Chitosan-Based Scaffold for Mineralized Tissues Regeneration
Abstract
:1. Introduction
2. Bone Tissue Engineering
3. BTE Scaffold
4. Chitin and Chitosan
5. Processing of Chitin and Chitosan for BTE
6. Applications of Chitosan Scaffolds and Their Limitations in BTE
7. Use of Chitosan Scaffolds in Growth Factors/Genes/Drug Delivery
8. Next-Generation Chitosan Scaffold for BTE
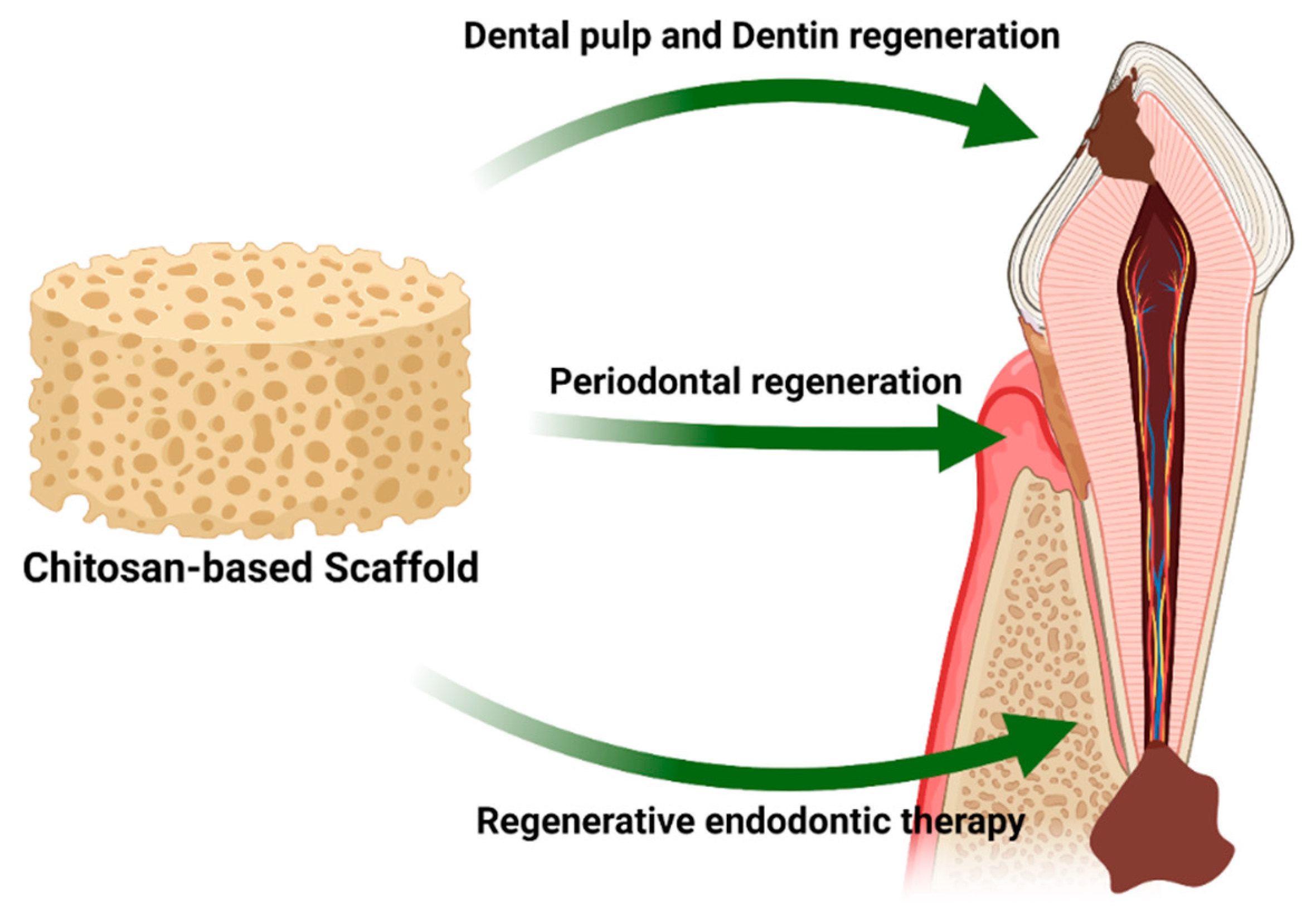
9. Use of Chitosan Scaffolds in Regenerative Dentistry
10. Conclusions and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Manzini, B.M.; Machado, L.M.R.; Noritomi, P.Y.; da Silva, J.V.L. Advances in Bone tissue engineering: A fundamental review. J. Biosci. 2021, 46, 1–18. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Pavia, F.C.; Brucato, V.; La Carrubba, V. Solution-Based Processing for Scaffold Fabrication in Tissue Engineering Applications: A Brief Review. Polymers 2021, 13, 2041. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; De Lima, M.A.B.; Franco, L.D.O.; De Campos-Takaki, G.M. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef] [PubMed]
- Satitsri, S.; Muanprasat, C. Chitin and Chitosan Derivatives as Biomaterial Resources for Biological and Biomedical Applications. Molecules 2020, 25, 5961. [Google Scholar] [CrossRef] [PubMed]
- Ebhodaghe, S.O. Natural Polymeric Scaffolds for Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2021, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Razak, S.I.A.; Al Arjan, W.S.; Nazir, S.; Anand, T.J.S.; Mehboob, H.; Amin, R. Recent Advances in Biopolymeric Composite Materials for Tissue Engineering and Regenerative Medicines: A Review. Molecules 2021, 26, 619. [Google Scholar] [CrossRef]
- Khosla, S.; Westendorf, J.J.; Mödder, U.I. Concise Review: Insights from Normal Bone Remodeling and Stem Cell-Based Therapies for Bone Repair. Stem Cells 2010, 28, 2124–2128. [Google Scholar] [CrossRef] [Green Version]
- Su, N.; Yang, J.; Xie, Y.; Du, X.; Chen, H.; Hong, Z.; Chen, L. Bone function, dysfunction and its role in diseases including critical illness. Int. J. Biol. Sci. 2019, 15, 776–787. [Google Scholar] [CrossRef] [Green Version]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burg, K.J.; Porter, S.; Kellam, J.F. Biomaterial developments for bone tissue engineering. Biomaterials 2000, 21, 2347–2359. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Lim, J.; Teoh, S.-H. Review: Development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol. Adv. 2013, 31, 688–705. [Google Scholar] [CrossRef] [PubMed]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.-Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Iijima, K.; Otsuka, H. Cell Scaffolds for Bone Tissue Engineering. Bioengineering 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Shi, C.; Zhu, Y.; Ran, X.; Wang, M.; Su, Y.; Cheng, T. Therapeutic Potential of Chitosan and Its Derivatives in Regenerative Medicine. J. Surg. Res. 2006, 133, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2016, 25, 1–13. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, S.; Mei, D.; Li, J.; Zhang, J.; Yang, S.; Guan, S. Application of 3D Printing Technology in Bone Tissue Engineering: A Review. Curr. Drug Deliv. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Limongelli, L.; Moreo, G.; Favia, G.; Carinci, F. Nanomaterials for Periodontal Tissue Engineering: Chitosan-Based Scaffolds. A Systematic Review. Nanomaterials 2020, 10, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Fasolino, I.; Raucci, M.G.; Soriente, A.; Demitri, C.; Madaghiele, M.; Sannino, A.; Ambrosio, L. Osteoinductive and anti-inflammatory properties of chitosan-based scaffolds for bone regeneration. Mater. Sci. Eng. C 2019, 105, 110046. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.S.; Sá, M.J.C.; Fook, M.V.L.; Neto, P.I.N.; Sousa, O.B.; Azevedo, S.S.; Teixeira, M.W.; Costa, F.S.; Araújo, A.L. Use of chitosan and β-tricalcium phosphate, alone and in combination, for bone healing in rabbits. J. Mater. Sci. Mater. Med. 2014, 25, 481–486. [Google Scholar] [CrossRef] [Green Version]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Suwattanachai, P.; Pimkhaokham, A.; Chirachanchai, S. Multi-functional carboxylic acids for chitosan scaffold. Int. J. Biol. Macromol. 2019, 134, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Sukpaita, T.; Chirachanchai, S.; Suwattanachai, P.; Everts, V.; Pimkhaokham, A.; Ampornaramveth, R.S. In Vivo Bone Regeneration Induced by a Scaffold of Chitosan/Dicarboxylic Acid Seeded with Human Periodontal Ligament Cells. Int. J. Mol. Sci. 2019, 20, 4883. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.; Li, M.; Yang, Y.; Wan, Y.; Wang, X.; Bi, N.; Li, C. Advancements and Frontiers in the High Performance of Natural Hydrogels for Cartilage Tissue Engineering. Front. Chem. 2020, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, S.; Vimalraj, S.; Lakshmanan, G.; Jindal, A.; Sundaramurthi, D.; Bhattacharya, J. Chitosan-Based Biocomposite Scaffolds and Hydrogels for Bone Tissue Regeneration. In Marine-Derived Biomaterials for Tissue Engineering Applications; Choi, A.H., Ben-Nissan, B., Eds.; Springer: Singapore, 2019; pp. 413–442. [Google Scholar]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Review of Bioactive Glass/Natural Polymer Composites: State of the Art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef]
- Soriente, A.; Fasolino, I.; Gomez-Sánchez, A.; Prokhorov, E.; Buonocore, G.G.; Luna-Barcenas, G.; Ambrosio, L.; Raucci, M.G. Chitosan/hydroxyapatite nanocomposite scaffolds to modulate osteogenic and inflammatory response. J. Biomed. Mater. Res. Part A 2021. [Google Scholar] [CrossRef] [PubMed]
- Chatzipetros, E.; Damaskos, S.; Tosios, K.I.; Christopoulos, P.; Donta, C.; Kalogirou, E.-M.; Yfanti, Z.; Tsiourvas, D.; Papavasiliou, A.; Tsiklakis, K. The effect of nano-hydroxyapatite/chitosan scaffolds on rat calvarial defects for bone regeneration. Int. J. Implant. Dent. 2021, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.; Li, J.; Cui, X.; Jiang, M.; Zhang, M.; Wang, X.; Zhang, W.; Liu, Z. Bilayer Membrane Composed of Mineralized Collagen and Chitosan Cast Film Coated with Berberine-Loaded PCL/PVP Electrospun Nanofiber Promotes Bone Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 684335. [Google Scholar] [CrossRef]
- Schoeller, J.; Itel, F.; Wuertz-Kozak, K.; Gaiser, S.; Luisier, N.; Hegemann, D.; Ferguson, S.; Fortunato, G.; Rossi, R. pH-Responsive Chitosan/Alginate Polyelectrolyte Complexes on Electrospun PLGA Nanofibers for Controlled Drug Release. Nanomaterials 2021, 11, 1850. [Google Scholar] [CrossRef]
- Aydogdu, M.O.; Oner, E.T.; Ekren, N.; Erdemir, G.; Kuruca, S.E.; Yuca, E.; Bostan, M.S.; Eroglu, M.S.; Ikram, F.; Uzun, M.; et al. Comparative characterization of the hydrogel added PLA/β-TCP scaffolds produced by 3D bioprinting. Bioprinting 2019, 13, e00046. [Google Scholar] [CrossRef]
- Shayganfard, M. A Review on Chitosan in Drug Delivery for the Treatment of Neurological and Psychiatric Disorders. Curr. Pharm. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Iacob, A.; Lupascu, F.; Apotrosoaei, M.; Vasincu, I.; Tauser, R.; Lupascu, D.; Giusca, S.; Caruntu, I.-D.; Profire, L. Recent Biomedical Approaches for Chitosan Based Materials as Drug Delivery Nanocarriers. Pharmaceutics 2021, 13, 587. [Google Scholar] [CrossRef]
- Aoki, K.; Saito, N. Biodegradable Polymers as Drug Delivery Systems for Bone Regeneration. Pharmaceutics 2020, 12, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, M.; Benoit, D.S. Local and targeted drug delivery for bone regeneration. Curr. Opin. Biotechnol. 2016, 40, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarigol-Calamak, E.; Hascicek, C. Tissue Scaffolds as a Local Drug Delivery System for Bone Regeneration. Adv. Exp. Med. Biol. 2018, 1078, 475–493. [Google Scholar] [CrossRef]
- Ogay, V.; Mun, E.A.; Kudaibergen, G.; Baidarbekov, M.; Kassymbek, K.; Zharkinbekov, Z.; Saparov, A. Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration. Polymers 2020, 12, 2881. [Google Scholar] [CrossRef]
- Shi, S.; Jiang, W.; Zhao, T.; Aifantis, K.E.; Wang, H.; Lin, L.; Fan, Y.; Feng, Q.; Cui, F.-Z.; Li, X. The application of nanomaterials in controlled drug delivery for bone regeneration. J. Biomed. Mater. Res. Part A 2015, 103, 3978–3992. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Ren, W.; Hou, R.; Liu, H.; Li, R.; Du, S.; Wang, L.; Liu, J. PEI-modified diatomite/chitosan composites as bone tissue engineering scaffold for sustained release of BMP-2. J. Biomater. Sci. Polym. Ed. 2021, 32, 1337–1355. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Guo, Q. Silk fibroin hydrogel scaffolds incorporated with chitosan nanoparticles repair articular cartilage defects by regulating TGF-β1 and BMP-2. Arthritis Res. Ther. 2021, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- Lara-Velazquez, M.; Alkharboosh, R.; Norton, E.S.; Ramirez-Loera, C.; Freeman, W.D.; Guerrero-Cazares, H.; Forte, A.J.; Quiñones-Hinojosa, A.; Sarabia-Estrada, R. Chitosan-Based Non-viral Gene and Drug Delivery Systems for Brain Cancer. Front. Neurol. 2020, 11, 740. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.J.; Venkatraman, S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef] [Green Version]
- Raftery, R.; O′Brien, F.J.; Cryan, S.-A. Chitosan for Gene Delivery and Orthopedic Tissue Engineering Applications. Molecules 2013, 18, 5611–5647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Lv, L.; Dai, Y.; Wu, G.; Zhao, H.; Zhang, F. Porous Chitosan Scaffolds with Embedded Hyaluronic Acid/Chitosan/Plasmid-DNA Nanoparticles Encoding TGF-β1 Induce DNA Controlled Release, Transfected Chondrocytes, and Promoted Cell Proliferation. PLoS ONE 2013, 8, e69950. [Google Scholar] [CrossRef]
- Montoya, C.; Du, Y.; Gianforcaro, A.L.; Orrego, S.; Yang, M.; Lelkes, P.I. On the road to smart biomaterials for bone research: Definitions, concepts, advances, and outlook. Bone Res. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Nafee, N.; Zewail, M.; Boraie, N. Alendronate-loaded, biodegradable smart hydrogel: A promising injectable depot formulation for osteoporosis. J. Drug Target. 2018, 26, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tanaka, M. Designing Smart Biomaterials for Tissue Engineering. Int. J. Mol. Sci. 2017, 19, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; He, J.; Yong, X.; Lu, J.; Xiao, J.; Liao, Y.; Li, Q.; Xiong, C. Biodegradable poly (lactic acid-co-trimethylene carbonate)/chitosan microsphere scaffold with shape-memory effect for bone tissue engineering. Colloids Surf. B Biointerfaces 2020, 195, 111218. [Google Scholar] [CrossRef]
- Fu, C.-Y.; Chuang, W.-T.; Hsu, S.-H. A Biodegradable Chitosan-Polyurethane Cryogel with Switchable Shape Memory. ACS Appl. Mater. Interfaces 2021, 13, 9702–9713. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef]
- Seo, J.W.; Shin, S.R.; Park, Y.J.; Bae, H. Hydrogel Production Platform with Dynamic Movement Using Photo-Crosslinkable/Temperature Reversible Chitosan Polymer and Stereolithography 4D Printing Technology. Tissue Eng. Regen. Med. 2020, 17, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-D.; Kim, K.-H.; Lee, Y.-M.; Ku, Y.; Seol, Y.-J. Periodontal Wound Healing and Tissue Regeneration: A Narrative Review. Pharmaceuticals 2021, 14, 456. [Google Scholar] [CrossRef]
- Mancini, L.; Romandini, M.; Fratini, A.; Americo, L.; Panda, S.; Marchetti, E. Biomaterials for Periodontal and Peri-Implant Regeneration. Materials 2021, 14, 3319. [Google Scholar] [CrossRef]
- Raveau, S.; Jordana, F. Tissue Engineering and Three-Dimensional Printing in Periodontal Regeneration: A Literature Review. J. Clin. Med. 2020, 9, 4008. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.; Vijayakumar, S.; Canciani, E.; Cochis, A.; De Nardo, L.; Lodi, G.; Rimondini, L.; Cerruti, M. Chitosan-Based Trilayer Scaffold for Multitissue Periodontal Regeneration. J. Dent. Res. 2018, 97, 303–311. [Google Scholar] [CrossRef]
- Liao, Y.; Li, H.; Shu, R.; Chen, H.; Zhao, L.; Song, Z.; Zhou, W. Mesoporous Hydroxyapatite/Chitosan Loaded with Recombinant-Human Amelogenin Could Enhance Antibacterial Effect and Promote Periodontal Regeneration. Front. Cell. Infect. Microbiol. 2020, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, X.; Zhao, S.; Wu, H.; Xu, H.H. Porous chitosan bilayer membrane containing TGF-β1 loaded microspheres for pulp capping and reparative dentin formation in a dog model. Dent. Mater. 2014, 30, 172–181. [Google Scholar] [CrossRef]
- Soares, D.G.; Bordini, E.; Cassiano, F.; Bronze-Uhle, E.S.; Pacheco, L.E.; Zabeo, G.; Hebling, J.; Lisboa-Filho, P.N.; Bottino, M.C.; Costa, C.A.D.S. Characterization of novel calcium hydroxide-mediated highly porous chitosan-calcium scaffolds for potential application in dentin tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2546–2559. [Google Scholar] [CrossRef]
- Raddall, G.; Mello, I.; Leung, B.M. Biomaterials and Scaffold Design Strategies for Regenerative Endodontic Therapy. Front. Bioeng. Biotechnol. 2019, 7, 317. [Google Scholar] [CrossRef]
- Aksel, H.; Mahjour, F.; Bosaid, F.; Calamak, S.; Azim, A.A. Antimicrobial Activity and Biocompatibility of Antibiotic-Loaded Chitosan Hydrogels as a Potential Scaffold in Regenerative Endodontic Treatment. J. Endod. 2020, 46, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
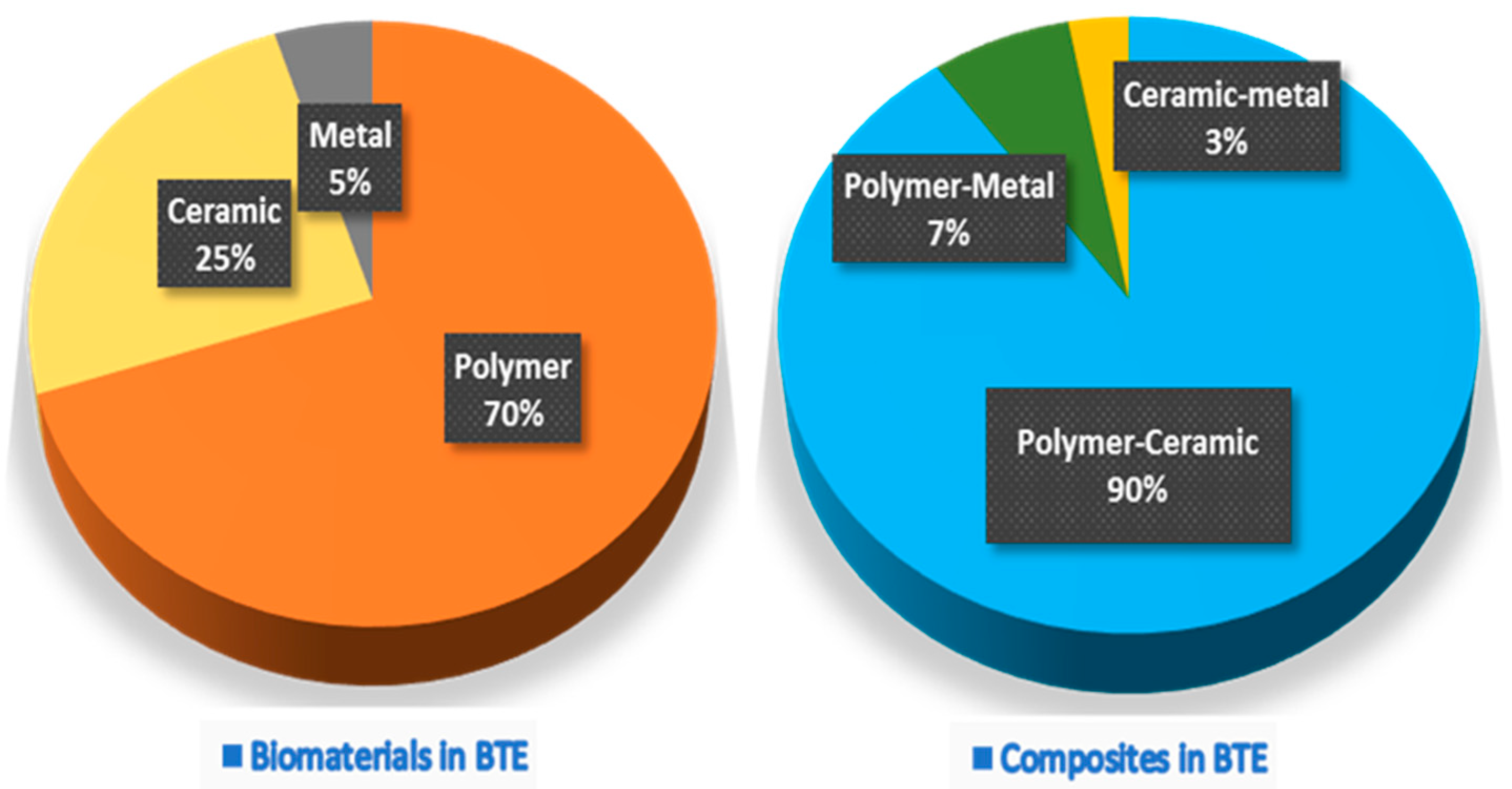
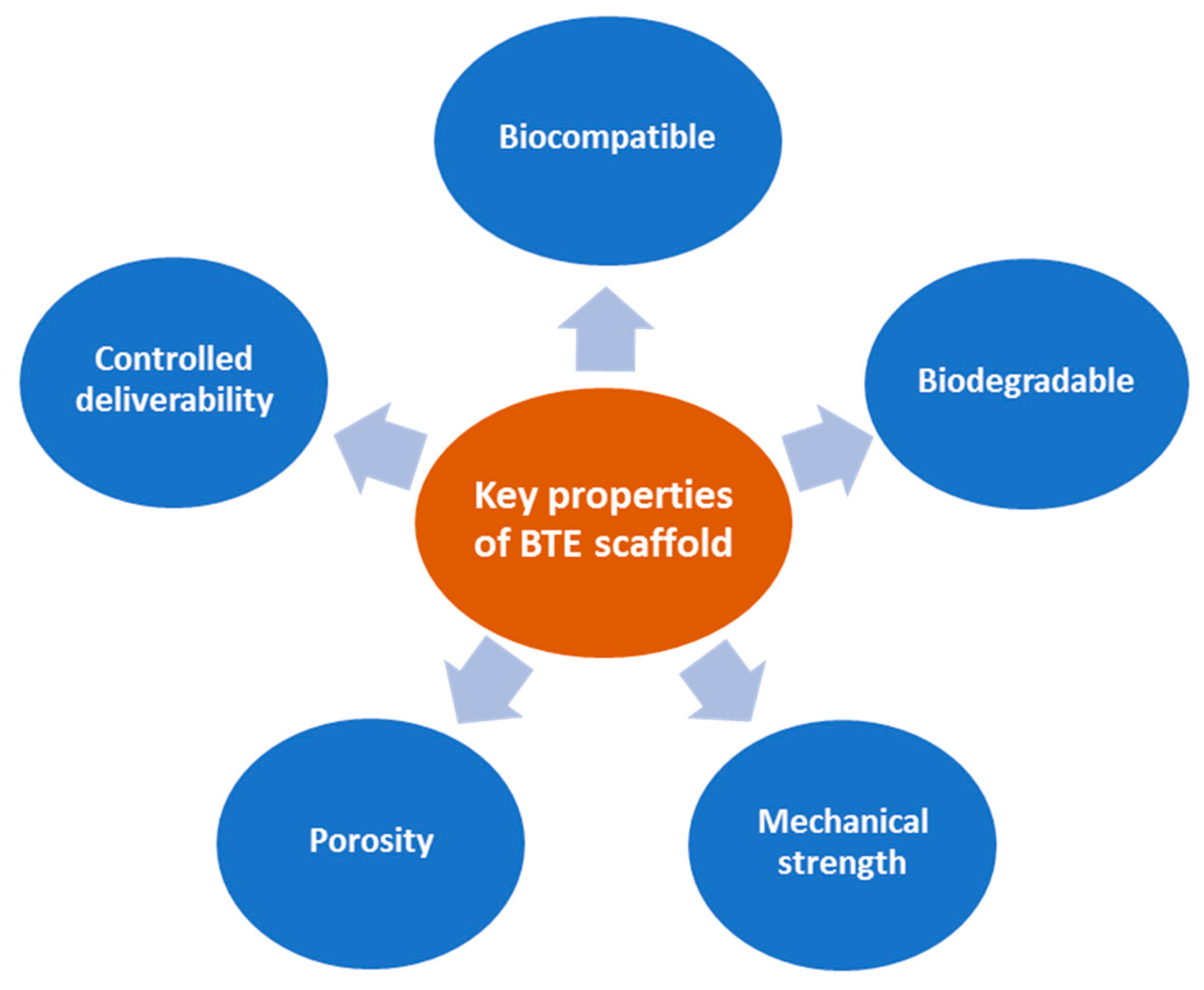

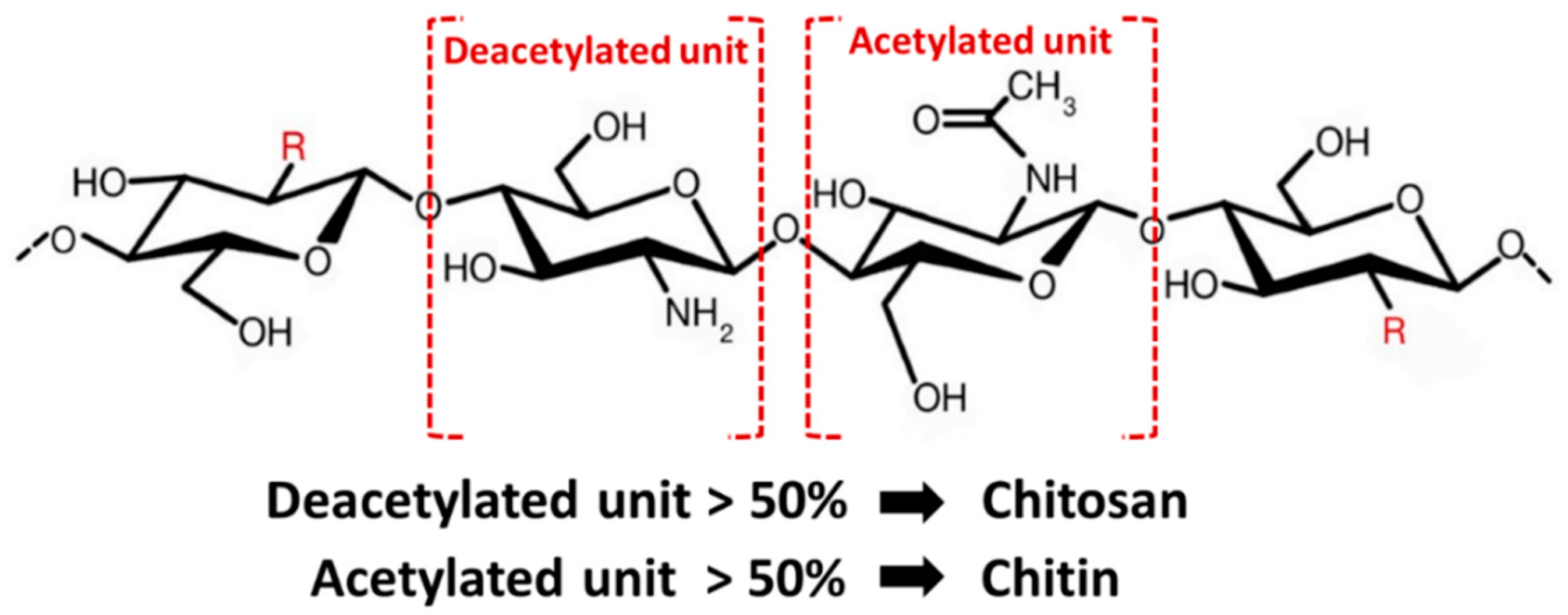
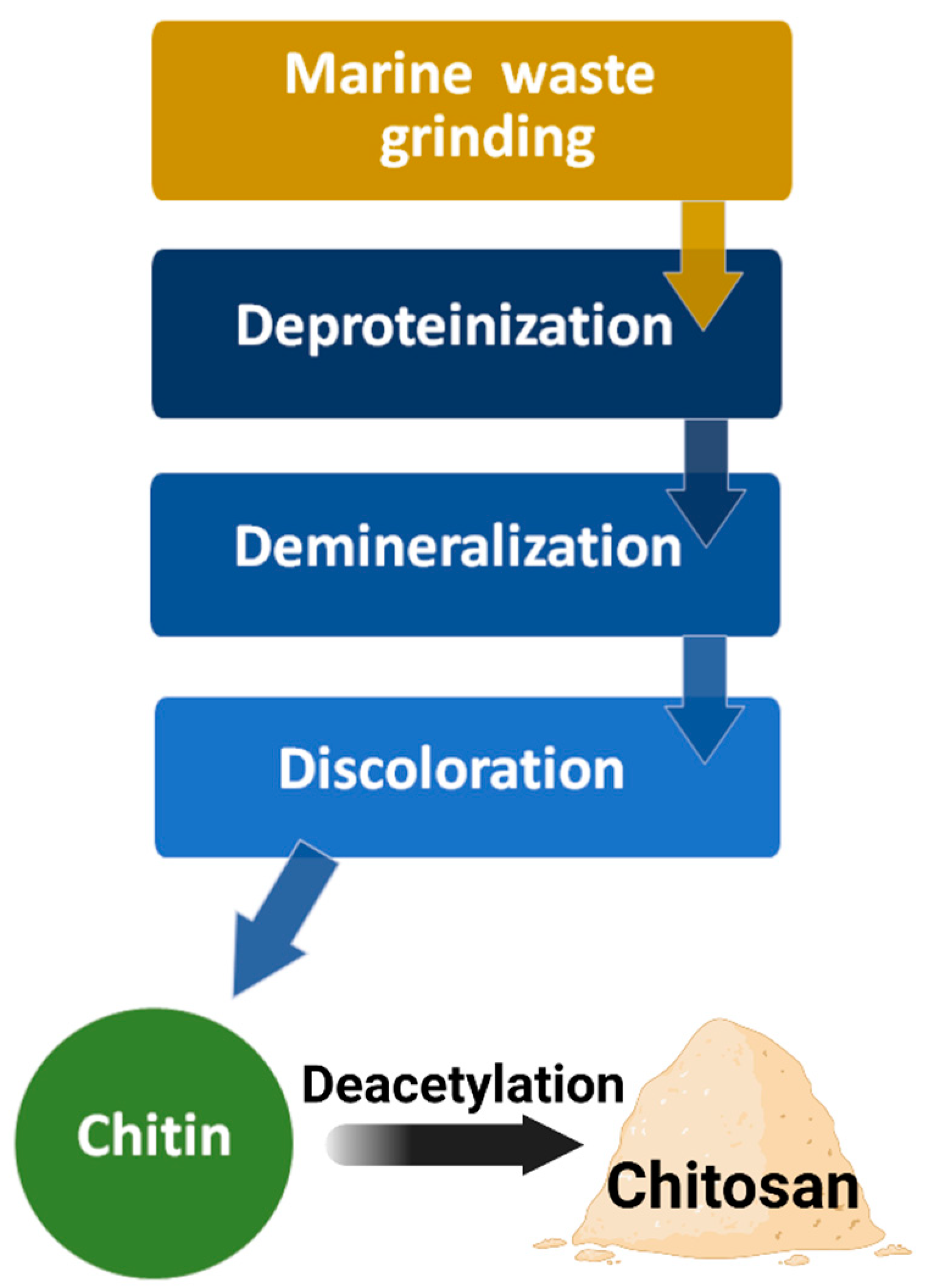

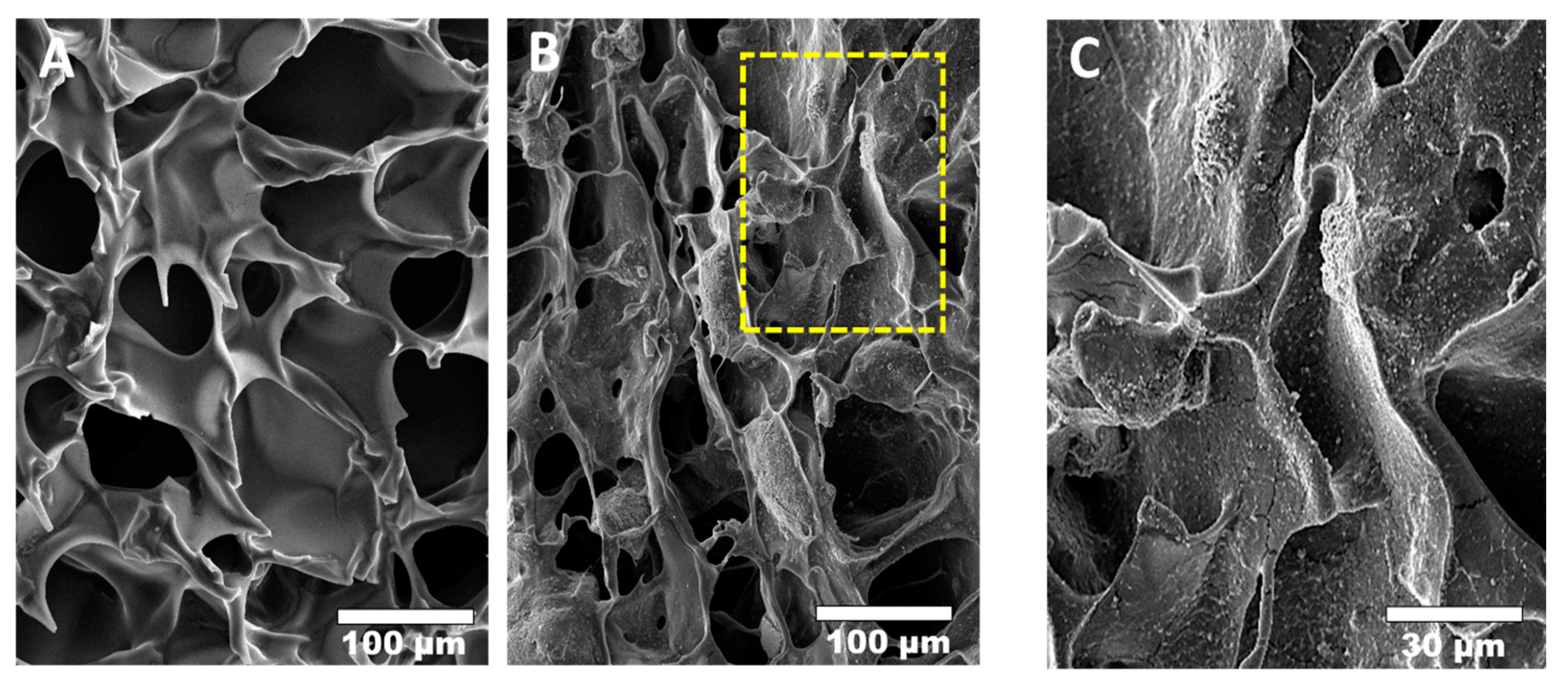

| Material Type | Advantage | Disadvantage | Example Materials |
|---|---|---|---|
| Metal | Biocompatibility Bioinert Good mechanical properties Fatigue resistance | Bioactive molecules cannot be integrated Not biodegradable Metal ion release Low elasticity | Titanium alloy Magnesium alloy Iron alloy |
| Ceramic | Biocompatibility Osteoinductive properties Good mechanical properties | Low fracture toughness High brittleness Difficult to manufacture Slow degradation | Hydroxyapatite (HA) Calcium carbonate (CC) Dicalcium phosphate (DCP) Octacalcium phosphate (OCP) β-Tricalcium phosphate (β-TCP) Biphasic calcium phosphate (BCP) |
| Polymer | Biocompatibility Low antigenicity response Easy formability Enzymatic biodegradability Easy chemical modification Crosslinking capacity | Low osteoinductive capacity Poor mechanical properties | Synthetic polymers Polylactic-co-glycolic acid (PLGA) Polylactic acid (PLA) Polyglycolides (PGA) Polycaprolactone (PCL) Natural polymers Collagen Cellulose Hyaluronan Fibrin Chitosan |
| Composite | Combines the advantages of each material type | Difficult to fabricate | β-TCP-Chitosan HA-Chitosan HA-Collagen HA-PLGA |
| Techniques | Description | Advantages | Disadvantages |
|---|---|---|---|
| Freeze-drying | Chitosan solutions are cooled down to a frozen state, allowed to form ice crystals followed by dehydration | Good pore interconnectivity Without high temperatures Few simple steps Easy control of porosity | Small pore size Low porosity Long fabrication time Expensive technique |
| Gas foaming | Chitosan is placed under pressure with an inert gas, usually carbon dioxide (CO2), resulting in the nucleation of gas bubbles within the structure | Organic solvents not required Inexpensive technique | Insufficient pore interconnectivity Insufficient mechanical strength Nonporous external surface |
| Solvent casting/particulate leaching (SCPL) | Chitosan solution is mixed with water-soluble salt particles and solidified; salt particles are then leached out | Controls the final pore size and porosity Minimal amount of material required Inexpensive technique | Insufficient pore interconnectivity Insufficient mechanical strength -Remaining toxic porogen |
| Electrospinning | Electrostatic forces are applied to draw charged threads of chitosan solutions into fine chitosan nanofibers | Very fine fiber thickness High surface-to-volume ratio Mimics the ECM structure | Limited cell seeding Mechanical strength and porosity decrease with fiber thickness |
| 3D-printing/ Rapid prototyping/Bioprinting | Computer-aided design (CAD) creates a layer-by-layer 3D chitosan scaffold | Complex 3D construct with controlled architecture and porosity Reproducible Easy incorporation of bioactive molecules | Use of high temperatures Insufficient mechanical strength Low-throughput technology Long fabrication time |
| Combination of Biomaterials | Bioactive Drugs/Cells | Fabrication Technique | Observations | Ref. |
|---|---|---|---|---|
| Pure chitosan | hPDLCs | Freeze-dry | In vitro and in vivo experiment No cytotoxicity with hPDLCs Enhanced bone regeneration in mouse calvarial defect model Low mechanical strength Speedy degradation rate | [31,32] |
| Chitosan/HA | - | Freeze-dry | In vitro experiment Chitosan/HA (60% and 70% v/v) scaffold can enhance differentiation of hMSC Can modulate the production of proinflammatory and anti-inflammatory cytokines | [37] |
| Chitosan/HA | - | Freeze-dry | In vivo experiment Chitosan/HA (25% w/w) scaffold provide suitable osteoconductive property Enhanced bone regeneration in rat calvarial defect model Good biodegradability | [38] |
| Chitosan/Mineralized collagen | Berberine | Electrospinning | In vitro and in vivo experiment Favorable mechanical properties Enhanced MC3T3-E1 cells proliferation and attachment Enhanced bone regeneration in rat femoral bone defect model Subsequent sustained release of bioactive drug | [39] |
| Chitosan/Alginate/PLGA | Ibuprofen | Electrospinning | In vitro experiment pH responsiveness for sustained drug release | [40] |
| Chitosan/PLA/β-TCP | Amoxicillin | 3D-bioprinted | In vitro experiment Favorable mechanical properties No cytotoxicity to Saos-2 (human osteosarcoma) cells Increase antimicrobial activity by amoxicillin | [41] |
| Chitosan/Diatomite | BMP-2 | Freeze-dry | In vitro experiment Enhance proliferation and osteogenic differentiation of the mesenchymal stem cells - Slow-release performance of BMP-2 | [50] |
| Silk scaffold /Chitosan nanoparticles | TGF-β1, BMP-2 | Freeze-dry | - In vitro and in vivo experiment - Favorable mechanical properties - No cytotoxicity with bone marrow stromal cells - Bioactive drugs from chitosan nanoparticles can continuously release up to 7 days - Enhanced chondrogenesis in a rabbit knee joint cartilage defect model | [51] |
| Pure Chitosan | Plasmid-DNA Encoding TGF-β1 | Freeze-dry | - In vitro experiment - Increased chondrocyte TGF-β1 expression and proliferation - Sustained release of nanoparticles up to 120 days | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. https://doi.org/10.3390/md19100551
Sukpaita T, Chirachanchai S, Pimkhaokham A, Ampornaramveth RS. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Marine Drugs. 2021; 19(10):551. https://doi.org/10.3390/md19100551
Chicago/Turabian StyleSukpaita, Teerawat, Suwabun Chirachanchai, Atiphan Pimkhaokham, and Ruchanee Salingcarnboriboon Ampornaramveth. 2021. "Chitosan-Based Scaffold for Mineralized Tissues Regeneration" Marine Drugs 19, no. 10: 551. https://doi.org/10.3390/md19100551
APA StyleSukpaita, T., Chirachanchai, S., Pimkhaokham, A., & Ampornaramveth, R. S. (2021). Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Marine Drugs, 19(10), 551. https://doi.org/10.3390/md19100551





