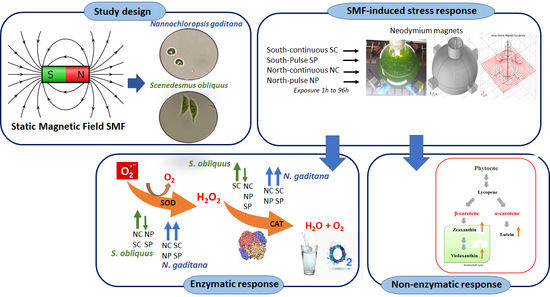
Response to Static Magnetic Field-Induced Stress in Scenedesmus obliquus and Nannochloropsis gaditana
Abstract
:1. Introduction
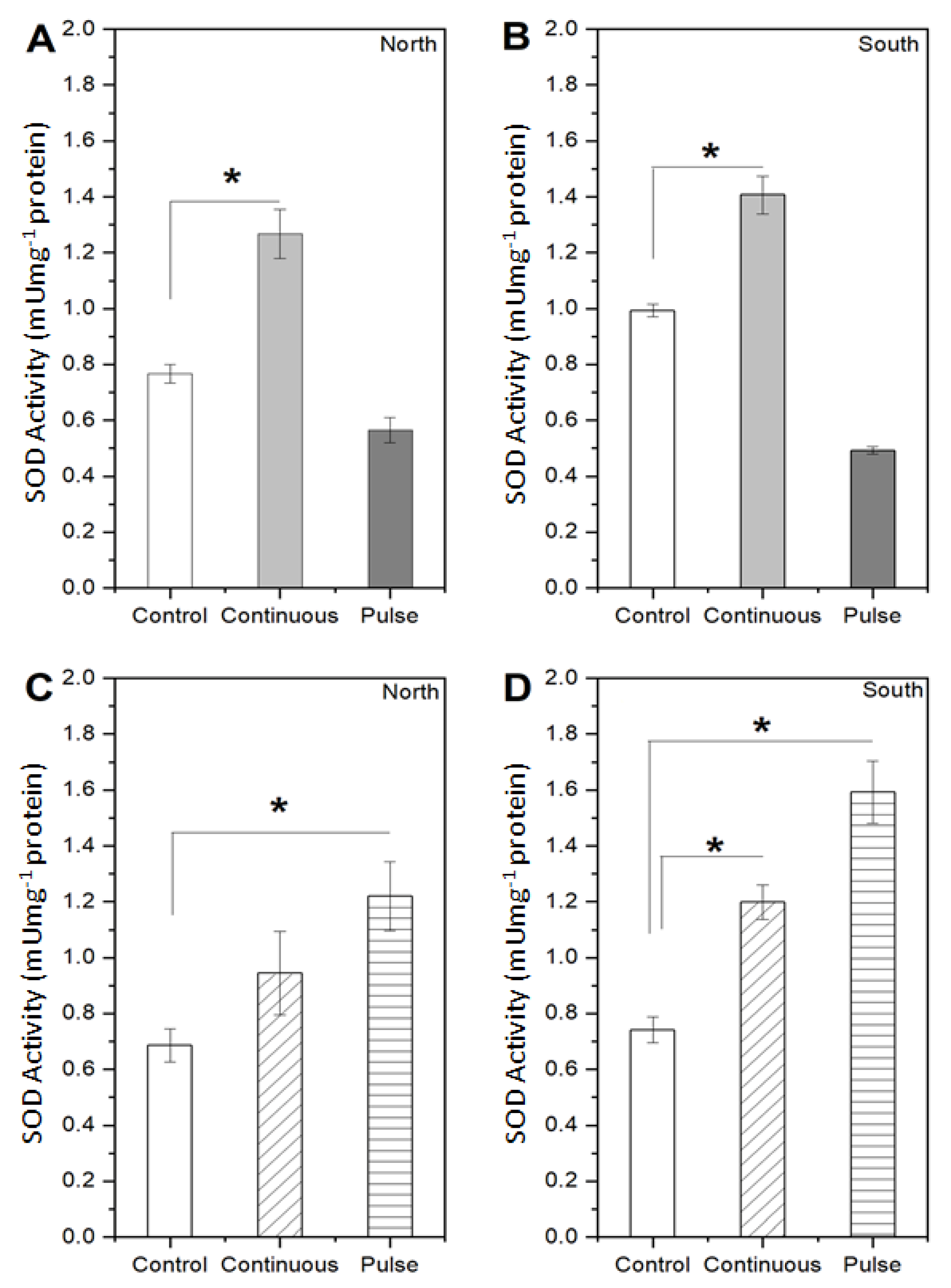
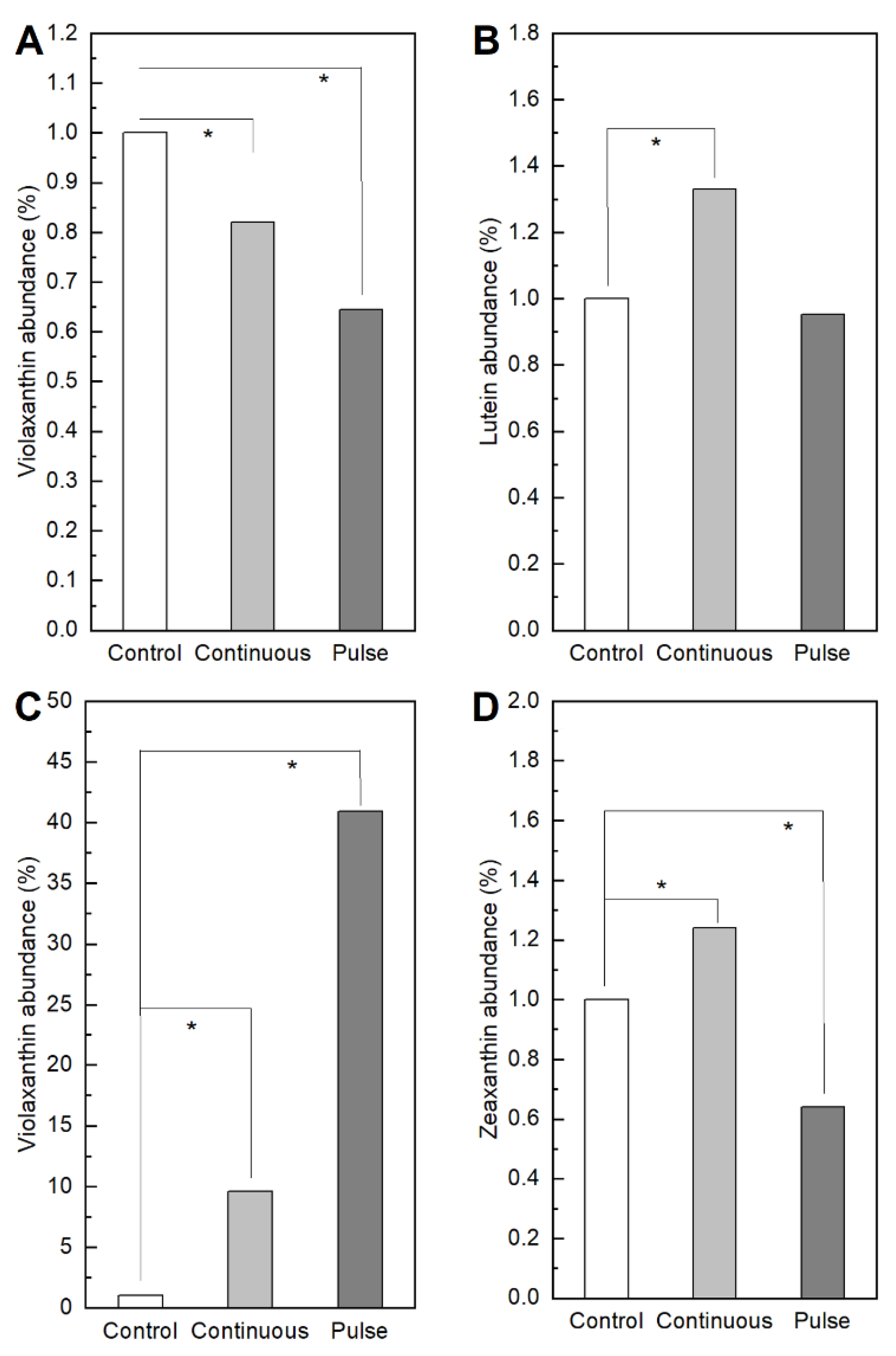
2. Results
2.1. Response of Microalgae to SMF
3. Discussion
4. Materials and Methods
4.1. Algal Strain and Growth Conditions
4.2. Experimental Setup
- Control (C): No magnetic field applied.
- South-continuous (SC): All south poles are oriented towards the center of the flask and continuous exposure to the magnetic flux density ().
- South-pulse (SP): All south poles oriented towards the center of the flask and pulsed exposure to .
- North-continuous (NC): All north poles oriented towards the center of the flask and continuous exposure to .
- North-pulse (NP): All north poles oriented towards the center of the flask and pulsed exposure to .
4.3. Measurement Procedures
4.3.1. Physicochemical Medium Parameters
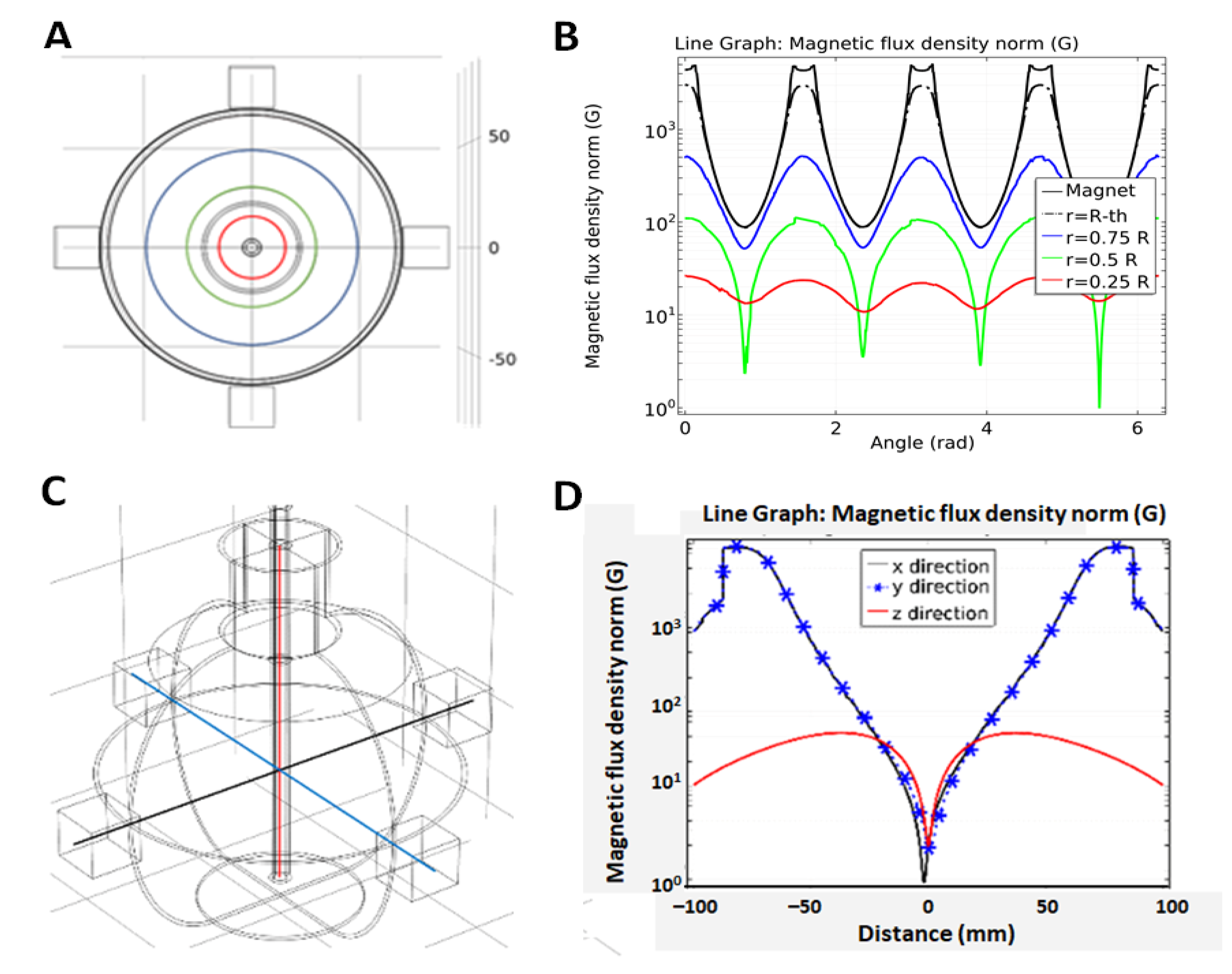
4.3.2. Magnetic Flux Density
4.3.3. Enzymatic Activity
4.3.4. Pigments Determination by Ultra High-Performance Liquid Chromatography (UHPLC) Mass-to-Mass
4.3.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nadkarni, R.; Barkley, S.; Fradin, C. A comparison of methods to measure the magnetic moment of magnetotactic bacteria through analysis of their trajectories in external magnetic fields. PLoS ONE 2013, 8, e82064. [Google Scholar] [CrossRef] [Green Version]
- Günther, A.; Einwich, A.; Sjulstok, E.; Feederle, R.; Bolte, P.; Koch, K.W.; Solov’yov, I.A.; Mouritsen, H. Double-cone localization and seasonal expression pattern suggest a role in magnetoreception for european robin cryptochrome 4. Curr. Biol. 2018, 28, 211–223.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschvink, J.L.; Kobayashi-Kirschvink, A.; Diaz-Ricci, J.C.; Kirschvink, S.J. Magnetite in human tissues: A mechanism for the biological effects of weak ELF magnetic fields. Bioelectromagnetics 1992, 13, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Ghodbane, S.; Lahbib, A.; Sakly, M.; Abdelmelek, H. Bioeffects of static magnetic fields: Oxidative stress, genotoxic effects, and cancer studies. BiomMed Res. Int. 2013, 2013, 602987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, P.; Williams, P.S.; Moore, L.R.; Caralla, T.; Boehm, C.; Muschler, G.; Zborowski, M. Circular halbach array for fast magnetic separation of hyaluronan-expressing tissue progenitors. Anal. Chem. 2015, 87, 9908–9915. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yarema, K.; Xu, A. Impact of static magnetic field (SMFs) on cells. In Biological Effects of Static Magnetic Fields; Springer: Singapore, 2017; pp. 81–132. [Google Scholar] [CrossRef]
- Colbert, A.P.; Wahbeh, H.; Harling, N.; Connelly, E.; Schiffke, H.C.; Forsten, C.; Gregory, W.L.; Markov, M.S.; Souder, J.J.; Elmer, P.; et al. Static magnetic field therapy: A critical review of treatment parameters. Evid. Based Complement. Altern. Med. 2009, 6, 133–139. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, H.; Sun, R.G.; Chen, W.F. An investigation into the combined effect of static magnetic fields and different anticancer drugs on K562 cell membranes. Tumori 2011, 97, 386–392. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T.; Lunov, O.; Dejneka, A. How a high-gradient magnetic field could affect cell life. Sci. Rep. 2016, 6, 37407. [Google Scholar] [CrossRef]
- Steiner, U.E.; Ulrich, T. Magnetic field effects in chemical kinetics and related phenomena. Chem. Rev. 1989, 89, 51–147. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, W.W.C.; Costa, R.M.P.B.; de Salazar e Fernandes, T.; Porto, A.L.F. Evidences of the static magnetic field influence on cellular systems. Prog. Biophys. Mol. Biol. 2016, 121, 16–28. [Google Scholar] [CrossRef]
- Small, D.P.; Hüner, N.P.A.; Wan, W. Effect of static magnetic fields on the growth photosynthesis and ultrastructure of Chlorella kessleri microalgae. Bioelectromagnetics 2012, 33, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M. Magnetic stimulation on the growth of the microalga Nannochloropsis oculata. In Electronic Thesis and Dissertation Repository; The University of Western Ontario: London, ON, Canada, 2017; p. 4597. Available online: http://ir.lib.uwo.ca/etd/4597 (accessed on 25 July 2021).
- Tu, R.; Jin, W.; Xi, T.; Yang, Q.; Han, S.F.; Abomohra, A.E.F. Effect of static magnetic field on the oxygen production of Scenedesmus obliquus cultivated in municipal wastewater. Water Res. 2015, 86, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zeng, X.; Guo, S.; Li, Z. Effects of magnetic field on the antioxidant defense system of recirculation-cultured Chlorella vulgaris. Bioelectromagnetics 2008, 29, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, H.; Li, Q.; Zhang, J. Effects of static magnetic field on Chlorella vulgaris: Growth and extracellular polysaccharide (EPS) production. J. Appl. Phycol. 2020, 32, 2819–2828. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Ajayan, K.V.; Selvaraju, M. Heavy metal induced antioxidant defense system of green microalgae and its effective role in phycoremediation of tannery effluent. Pak. J. Biol. Sci. 2012, 15, 1056–1062. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Mandotra, S.K.; Kumar, P.; Suseela, M.R.; Ramteke, P.W. Fresh water green microalga Scenedesmus abundans: A potential feedstock for high quality biodiesel production. Bioresour. Technol. 2014, 156, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Chan, M.C.; Liu, C.C.; Chen, C.Y.; Lee, W.L.; Lee, D.J.; Chang, J.S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Deepika, G.; Ravishankar, G.; Sarada, R.; Panduranga, N.B.; Lei, B.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef]
- Solovchenko, A.; Lukyanov, A.; Solovchenko, O.; Didi-Cohen, S.; Boussiba, S.; Khozin-Goldberg, I. Interactive effects of salinity, high light, and nitrogen starvation on fatty acid and carotenoid profiles in Nannochloropsis oceanica CCALA 804. Eur. J. Lipid Sci. Technol. 2014, 166, 635–644. [Google Scholar] [CrossRef]
- Ferrada, P.; Rodríguez, S.; Serrano, G.; Miranda-Ostojic, C.; Maureira, A.; Zapata, M. An analytical–experimental Approach to quantifying the effects of static magnetic fields for cell culture applications. Appl. Sci. 2020, 10, 531. [Google Scholar] [CrossRef] [Green Version]
- Yamaoka, Y.; Takimura, O.; Fuse, H.; Kaminura, K. Effect of magnetism on growth of Dunaliella salina. Res. Photosynth. 1992, 3, 87–90. [Google Scholar]
- Hunt, R.W.; Zavalin, A.; Bhatnagar, A.; Chinnasamy, S.; Das, K.C. Electromagnetic biostimulation of living cultures for biotechnology, biofuel and bioenergy applications. Int. J. Mol. Sci. 2009, 10, 4515–4558. [Google Scholar] [CrossRef] [PubMed]
- Gebicka, L.; Krych-Madej, J. The role of catalases in the prevention/promotion of oxidative stress. J. Inorg. Biochem. 2019, 197, 110699. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Barroso, J.B. Lead-induced stress, which triggers the production of nitric oxide (NO) and superoxide anion (O2−) in Arabidopsis peroxisomes, affects catalase activity. Nitric Oxide Biol. Chem. 2017, 68, 103–110. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Salinity induced oxidative stress alters the physiological responses and improves the biofuel potential of green microalgae Acutodesmus dimorphus. Bioresour. Technol. 2017, 244, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Bux, F.; Chisti, Y. Algae Biotechnology: Products and Processes; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-12334-9. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zeng, X.B.; Guo, S.Y. Growth of Chlorella vulgaris under different magnetic treatments. Prog. Mod. Biomed. 2006, 6, 106–108. [Google Scholar]
- Katz, E.; Lioubashevski, O.; Willner, I. Magnetic field effects on bioelectrocatalytic reactions of surface-confined enzyme systems: enhanced performance of biofuel cells. J. Am. Chem. Soc. 2005, 127, 3979–3988. [Google Scholar] [CrossRef]
- Lu, H.; Wang, X.; Hu, S.; Han, T.; He, S.; Zhang, G.; He, M.; Lin, X. Bioeffect of static magnetic field on photosynthetic bacteria: Evaluation of bioresources production and wastewater treatment efficiency. Water Environ Res. 2020, 92, 1131–1141. [Google Scholar] [CrossRef]
- Guo, J.; Peng, J.; Lei, Y.; Kanerva, M.; Li, Q.; Song, J.; Guo, J.; Sun, H. Comparison of oxidative stress induced by clarithromycin in two freshwater microalgae Raphidocelis subcapitata and Chlorella vulgaris. Aquat. Toxicol. 2020, 219, 105376. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; Trombini, C.; Crespo, E.; Blasco, J.; Moreno-Garrido, I. ROI-scavenging enzyme activities as toxicity biomarkers in three species of marine microalgae exposed to model contaminants (copper, Irgarol and atrazine). Ecotoxicol. Environ. Saf. 2014, 104, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front. Microbiol. 2016, 7, 546. [Google Scholar] [CrossRef] [Green Version]
- Bold, H.C. The morphology of Chlamydomonas chlamydogama, sp. nov. Bull. Torrey Bot. Club 1949, 76, 101. [Google Scholar] [CrossRef]
- Bischoff, H.W.; Bold; Harold, C. Some Soil Algae from Enchanted Rock and Related Algal Species; University of Texas: Austin, TX, USA, 1963. [Google Scholar]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Bottura, L.; Henrichsen, K.N. Field Measurements; CAS–CERN Accelerator School: Superconductivity and Cryogenics for Accelerators and Detectors: Geneva, Switzerland, 2002; pp. 118–148. [Google Scholar] [CrossRef]
- Popovic, R. Not-plate-like Hall magnetic sensors and their applications. Sens. Actuators A Phys. 2000, 85, 9–17. [Google Scholar] [CrossRef]
- Yang, Z.J.; Johansen, T.H.; Bratsberg, H.; Helgesen, G.; Skjeltorp, A.T. Potential and force between a magnet and a bulk Y1Ba2Cu3O7-δsuperconductor studied by a mechanical pendulum. Supercond. Sci. Technol. 1990, 3, 591–597. [Google Scholar] [CrossRef]
- Camacho, J.M.; Sosa, V. Alternative method to calculate the magnetic field of permanent magnets with azimuthal symmetry. Rev. Mex. Fis. E 2013, 59, 8–17. [Google Scholar]
- Furlani, E.P.; Furlani, E.P. Permanent magnet applications. In Perm. Magn. Electromech. Devices; Elsevier, B.V.: Amsterdam, The Netherlands, 2001; pp. 207–333. ISBN 978-0-12-269951-1. [Google Scholar] [CrossRef]
- ITTC Recommended Procedures. The Specialist Committee on Uncertainty Analysis. In Proceedings of 26th International Towing Tank Conference, Rio de Janeiro, Brazil, 28 August–3 September 2011; Fresh Water and Seawater Properties. Available online: http://ittc.info/media/5526/08.pdf (accessed on 25 July 2021).
- Schlichting, H.; Gersten, K. Boundary Layer Theory, 9th ed.; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9783662570951. [Google Scholar]
- Davidson, P.A. Kinematics of MHD: Advection and diffusion of a magnetic field. In An Introduction to Magnetohydrodynamics; Cambridge University Press: Cambridge, UK, 2010; pp. 102–116. ISBN 9780511626333. [Google Scholar]
- Janknegt, P.J.; Rijstenbil, J.W.; van de Poll, W.H.; Gechev, T.S.; Buma, A.G.J. A comparison of quantitative and qualitative superoxide dismutase assays for application to low temperature microalgae. J. Photochem. Photobiol. B Biol. 2007, 87, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Cartes, P.; McManus, M.; Wulff-Zottele, C.; Leung, S.; Gutiérrez-Moraga, A.; de la Luz Mora, M. Differential superoxide dismutase expression in ryegrass cultivars in response to short term aluminium stress. Plant Soil 2012, 350, 353–363. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Donahue, J.L.; Okpodu, C.M.; Cramer, C.L.; Grabau, E.A.; Alscher, R.G. Responses of antioxidants to paraquat in pea leaves (relationships to resistance). Plant Physiol. 1997, 113, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Pinhero, R.G.; Rao, M.V.; Paliyath, G.; Murr, D.P.; Fletcher, R.A. Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings. Plant Physiol. 1997, 114, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Fu, W.; Magnúsdóttir, M.; Brynjólfson, S.; Palsson, B.; Paglia, G. UPLC-UV-MSE analysis for quantification and identification of major carotenoid and chlorophyll species in algae. Anal. Bioanal. Chem. 2012, 404, 3145–3154. [Google Scholar] [CrossRef]



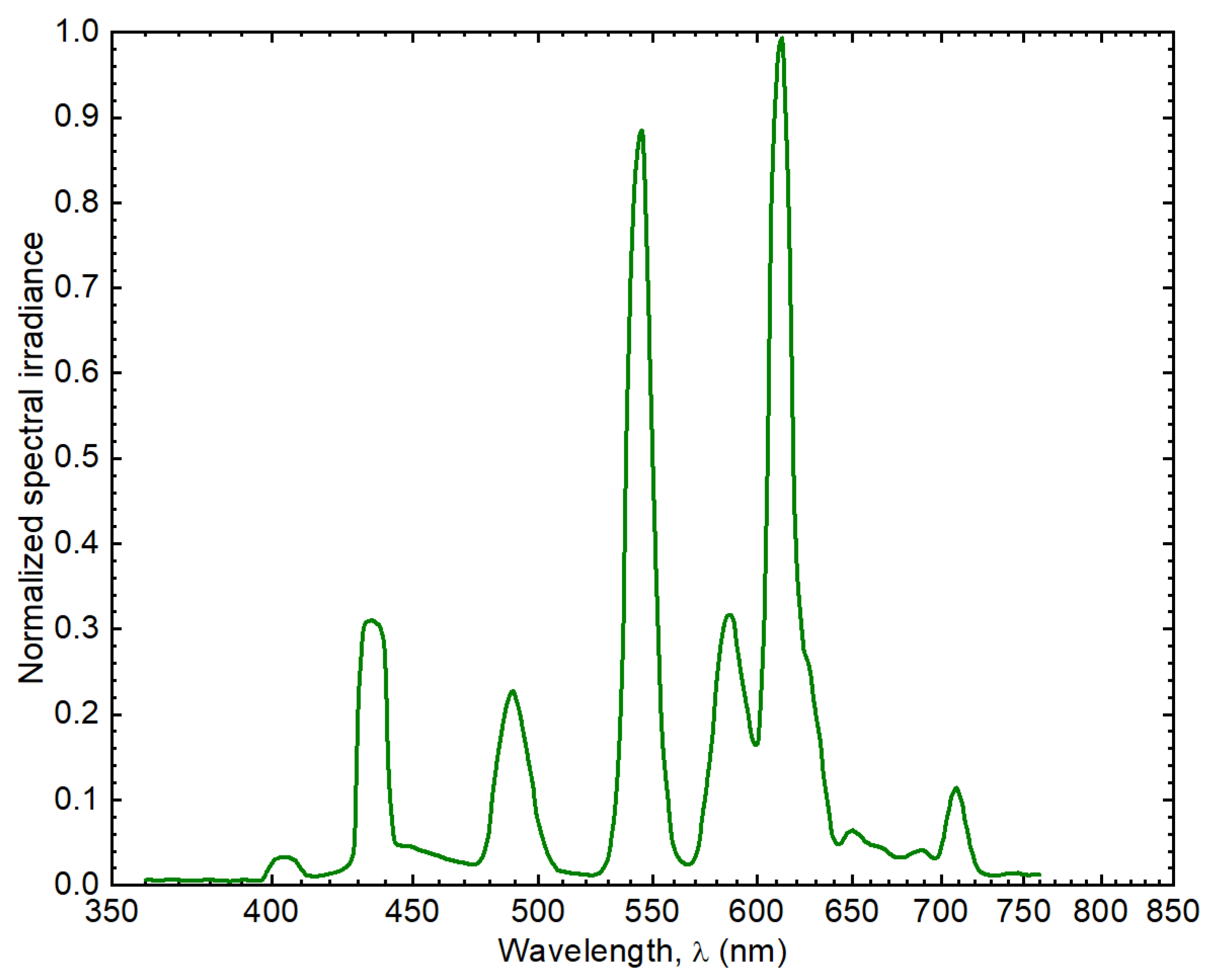
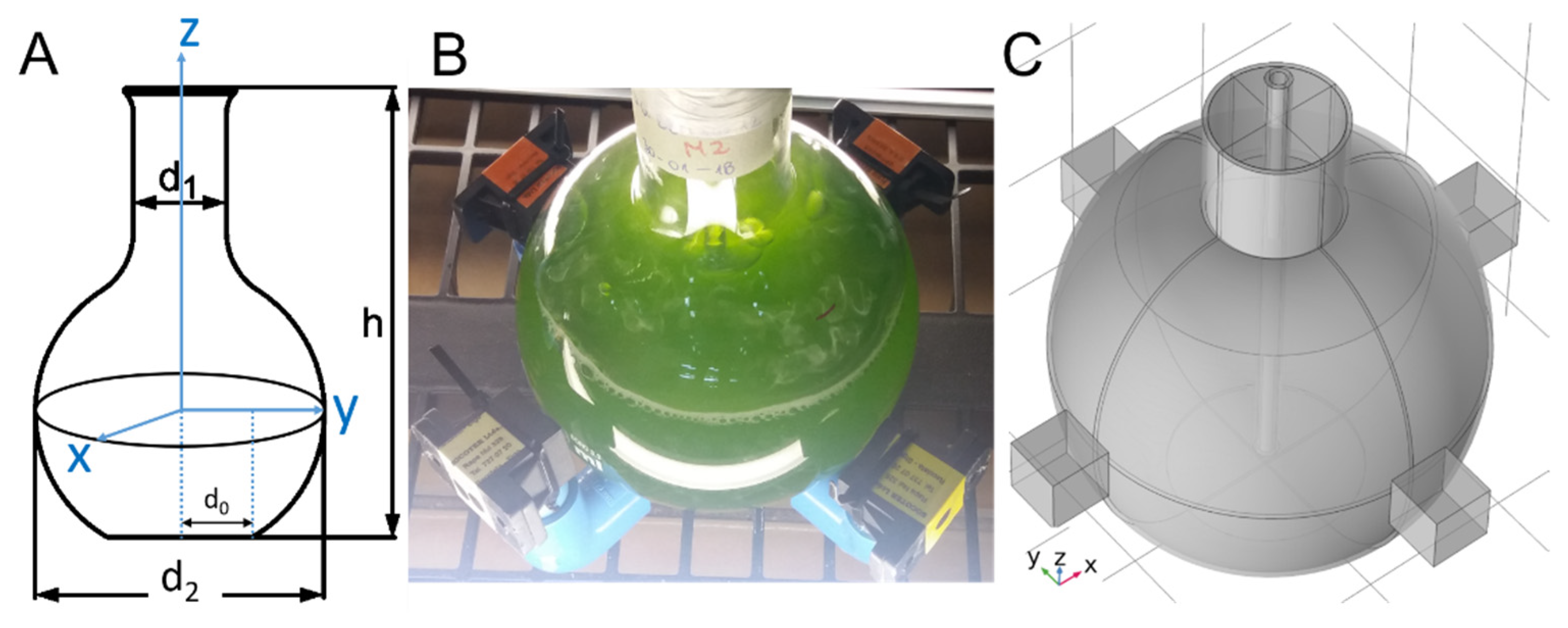
| Species | Treatment | T (°C) | pH | σ (S/m) | S (PSU) | DO (%) |
|---|---|---|---|---|---|---|
| S. obliquus | Control for North | 21.1 ± 2.3 | 8.7 ± 0.8 | 0.09 ± 0.01 | 0.47 ± 0.07 | 6.5 ± 0.9 |
| NC | 21.0 ± 2.4 | 8.5 ± 0.8 | 0.1 ± 0.01 | 0.49 ± 0.05 | 6.8 ± 0.9 | |
| NP | 21.0 ± 2.5 | 9.0 ± 1.1 | 0.09 ± 0.01 | 0.46 ± 0.08 | 6.6 ± 1.1 | |
| Control for South | 22.8 ± 0.3 | 8.9 ± 0.5 | 0.10 ± 0.01 | 0.48 ± 0.05 | 5.7 ± 0.3 | |
| SC | 23.0 ± 0.5 | 8.6 ± 0.7 | 0.09 ± 0.03 | 0.42 ± 0.14 | 5.9 ± 0.6 | |
| SP | 23.0 ± 0.8 | 8.8 ± 0.8 | 0.09 ± 0.02 | 0.47 ± 0.11 | 6.0 ± 0.6 | |
| N. gaditana | Control for North | 21.2 ± 1.4 | 8.4 ± 0.4 | 3.7 ± 0.4 | 23.7 ± 2.5 | 5.2 ± 0.3 |
| NC | 21.3 ± 1.8 | 8.6 ± 0.2 | 4.4 ± 0.6 | 28.5 ± 4.6 | 5.4 ± 0.3 | |
| NP | 21.3 ± 1.5 | 8.6 ± 0.2 | 4.4 ± 0.6 | 28.9 ± 4.5 | 5.2 ± 0.4 | |
| Control for South | 21.1 ± 0.2 | 8.6 ± 0.2 | 4.2 ± 0.9 | 27.5 ± 6.3 | 5.0 ± 0.3 | |
| SC | 21.3 ± 0.1 | 8.6 ± 0.2 | 4.3 ± 0.6 | 28.2 ± 4.1 | 5.1 ± 0.2 | |
| SP | 21.2 ± 0.2 | 8.6 ± 0.2 | 5.0 ± 0.6 | 33.0 ± 4.4 | 4.9 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, G.; Miranda-Ostojic, C.; Ferrada, P.; Wulff-Zotelle, C.; Maureira, A.; Fuentealba, E.; Gallardo, K.; Zapata, M.; Rivas, M. Response to Static Magnetic Field-Induced Stress in Scenedesmus obliquus and Nannochloropsis gaditana. Mar. Drugs 2021, 19, 527. https://doi.org/10.3390/md19090527
Serrano G, Miranda-Ostojic C, Ferrada P, Wulff-Zotelle C, Maureira A, Fuentealba E, Gallardo K, Zapata M, Rivas M. Response to Static Magnetic Field-Induced Stress in Scenedesmus obliquus and Nannochloropsis gaditana. Marine Drugs. 2021; 19(9):527. https://doi.org/10.3390/md19090527
Chicago/Turabian StyleSerrano, Génesis, Carol Miranda-Ostojic, Pablo Ferrada, Cristian Wulff-Zotelle, Alejandro Maureira, Edward Fuentealba, Karem Gallardo, Manuel Zapata, and Mariella Rivas. 2021. "Response to Static Magnetic Field-Induced Stress in Scenedesmus obliquus and Nannochloropsis gaditana" Marine Drugs 19, no. 9: 527. https://doi.org/10.3390/md19090527
APA StyleSerrano, G., Miranda-Ostojic, C., Ferrada, P., Wulff-Zotelle, C., Maureira, A., Fuentealba, E., Gallardo, K., Zapata, M., & Rivas, M. (2021). Response to Static Magnetic Field-Induced Stress in Scenedesmus obliquus and Nannochloropsis gaditana. Marine Drugs, 19(9), 527. https://doi.org/10.3390/md19090527