolipemic (0.849) Antineoplastic (0.849) Antihyperlipoproteinemic (0.801) Cholesterol synthesis inhibitor (0.671) Atherosclerosis treatment (0.610) Prostate cancer treatment (0.601) | Cytotoxic Anticancer | [211,212,213,214] |
| 75 | Antihypercholesterolemic (0.923) Hypolipemic (0.732) Atherosclerosis treatment (0.643) Cholesterol synthesis inhibitor (0.640) | Activity not studied | |
| 76 | Antihypercholesterolemic (0.935) Hypolipemic (0.731) Antihyperlipoproteinemic (0.689) Cholesterol synthesis inhibitor (0.600) | Activity not studied | |
| 77 | Antihypercholesterolemic (0.908) Hypolipemic (0.726) Cholesterol synthesis inhibitor (0.589) Antihyperlipoproteinemic (0.587) | Activity not studied | |
| 78 | Antihypercholesterolemic (0.969) Hypolipemic (0.810) Lipid metabolism regulator (0.716) Cholesterol synthesis inhibitor (0.707) Atherosclerosis treatment (0.586) | Activity not studied |
| No. | Discovered Activity, (Pa) * | Reported Activity | Ref. |
|---|---|---|---|
| 79 | Antihypercholesterolemic (0.964) Atherosclerosis treatment (0.717) | Activity not studied | |
| 80 | Antihypercholesterolemic (0.956) | Activity not studied | |
| 81 | Antihypercholesterolemic (0.989) Hypolipemic (0.808) | Activity not studied | |
| 82 | Antihypercholesterolemic (0.960) Atherosclerosis treatment (0.683) | Activity not studied | |
| 83 | Antihypercholesterolemic (0.961) Cholesterol synthesis inhibitor (0.745) | Activity not studied | |
| 84 | Antihypercholesterolemic (0.956) Cholesterol synthesis inhibitor (0.747) | Activity not studied | |
| 85 | Antihypercholesterolemic (0.964) | Activity not studied | |
| 86 | Antihypercholesterolemic (0.969) | Activity not studied | |
| 87 | Antihypercholesterolemic (0.952) Atherosclerosis treatment (0.710) | Activity not studied | |
| 88 | Antihypercholesterolemic (0.965) Atherosclerosis treatment (0.677) | Activity not studied | |
| 89 | Antihypercholesterolemic (0.969) | Activity not studied | |
| 90 | Antihypercholesterolemic (0.965) Atherosclerosis treatment (0.700) | Activity not studied | |
| 91 | Antihypercholesterolemic (0.969) | Activity not studied | |
| 92 | Antihypercholesterolemic (0.962) Atherosclerosis treatment (0.704) Lipid metabolism regulator (0.702) | Activity not studied | |
| 93 | Antihypercholesterolemic (0.974) Antimicrobial treatment (0.717) | Antimicrobial | [237] |
| 94 | Antihypercholesterolemic (0.961) Antineoplastic (0.863) Anticarcinogenic (0.828) Lipid metabolism regulator (0.747) Antimetastatic (0.590) | Anticancer | [238,239] |
| 95 | Antihypercholesterolemic (0.936) Antineoplastic (0.870) Anticarcinogenic (0.807) Antimetastatic (0.598) | Anticancer | [238,239] |
| No. | Discovered Activity, (Pa) * | Reported Activity | Ref. |
|---|---|---|---|
| 96 | Neuroprotector (0.983) Antihypercholesterolemic (0.919) Acute neurologic disorders treatment (0.636) Hypolipemic (0.626) | Anticancer | [243] |
| 97 | Antihypercholesterolemic (0.954) Neuroprotector (0.754) Hypolipemic (0.704) | Activity not studied | |
| 98 | Antihypercholesterolemic (0.927) Neuroprotector (0.773) Hypolipemic (0.584) | Activity not studied | |
| 99 | Antihypercholesterolemic (0.962) | Activity not studied | |
| 100 | Antihypercholesterolemic (0.918) Anti-inflammatory (0.903) Antineoplastic (0.875) Proliferative diseases treatment (0.856) Atherosclerosis treatment (0.618) | Anti-inflammatory Anticancer | [247] |
| 101 | Antihypercholesterolemic (0.976) Hypolipemic (0.735) Lipid metabolism regulator (0.707) | Activity not studied | |
| 102 | Antihypercholesterolemic (0.920) Hypolipemic (0.798) Atherosclerosis treatment (0.637) Hyperparathyroidism treatment (0.523) | Activity not studied | |
| 103 | Antihypercholesterolemic (0.955) Atherosclerosis treatment (0.596) | Activity not studied | |
| 104 | Proliferative diseases treatment (0.967) Chemopreventive (0.958) Antihypercholesterolemic (0.947) Anticarcinogenic (0.907) Antineoplastic (0.902) Hypolipemic (0.781) Atherosclerosis treatment (0.609) | Anticancer | [251] |
| 105 | Antihypercholesterolemic (0.957) Hypolipemic (0.809) Atherosclerosis treatment (0.592) | Activity not studied | |
| 106 | Antihypercholesterolemic (0.955) Hypolipemic (0.878) Atherosclerosis treatment (0.658) | Activity not studied | |
| 107 | Neuroprotector (0.938) Antihypercholesterolemic (0.912) Atherosclerosis treatment (0.548) | Activity not studied |
| No. | Discovered Activity, (Pa) * | Reported Activity | Ref. |
|---|---|---|---|
| 108 | Antineoplastic (0.964) Antisecretoric (0.948) Estrogen antagonist (0.860) Cardiotonic (0.729) Prostate disorders treatment (0.709) Neuroprotector (0.723) Bone diseases treatment (0.693) Antineoplastic (breast cancer) (0.598) | Anti-breast cancer Estrogen receptor antagonist | [294] |
| 109 | Antineoplastic (0.966) Antisecretoric (0.952) Estrogen antagonist (0.832) Anti-inflammatory (0.754) Prostate disorders treatment (0.736) Prostatic (benign) hyperplasia treatment (0.673) | Estrogen antagonist | [294] |
| 110 | Antineoplastic (0.966) Antisecretoric (0.952) Estrogen antagonist (0.832) Anti-inflammatory (0.754) Prostate disorders treatment (0.736) Prostatic (benign) hyperplasia treatment (0.673) | Estrogen antagonist | [294] |
| 111 | Antineoplastic (0.932) Antihypercholesterolemic (0.759) Bone diseases treatment (0.729) Hypolipemic (0.676) Estrogen antagonist (0.660) | Anabolic | [294] |
| 112 | Antisecretoric (0.967) Estrogen antagonist (0.946) Antineoplastic (0.939) Anabolic (0.823) | Anabolic | [284,285,286] |
| 113 | Cholesterol antagonist (0.933) Antihypercholesterolemic (0.929) Hypolipemic (0.818) Estrogen antagonist (0.443) | Antiseptic, Germicidal, Fungicidal | [294] |
| 114 | Cholesterol antagonist (0.946) Antihypercholesterolemic (0.930) Hypolipemic (0.781) Estrogen antagonist (0.465) | Antiseptic, Germicidal, Fungicidal | [294] |
| 115 | Cholesterol antagonist (0.932) Antihypercholesterolemic (0.900) Cardiotonic (0.886) Choleretic (0.871) Atherosclerosis treatment (0.838) Antineoplastic (0.775) Hypolipemic (0.746) Estrogen antagonist (0.611) | DOCA inhibitor | [294] |
| 116 | Lipid metabolism regulator (0.954) Antineoplastic (0.924) Apoptosis agonist (0.869) Estrogen antagonist (0.505) | Anticancer | [292,295] |
| 117 | Cholesterol antagonist (0.916) Antihypercholesterolemic (0.836) Hypolipemic (0.801) | Activity not studied |
| No. | Discovered Activity, (Pa) * | Reported Activity | Ref. |
|---|---|---|---|
| 118 | Antihypercholesterolemic (0.905) | Activity not studied | |
| 119 | Choleretic (0.909) Antihypercholesterolemic (0.905) | Activity not studied | |
| 120 | Hypolipemic (0.995) Atherosclerosis treatment (0.991) Lipoprotein disorders treatment (0.982) Antioxidant (0.973) Erythropoiesis stimulant (0.823) Biliary tract disorders treatment (0.808) Laxative (0.709) | Activity not studied | |
| 121 | Hypolipemic (0.995) Atherosclerosis treatment (0.989) Lipoprotein disorders treatment (0.980) Antioxidant (0.970) Biliary tract disorders treatment (0.808) Erythropoiesis stimulant (0.730) Laxative (0.683) | Activity not studied | |
| 122 | Hypolipemic (0.996) Atherosclerosis treatment (0.995) Lipoprotein disorders treatment (0.991) Antioxidant (0.978) Erythropoiesis stimulant (0.756) | Activity not studied | |
| 123 | Hypolipemic (0.913) Antihypercholesterolemic (0.884) Atherosclerosis treatment (0.822) | Activity not studied | |
| 124 | Antihypercholesterolemic (0.902) Hypolipemic (0.899) Atherosclerosis treatment (0.791) | Activity not studied | |
| 125 | Antihypercholesterolemic (0.905) | Activity not studied | |
| 126 | Antihypercholesterolemic (0.908) Bone diseases treatment (0.772) Hypolipemic (0.769) | Activity not studied | |
| 127 | Antihypercholesterolemic (0.912) Antineoplastic (0.884) Anticarcinogenic (0.753) | Cytotoxic activity Agent for Alzheimer’s disease | [303,310] |
| 128 | Antihypercholesterolemic (0.953) Antineoplastic (0.856) Anticarcinogenic (0.804) | Cytotoxic activity Agent for Alzheimer’s disease | [303,310] |
| No. | Discovered Activity, (Pa) * | Reported Activity |
|---|---|---|
| 129 | Atherosclerosis treatment (0.977) Antioxidant (0.963) Antihypercholesterolemic (0.956) Antiparkinsonian (0.955) Neurodegenerative diseases treatment (0.954) Alzheimer’s disease treatment (0.940) Antihyperlipoproteinemic (0.811) | Activity not studied |
| 130 | Antihypercholesterolemic (0.958) Atherosclerosis treatment (0.890) Alzheimer’s disease treatment (0.838) Antioxidant (0.807) Neurodegenerative diseases treatment (0.801) | Activity not studied |
| 131 | Antioxidant (0.922) Atherosclerosis treatment (0.908) Neurodegenerative diseases treatment (0.877) Antihypercholesterolemic (0.869) Alzheimer’s disease treatment (0.868) Antiparkinsonian (0.848) | Activity not studied |
| 132 | Antihypercholesterolemic (0.909) Atherosclerosis treatment (0.876) Alzheimer’s disease treatment (0.828) Neurodegenerative diseases treatment (0.808) Biliary tract disorders treatment (0.807) | Activity not studied |
| No. | Discovered Activity, (Pa) * | Reported Activity |
|---|---|---|
| 133 | Antihypercholesterolemic (0.967) Antineoplastic (0.824) Bone diseases treatment (0.796) Hypolipemic (0.785) Neuroprotector (0.758) Antipsoriatic (0.739) Anti-inflammatory (0.728) Apoptosis agonist (0.724) Prostate disorders treatment (0.719) | Activity not studied |
| 134 | Antihypercholesterolemic (0.927) Bone diseases treatment (0.784) Hypolipemic (0.740) | Activity not studied |
| 135 | Antihypercholesterolemic (0.920) Growth stimulant (0.805) Bone diseases treatment (0.743) | Activity not studied |
| 136 | Antihypercholesterolemic (0.901) Bone diseases treatment (0.720) Growth stimulant (0.703) | Activity not studied |
| 137 | Antihypercholesterolemic (0.912) Bone diseases treatment (0.777) | Activity not studied |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembitsky, V.M. In Silico Prediction of Steroids and Triterpenoids as Potential Regulators of Lipid Metabolism. Mar. Drugs 2021, 19, 650. https://doi.org/10.3390/md19110650
Dembitsky VM. In Silico Prediction of Steroids and Triterpenoids as Potential Regulators of Lipid Metabolism. Marine Drugs. 2021; 19(11):650. https://doi.org/10.3390/md19110650
Chicago/Turabian StyleDembitsky, Valery M. 2021. "In Silico Prediction of Steroids and Triterpenoids as Potential Regulators of Lipid Metabolism" Marine Drugs 19, no. 11: 650. https://doi.org/10.3390/md19110650
APA StyleDembitsky, V. M. (2021). In Silico Prediction of Steroids and Triterpenoids as Potential Regulators of Lipid Metabolism. Marine Drugs, 19(11), 650. https://doi.org/10.3390/md19110650