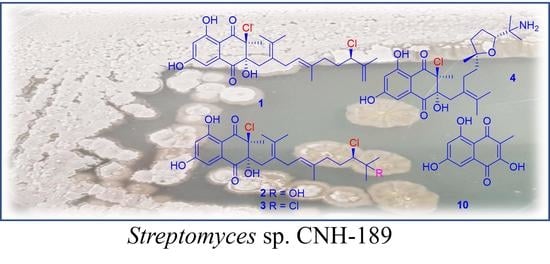
Antibacterial Meroterpenoids, Merochlorins G–J from the Marine Bacterium Streptomyces sp.
Abstract
:1. Introduction
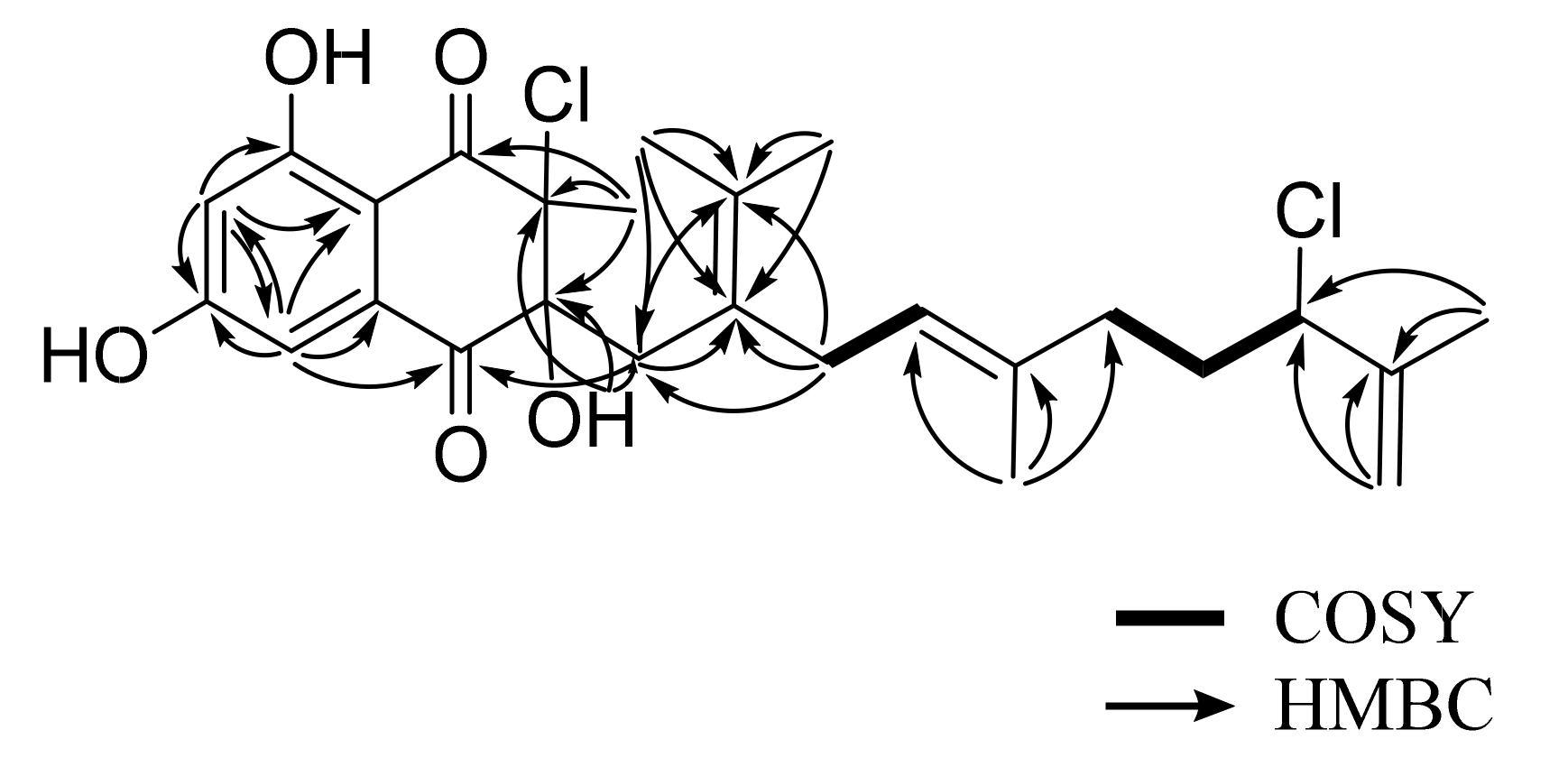
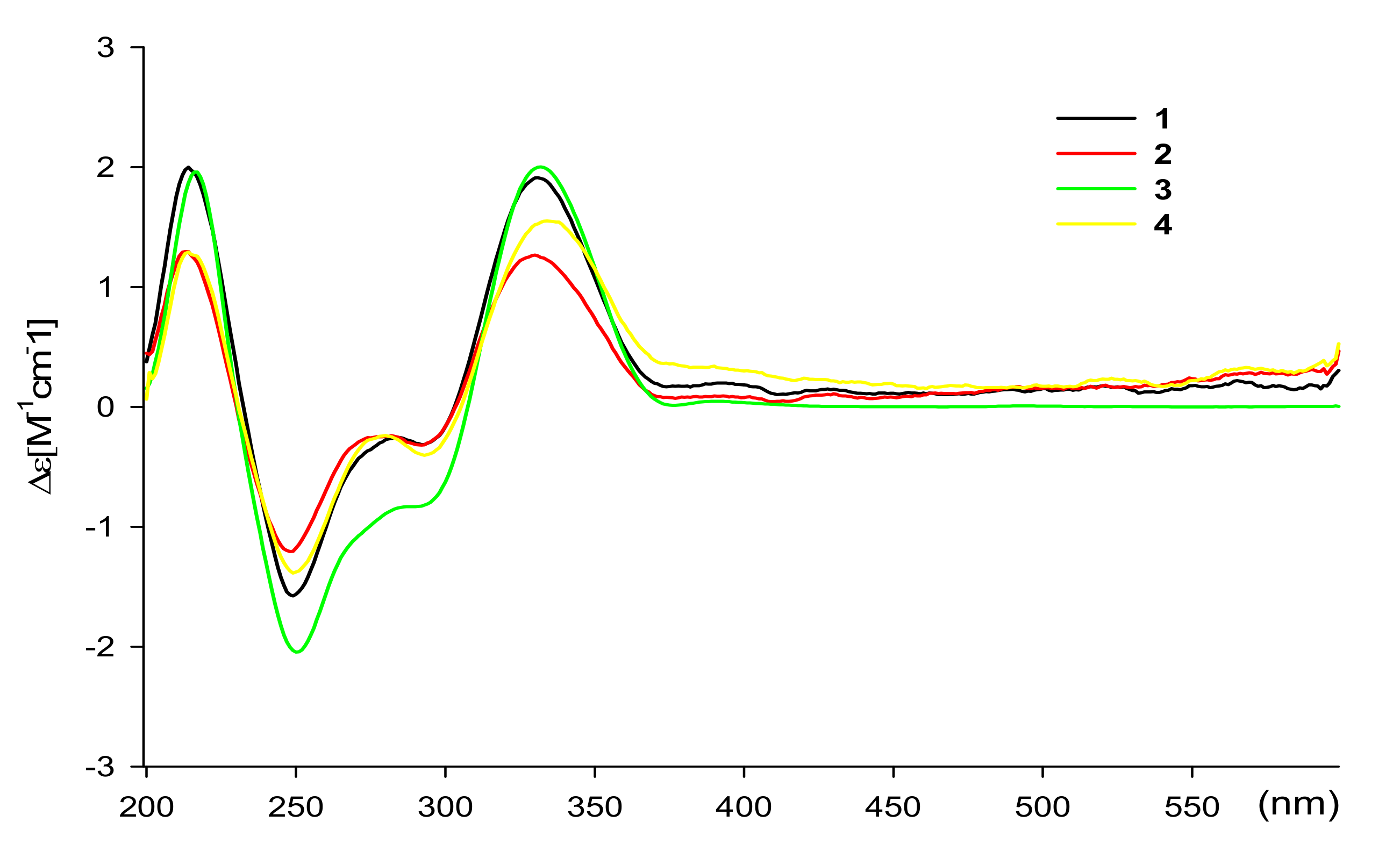
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Collection and Phylogenetic Analysis of Strain CNH-189
3.3. Fermentation
3.4. Extraction and Isolation
3.5. Conformational Search and DP4 Calculations
3.6. Antibacterial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nett, M.; Ikeda, H.; Moore, B.S. Genomic Basis for Natural Product Biosynthetic Diversity in the Actinomycetes. Nat. Prod. Rep. 2009, 26, 1362–1384. [Google Scholar] [CrossRef]
- Almasi, F.; Mohammadipanah, F.; Adhami, H.R.; Hamedi, J. Introduction of Marine-Derived Streptomyces sp. UTMC 1334 as A Source of Pyrrole Derivatives with Anti-Acetylcholinesterase Activity. J. Appl. Microbiol. 2018, 125, 1370–1382. [Google Scholar] [CrossRef]
- Mohammadipanah, F.; Matasyoh, J.; Hamedi, J.; Klenk, H.P.; Laatsch, H. Persipeptides A and B, Two Cyclicpeptides from Streptomyces sp. UTMC 1154. Bioorganic Med. Chem. 2012, 20, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Imada, C. Enzyme Inhibitors and Other Bioactive Compounds from Marine Actinomycetes. Antonie Van Leeuwenhoek 2005, 87, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Salimi, F.; Hamedi, J.; Motevaseli, E.; Mohammadipanah, F. Isolation and Screening of Rare-Actinobacteria, A New Insight for Finding Natural Products with Anti-Vascular Calcification Activity. J. Appl. Microbiol. 2017, 124, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, R.l.G.a.; Olano, C.; Gomez, C.; Fernandez, R.; Brana, A.F.; Mendez, C.; Calle, F.; Salas, J.A. Characterization and Engineering of the Biosynthesis Gene Cluster for Antitumor Macrolides PM100117 and PM100118 from A Marine Actinobacteria: Generation of A Novel Improved Derivative. Microb. Cell Fact. 2017, 124, 254–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azarakhsh, Y.; Mohammadipanah, F.; Nassiri, S.M.; Siavashi, V.; Hamedi, J. Isolation and Screening of Proangiogenic and Antiangiogenic Metabolites Producing Rare Actinobacteria from Soil. J. Appl. Microbiol. 2017, 122, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Dharmaraj, S. Marine Streptomyces as a Novel Source of Bioactive Substances. World J. Microbiol. Biotechnol. 2010, 26, 2123–2139. [Google Scholar] [CrossRef]
- Prieto-Davó, A.; Fenical, W.; Jensen, P.R. Comparative Actinomycete Diversity in Marine Sediments. Aquat. Microb. Ecol. 2008, 52, 1–11. [Google Scholar] [CrossRef]
- Udwary, D.W.; Zeigler, L.; Asolkar, R.N.; Singan, V.; Lapidus, A.; Fenical, W.; Jensen, P.R.; Moore, B.S. Genome Sequencing Reveals Complex Secondary Metabolome in the Marine Actinomycete Salinispora Tropica. Proc. Natl. Acad. Sci. USA 2007, 104, 10376–10381. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.J.; Kauffman, C.A.; Paul, L.A.; Jensen, P.R.; Fenical, W. Actinoranone, A Cytotoxic Meroterpenoid of Unprecedented Structure from A Marine Adapted Sterptomyces sp. Org. Lett. 2013, 15, 5400–5403. [Google Scholar] [CrossRef] [Green Version]
- Sproule, A.; Correa, H.; Decken, A.; Haltli, B.; Berrue, F.; Overy, D.P.; Kerr, R.G. Terrosamycins A and B, Bioactive Polyether Ionophores from Streptomyces sp. RKND004 from Prince Edward Island Sediment. Mar. Drug. 2019, 17, 347. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.C.; Cullum, R.; Hebishy, A.M.S.; Mohamed, H.A.; Faraag, A.H.I.; Salah, N.M.; Abdelfattah, M.S.; Fenical, W. Mersaquinone, A New Tetracene Derivative from The Marine-Derived Streptomyces sp. EG1 Exhibiting Activity Against Methicillin-Resistance Staphylococcus aureus (MRSA). Antibiotics 2020, 9, 252. [Google Scholar] [CrossRef]
- Paderog, M.J.V.; Suarez, A.F.L.; Sabido, E.M.; Low, Z.J.; Saludes, J.P.; Dalisay, D.S. Anthracycline Shunt Metabolites from Philippine Marine Sediment-Derived Streptomyces Destroy Cell Membrane Integrity of Multidrug-Resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 743. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Yang, J.; Yu, J.; Li, J.; Yuan, J.; Wong, N.; Ju, J. Chlorinated Bis-indole Alkaloids from Deep-Sea Derived Streptomyces sp. SCSIO 11791 with Antibacterial and Cytotoxic Activities. J. Antibiot. 2020, 73, 542–547. [Google Scholar] [CrossRef]
- Wilson, M.C.; Nam, S.J.; Gulder, T.A.M.; Kauffman, C.A.; Jensen, P.R.; Fenical, W.; Moore, B.S. Structure and Biosynthesis of the Marine Streptomycete Ansamycin Ansalactam A and Its Distinctive Branched Chain Polyketide Extender Unit. J. Am. Chem. Soc. 2011, 133, 1971–1977. [Google Scholar] [CrossRef] [Green Version]
- Le, T.C.; Yang, I.; Yoon, Y.J.; Nam, S.J.; Fenical, W. Ansalactams B-D Illustrate Further Biosynthetic Plasticity within the Ansamycin Pathway. Org. Lett. 2016, 18, 2256–2259. [Google Scholar] [CrossRef]
- Kaysser, L.; Bernhardt, P.; Nam, S.J.; Loesgen, S.; Ruby, J.G.; Skewes-Cox, P.; Jensen, P.R.; Fenical, W.; Moore, B.S. Merochlorins A-D, Cyclic Meroterpenoid Antibiotics Biosynthesized in Divergent Pathways with Vanadium-Dependent Chloroperoxidases. J. Am. Chem. Soc. 2012, 134, 11988–11991. [Google Scholar] [CrossRef] [Green Version]
- Ryu, M.J.; Hwang, S.; Kim, S.; Yang, I.; Oh, D.C.; Nam, S.J.; Fenical, W. Meroindenon and Merochlorins e and F, Antibacterial Meroterpenoids from a Marine-Derived Sediment Bacterium of the Genus Streptomyces. Org. Lett. 2019, 21, 5779–5783. [Google Scholar] [CrossRef]
- Sabutskii, Y.E.; Polonik, S.G.; Denisenko, V.A.; Dmitrenok, P.S. A New Method for Thiomethylation of Hydroxy-1,4-naphthoquinones with N-Acetyl-L-cystein; First Synthesis of Fibrostatin, B,C, and D. Synthesis 2014, 46, 2763–2770. [Google Scholar]
- Zanardi, M.M.; Suarez, A.G.; Sarotti, A.M. Determination of the Relative Configuration of Terminal and Spiroepoxides by Computational Methods. Advantages of the Inclusion of Unscaled Data. J. Org. Chem. 2020, 2020. 85, 11566–11570. [Google Scholar] [CrossRef]
- Maxwell, A. and Rampersad, D. Novel Prenylated Hydroxybenzoic Acid Derivatives from Piper Saltuum. J. Nat. Prod. 1989, 52, 614–618. [Google Scholar] [CrossRef]
- Miles, Z.D.; Diethelm, S.; Pepper, H.P.; Huang, D.M.; George, J.H.; Moore, B.S. A Unifying Paradigm for Naphthoquinone-Based Meroterpenoid (Bio)Synthesis. Nat. Chem. 2017, 9, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Iwai, T.; Kinoshita, T. Revised Structure of Squalene-Derived PentaTHF Polyether, Glabrescol, through Its Enantioselective Total Synthesis: Biogenetically Intriguing C(s) vs C2 Symmetric Relationships. J. Am. Chem. Soc. 2000, 122, 7124–7125. [Google Scholar] [CrossRef]
- Lorente, A.; Lamariano-Merketegi, J.; Albericio, F.; Álvarez, M. Tetrahydrofuran-Containing Macrolides: A Fascinating Gift from the Deep Sea. Chem. Rev. 2013, 113, 4567–4610. [Google Scholar] [CrossRef] [PubMed]
- Bahn, S.; Imm, S.; Neubert, L.; Zhang, M.; Neumann, H.; Beller, M. The Catalytic amination of Alcohols. ChemCatChem. 2011, 3, 1853–1864. [Google Scholar] [CrossRef]
- Saha, N.; Muller, M.; Husain, S.S. Asymmetric Synthesis of Natural cis-Dihydroarenediols Using Tetrahydroxynaphthalene Reductase and Its Biosynthetic Implications. Org. Lett. 2019, 21, 2204–2208. [Google Scholar] [CrossRef] [PubMed]
- López-Pérez, B.; Pepper, H.P.; Ma, R.; Fawcett, B.J.; Pehere, A.D.; Wei, Q.; Ji, Z.; Polyak, S.W.; Dai, H.; Song, F.; et al. Biosynthetically Guided Structure–Activity Relationship Studies of Merochlorin A, an Antibiotic Marine Natural Product. ChemMedChem 2017, 12, 1969–1976. [Google Scholar] [CrossRef]
- Yang, H.; Liu, X.; Li, Q.; Li, L.; Zhang, J.R.; Tang, Y. Total Synthesis and Preliminary SAR Study of (±)-Merochlorins A and B. Org. Biomol. Chem. 2015, 14, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Sakoulas, G.; Nam, S.J.; Loesgen, S.; Fenical, W.; Jensen, P.R.; Nizet, V.; Hensler, M. Novel Bacterial Metabolite Merochlorin A Demonstrates in Vitro Activity against Multi-Drug Resistant Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2012, 7, 1–6. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]


| No. | 1 a | 2 b | 3 b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| δC, Mult. c | δH (J in Hz) | COSY | HMBC | δC, Mult. c | δH (J in Hz) | δC, Mult. c | δH (J in Hz) | ||
| 1 | 196.1, C | 195.2, C | 195.1, C | ||||||
| 2 | 134.7, C | 135.1, C | 135.0, C | ||||||
| 3 | 107.4, CH | 7.01, d (2.0) | H-5 | 1, 2, 4, 5, 7 | 107.3, CH | 6.84, d (2.0) | 107.3, CH | 6.94, d (2.0) | |
| 4 | 162.9, C | 165.7, C | 164.2, C | ||||||
| 5 | 108.9, CH | 6.71, d (2.0) | H-3 | 3, 4, 6, 7 | 107.8, CH | 6.64, d (2.0) | 107.7, CH | 6.62, d (2.0) | |
| 6 | 165.3, C | 164.2, C | 165.7, C | ||||||
| 7 | 109.9, C | 108.0, C | 108.0, C | ||||||
| 8 | 194.8, C | 193.4, C | 193.3, C | ||||||
| 9 | 74.5, C | 76.2, C | 76.2, C | ||||||
| 10 | 84.0, C | 84.0, C | 84.0, C | ||||||
| 11 | 38.6, CH2 | 2.61, d (14.0), 2.37, d (14.0) | 1, 13 | 30.9, CH2 | 1.38, s | 31.1, CH2 | 2.13, m | ||
| 12 | 131.6, C | 125.9, C | 125.7, C | ||||||
| 13 | 31.3, CH2 | 2.84, dd (15.2, 6.7), 2.45, dd (15.2, 6.7) | H-14 | 11, 14, 24 | 30.3, CH2 | 2.88, dd (15.2, 7.2), 2.42, dd (15.2, 7.2) | 30.3, CH2 | 2.86, dd (15.2, 7.2), 2.40, dd (15.2, 7.2) | |
| 14 | 123.5, CH | 4.87, t (7.0) | H-13 | 13, 16, 24, 26 | 123.2, CH | 4.89, t (7.0) | 123.9, CH | 4.90, t (7.0) | |
| 15 | 134.0, C | 133.5, C | 132.9, C | ||||||
| 16 | 36.6, CH2 | 2.05, m d, 1.95, m d | H-17 | 14, 17, 18, 26 | 36.8, CH2 | 2.15, m d, 1.95, m d | 36.3, CH2 | 2.18, m d, 2.00, m d | |
| 17 | 34.8, CH2 | 1.92, m d, 1.87, m d | H-16, H-18 | 16, 18 | 31.3, CH2 | 1.28, d (9.2) | 39.2, CH2 | 2.45, d (14.6), 2.27, d (14.6) | |
| 18 | 66.4, CH | 4.33, t (7.0) | H-17 | 17, 20, 21 | 69.8, CH | 3.51, t (2.0) | 70.7, CH | 3.93, t (2.0) | |
| 19 | 144.3, C | 71.7, C | 72.9, C | ||||||
| 20 | 114.2, CH2 | 4.99, s, 4.90, quint (1.4) | 18, 21 | 24.4, CH3 | 1.12, s | 29.1, CH3 | 1.59, s | ||
| 21 | 17.0, CH3 | 1.82, s | 18, 19 | 27.6, CH3 | 1.18, s | 30.3, CH3 | 1.63, s | ||
| 22 | 125.1, C | 129.5, C | 129.7, C | ||||||
| 23 | 20.9, CH3 | 1.20, s | 10, 11, 12, 13, 14, 22, 24 | 20.3, CH3 | 1.05, s | 20.7, CH3 | 1.09, s | ||
| 24 | 20.6, CH3 | 1.58, s | 10, 11, 12, 22, 23 | 20.2, CH3 | 1.49, s | 20.3, CH3 | 1.49, s | ||
| 25 | 18.1, CH3 | 1.94, s | 17 | 15.6, CH3 | 1.47, s | 15.4, CH3 | 1.45, s | ||
| 26 | 15.9, CH3 | 1.51, s | 8, 9, 10 | 18.0, CH3 | 1.80, s | 18.1, CH3 | 1.79, s | ||
| 4-OH | 5.77, s | 3, 4, 5 | 11.43, s | 11.48, s | |||||
| 6-OH | 11.70, s | 4, 5, 6, 7, 8 | 11.52, s | 11.52, s | |||||
| 10-OH | 4.08, s | 9, 10, 11 | 6.14, s | 6.13, s | |||||
| No. | 4 | |||
|---|---|---|---|---|
| δC, Mult. b | δH (J in Hz) | COSY | HMBC | |
| 1 | 195.3, C | |||
| 2 | 134.9, C | |||
| 3 | 107.2, CH | 6.81, d (2.0) | H-5 | 1, 2, 4, 5, 7 |
| 4 | 164.1, C | |||
| 5 | 107.7, CH | 6.62, d (2.0) | H-3 | 3, 4, 6, 7 |
| 6 | 165.2, C | |||
| 7 | 107.8, C | |||
| 8 | 193.6, C | |||
| 9 | 76.1, C | |||
| 10 | 84.0, C | |||
| 11 | 37.9, CH2 | 2.45, d (16.0), 2.31, d (16.0) | 9, 10, 12, 13, 23 | |
| 12 | 126.4, C | |||
| 13 | 26.3, CH2 | 2.23, d (14.0), 1.65, m c | H-14 | 11, 23 |
| 14 | 39.7, CH2 | 1.42, m c, 1.17, m c | H-13 | 15, 25 |
| 15 | 83.4, C | |||
| 16 | 36.3, CH2 | 1.56, m c | H-17 | 14, 15, 17, 18, 25 |
| 17 | 25.9, CH2 | 1.88, m c 1.64, m c | H-16, H-18 | 15, 16, 18, 19 |
| 18 | 81.0, CH | 3.82, t (7.6) | H-17 | 20, 21 |
| 19 | 55.5, C | |||
| 20 | 19.7, CH3 | 1.14, s | 18, 19, 21 | |
| 21 | 22.8, CH3 | 1.16, s | 18, 19, 20 | |
| 22 | 129.2, C | |||
| 23 | 20.0, CH3 | 1.05, s | 12, 22, 24 | |
| 24 | 20.0, CH3 | 1.47, s | 12, 22, 23 | |
| 25 | 24.7, CH3 | 1.06, s | 14, 15, 16 | |
| 26 | 18.1, CH3 | 1.81, s | 8, 9, 10 | |
| 10-OH | 6.16, br s | 1, 9, 10, 11 | ||
| MIC (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Gram-Positive Bacteria | Gram-Negative Bacteria | ||||||
| B. subtilis KCTC 1021 | K. rhizophila KCTC 1915 | S. aureus KCTC 1927 | E. coli KCTC 2441 | S. typhimurium KCTC 2515 | K. pneumonia KCTC 2690 | ||
| 1 | 16 | 32 | 16 | >128 | >128 | >128 | |
| 2 | 64 | >128 | >128 | >128 | >128 | >128 | |
| 3 | 1 | 2 | 2 | >128 | >128 | >128 | |
| 4 | >128 | >128 | >128 | >128 | >128 | >128 | |
| 5 | 2 | >128 | 4 | >128 | >128 | >128 | |
| 6 b | 16 | 32 | 32 | >128 | >128 | >128 | |
| 7 | 1 | 0.5 | 1 | >128 | >128 | >128 | |
| 8 b | 1 | 2 | 2 | >128 | >128 | >128 | |
| 9 b | 1 | 2 | 1 | >128 | >128 | >128 | |
| 10 | 32 | 2 | >128 | >128 | >128 | >128 | >128 |
| Ampicillin | 0.25 | 0.25 | 0.25 | 4 | 2 | >128 | |
| Vancomycin | 0.25 | 0.5 | 0.5 | >128 | >128 | >128 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, M.-J.; Hillman, P.F.; Lee, J.; Hwang, S.; Lee, E.-Y.; Cha, S.-S.; Yang, I.; Oh, D.-C.; Nam, S.-J.; Fenical, W. Antibacterial Meroterpenoids, Merochlorins G–J from the Marine Bacterium Streptomyces sp. Mar. Drugs 2021, 19, 618. https://doi.org/10.3390/md19110618
Ryu M-J, Hillman PF, Lee J, Hwang S, Lee E-Y, Cha S-S, Yang I, Oh D-C, Nam S-J, Fenical W. Antibacterial Meroterpenoids, Merochlorins G–J from the Marine Bacterium Streptomyces sp. Marine Drugs. 2021; 19(11):618. https://doi.org/10.3390/md19110618
Chicago/Turabian StyleRyu, Min-Ji, Prima F. Hillman, Jihye Lee, Sunghoon Hwang, Eun-Young Lee, Sun-Shin Cha, Inho Yang, Dong-Chan Oh, Sang-Jip Nam, and William Fenical. 2021. "Antibacterial Meroterpenoids, Merochlorins G–J from the Marine Bacterium Streptomyces sp." Marine Drugs 19, no. 11: 618. https://doi.org/10.3390/md19110618
APA StyleRyu, M.-J., Hillman, P. F., Lee, J., Hwang, S., Lee, E.-Y., Cha, S.-S., Yang, I., Oh, D.-C., Nam, S.-J., & Fenical, W. (2021). Antibacterial Meroterpenoids, Merochlorins G–J from the Marine Bacterium Streptomyces sp. Marine Drugs, 19(11), 618. https://doi.org/10.3390/md19110618