Abstract
The demand for natural products isolated from microalgae has increased over the last decade and has drawn the attention from the food, cosmetic and nutraceutical industries. Among these natural products, the demand for natural antioxidants as an alternative to synthetic antioxidants has increased. In addition, microalgae combine several advantages for the development of biotechnological applications: high biodiversity, photosynthetic yield, growth, productivity and a metabolic plasticity that can be orientated using culture conditions. Regarding the wide diversity of antioxidant compounds and mode of action combined with the diversity of reactive oxygen species (ROS), this review covers a brief presentation of antioxidant molecules with their role and mode of action, to summarize and evaluate common and recent assays used to assess antioxidant activity of microalgae. The aim is to improve our ability to choose the right assay to assess microalgae antioxidant activity regarding the antioxidant molecules studied.
1. Introduction
The demand for natural products isolated from microalgae has increased over the last decade and has drawn attention from the food, cosmetic and nutraceutical industries. Microalgae are eukaryotic unicellular cells that combine several advantages for the development of biotechnological applications: high biodiversity, photosynthetic yield, growth, productivity and a metabolic plasticity that can be orientated using culture conditions [1,2]. Some of these metabolites are molecules of interest such as pigments (e.g., carotenoids), polyunsaturated fatty acids (PUFAs, e.g., the omega-3 or -6 fatty acids), polysaccharides, vitamins and sterols which can be introduced as dietary supplements in human nutrition and animal feed e.g., [3,4]. In addition, most of them are bioactive molecules with anti-inflammatory, antibacterial, anti-UV, antifungal, anticancer, and/or antioxidant activities which may bring added value to cosmetics, nutraceuticals or food products e.g., [5,6,7,8,9].
The demand for natural antioxidants as an alternative to synthetic antioxidants has increased [6,10]. Indeed, many synthetic antioxidants (e.g., butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT)) are considered to have a carcinogenic and/or toxic effect on animal models [11,12,13,14]. Although, most natural antioxidants currently available on the market are derived from terrestrial plants, microalgae are being more and more considered as a potential source of natural antioxidant compounds by the food industry [15,16,17] and by the cosmetic and nutraceutical industries [4,18].
Regarding the wide diversity of antioxidant compounds and mode of action combined with the diversity of ROS, this review first covers a global presentation of antioxidant molecules with their role and mode of action, to finally summarize and evaluate common and recent assays used to assess antioxidant activity of microalgae. The aim of this review is to improve our ability to choose the right assay to assess microalgae antioxidant activity regarding the antioxidant molecules studied. It also emphasizes and discusses the potential use of microalgae by the food industry for their antioxidant activity.
2. Antioxidant and Reactive Oxygen Species (ROS)
An antioxidant is defined as “a substance that, when present at low concentrations compared with those of an oxidizable substrate, significantly delays or prevents oxidation of that substrate” [19]. Antioxidant molecules produced by microalgae are used to protect the cell against reactive oxygen species (ROS) produced in response to biotic or abiotic stressors. Indeed, irradiance, UV, temperature, pH, metals, and nutrient can directly influence the production of antioxidant molecules in response to their availability, either through an excess or a limitation [7,20,21,22,23,24,25,26].
Antioxidants used for ROS detoxification have enzymatic and nonenzymatic origins with intracellular or extracellular mode of action (e.g., singlet O2 quencher, radical scavenger, electron donor, hydrogen donor, peroxide decomposer, enzyme inhibitor, gene expression regulation, synergist, and metal-chelating agents) [27].
In microalgae, ROS are produced by electron transport chains in chloroplasts and mitochondria, by the activity of some enzymes such as peroxidases and oxidases and also by the activity of some photosensitizers such as the chlorophyll [28]. The reactive oxygen species are therefore essentially generated in the chloroplasts and mitochondria but also in the peroxisomes [29]. More generally, ROS refer to O2 derivatives that are more reactive than O2 itself. This includes free radicals that contain at least one unpaired electron, as well as nonradical molecules [30]. Briefly, the activation of O2, in its stable state triplet oxygen (3O2), takes place (i) either by a transfer of energy large enough to reverse the spin of one of the electrons, which leads to the formation of singlet oxygen (1O2), or (ii) by an electron transfer that leads to the sequential reduction of 3O2 to superoxide radical (O2−•), hydrogen peroxide (H2O2) and hydroxyl radical (OH•).
In plants and algae, singlet oxygen 1O2 is produced under high light by chloroplasts in the reaction center of the photosystem II (PSII) and to a lower extent in the antenna complex [31]. In the antenna complex, triplet-excited chlorophyll (3Chl *) is formed from singlet-excited chlorophyll (1Chl *) by intersystem conversion [32]. The chlorophyll in the triplet state has a longer lifespan than in the singlet state and can react with 3O2 to form the highly reactive 1O2 [33]. The singlet oxygen is responsible for extensive cell damage (e.g., protein, lipid and nucleic acid oxidation, chloroplasts and thylakoids membranes disruption and photoinhibition) around the production area [34,35]. The reaction center of PSII is thus particularly threatened. The superoxide radical (O2−•) generation takes place in the chloroplast during photosynthesis, in the mitochondria during oxidative phosphorylation and in cell membranes through the activity of the NADPH oxidase [30]. The superoxide radical is poorly reactive because it lacks the ability to modify macromolecules and is quickly transformed into hydrogen peroxide (H2O2) [34]. However, its protonated form is the precursor of much more reactive radicals [30]. The hydrogen peroxide is formed by disproportionation of the O2−• a redox reaction that can be spontaneous or catalyzed by the superoxide dismutase (SOD). The hydrogen peroxide is also poorly reactive; however, it remains particularly toxic, as it can cross membranes, diffuse throughout the cell and oxidize sulfhydryl groups, causing the deactivation of essential enzymes [36]. It can also react with DNA and more specifically with some transition metals (e.g., iron and copper) inducing the formation of highly reactive hydroxyl radicals by the Haber–Weiss reaction [36,37]. The hydroxyl radical (OH•) is formed in the same cell compartments as the H2O2, i.e., in the stroma of the chloroplasts using the H2O2 generated by the photosystems, but needs the presence of reduced metal of transition [30]. The hydroxyl radicals can induce lipid peroxidation, protein and nucleic acid denaturation. In addition, there are no enzymes that can detoxify these radicals; in excess, it might lead to cell death [38], and lipid peroxidation may also generate other very reactive free radicals (e.g., the perhydroxyl HO2•, alkyl radical, reactive aldehydes malondialdehyde (MDA) and 4-hydroxy-2-nonenal (HNE)) [33,35]. Thus, the lipid-rich membranes and their functions are particularly affected by lipid peroxidation mainly through a decrease in membrane fluidity, an increase in their permeability and by enzyme, protein, ion channel and membrane receptor inactivation, which could lead to cell damage [33].
3. The Antioxidants Molecules of Microalgae
3.1. Ascorbic Acid
Ascorbic acid or vitamin C (1) is one of the most abundant water-soluble antioxidants synthesized by plants (Figure 1). It is mainly present in the cytosol and chloroplasts where it can directly neutralize superoxide and hydroxyl radicals as well as singlet oxygen by electron transfer, in addition to its role in the detoxification of hydrogen peroxide during the ascorbate-glutathione cycle [39]. Ascorbic acid is also involved in the protection of the photosynthetic apparatus through its participation in the regeneration of carotenoids of the xanthophyll cycle (cofactor of violaxanthin de-epoxidase) and α-tocopherol linked to membranes [39]. It has been shown that ascorbate can also have a pro-oxidant action by the reduction of transition metals (Fe3+ to Fe2+ and Cu2+ to Cu+) which can reduce hydrogen peroxide to hydroxyl radical by the Fenton reaction [40].
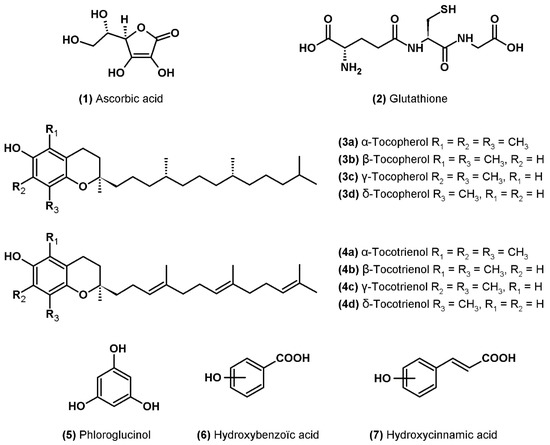
Figure 1.
Molecular structure of ascorbic acid, glutathione, tocopherols and phenolic compounds.
3.2. Glutathione
Glutathione (2) is a water-soluble tripeptide (l-γ-glutamyl-l-cysteinylglycine) present in all cellular compartments that play a crucial role in the antioxidant response (Figure 1). In addition to its role as a cofactor in the neutralization of hydrogen peroxide by glutathione peroxidase and in the regeneration of ascorbate in reduced form via the ascorbate-glutathione cycle, glutathione can directly deactivate superoxide and hydroxyl radicals as well as singlet oxygen. In addition, like ascorbate, glutathione participates in the regeneration of α-tocopherol in its reduced form [37].
3.3. Tocopherols
Tocopherols or vitamin E are fat-soluble molecules only synthesized by photosynthetic organisms and located in the lipid bilayers of membranes, mainly in those of chloroplasts [41]. The name “vitamin E” groups together four natural forms of tocopherols (α-, β-, γ- and δ-) (3a–d) to which are added the four forms of tocotrienols (α-, β-, γ- and δ-) (4a–d) (Figure 1). Tocopherols and tocotrienols consist of a chromanol ring and a hydrophobic phytyl side chain, tocotrienols differing from tocopherols by the presence of three double bonds on the side chain [41].
Tocopherols and tocotrienols have the capacity to neutralize lipid peroxyl radicals by giving a hydrogen atom from the hydroxyle group of the chromanol ring, thus making it possible to stop the chain reaction of lipid peroxidation [41]. The reaction results in the formation of a hydroperoxide, which can be neutralized by the action of glutation peroxidase, and of a tocopheroxyl radical (for tocopherols) or tocotrienoxyl (for tocotrienols), which are less reactive. Tocopherols and tocotrienols can then be regenerated by the action of ascorbate and glutathione at the interface of the membrane and cytosol or by coenzyme Q (UQH2) in the membrane [41]. Tocopherols can also deactivate singlet oxygen by two mechanisms: a physical quenching by charge transfer and a chemical reaction resulting in the formation of tocopherol quinone by irreversible opening of the chromanol ring [42].
3.4. Phenolic Compounds
Phenolic compounds are a large family of molecules: more than 8000 phenolic structures have been described to date in the plant kingdom [43]. These molecules contain at least one aromatic ring carrying one or more hydroxyl groups (Figure 1). The main families of compounds are phenolic acids, tocopherols described above, flavonoids and tannins as well as stilbenes and lignans [43]. Phenolics are an important class of antioxidants in higher plants and macroalgae but have only recently been studied in microalgae. However, the total content of phenolic compounds has been shown to contribute to the antioxidant activity of microalgae extracts [10,44,45,46,47]. The main molecules identified to date in microalgae are phloroglucinol (5) and phenolic acids derived from hydroxybenzoic acid (6) and hydroxycinnamic acid (7). Several studies have also shown the presence of weak concentrations of flavonoids e.g., [8,47,48,49,50,51,52,53,54]. All of these molecules are found in higher plants where their concentration is generally higher than in microalgae [55].
Phenolic acids can neutralize ROS primarily by hydrogen atom transfer. The antioxidant activity of the different molecules is directly linked to their chemical structure such as the number of hydroxyl groups or their position on the aromatic cycle [55]. The reaction results in the formation of a phenoxyl radical which is stabilized by the delocalization of the single electron around the aromatic ring (resonance stabilization). Phenolic acids also have the ability to inactivate radicals by monoelectronic transfer, and some can chelate the transition metals involved in the Fenton reaction thus preventing the formation of the highly reactive hydroxyl radical [55,56].
Among the pigments, we can also mention marennine, a blue-green pigment produced by Haslea ostrearia, which shows particularly interesting anti-free radical and antioxidant properties [57].
3.5. Carotenoids
Carotenoids are the most common pigments in nature, and more than 750 molecules have been described in algae, higher plants, bacteria and fungi [58] (Figure 2). They are fat-soluble molecules belonging to the terpenoids family containing a central chain with a system of conjugated double bonds, which can carry cyclic end groups. Carotenoids are separated into two groups: carotenes which contain only carbon and hydrogen atoms, and xanthophylls which contain at least one oxygen atom (hydroxyl, epoxy, ketone functions, for example) [59].
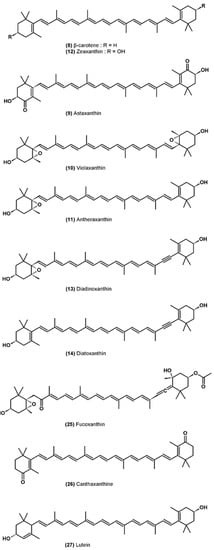
Figure 2.
Molecular structure of carotenoids.
Carotenoids are mainly present in the pigment-protein complexes of the thylakoid membrane, but certain species of microalgae can also accumulate carotenoids (β-carotene (8) and astaxanthin (9)) in lipid globules located in the stroma of the chloroplast or in the cytoplasm [60]. Some carotenoids are only found in specific classes of algae and so be used as chemotaxonomic markers [58].
The role of carotenoids is on the one hand to transfer light energy to chlorophylls and on the other hand to protect the photosynthetic system by deactivating ROS and preventing their formation [61]. The first photoprotection mechanism involves xanthophylls associated with the antennal complexes of the PSII which allows the dissipation of an excess of light energy without damage, according to a series of reactions called the “xanthophyll cycle” [62]. In excess light, violaxanthin (10) is converted to antheraxanthin (11) and then to zeaxanthin (12) by de-epoxidation provided by violaxanthin de-epoxidase, which uses ascorbate as cofactor. This enzyme, bound to thylakoids in the lumen, is activated by an acidic pH, an excess of proton in the lumen signaling that the light energy absorbed exceeds the capacity of the electron transport chain. The de-epoxidation of violaxanthin to zeaxanthin is a very rapid phenomenon on the order of a few minutes and reversible at low light intensity or in darkness by the action of zeaxanthin epoxidase. Zeaxanthin, unlike violaxanthin, can deactivate 1Chl* by dissipating its energy by heat [63]. This nonphotochemical quenching (NPQ) mechanism decreases the lifespan of 1Chl* and therefore prevents the formation of 3Chl* and then singlet oxygen in the PSII. In addition, by dissipating the excess energy, the possibilities of reducing O2 to the superoxide radical O2−• in the PSI are minimized (less electron leakage in the transport chain) [32]. The violaxanthin cycle takes place primarily in chlorophytes. There is an alternative xanthophyll cycle, with similar photoprotective functions, in certain classes of microalgae (heterokonts, haptophytes, euglenophytes and dinophyceae) for which diadinoxanthin (13) is converted to diatoxanthin (14) [62].
At high light intensity, the probability of 3Chl* formation is high despite the action of the xanthophyll cycle [32]. In antenna complexes, carotenoids are located near chlorophylls and can thus quickly neutralize 3Chl* by triplet–triplet transfers before they react with 3O2 to form 1O2 [32]. Carotenoids can also directly deactivate singlet oxygen if it is formed [64]. This ability to deactivate 1O2 is particularly important in the reaction center of PSII where there are no carotenoids in close proximity to the special pair of chlorophylls which can change to the triplet state and then react with the 3O2 without that the reaction is not neutralized beforehand by the carotenoids [32]. Carotenoids therefore deactivate the 1O2 that is formed in the reaction center, thus protecting the photosynthetic system from oxidative damage. The deactivation of 3Chl* and of 1O2 results in the formation of triplet carotenoids (3CAR*) which de-excite without damage by dissipating the excess energy absorbed in the form of heat and can again intervene in a deactivation cycle [32].
Carotenoids are considered to be the most efficient molecules in deactivating 1O2 owing to their system of conjugated double bonds. Thus, the greater the number of conjugated double bonds is, the more effective the carotenoid will be [64]. Carotenoids also have the ability to react with free radicals through three mechanisms: hydrogen atom transfer, monoelectronic transfer and adduct formation [65].
The interactions between carotenoids and free radicals are complex. Indeed, many parameters are involved, such as the nature of the radical, the polarity of the reaction medium, the partial pressure of oxygen, the interactions with other antioxidants, such as ascorbate or tocopherols, and the concentration and structure of the carotenoid (number of conjugated double bonds, presence and types of oxygen functions, presence of end groups, cis- or trans-configuration, etc.) [65]. Carotenoids can, for example, react with a peroxyl radical (ROO•), which is added to the polyene chain of the carotenoid forming an adduct ROO-CAR• which can react with another peroxyl radical forming a nonradical product ROO-CAR-OOR, thus allowing one to break the reaction chain of lipid peroxidation. This phenomenon takes place at low partial pressure of oxygen; however, at higher partial pressure, the ROO-CAR• radical can react with 3O2 to form a ROO-CAR-OO• radical which acts as a pro-oxidant and could in this case contribute to the spread of lipid peroxidation [65,66].
3.6. Miscellaneous Antioxidants
There are other more specific antioxidant molecules produced by certain microalgae:
Mycosporins-like amino acids (MAA) form a family of thirty-five molecules. They are colorless, water-soluble molecules found in a wide variety of marine organisms [67]. In microalgae, the most abundant MAAs are mycosporin-glycine (15), porphyra 334 (16), shinorin (17), asterina-330 (18), palythene (19) and palythine (20) [68,69] (Figure 3). The main function of these molecules is UV protection, but some of them have also been shown to have antioxidant properties. In particular, they can inhibit lipid peroxidation and neutralize singlet oxygen and certain free radicals [67].
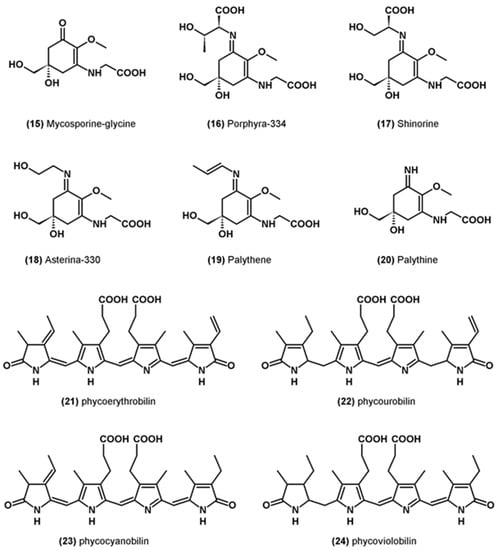
Figure 3.
Molecular structure of other miscellaneous molecules with antioxidant activity.
Polysaccharides are polymers composed of osidic units linked to glycosidic bonds attached to the cell wall or released into the medium (exopolysaccharides) [70]. Several polysaccharides derived from microalgae have shown antioxidant activity against free radicals; however, this in vitro activity remains quite low [71,72,73,74,75].
Phycobiliproteins are water-soluble pigments participating in the photosynthesis of certain groups of microalgae. They are composed of a protein and a chromophore called phycobilin particularly effective at absorbing red, orange, yellow and green light, which is not optimally absorbed by chlorophyll a [76]. There are four different structures: phycoerythrobilin (21), phycourobilin (22), phycocyanobilin (23) and phycoviolobilin (24) (Figure 3). They can neutralize ROS and chelate or reduce ferrous ions [77].
4. Common and Recent Assays Used to Evaluate Antioxidant Activity of Microalgae
Many antioxidant assays have been developed with different types of reactions to highlight the wide variety of antioxidant molecules and ROS, which act with different mechanisms. It is important to note that there is no single ideal test, and it is necessary to use several tests with different mechanisms of action to evaluate the whole antioxidant capacity of an extract or molecule [7,78,79,80].
The majority of the assays are based on the two main mechanisms of action of antioxidants (AH) to deactivate radicals (X•):
Hydrogen atom transfer (or HAT):
These reactions are generally fast; they are completed in seconds to minutes. The effectiveness of the antioxidant is determined by its ability to give a hydrogen atom (homolytic dissociation energy); therefore, the weaker the A-H bond, the more effective the antioxidant is [80].
Single electron transfer (SET):
These reactions are slower than hydrogen transfer reactions. The reaction is pH dependent, and the effectiveness of the antioxidant is mainly determined by its ionization potential. In general, the ionization potential decreases with increasing pH leading to an increase in the ability to donate an electron by deprotonation [80].
Other methods can also be used to evaluate the capacity of antioxidants to chelate transition metals or to inhibit the lipid peroxidation chain reaction. The most commonly used methods to evaluate the antioxidant activity of microalgae are presented in Table 1, and the most relevant results to assess antioxidant activity of microalgae extracts by in vitro chemical methods are presented in Table 2. Some cell-based antioxidant activity assays are presented, although few results using microalgae are found in the literature (Table 3). In addition, there does not seem to be any specific assays to evaluate antioxidant activity of microalgae on an animal model. Indeed, in most cases, microalgae are administrated to animals by food with a defined period and dosage; the testing animals are then sacrificed, and common in vitro chemical antioxidant activity assays (TBARS mostly) are used on animal tissues or blood by comparing with animals that did not consume microalgae (Table 4).

Table 1.
Main methods used for antioxidant activity evaluation of microalgae.

Table 2.
Antioxidant activity evaluation of microalgae extracts by in vitro chemical methods (AA: ascorbic acid, AAE: ascorbic acid equivalent, ABS: absorbance, Ac: acetone, AcOH: acetic acid, AIOLA: AAPH induced oxidation of linoleic acid, BHA: butylated hydroxyanisole, BHT: butylated hydroxytoluene, CCA: copper-chelating activity, CHCl3: chloroform, conc.: concentration, Co-Q10: co-enzyme Q10, DCM: dichloromethane, DPPH: 2,2-diphenyl-1-picrylhydrazyl, DW: dry weight, Eq: equivalent, EtOAC: ethyl acetate, EtOH: ethanol, FA: fatty acid, FCA: ferrous-chelating activity, FRAP: ferric-reducing antioxidant power, FTC: ferric thiocyanate assay, FW: fresh weight, GC-MS: gas chromatography–mass spectroscopy, Hex: hexane, IC50: inhibition concentration 50, inhib.: inhibition, i-PrOH: isopropanol, MeOH: methanol, ORAC: oxygen radical absorbance capacity, PBS: phosphate buffer saline, PE: petroleum ether, PLE: pressurized liquid extraction, PUFA: polyunsaturated fatty acid, TAC: total antioxidant capacity, TBARS: thiobarbituric acid reactive substance, TE: trolox equivalent, TEAC: trolox equivalent antioxidant capacity, temp.: temperature, TPC: total phenolic compounds, US: ultrasounds, α-toco.: α-tocopherol).

Table 3.
Antioxidant activity evaluation of microalgae extracts by cellular assays (AA: ascorbic acid, Ac: acetone, CAA: cellular antioxidant activity, CHCl3: chloroform, CLPAA: cellular lipid peroxidation antioxidant activity, EtOH: ethanol, Hex: hexane, IC50: inhibition concentration 50, inhib.: inhibition, MeOH: methanol, NMR: nuclear magnetic resonance, PBS: phosphate buffer saline, ROS: reactive oxygen species, TPC: total phenolic compounds, US: ultrasounds).

Table 4.
Antioxidant activity evaluation of microalgae extracts by in vivo experimentations (CAT: catalase, DNPH: 2,4-dinitrophenyl hydrazine, FA: fatty acid, FRAP: ferric-reducing antioxidant power, GPX: glutathione peroxidase, GSH: reduced glutathione, MDA: malondialdehyde, PX: peroxidase SOD: superoxide dismutase, TAC: total antioxidant capacity, and TBARS: thiobarbituric acid reactive substance).
5. Antioxidant Activity of Microalgae
The studies included in this part have been selected using Scopus and Google Scholar databases, using the terms “microalgae” in combination with “antioxidant”, “antioxidant activity”, “antioxidant capacity” or “antioxidant properties” as keywords. The research was limited to publication with an impact factor higher than 0.5, published until 2020. The studies have been selected based on these criteria: studies using in vitro (Table 2) or in cellular assays (Table 3) reporting the antioxidant activity of crude extract of eukaryotic microalgae. Studies focusing in the antioxidant activity of specific purified metabolite(s), or antioxidant enzyme activity have not been considered.
In addition, we have included a nonexhaustive selection of studies evaluating the antioxidant activity of microalgae in different animal models (Table 4).
The main publications evaluating the antioxidant activity of crude microalgal extracts by in vitro chemical tests are presented in Table 2. In these studies, more than two hundred strains of microalgae were evaluated. The most studied genera are Chlorella (29 strains), Scenedesmus and Tetraselmis (14 strains) (Supplementary Materials Figure S1).
The most commonly used assays to evaluate the antioxidant activity of microalgae are the DPPH (36 studies out of 52 referenced), ABTS (20 studies) and FCA assays (13 studies) (Supplementary Materials Figure S2). Overall, the results are very heterogeneous depending on the species of microalgae studied and the tests used to measure antioxidant activity. The protocols of the assays vary from one study to another, notably in terms of the extraction method, the solvents used, the reaction time and the concentrations tested. In addition, the results are expressed in different ways, making it difficult to compare the results. For example, for the DPPH assay, the results are expressed in percentage of inhibition for a given concentration (at different concentrations according to the studies), in IC50, in equivalent trolox (per unit of weight of extract or per unit of dry weight) or in equivalent ascorbic acid. Finally, the use of a reference product as a point of comparison is not systematic, and the choice of the reference product is not always relevant according to the assay used.
Nevertheless, several studies highlight the potential of microalgae as a source of antioxidants:
Chloromonas sp. and Botryidiopsidaceae sp. (ethanolic extracts) show a strong ability to neutralize DPPH radicals (IC50 of 0.97 and 1.53 µg mL−1, respectively) and ABTS (IC50 of 0.95 µg mL−1 and 1.79 µg mL−1) similar to vitamin C [118,119]. The ABTS assay also revealed interesting activities of Scenedesmus obliquus (IC50 of 41 µg mL−1, [96]), Haematococcus pluvialis (activity up to 1974 µmol TE g−1 extract for supercritical H2O extraction, [114]) and Dunaliella salina (activity up to 1118 µmol TE g−1 extract with hexane extraction, [83]). Interesting results are also obtained with the DPPH assay for Galdieria sulphuraria, Ettlia carotinosa, Neochloris texensis, Chlorella minutissima, Chlorella vulgaris, Schizochytrium limacinum, Stichococcus bacillaris and Crypthecodinium cohnii with inhibition percentages between 89% and 95% with aqueous or methanolic extracts at concentrations of 250 µg mL−1 [106].
Natrah et al. [84] showed that Chaetoceros calcitrans, Scenedesmus quadricauta, Isochrysis galbana, Chlorella vulgaris, Nannochloropsis oculata, and Tetraselmis tetrahele had a strong ability to inhibit lipid peroxidation with inhibition percentages ranging from 88% to 98% for methanolic extracts at 80 µg mL−1 with the TBARS assay and between 88.4 and 97% for extracts at 200 µg mL−1 with the FTC assay (Ferric ThioCyanate assay, indirect measurement of the quantity of hydroperoxides formed during the first stages of lipid oxidation). The ability of the genera Tetraselmis to inhibit lipid peroxidation is confirmed by Coulombier et al. [21] who have obtained an IC50 up to 3,4 µg mL−1 with a methanol-dichloromethane extract. Euglena tuba also seems to be an interesting species for its ability to inhibit lipid peroxidation (IC50 with TBARS assay = 42 µg mL−1) and to neutralize the superoxide radical (IC50 = 5.2 µg mL−1, [49]). Some species show good ability to neutralize superoxide radical such as Chaetoceros sp. (1029 µmol TE g−1 dichloromethane extract), Nannochloropsis sp. (3224 µmol TE g−1 methanol extract), Chlorella stigmatophora and Phaeodactylum tricornutum (IC50 of 48.37 and 68.61 µg mL−1 with aqueous extracts, [87]). Chloroform and methanol extracts of Chaetoceros sp. also show interesting results with the FRAP assay (610 and 492.50 µmol TE g−1, [86]). Good results are also obtained with the TAC assay with IC50 below 100 µg mL−1 for methanolic extracts of Chlorella vulgaris and Chlamydomonas reinhardtii [47].
The antioxidant activity of the genus Chlorella has been demonstrated by several authors with different antioxidant assays. In addition to the results obtained with the DPPH, TBARS, FTC, TAC, and superoxide radical neutralization assays presented above, Aremu et al. [44,48] obtained IC50 up to 25 µg mL−1 with the β-carotene bleaching assay for Chlorella minutissima and Chlorella sp. and Plaza et al. [81] showed activities up to 1008 µmol TE g−1 of Chlorella vulgaris extract with the ORAC assay.
Overall, few links are made between these antioxidant activities and the metabolites involved. Still, correlations have been shown with carotenoid content [44,83], phenolic compound content [44,106] including flavonoids [47] and gallic acid and vitamin E content [114].
Despite cellular assays potentially giving more biological relevant information, as they take into account the bioavailability and metabolism of the tested compounds, we found only four studies using cellular assays to determine antioxidant activity of microalgae extract (Table 3). Those studies use different antioxidant cellular assays and different cell models (mouse fibroblast, macrophage or lymphoma cells and human liver cancer cell line).
Chloroform, methanol, acetone and 70% ethanol extracts of Chaetoceros calcitrans showed high nitric oxide scavenging activity in mouse macrophage with IC50 values of 3.46, 3.83, 15.35 and 17.94 µg mL−1, respectively, that is closed to reference compounds (IC50 of 4.7 and 6.1 µg mL−1 for quercetin and curcumin, [88]). This strong inhibitory activity of nitric oxide was attributed to the carotenoid content of Chaetoceros calcitrans (fucoxanthin, astaxanthin, violaxanthin, zeaxanthin, canthaxanthin and lutein). Karawita et al. [92] showed that Pediastrum duplex extract has a good protective effect against DNA damage induced by hydrogen peroxyde exposure (Comet assay). Indeed, a decrease of 80% of DNA damage on mouse lymphoma cells was measured with Pediastrum extract at 100 µg mL−1 compared to control with no microalgae extract. Good antioxidant activity was also measured with CAA (cellular antioxidant activity) and CLPAA (cellular lipid peroxidation antioxidant activity) assays on human liver cancer cell line with Ostreopsis ovata and Alexandrium minutum; however, both species extracts showed toxicity in cytotoxicity assay [91].
Similarly to cellular assays, the evaluation of the antioxidant activity of microalgae extracts by in vivo experimentations are limited compared to in vitro assays (Table 4). Those studies used different antioxidant in vitro assays couple with other physiological measurement, such as antioxidant enzyme activity, on various animal models (e.g., shrimps, chicken, catfish, rats, turbots or trouts, Table 4) to assess the effect of microalgae. The microalgae (Schizochytrium sp., Chlorella vulgaris, Amphora coffeaformis, Schizochytrium limacinum, Acutodesmus obliquus, Nannochloropsis spp., Tetraselmis chuii and Botryococcus braunii) were mostly included in the animal feed as dry microalgae with a percentage of inclusion mainly going from 1–10% or as a molecule equivalent of given antioxidant compounds. The results are variable depending on species from no effect of the microalgae tested [120,123] to a decrease in oxidative stress measurements such as the malondialdehyde or hydrogen peroxide content [124,125,126,127,128,129] or a decrease in DNA damage [123]. In most cases, it seems that the inclusion of microalgae directly in the fed has a positive effect on the animal physiology, which is promising regarding further used of microalgae in the food industry either in human or animal nutrition as functional ingredients. It also raises the question of the bioavailability of an antioxidant compound in the algal matrices and thus of the digestibility of the microalgae tested.
6. Applications in the Food Industry
In the food industry, antioxidants are used for human and animal nutrition as functional ingredients to provide nutritional benefits to a product (e.g., orange juice enriched with vitamin C), and as preservatives to extend the shelf life of foods and beverages to prevent their degradation by oxidation [130,131]. The use of antioxidant ingredients in food products intended for humans is highly regulated by country-specific laws owing to their potential toxicity. In the European Union, there is a list of authorized antioxidant additives, some of which may be of natural origin such as vitamin C (E300-E304), vitamin E (E306-E309), guaiac resin (E314) and rosemary extract (E392). Certain carotenoids are also authorized as dyes but can have an antioxidant role such as β-carotene (E160a), lycopene (E160d), lutein (E161b), violaxanthin (E161e), zeaxanthin (E161h), canthaxanthin (E161g) or astaxanthin (E161j) [130]. For foods and ingredients that were not significantly consumed before 1997, such as most microalgae, the "Novel Food" regulation framework was to be applied in Europe [132]. New microalgae on the market must obtain this authorization; however, to receive it, it has to be demonstrated that the product does not present any risk in terms of safety for human health [133] as some microalgae are known to produce phytotoxins [134,135,136]. In addition, and beyond the regulatory framework, to be of interest to the food industry, an antioxidant should not affect the color, smell and taste of the food and should be effective at low concentrations (0.001–0.01%), be easily usable, stable during processing and storage and be inexpensive [130,131]. The use of microalgae may thus be regarded as promising additive for human food, livestock feed and shelf life; however, it greatly depends on the microalgae productivity and nutrient compositions in protein, carbohydrates, lipids, vitamins, and antioxidants, which also strongly depend on species, mode of cultivation and culture medium composition e.g., [7,21,22]. Currently, around 10 species of microalgae or microalgae extract are authorized for human consumption in Europe as a food or food ingredient [137].
For livestock feed, antioxidant additives are subject to authorization before going on the market, an authorization that remains only valid ten years. On the other hand, raw materials are not subject to authorization, but a contribution of microalgae as an antioxidant in animal feed could only be considered as a raw material if it also provides proteins, minerals, fats, fibers, energy or carbohydrates [138]. Microalgae presents growth rate and dietary value of interest (e.g., polyunsaturated fatty acids, vitamins, pigments, polysaccharides) for livestock feed or aquaculture feed either fish, live feed and shellfish applications (e.g., in Table 4). Indeed, in aquaculture, the polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid (DHA) and docosahexanoic acid (DHA) are of nutritional importance in aquafeeds and are hitherto ensured by inclusion of fish oil in aquafeeds. However, this resource is limited, and microalgae offer an alternative to fish oil. In addition, microalgae are not only seen as a source of PUFAs but also as source of other metabolites of interest such as pigments, polysaccharides, vitamins (e.g., vitamin E and C) and sterols which are introduced as dietary supplements for dietetic and therapeutic purposes [3,129]. In terms of applications, antioxidant molecules (asthaxanthin, lutein, β-carotene) carotenoids are produced by a wide variety of microalgae (see Table 2).
7. Conclusion
Antioxidant molecules from microalgae are more and more considered as a potential source of natural antioxidant compounds by the food, the cosmetic and nutraceutical industries as they may bring benefits to their products.
However, it is very crucial to assess properly the antioxidant activity of an algal extract owing to the wide diversity of antioxidant compounds and the mode of action combined with the diversity of ROS involved. This review highlights the lack of standardization between extractions procedures used to assess antioxidant activity from microalgae matrices, and more disturbingly, it highlights the inappropriateness between the assay used and the molecules studied. These often hamper the comparison between studies and bring the authors to false or incorrect interpretation of their results.
Therefore, although all the assays have their merits and demerits, the appropriate selection of a given assay was to be made based on the mode of action of a studied molecule in front of the principle and mechanism of an assay, especially in vitro assays. In addition to the need of normalization of the extraction procedures and to the appropriate use of an assay, we conclude that it is crucial to combine many assays to assess microalgae full antioxidant activity.
This review also highlights that microalgae are rich in antioxidant molecules with more or less potent activities, which can be used as an ingredient in food, cosmetic and nutraceutical industries. In addition, research publications are available on modern in vitro chemical methods, but application on cellular assays and in vivo experimentations are still lacking. There is a need to develop models to improve our ability to assess the activity of antioxidant molecule on these kinds of models to further improve industrial adaption and application.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/md19100549/s1, Figure S1. Top 15 microalgae genus studied for their antioxidant activity, Figure S2. In vitro chemical assays used to evaluate the antioxidant activity of microalgae crude extracts.
Author Contributions
N.C. built the bibliographic database. N.C., N.L. and T.J. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Province Nord, the Province Sud, the Government of New Caledonia and the Comité Interministériel de l’Outre-Mer (CIOM) through the AMICAL (Aquaculture of Microalgae in NewCALedonia) 1 and 2 research programs.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.-P.; Morant-Manceau, A.; Schoefs, B. The Potential of Microalgae for the Production of Bioactive Molecules of Pharmaceutical Interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Microalgae for the Production of Bulk Chemicals and Biofuels. Biofuels. Bioprod. Biorefining 2010, 4, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Aklakur, M. Natural Antioxidants from Sea: A Potential Industrial Perspective in Aquafeed Formulation. Rev. Aquacult. 2016, 10, 385–399. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of High Added-Value Compounds—a Brief Review of Recent Work. Biotechnol Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Natural Marine Anti-Inflammatory Products. Mini-Rev. Med. Chem. 2008, 8, 740–754. [Google Scholar] [CrossRef]
- Assunção, M.F.G.; Amaral, R.; Martins, C.B.; Ferreira, J.D.; Ressurreição, S.; Santos, S.D.; Varejão, J.M.T.B.; Santos, L.M.A. Screening Microalgae as Potential Sources of Antioxidants. J. Appl. Phycol. 2016, 29, 865–877. [Google Scholar] [CrossRef]
- Coulombier, N.; Nicolau, E.; Le Déan, L.; Antheaume, C.; Jauffrais, T.; Lebouvier, N. Impact of Light Intensity on Antioxidant Activity of Tropical Microalgae. Mar. Drugs 2020, 18, 122. [Google Scholar] [CrossRef] [Green Version]
- Safafar, H.; van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [Green Version]
- Sathasivam, R.; Ki, J.-S. A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef] [Green Version]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; Brabanter, J.D.; Cooman, L.D. Antioxidant Potential of Microalgae in Relation to Their Phenolic and Carotenoid Content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Cezare-Gomes, E.A.; del Carmen Mejia-da-Silva, L.; Pérez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; de Carvalho, J.C.M. Potential of Microalgae Carotenoids for Industrial Application. Appl. Biochem. Biotechnol. 2019, 188, 602–634. [Google Scholar] [CrossRef]
- Ito, N.; Hirose, M.; Fukushima, S.; Tsuda, H.; Shirai, T.; Tatematsu, M. Studies on Antioxidants: Their Carcinogenic and Modifying Effects on Chemical Carcinogenesis. Food Chem. Toxicol. 1986, 24, 1071–1082. [Google Scholar] [CrossRef]
- Li, H.-B.; Cheng, K.-W.; Wong, C.-C.; Fan, K.-W.; Chen, F.; Jiang, Y. Evaluation of Antioxidant Capacity and Total Phenolic Content of Different Fractions of Selected Microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Safer, A.M.; Al-Nughamish, A.J. Hepatotoxicity Induced by the Anti-Oxidant Food Additive, Butylated Hydroxytoluene (BHT), in Rats: An Electron Microscopical Study. Histol. Histopathol. 1999, 14, 391–406. [Google Scholar]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae Biomass as an Alternative Ingredient in Cookies: Sensory, Physical and Chemical Properties, Antioxidant Activity and in Vitro Digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as Functional Ingredients in Savory Food Products: Application to Wheat Crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef] [Green Version]
- Goiris, K.; Muylaert, K.; De Cooman, L. Microalgae as a Novel Source of Antioxidants for Nutritional Applications. In Handbook of Marine Microalgae; Kim, S.-K., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 269–280. ISBN 978-0-12-800776-1. [Google Scholar]
- Sansone, C.; Brunet, C. Promises and Challenges of Microalgal Antioxidant Production. Antioxidants 2019, 8, 199. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. How to Characterize an Antioxidant: An Update. Biochem. Soc. Symp. 1995, 61, 73–101. [Google Scholar] [CrossRef]
- Chen, B.; Wan, C.; Mehmood, M.A.; Chang, J.-S.; Bai, F.; Zhao, X. Manipulating Environmental Stresses and Stress Tolerance of Microalgae for Enhanced Production of Lipids and Value-Added Products–A Review. Bioresour. Technol. 2017, 244, 1198–1206. [Google Scholar] [CrossRef]
- Coulombier, N.; Blanchier, P.; Le Dean, L.; Barthelemy, V.; Lebouvier, N.; Jauffrais, T. The Effects of CO2-Induced Acidification on Tetraselmis Biomass Production, Photophysiology and Antioxidant Activity: A Comparison Using Batch and Continuous Culture. J. Biotechnol. 2021, 325, 312–324. [Google Scholar] [CrossRef]
- Coulombier, N.; Nicolau, E.; Le Déan, L.; Barthelemy, V.; Schreiber, N.; Brun, P.; Lebouvier, N.; Jauffrais, T. Effects of Nitrogen Availability on the Antioxidant Activity and Carotenoid Content of the Microalgae Nephroselmis sp. Mar. Drugs 2020, 18, 453. [Google Scholar] [CrossRef]
- Gauthier, M.R.; Senhorinho, G.N.A.; Scott, J.A. Microalgae under Environmental Stress as a Source of Antioxidants. Algal Res. 2020, 52, 102104. [Google Scholar] [CrossRef]
- Goiris, K.; Van Colen, W.; Wilches, I.; León-Tamariz, F.; De Cooman, L.; Muylaert, K. Impact of Nutrient Stress on Antioxidant Production in Three Species of Microalgae. Algal Res. 2015, 7, 51–57. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Pereira, R.D.; Malcata, F.X. Effects of Temperature and PH on Growth and Antioxidant Content of the Microalga Scenedesmus obliquus. Biotechnol. Prog. 2011, 27, 1218–1224. [Google Scholar] [CrossRef]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, V.S.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic Stresses as Tools for Metabolites in Microalgae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Diplock, A.T. Current Status of Antioxidant Therapy. Free Radic. Biol. Med. 1993, 15, 77–96. [Google Scholar] [CrossRef]
- Saed-Moucheshi, A.; Shekoofa, A.; Pessarakli, M. Reactive Oxygen Species (ROS) Generation and Detoxifying in Plants. J. Plant. Nutr. 2014, 37, 1573–1585. [Google Scholar] [CrossRef]
- Cirulis, J.T.; Scott, J.A.; Ross, G.M. Management of Oxidative Stress by Microalgae. Can. J. Physiol. Pharmacol. 2013, 91, 15–21. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of Oxidative Stress in Plants: From Classical Chemistry to Cell Biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Triantaphylidès, C.; Havaux, M. Singlet Oxygen in Plants: Production, Detoxification and Signaling. Trends Plant. Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef]
- Telfer, A.; Pascal, A.; Gall, A. Carotenoids in Photosynthesis. In Carotenoids: Volume 4: Natural Functions; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2008; pp. 265–308. ISBN 978-3-7643-7499-0. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant. Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ledford, H.K.; Niyogi, K.K. Singlet Oxygen and Photo-Oxidative Stress Management in Plants and Algae. Plant. Cell Environ. 2005, 28, 1037–1045. [Google Scholar] [CrossRef]
- Pospíšil, P.; Yamamoto, Y. Damage to Photosystem II by Lipid Peroxidation Products. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 457–466. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 21703. [Google Scholar] [CrossRef] [Green Version]
- Richards, S.L.; Wilkins, K.A.; Swarbreck, S.M.; Anderson, A.A.; Habib, N.; Smith, A.G.; McAinsh, M.; Davies, J.M. The Hydroxyl Radical in Plants: From Seed to Seed. J. Exp. Bot. 2015, 66, 37–46. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Duarte, T.L.; Lunec, J. When Is an Antioxidant Not an Antioxidant? A Review of Novel Actions and Reactions of Vitamin C. Free Radic. Res. 2005, 39, 671–686. [Google Scholar] [CrossRef]
- Herrera, E.; Barbas, C. Vitamin E: Action, Metabolism and Perspectives. J. Physiol. Biochem. 2001, 57, 43–56. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. The Function of Tocopherols and Tocotrienols in Plants. Crit. Rev. Plant. Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Aremu, A.O.; Neményi, M.; Stirk, W.A.; Ördög, V.; van Staden, S. Manipulation of Nitrogen Levels and Mode of Cultivation Are Viable Methods to Improve the Lipid, Fatty Acids, Phytochemical Content, and Bioactivities in Chlorella minutissima. J. Phycol. 2015, 51, 659–669. [Google Scholar] [CrossRef]
- Custódio, L.; Justo, T.; Silvestre, L.; Barradas, A.; Duarte, C.V.; Pereira, H.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Microalgae of Different Phyla Display Antioxidant, Metal Chelating and Acetylcholinesterase Inhibitory Activities. Food Chem. 2012, 131, 134–140. [Google Scholar] [CrossRef]
- Hajimahmoodi, M.; Faramarzi, M.A.; Mohammadi, N.; Soltani, N.; Oveisi, M.R.; Nafissi-Varcheh, N. Evaluation of Antioxidant Properties and Total Phenolic Contents of Some Strains of Microalgae. J. Appl. Phycol. 2010, 22, 43–50. [Google Scholar] [CrossRef]
- Jayshree, A.; Jayashree, S.; Thangaraju, N. Chlorella vulgaris and Chlamydomonas reinhardtii: Effective Antioxidant, Antibacterial and Anticancer Mediators. Indian J. Pharm. Sci. 2016, 78, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Aremu, A.O.; Masondo, N.A.; Molnár, Z.; Stirk, W.A.; Ördög, V.; Van Staden, S. Changes in Phytochemical Content and Pharmacological Activities of Three Chlorella Strains Grown in Different Nitrogen Conditions. J. Appl. Phycol. 2016, 28, 149–159. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Ghate, N.B.; Deb, S.; Panja, S.; Sarkar, R.; Rout, J.; Mandal, N. Assessment of the Phytochemical Constituents and Antioxidant Activity of a Bloom Forming Microalgae Euglena tuba. Biol. Res. 2014, 47, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; Paepe, D.D.; Baart, G.J.E.; Cooman, L.D. Detection of Flavonoids in Microalgae from Different Evolutionary Lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef]
- Haoujar, I.; Cacciola, F.; Abrini, J.; Mangraviti, D.; Giuffrida, D.; Oulad El Majdoub, Y.; Kounnoun, A.; Miceli, N.; Fernanda Taviano, M.; Mondello, L.; et al. The Contribution of Carotenoids, Phenolic Compounds, and Flavonoids to the Antioxidative Properties of Marine Microalgae Isolated from Mediterranean Morocco. Molecules 2019, 24, 4037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, A.; Rico, M.; Santana-Casiano, J.M.; González, A.G.; González-Dávila, M. Phenolic Profile of Dunaliella Tertiolecta Growing under High Levels of Copper and Iron. Environ. Sci. Pollut. Res. 2015, 22, 14820–14828. [Google Scholar] [CrossRef]
- Smerilli, A.; Balzano, S.; Maselli, M.; Blasio, M.; Orefice, I.; Galasso, C.; Sansone, C.; Brunet, C. Antioxidant and Photoprotection Networking in the Coastal Diatom Skeletonema marinoi. Antioxidants 2019, 8, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Wang, Y.; Sun, J.; Bian, F.; Chen, G.; Zhang, Y.; Bi, Y.; Wu, Y. Antioxidant Activity of Alcohol Aqueous Extracts of Crypthecodinium cohnii and Schizochytrium sp. J. Zhejiang Univ.-Sci. B 2017, 18, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The Molecular Basis of Working Mechanism of Natural Polyphenolic Antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Pouvreau, J.-B.; Morançais, M.; Taran, F.; Rosa, P.; Dufossé, L.; Guérard, F.; Pin, S.; Fleurence, J.; Pondaven, P. Antioxidant and Free Radical Scavenging Properties of Marennine, a Blue-Green Polyphenols Pigment from the Diatom Haslea ostrearia (Gaillon/Bory) Simonsen Responsible for the Natural Greening of Cultured Oysters. J. Agric. Food Chem. 2008, 56, 6278–6286. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant Activity of Carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Solovchenko, A.E. Physiology and Adaptive Significance of Secondary Carotenogenesis in Green Microalgae. Russ. J. Plant. Physiol. 2013, 60, 167–176. [Google Scholar] [CrossRef]
- Huang, J.J.; Lin, S.; Xu, W.; Cheung, P.C.K. Occurrence and Biosynthesis of Carotenoids in Phytoplankton. Biotechnol. Adv. 2017, 35, 597–618. [Google Scholar] [CrossRef] [PubMed]
- Goss, R.; Jakob, T. Regulation and Function of Xanthophyll Cycle-Dependent Photoprotection in Algae. Photosynth. Res. 2010, 106, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Jahns, P.; Holzwarth, A.R. The Role of the Xanthophyll Cycle and of Lutein in Photoprotection of Photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Edge, R.; McGarvey, D.J.; Truscott, T.G. The Carotenoids as Antioxidants—A Review. J. Photochem. Photobiol. B Biol. 1997, 41, 189–200. [Google Scholar] [CrossRef]
- El-Agamey, A.; McGarvey, D.J. Carotenoid Radicals and Radical Ions. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2008; Volume 4, pp. 119–154. ISBN 978-3-7643-7498-3. [Google Scholar]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and Pro-Oxidant Activities of Carotenoids and Their Oxidation Products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-Like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef]
- Llewellyn, C.A.; Airs, R.L. Distribution and Abundance of MAAs in 33 Species of Microalgae across 13 Classes. Mar. Drugs 2010, 8, 1273–1291. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.P.; Singh, S.P.; Häder, D.-P. Database on Mycosporines and Mycosporine-like Amino Acids (MAAs) in Fungi, Cyanobacteria, Macroalgae, Phytoplankton and Animals. J. Photochem. Photobiol. B Biol. 2007, 89, 29–35. [Google Scholar] [CrossRef]
- Raposo, M.F.D.J.; De Morais, R.M.S.C.; de Morais, B.A.M.M. Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [Green Version]
- Balavigneswaran, C.K.; Sujin Jeba Kumar, T.; Moses Packiaraj, R.; Veeraraj, A.; Prakash, S. Anti-Oxidant Activity of Polysaccharides Extracted from Isochrysis galbana Using RSM Optimized Conditions. Int. J. Biol. Macromol. 2013, 60, 100–108. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Guo, G.; Pu, Y.; Yan, B. The Isolation and Antioxidant Activity of Polysaccharides from the Marine Microalgae Isochrysis galbana. Carbohydr. Polym. 2014, 113, 22–31. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Bergman, M.; Van-Moppes, D.; Grossman, S.; Arad, S. Antioxidant Activity of the Polysaccharide of the Red Microalga Porphyridium sp. J. Appl. Phycol. 2005, 17, 215–222. [Google Scholar] [CrossRef]
- Yu, M.; Chen, M.; Gui, J.; Huang, S.; Liu, Y.; Shentu, H.; He, J.; Fang, Z.; Wang, W.; Zhang, Y. Preparation of Chlorella vulgaris Polysaccharides and Their Antioxidant Activity in Vitro and in Vivo. Int. J. Biol. Macromol. 2019, 137, 139–150. [Google Scholar] [CrossRef]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The Antioxidant Activity of Polysaccharides Derived from Marine Organisms: An Overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glazer, A.N. Phycobiliproteins—A Family of Valuable, Widely Used Fluorophores. J. Appl. Phycol. 1994, 6, 105–112. [Google Scholar] [CrossRef]
- Sonani, R.R.; Rastogi, R.P.; Madamwar, D. Antioxidant Potential of Phycobiliproteins: Role in Anti-Aging Research. Biochem. Anal. Biochem. 2015, 4, 1009–2161. [Google Scholar] [CrossRef] [Green Version]
- Carocho, M.; Ferreira, I.C.F.R. A Review on Antioxidants, Prooxidants and Related Controversy: Natural and Synthetic Compounds, Screening and Analysis Methodologies and Future Perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Frankel, E.N.; Meyer, A.S. The Problems of Using One-Dimensional Methods to Evaluate Multifunctional Food and Biological Antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; García-Blairsy Reina, G.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for Bioactive Compounds from Algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; Stirk, W.A.; Ördög, V.; Staden, J.V. Influence of Culture Age on the Phytochemical Content and Pharmacological Activities of Five Scenedesmus Strains. J. Appl. Phycol. 2014, 26, 407–415. [Google Scholar] [CrossRef]
- Herrero, M.; Jaime, L.; Martín-Alvarez, P.J.; Cifuentes, A.; Ibáñez, E. Optimization of the Extraction of Antioxidants from Dunaliella salina Microalga by Pressurized Liquids. J. Agric. Food Chem. 2006, 54, 5597–5603. [Google Scholar] [CrossRef]
- Natrah, F.M.I.; Yusoff, F.M.; Shariff, M.; Abas, F.; Mariana, N.S. Screening of Malaysian Indigenous Microalgae for Antioxidant Properties and Nutritional Value. J. Appl. Phycol. 2007, 19, 711–718. [Google Scholar] [CrossRef]
- Simic, S.; Kosanic, M.; Rankovic, B. Evaluation of In Vitro Antioxidant and Antimicrobial Activities of Green Microalgae Trentepohlia umbrina. Not. Bot. Horti Agrobot. 2012, 40, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Goh, S.-H.; Yusoff, F.M.; Loh, S.P. A Comparison of the Antioxidant Properties and Total Phenolic Content in a Diatom, Chaetoceros sp. and a Green Microalga, Nannochloropsis sp. J. Agric. Sci. 2010, 2, 123. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, S.; Gato, A.; Calleja, J.M. Antiinflammatory, Analgesic and Free Radical Scavenging Activities of the Marine Microalgae Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2001, 15, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Azizan, A.; Ahamad Bustamam, M.S.; Maulidiani, M.; Shaari, K.; Ismail, I.S.; Nagao, N.; Abas, F. Metabolite Profiling of the Microalgal Diatom Chaetoceros calcitrans and Correlation with Antioxidant and Nitric Oxide Inhibitory Activities via 1H NMR-Based Metabolomics. Mar. Drugs 2018, 16, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Lee, J.-B.; Lee, K.-W.; Jeon, Y.-J. Antioxidant Properties of Tidal Pool Microalgae, Halochlorococcum porphyrae and Oltamannsiellopsis unicellularis from Jeju Island, Korea. Algae 2010, 25, 45–56. [Google Scholar] [CrossRef]
- Buono, S.; Langellotti, A.L.; Martello, A.; Bimonte, M.; Tito, A.; Carola, A.; Apone, F.; Colucci, G.; Fogliano, V. Biological Activities of Dermatological Interest by the Water Extract of the Microalga Botryococcus braunii. Arch. Derm. Res. 2012, 304, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef] [Green Version]
- Karawita, R.; Senevirathne, M.; Athukorala, Y.; Affan, A.; Lee, Y.-J.; Kim, S.-K.; Lee, J.-B.; Jeon, Y.-J. Protective Effect of Enzymatic Extracts from Microalgae against DNA Damage Induced by H2O2. Mar. Biotechnol. 2007, 9, 479–490. [Google Scholar] [CrossRef]
- Affan, A.; Karawita, R.; Jeon, Y.-J.; Kim, B.-Y.; Lee, J.-B. Growth Characteristics, Bio-Chemical Composition and Antioxidant Activities of Benthic Diatom Grammatophora marina from Jeju Coast, Korea. Algae 2006, 21, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Barba, F.J.; Lorenzo, J.M. Antioxidant Potential of Extracts Obtained from Macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and Micro-Algae (Chlorella vulgaris and Spirulina platensis) Assisted by Ultrasound. Medicines 2018, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.; Fanning, K.; Netzel, M.; Turner, W.; Li, Y.; Schenk, P.M. Profiling of Carotenoids and Antioxidant Capacity of Microalgae from Subtropical Coastal and Brackish Waters. Food Chem. 2014, 165, 300–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaro, H.M.; Fernandes, F.; Valentão, P.; Andrade, P.B.; Sousa-Pinto, I.; Malcata, F.X.; Guedes, A.C. Effect of Solvent System on Extractability of Lipidic Components of Scenedesmus obliquus (M2-1) and Gloeothece sp. on Antioxidant Scavenging Capacity Thereof. Mar. Drugs 2015, 13, 6453–6471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J.B. Antioxidant Properties and Lipid Composition of Selected Microalgae. J. Appl. Phycol. 2018, 31, 309–318. [Google Scholar] [CrossRef]
- Bauer, L.M.; Costa, J.A.V.; da Rosa, A.P.C.; Santos, L.O. Growth Stimulation and Synthesis of Lipids, Pigments and Antioxidants with Magnetic Fields in Chlorella Kessleri Cultivations. Bioresour. Technol. 2017, 244, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Bulut, O.; Akın, D.; Sönmez, Ç.; Öktem, A.; Yücel, M.; Öktem, H.A. Phenolic Compounds, Carotenoids, and Antioxidant Capacities of a Thermo-Tolerant Scenedesmus sp. (Chlorophyta) Extracted with Different Solvents. J. Appl. Phycol. 2019, 31, 1675–1683. [Google Scholar] [CrossRef]
- Choochote, W.; Suklampoo, L.; Ochaikul, D. Evaluation of Antioxidant Capacities of Green Microalgae. J. Appl. Phycol. 2014, 26, 43–48. [Google Scholar] [CrossRef]
- Custódio, L.; Soares, F.; Pereira, H.; Barreira, L.; Vizetto-Duarte, C.; Rodrigues, M.J.; Rauter, A.P.; Alberício, F.; Varela, J. Fatty Acid Composition and Biological Activities of Isochrysis galbana T-ISO, Tetraselmis sp. and Scenedesmus sp.: Possible Application in the Pharmaceutical and Functional Food Industries. J. Appl. Phycol. 2014, 26, 151–161. [Google Scholar] [CrossRef]
- Elshobary, M.E.; El-Shenody, R.A.; Ashour, M.; Zabed, H.M.; Qi, X. Antimicrobial and Antioxidant Characterization of Bioactive Components from Chlorococcum minutum. Food Biosci. 2020, 35, 100567. [Google Scholar] [CrossRef]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Khong, N.M.H.; Chan, K.W.; Yau, S.K. Efficient Solvent Extraction of Antioxidant-Rich Extract from a Tropical Diatom, Chaetoceros calcitrans (Paulsen) Takano 1968. Asian Pac. J. Trop. Biomed. 2015, 5, 834–840. [Google Scholar] [CrossRef]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Yau, S.K.; Khong, N.M.H.; Chan, K.W.; Ebrahimi, M. Antioxidant Capacities of Fucoxanthin-Producing Algae as Influenced by Their Carotenoid and Phenolic Contents. J. Biotechnol. 2017, 241, 175–183. [Google Scholar] [CrossRef]
- Guedes, A.C.; Gião, M.S.; Seabra, R.; Ferreira, A.C.S.; Tamagnini, P.; Moradas-Ferreira, P.; Malcata, F.X. Evaluation of the Antioxidant Activity of Cell Extracts from Microalgae. Mar. Drugs 2013, 11, 1256–1270. [Google Scholar] [CrossRef] [Green Version]
- Gürlek, C.; Yarkent, Ç.; Köse, A.; Tuğcu, B.; Gebeloğlu, I.K.; Öncel, S.Ş.; Elibol, M. Screening of Antioxidant and Cytotoxic Activities of Several Microalgal Extracts with Pharmaceutical Potential. Health Technol. 2020, 10, 111–117. [Google Scholar] [CrossRef]
- Jaime, L.; Mendiola, J.A.; Ibáñez, E.; Martin-Álvarez, P.J.; Cifuentes, A.; Reglero, G.; Señoráns, F.J. β-Carotene Isomer Composition of Sub- and Supercritical Carbon Dioxide Extracts. Antioxidant Activity Measurement. J. Agric. Food Chem. 2007, 55, 10585–10590. [Google Scholar] [CrossRef]
- Jerez-Martel, I.; García-Poza, S.; Rodríguez-Martel, G.; Rico, M.; Afonso-Olivares, C.; Gómez-Pinchetti, J.L. Phenolic Profile and Antioxidant Activity of Crude Extracts from Microalgae and Cyanobacteria Strains. J. Food Qual. 2017, 2017, 2924508. [Google Scholar] [CrossRef] [Green Version]
- Maadane, A.; Merghoub, N.; Ainane, T.; El Arroussi, H.; Benhima, R.; Amzazi, S.; Bakri, Y.; Wahby, I. Antioxidant Activity of Some Moroccan Marine Microalgae: Pufa Profiles, Carotenoids and Phenolic Content. J. Biotechnol. 2015, 215, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Cardoso, C.; Gomes, A.; Campos, A.M.; Falé, P.; Afonso, C.; Bandarra, N.M. Bioprospection of Isochrysis galbana and Its Potential as a Nutraceutical. Food Funct. 2019, 10, 7333–7342. [Google Scholar] [CrossRef] [PubMed]
- Millao, S.; Uquiche, E. Antioxidant Activity of Supercritical Extracts from Nannochloropsis Gaditana: Correlation with Its Content of Carotenoids and Tocopherols. J. Supercrit. Fluids 2016, 111, 143–150. [Google Scholar] [CrossRef]
- Morowvat, M.H.; Ghasemi, Y. Evaluation of Antioxidant Properties of Some Naturally Isolated Microalgae: Identification and Characterization of the Most Efficient Strain. Biocatal. Agric. Biotechnol. 2016, 8, 263–269. [Google Scholar] [CrossRef]
- Pereira, H.; Custódio, L.; Rodrigues, M.J.; De, S.; Oliveira, M.; Barreira, L.; Neng, N.D.R.; Nogueira, J.M.F.; Alrokayan, S.A.; Mouffouk, F.; et al. Biological Activities and Chemical Composition of Methanolic Extracts of Selected Autochthonous Microalgae Strains from the Red Sea. Mar. Drugs 2015, 13, 3531–3549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Subcritical Water Extraction and Characterization of Bioactive Compounds from Haematococcus pluvialis Microalga. J. Pharm. Biomed. Anal. 2010, 51, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Sansone, C.; Galasso, C.; Orefice, I.; Nuzzo, G.; Luongo, E.; Cutignano, A.; Romano, G.; Brunet, C.; Fontana, A.; Esposito, F.; et al. The Green Microalga Tetraselmis suecica Reduces Oxidative Stress and Induces Repairing Mechanisms in Human Cells. Sci. Rep. 2017, 7, 41215. [Google Scholar] [CrossRef]
- Sharma, A.K.; General, T. Variation of Both Chemical Composition and Antioxidant Properties of Newly Isolated Parachlorella kessleri GB1, by Growing in Different Culture Conditions. LWT 2019, 112, 108205. [Google Scholar] [CrossRef]
- Singh, P.; Baranwal, M.; Reddy, S.M. Antioxidant and Cytotoxic Activity of Carotenes Produced by Dunaliella Salina under Stress. Pharm. Biol. 2016, 54, 2269–2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, S.-S.; Yang, E.J.; Lee, S.G.; Youn, U.J.; Han, S.J.; Kim, I.-C.; Kim, S. Bioactivities of Ethanol Extract from the Antarctic Freshwater Microalga, Chloromonas sp. Int. J. Med. Sci. 2017, 14, 560–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, S.-S.; Kim, S.-M.; Kim, J.E.; Hong, J.-M.; Lee, S.G.; Youn, U.J.; Han, S.J.; Kim, I.-C.; Kim, S. Anticancer Activities of Ethanol Extract from the Antarctic Freshwater Microalga, Botryidiopsidaceae sp. BMC Complemen. Altern. Med. 2017, 17, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, K.M.; Habte-Tsion, H.-M.; Thompson, K.R.; Filer, K.; Tidwell, J.H.; Kumar, V. Freshwater Microalgae (Schizochytrium sp.) as a Substitute to Fish Oil for Shrimp Feed. Sci. Rep. 2019, 9, 6178. [Google Scholar] [CrossRef] [Green Version]
- El-Bahr, S.; Shousha, S.; Shehab, A.; Khattab, W.; Ahmed-Farid, O.; Sabike, I.; El-Garhy, O.; Albokhadaim, I.; Albosadah, K. Effect of Dietary Microalgae on Growth Performance, Profiles of Amino and Fatty Acids, Antioxidant Status, and Meat Quality of Broiler Chickens. Animals 2020, 10, 761. [Google Scholar] [CrossRef]
- Long, S.F.; Kang, S.; Wang, Q.Q.; Xu, Y.T.; Pan, L.; Hu, J.X.; Li, M.; Piao, X.S. Dietary Supplementation with DHA-Rich Microalgae Improves Performance, Serum Composition, Carcass Trait, Antioxidant Status, and Fatty Acid Profile of Broilers. Poult. Sci. 2018, 97, 1881–1890. [Google Scholar] [CrossRef]
- Marques, A.E.M.L.; Balen, R.E.; da Silva Pereira Fernandes, L.; Motta, C.M.; de Assis, H.C.S.; Taher, D.M.; Meurer, F.; Vargas, J.V.C.; Mariano, A.B.; Cestari, M.M. Diets Containing Residual Microalgae Biomass Protect Fishes against Oxidative Stress and DNA Damage. J. Appl. Phycol. 2019, 31, 2933–2940. [Google Scholar] [CrossRef]
- Nacer, W.; Baba Ahmed, F.Z.; Merzouk, H.; Benyagoub, O.; Bouanane, S. Evaluation of the Anti-Inflammatory and Antioxidant Effects of the Microalgae Nannochloropsis gaditana in Streptozotocin-Induced Diabetic Rats. J. Diabetes Metab. Disord. 2020, 19, 1483–1490. [Google Scholar] [CrossRef]
- Qiao, H.; Hu, D.; Ma, J.; Wang, X.; Wu, H.; Wang, J. Feeding Effects of the Microalga Nannochloropsis sp. on Juvenile Turbot (Scophthalmus maximus L.). Algal Res. 2019, 41, 101540. [Google Scholar] [CrossRef]
- Rahman, N.A.; Khatoon, H.; Yusuf, N.; Banerjee, S.; Haris, N.A.; Lananan, F.; Tomoyo, K. Tetraselmis Chuii Biomass as a Potential Feed Additive to Improve Survival and Oxidative Stress Status of Pacific White-Leg Shrimp Litopenaeus Vannamei Postlarvae. Int. Aquat. Res. 2017, 9, 235–247. [Google Scholar] [CrossRef] [Green Version]
- Ranga Rao, A.; Raghunath Reddy, R.L.; Baskaran, V.; Sarada, R.; Ravishankar, G.A. Characterization of Microalgal Carotenoids by Mass Spectrometry and Their Bioavailability and Antioxidant Properties Elucidated in Rat Model. J. Agric. Food Chem. 2010, 58, 8553–8559. [Google Scholar] [CrossRef] [PubMed]
- Ranga Rao, A.; Baskaran, V.; Sarada, R.; Ravishankar, G.A. In Vivo Bioavailability and Antioxidant Activity of Carotenoids from Microalgal Biomass—A Repeated Dose Study. Food Res. Int. 2013, 54, 711–717. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Tayefi-Nasrabadi, H.; Khani Oushani, A.; Najafi Enferadi, M.H. Effects of Haematococcus pluvialis Supplementation on Antioxidant System and Metabolism in Rainbow Trout (Oncorhynchus mykiss). Fish. Physiol. Biochem. 2012, 38, 413–419. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the Chemistry, Food Applications, Legislation and Role as Preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission Règlement (UE). 2015/2283 du Parlement Européen et du Conseil du 25 Novembre 2015 Relatif aux Nouveaux Aliments, Modifiant le Règlement (UE) No. 1169/2011 du Parlement Européen et du Conseil et Abrogeant le Règlement (CE) n° 258/97 du Parlement Européen et du Conseil et le Règlement (CE) No. 1852/2001 de la Commission (Texte Présentant de L’intérêt pour l’EEE). Available online: http://data.europa.eu/eli/reg/2015/2283/oj/fra (accessed on 15 May 2020).
- Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; Naska, A.; et al. Guidance on the Preparation and Presentation of an Application for Authorisation of a Novel Food in the Context of Regulation (EU) 2015/2283. EFSA J. 2016, 14, e04594. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, K.A.; Robertson, A. Domoic Acid and Human Exposure Risks: A Review. Toxicon 2010, 56, 218–230. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, C.H.; Bates, S.S.; Martin, J.L.; Haigh, N.; Howland, K.L.; Lewis, N.I.; Locke, A.; Peña, A.; Poulin, M.; Rochon, A.; et al. Three Decades of Canadian Marine Harmful Algal Events: Phytoplankton and Phycotoxins of Concern to Human and Ecosystem Health. Harmful Algae 2021, 102, 101852. [Google Scholar] [CrossRef]
- Kilcoyne, J.; Nulty, C.; Jauffrais, T.; McCarron, P.; Herve, F.; Foley, B.; Rise, F.; Crain, S.; Wilkins, A.L.; Twiner, M.J.; et al. Isolation, Structure Elucidation, Relative LC-MS Response, and in Vitro Toxicity of Azaspiracids from the Dinoflagellate Azadinium Spinosum. J. Nat. Prod. 2014, 77, 2465–2474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CEVA. Macroalgues et Microalgues Alimentaires—Statut Règlementaire en France et en Europe; CEVA: Marseille, France, 2019; p. 15. [Google Scholar]
- European Commission Règlement (UE). No. 68/2013 de la Commission du 16 Janvier 2013 Relatif au Catalogue des Matières Premières pour Aliments des Animaux Texte Présentant de L’intérêt pour l’EEE. Available online: https://eur-lex.europa.eu/legal-content/FR/ALL/?uri=CELEX%3A32013R0068 (accessed on 15 May 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).